1. Introduction
A plant microbiome consists of a diverse community of microorganisms, including bacteria, fungi, viruses, and archaea, that live are associated with various plant species. These microorganisms have distinct habitats in and around the plant, such as the rhizosphere (the soil near the roots), the phyllosphere (leaf surfaces), the endosphere (within plant tissues), and the spermosphere (around seeds) [
1]. The plant microbiome significantly impacts plant health, growth, and development, contributing to various processes including soil health, nutrient management, abiotic/biotic stress tolerance, and disease resistance, while supporting a more balanced and sustainable agroecosystem [
2,
3,
4].
A sustainable agroecosystem is a complex and dynamic system that integrates ecological processes with an agricultural landscape to optimize resource utilization, enhance biodiversity, and maintain long-term productivity while ensuring economic viability and environmental stewardship [
5,
6,
7,
8]. Mycorrhizal fungi are particularly significant among various microorganisms due to their symbiotic associations with host plants [
9]. Mycorrhizal fungi have been demonstrated to confer protection to host plants against various environmental stressors, including toxic metal toxicity [
10], root pathogens [
11], salinity, drought [
12], acidity [
13], and temperature fluctuations [
10]. Furthermore, mycorrhizal fungi enhance plant nutrient uptake and promote overall plant growth and development [
14]. The multifaceted benefits provided by mycorrhizal fungi to their host plants contribute significantly to the resilience and sustainability of agroecosystems.
The term ‘mycorrhiza’ comes from the Greek words for “fungus” and “root”, which describes various diverse associations between plant roots and fungi [
15]. The most common and well-known type of mycorrhizal association is a symbiotic relationship, where host plants provide sugar to the fungi, and the fungi provide the mineral nutrients to the plants [
16]. Mycorrhizal fungi have colonized the roots of about 240,000 plant species so far, and the association between them is identified as an important phenomenon in the biology and ecology of many terrestrial plants as they affect the growth of plants, water and nutrient uptake and interact with the root diseases [
17]. There are two types of mycorrhizas, ‘endomycorrhiza’ and ‘ectomycorrhiza,’ which are differentiated according to the plant's taxonomic status and type of fungi. Ectomycorrhiza are especially found in trees and shrubs. The hyphae are extracellular, causing little changes in the epidermis. At the same time, endomycorrhizas i.e. arbuscular (AM), ericoid, and orchid mycorrhizas are intracellular, and the hyphae penetrate the root cells [
15]. The mycorrhiza plays an important role in giving plants access to growth-limiting nutrients that help in their proper growth and development [
18]. They can directly affect the mineral nutrient regime of the host plants through nutrient supplements to the plants by fungus or indirectly by modification in the rate of transpiration and composition of rhizosphere microflora [
19]. Vesicular-arbuscular mycorrhizal associations (VAM), also called Arbuscular mycorrhiza (AM), are the most common root-fungus that can uptake the mineral nutrients from the soils and provide those nutrients to plants in compensation for photosynthetically fixed carbon [
9,
20]. They also protect plants from pathogens and biotic and abiotic stresses [
21]. VAM can take and deliver up to 80% of plant P, 25% of plant N, 10% of plant K, 25% of plant Zn, and 60% of plant Cu [
19]. Ectomycorrhizal Fungi increase the uptake rates of nutrients by the plant roots with the help of various mechanisms like the release of organic acids and production of enzymes that will change the chemistry of the mycorrhizosphere, increasing physical access to soil and by changing the microbial community in the mycorrhizosphere [
22]. Mycorrhizal fungi can affect soil fertility in different aspects and can be influenced by soil fertility as well [
23]. Thus, the objective of this review paper is to discuss the mechanism through which these mycorrhizas contribute to soil fertility and sustainable agroecosystems.
2. Types of Mycorrhizal Fungi
Mycorrhizal associations are typically categorized into three main types: arbuscular mycorrhiza (AM), ectomycorrhiza (ECM), and ericoid mycorrhiza (ERM), each with distinct characteristics and host ranges [
24].
2.1. Arbuscular mycorrhiza (AM) is primarily associated with the fungal phylum ‘Zygomycota’, forms symbioses with a diverse range of plant taxa, including angiosperms, gymnosperms, pteridophytes, and some lower plants [
25]. The defining feature of AM fungi is the formation of highly branched intracellular structures called arbuscules within the cortical cells of the host plant [
26]. These fungi also develop an extensive extraradical hyphal network extending from the root surface into the surrounding soil matrix, significantly enhancing the plant's capacity for nutrient absorption [
27].
2.2 Ectomycorrhizal (ECM) is associations involve fungal species predominantly from the phyla Basidiomycota, Ascomycota, and Zygomycota [
28]. ECM fungi primarily colonize woody perennials, including trees and shrubs, as well as certain herbaceous plants [
25]. The main feature of ECM associations is the formation of a dense hyphal sheath, or mantle, encasing the fine roots of the host plant. Additionally, these fungi develop a complex intercellular network known as the Hartig net, which penetrates between the epidermis and cortical cells [
29]. ECM fungi also establish an extensive extraradical mycelium in the soil. Beyond increasing the surface area for nutrient uptake, ECM fungi secrete proteases and phosphatases, facilitating the acquisition of organic nitrogen and phosphorus [
30]. Moreover, they produce plant cell wall-degrading enzymes, further enhancing access to these organic nutrient sources [
31].
2.3 Ericoid mycorrhiza (ERM) froms the fungal phylum ‘Ascomycota’, which are specific to plants within the order Ericales [
25]. The distinguishing feature of ERM is the development of extensive hyphal coils within the epidermal cells of the host plant roots [
32]. Unlike AM and ECM, ERM fungi exhibit limited extraradical hyphal growth. However, they secrete proteases and phosphatases, that enable the mobilization of organic nitrogen and phosphorus [
33]. Furthermore, ERM fungi produce an array of enzymes, including those capable of degrading plant cell walls and oxidizing phenolic compounds, which are particularly effective in accessing organic nutrients sequestered in humic substances [
34].
3. Formation of AM Symbiosis
The AM fungi form endosymbiosis with most flowering plants, where the branched hyphae called arbuscules are formed within the cortical cells and colonize the root cortex. The symbiotic interface between the symbionts, fungus, and host plants is the pathway for nutrient exchange [
35]. The lifecycle of a mycorrhizal association begins with the dispersal of fungal propagules in the soil. Plant roots release soluble or volatile substances that attract the fungal propagules toward them. Active soil hyphae proliferate on the root surface, aided by appressoria in VAM and mantle in ectomycorrhiza (ECM). They penetrate into or between root cells, forming an exchange site with branched structures like arbuscules in VAM and Hartig net in ECM. The exchange of nutrients between host and fungus is a complex process, heavily influenced by the interaction between host, fungus, and environment. This interaction dictates the duration of the exchange processes, leading to the eventual senescence of hyphal structures and the formation of the resting spores by the fungal propagule in soil or root [
36].
At the cellular level, the exchange of nutrients between the plant host and the mycorrhizal fungi is mediated by transport proteins present in the cell membranes of both organisms. The AM fungi were found to induce the expression of phosphate transporters that help in the phosphate acquisition from the soil and transfer it to the plant [
37]. The host plants, in turn, induce the expression of sugar transporters that facilitate carbohydrate/sucrose flux from the host plants to the fungus [
38]. The plants maintain fungal colonization by regulating the expression of genes responsible for defense mechanisms [
37], while the AM fungi regulate the nutrient uptake and transfer based on the nutrient status of the host plants [
39]. This regulation at the cellular level makes the plant-fungal symbiosis efficient and adapts dynamically to meet the requirements of each organism.
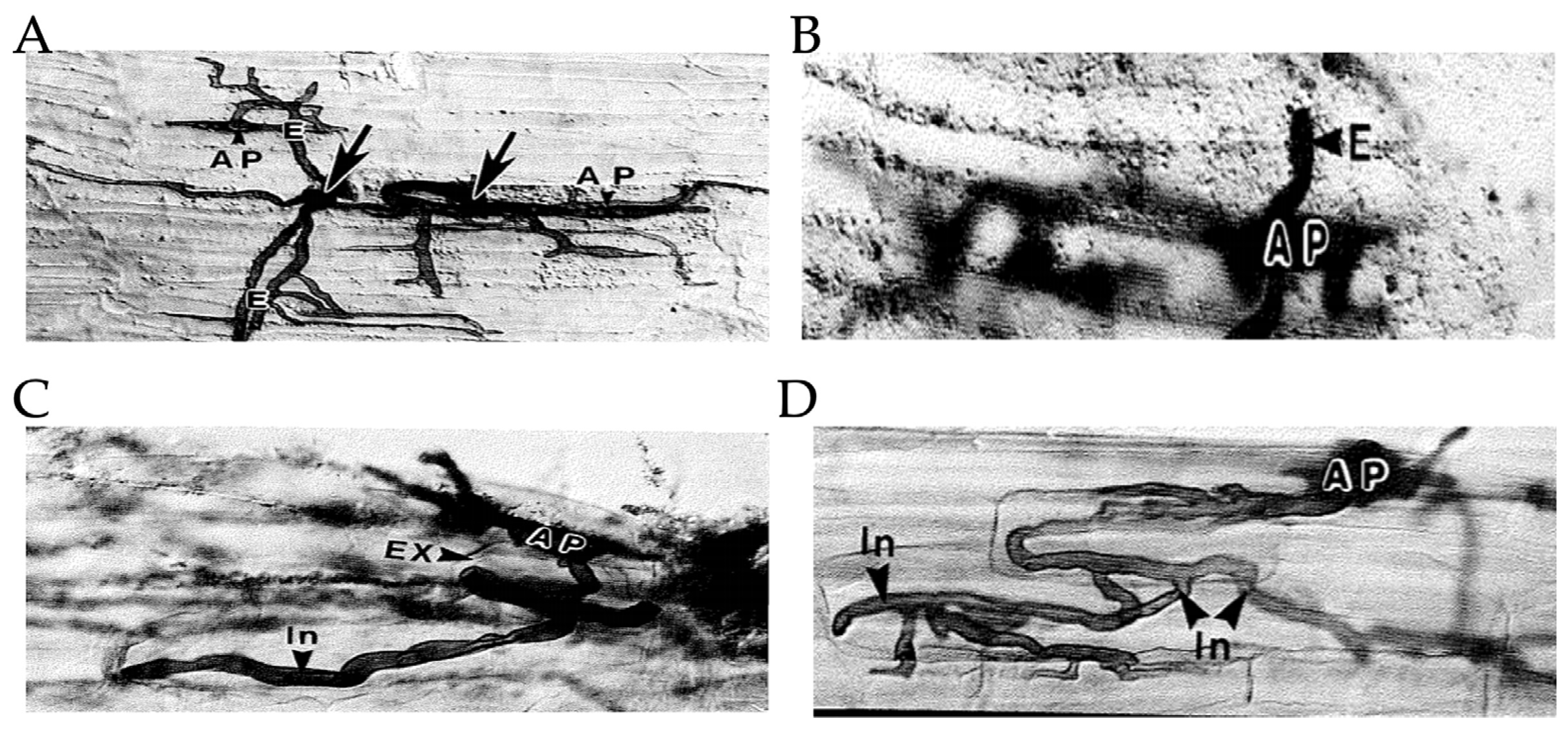
Brundrett et al. [
40] experimented with leek roots to study the early stages of VAM formation. They transplanted the leek seedlings into a pot culture containing the inoculum, and data on the different stages of the VAM formation was collected in 2-day intervals after exposure of the leek seedlings with inoculum. They found infection initiation in the roots by external hyphae in about 1 day (
Figure 1A), penetration of hyphae in two days (
Figure 1B), arbuscule formation in 3-4 days (
Figure 1C), and vesicle formation in 4-5 days (
Figure 1D). The following figure shows the formation of VAM as observed with Nomarski interference contrast optics.
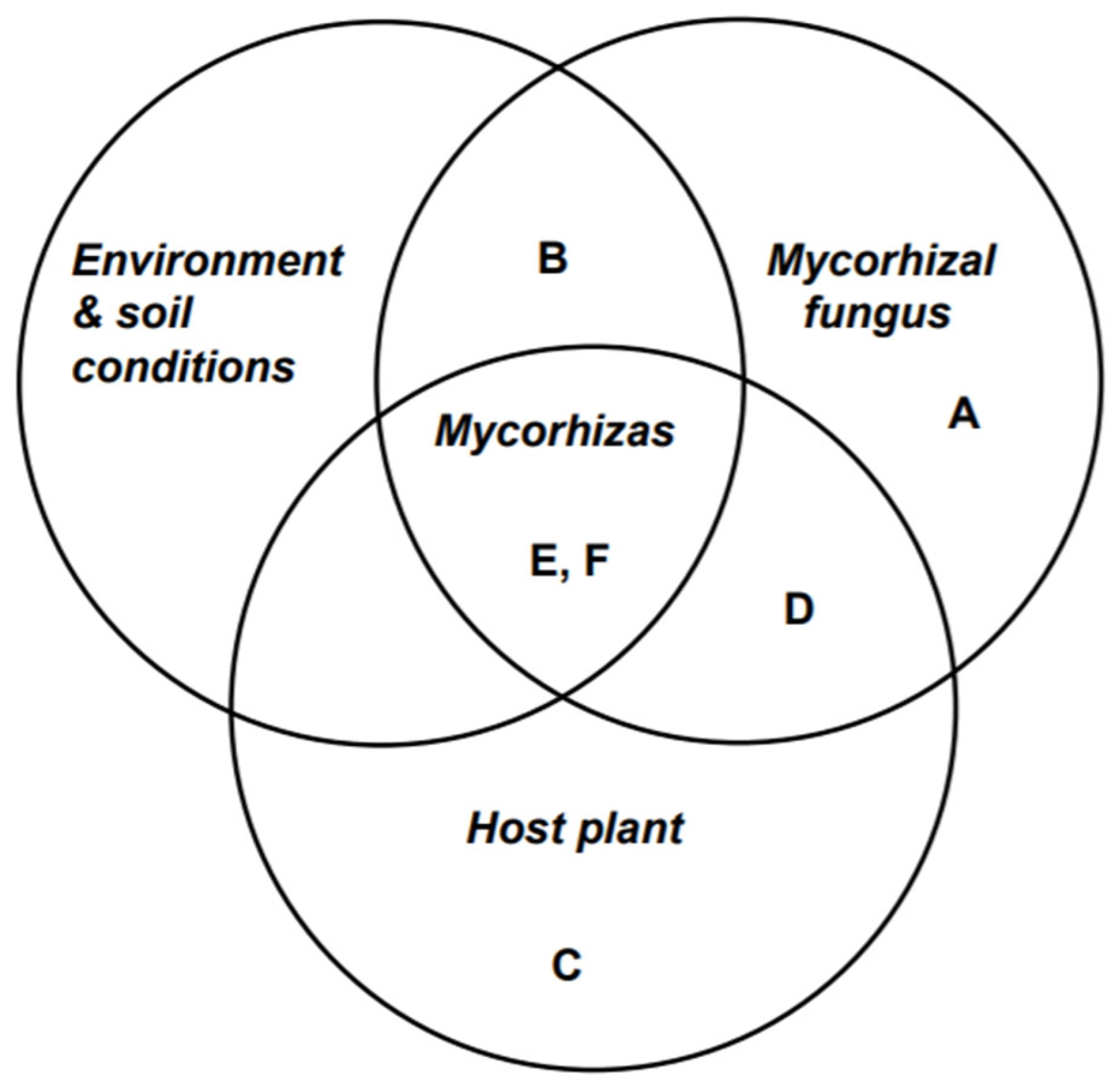
4. Factors Affecting Mycorrhizal Association
Some factors that affect the effectiveness of mycorrhizal associations are climate, root properties, organisms present in the soil, soil condition, host-fungus relationship, nutrient competition between mycorrhizal and non-mycorrhizal plants, mycorrhizal interaction with rhizosphere, allelopathy, pollution and other stresses [
36]. In general, this relation is shown through a three-way interaction Venn diagram as in
Figure 2 [
36].
4.1. Effect of Host Plant on Mycorrhizal Interaction
Mycorrhizal fungi are the group of fungi present in or within the roots of host plants quite consistently sharing the common space with the exchange of water and nutrients called mutualistic symbiosis [
41]. Arbuscular mycorrhizal fungi are the most common type of mycorrhiza and are named after the arbuscules and vesicles. Arbuscules are the penetrating hyphae that branch to form a complex branched structure giving a bush-like structure. Arbuscules act as a passage to pass the materials between the symbionts along with other simpler hyphae [
42]. The hyphae within the roots are connected to the external mycelium, which acts as an infection source and probably helps exchange materials between symbionts. The fungus gets carbon compounds from its host, absorbs nutrients from the soil (especially phosphate), and passes a certain amount of that nutrient to the host plants. Also, plant roots produce a hormone called strigolactones that helps in fungal metabolism and branching [
26,
43]. Strigolactones are a class of sesquiterpene lactones derived from carotenoids [
44]. AM fungi can detect these molecules very easily [
45] and respond by increasing their metabolic activity growing towards them and branching extensively [
46]. Different plant species' affinity to attract AM fungi varies depending on the types and amount of strigolactones they release [
47]. Crops like rice and sorghum [
48,
49] were found to be more attractive to AM fungi than tomato and lettuce [
50,
51] which correlated with their higher strigolactone exudation. Also, the amount of N and P around the rhizosphere affects the production of strigolactones. Strigolactone production was higher in rice under mineral deficiency conditions while an increase in the N and P decreased the amount of strigolactones in the exudates(
Table 1) [
52].
Table 1 summarize the root exudate compounds and AM fungi species attracted by them.
The mycorrhizal fungi are non-specific and infect various host plants where some hosts are also found to be related to more than one species of fungus [
42]. Various research has found a significant positive correlation between mycorrhizal root colonization and plant responsiveness, particularly in terms of root hair length and incidence while a negative correlation was observed with root diameter in early successional species. This relationship suggests that an increase in AM fungi hyphae in soil is likely with an increase in the root area for nutrient uptake. Conversely, late successional species were found to have larger root tissue densities that were more resistant to AM hyphae penetration hence causing the decrement in mycorrhizal root colonization [
56].
Liu et al. [
57] found that high fertilizer application in an alpine meadow ecosystem caused a dramatic loss of
Glomus species, but a significant increase in genus richness. This was attributed to the competition between the AM fungal communities for photosynthate from host plants. The response of plant growth to mycorrhizal inoculation varies within an ecosystem, ranging from parasitism to mutualism. The sensitivity of mycorrhizal species is more pronounced in native AMF than in exotic AMF, underscoring the crucial role of mycorrhizal fungi in the ecosystem [
58].
4.2. Effect of Abiotic Factors on Plant-Mycorrhiza Interactions
Abiotic factors such as climate, drought, pollution, pH, and organic matter content can affect mycorrhizal populations, and ultimately, soil health and crop production [
59]. Climate change and conventional agricultural practices like synthetic fertilizers and pesticides have increased the effects of abiotic stresses on crop plants and hampered the quality and productivity of the crop [
10]. Lenoir et al. [
60] discussed the role of AM fungi in tolerance against various abiotic stresses (pollutants, salinity, drought, extreme temperatures, CO
2, acidity) through various mechanisms like morphological adaptation, production of antioxidants, chaperone proteins, and trehalose to protect cells against damage.
A comprehensive evaluation of the population of AM fungi in the semi-arid agroecosystem of North Jordan revealed that abiotic factors and cropping patterns significantly influenced the population of AM fungal species. They discovered a noteworthy positive correlation between the spore density and organic matter (OM) and CaCO
3 percentages. Additionally, they observed a weak correlation between spore density with decreasing soil pH and increasing electrical conductivity (EC). Conversely, they found a negative correlation between spore density and soil phosphorus (
Table 2) [
61].
AMF protects host plants against salt stress through various mechanisms, such as the accumulation of osmolytes to reduce the osmotic potential of the cell sap, increase in nutrient uptake, increase in water uptake, high K
+/Na
+ ratio that helps in ionic balance, and antioxidant production that helps host plants from oxidative damage (
Table 2) [
12].
Porcel et al. [
62] discussed the mechanisms like improved host plant nutrition, K
+/Na
+ ratios, osmotic adjustment, and accumulation of solutes like proline and soluble sugars exhibited by mycorrhizal plants that help host plants from salinity stress. They also discussed the regulation of plant genes involved in the biosynthesis of proline and aquaporins by AM symbiosis that helps maintain water status in the tissues of mycorrhizal plants. Hajiboland et al. [
63] found that mycorrhiza-inoculated tomato plants have higher Ca/Na and K/Na ratios and elevated stomatal conductance that protects host plants against salt tolerance. Mycorrhization was found to increase the uptake of P, Ca, and K, which has been reduced by the salt stress in tomato plants (
Table 2).
An experiment by Mosse [
13] found that the plant growth response of phosphorus applied to soil depends on the soil type. Toxic phosphorus concentration was found to be lower in light or sandy soil where added phosphate quickly becomes unavailable to plants. Plant P was found to increase slowly with an increase in the amount of phosphate in mycorrhizal plants in clayey soil and is found to perform better in mycorrhizal plants than non-mycorrhizal plants. On the other hand, there was a rapid increase of plant P in mycorrhizal plants in sandy soils which were only better with small amounts of added phosphate, which would cause toxic P concentration too quickly. It shows that mycorrhizal plants' roots absorb more phosphate to reach the optimum level of P even with a small addition of phosphate (
Table 2).
A report on the differences in the accumulation of proline in leaves of lettuce inoculated with different fungal species suggests that the fungi were able to induce different degrees of osmotic adjustment and help the host plant tolerate drought stress (
Table 2) [
64]
Begum et al. [
10] discussed the potential use of AM fungi in alleviating abiotic stresses like heat, salinity, drought, nutrient stress, heavy metal toxicity, and extreme temperatures in host plants. AM fungi being a natural root symbiont provide host plants an easy access to various inorganic nutrients and thus act like a bio-fertilizer. It has also been found to increase the phyto-availability of micronutrients like Zn and Cu. AM fungi regulate various biochemical processes in plants such as osmotic regulation, stomatal regulation, and accumulation of proline and glutathione which help maintain the drought stress of the host plants. Fungal hyphae of AM fungi have the ability to fix heavy metals in their cell wall, thus reducing the metal toxicity in plants (
Table 2). Thus, the inclusion of AM fungi in farming is directed toward better plant growth and sustainable farming.
Table 2.
Adaptation of mycorrhizal fungi to localized soil or environmental conditions.
Table 2.
Adaptation of mycorrhizal fungi to localized soil or environmental conditions.
| Soil or Environmental Conditions |
AM Fungi |
References |
Salinity/salt stress
Drought
High or low soil P levels
Acidity
Metal toxicity
Extreme temperatures |
Glomus intraradices, G. versiform, G. etunicatum
G. deserticola, G. fasciculatum, G. mosseae, G. etunicatum, G. intraradices
G. intraradices, Gigaspora rosea Nicol. & Schenck
G. mosseae
Gigaspora margarita, Rhizophagus irregularis, G. mosseae, G. monosporum
G. fasciculatum, R. irregularis, R. intraradices
|
Evelin et al. [12]; Hajiboland et al. [63]; Porcel et al. [62]
Evelin et al. [12]; Ruiz-Lozano et al. [64]
Mosse, [13]; Cardosso and Kuyper, [65]
Mohammed et al. [61]
Begum et al. [10]; Lenoir et al. [60]
Begum et al. [10] |
4.3. Effect of Biotic Factors on Plant-Mycorrhiza Interactions
Mycorrhizal fungi interact with various soil organisms found in roots, the rhizosphere, and bulk soil. These interactions can be inhibitive, stimulative, competitive, or mutualistic [
66]. Microbial interaction is an important factor in soil fertility and various mycorrhizal fungi are found to interact with the host plant species in symbiotic relationships exchanging nutrients. The presence of mycorrhizal fungi affects the microbial community of the soil both qualitatively and quantitatively and results from the changes in the root and fungal exudates. AM fungi benefit from other microorganisms present in the rhizosphere like potassium solubilizing microorganisms (KSMs) as they increase the bioavailability of K in the soil hence increasing the capacity of K absorption by AMF hyphae [
67].
It was found that the root nematode
Meloidogyne incognita penetration was significantly decreased in the mycorrhizal roots than the control roots and the application of mycorrhizal root exudates further decreased nematode penetration and paralyzed the nematodes temporarily (
Table 3) [
68].
A study on the response of nitrogen-transforming microorganisms to AM fungi in pot cultures of mycorrhizal and non-mycorrhizal maize found that the number of autotrophic ammonium oxidizers in pot cultures of mycorrhizal fungi was significantly higher than in non-mycorrhizal cultures. They also found that these bacteria were seen only after 15 days in non-mycorrhizal cultures as compared to mycorrhizal cultures. The number of ammonifying and denitrifying bacteria was significantly reduced in the mycorrhizal pot cultures as compared to the control. This shows the variable effects of mycorrhizal fungi in different microbial groups (
Table 3) [
69].
Another study reported the growth of
Pseudomonas chlororaphis and the conidial germination of
Trichoderma harzianum in the presence of the
Glomus intraradices AM fungal extract. In contrast, the conidial germination of
Fusarium oxysporum f. sp.
Chrysanthemi was reduced and growth of
Clavibacter michiganensis subsp.
michiganensis was unaffected. These results suggest the possible interactions between AM fungi and soil microorganisms (
Table 3) [
70].
Table 3.
Common mycorrhizal fungi and how they interact with other micro-organisms.
Table 3.
Common mycorrhizal fungi and how they interact with other micro-organisms.
| Organisms |
Mycorrhizal fungi |
Type of association |
References |
Bacteria
Rhizobacteria
Paenibacillus validus
Bacillus subtilis
Enterobacter species
Pseudonomas species
Corynebacterium species
Ammonifying and denitrifying bacteria
P. chlororaphis
|
Arbuscular mycorrhizal fungi
G. intraradices
G. intraradices
G. intraradices
G. versiforme
G. versiforme
Arbuscular mycorrhizal fungi
G. intraradices
|
AM fungi enrich the bacterial flora
Forms new spores, support growth of fungus
Increases root colonization, P solubilization
Increases root colonization, P solubilization
Spore formation
Spore formation
Presence of mycorrhizal hyphae reduce the number of ammonifying and denitrifying bacteria
Growth of bacteria stimulated |
Andrade et al. [71]
Hildebrandt et al. [72]
Toro et al. [73]
Toro et al. [73]
Mayo et al. [74]
Mayo et al. [74]
Amora-Lazcano et al. [69]
Filion et al. [70] |
Fungi
T. harzianum
F. oxysporum f. sp. Chrysanthemi
|
G. intraradices
G. intraradices
|
Conidial germination stimulation in presence of AM fungal extract
Conidial germination reduced by AM fungal extract |
Filion et al. [70]
Filion et al. [70] |
Nematode
M. incognita
|
Arbuscular mycorrhizal fungi |
Mycorrhizal hyphae decrease nematode penetration |
Vos et al. [68] |
5. Contribution of Mycorrhiza in Maintaining Soil Health
The capacity of the soil to optimize crop production, along with balanced soil functional activities like carbon transformations, nutrient cycles, soil structure maintenance, and insect and disease regulation, determines soil health [
75]. Mycorrhizal fungi are naturally present in the roots of most plants and have unique abilities to assist plants in nutrient uptake, and maintaining plant stress [
76], and improving the soil structure by forming stable soil aggregates [
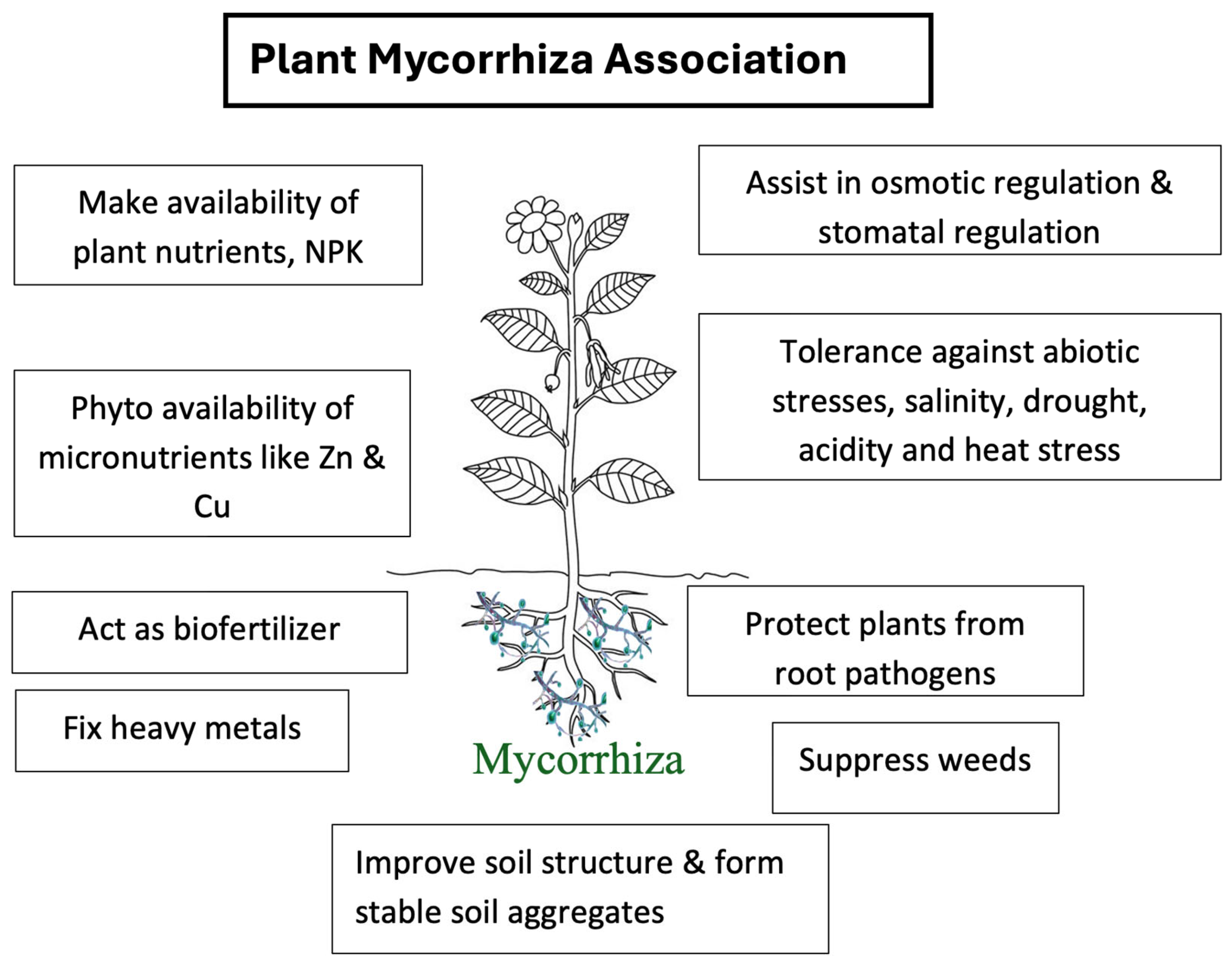
77]. The formation of stable soil aggregates includes the production of glycoprotein and glomalin deposited on the hyphal walls and adjacent soils. Glomalin acts as a hydrophobic glue that prevents macroaggregate disruption during the drying and wetting events by decreasing the water movement of water into the pores within the aggregate structure. This way, AMF forms stable soil aggregates, which are the building blocks of soil structure (
Figure 3) [
78]. Miransari et al. [
11] also found AM fungi to reduce the effects of soil compaction on wheat growth and increase the root, shoot, and grain dry weight. Similarly, mycorrhizal fungi (
Melanogaster variegatus s.l.) inoculated White Alder (
Alnus incana) had significantly more roots and higher soil aggregate stability than non-mycorrhizal White Alder[
79]. Chibuike [
80] discussed mycorrhiza’s use to treat polluted soils, a process known as mycorrhiza-assisted remediation (MAR). He concluded MAR is a suitable method for detoxification of organic and inorganic pollutants of the soil that are intended for crop production. However, the efficiency of the MAR method depends on carefully selecting the species and origin of fungi used, the type of plant colonized, and the type and concentration of pollutants.
Mycorrhizal fungi play a significant role in protecting host plants from root pathogens, increasing the availability of plant nutrients NPK along with Zn, Cu, Fe, Ca, and Mg, controlling the soil insects, and aiding in phytoremediation (
Figure 3) [
81]. AM fungi were found to decrease the stress of soil pathogens in corn plants (
Figure 3) and were also found effective in nutrient uptake like N and P even in higher soil compaction [
11]. Mycorrhizal associations enable better use of sparingly soluble phosphorus pools, increasing the efficiency of added phosphorus fertilizer and immobile P pools [
65]. Milleret et al. [
82] found that AM fungi increase the P acquisition by leek plants from the soil and increase the plant biomass. They also found the interaction of leek roots and AM fungi to improve the water-stable macroaggregates which is supposed to be the combined effects of root exudates and glomalin secretion from AM fungi. Astiko et al. [
83] found that inoculation of mycorrhiza into soil and combination of cattle manure had significantly higher concentrations of N, P, K, and organic-C and higher yield than other treatments like mycorrhiza alone, mycorrhiza combined with rock phosphate, mycorrhiza combined with inorganic fertilizers and control (soil without any inoculation). These results showed that the use of mycorrhiza along with cattle manure could be a suitable soil amendment option for sustainable soil health and productivity in the soybean growing system.
The use of chemicals and heavy machinery has hampered the population of mycorrhizal fungi leading to the degradation of soil health [
76], thus there is a need for awareness among producers about the role of mycorrhiza in maintaining soil health and teaching them the ways to maintain their populations in the soil.
6. Contribution of Mycorrhiza in Maintaining Sustainable Agroecosystem
An agroecosystem is a complex, interconnected network of biotic and abiotic components within a defined agricultural area [
5]. A sustainable agroecosystem maintains long-term productivity by balancing resource conservation, economic feasibility, social acceptance, and environmental integrity.[
6,
7,
8]. The symbiotic association between the host plants and mycorrhizal fungi is nature’s gift for maintaining a sustainable agroecosystem in today’s dynamic environment [
84]. Arbuscular mycorrhiza has been found to increase host plant nutrient uptake and growth while suppressing the growth of the non-mycorrhizal species [
14]. Munyanziza et al. [
85] discussed the relation between mycorrhizal fungi and host plants. Mycorrhizal fungi are found to have greater absorptive surfaces and hence increase the uptake of nutrients by host plants. They also have greater tolerance to toxic metals, root pathogens, drought, higher soil temperatures, adverse pH conditions, and transplant shock and hence protect host plants from all these adversities (
Figure 3).
Mycorrhizal fungi can act as a tool to suppress weed species and can be a potential agroecosystem engineer that can replace herbicides in controlling weed species (
Figure 3) [
14]. Rinaudo et al.[
86] found 47% reduced total weed biomass with the presence of AM fungi in microcosms where weeds and sunflowers were grown together whereas only 25% reduced total weed biomass where weeds were grown alone. Also, the biomass of two among six weed species was significantly reduced by the presence of AM fungi and the biomass of remaining weeds was only slightly reduced.
Medina and Azcon [
87]also discussed the role of AM fungi in reclamating degraded soils through the maintenance of microbial soil status and tolerance to heavy metals and/or drought. They found AM fungi a promising option for the successful restoration of degraded ecosystems. Experiment on the role of AM fungi in cadmium immobilization by Janouskova et al. [
88] found lower cadmium toxicity in mycorrhizal plants than in non-mycorrhizal plants and concluded that AM fungi play an important role in reducing cadmium toxicity to plants by cadmium immobilization in soil.
7. Challenges of Mycorrhizal Fungi in Maintaining Soil Health and Sustainable Agroecosystem
Mycorrhizal fungi are crucial players in maintaining soil health and a sustainable agroecosystem through the formation of symbiotic associations with plant roots, enhancing plant nutrient uptake, and improving soil structure. However, maintaining the health and functionality of mycorrhizal fungi faces several challenges. Intensive agricultural practices such as heavy tillage, continuous monoculture, and overuse of chemical fertilizers can significantly impact mycorrhizal populations and diversity [
89,
90].
Heavy tillage fragments the vital hyphal networks that mycorrhizal fungi depend on to support plant health and nutrient uptake, thus diminishing the symbiotic advantages they offer to crops that follow. The breaking apart of these networks by tillage not only impedes the mycorrhizae's functionality but also impacts the broader soil ecosystem, reducing soil organic matter and disrupting essential nutrient cycling processes. These changes are detrimental to soil health and plant development, impairing the soil's ability to support robust plant growth and maintain ecological balance [
91]. Jansa et al. [
92] found a reduced number of non-
Glomus AMF species in tilled soil compared to no-tilled soil and decreased mycorrhizal diversity of certain AMF species.
The widespread application of chemical fertilizers and pesticides in traditional farming practices has detrimental impacts on the populations and functions of mycorrhizal fungi. Additionally, the use of pesticides and herbicides can disrupt the composition of soil microbial communities, posing a risk to the advantageous mycorrhizal fungi [
93]. Helander et al. [
94] found that using herbicides containing glyphosate diminished the colonization of arbuscular mycorrhizal fungi in perennial grass species, leading to changes in the composition and productivity of plant communities. Munyanziza et al. [
85] also discussed the effects of the conversion of natural systems into agricultural systems on AM fungi. They concluded that high-input agricultural methods are harmful to AM fungi and hence low-input sustainable agriculture practices should be encouraged among growers to enhance the population of AM fungi in soil and to maintain a sustainable agroecosystem.
The dominance of crop monocultures presents significant obstacles to the diversity and efficacy of mycorrhizal fungi. A rich array of plant species typically fosters a correspondingly diverse community of mycorrhizal fungi. Conversely, the homogeneity of monocultures can result in a diminished variety of mycorrhizal fungi, adversely impacting the symbiotic relationships that sustain soil vitality and plant health. This reduction in mycorrhizal diversity can undermine soil structure, nutrient availability, and the overall sustainability of agricultural systems [
4]. The study by Fu et al. [
95] revealed that with the increase in continuous monoculture cycles of tomatoes, soil quality indicators such as microbial diversity and enzyme activities initially improved but then deteriorated. This pattern was linked to decreased tomato yields, emphasizing that long-term monoculture, significantly beyond 11 cycles, negatively impacts soil health and agricultural productivity.
8. Conclusion
A sustainable plant ecosystem occurs when the soil’s productive capacity is conserved with minimum use of energy and resources without the degradation of the ecosystem. It can be gained by the efficient utilization of nutrients by the plants, which the symbiotic association of the mycorrhizal fungi facilitates. These fungi also help to form soil structures that improve water retention and reduce erosion. They produce a substance called glomalin, which binds soil particles together to form aggregates. These aggregates can hold water and nutrients and support robust plant growth. Mycorrhizal fungi are also important in helping plants cope with stress such as drought, salinity, and heavy metals. They assist plants by enhancing their water uptake and helping them with internal water management and protection mechanisms. Furthermore, mycorrhizal fungi contribute to the natural control of pests, diseases, and weeds. They can outcompete harmful pathogens and suppress weed growth by making conditions less favorable for weeds and more favorable for crops. This helps in the reduction of chemical pesticides and herbicides, which can be detrimental to the environment and human health.
However, the effectiveness of these fungi can be diminished by intensive farming practices such as excessive tillage, increased use of fertilizer, and monoculture. These practices can harm the fungi’s surroundings and reduce their ability to work effectively with plant roots. To maintain a healthy soil environment that supports diverse microbial life, including mycorrhizal fungi growers can adopt sustainable practices like reduced tillage, organic farming, crop rotation, and cover crops.
In conclusion, mycorrhizal fungi are crucial in agriculture and environmental sustainability. They help improve soil health, increase plant nutrient uptake, and reduce chemical inputs. Thus, it is important to understand the interactions of soil biotic and abiotic factors with mycorrhizal fungi, and their impacts on soil health, crop production, and the agroecosystem. Future research can be done to optimize the conditions beneficial to mycorrhizal associations with plants, potentially leading to more resilient agricultural systems.
Author Contributions
AC and AK conceived the concept. AC wrote the original draft, AK and SP edited and reviewed this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat Rev Microbiol 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Burlakoti, S.; Devkota, A.R.; Poudyal, S.; Kaundal, A. Beneficial Plant–Microbe Interactions and Stress Tolerance in Maize. Appl Microbiol 2024, 4, 1000–1015. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The Unseen Majority: Soil Microbes as Drivers of Plant Diversity and Productivity in Terrestrial Ecosystems. Ecol Lett 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Marten, G.G. Productivity, Stability, Sustainability, Equitability and Autonomy as Properties for Agroecosystem Assessment. Agric Syst 1988, 26, 291–316. [Google Scholar] [CrossRef]
- Bellon, M. Farmers’ Knowledge and Sustainable Agroecosystem Management: An Operational Definition and an Example from Chiapas, Mexico. Hum Organ 1995, 54, 263–272. [Google Scholar] [CrossRef]
- Brown, B.J.; Hanson, M.E.; Liverman, D.M.; Merideth, R.W. Global Sustainability: Toward Definition. Environ Manage 1987, 11, 713–719. [Google Scholar] [CrossRef]
- Douglass, G.K. Agricultural Sustainability in a Changing World. Westview Press 1984. [Google Scholar]
- Brundrett, M. Diversity and Classification of Mycorrhizal Associations. Biological Reviews 2004, 79, 473–495. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front Plant Sci 2019, 10. [Google Scholar] [CrossRef]
- Miransari, M.; Bahrami, H.A.; Rejali, F.; Malakouti, M.J. Effects of Soil Compaction and Arbuscular Mycorrhiza on Corn (Zea Mays L.) Nutrient Uptake. Soil Tillage Res 2009, 103, 282–290. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular Mycorrhizal Fungi in Alleviation of Salt Stress: A Review. Ann Bot 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- MOSSE, B. PLANT GROWTH RESPONSES TO VESICULAR-ARBUSCULAR MYCORRHIZA. New Phytologist 1973, 72, 127–136. [Google Scholar] [CrossRef]
- Cameron, D.D. Arbuscular Mycorrhizal Fungi as (Agro)Ecosystem Engineers. Plant Soil 2010, 333, 1–5. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.-A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu Rev Microbiol 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal Fungi Can Dominate Phosphate Supply to Plants Irrespective of Growth Responses. Plant Physiol 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Bonfante, P. At the Interface Between Mycorrhizal Fungi and Plants: The Structural Organization of Cell Wall, Plasma Membrane and Cytoskeleton. In Fungal Associations; Springer Berlin Heidelberg: Berlin, Heidelberg, 2001; pp. 45–61. [Google Scholar]
- Read, D.J. Mycorrhizas in Ecosystems. Experientia 1991, 47, 376–391. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient Uptake in Mycorrhizal Symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen Transfer in the Arbuscular Mycorrhizal Symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef]
- Jeffries, P.; Barea, J.M. Arbuscular Mycorrhiza — a Key Component of Sustainable Plant-Soil Ecosystems. In Fungal Associations; Springer Berlin Heidelberg: Berlin, Heidelberg, 2001; pp. 95–113. [Google Scholar]
- Simard, S.W.; Jones, M.D.; Durall, D.M. Carbon and Nutrient Fluxes Within and Between Mycorrhizal Plants. 2003; 33–74. [Google Scholar]
- Abbott, L.K.; Johnson, N.C. Introduction: Perspectives on Mycorrhizas and Soil Fertility. In Mycorrhizal Mediation of Soil; Elsevier, 2017; pp. 93–105. [Google Scholar]
- Cairney, J.W.G. Evolution of Mycorrhiza Systems. Naturwissenschaften 2000, 87, 467–475. [Google Scholar] [CrossRef]
- Sally E. Smith; David Read. In Mycorrhizal Symbiosis; Elsevier, 2008; ISBN 9780123705266.
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat Rev Microbiol 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Leake, J.; Johnson, D.; Donnelly, D.; Muckle, G.; Boddy, L.; Read, D. Networks of Power and Influence: The Role of Mycorrhizal Mycelium in Controlling Plant Communities and Agroecosystem Functioning. Canadian Journal of Botany 2004, 82, 1016–1045. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A Phylum-Level Phylogenetic Classification of Zygomycete Fungi Based on Genome-Scale Data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Bucking, H.; Liepold, E.; Ambilwade, P. The Role of the Mycorrhizal Symbiosis in Nutrient Uptake of Plants and the Regulatory Mechanisms Underlying These Transport Processes. In Plant Science; InTech, 2012. [Google Scholar]
- Courty, P.-E.; Buée, M.; Diedhiou, A.G.; Frey-Klett, P.; Le Tacon, F.; Rineau, F.; Turpault, M.-P.; Uroz, S.; Garbaye, J. The Role of Ectomycorrhizal Communities in Forest Ecosystem Processes: New Perspectives and Emerging Concepts. Soil Biol Biochem 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal Fungi – Potential Organic Matter Decomposers, yet Not Saprotrophs. New Phytologist 2015, 205, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Perotto, S.; Martino, E.; Abbà, S.; Vallino, M. 14 Genetic Diversity and Functional Aspects of Ericoid Mycorrhizal Fungi. In Fungal Associations; Springer Berlin Heidelberg: Berlin, Heidelberg, 2012; pp. 255–285. [Google Scholar]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and Nutrient Cycling in Ecosystems – a Journey towards Relevance? New Phytologist 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Read, D.J.; Leake, J.R.; Perez-Moreno, J. Mycorrhizal Fungi as Drivers of Ecosystem Processes in Heathland and Boreal Forest Biomes. Canadian Journal of Botany 2004, 82, 1243–1263. [Google Scholar] [CrossRef]
- Floss, D.S.; Levy, J.G.; Lévesque-Tremblay, V.; Pumplin, N.; Harrison, M.J. DELLA Proteins Regulate Arbuscule Formation in Arbuscular Mycorrhizal Symbiosis. Proceedings of the National Academy of Sciences 2013, 110. [Google Scholar] [CrossRef]
- Brundrett, M. Mycorrhizas in Natural Ecosystems. 1991; 171–313. [Google Scholar]
- JAVOT, H.; PUMPLIN, N.; HARRISON, M.J. Phosphate in the Arbuscular Mycorrhizal Symbiosis: Transport Properties and Regulatory Roles. Plant Cell Environ 2007, 30, 310–322. [Google Scholar] [CrossRef]
- Doidy, J. The Medicago Truncatula Sucrose Transporter Family: Sugar Transport from Plant Source Leaves towards the Arbuscular Mycorrhizal Fungus., 2012.
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon Availability Triggers Fungal Nitrogen Uptake and Transport in Arbuscular Mycorrhizal Symbiosis. Proceedings of the National Academy of Sciences 2012, 109, 2666–2671. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Piché, Y.; Peterson, R.L. A Developmental Study of the Early Stages in Vesicular–Arbuscular Mycorrhiza Formation. Canadian Journal of Botany 1985, 63, 184–194. [Google Scholar] [CrossRef]
- Harley, J.L. The Significance of Mycorrhiza. Mycol Res 1989, 92, 129–139. [Google Scholar] [CrossRef]
- Newman, E.I.; Harley, J.L.; Harley, E.L. A Check-List of Mycorrhiza in the British Flora. J Ecol 1988, 76, 292. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant Sesquiterpenes Induce Hyphal Branching in Arbuscular Mycorrhizal Fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Matusova, R.; Zhongkui, S.; Beale, M.H. Secondary Metabolite Signalling in Host–Parasitic Plant Interactions. Curr Opin Plant Biol 2003, 6, 358–364. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.-C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones Stimulate Arbuscular Mycorrhizal Fungi by Activating Mitochondria. PLoS Biol 2006, 4, e226. [Google Scholar] [CrossRef]
- Besserer, A.; Bécard, G.; Jauneau, A.; Roux, C.; Séjalon-Delmas, N. GR24, a Synthetic Analog of Strigolactones, Stimulates the Mitosis and Growth of the Arbuscular Mycorrhizal Fungus Gigaspora Rosea by Boosting Its Energy Metabolism. Plant Physiol 2008, 148, 402–413. [Google Scholar] [CrossRef]
- Conn, C.E.; Bythell-Douglas, R.; Neumann, D.; Yoshida, S.; Whittington, B.; Westwood, J.H.; Shirasu, K.; Bond, C.S.; Dyer, K.A.; Nelson, D.C. Convergent Evolution of Strigolactone Perception Enabled Host Detection in Parasitic Plants. Science (1979) 2015, 349, 540–543. [Google Scholar] [CrossRef]
- Yoneyama, K.; Awad, A.A.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones as Germination Stimulants for Root Parasitic Plants. Plant Cell Physiol 2010, 51, 1095–1103. [Google Scholar] [CrossRef]
- Samejima, H.; Babiker, A.G.; Mustafa, A.; Sugimoto, Y. Identification of Striga Hermonthica-Resistant Upland Rice Varieties in Sudan and Their Resistance Phenotypes. Front Plant Sci 2016, 7. [Google Scholar] [CrossRef]
- Vogel, J.T.; Walter, M.H.; Giavalisco, P.; Lytovchenko, A.; Kohlen, W.; Charnikhova, T.; Simkin, A.J.; Goulet, C.; Strack, D.; Bouwmeester, H.J.; et al. SlCCD7 Controls Strigolactone Biosynthesis, Shoot Branching and Mycorrhiza-Induced Apocarotenoid Formation in Tomato. The Plant Journal 2009, 61, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Kim, H. Il; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. How Do Nitrogen and Phosphorus Deficiencies Affect Strigolactone Production and Exudation? Planta 2012, 235, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- JAMIL, M.; CHARNIKHOVA, T.; CARDOSO, C.; JAMIL, T.; UENO, K.; VERSTAPPEN, F.; ASAMI, T.; BOUWMEESTER, H.J. Quantification of the Relationship between Strigolactones and Striga Hermonthica Infection in Rice under Varying Levels of Nitrogen and Phosphorus. Weed Res 2011, 51, 373–385. [Google Scholar] [CrossRef]
- SIQUEIRA, J.O.; SAFIR, G.R.; NAIR, M.G. Stimulation of Vesicular-arbuscular Mycorrhiza Formation and Growth of White Clover by Flavonoid Compounds. New Phytologist 1991, 118, 87–93. [Google Scholar] [CrossRef]
- Tsai, S.M.; Phillips, D.A. Flavonoids Released Naturally from Alfalfa Promote Development of Symbiotic Glomus Spores In Vitro. Appl Environ Microbiol 1991, 57, 1485–1488. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds, D.D.; Ferhatoglu, Y. Functional Categories of Root Exudate Compounds and Their Relevance to AM Fungal Growth. In Arbuscular Mycorrhizas: Physiology and Function; Springer Netherlands: Dordrecht, 2010; pp. 33–56. [Google Scholar]
- Zangaro, W.; Nishidate, F.R.; Vandresen, J.; Andrade, G.; Nogueira, M.A. Root Mycorrhizal Colonization and Plant Responsiveness Are Related to Root Plasticity, Soil Fertility and Successional Status of Native Woody Species in Southern Brazil. J Trop Ecol 2007, 23, 53–62. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, N.C.; Mao, L.; Shi, G.; Jiang, S.; Ma, X.; Du, G.; An, L.; Feng, H. Phylogenetic Structure of Arbuscular Mycorrhizal Community Shifts in Response to Increasing Soil Fertility. Soil Biol Biochem 2015, 89, 196–205. [Google Scholar] [CrossRef]
- Klironomos, J.N. VARIATION IN PLANT RESPONSE TO NATIVE AND EXOTIC ARBUSCULAR MYCORRHIZAL FUNGI. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The Contribution of Arbuscular Mycorrhizal Fungi in Sustainable Maintenance of Plant Health and Soil Fertility. Biol Fertil Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Arbuscular Mycorrhizal Fungal Responses to Abiotic Stresses: A Review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Hamad, S.R.; Malkawi, H.I. Population of Arbuscular Mycorrhizal Fungi in Semi-Arid Environment of Jordan as Influenced by Biotic and Abiotic Factors. J Arid Environ 2003, 53, 409–417. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity Stress Alleviation Using Arbuscular Mycorrhizal Fungi. A Review. Agron Sustain Dev 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with Arbuscular Mycorrhizal Fungi Improves Salinity Tolerance of Tomato (Solanum Lycopersicum L.) Plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcon, R.; Gomez, M. Effects of Arbuscular-Mycorrhizal Glomus Species on Drought Tolerance: Physiological and Nutritional Plant Responses. Appl Environ Microbiol 1995, 61, 456–460. [Google Scholar] [CrossRef] [PubMed]
- CARDOSO, I.; KUYPER, T. Mycorrhizas and Tropical Soil Fertility. Agric Ecosyst Environ 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Fitter, A.H.; Garbaye, J. Interactions between Mycorrhizal Fungi and Other Soil Organisms. Plant Soil 1994, 159, 123–132. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. Interactions Between Arbuscular Mycorrhizal Fungi and Potassium-Solubilizing Microorganisms on Agricultural Productivity. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer India: New Delhi, 2016; pp. 111–125. [Google Scholar]
- Vos, C.; Claerhout, S.; Mkandawire, R.; Panis, B.; De Waele, D.; Elsen, A. Arbuscular Mycorrhizal Fungi Reduce Root-Knot Nematode Penetration through Altered Root Exudation of Their Host. Plant Soil 2012, 354, 335–345. [Google Scholar] [CrossRef]
- Amora-Lazcano, E.; Vázquez, M.M.; Azcón, R. Response of Nitrogen-Transforming Microorganisms to Arbuscular Mycorrhizal Fungi. Biol Fertil Soils 1998, 27, 65–70. [Google Scholar] [CrossRef]
- FILION, M.; ST-ARNAUD, M.; FORTIN, J.A. Direct Interaction between the Arbuscular Mycorrhizal Fungus Glomus Intraradices and Different Rhizosphere Microorganisms. New Phytologist 1999, 141, 525–533. [Google Scholar] [CrossRef]
- Andrade, G.; Mihara, K.L.; Linderman, R.G.; Bethlenfalvay, G.J. Bacteria from Rhizosphere and Hyphosphere Soils of Different Arbuscular-Mycorrhizal Fungi. Plant Soil 1997, 192, 71–79. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Janetta, K.; Bothe, H. Towards Growth of Arbuscular Mycorrhizal Fungi Independent of a Plant Host. Appl Environ Microbiol 2002, 68, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- TORO, M.; AZCÓN, R.; BAREA, J.M. The Use of Isotopic Dilution Techniques to Evaluate the Interactive Effects of Rhizobium Genotype, Mycorrhizal Fungi, Phosphate-solubilizing Rhizobacteria and Rock Phosphate on Nitrogen and Phosphorus Acquisition by Medicago Sativa. New Phytologist 1998, 138, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.; Davis, R.E.; Motta, J. Stimulation of Germination of Spores of Glomus Versiforme by Spore-Associated Bacteria. Mycologia 1986, 78, 426–431. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil Health in Agricultural Systems. Philosophical Transactions of the Royal Society B: Biological Sciences 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Menge, J.A. Utilization of Vesicular–Arbuscular Mycorrhizal Fungi in Agriculture. Canadian Journal of Botany 1983, 61, 1015–1024. [Google Scholar] [CrossRef]
- Celik, I.; Ortas, I.; Kilic, S. Effects of Compost, Mycorrhiza, Manure and Fertilizer on Some Physical Properties of a Chromoxerert Soil. Soil Tillage Res 2004, 78, 59–67. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Mycorrhizal Fungi Influence Soil Structure. In Arbuscular Mycorrhizas: Physiology and Function; Springer Netherlands: Dordrecht, 2000; pp. 3–18. [Google Scholar]
- Graf, F.; Frei, M. Soil Aggregate Stability Related to Soil Density, Root Length, and Mycorrhiza Using Site-Specific Alnus Incana and Melanogaster Variegatus s.l. Ecol Eng 2013, 57, 314–323. [Google Scholar] [CrossRef]
- Chibuike, G.U. Use of Mycorrhiza in Soil Remediation: A Review. Scientific Research and Essays 2013, 8, 679–1687. [Google Scholar] [CrossRef]
- ud din Khanday, M.; Bhat, R.A.; Haq, S.; Dervash, M.A.; Bhatti, A.A.; Nissa, M.; Mir, M.R. Arbuscular Mycorrhizal Fungi Boon for Plant Nutrition and Soil Health. In Soil Science: Agricultural and Environmental Prospectives; Springer International Publishing: Cham, 2016; pp. 317–332. [Google Scholar]
- Milleret, R.; Le Bayon, R.-C.; Gobat, J.-M. Root, Mycorrhiza and Earthworm Interactions: Their Effects on Soil Structuring Processes, Plant and Soil Nutrient Concentration and Plant Biomass. Plant Soil 2009, 316, 1–12. [Google Scholar] [CrossRef]
- Astiko, W.; Sastrahidayat, I.R.; Djauhari, S.; Muhibuddin, A. The Role of Indigenous Mycorrhiza in Combination with Cattle Manure in Improving Maize Yield (Zea Mays L) on Sandy Loam of Northern Lombok, Eastern of Indonesia. Jurnal TANAH TROPIKA (Journal of Tropical Soils) 2013, 18, 53–58. [Google Scholar] [CrossRef]
- Barman, J.; Samanta, A.; Saha, B.; Datta, S. Mycorrhiza. Resonance 2016, 21, 1093–1104. [Google Scholar] [CrossRef]
- Munyanziza, E.; Kehri, H.K.; Bagyaraj, D.J. Agricultural Intensification, Soil Biodiversity and Agro-Ecosystem Function in the Tropics: The Role of Mycorrhiza in Crops and Trees. Applied Soil Ecology 1997, 6, 77–85. [Google Scholar] [CrossRef]
- Rinaudo, V.; Bàrberi, P.; Giovannetti, M.; van der Heijden, M.G.A. Mycorrhizal Fungi Suppress Aggressive Agricultural Weeds. Plant Soil 2010, 333, 7–20. [Google Scholar] [CrossRef]
- Medina, A.; Azcón, R. EFFECTIVENESS OF THE APPLICATION OF ARBUSCULAR MYCORRHIZA FUNGI AND ORGANIC AMENDMENTS TO IMPROVE SOIL QUALITY AND PLANT PERFORMANCE UNDER STRESS CONDITIONS. J Soil Sci Plant Nutr 2010, 10. [Google Scholar] [CrossRef]
- Janoušková, M.; Pavlíková, D.; Vosátka, M. Potential Contribution of Arbuscular Mycorrhiza to Cadmium Immobilisation in Soil. Chemosphere 2006, 65, 1959–1965. [Google Scholar] [CrossRef]
- Menéndez, A.; Scervino, J.; Godeas, A. Arbuscular Mycorrhizal Populations Associated with Natural and Cultivated Vegetation on a Site of Buenos Aires Province, Argentina. Biol Fertil Soils 2001, 33, 373–381. [Google Scholar] [CrossRef]
- Verbruggen, E.; Toby Kiers, E. Evolutionary Ecology of Mycorrhizal Functional Diversity in Agricultural Systems. Evol Appl 2010, 3, 547–560. [Google Scholar] [CrossRef]
- Kabir, Z. Tillage or No-Tillage: Impact on Mycorrhizae. Canadian Journal of Plant Science 2005, 85, 23–29. [Google Scholar] [CrossRef]
- Jansa, J.; Mozafar, A.; Anken, T.; Ruh, R.; Sanders, I.; Frossard, E. Diversity and Structure of AMF Communities as Affected by Tillage in a Temperate Soil. Mycorrhiza 2002, 12, 225–234. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The Key Role of Arbuscular Mycorrhizas in Ecosystem Services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.-P.; Saikkonen, K. Glyphosate Decreases Mycorrhizal Colonization and Affects Plant-Soil Feedback. Science of The Total Environment 2018, 642, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, G.; Zhang, F.; Sun, Z.; Geng, G.; Li, T. Effects of Continuous Tomato Monoculture on Soil Microbial Properties and Enzyme Activities in a Solar Greenhouse. Sustainability 2017, 9, 317. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).