Submitted:
22 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
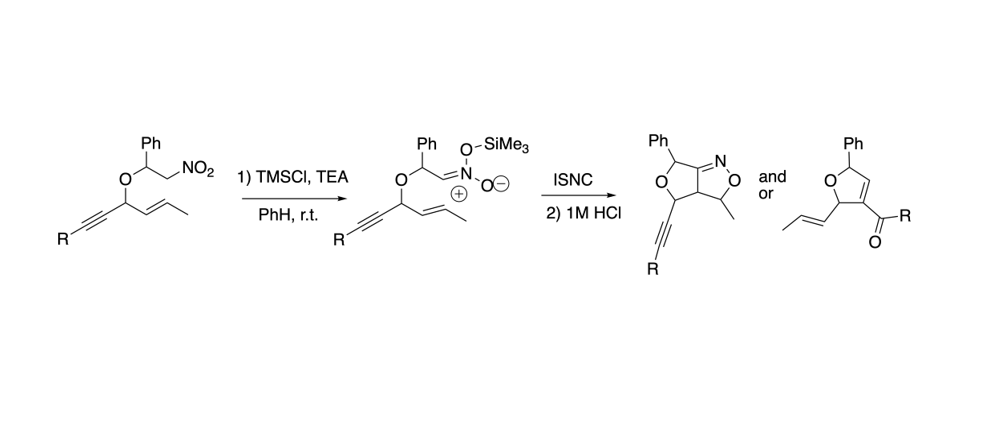
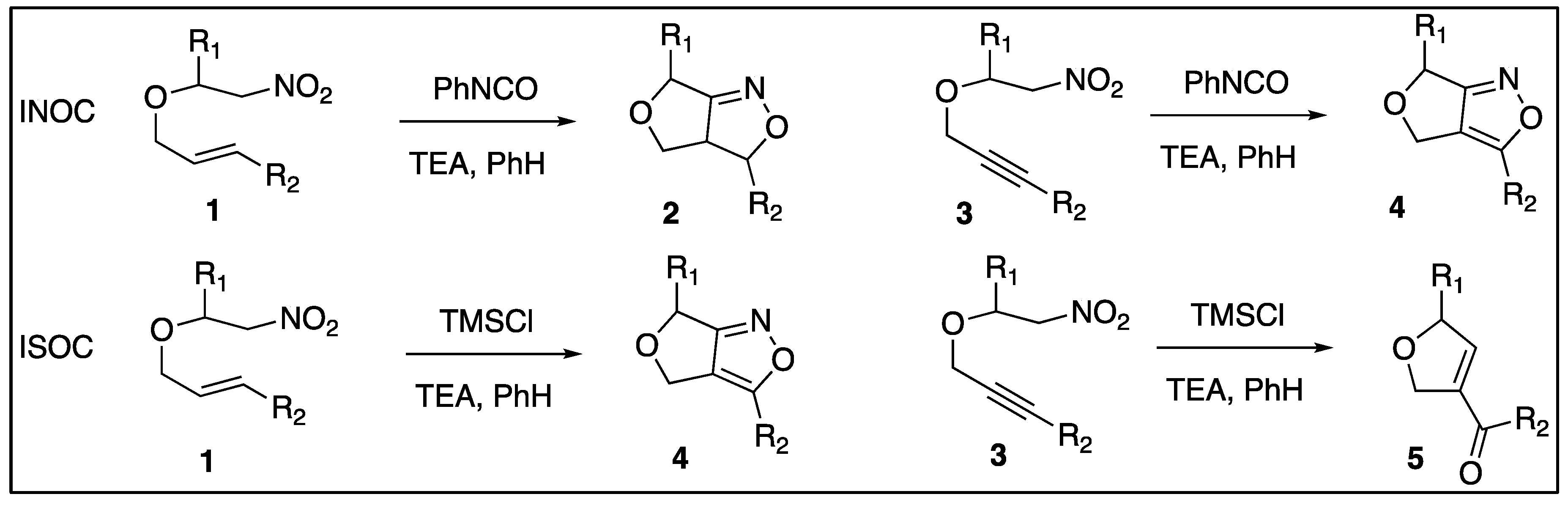
1. Introduction
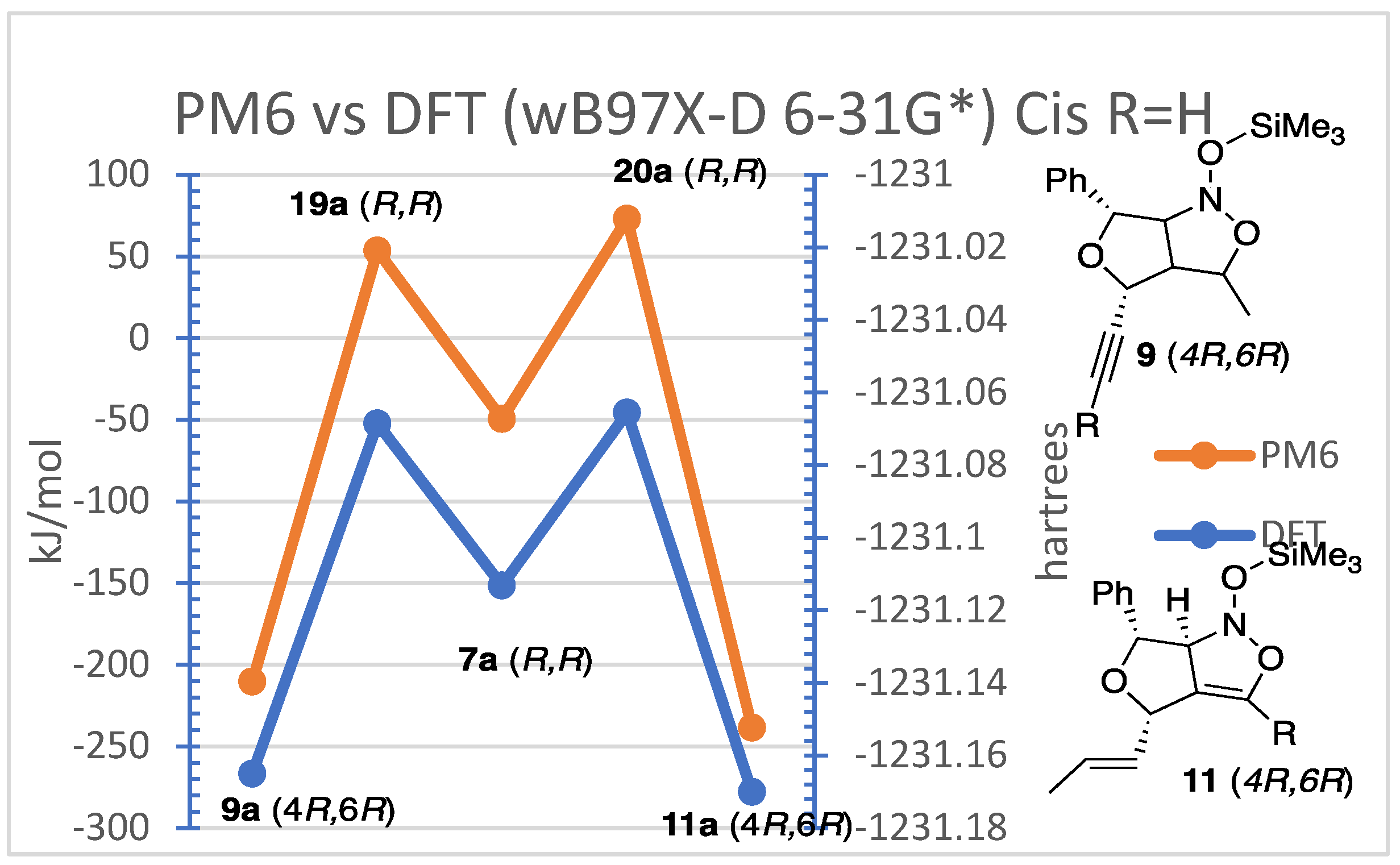
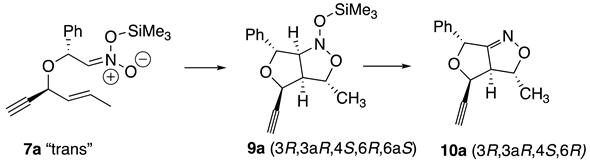
2. Results
3. Discussion
4. Materials and Methods
4.1. Physical and Spectral Data
4.2. Chromatography
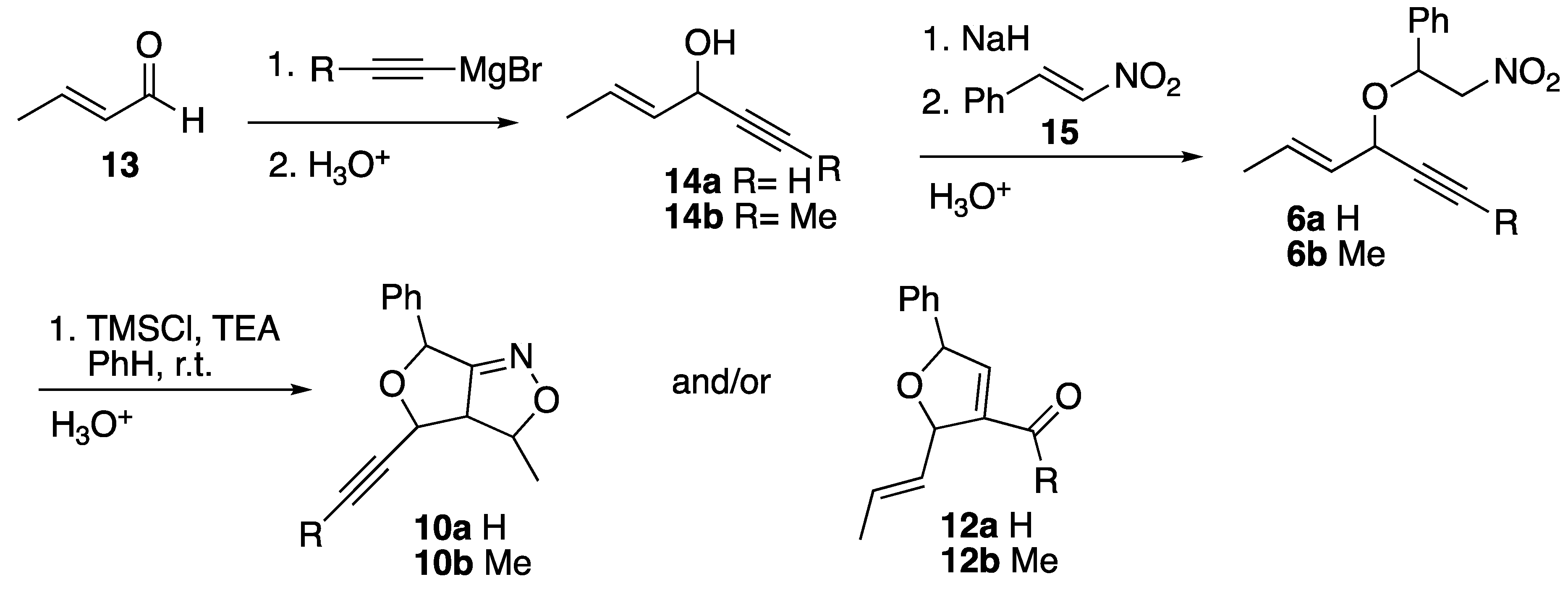
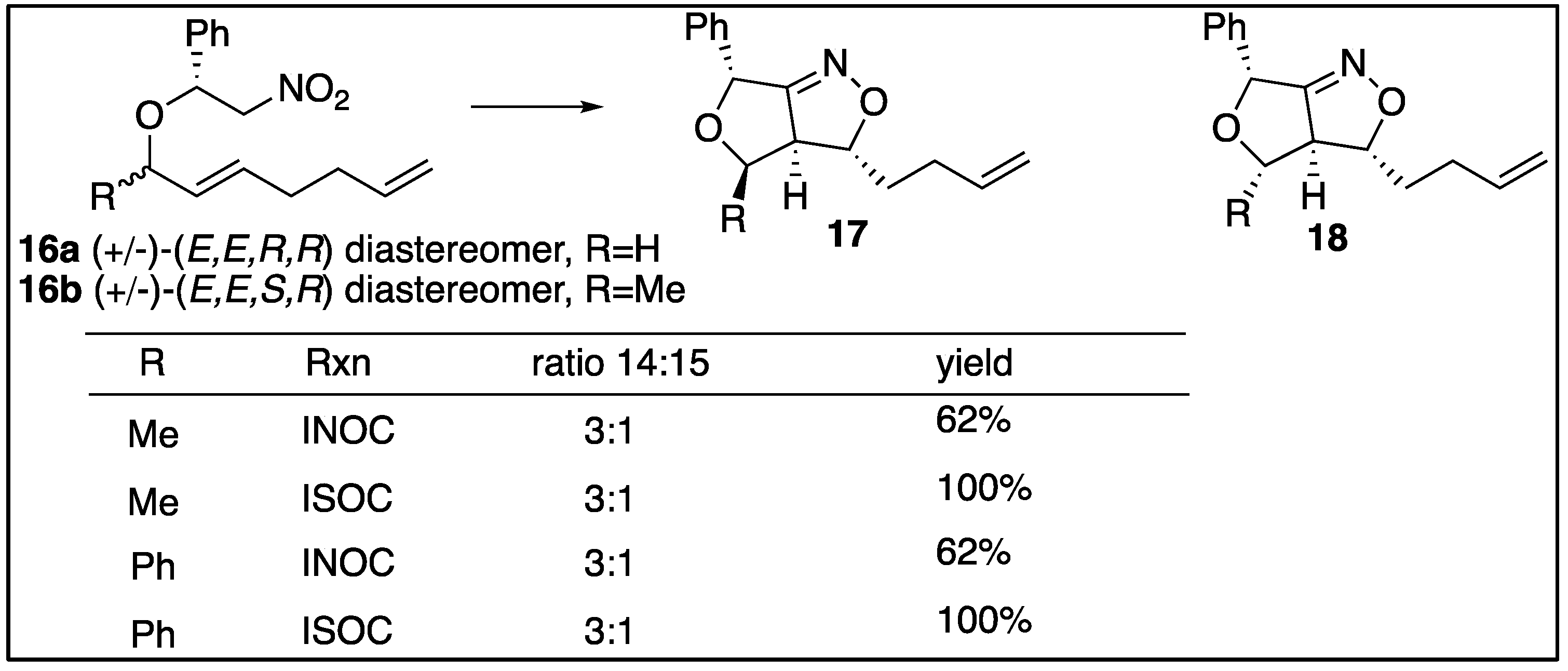
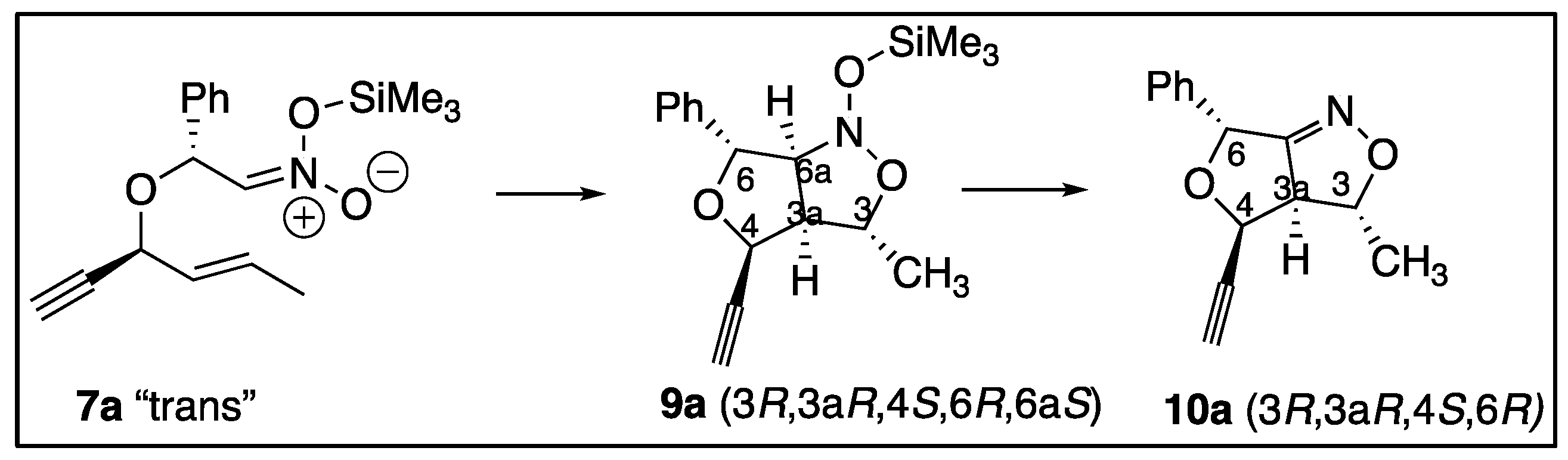
4.3. Reactions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, D.G.J.; Gomez-Bengoa, E.; Hoveyda, A.H. Diastereoselective Intramolecular Cycloaddition of Vinylsilanes and Silyl Nitronates. Effective Control of Remote Acyclic Asymmetry. J. Org. Chem. 1999, 64, 692–693. [CrossRef]
- Marrugo, H.; Dogbéavou, R.; Breau, L. Diastereoselective synthesis of 2-isoxazolines via silaketal tethered 1,3-dipolar cycloadditions. Tetrahedron Lett. 1999, 40, 8979–8983. [CrossRef]
- Hassner, A.; Friedman, O.; Dehaen, W. Cycloadditions, 55. – Substituent Effects in Tandem Intramolecular Silyl Nitronate Olefin Cycloadditions (ISOC) Leading to Functionalized Tetrahydrofurans. Eur. J. Org. Chem. 1997, 1997, 587–594. [CrossRef]
- Galley, G.; Jones, P.G.; Pätzel, M. Enantiomerically pure isoxazolines by stereoselective 1,3-dipolar cycloaddition of silyl nitronates. Tetrahedron: Asymmetry 1996, 7, 2073–2082. [CrossRef]
- Kim, B.H.; Lee, J.Y.; Kim, K.; Whang, D. Asymmetric induction in silyl nitronate cycloadditions to Oppolzer's chiral sultam derivatives. Tetrahedron: Asymmetry 1991, 2, 27–30. [CrossRef]
- Torssell, K.; Zeuthen, O. ChemInform Abstract: REACTIONS OF TERT-BUTYL NITRONES AND TRIMETHYLSILYL NITRONATES. SYNTHESIS AND REACTIONS OF ISOXAZOLIDINES AND 2-ISOXAZOLINES. Chem. Informationsdienst 1978, 9. [CrossRef]
- Gottlieb, L.; Hassner, A. Cycloadditions. 53. Stereoselective Synthesis of Functionalized Pyrrolidines via Intramolecular 1,3-Dipolar Silyl Nitronate Cycloaddition. J. Org. Chem. 1995, 60, 3759–3763. [CrossRef]
- Dehaen, W.; Hassner, A. Stereoselectivity in intramolecular 1,3-dipolar cycloadditions. Nitrile oxides versus silyl nitronates. Tetrahedron Lett. 1990, 31, 743–746. [CrossRef]
- Kumar, A.; Fernandes, J.; Kumar, P. Synthesis and Biological Evaluation of Some Novel Isoxazoline Derivatives of Carbostyril. World J. Pharm. Pharmacuetical Sci. 2014, 3 (2), 1267–1277.
- Kaur, K.; Kumar, V.; Sharma, A.K.; Gupta, G.K. Isoxazoline containing natural products as anticancer agents: A review. Eur. J. Med. Chem. 2014, 77, 121–133. [CrossRef]
- Zhou, X.; Hohman, A.E.; Hsu, W.H. Current review of isoxazoline ectoparasiticides used in veterinary medicine. J. Veter- Pharmacol. Ther. 2021, 45, 1–15. [CrossRef]
- Indorkar, D.; Chourasia, O. P.; Limaye, S. N. Synthesis, Characterization, Antimicrobial, Antifungal Activity of Some s-Triazine Derivatives of Isoxazoline, Pyrazoline and PC Model Computational Studies. Res. J. Pharm. Sci. 2012, 1 (4), 10–16.
- Duffy, J.L.; Kurth, M.J. A Novel Intramolecular Silyl Nitronate Cycloaddition Route to Dihydrofuraldehydes and Dihydropyranaldehydes. J. Org. Chem. 1994, 59, 3783–3785. [CrossRef]
- Grandbois, M.L.; Betsch, K.J.; Buchanan, W.D.; Duffy-Matzner, J.L. Synthesis of novel 2H,5H-dihydrofuran-3-yl ketones via ISNC reactions. Tetrahedron Lett. 2009, 50, 6446–6449. [CrossRef]
- Namboothiri, I.N.N.; Hassner, A.; Gottlieb, H.E. A Highly Stereoselective One-Pot Tandem Consecutive 1,4-Addition−Intramolecular 1,3-Dipolar Cycloaddition Strategy for the Construction of Functionalized Five- and Six-Membered Carbocycles,1. J. Org. Chem. 1997, 62, 485–492. [CrossRef]
- Cheng, Y.; Wang, J.; Hu, Z.; Zhong, S.; Huang, N.; Zhao, Y.; Tao, Y.; Liang, Y. Preparation of norfloxacin-grafted chitosan antimicrobial sponge and its application in wound repair. Int. J. Biol. Macromol. 2022, 210, 243–251. [CrossRef]
- Duffy, J.L.; Kurth, J.A.; Kurth, M.J. Lithium, potassium and sodium alkoxides: Donors in the Michael addition reaction of α-nitroolefins.. Tetrahedron Lett. 1993, 34, 1259–1260. [CrossRef]
- Kim, H.R.; Kim, H.J.; Duffy, J.L.; Olmstead, M.M.; Ruhlandt-Senge, K.; Kurth, M.J. Double diastereoselectivity in the intramolecular nitrile oxide-olefin cycloaddition (INOC) reaction.. Tetrahedron Lett. 1991, 32, 4259–4262. [CrossRef]
- Duffy, Jetty L. An Investigation into the Diastereoselectivity of the Intramolecular Nitrile Oxide Olefin Cycloaddition vs. the Intramolecular Silyl Nitronate Olefin Cycloaddition. Ph.D. Thesis, University of California, Davis, Davis, CA, 1993.
- Kim, H.R.; Kim, K.M.; Kim, J.N.; Ryu, E.K. Regioselectivity and Stereoselectivity in the Intramolecular Nitrile Oxide-Olefin Cycloaddition (INOC) Reaction and the Intramolecular Silyl Nitronate-Olefin Cycloaddition (ISOC) Reaction. Synth. Commun. 1994, 24, 1107–1116. [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2012, 19, 1–32. [CrossRef]
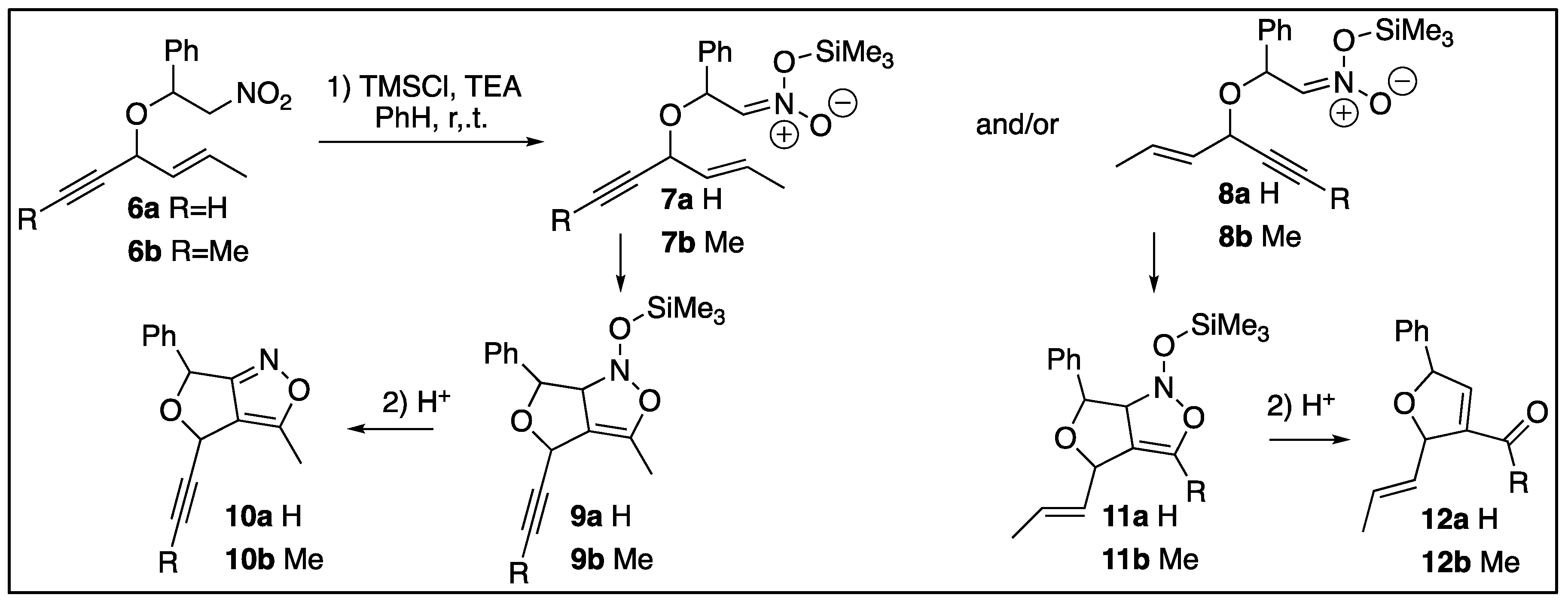
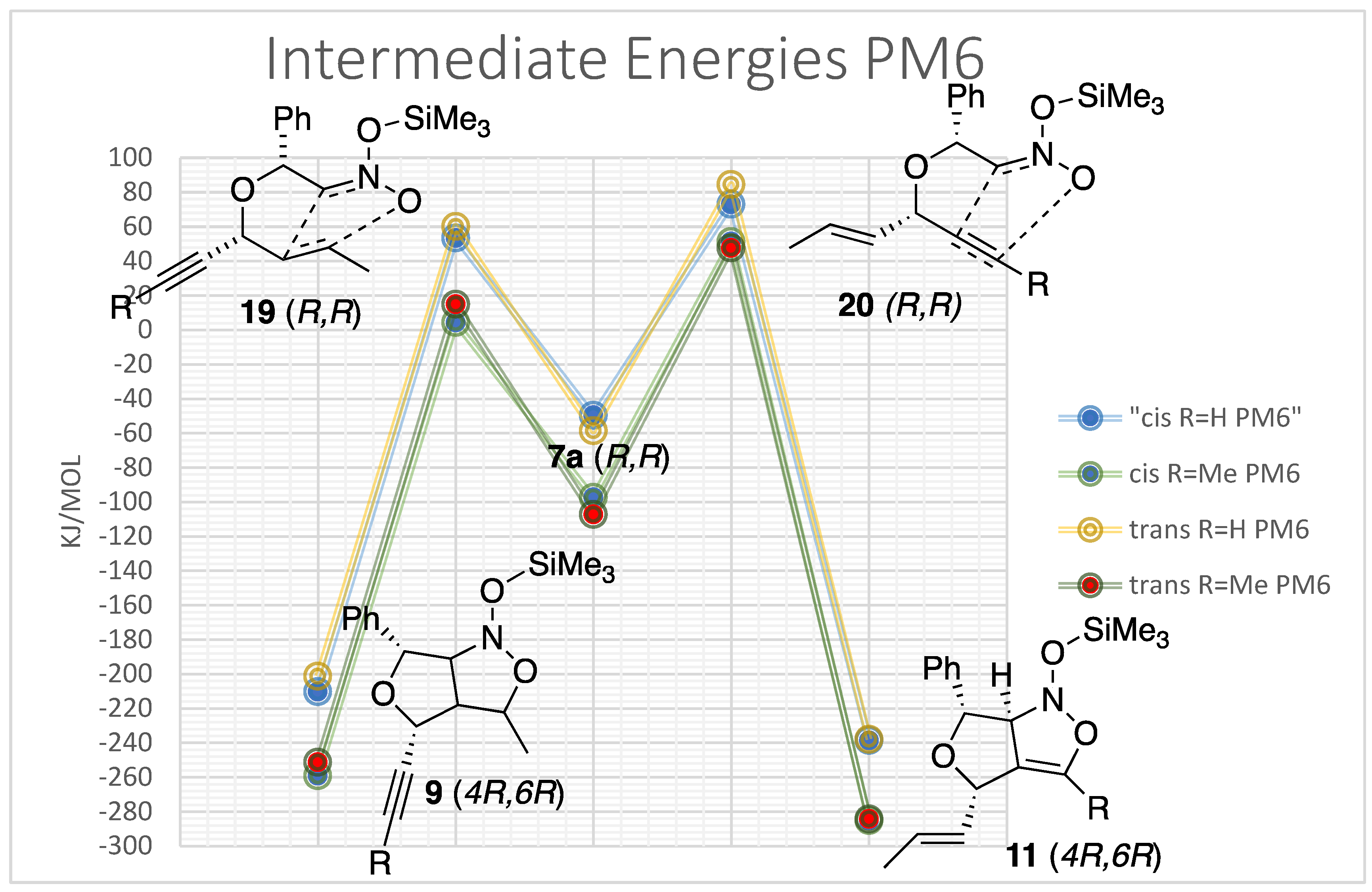
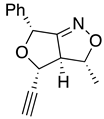
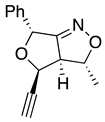
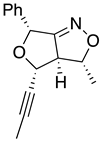
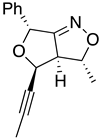
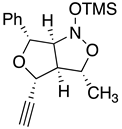
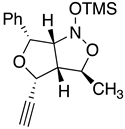
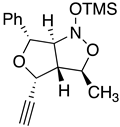
| 10a major | 10a minor | 10b major | 10b minor | |
 |
 |
 |
 |
|
| (±)-(3R,3aR,4R,6R) | (±)-(3R,3aR,4S,6R) | (±)-(3R,3aR,4R,6R) | (±)-(3R,3aR,4S,6R) |
| I 3R 3aR 4R 6R 6aS | II 3S 3aS4R 6R 6aR | III 3S 3aS 4R 6R 6aS | IV 3R 3aR 4R 6R6aR |
| Intermediates | |||
| ∆Hf˙= -210.26 kJ/mol | -189.11 kJ/mol | -132.63 kJ/mol | -117.22 kJ/mol |
 |
 |
 |
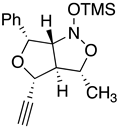 |
| Transition states | |||
| ‡alkene up/imino up | ‡alkene down/imino down | ‡alkene down/imino up | ‡alkene up/imino down |
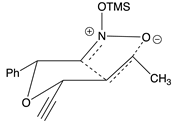 |
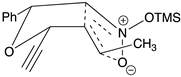 |
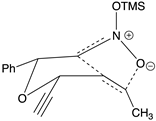 |
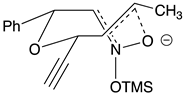 |
| Rxn scheme | Transition state |
 |
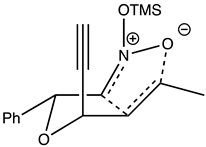 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





