1. Introduction
1.1. Brief Overview of Cancer Drug Discovery
The field of cancer drug discovery has undergone transformative advancements in recent years, driven by technological innovations and a deeper understanding of molecular cancer biology. This progress spans from improvements in HTS and small molecule (SM) therapies to new developments in artificial intelligence (AI) applications and targeted therapies. The integration of SMs into targeted cancer therapies, phenotypic screenings, and structural biology has significantly expanded the scope and efficacy of cancer treatments [
1,
2,
3].
HTS remains a pivotal tool in the drug discovery process, enabling the rapid evaluation of thousands of compounds. Several methods have been used for HTS, including label free assays [e.g. surface plasmon resonance (SPR), Biocore, isothermal titration calorimetry (ITC)], fluorescence based assays [e.g. fluorescence polarization (FP), and anisotropy, fluorescent resonance energy transfer (FRET), time-resolved FRET (TR-FRET), and fluorescence lifetime analysis], bioluminescent based assays (e.g. NanoBiT, NanoBret, AlphaScreen, and luciferase reporter), binding based assays [e.g. proteolysis targeting chimera (PROTAC), covalent drug, mass-spec technology, and DNA encoding library (DEL)], and cell based assays [
4,
5,
6]. Traditionally, HTS involved two-dimensional (2D) cultures, which often fell short in mimicking the complex tumor microenvironment. However, more recent developments incorporate 3D multicellular spheroids or animal models, which offer more physiologically relevant models for studying drug efficacy and resistance [
7]. The ability to simulate the tumor microenvironment has dramatically improved the predictability of therapeutic outcomes [
5,
7,
8]. This is particularly important for targeting cancer cells within the tumor's unique milieu, which often influences the effectiveness of treatments.
SMs in targeted cancer therapies represent one of the most significant advancements in oncology over the past two decades. Due to their small size, these compounds can penetrate cells and inhibit intracellular signaling pathways, offering advantages over monoclonal antibodies, which typically act on extracellular targets [
3,
9]. The FDA has approved more than 43 SM inhibitors for oncology indications, with many of these drugs exhibiting fewer side effects and higher efficacy compared to traditional cytotoxic chemotherapies [
3,
10]. Selective kinase inhibitors, such as sorafenib and sunitinib, exemplify the success of these therapies, targeting multiple kinases across various cancer types. This class of drugs has evolved to include multitargeted agents that inhibit a broad range of kinases, as well as selective inhibitors that focus on specific components of cancer signaling pathways. This approach has allowed for more personalized treatment strategies, tailored to the genetic makeup of individual tumors [
3,
9]. Selective EGFR inhibitors like erlotinib and gefitinib have revolutionized the treatment of non-small cell lung cancer (NSCLC), particularly in patients with EGFR mutations. Similarly, BRAF inhibitors such as vemurafenib have shown efficacy in melanoma patients with BRAF V600E mutations, further emphasizing the role of genomic markers in guiding therapy [
11].
One of the ongoing challenges in cancer drug discovery is overcoming drug resistance, which often arises through mechanisms such as secondary mutations in target proteins, activation of compensatory pathways, or drug efflux [
1,
2,
3]. Researchers are addressing this issue by developing next-generation inhibitors that target resistance mechanisms and by exploring combination therapies that prevent cancer cells from bypassing the effects of single-agent treatments [
9]. Additionally, phenotypic screening and pooled CRISPR approaches are increasingly being used to uncover new targets and mechanisms of drug resistance. These strategies allow for the identification of compounds that modulate cancer cell behavior based on observable traits rather than predefined molecular targets [
6,
11]. CRISPR-based screens have been critical in identifying genes that contribute to drug resistance, providing valuable insights into potential therapeutic targets [
6].
Artificial intelligence (AI) has also significantly impacted cancer drug discovery, particularly in protein structure prediction. AI-powered tools, such as AlphaFold2, have dramatically improved the accuracy of protein structure models, which are crucial for rational drug design. This advancement has accelerated the discovery of new SM inhibitors by enabling researchers to target previously "undruggable" proteins with greater precision [
2]. AI-driven structural predictions are helping to identify new binding sites on oncogenic proteins, offering fresh avenues for therapeutic intervention. Additionally, the drugging of "undruggable" targets, including key oncogenes like RAS and MYC, has advanced through approaches such as PROTACs (Proteolysis Targeting Chimeras). PROTACs function by tagging disease-causing proteins for degradation rather than simply inhibiting them, providing a new modality for addressing proteins that were previously difficult to target with traditional SMs [
12].
The integration of molecular screening techniques, such as next-generation sequencing (NGS) and connectivity mapping, has further enhanced the drug discovery process. These techniques allow researchers to match patients with therapies based on their unique molecular profiles, ushering in an era of precision medicine. Connectivity mapping, in particular, has accelerated the discovery of effective treatments by linking transcriptomic data with existing drug compounds, leading to novel uses for established drugs [
3,
6]. The repurposing of existing drugs for new cancer indications, guided by these tools, has reduced the time and cost associated with developing new therapies [
11].
In conclusion, the field of cancer drug discovery is experiencing rapid advancements driven by the convergence of HTS, AI-driven insights, and novel SM therapies. These developments are pushing the boundaries of what is possible, allowing for more targeted, effective, and personalized cancer treatments. Despite ongoing challenges like drug resistance and the complexity of cancer biology such as heterogenicity, the collective progress highlighted in these studies offers a promising foundation for future breakthroughs in oncology.
1.2. Significance of Biosensors in Cancer Research
Biosensors have revolutionized cancer research by offering high sensitivity, specificity, and real-time monitoring of molecular levels, structure and interactions, significantly advancing the fields of diagnostics, therapeutic monitoring, and drug discovery [
13,
14,
15]. These technologies have provided invaluable tools for early cancer detection, particularly in cases where traditional diagnostic methods fall short due to complexity, invasiveness, or cost [
16,
17,
18,
19]. One of the most impactful roles of biosensors in cancer research is in early-stage diagnosis, particularly through the detection of circulating tumor biomarkers. Biomarkers such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and various proteins can be detected with high precision using biosensors. For example, studies have demonstrated that biosensors, including label-free electrochemical platforms, are effective in detecting these biomarkers in body fluids like blood, offering non-invasive methods for early cancer detection [
16,
18]. These biosensors eliminate the need for complex sample preparation and labeling, making them more accessible and faster to deploy in clinical settings. They allow for the diagnosis of cancer such as breast cancer, by identifying key biomarkers like HER2, CA15-3, and circulating microRNAs [
16,
17].
Moreover, biosensors have transformed the landscape of drug discovery and therapeutic monitoring in cancer research [
20,
21]. High-throughput biosensor assays, including split-luciferase complementation assays (SLCA), have been critical in screening potential drug candidates by measuring protein-protein interactions (PPIs) and receptor activity in cancer cells. These biosensors enable researchers to identify compounds that modulate oncogenic signaling pathways, such as the Hippo or Wnt pathways, which are central to cancer cell proliferation and survival [
22,
23,
24,
25]. The use of these advanced sensor technologies helps accelerate the identification and optimization of SM inhibitors, significantly shortening the drug discovery process. In addition, biosensors are being used for their role in personalized cancer therapy. With their ability to measure real-time responses to drugs, biosensors allow for precise monitoring of therapeutic efficacy, enabling adjustments in treatment regimens based on how a patient’s cancer cells respond. This approach is especially promising in targeted therapies, where biosensors can help identify resistance mechanisms, such as mutations in ctDNA or protein alterations, allowing clinicians to tailor treatments to individual patients [
17,
18].
In summary, biosensors have become integral to the advancement of cancer research, particularly in the realms of early detection and personalized medicine. By providing highly sensitive, non-invasive, and real-time diagnostic tools, biosensors enhance our ability to detect cancer earlier, monitor disease progression more accurately, and optimize therapeutic interventions. As biosensor technology continues to evolve, it promises to further revolutionize the way cancer is diagnosed, treated, and monitored, ultimately improving patient outcomes across various cancer types.
2. Types of Biosensors
Biosensors have evolved significantly in recent years, with various types being developed for different applications in fields such as healthcare, environmental monitoring, and drug discovery. These biosensors can be classified based on the type of transducer or biorecognition element used.
2.1. Electrochemical Biosensors
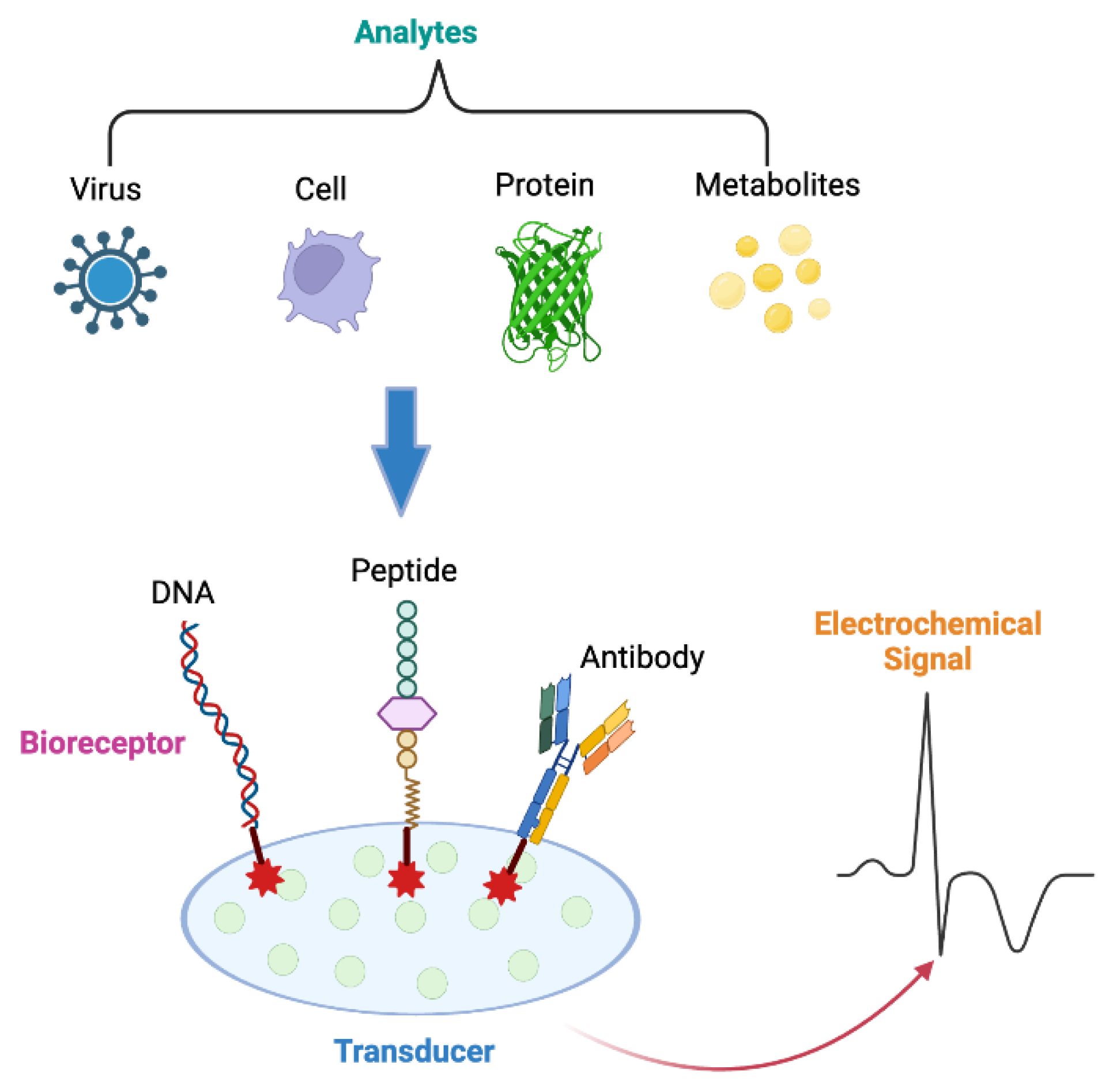
Electrochemical biosensors are among the most widely used due to their high sensitivity and relatively low cost. These sensors measure the electrical signals generated by a biochemical reaction between the analyte (e.g. virus, cell, protein, metabolites, etc) and a biological element called bioreceptor, such as DNA, peptides or antibodies (
Figure 1) [
26]. A common example is the glucose biosensor, which uses glucose oxidase to detect glucose levels in blood samples. The enzyme reaction produces hydrogen peroxide, which is then electrochemically detected [
27,
28]. Advances in nanomaterials, such as carbon nanotubes and metal nanoparticles, have improved the sensitivity and detection limits of these sensors [
29].
2.2. Nanomaterial-Enabled Biosensors
Nanomaterials have played a significant role in advancing biosensor technology. The use of nanomaterials like quantum dots, carbon nanotubes, and gold nanoparticles in biosensors has improved their sensitivity, selectivity, and response time. Nanomaterial-enabled biosensors are particularly effective in detecting small amounts of biomolecules, even at the nanomolar and picomolar levels, making them highly useful for early disease detection, including cancer [
29].
2.3. Fluorescence Biosensors
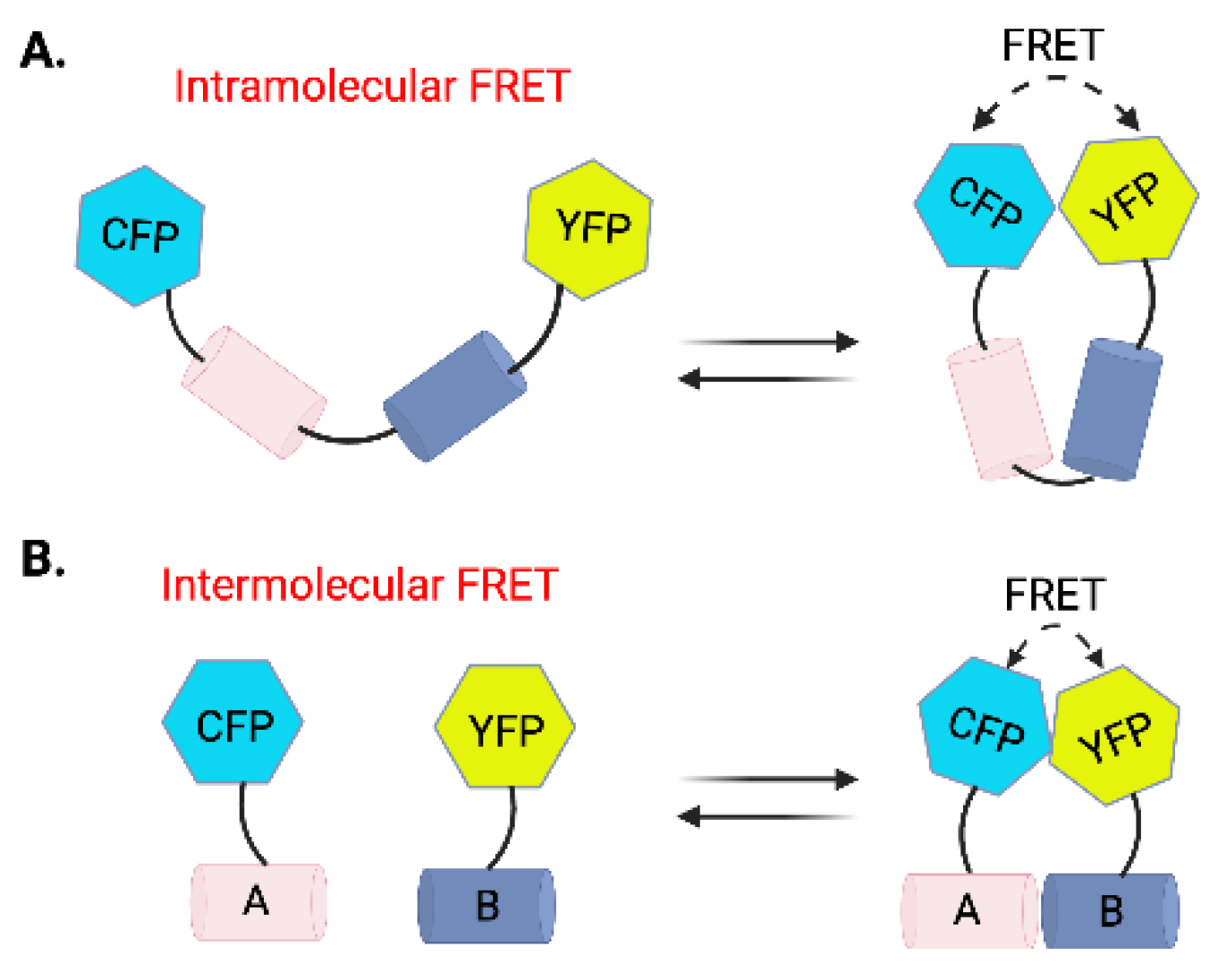
Fluorescence biosensors rely on the excitation of fluorescent molecules, or fluorophores by an external light source, followed by the emission of light at a different wavelength. These biosensors are commonly used for detecting biomolecular interactions, conformational changes, and enzyme activities. Fluorescence biosensors typically involve a donor fluorophore [e.g. light emitted from cyan fluorescent protein (CFP)] that, upon excitation, transfers energy to an acceptor fluorophore [e.g. light, either directly or through a process known as Förster resonance energy transfer (FRET)]. Intramolecular FRET and intermolecular FRET biosensors (
Figure 2) are particularly valuable for studying dynamic changes from a singular protein and interactions between proteins, respectively, as the efficiency of energy transfer between the donor and acceptor depends on their proximity, typically within 1-10 nanometers.
By measuring changes in FRET efficiency, researchers can infer molecular distances and detect interactions in real-time [
30,
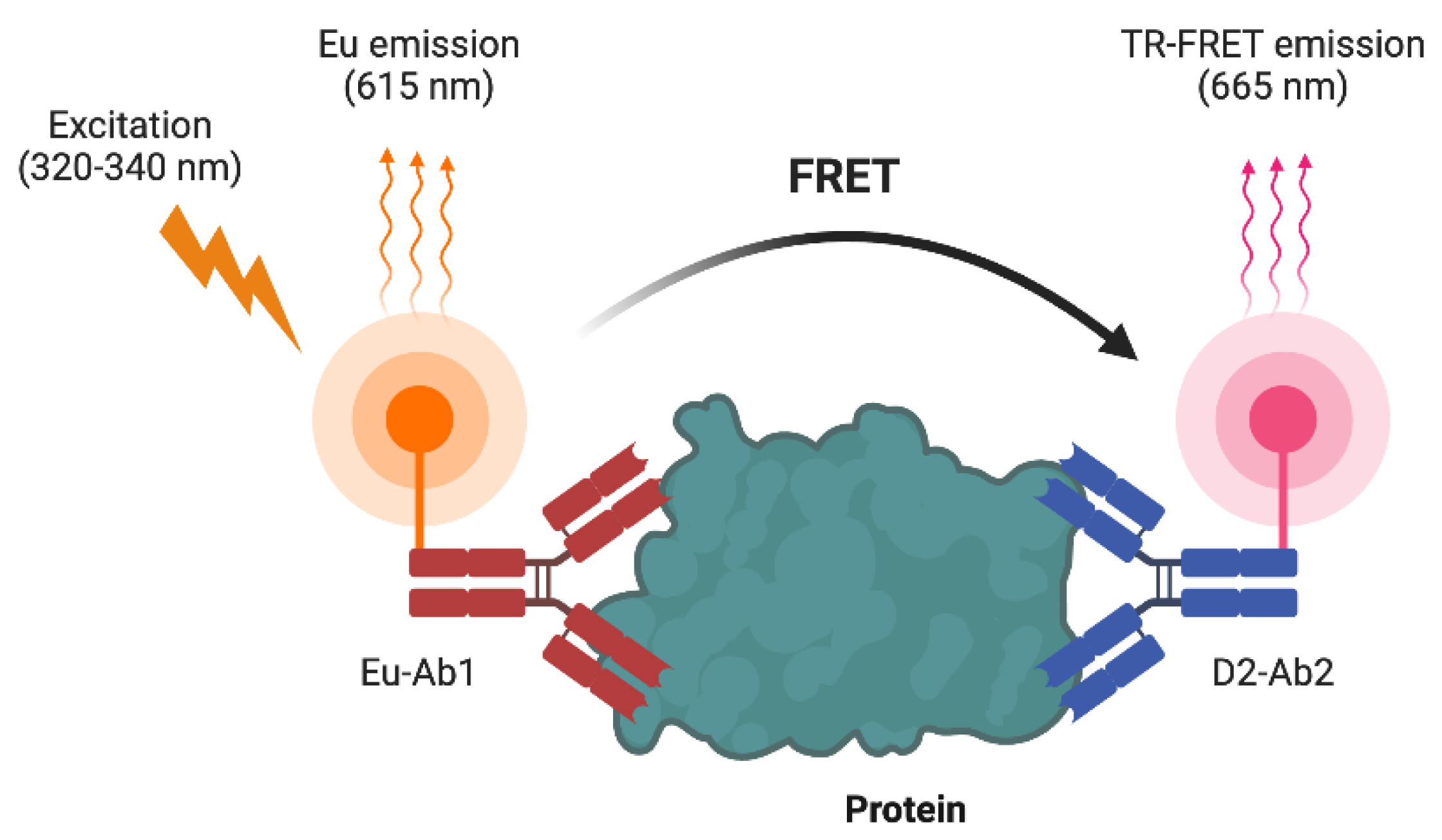
31]. Recently, another FRET method called Time-Resolved FRET (TR-FRET) was developed. TR-FRET utilizes a donor, often a lanthanide (e.g., terbium), which emits light after excitation (320-340 nm) at wavelengths around 615 nm. If the donor and acceptor are close, energy transfer causes the acceptor to emit light at 665 nm (
Figure 3). The delayed detection, usually 50-100 microseconds, reduces background noise from short-lived fluorescence. Both FRET and TR-FRET fluorescent biosensors are used extensively to monitor intracellular events, such as calcium signaling, kinase activity, and protein folding, providing valuable insights into cellular processes at a molecular level [
31,
32,
33]. Fluorescence biosensors also offer high temporal resolution, making them suitable for applications where precise, real-time tracking of fast cellular events is required. For example, green fluorescent protein (GFP) and its variants have been engineered into biosensors to visualize the localization and activity of proteins within live cells. The real-time imaging capabilities of fluorescence biosensors have been instrumental in studying cancer cell behavior, drug responses, and the dynamics of various oncogenic signaling pathways [
27]. Fluorescence biosensors are widely used in HTS to identify potential drug candidates by detecting changes in fluorescent signal corresponding to the binding or inhibition of target proteins. This is particularly important in cancer drug discovery, where rapid screening of large chemical libraries is essential for identifying new therapeutic molecules.
Bioluminescence Resonance Energy Transfer (BRET) and its enhanced version, NanoBRET (NanoLuc BRET), are powerful techniques used to study molecular interactions in live cells. These proximity-based assays involve the transfer of energy from a luciferase enzyme as donor [e.g. Renilla luciferase (RLuc)] to a fluorescent acceptor, generating a measurable light signal (
Figure 4). NanoBRET improves classical BRET by using NanoLuc luciferase, a smaller and more stable luciferase, which provides higher luminescence intensity and better spectral resolution. These advancements reduce background interference and enhance sensitivity, making NanoBRET ideal for HTS in drug discovery. BRET/NanoBRET is widely used to detect PPIs and to screen for small-molecule inhibitors, as it preserves the physiological relevance of cellular environments [
34,
35,
36,
37].
2.4. Bioluminescence Biosensors
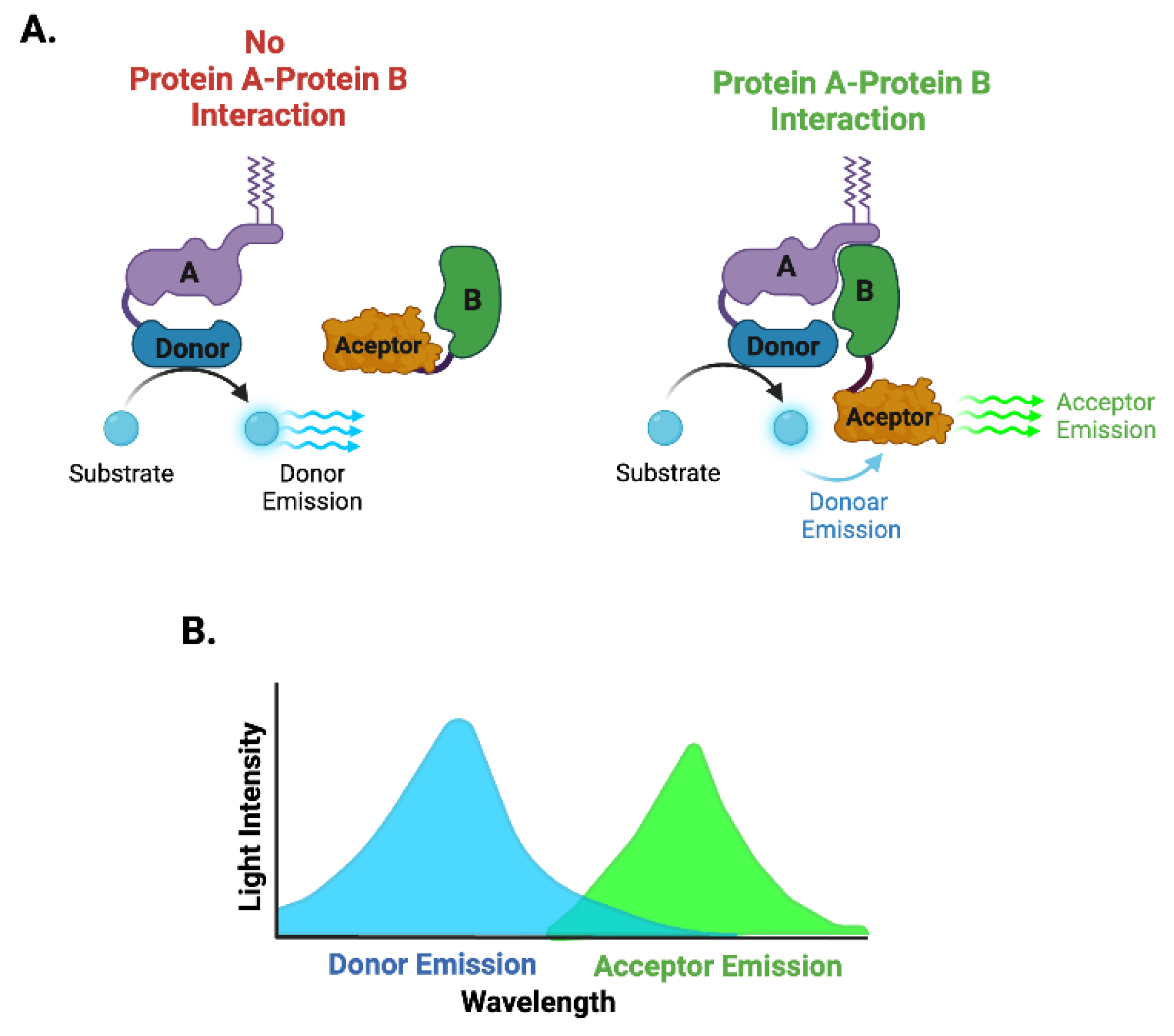
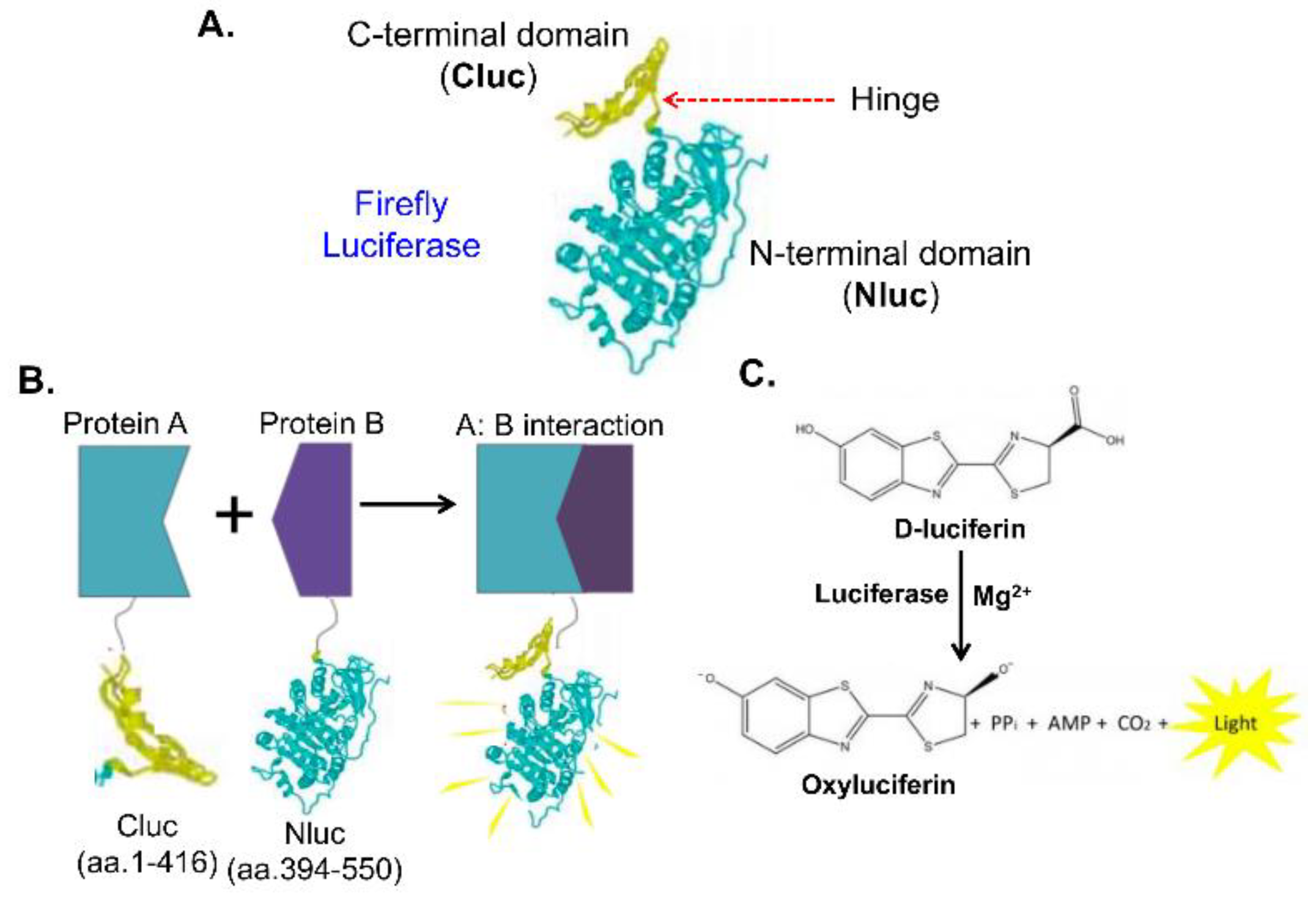
Bioluminescence biosensors are based on the emission of light resulting from a biochemical reaction. The most commonly used bioluminescent system is the enzyme luciferase, which catalyzes the oxidation of a substrate (e.g., luciferin), emitting photons as a byproduct. Unlike fluorescence, bioluminescence does not require external light excitation, which reduces background noise and makes these biosensors highly sensitive. The two most widely used bioluminescence biosensor systems are based on firefly luciferase and the NanoLuc luciferase. A SLCA system involves splitting the firefly luciferase into two non-functional halves, N-terminal (NLuc) and C-terminal (CLuc) luciferase (
Figure 5A), which only reconstitute into an active firefly luciferase by PPI of two proteins (Protein A and Protein B) (
Figure 5B-5C). The firefly biosensors have been widely used to study signaling transduction, and tumor growth in xenograft mouse model [
13].
NanoLuc is a small, bright luciferase enzyme that generates a robust light signal that is over 100 time stronger than firefly luciferases [
38,
39,
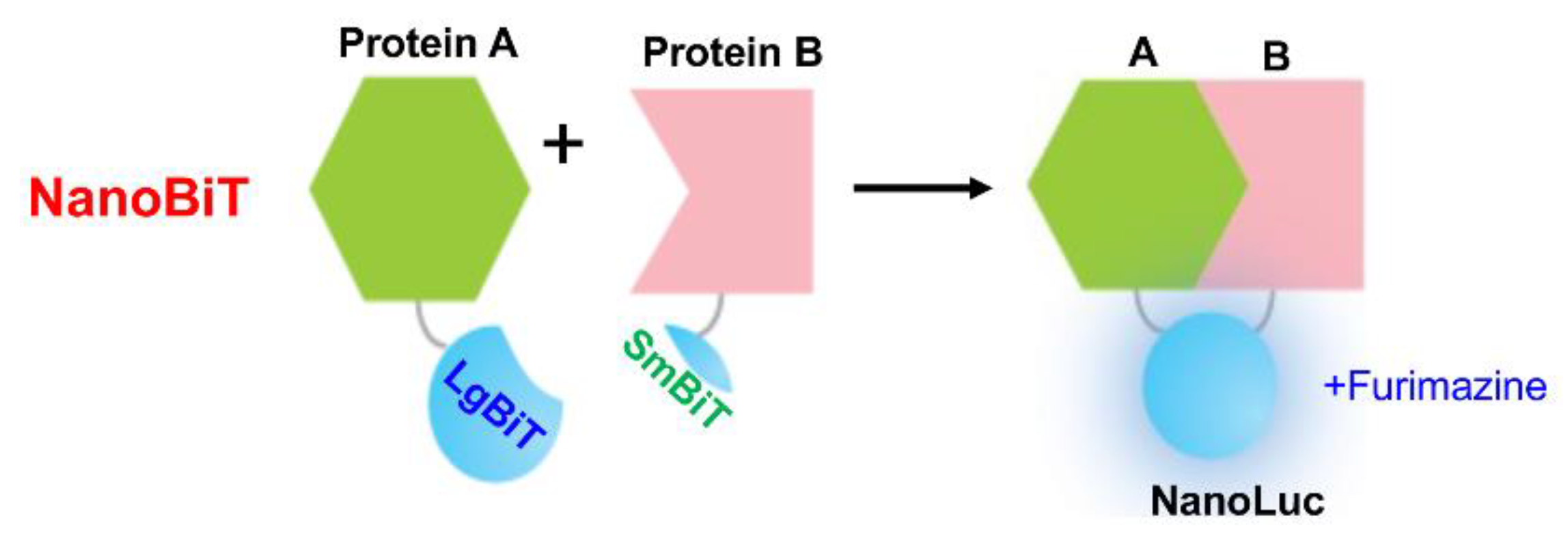
40], making it ideal for detecting molecular interactions and cellular events in real-time. This technology is particularly valuable in drug discovery for monitoring PPIs, receptor-ligand binding, and cellular signaling events. For example, NanoBiT, a SLCA system, involves splitting the NanoLuc luciferase enzyme into two non-functional halves, LgBiT (18 kDa) and SmBiT (1.3 kDa, 11 amino acids), which only reconstitute into an active NanoLuc enzyme when brought together by a specific interaction, such as a PPI (
Figure 6) [
23]. This approach allows for the real-time detection of PPIs in live cells with high sensitivity [
23,
30]. Due to their high sensitivity and low background interference, bioluminescent biosensors are especially useful in
in vitro applications. For instance, they can be used to track tumor growth, metastasis, or therapeutic responses in animal models by tagging cancer cells or specific proteins with bioluminescent markers [
13,
14,
15,
27,
41,
42,
43]. The ability to monitor biological processes non-invasively over time makes bioluminescence biosensors a powerful tool in cancer research and drug discovery.
3. Application of Biosensors in HTS
3.1. HTS Using Fluorescence-Based Biosensors
Fluorescence-based biosensors have become integral to HTS in drug discovery, particularly in cancer research. Techniques like FRET, TR-FRET, BRET, and NanoBRET offer exceptional sensitivity in monitoring PPIs, kinase activities, and other cellular processes critical for cancer progression. These methods accelerate the identification of small-molecule inhibitors targeting key signaling pathways. Below, we explore the various fluorescence-based biosensors and their applications in HTS, drawing insights from recent studies.
3.1.1. FRET Biosensors
FRET-based assays are widely recognized as powerful tools in HTS, offering real-time insights into molecular interactions and protein dynamics within live cells. By leveraging non-radiative energy transfer between two fluorophores, these assays detect subtle changes in molecular proximity (
Figure 2), making them invaluable across therapeutic areas, including cancer, neurodegenerative diseases, and inflammation.
Several studies have showcased the versatility of FRET biosensors in drug screening. He
et al. (2019) employed FRET-based biosensors to measure ERK and AKT kinase activities in triple-negative breast cancer (TNBC) cells, providing insights into differential kinase dependencies and helping identifying inhibitors to overcome resistance [
44]. Similarly, Senarisoy
et al. (2020) utilized FRET technology to study interactions between hNTH1 and YB1, crucial drivers of cancer progression, and identify molecules sensitizing tumors to chemotherapy [
45]. Recent advancements have further enhanced FRET’s utility. Liu
et al. (2021) integrated FRET with next-generation sequencing (NGS) to detect subtle kinase activities, such as ZAP70, in immune cells, improving biosensor sensitivity for HTS [
46]. Hao
et al. (2022) developed a peroxiredoxin-based FRET sensor for screening cancer therapeutics targeting H
2O
2-mediated pathways [
47]. In addition, Vunnam
et al. (2023) employed FRET assays for HTS to identify selective inhibitors of TNFR1, offering potential anti-inflammatory treatments without off-target effects [
48].
3.1.2. TR-FRET Biosensors
TR-FRET assays build on conventional FRET by reducing background noise through time-gated measurements, improving signal stability and sensitivity (
Figure 3). This method has proven effective in HTS across a range of biological applications. Zhang
et al. (2020) employed TR-FRET to discover inhibitors targeting the CBP bromodomain, disrupting oncogenic transcriptional activity and inhibiting MYC expression [
49]. Loss of tumor suppressor genes by mutations is a hallmark of cancers. However, direct targeting of tumor suppressors remains challenging. To address this gap, Tang
et al developed a TR-FRET biosensor that can recapitulate the dynamic differential interaction of SMAD4 and SMAD4R361H mutant with SMAD3 [
50]. By using this biosensor for HTS, they identified Ro-31-8220, a bisindolylmaleimide derivative, as a SMAD4R361H/SMAD3 interaction inducer. Ro-31-8220 reactivated the dormant SMAD4R361H-mediated transcriptional activity and restored TGF-β-induced tumor suppression activity in SMAD4 mutant cancer cells [
50]. TR-FRET has also widely used to develop biosensor monitoring PPIs. For example, Xiong
et al. (2018) used TR-FRET to develop a platform for ultra HTS (uHTS) of NSD3-MYC interactions, and identified inhibitors that could block NSD2-MYC-induced oncogenic activation [
51]. In addition, Yang
et al. and Ouyang
et al. developed a cell lysate based TR-FRET assay to monitor MKK2-MYC and SMAD4-SMAD2 PPI, respectively, and identified a quinoline derivative SGI-1027 and gambogic/gambogenic acid as potent inhibitors for MKK2-MYC and SMAD4-SMD3 PPIs, respectively [
33,
52]. Moreover, Du
et al. (2024) adapted TR-FRET for screening immune-related targets by designing a biosensor for the SYK-FCER1G interaction. Their miniaturized 1536-well uHTS assay identified hematoxylin as a disruptor of this pathway, highlighting its potential for treating immune-related cancers [
53].
Applications of TR-FRET extend to other signaling pathways as well. Singh
et al. developed a TR-FRET demethylation screen assay for HTS and identified geldanamycin and its analog 17-DMAG as histone lysine demethylase (KDM) inhibitor, which inhibits tumor growth of alveolar rhabdomyosarcoma [
54]. In addition, Larson
et al. (2023) employed the method to identify modulators of the KRAS A146T mutant, a challenging target in oncology. They developed a novel high throughput TR-FRET assay that leverages the reduced nucleotide affinity of KRAS A146T. This assay is capable of detecting SMs that act to allosterically modulate GDP affinity or directly compete with the bound nucleotide. By HTS for a diversity library totaling over 83,000 compounds and further validation, they identified UNC10104889 as a novel compound that inhibit KRAS GTPase activity, which provides new therapeutic opportunities for colorectal and pancreatic cancers [
55].
While TR-FRET is a powerful tool, challenges in assay design persist, including donor-acceptor pair optimization and fluorophore stability. However, innovations such as machine learning for data analysis are improving assay reliability and scalability, as reported by Shimizu
et al. (2021) [
56].
3.1.3. BRET and NanoBRET Biosensors
BRET and its advanced version NanoBRET (
Figure 4) have become pivotal in HTS, offering unparalleled sensitivity for studying PPIs in live cells. NanoBRET has been instrumental in monitoring the RAS-RAF PPI, critical in cancer progression, identifying both inhibitors and pathway-specific modulators [
36]. Similarly, this technology has been used for HTS for drugs targeting the PTK7-β-catenin interaction, a key driver in colorectal cancer [
57]. Researchers have used it to screen inhibitors of TNFR interactions, identifying molecules that modulate receptor assembly with precision [
34]. NanoBRET has also provided insights into neo-protein interactions in cancers, revealing vulnerabilities associated with oncogenic mutations, such as BRAF V600E [
34].
The integration of DELs with BRET assays offers a powerful workflow for drug discovery. DELs provide chemical diversity, while BRET-based assays validate target engagement in live cells, streamlining the identification of lead compounds. Teske
et al. (2023) demonstrated this by converting DEL ligands, particularly from an Aurora kinase A screen, into cell-active functional BRET probes. These probes enable stratification of cell permeability and are useful for prioritizing DEL-derived hits without requiring detailed structure-activity relationship (SAR) data. The DEL/BRET approach is generalizable across multiple target classes, accelerating the hit-to-lead process in live-cell assays during HTS [
37].
3.2. HTS Using Bioluminescent NanoBiT Biosensors
NanoBiT is a cutting-edge bioluminescent tool increasingly used in drug discovery, specifically for monitoring protein levels and PPIs in cells (
Figure 6). The small size of the fragments and the high sensitivity of the NanoLuc system make NanoBiT highly suitable for dynamic, real-time applications across diverse drug discovery platforms. In addition, NanoBiT retains high specificity and sensitivity even in the presence of widely used kinase inhibitors. This resilience is critical for screening chemical libraries, where assay interference from certain compounds can lead to false-positive or false-negative results [
58].
NanoBiT biosensors have been used for screening drugs disrupting PPIs. As demonstrated by Miyamoto
et al. (2019), NanoBiT-based biosensors were developed to detect RAF dimerization, a process that contributes to resistance in cancer therapies targeting RAF kinases [
59]. Their study highlighted that split luciferase complementation can be used for HTS of drug candidates, identifying inhibitors that specifically modulate RAF dimerization. In a similar context, Claes and Bollen (2023) demonstrated the utility of NanoBiT for screening SMs that modulate phosphatase subunit interactions [
60]. Their SLCA provided a robust platform for HTS of compounds that interfere with phosphatase holoenzyme formation, which has therapeutic implications for diseases such as cancer and neurodegenerative disorders. To enable screening for SM compounds that disrupt the YAP-TEAD interaction, we recently developed an ultra-bright NanoLuc biosensor to quantify YAP/TAZ-TEAD PPIs both in living cells and
in vitro using biosensor fusion proteins purified from bacteria. With this biosensor, we conducted an
in vitro HTS of SM compounds and identified and validated Celastrol as a novel inhibitor of the YAP/TAZ-TEAD interaction. Additionally, we demonstrated that Celastrol can inhibit cancer cell proliferation, transformation, and migration by disrupting YAP/TAZ-TEAD PPI [
24]. Using a similar cell-free approach, in another study, Cooley
et al. (2020) developed NanoBiT biosensor to detect weak protein interactions between RAS and its effectors [
61]. This allowed for the screening of poorly soluble protein domains, demonstrating that the flexibility of NanoBiT enables its use in challenging drug discovery applications. Such versatility has made NanoBiT a valuable asset for identifying inhibitors that target PPIs relevant to cancer and other diseases.
Targeted protein degradation (TPD) is a promising therapeutic strategy that involves the selective destruction of disease-related proteins [
62,
63]. NanoBiT technology has been adapted to a HiBiT system for use in TPD studies, providing a sensitive and high-throughput platform for monitoring protein levels in real-time. In this HiBiT system, the 11-amino acid SmBiT sequence is modified to HiBiT with the same size but has much higher affinity to LgBiT, another component of the NanoBiT system (
Figure 6). When HiBiT is fused to a protein of interest and expressed in cells, it can spontaneously associate with the LgBiT fragment, forming a functional luciferase enzyme. This reconstituted enzyme emits light in the presence of a substrate, allowing for the quantification of HiBiT-tagged protein levels. Moreover, Lankford
et al. (2024) developed a protocol for HiBiT tagging endogenous proteins using CRISPR-Cas9, which enhances the ability to study endogenous proteins in real-time, which is essential for understanding the effects of drug candidates on physiologically relevant protein targets [
64].
The HiBiT system is particularly useful for HTS for SMs causing protein degradation in living cells due to its high sensitivity and simplicity [
65]. For example, Uchida
et al. (2021) used HiBiT-tagged PD-L1 proteins to screen chemical libraries for compounds that modulate PD-L1 expression [
66]. This approach identified several compounds that upregulate or downregulate PD-L1, a key immune checkpoint molecule. Modulating PD-L1 expression is central to the development of immune checkpoint inhibitors, a class of drugs that has revolutionized cancer immunotherapy. In addition, we recently utilized HiBiT biosensors to monitor the stability of YAP/TAZ proteins in breast cancer cells [
43]. Our HTS identified many novel SMs causing degradation of oncogenic YAP/TAZ proteins, providing a valuable tool for developing YAP/TAZ-targeted TPD anti-cancer therapeutics. Moreover, Lin
et al. (2024) introduced lysine-deficient HiBiT and NanoLuc variants to eliminate potential degradation artifacts caused by traditional tagging systems [
67]. Their study highlighted that these variants maintain the sensitivity and specificity of the original NanoBiT system, making it an ideal tool for studying the effects of protein degraders like PROTACs.
4. Application of Biosensors in Drug Validation
Biosensors have revolutionized drug discovery by enabling real-time, sensitive monitoring of biological processes, providing critical insights into the efficacy, target engagement, and mechanism of action of drug candidates. In drug validation, various biosensors—including FRET, NanoBRET, and NanoBiT—play pivotal roles in assessing SM drugs, including PROTACs. This section discusses the roles of these biosensors in drug validation.
4.1. FRET and TR-FRET Biosensors
FRET biosensors are widely used to investigate PPIs and protein conformational dynamics upon ligand binding. These assays are crucial in validating drug efficacy by tracking how SMs modulate these interactions. For example, Sahin
et al. (2021) applied FRET biosensors to validate novel inhibitors disrupting anti-apoptotic BCL-2 complexes targeting the apoptotic pathway in cancer [
68]. Similarly, Borysko
et al. (2018) utilized FRET to visualize drug-induced dissociation of BRD4 from its interaction partners, contributing to the identification of novel BRD4 inhibitors [
69]. FRET biosensors are also highly effective in live-cell assays, facilitating the study of drug effects in physiological conditions. For example, Farmer
et al. (2022) used FRET-based peptide biosensors to monitor GPCR activation and quantify the influence of intracellular allosteric modulators, broadening the application of FRET in GPCR-targeting drug validation [
70].
TR-FRET builds on FRET principles by using time-resolved detection to minimize background fluorescence (
Figure 3). This enhanced sensitivity makes TR-FRET particularly valuable in studying binding affinities and protein conformational changes in drug validation. For example, Lin
et al. (2021) developed a TR-FRET assay to quantify ternary complex formation between BRD4, PROTAC molecules, and CRBN ligase, providing insights into PROTAC mechanism of action [
71]. In addition, Ali Abed
et al. (2023) applied TR-FRET assays to evaluate novel inhibitors disrupting the Keap1-Nrf2 interaction, a key target for oxidative stress modulation [
72]. Moreover, Payne
et al. (2023) further demonstrated the versatility of TR-FRET by using it to quantify endogenous BRD4 protein levels in cancer cells, enabling the rapid validation of small-molecule degraders of BRD4 [
73]. TR-FRET's adaptability makes it a valuable tool for validating a wide range of therapeutic candidates beyond PROTACs.
4.2. BRET and NanoBRET Biosensors
BRET and NanoBRET biosensors offer a non-invasive, highly sensitive platform for studying molecular interactions in living cells (
Figure 4). These techniques leverage energy transfer from luciferase to a fluorophore, providing real-time, quantitative insights into drug-target engagement. NanoBRET, with its improved luminescence and reduced background noise, has become particularly valuable in drug validation.
4.2.1. Ligand-Receptor Binding Inhibitor Validation
NanoBRET assays have been widely applied to study ligand-receptor interactions. Kozielewicz
et al. (2022) used NanoBRET to evaluate the binding affinities of SMs targeting the SMO receptor in Hedgehog signaling, providing critical insights into competition with known agonists [
74]. Similarly, Lay
et al. (2022) developed a NanoBRET assay to study intracellular binding kinetics of BET inhibitors, helping to optimize drug efficacy by measuring dissociation rates [
75].
4.2.2. Kinase Inhibitor Validation
NanoBRET is increasingly used to study kinase inhibitors. Yang
et al. (2023) developed cell-permeable NanoBRET probes to monitor Polo-like kinase 1 (PLK1) engagement, providing critical insights into kinase inhibitor potency in real-time [
76]. Moreover, Kong
et al. (2024) use NanoBRET biosensor to validate inhibitors targeting MERTK and AXL kinases, essential in overcoming chemoresistance in lung cancer cells [
77].
4.2.3. PROTAC and Molecular Glue Validation
NanoBRET is also used for the validation of PROTAC, offering valuable insights into their intracellular target engagement, bioavailability, and mechanisms of action. By measuring real-time interactions between PROTACs, target proteins, and E3 ligases, NanoBRET allows for the quantitative analysis of these interactions in living cells. This approach provides detailed data on ternary complex formation, intracellular accumulation, and target degradation, which are essential for optimizing PROTAC design. One of the primary applications of NanoBRET in PROTAC validation is the assessment of target engagement within live cells. The technology enables the measurement of the binding affinity between PROTACs and their targets by monitoring the energy transfer between NanoLuc-tagged proteins and fluorescent tracers. For instance, Vasta
et al. (2021) presented a high-throughput NanoBRET-based assay to evaluate the intracellular permeability and target engagement of PROTACs targeting CRBN and VHL E3 ligases [
78]. This approach helps prioritize PROTAC candidates based on their relative intracellular availability and engagement with E3 ligases, making NanoBRET a critical tool for drug discovery.
In addition to target engagement, NanoBRET plays a significant role in understanding the ubiquitination process, a key step in PROTAC-induced protein degradation. Bai
et al. (2022) highlighted how NanoBRET assays can be used to model and predict target protein ubiquitination efficiency [
79]. These insights are crucial for evaluating whether ternary complex formation leads to productive ubiquitination and subsequent proteasomal degradation. The ability of NanoBRET to measure such interactions in a live-cell context provides a comprehensive view of how PROTACs induce degradation, aiding in the optimization of their design.
NanoBRET is also useful for exploring new E3 ligase ligands in PROTAC design. Pei
et al. (2023) demonstrated the use of NanoBRET to confirm that a Piperlongumine-based PROTAC recruits KEAP1 as its E3 ligase to degrade CDK9 [
80]. This approach expands the toolkit for PROTAC design by identifying novel E3 ligases that can be leveraged for targeted degradation, thus broadening the applicability of PROTACs to different cellular contexts and protein targets. Furthermore, NanoBRET helps assess the bioavailability and intracellular accumulation of PROTACs, which are critical parameters influencing drug efficacy. Yu
et al. (2023) developed a NanoBRET-based platform to measure the intracellular accumulation of PROTACs, providing a quantitative method to evaluate their cellular permeability [
81]. This information is essential for improving the pharmacokinetics of PROTACs and ensuring that they reach sufficient intracellular concentrations to induce target degradation effectively. Zerfas
et al. (2023) further advanced the application of NanoBRET in drug validation by developing a CRBN-specific NanoBRET assay [
82]. This assay measures the occupancy of the CRBN binding site, allowing researchers to study the relationship between CRBN engagement and target degradation. Such data are vital for optimizing PROTACs that rely on CRBN-mediated degradation pathways, providing a detailed understanding of the interaction dynamics that drive their efficacy.
4.2.4. Covalent Inhibitor Validation
Covalent inhibitors, designed to form irreversible bonds with target proteins, benefit from NanoBRET’s ability to monitor real-time interactions and verify the specificity of drug binding. In their 2022 study, Borsari
et al. used NanoBRET to confirm covalent binding of phosphoinositide 3-kinase α (PI3Kα) inhibitors to a distal cysteine residue, highlighting how this method allows for precise assessment of target engagement and off-target interactions in live cells [
83]. By combining NanoBRET with X-ray crystallography and mass spectrometry, the authors validated the covalent interaction of acrylamide-based inhibitors with PI3Kα, providing a comprehensive view of drug action. In addition, Weeks
et al. (2022) also demonstrated the utility of NanoBRET biosensor in live-cell validation of Ras covalent inhibitors. Their study leveraged a Ras activity biosensor to track the inhibition kinetics of KRasG12C inhibitors in living cells [
84]. This approach enabled real-time observation of Ras activity modulation, thus facilitating the validation of covalent inhibitors and their dynamic effects within a cellular environment. Together, these studies underscore the effectiveness of using NanoBRET in validating covalent drug mechanisms and refining therapeutic strategies.
4.2.5. Validation of Candidate Inhibitors from DEL Screening
DEL Screening is a high-throughput technology used in drug discovery to identify SMs that can bind to target proteins or other biological macromolecules. The method involves creating large libraries of SMs, each attached to a unique DNA barcode that serves as an identifier. This DNA tag encodes the identity of the SM and allows for the rapid screening of millions to billions of compounds in a single experiment [
85]. NanoBRET biosensors are increasingly used to validate SMs identified from DEL screening. Teske
et al. (2023) developed cell-permeable BRET probes from DEL hits targeting aurora kinase A, allowing real-time assessment of target engagement [
37]. In addition, Madasu
et al. (2024) extended this approach to EPH receptor kinase inhibitors, using NanoBRET to evaluate cellular selectivity and potency, guiding further optimization [
86]. These studies suggest that NanoBRET can be used for validation of hits from DEL screening.
4.2. NanoBiT Biosensors
NanoBiT biosensors have become an essential tool in drug validation, particularly for monitoring real-time PPI in cellular processes. For instance, Hinz
et al. (2021) developed a NanoBiT assay to monitor the interaction between human Geranylgeranyltransferase Type I (GGTase-I) and its substrate Rap1B, providing real-time insights into interaction dynamics [
87]. This sensitive platform is instrumental for screening and validation of GGTase-I inhibitors, potential therapeutic agents in cancer treatment. NanoBiT effectively captures subtle changes in protein interactions caused by drug candidates, facilitating the assessment of drug efficacy in modulating these interactions. Similarly, Reyes-Alcaraz
et al. (2022) designed a NanoBiT-based assay to quantify membrane protein internalization and recycling, a process critical to both physiological and pathological mechanisms. This assay is particularly valuable for validating drug candidates targeting membrane proteins, especially in the context of drug-receptor interactions [
21].
Moreover, NanoBiT has been applied in diverse areas such as receptor oligomerization, as demonstrated by Morató
et al. (2023), where it was used to study the heterodimerization of S1R with the binding immunoglobulin protein (BiP) [
88]. This application highlights NanoBiT’s utility in validating compounds that affect PPIs, proving its relevance in drug discovery for cancer and neurodegenerative diseases. The versatility of NanoBiT extends to therapeutic drug monitoring (TDM) of monoclonal antibodies, as shown by Campbell
et al. (2023) [
89], enabling real-time monitoring of drug levels to improve treatment outcomes. Additionally, Claes and Bollen (2023) employed NanoBiT for validation of phosphatase subunit modulators [
60], while Rohrer et al. (2023) utilized NanoBiT to investigate RAF kinase dimerization, further showcasing its value in drug validation for key signaling pathways like RAF/MEK/ERK [
90].
Lastly, We developed a NanoBiT biosensor for GSDMD, a critical effector in pyroptosis linked to cancer and inflammation-related diseases [
91]. This biosensor allowed for the quantification of GSDMD’s intramolecular interaction and levels both
in vitro and
in vitro, exemplifying NanoBiT’s potential in cancer research and therapeutic validation.
5. Biosensors in Studying Cancer Cell Signaling Pathways
Advances in biosensor technologies have significantly enhanced our understanding of cancer cell signaling pathways by enabling the study of PPIs, ligand binding, enzyme activities, protein levels, and conformational changes. These biosensors provide insights both in vitro and in real-time within living cells or in vivo cancer xenograft mouse models. This section discusses various biosensor technologies, such as NanoBRET, NanoBiT, and FRET, and their applications in uncovering key aspects of cancer signaling pathways.
5.1. FRET-Based Biosensors
FRET-based biosensors provide valuable insights into kinase activities and protein interactions within specific cellular compartments. These biosensors leverage energy transfer between fluorophores to track molecular interactions with high temporal resolution. Hsu
et al. (2014) applied a split-luciferase complementation assay to identify multiple kinases involved in the assembly of ion channel complexes [
92]. Ouyang
et al. (2024) further demonstrated the power of FRET biosensors by developing a tool to monitor C-terminal Src kinase (CSK) activity in live cells, tracking the kinase’s activity within distinct membrane regions. This approach highlights how FRET biosensors can provide insights into spatial dynamics of signaling molecules.
5.2. NanoBRET Biosensors
NanoBRET has emerged as a powerful tool for studying ligand binding and protein interactions with high spatiotemporal resolution. This technology enables researchers to monitor drug-target engagement both in live cells and
in vitro. Alcobia
et al. (2018) used NanoBRET to visualize ligand binding to β2-adrenoceptors in real-time, demonstrating the method's effectiveness in providing dynamic insights into receptor-ligand interactions [
93]. Stoddart
et al. (2015) further improved ligand binding assays using NanoBRET to monitors GPCR activity in real-time, surpassing the sensitivity of traditional radioligand binding techniques [
94].
Dosquet
et al. (2021) showcased the versatility of NanoBRET by developing biosensors to monitor receptor tyrosine kinase (RTK) activity, focusing on EGFR and AXL [
95]. Building on this, Boon
et al. (2023) developed REGA-SIGN, a suite of NanoBRET-based biosensors capable of tracking G protein activation across multiple G protein families, illustrating the broad applicability of NanoBRET in signaling pathway studies [
96].
5.3. Firefly luciferase Biosensor and NanoBiT Biosensors
Firefly luciferase biosensor and NanoBiT biosensors are widely employed to study protein interactions and pathway regulation due to their ability to monitor real-time interactions with high sensitivity. We have recently developed a bioluminescence-based biosensor using firefly split luciferase assays to monitor the activity of LATS kinase, a core component of the Hippo signaling pathway [
41]. This LATS biosensor (LATS-BS) allowed non-invasive, real-time measurement of LATS activity
in vitro and
in vitro with high sensitivity and quantification. By using the LATS-BS and a library of kinase inhibitors, we performed a screen to identify kinases modulating LATS activity. This screen revealed VEGFR as an upstream regulator of the Hippo signaling pathway. We found that VEGFR activation by VEGF triggers PI3K/MAPK signaling, which subsequently inhibits LATS and activates the Hippo effectors YAP and TAZ. Further experiments showed the Hippo pathway is a critical mediator of VEGF-induced angiogenesis and tumor vasculogenic mimicry. Inhibition of YAP/TAZ reduced VEGF-stimulated angiogenesis in multiple
in vitro and
in vitro models. We have also developed a more sensitive NanoBiT biosensors that can monitor LATS activity [
97]. Gain-of-functional and loss-of-functional screenings using this LATS biosensor identified many receptor tyrosine kinases (e.g. ALK, FGFR, AXL, MERTK, and RET) as novel regulators of the Hippo signaling pathway in tumorigenesis, metastasis, and immune evasion [
97,
98]. By using this biosensor for a gain-of-functional screening, we have also identified several tyrosine phosphatases including PTPN12 as novel regulator of the LATS kinase and the Hippo pathway [
99]. Similarly, Poti
et al. (2023) further expanded NanoBiT applications with PhALC (Phosphorylation-Assisted Luciferase Complementation) [
100], enabling real-time monitoring of kinase activity and PPIs. In addition, Kupcho
et al. (2019) developed a real-time bioluminescent annexin V assay using NanoBiT to detect apoptosis [
101], while Inoue
et al. (2019) applied this technology to comprehensively profile GPCR-G protein coupling selectivity [
102]. Moreover, Zeghal
et al. (2023) and Pipchuk
et al. (2024) demonstrated the utility of NanoBiT biosensor in studying GPCR signaling and Merlin tumor suppressor protein conformation, respectively [
22,
103].
6. Challenges and Future Perspectives
The application of biosensors in drug discovery, particularly in cancer research, presents several challenges. Despite their precision and ability to monitor real-time molecular interactions, the integration of biosensors into HTS for drug compounds faces technical and operational hurdles. One significant challenge is the complexity of designing biosensors that accurately mimic the physiological conditions of cancer cells. This includes ensuring biosensors can function effectively in complex environments, such as 3D tumor models, where traditional 2D cultures fail to replicate the intricate interactions of the tumor microenvironment. Another challenge lies in the scalability of biosensors for HTS. The sensitivity of biosensors often requires sophisticated instrumentation and precise calibration, limiting their accessibility for large-scale drug screening. Additionally, biosensors sometimes exhibit limited stability over long periods, complicating their use in extended screening processes. Achieving high specificity while avoiding cross-reactivity between biomolecules is also a challenge, as unintended interactions can lead to false positives, reducing the reliability of the results. Moreover, validation of hits from HTS using biosensor can be also challenging. Multiple approaches have to be employed to further exclude the off-target hits obtained due to quenching of bioluminescent or fluorescent signals.
Looking ahead, the future of biosensors in drug discovery is promising. Advances in nanomaterials, such as carbon nanotubes and quantum dots, offer the potential to enhance biosensor sensitivity and specificity. These materials can improve signal transduction and lower detection limits, making biosensors more efficient for early disease detection and personalized medicine. Additionally, integrating artificial intelligence (AI) with biosensor technologies could help analyze large datasets generated by HTS, facilitating the identification of novel drug candidates and optimizing biosensor design.
7. Conclusion
Biosensors such as FRET, TR-FRET, NanoBRET, and NanoBiT have significantly advanced our understanding of cancer cell signaling pathways. These technologies enable real-time, sensitive, and specific detection of protein levels, PPI, ligand binding, and enzyme activities in live cells. By uncovering novel regulatory mechanisms, identifying new drug targets, and providing valuable tools for drug discovery and validation, biosensors play an essential role in modern cancer research and drug discovery. As these technologies continue to evolve, they promise to yield deeper insights into the complex signaling networks that underlie cancer biology, facilitating the development of innovative therapeutic strategies.
Author Contributions
Conceptualization, X.Y.; writing—original draft preparation, T.K, X.Y.; writing—review and editing, T.K, X.Y.; visualization, TK, X.Y.; supervision, X.Y.; project administration, X.Y.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Canadian Cancer Society-Challenge grant (Grant#369904) and Canadian Institute of Health Research (CIHR), grant numbers 186142 and 148629 .
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gershell, L.J.; Atkins, J.H. A brief history of novel drug discovery technologies. Nat Rev Drug Discov 2003, 2, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, H.; Ver Steeg, G.; Godzik, A. Advances in AI for Protein Structure Prediction: Implications for Cancer Drug Discovery and Development. Biomolecules 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cuozzo, J. Review article: High-throughput affinity-based technologies for small-molecule drug discovery. J Biomol Screen 2009, 14, 1157–1164. [Google Scholar] [CrossRef]
- Blay, V.; Tolani, B.; Ho, S.P.; Arkin, M.R. High-Throughput Screening: today's biochemical and cell-based approaches. Drug Discov Today 2020, 25, 1807–1821. [Google Scholar] [CrossRef]
- Salame, N.; Fooks, K.; El-Hachem, N.; Bikorimana, J.P.; Mercier, F.E.; Rafei, M. Recent Advances in Cancer Drug Discovery Through the Use of Phenotypic Reporter Systems, Connectivity Mapping, and Pooled CRISPR Screening. Front Pharmacol 2022, 13, 852143. [Google Scholar] [CrossRef]
- Guo, H.; Xu, X.; Zhang, J.; Du, Y.; Yang, X.; He, Z.; Zhao, L.; Liang, T.; Guo, L. The Pivotal Role of Preclinical Animal Models in Anti-Cancer Drug Discovery and Personalized Cancer Therapy Strategies. Pharmaceuticals (Basel) 2024, 17. [Google Scholar] [CrossRef]
- Calpe, B.; Kovacs, W.J. High-throughput screening in multicellular spheroids for target discovery in the tumor microenvironment. Expert Opin Drug Discov 2020, 15, 955–967. [Google Scholar] [CrossRef]
- Sun, G.; Rong, D.; Li, Z.; Sun, G.; Wu, F.; Li, X.; Cao, H.; Cheng, Y.; Tang, W.; Sun, Y. Role of Small Molecule Targeted Compounds in Cancer: Progress, Opportunities, and Challenges. Front Cell Dev Biol 2021, 9, 694363. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct Target Ther 2021, 6, 201. [Google Scholar] [CrossRef]
- Brown, D.G.; Boström, J. Where Do Recent Small Molecule Clinical Development Candidates Come From? J Med Chem 2018, 61, 9442–9468. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Crown, J. Drugging "undruggable" genes for cancer treatment: Are we making progress? Int J Cancer 2021, 148, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.; Tashakor, A.; Hosseinkhani, S. Split-luciferase complementary assay: Applications, recent developments, and future perspectives. Analytical and Bioanalytical Chemistry 2014, 406, 5541–5560. [Google Scholar] [CrossRef] [PubMed]
- Quazi, S. Application of biosensors in cancers, an overview. Front Bioeng Biotechnol 2023, 11, 1193493. [Google Scholar] [CrossRef]
- Wehr, M.C.; Rossner, M.J. Split protein biosensor assays in molecular pharmacological studies. Drug Discov Today 2016, 21, 415–429. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Zhou, M.; Wu, B.; Zhou, J. Application of Biosensors in Detecting Breast Cancer Metastasis. Sensors (Basel) 2023, 23. [Google Scholar] [CrossRef]
- Abdul Wahab, M.R.; Palaniyandi, T.; Viswanathan, S.; Baskar, G.; Surendran, H.; Gangadharan, S.G.D.; Sugumaran, A.; Sivaji, A.; Kaliamoorthy, S.; Kumarasamy, S. Biomarker-specific biosensors revolutionise breast cancer diagnosis. Clin Chim Acta 2024, 555, 117792. [Google Scholar] [CrossRef]
- Sanko, V.; Kuralay, F. Label-Free Electrochemical Biosensor Platforms for Cancer Diagnosis: Recent Achievements and Challenges. Biosensors (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Das, S.; Devireddy, R.; Gartia, M.R. Surface Plasmon Resonance (SPR) Sensor for Cancer Biomarker Detection. Biosensors (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Simard, J.R.; Lee, L.; Vieux, E.; Improgo, R.; Tieu, T.; Phillips, A.J.; Fisher, S.L.; Pollock, R.M.; Park, E. High-Throughput Quantitative Assay Technologies for Accelerating the Discovery and Optimization of Targeted Protein Degradation Therapeutics. SLAS Discov 2021, 26, 503–517. [Google Scholar] [CrossRef]
- Reyes-Alcaraz, A.; Lucero Garcia-Rojas, E.Y.; Merlinsky, E.A.; Seong, J.Y.; Bond, R.A.; McConnell, B.K. A NanoBiT assay to monitor membrane proteins trafficking for drug discovery and drug development. Commun Biol 2022, 5, 212. [Google Scholar] [CrossRef] [PubMed]
- Pipchuk, A.; Kelly, T.; Carew, M.; Nicol, C.; Yang, X. Development of Novel Bioluminescent Biosensors Monitoring the Conformation and Activity of the Merlin Tumour Suppressor. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Pipchuk, A.; Yang, X. Using Biosensors to Study Protein-Protein Interaction in the Hippo Pathway. Front Cell Dev Biol 2021, 9, 660137. [Google Scholar] [CrossRef] [PubMed]
- Nouri, K.; Azad, T.; Ling, M.; van Rensburg, H.J.J.; Pipchuk, A.; Shen, H.; Hao, Y.; Zhang, J.; Yang, X. Identification of celastrol as a novel YAP-TEAD inhibitor for cancer therapy by high throughput screening with ultrasensitive YAP/TAZ-TEAD biosensors. Cancers (Basel) 2019, 11, 1596. [Google Scholar] [CrossRef]
- Kozielewicz, P.; Schihada, H.; Schulte, G. Employing Genetically Encoded, Biophysical Sensors to Understand WNT/Frizzled Interaction and Receptor Complex Activation. Handb Exp Pharmacol 2021, 269, 101–115. [Google Scholar] [CrossRef]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat Rev Bioeng 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications - An Overview. Front Bioeng Biotechnol 2016, 4, 11. [Google Scholar] [CrossRef]
- Tetyana, P.S. Poslet Morgan. Biosensors: Design, development and applications. IntechOpen 2021. [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors (Basel) 2021, 21. [Google Scholar] [CrossRef]
- Dale, N.C.; Johnstone, E.K.M.; White, C.W.; Pfleger, K.D.G. NanoBRET: The Bright Future of Proximity-Based Assays. Front Bioeng Biotechnol 2019, 7, 56. [Google Scholar] [CrossRef]
- Verma, A.K.; Noumani, A.; Yadav, A.K.; Solanki, P.R. FRET Based Biosensor: Principle Applications Recent Advances and Challenges. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Ouyang, W.; Niu, Q.; Qui, M.; Fu, H.; Du, Y.; Mo, X. A multiplexed time-resolved fluorescence resonance energy transfer ultrahigh-throughput screening assay for targeting SMAD4-SMAD3-DNA complex. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, D.; Troha, A.H.; Ahn, H.M.; Qian, K.; Liang, B.; Du, Y.; Fu, H.; Ivanov, A.A. Discovery of the first chemical tools to regulate MKK3-mediated MYC activation in cancer. Bioorg Med Chem 2021, 45, 116324. [Google Scholar] [CrossRef] [PubMed]
- Machleidt, T.; Woodroofe, C.C.; Schwinn, M.K.; Méndez, J.; Robers, M.B.; Zimmerman, K.; Otto, P.; Daniels, D.L.; Kirkland, T.A.; Wood, K.V. NanoBRET--A Novel BRET Platform for the Analysis of Protein-Protein Interactions. ACS Chem Biol 2015, 10, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Vunnam, N.; Lewis, A.K.; Chiu, T.L.; Brummel, B.E.; Schaaf, T.M.; Grant, B.D.; Bawaskar, P.; Thomas, D.D.; Sachs, J.N. An Innovative High-Throughput Screening Approach for Discovery of Small Molecules That Inhibit TNF Receptors. SLAS Discov 2017, 22, 950–961. [Google Scholar] [CrossRef]
- Durrant, D.E.; Smith, E.A.; Goncharova, E.I.; Sharma, N.; Alexander, P.A.; Stephen, A.G.; Henrich, C.J.; Morrison, D.K. Development of a High-throughput NanoBRET Screening Platform to Identify Modulators of the RAS/RAF Interaction. Mol Cancer Ther 2021, 20, 1743–1754. [Google Scholar] [CrossRef]
- Teske, K.A.; Su, W.; Corona, C.R.; Wen, J.; Deng, J.; Ping, Y.; Zhang, Z.; Zhang, Q.; Wilkinson, J.; Beck, M.T.; et al. DELs enable the development of BRET probes for target engagement studies in cells. Cell Chem Biol 2023, 30, 987–998.e924. [Google Scholar] [CrossRef]
- Dixon, A.S.; Schwinn, M.K.; Hall, M.P.; Zimmerman, K.; Otto, P.; Lubben, T.H.; Butler, B.L.; Binkowski, B.F.; Machleidt, T.; Kirkland, T.A.; et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chemical Biology 2016, 11, 400–408. [Google Scholar] [CrossRef]
- England, C.G.; Ehlerding, E.B.; Cai, W. NanoLuc: A Small Luciferase Is Brightening Up the Field of Bioluminescence. Bioconjugate chemistry 2016, 27, 1175–1187. [Google Scholar] [CrossRef]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS chemical biology 2012, 7, 1848–1857. [Google Scholar] [CrossRef]
- Azad, T.; Janse van Rensburg, H.J.; Lightbody, E.D.; Neveu, B.; Champagne, A.; Ghaffari, A.; Kay, V.R.; Hao, Y.; Shen, H.; Yeung, B.; et al. A LATS biosensor functional screen identifies VEGFR as a novel regulator of the Hippo pathway in angiogenesis. Nat.Commun. 2018, 9, 1061. [Google Scholar] [CrossRef]
- Azad, T.; Nouri, K.; Janse van Rensburg, H.J.; Hao, Y.; Yang, X. Monitoring Hippo signaling pathway activity using a luciferase-based large tumor suppressor (LATS) biosensor. J Vis Exp 2018. [Google Scholar] [CrossRef]
- Wu, L.; Ge, A.; Hao, Y.; Yang, X. Development of a New HiBiT Biosensor Monitoring Stability of YAP/TAZ Proteins in Cells. Chemosensors 2023, 11, 492. [Google Scholar] [CrossRef]
- He, J.; Wink, S.; de Bont, H.; Le Dévédec, S.; Zhang, Y.; van de Water, B. FRET biosensor-based kinase inhibitor screen for ERK and AKT activity reveals differential kinase dependencies for proliferation in TNBC cells. Biochem Pharmacol 2019, 169, 113640. [Google Scholar] [CrossRef]
- Senarisoy, M.; Barette, C.; Lacroix, F.; De Bonis, S.; Stelter, M.; Hans, F.; Kleman, J.P.; Fauvarque, M.O.; Timmins, J. Förster Resonance Energy Transfer Based Biosensor for Targeting the hNTH1-YB1 Interface as a Potential Anticancer Drug Target. ACS Chem Biol 2020, 15, 990–1003. [Google Scholar] [CrossRef]
- Liu, L.; Limsakul, P.; Meng, X.; Huang, Y.; Harrison, R.E.S.; Huang, T.S.; Shi, Y.; Yu, Y.; Charupanit, K.; Zhong, S.; et al. Integration of FRET and sequencing to engineer kinase biosensors from mammalian cell libraries. Nat Commun 2021, 12, 5031. [Google Scholar] [CrossRef]
- Hao, Y.; Langford, T.F.; Moon, S.J.; Eller, K.A.; Sikes, H.D. Screening compound libraries for H(2)O(2)-mediated cancer therapeutics using a peroxiredoxin-based sensor. Cell Chem Biol 2022, 29, 625–635.e623. [Google Scholar] [CrossRef]
- Vunnam, N.; Yang, M.; Lo, C.H.; Paulson, C.; Fiers, W.D.; Huber, E.; Been, M.; Ferguson, D.M.; Sachs, J.N. Zafirlukast Is a Promising Scaffold for Selectively Inhibiting TNFR1 Signaling. ACS Bio Med Chem Au 2023, 3, 270–282. [Google Scholar] [CrossRef]
- Zhang, F.C.; Sun, Z.Y.; Liao, L.P.; Zuo, Y.; Zhang, D.; Wang, J.; Chen, Y.T.; Xiao, S.H.; Jiang, H.; Lu, T.; et al. Discovery of novel CBP bromodomain inhibitors through TR-FRET-based high-throughput screening. Acta Pharmacol Sin 2020, 41, 286–292. [Google Scholar] [CrossRef]
- Tang, C.; Mo, X.; Niu, Q.; Wahafu, A.; Yang, X.; Qui, M.; Ivanov, A.; Du, Y.; Fu, H. Hypomorph mutation-directed small-molecule protein-protein interaction inducers to restore mutant SMAD4-suppressed TGF-β signaling. Cell Chem. Biol. 2021, 28, 636–647. [Google Scholar] [CrossRef]
- Xiong, J.; Pecchi, V.G.; Qui, M.; Ivanov, A.A.; Mo, X.; Niu, Q.; Chen, X.; Fu, H.; Du, Y. Development of a Time-Resolved Fluorescence Resonance Energy Transfer Ultrahigh-Throughput Screening Assay for Targeting the NSD3 and MYC Interaction. Assay Drug Dev Technol 2018, 16, 96–106. [Google Scholar] [CrossRef]
- Ouyang, W.; Li, Q.; Niu, Q.; Qui, M.; Fu, H.; Du, Y.; Mo, X. A multiplexed time-resolved fluorescence resonance energy transfer ultrahigh-throughput screening assay for targeting the SMAD4-SMAD3-DNA complex. J Mol Cell Biol 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, D.; Katis, V.L.; Zoeller, E.L.; Qui, M.; Levey, A.I.; Gileadi, O.; Fu, H. Development of a time-resolved fluorescence resonance energy transfer ultra-high throughput screening assay targeting SYK and FCER1G interaction. SLAS Discov 2024, 29, 100177. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Abu-Zaid, A.; Lin, W.; Low, J.; Abdolvahabi, A.; Jin, H.; Wu, Q.; Cooke, B.; Fang, J.; Bowling, J.; et al. 17-DMAG dually inhibits Hsp90 and histone lysine demethylases in alveolar rhabdomyosarcoma. iScience 2021, 24, 101996. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.E.; Hardy, P.B.; Schomburg, N.K.; Wang, X.; Kireev, D.; Rossman, K.L.; Pearce, K.H. Development of a high-throughput TR-FRET screening assay for a fast-cycling KRAS mutant. SLAS Discov 2023, 28, 39–47. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yonezawa, T.; Sakamoto, J.; Furuya, T.; Osawa, M.; Ikeda, K. Identification of novel inhibitors of Keap1/Nrf2 by a promising method combining protein-protein interaction-oriented library and machine learning. Sci Rep 2021, 11, 7420. [Google Scholar] [CrossRef]
- Ganier, L.; Betzi, S.; Derviaux, C.; Roche, P.; Dessaux, C.; Muller, C.; Hoffer, L.; Morelli, X.; Borg, J.P. Discovery of Small-Molecule Inhibitors of the PTK7/β-Catenin Interaction Targeting the Wnt Signaling Pathway in Colorectal Cancer. ACS Chem Biol 2022, 17, 1061–1072. [Google Scholar] [CrossRef]
- Cartwright, T.N.; Meyer, S.K.; Higgins, J.M.G. Robustness of NanoBiT luciferase complementation technology in the presence of widely used kinase inhibitors. SLAS Discov 2022, 27, 471–475. [Google Scholar] [CrossRef]
- Miyamoto, K.; Sawa, M. Development of Highly Sensitive Biosensors of RAF Dimerization in Cells. Sci Rep 2019, 9, 636. [Google Scholar] [CrossRef]
- Claes, Z.; Bollen, M. A split-luciferase lysate-based approach to identify small-molecule modulators of phosphatase subunit interactions. Cell Chem Biol 2023, 30, 1666–1679.e1666. [Google Scholar] [CrossRef]
- Cooley, R.; Kara, N.; Hui, N.S.; Tart, J.; Roustan, C.; George, R.; Hancock, D.C.; Binkowski, B.F.; Wood, K.V.; Ismail, M.; et al. Development of a cell-free split-luciferase biochemical assay as a tool for screening for inhibitors of challenging protein-protein interaction targets. Wellcome Open Res 2020, 5, 20. [Google Scholar] [CrossRef]
- Kong, N.R.; Jones, L.H. Clinical Translation of Targeted Protein Degraders. Clin Pharmacol Ther 2023, 114, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Bhole, R.P.; Kute, P.R.; Chikhale, R.V.; Bonde, C.G.; Pant, A.; Gurav, S.S. Unlocking the potential of PROTACs: A comprehensive review of protein degradation strategies in disease therapy. Bioorg Chem 2023, 139, 106720. [Google Scholar] [CrossRef] [PubMed]
- Lankford, K.P.; Hulleman, J.D. Protocol for HiBiT tagging endogenous proteins using CRISPR-Cas9 gene editing. STAR Protoc 2024, 5, 103000. [Google Scholar] [CrossRef] [PubMed]
- Schwinn, M.K.; Machleidt, T.; Zimmerman, K.; Eggers, C.T.; Dixon, A.S.; Hurst, R.; Hall, M.P.; Encell, L.P.; Binkowski, B.F.; Wood, K.V. CRISPR-Mediated Tagging of Endogenous Proteins with a Luminescent Peptide. ACS chemical biology 2018, 13, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Matsushima, T.; Kurimoto, R.; Chiba, T.; Inutani, Y.; Asahara, H. Identification of chemical compounds regulating PD-L1 by introducing HiBiT-tagged cells. FEBS Lett 2021, 595, 563–576. [Google Scholar] [CrossRef]
- Lin, H.; Riching, K.; Lai, M.P.; Lu, D.; Cheng, R.; Qi, X.; Wang, J. Lysineless HiBiT and NanoLuc Tagging Systems as Alternative Tools for Monitoring Targeted Protein Degradation. ACS Med Chem Lett 2024, 15, 1367–1375. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, M.D.; Avsar, T.; Durdagi, S. Hybrid In Silico and TR-FRET-Guided Discovery of Novel BCL-2 Inhibitors. ACS Pharmacol Transl Sci 2021, 4, 1111–1123. [Google Scholar] [CrossRef]
- Borysko, P.; Moroz, Y.S.; Vasylchenko, O.V.; Hurmach, V.V.; Starodubtseva, A.; Stefanishena, N.; Nesteruk, K.; Zozulya, S.; Kondratov, I.S.; Grygorenko, O.O. Straightforward hit identification approach in fragment-based discovery of bromodomain-containing protein 4 (BRD4) inhibitors. Bioorg Med Chem 2018, 26, 3399–3405. [Google Scholar] [CrossRef]
- Farmer, J.P.; Mistry, S.N.; Laughton, C.A.; Holliday, N.D. Development of fluorescent peptide G protein-coupled receptor activation biosensors for NanoBRET characterization of intracellular allosteric modulators. Faseb j 2022, 36, e22576. [Google Scholar] [CrossRef]
- Lin, W.; Chen, T. General Stepwise Approach to Optimize a TR-FRET Assay for Characterizing the BRD/PROTAC/CRBN Ternary Complex. ACS Pharmacol Transl Sci 2021, 4, 941–952. [Google Scholar] [CrossRef]
- Abed, D.A.; Ali, A.R.; Lee, S.; Nguyen, M.U.; Verzi, M.P.; Hu, L. Optimization of the C2 substituents on the 1,4-bis(arylsulfonamido)naphthalene-N,N'-diacetic acid scaffold for better inhibition of Keap1-Nrf2 protein-protein interaction. Eur J Med Chem 2023, 252, 115302. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.C.; Maksoud, S.; Tannous, B.A.; Mazitschek, R. A direct high-throughput protein quantification strategy facilitates discovery and characterization of a celastrol-derived BRD4 degrader. Cell Chem Biol 2022, 29, 1333–1340.e1335. [Google Scholar] [CrossRef] [PubMed]
- Kozielewicz, P.; Schulte, G. NanoBRET and NanoBiT/BRET-Based Ligand Binding Assays Permit Quantitative Assessment of Small Molecule Ligand Binding to Smoothened. Methods Mol Biol 2022, 2374, 195–204. [Google Scholar] [CrossRef]
- Lay, C.S.; Thomas, D.A.; Evans, J.P.; Campbell, M.; McCombe, K.; Phillipou, A.N.; Gordon, L.J.; Jones, E.J.; Riching, K.; Mahmood, M.; et al. Development of an intracellular quantitative assay to measure compound binding kinetics. Cell Chem Biol 2023, 30, 1692. [Google Scholar] [CrossRef]
- Yang, X.; Smith, J.L.; Beck, M.T.; Wilkinson, J.M.; Michaud, A.; Vasta, J.D.; Robers, M.B.; Willson, T.M. Development of Cell Permeable NanoBRET Probes for the Measurement of PLK1 Target Engagement in Live Cells. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Kong, D.; Tian, Q.; Chen, Z.; Zheng, H.; Stashko, M.A.; Yan, D.; Earp, H.S.; Frye, S.V.; DeRyckere, D.; Kireev, D.; et al. Discovery of Novel Macrocyclic MERTK/AXL Dual Inhibitors. J Med Chem 2024, 67, 5866–5882. [Google Scholar] [CrossRef]
- Chi, Z.; Chen, S.; Yang, D.; Cui, W.; Lu, Y.; Wang, Z.; Li, M.; Yu, W.; Zhang, J.; Jiang, Y.; et al. Gasdermin D-mediated metabolic crosstalk promotes tissue repair. Nature 2024. [Google Scholar] [CrossRef]
- Bai, N.; Riching, K.M.; Makaju, A.; Wu, H.; Acker, T.M.; Ou, S.C.; Zhang, Y.; Shen, X.; Bulloch, D.N.; Rui, H.; et al. Modeling the CRL4A ligase complex to predict target protein ubiquitination induced by cereblon-recruiting PROTACs. J Biol Chem 2022, 298, 101653. [Google Scholar] [CrossRef]
- Pei, J.; Xiao, Y.; Liu, X.; Hu, W.; Sobh, A.; Yuan, Y.; Zhou, S.; Hua, N.; Mackintosh, S.G.; Zhang, X.; et al. Piperlongumine conjugates induce targeted protein degradation. Cell Chem Biol 2023, 30, 203–213.e217. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J. Quantitative measurement of PROTAC intracellular accumulation. Methods Enzymol 2023, 681, 189–214. [Google Scholar] [CrossRef]
- Zerfas, B.L.; Huerta, F.; Liu, H.; Du, G.; Gray, N.S.; Jones, L.H.; Nowak, R.P. Advancing targeted protein degrader discovery by measuring cereblon engagement in cells. Methods Enzymol 2023, 681, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Borsari, C.; Keles, E.; McPhail, J.A.; Schaefer, A.; Sriramaratnam, R.; Goch, W.; Schaefer, T.; De Pascale, M.; Bal, W.; Gstaiger, M.; et al. Covalent Proximity Scanning of a Distal Cysteine to Target PI3Kα. J Am Chem Soc 2022, 144, 6326–6342. [Google Scholar] [CrossRef]
- Weeks, R.; Zhou, X.; Yuan, T.L.; Zhang, J. Fluorescent Biosensor for Measuring Ras Activity in Living Cells. J Am Chem Soc 2022, 144, 17432–17440. [Google Scholar] [CrossRef]
- Sunkari, Y.K.; Siripuram, V.K.; Nguyen, T.L.; Flajolet, M. High-power screening (HPS) empowered by DNA-encoded libraries. Trends Pharmacol Sci 2022, 43, 4–15. [Google Scholar] [CrossRef]
- Madasu, C.; Liao, Z.; Parks, S.E.; Sharma, K.L.; Bohren, K.M.; Ye, Q.; Li, F.; Palaniappan, M.; Tan, Z.; Yuan, F.; et al. Identification of potent pan-ephrin receptor kinase inhibitors using DNA-encoded chemistry technology. Proc Natl Acad Sci U S A 2024, 121, e2322934121. [Google Scholar] [CrossRef]
- Hinz, S.; Jung, D.; Hauert, D.; Bachmann, H.S. Molecular and Pharmacological Characterization of the Interaction between Human Geranylgeranyltransferase Type I and Ras-Related Protein Rap1B. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Morató, X.; Fernández-Dueñas, V.; Pérez-Villamor, P.; Valle-León, M.; Vela, J.M.; Merlos, M.; Burgueño, J.; Ciruela, F. Development of a Novel σ(1) Receptor Biosensor Based on Its Heterodimerization with Binding Immunoglobulin Protein in Living Cells. ACS Chem Neurosci 2023, 14, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.; Adamson, H.; Luxton, T.; Tiede, C.; Wälti, C.; Tomlinson, D.C.; Jeuken, L.J.C. Therapeutic drug monitoring of immunotherapies with novel Affimer-NanoBiT sensor construct. Sens Diagn 2024, 3, 104–111. [Google Scholar] [CrossRef]
- Rohrer, L.; Spohr, C.; Beha, C.; Griffin, R.; Braun, S.; Halbach, S.; Brummer, T. Analysis of RAS and drug induced homo- and heterodimerization of RAF and KSR1 proteins in living cells using split Nanoluc luciferase. Cell Commun Signal 2023, 21, 136. [Google Scholar] [CrossRef]
- Kelly, T.; Bhandari, S.; Carew, M.; Rubino, R.; Nicol, C.; Yang, X. A Novel Bioluminescent Biosensor Quantifying Intramolecular Interaction and Levels of Pyroptosis Effector GSDMD. Cells 2024, 13. [Google Scholar] [CrossRef]
- Hsu, W.C.; Nenov, M.N.; Shavkunov, A.; Panova, N.; Zhan, M.; Laezza, F. Identifying a kinase network regulating FGF14:Nav1.6 complex assembly using split-luciferase complementation. PLoS ONE 2015, 10, e0117246. [Google Scholar] [CrossRef] [PubMed]
- Alcobia, D.C.; Ziegler, A.I.; Kondrashov, A.; Comeo, E.; Mistry, S.; Kellam, B.; Chang, A.; Woolard, J.; Hill, S.J.; Sloan, E.K. Visualizing Ligand Binding to a GPCR In Vivo Using NanoBRET. iScience 2018, 6, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, L.A.; Kilpatrick, L.E.; Hill, S.J. NanoBRET Approaches to Study Ligand Binding to GPCRs and RTKs. Trends Pharmacol Sci 2018, 39, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Dosquet, H.; Neirinckx, V.; Meyrath, M.; Wantz, M.; Haan, S.; Niclou, S.P.; Szpakowska, M.; Chevigné, A. Nanoluciferase-based complementation assays to monitor activation, modulation and signaling of receptor tyrosine kinases (RTKs). Methods Enzymol 2023, 682, 1–16. [Google Scholar] [CrossRef]
- Boon, K.; Vanalken, N.; Meyen, E.; Schols, D.; Van Loy, T. REGA-SIGN: Development of a Novel Set of NanoBRET-Based G Protein Biosensors. Biosensors (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Azad, T.N.K.; Janse van Rensburg, H.J.; Maritan, S.M.; Wu, L.; Hao, Y.; Montminy, T.; Yu, J.; Khanal, K.; Mulligan, L.M.; Yang, X. A gain-of-functional screen identifies the Hippo pathway as a central mediator of receptor tyrosine kinases during tumorigenesis. Oncogene 2020, 39, 334–355. [Google Scholar] [CrossRef]
- Nouri, K.A.T.; Lightbody, E.; Khanal, P.; Nicol, C.J.; Yang, X. A kinome-wide screen using a NanoLuc LATS luminescent biosensor identifies ALK as a novel regulator of the Hippo pathway in tumorigenesis and immune evasion. FASEB Journal 2019, 33, 12487–12499. [Google Scholar] [CrossRef]
- Sarmasti Emami, S.; Ge, A.; Zhang, D.; Hao, Y.; Ling, M.; Rubino, R.; Nicol, C.J.B.; Wang, W.; Yang, X. Identification of PTPN12 Phosphatase as a Novel Negative Regulator of Hippo Pathway Effectors YAP/TAZ in Breast Cancer. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Póti, Á.L.; Dénes, L.; Papp, K.; Bató, C.; Bánóczi, Z.; Reményi, A.; Alexa, A. Phosphorylation-Assisted Luciferase Complementation Assay Designed to Monitor Kinase Activity and Kinase-Domain-Mediated Protein-Protein Binding. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef]
- Inoue, A.; Raimondi, F.; Kadji, F.M.N.; Singh, G.; Kishi, T.; Uwamizu, A.; Ono, Y.; Shinjo, Y.; Ishida, S.; Arang, N.; et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 2019, 177, 1933–1947.e1925. [Google Scholar] [CrossRef]
- Zeghal, M.; Matte, K.; Venes, A.; Patel, S.; Laroche, G.; Sarvan, S.; Joshi, M.; Rain, J.C.; Couture, J.F.; Giguère, P.M. Development of a V5-tag-directed nanobody and its implementation as an intracellular biosensor of GPCR signaling. J Biol Chem 2023, 299, 105107. [Google Scholar] [CrossRef]
Figure 1.
Electrochemical biosensors.
Figure 1.
Electrochemical biosensors.
Figure 2.
Schematic representation of intramolecular and intermolecular FRET biosensors. (A) Intramolecular FRET. A single protein with CFP and yellow fluorescent protein (YFP) attached at two ends undergoes conformational changes, bringing CFP and YFP into proximity. Energy transfer occurs when CFP is excited, leading to emission from YFP. (B) Intermolecular FRET. Two interacting proteins, A and B, are tagged with CFP and YFP, respectively. When the proteins interact closely, FRET occurs as energy from the donor (CFP) is transferred to the acceptor (YFP), resulting in fluorescence from YFP. These FRET configurations allow the monitoring of protein interactions and conformational changes in live cells.
Figure 2.
Schematic representation of intramolecular and intermolecular FRET biosensors. (A) Intramolecular FRET. A single protein with CFP and yellow fluorescent protein (YFP) attached at two ends undergoes conformational changes, bringing CFP and YFP into proximity. Energy transfer occurs when CFP is excited, leading to emission from YFP. (B) Intermolecular FRET. Two interacting proteins, A and B, are tagged with CFP and YFP, respectively. When the proteins interact closely, FRET occurs as energy from the donor (CFP) is transferred to the acceptor (YFP), resulting in fluorescence from YFP. These FRET configurations allow the monitoring of protein interactions and conformational changes in live cells.
Figure 3.
Schematic presentation of TR-FRET biosensors. The target protein is bound by two antibodies: Eu-Ab1 labeled with a europium (Eu) donor fluorophore, and D2-Ab2 labeled with the D2 acceptor fluorophore. Upon excitation at 320-340 nm, the Eu donor emits at 615 nm. When the antibodies are in close proximity due to binding the same protein or two interacting proteins, FRET occurs from the Eu donor to the acceptor, resulting in a TR-FRET emission at 665 nm.
Figure 3.
Schematic presentation of TR-FRET biosensors. The target protein is bound by two antibodies: Eu-Ab1 labeled with a europium (Eu) donor fluorophore, and D2-Ab2 labeled with the D2 acceptor fluorophore. Upon excitation at 320-340 nm, the Eu donor emits at 615 nm. When the antibodies are in close proximity due to binding the same protein or two interacting proteins, FRET occurs from the Eu donor to the acceptor, resulting in a TR-FRET emission at 665 nm.
Figure 4.
Schematic representation of the BRET and NanoBRET biosensor. The energy donor such as a luciferase enzyme (RLuc or NanoLuc), is fused to one protein of interest (Protein A), while a fluorescent acceptor is attached to the interacting partner (Protein B)(A). When the Protein A and B interact within a close distance (~5-10 nm), the luciferase emits energy in the presence of its substrate (e.g. coelenteramide), which excites the acceptor fluorophore (A), producing a measurable light signal (B).
Figure 4.
Schematic representation of the BRET and NanoBRET biosensor. The energy donor such as a luciferase enzyme (RLuc or NanoLuc), is fused to one protein of interest (Protein A), while a fluorescent acceptor is attached to the interacting partner (Protein B)(A). When the Protein A and B interact within a close distance (~5-10 nm), the luciferase emits energy in the presence of its substrate (e.g. coelenteramide), which excites the acceptor fluorophore (A), producing a measurable light signal (B).
Figure 5.
Schematic representation of bioluminescent biosensors using firefly SLCA monitoring PPIs. A) Representation of the SLCA components in luciferase structure. B) Split luciferase assays in monitoring PPIs. In this biosensor system, the firefly luciferase enzyme is split into two non-functional fragments: the N-terminal domain (Nluc, amino acids 1-416) and the C-terminal domain (Cluc, amino acids 394-550; A). These fragments are fused to two proteins of interest, Protein A and Protein B. When Protein A and Protein B interact, the two luciferase fragments are brought into close proximity, allowing them to reconstitute the functional enzyme. C) Luciferase assays. In the presence of the substrate D-luciferin and cofactors such as Mg²⁺, the restored luciferase catalyzes the oxidation of D-luciferin to oxyluciferin, producing light. This luminescence signal indicates the interaction between the two proteins and can be quantified to measure the strength and dynamics of the PPIs.
Figure 5.
Schematic representation of bioluminescent biosensors using firefly SLCA monitoring PPIs. A) Representation of the SLCA components in luciferase structure. B) Split luciferase assays in monitoring PPIs. In this biosensor system, the firefly luciferase enzyme is split into two non-functional fragments: the N-terminal domain (Nluc, amino acids 1-416) and the C-terminal domain (Cluc, amino acids 394-550; A). These fragments are fused to two proteins of interest, Protein A and Protein B. When Protein A and Protein B interact, the two luciferase fragments are brought into close proximity, allowing them to reconstitute the functional enzyme. C) Luciferase assays. In the presence of the substrate D-luciferin and cofactors such as Mg²⁺, the restored luciferase catalyzes the oxidation of D-luciferin to oxyluciferin, producing light. This luminescence signal indicates the interaction between the two proteins and can be quantified to measure the strength and dynamics of the PPIs.
Figure 6.
Schematic representation of the NanoBiT biosensors. The NanoBiT system utilizes two complementary luciferase fragments, LgBiT and SmBiT, which are fused to proteins of interest (Protein A and Protein B). Upon interaction between the two proteins, the fragments reassemble to form an active NanoLuc luciferase, generating bioluminescence in the presence of the substrate furimazine.
Figure 6.
Schematic representation of the NanoBiT biosensors. The NanoBiT system utilizes two complementary luciferase fragments, LgBiT and SmBiT, which are fused to proteins of interest (Protein A and Protein B). Upon interaction between the two proteins, the fragments reassemble to form an active NanoLuc luciferase, generating bioluminescence in the presence of the substrate furimazine.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).