Submitted:
23 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Wearable Devices
1.2. Design Perspective
1.3. Research Questions
- What are the key factors influencing the wearability of wearable devices?
- What methodologies and measures are used to assess the wearability of wearable devices?
- What are the reported user experiences and satisfaction levels regarding the wearability of different types of wearable devices?
1.4. Design Considerations
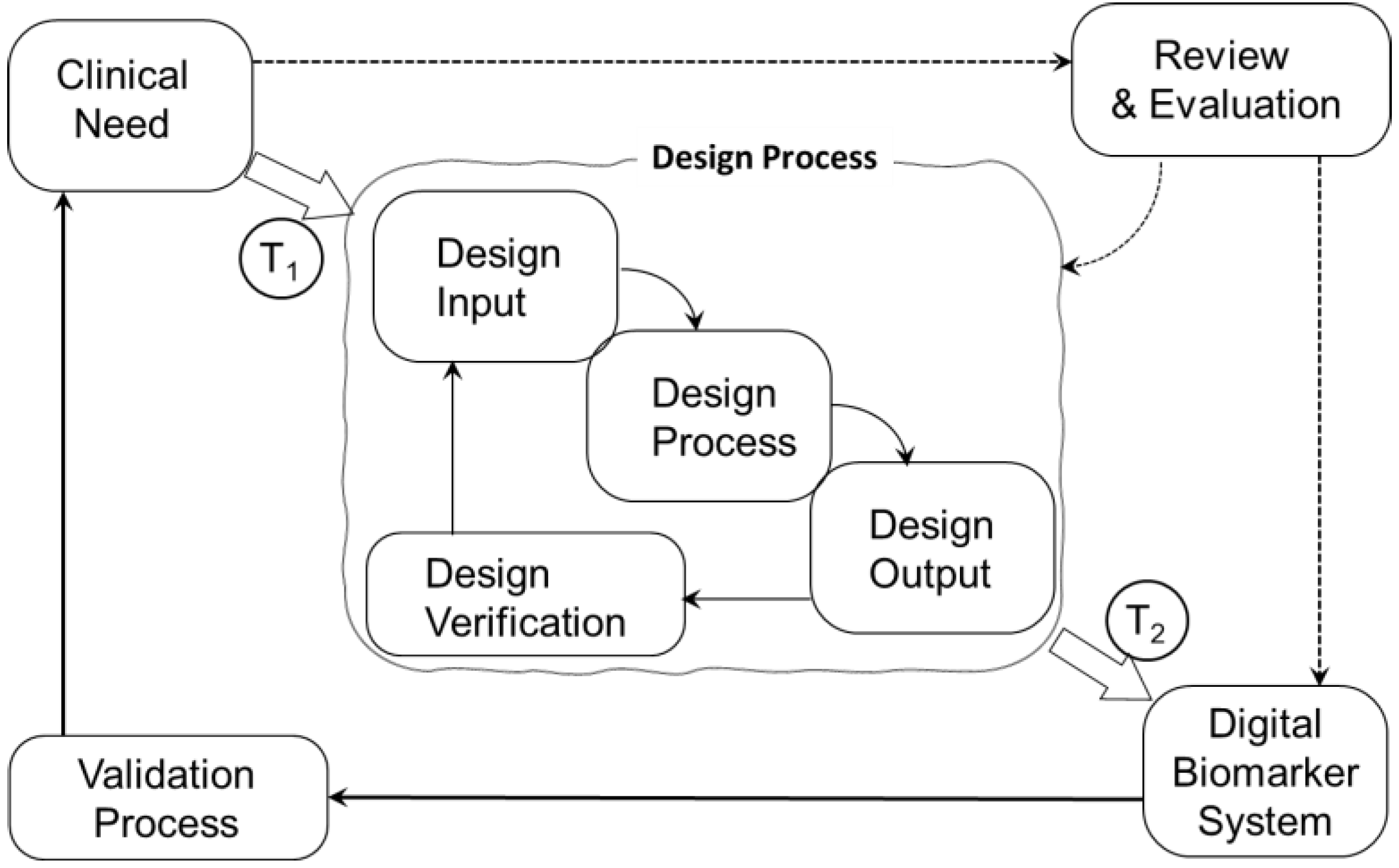
2. Materials and Methods
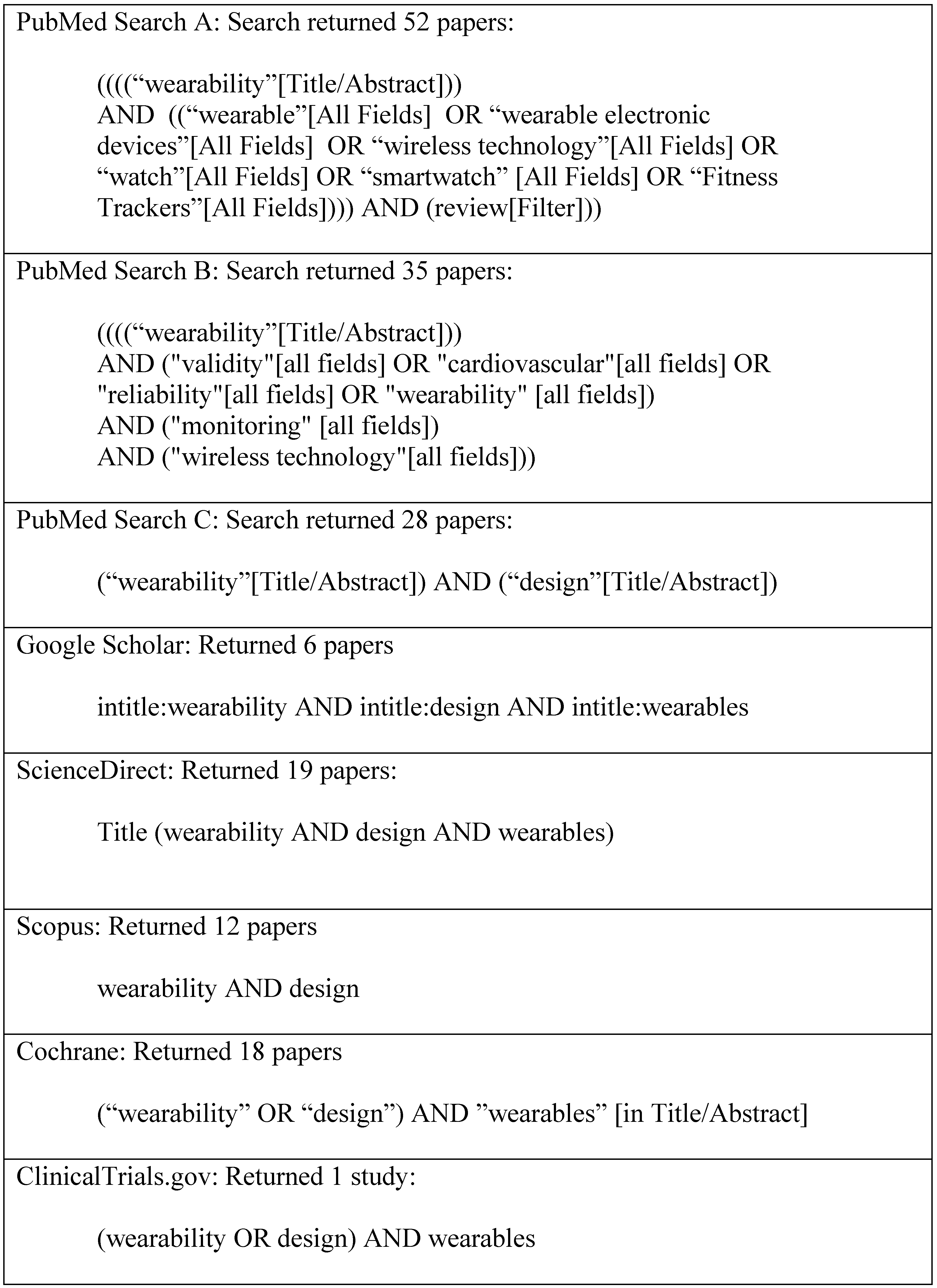
2.1. Search Strategy
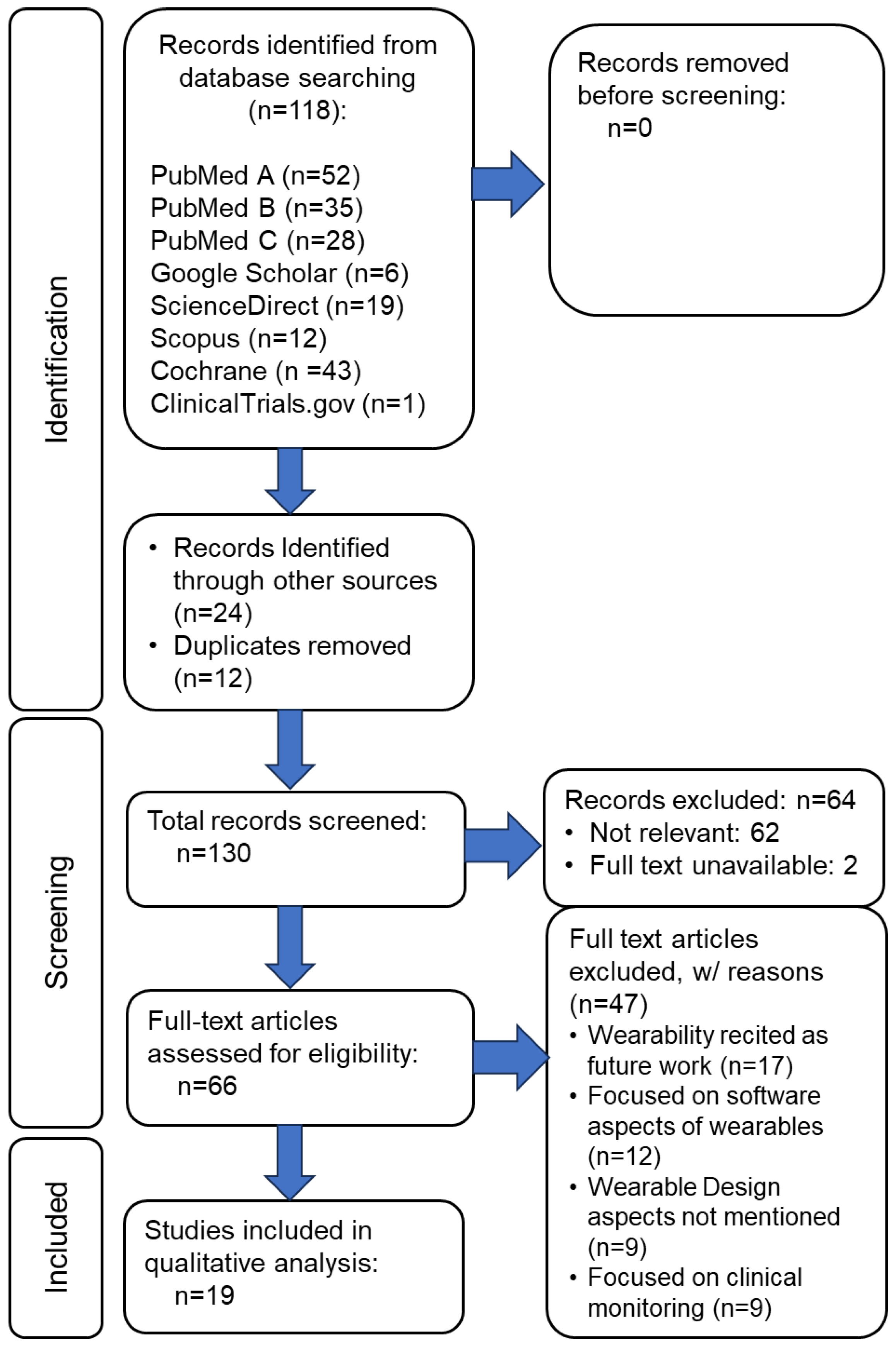
2.2. Eligibility Criteria and Screening
3. Results
4. Discussion
4.1. Limitations
- Despite providing insight into the limited data available to support design for wearability, the sample size for the scoping review was small and the search terms may not have been adequate to tease out design data. The field of digital medicine continues to evolve rapidly, and investigators may use terms in their studies that we did not use in our search.
- Our search was limited to peer-reviewed literature. It seems reasonable that we are unaware of many usability studies are undertaken by technology manufacturers - clinical trials we reviewed that were sponsored by industry appeared to be more for the goal of collecting marketing data, not providing design insight.
5. Conclusions
- Lack of Standardized Assessment Methods: The absence of an accepted and standardized method for assessing wearability has resulted in inconsistent evaluations across studies. Researchers often rely on subjective criteria, leading to variability in how wearability is measured and reported.
- Qualitative Nature of Assessments: Most existing assessments of wearability remain qualitative, lacking objective metrics for rigorous analysis. While self-report scales provide valuable insights, they fall short of quantifying wearability in a consistent and comparable manner.
- Limited Utility for Design: Despite the wealth of existing studies, their qualitative nature and lack of quantifiable data hinder their practical utility in designing wearable devices. Insights gleaned from these studies do not directly inform design decisions or address the specific needs of users.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, C. Wearable and Medical Device Litigation Is on the Rise. Available online: https://www.witlegal.com/insights/blog/wearable-and-medical-device-litigation-is-on-the-rise/ (accessed on 20 March 2024).
- Spatz, E.S.; Ginsburg, G.S.; Rumsfeld, J.S.; Turakhia, M.P. Wearable Digital Health Technologies for Monitoring in Cardiovascular Medicine. New England Journal of Medicine 2024, 390, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Friend, S.H.; Ginsburg, G.S.; Picard, R.W. Wearable Digital Health Technology. New England Journal of Medicine 2023, 389, 2100–2101. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, G.S.; Picard, R.W.; Friend, S.H. Key Issues as Wearable Digital Health Technologies Enter Clinical Care. N Engl J Med 2024, 390, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Varma, N.; Han, J.K.; Passman, R.; Rosman, L.A.; Ghanbari, H.; Noseworthy, P.; Avari Silva, J.N.; Deshmukh, A.; Sanders, P.; Hindricks, G.; et al. Promises and Perils of Consumer Mobile Technologies in Cardiovascular Care: JACC Scientific Statement. J Am Coll Cardiol 2024, 83, 611–631. [Google Scholar] [CrossRef]
- Velasco, E. How Wearable Sensors Will Transform the Practice of Medicine. Available online: https://magazine.caltech.edu/post/how-wearable-sensors-will-transform-the-practice-of-medicine (accessed on 30 June 2024).
- Xian, X. Frontiers of Wearable Biosensors for Human Health Monitoring. Biosensors 2023, 13, 964. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping Healthcare with Wearable Biosensors. Sci Rep 2023, 13, 4998. [Google Scholar] [CrossRef]
- Lopez, X.; Afrin, K.; Nepal, B. Examining the Design, Manufacturing and Analytics of Smart Wearables. MEDICAL DEVICES & SENSORS 2020, 3, e10087. [Google Scholar] [CrossRef]
- Ferguson, C.; Hickman, L.D.; Turkmani, S.; Breen, P.; Gargiulo, G.; Inglis, S.C. “Wearables Only Work on Patients That Wear Them”: Barriers and Facilitators to the Adoption of Wearable Cardiac Monitoring Technologies. Cardiovasc Digit Health J 2021, 2, 137–147. [Google Scholar] [CrossRef]
- Slater, K. Human Comfort. Available online: https://www.abebooks.com/9780398051280/Human-Comfort-Slater-Keith-0398051283/plp (accessed on 21 February 2024).
- Coravos, A.; Doerr, M.; Goldsack, J.; Manta, C.; Shervey, M.; Woods, B.; Wood, W.A. Modernizing and Designing Evaluation Frameworks for Connected Sensor Technologies in Medicine. NPJ Digit Med 2020, 3, 37. [Google Scholar] [CrossRef]
- Olaye, I.M.; Belovsky, M.P.; Bataille, L.; Cheng, R.; Ciger, A.; Fortuna, K.L.; Izmailova, E.S.; McCall, D.; Miller, C.J.; Muehlhausen, W.; et al. Recommendations for Defining and Reporting Adherence Measured by Biometric Monitoring Technologies: Systematic Review. Journal of Medical Internet Research 2022, 24, e33537. [Google Scholar] [CrossRef]
- Tandon, A.; de Ferranti, S.D. Wearable Biosensors in Pediatric Cardiovascular Disease. Circulation 2019, 140, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Ke, H.-L.; Chou, W.-C.; Chang, P.-C.; Tsai, T.-H.; Lee, M.-Y. Realization and Technology Acceptance Test of a Wearable Cardiac Health Monitoring and Early Warning System with Multi-Channel MCGs and ECG. Sensors (Basel) 2018, 18, E3538. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.F.; Baber, C.; Schwirtz, A.; Bristow, H. The Comfort Assessment of Wearable Computers. Proceedings. Sixth International Symposium on Wearable Computers, 2002. [CrossRef]
- Francés-Morcillo, L.; Morer-Camo, P.; Rodríguez-Ferradas, M.I.; Cazón-Martín, A. Wearable Design Requirements Identification and Evaluation. Sensors 2020, 20, 2599. [Google Scholar] [CrossRef]
- Ferraro, V.; Ugur, S. Designing Wearable Technologies through a User Centered Approach. In Proceedings of the Proceedings of the 2011 Conference on Designing Pleasurable Products and Interfaces; Association for Computing Machinery: New York, NY, USA, June 22 2011; pp. 1–8.
- Nuske, H.J.; Goodwin, M.S.; Kushleyeva, Y.; Forsyth, D.; Pennington, J.W.; Masino, A.J.; Finkel, E.; Bhattacharya, A.; Tan, J.; Tai, H.; et al. Evaluating Commercially Available Wireless Cardiovascular Monitors for Measuring and Transmitting Real-Time Physiological Responses in Children with Autism. Autism Res 2022, 15, 117–130. [Google Scholar] [CrossRef]
- Sana, F.; Isselbacher, E.M.; Singh, J.P.; Heist, E.K.; Pathik, B.; Armoundas, A.A. Wearable Devices for Ambulatory Cardiac Monitoring. J Am Coll Cardiol 2020, 75, 1582–1592. [Google Scholar] [CrossRef]
- Hochstadt, A.; Chorin, E.; Viskin, S.; Schwartz, A.L.; Lubman, N.; Rosso, R. Continuous Heart Rate Monitoring for Automatic Detection of Atrial Fibrillation with Novel Bio-Sensing Technology. J Electrocardiol 2019, 52, 23–27. [Google Scholar] [CrossRef]
- Yock, P. Needs-Based Innovation: The Biodesign Process. BMJ Innovations 2015, 1. [Google Scholar] [CrossRef]
- Yock, P.; Zenios, S.; Makower, J.; Brinton, T.J.; Kumar, U.N.; Watkins, F.T.J.; Denend, L.; Krummel, T.; Kurihara, C.Q. BioDesign: The Process of Innovating New Medical Technologies; Cambridge University Press, 2015.
- Mouyal, N. New Standards for Wearable Technologies. Available online: https://etech.iec.ch/issue/2021-04/new-standards-for-wearable-technologies (accessed on 19 March 2024).
- Underwriters Laboratory Keeping Wearable Technology Safe at Any Speed. Available online: https://www.ul.com/insights/keeping-wearable-technology-safe-any-speed (accessed on 21 March 2024).
- de Vries, M.J. Translating Customer Requirements into Technical Specifications. In Philosophy of Technology and Engineering Sciences; Meijers, A., Ed.; Handbook of the Philosophy of Science; North-Holland: Amsterdam, 2009; pp. 489–512. [Google Scholar]
- Göhler, S.; Husung, S.; Howard, T. The Translation between Functional Requirements and Design Parameters for Robust Design. Procedia CIRP 2016, 43, 106–111. [Google Scholar] [CrossRef]
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J Grad Med Educ 2022, 14, 565–567. [Google Scholar] [CrossRef]
- Cochrane Cochrane Database of Systematic Reviews. Available online: https://www.cochranelibrary.com/cdsr/about-cdsr (accessed on 30 June 2024).
- Canali, S.; Schiaffonati, V.; Aliverti, A. Challenges and Recommendations for Wearable Devices in Digital Health: Data Quality, Interoperability, Health Equity, Fairness. PLOS Digit Health 2022, 1, e0000104. [Google Scholar] [CrossRef]
- Cho, S.; Chang, T.; Yu, T.; Lee, C.H. Smart Electronic Textiles for Wearable Sensing and Display. Biosensors 2022, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Gemperle, F.; Kasabach, C.; Stivoric, J.; Bauer, M.; Martin, R. Design for Wearability. In Proceedings of the Digest of Papers. Second International Symposium on Wearable Computers (Cat. No.98EX215); IEEE Comput. Soc: Pittsburgh, PA, USA, 1998; pp. 116–122.
- Haghi, M.; Danyali, S.; Ayasseh, S.; Wang, J.; Aazami, R.; Deserno, T.M. Wearable Devices in Health Monitoring from the Environmental towards Multiple Domains: A Survey. Sensors 2021, 21, 2130. [Google Scholar] [CrossRef]
- Jamshidi, M.; Park, C.B.; Azhari, F. The Design and Fabrication of a Wearable Lattice-Patterned 3D Sensing Skin. Sensors and Actuators A: Physical 2024, 369, 115143. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable Biosensors for Healthcare Monitoring. Nat Biotechnol 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Lee, R.; James, C.; Edwards, S.; Skinner, G.; Young, J.L.; Snodgrass, S.J. Evidence for the Effectiveness of Feedback from Wearable Inertial Sensors during Work-Related Activities: A Scoping Review. Sensors (Basel) 2021, 21, 6377. [Google Scholar] [CrossRef]
- Lind, C.M.; Abtahi, F.; Forsman, M. Wearable Motion Capture Devices for the Prevention of Work-Related Musculoskeletal Disorders in Ergonomics-An Overview of Current Applications, Challenges, and Future Opportunities. Sensors (Basel) 2023, 23, 4259. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L.; Gou, G.; Fang, Z.; Sun, J.; Chen, J.; Cheng, J.; Han, M.; Ma, T.; Liu, C.; et al. Recent Advancements in Physiological, Biochemical, and Multimodal Sensors Based on Flexible Substrates: Strategies, Technologies, and Integrations. ACS Appl. Mater. Interfaces 2023, 15, 21721–21745. [Google Scholar] [CrossRef]
- Shah, M.A.; Pirzada, B.M.; Price, G.; Shibiru, A.L.; Qurashi, A. Applications of Nanotechnology in Smart Textile Industry: A Critical Review. Journal of Advanced Research 2022, 38, 55–75. [Google Scholar] [CrossRef]
- Tandon, A.; Cobb, B.R.; Centra, J.; Izmailova, E.; Manyakov, N.V.; McClenahan, S.; Patel, S.; Sezgin, E.; Vairavan, S.; Vrijens, B.; et al. A Systematic Scoping Review of Studies Describing Human Factors, Human-Centered Design, and Usability of Sensor-Based Digital Health Technologies 2024, 2024.02.23.24303220.
- Uchitel, J.; Vidal-Rosas, E.E.; Cooper, R.J.; Zhao, H. Wearable, Integrated EEG-fNIRS Technologies: A Review. Sensors (Basel) 2021, 21, 6106. [Google Scholar] [CrossRef]
- Zhao, H.; Su, R.; Teng, L.; Tian, Q.; Han, F.; Li, H.; Cao, Z.; Xie, R.; Li, G.; Liu, X.; et al. Recent Advances in Flexible and Wearable Sensors for Monitoring Chemical Molecules. Nanoscale 2022, 14, 1653–1669. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, C.; Huang, Y.; Zhou, G.; Xiao, Y.; Ji, N.; Zhang, Y.-T.; Zhao, N. Emerging Sensing and Modeling Technologies for Wearable and Cuffless Blood Pressure Monitoring. npj Digit. Med. 2023, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Waks, Z.; Elm, J.J.; Gordon, M.F.; Grachev, I.D.; Navon-Perry, L.; Fine, S.; Grossman, I.; Papapetropoulos, S.; Savola, J.-M. Characterizing Patient Compliance over Six Months in Remote Digital Trials of Parkinson’s and Huntington Disease. BMC Med Inform Decis Mak 2018, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Nasirzadeh, F.; Karmakar, C.; Habib, A.; Benny Neelangal, K.; Mir, M.; Lee, S.; Arnel, T. Continuous Monitoring of Body Temperature for Objective Detection of Health and Safety Risks in Construction Sites: An Analysis of the Accuracy and Comfort of off-the-Shelf Wearable Sensors. Heliyon 2024, 10, e26947. [Google Scholar] [CrossRef]
- Su, M.; Hua, J.; Sun, X.; Liu, Z.; Shi, Y.; Pan, L. Wireless Wearable Devices and Recent Applications in Health Monitoring and Clinical Diagnosis. Biomedical Materials & Devices 2024, 2, 669–694. [Google Scholar] [CrossRef]
- Teng, S.; Kim, J.-Y.; Jeon, S.; Gil, H.-W.; Lyu, J.; Chung, E.H.; Kim, K.S.; Nam, Y. Analyzing Optimal Wearable Motion Sensor Placement for Accurate Classification of Fall Directions. Sensors 2024, 24, 6432. [Google Scholar] [CrossRef]
- Schaefer, S.E.; Van Loan, M.; German, J.B. A Feasibility Study of Wearable Activity Monitors for Pre-Adolescent School-Age Children. Prev Chronic Dis 2014, 11, E85. [Google Scholar] [CrossRef]
- Bouwstra, S.; Chen, W.; Feijs, L.M.G.; Bambang Oetomo, S. Smart Jacket Design for Neonatal Monitoring with Wearable Sensors: 6th International Workshop on Wearable and Implantable Body Sensor Networks (BSN 2009). Proceedings of the sixth International Workshop on Wearable and Implantable Body Sensor Networks 2009 2009, 162–167.
- Evans, E.W.; Abrantes, A.M.; Chen, E.; Jelalian, E. Using Novel Technology within a School-Based Setting to Increase Physical Activity: A Pilot Study in School-Age Children from a Low-Income, Urban Community. Biomed Res Int 2017, 2017, 4271483. [Google Scholar] [CrossRef]
- Martinez-Tabares, F.J.; Gaviria-Gomez, N.; Castellanos-Dominguez, G. Very Long-Term ECG Monitoring Patch with Improved Functionality and Wearability. Annu Int Conf IEEE Eng Med Biol Soc 2014, 2014, 5964–5967. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Katthula, V.; Moustakas, E. Patterns of Use and Key Predictors for the Use of Wearable Health Care Devices by US Adults: Insights from a National Survey. J Med Internet Res 2020, 22, e22443. [Google Scholar] [CrossRef]
| User Need | Design Considerations | |
| A. | Comfort | Evaluate the comfort of the device when worn for extended periods. This includes assessing the materials used, fit, weight, and overall ergonomic design. |
| B. | Fit and Adjustability | Ensure the device can fit various body types and sizes. This includes the design of adjustable straps, bands, or other mechanisms to secure the device properly. |
| C. | Battery Life: | Assess the duration the device can operate before needing a recharge. Longer battery life is preferable to reduce the frequency of recharging, which can affect wearability and user compliance. |
| D. | Durability and Robustness: | The device should withstand daily wear and tear, including exposure to different environmental conditions like moisture, dust, and physical impact. |
| E. | Ease of Use: | Evaluate how easy it is for users to operate the device. This includes the simplicity of putting it on and taking it off, as well as the user interface for any necessary interactions. |
| F. | Data Accuracy and Reliability: | Assess the precision and consistency of the data collected by the device. Reliable sensors and accurate data collection are crucial for clinical trial validity. |
| G. | Mobility and Range of Motion | Determine how the device affects natural movement and range of motion. Assess whether it restricts movement during various activities. |
| H. | Integration with Clothing and Accessories: | Determine how well the device integrates with different types of clothing and accessories. Assess whether it can be worn discreetly or if it interferes with other wearable items. |
| I. | Aesthetic Appeal: | Consider the visual design of the device. It should be appealing or at least unobtrusive to encourage regular wear. |
| J. | Skin Compatibility | Ensure the materials used do not cause skin irritation or allergies. This includes testing for hypoallergenic properties and breathability of materials in contact with the skin. |
| K. | Connectivity and Data Transfer: | Evaluate how the device connects to other systems or devices for data transfer (includes reliability and security of connections). |
| L. | Regulatory Compliance: | Ensure the device meets all necessary regulatory standards and guidelines for medical devices. This includes certifications and compliance with relevant health and safety standards. |
| Assessment of user need | Score |
|---|---|
| Reference does not mention or only briefly mentions, without detail | 1-2 |
| Reference mentions with some detail. | 3-4 |
| Reference discusses with moderate detail and some context | 5-6 |
| Reference provides detailed discussion w/ relevant data/examples. | 7-8 |
| Reference extensively comprehensive data, examples, and critical analysis. | 9-10 |
| Author | Year | Type | Title | |
|---|---|---|---|---|
| 1. | Canali et. al.[30] | 2022 | R | Challenges and recommendations for wearable devices in digital health: Data quality, interoperability, health equity, fairness |
| 2. | Cho et. al.[31] | 2022 | R | Smart electronic textiles for wearable sensing and display |
| 3. | Ferguson et. al.[10] | 2021 | R | Wearables only work on patients that wear them: Barriers and facilitators to the adoption of wearable cardiac monitoring technologies |
| 4. | Ferraro and Yavuz [18] | 2011 | D | Designing wearable technologies through a user centered approach |
| 5. | Frances-Morcillo et al.[17] | 2020 | D | Wearable design requirements identification and evaluation. |
| 6. | Friend et al.[3] | 2023 | R | Wearable digital health technology. |
| 7. | Ginsburg et al. [4] | 2024 | D | Key Issues as Wearable Digital Health Technologies Enter Clinical Care |
| 8. | Gemperle et al.[32] | 1998 | D | Design for wearability |
| 9. | Haghi et. al.[33] | 2021 | R | Wearable Devices in Health Monitoring from the Environmental towards Multiple Domains: A Survey |
| 10. | Jamshidi et. al.[34] | 2024 | E | The design and fabrication of a wearable lattice-patterned 3D sensing skin |
| 11. | Kim et. al.[35] | 2019 | R | Wearable biosensors for healthcare monitoring |
| 12. | Lee et. al.[36] | 2021 | R | Evidence for the Effectiveness of Feedback from Wearable Inertial Sensors during Work-Related Activities: A Scoping Review |
| 13. | Lind et. al.[37] | 2023 | D | Wearable Motion Capture Devices for the Prevention of Work-Related Musculoskeletal Disorders in Ergonomics |
| 14. | Liu et. al.[38] | 2023 | R | Recent Advancements in Physiological, Biochemical, and Multimodal Sensors Based on Flexible Substances: Strategies, Technologies, and Integrations. |
| 15. | Shah et. al.[39] | 2022 | R | Applications of nanotechnology in smart textile industry: A critical review. |
| 16. | Tandon et. al.[40] | 2024 | R | A systematic scoping review of studies describing human factors, human-centered design, and usability of sensor-based digital health technologies |
| 17. | Uchitel et. al.[41] | 2021 | R | Wearable, integrated EEG-fNIRS technologies: A review |
| 18. | Zhao et. al.[42] | 2022 | R | Recent advances in flexible and wearable sensors for monitoring chemical molecules |
| 19. | Zhao et. al.[43] | 2023 | D | Emerging sensing and modeling technologies for wearable and cuffless blood pressure monitoring |
| User Need Design Criteria | Average | |||||||||||||
| Reference | A | B | C | D | E | F | G | H | I | J | K | L | Score | |
| 1. | Canali et. al. (2022) | 2 | 2 | 1 | 3 | 2 | 4 | 2 | 1 | 2 | 2 | 2 | 1 | 2.0 |
| 2. | Cho et. al. (2022) | 2 | 2 | 1 | 1 | 5 | 7 | 3 | 1 | 1 | 1 | 3 | 1 | 2.3 |
| 3. | Ferguson et. al. (2021) | 4 | 4 | 4 | 4 | 8 | 5 | 1 | 3 | 7 | 1 | 3 | 1 | 3.8 |
| 4. | Ferraro and Yavuz (2011) | 6 | 6 | 2 | 2 | 6 | 3 | 6 | 5 | 4 | 3 | 2 | 2 | 3.9 |
| 5. | Frances-Morcillo et al. (2020) | 7 | 7 | 5 | 8 | 8 | 5 | 5 | 5 | 5 | 3 | 3 | 1 | 5.2 |
| 6. | Friend et al. (2023) | 3 | 5 | 5 | 6 | 5 | 7 | 2 | 2 | 2 | 6 | 8 | 7 | 4.8 |
| 7. | Ginsburg et al. (2024) | 1 | 1 | 5 | 3 | 7 | 9 | 5 | 3 | 3 | 6 | 9 | 9 | 5.1 |
| 8. | Gemperle et al. (1998) | 3 | 8 | 1 | 7 | 1 | 1 | 9 | 2 | 2 | 6 | 1 | 1 | 3.5 |
| 9. | Haghi et. al. (2021) | 2 | 2 | 8 | 6 | 4 | 9 | 2 | 2 | 2 | 4 | 9 | 4 | 4.5 |
| 10. | Jamshidi et. al. (2024) | 1 | 1 | 1 | 4 | 4 | 6 | 1 | 1 | 5 | 10 | 5 | 1 | 3.3 |
| 11. | Kim et. al. (2019) | 2 | 2 | 5 | 4 | 4 | 7 | 4 | 4 | 4 | 10 | 10 | 1 | 4.8 |
| 12. | Lee et. al. (2021) | 3 | 5 | 5 | 6 | 5 | 6 | 10 | 7 | 2 | 4 | 7 | 1 | 5.1 |
| 13. | Lind et. al. (2023) | 1 | 3 | 1 | 3 | 1 | 6 | 9 | 9 | 2 | 2 | 3 | 2 | 3.5 |
| 14. | Liu et. al. (2023) | 2 | 2 | 6 | 6 | 1 | 3 | 1 | 1 | 2 | 6 | 5 | 1 | 3.0 |
| 15. | Shah et. al. (2022) | 1 | 1 | 4 | 4 | 4 | 4 | 1 | 9 | 6 | 4 | 5 | 1 | 3.7 |
| 16. | Tandon et. al. (2024) | 5 | 5 | 1 | 1 | 5 | 6 | 5 | 1 | 2 | 2 | 2 | 2 | 3.1 |
| 17. | Uchitel et. al. (2021) | 2 | 2 | 3 | 5 | 1 | 5 | 1 | 1 | 1 | 6 | 5 | 1 | 2.8 |
| 18. | Zhao et. al. (2022) | 1 | 2 | 2 | 2 | 1 | 7 | 1 | 1 | 2 | 5 | 5 | 1 | 2.5 |
| 19. | Zhao et. al. (2023) | 2 | 2 | 2 | 3 | 2 | 4 | 1 | 1 | 1 | 3 | 3 | 1 | 2.1 |
| Assessment | Average score | Impact |
|---|---|---|
| Minimal value as a data source for design. | Under 3 | Low |
| Provides indirect linkage to studies or reviews with data | 3 – 5 | Medium |
| Recites data that has potential support for device design. | Over 5 | High |
| Paper | Author | Scale | Summary Description |
| 1 | Canali et. al. | L | The paper maps out the principles for which wearable devices can be measured and the ethical problems they present. The paper recognized the gap in equity and medical literacy when it comes to wearable devices. Though the paper recognizes and comments on ethical topics/issues, it does not touch on the design and engineering process of them. |
| 2 | Cho et. al. | L | The paper focuses on the aspects of design and use of e-textiles. Though the paper mentions many issues related to the use of textiles for wearable technology, specific links to device design are absent. |
| 3 | Ferguson et. al. | M | This paper focuses not only on the developmental and design aspects of wearable technology; it also addresses the ethical and social barriers that are presented that may hinder the use of them. Cardiac devices are used as an example and design for specific end-users is discussed, such as the elderly. |
| 4 | Ferraro and Yavuz | M | The paper is directed towards designers who are responsible for upcoming wearable technology to consider the human aspects in design. The paper has a portion focused on the wearability of products and suggests a user adjustable approach to this issue. The paper also mentions how devices affect human life and what the body “senses” from these new technologies. The paper informs on psychological aspects of rejection reactions that might occur by patients of older age. |
| 5 | Frances-Morcillo et al. | H | The paper is directed towards identifying the design features of wearability of wearables. The paper included surveys from experts who are developing wearable technology and presents the results of questionnaires. |
| 6 | Friend et al. | M | The paper focuses on clinical insight that could result from clinical trials of wearable devices. Although the paper identified challenges in the field of wearables, no specific data is provided. |
| 7 | Ginsburg et al. | H | The paper successfully mentions the challenges encountered while pursuing the application of wearable technologies to healthcare. It is ranked high due to the inclusion of regulatory issues and the need for design standards. |
| 8 | Gemperle et al. | M | The paper is directed towards identifying specific design considerations that prevail when wearables are used in high-activity scenarios. The paper does not include design data but addresses device detachment during motion. |
| 9 | Haghi et. al. | M | The paper highlights the psychological aspect of utilizing wearability and the patient’s responses towards implanting or using or wearing wearables. Although the design specifications were not discussed, the specifications to be considered when applying wearable sensors to clinical settings are presented. |
| 10 | Jamshidi et. al. | M | With a focus on the interaction between wearable devices and body location, the paper provides ideas to consider when studying the interaction itself. The paper focuses on the joints and appropriate location of wearables. |
| 11 | Kim et. al. | M | The paper provides a comprehensive study on the different types of wearables sensor technology and the nature of the data that can be collected. This is an ideal manuscript to identify wearability relative to sensor data. |
| 12 | Lee et. al. | H | The paper highlights the influences of wearability towards the biosensor field. Although the paper did not discuss about the “design aspects” of wearability directly, it did discuss the guidelines of wearability. They introduce a “Technological and Design Checklist” of wearable inertial sensor and also underlines the importance of personalized designs to enhance wearability experience for patients |
| 13 | Lind et. al. | M | The paper highlights human movements in the context of typical work-related musculoskeletal activities. The paper recites issues in current use-case applications, challenges and detailed future opportunities. The paper is focused on muscular data collection. |
| 14 | Liu et. al. | M | The paper is directed towards the materials that can enhance the wearability experience of the patient. The paper then focuses highly on the improvements that should be made to utilize the fiber contained devices to become practical in the field. |
| 15 | Shah et. al. | M | The paper focuses on the different smart textiles that can be utilized to enhance the wearability of sensor clothes. Additionally, it categorized different textiles for different usage of clothing sensors such as electrically conductive textiles and energy storing textiles. |
| 16 | Tandon et. al. | M | The paper offers a comprehensive review of digital health technologies relative to approaches to evaluating sensor wearability, with a special focus on human factors, human-centered design and usability. Suggestions are included on how to improve the digital health technologies field, but no data is provided. |
| 17 | Uchitel et. al. | L | The paper emphasizes the integration of EEG and fNIRS for portable, affordable, and appropriate long-term monitoring. The paper highlights evaluation of the types of EEG electrode and amplifiers though only peripherally mentions device design aspects of the technologies. |
| 18 | Zhao et. al. | L | The paper emphasizes flexible wearable devices for real-time health monitoring based on small, soft and low-cost materials. The paper focuses on the wearability of chemical sensors for various biomarkers, commenting on the advantages and disadvantages. |
| 19 | Zhao et. al. | L | The emphasizes the challenges of monitoring blood pressure and focuses on topics such as flexible sensing, signal collecting and processing, noise reducing and estimation models for blood pressure extraction. Accuracy of continuous data collection is discussed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





