Submitted:
23 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Human Gut Microbiome Composition
3. Methods of the Gut Microbiota Identification
4. Products of the Gut Microbiome Metabolism
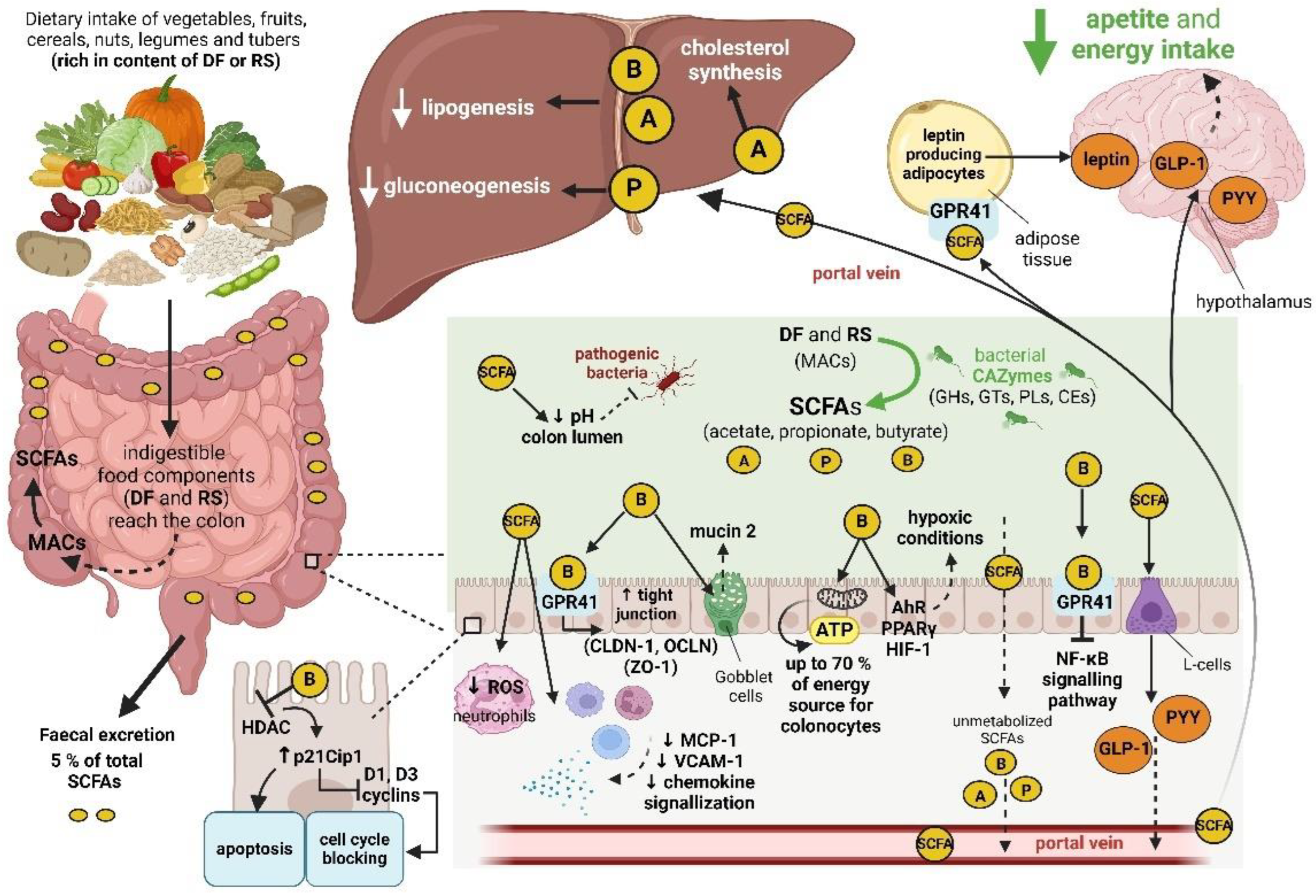
4.1. Short-Chain Fatty Acids
4.1.1. Butyrate, Propionate and Acetate Functions
4.1.2. Production of Short-Chain Fatty Acids by the Gut Microbiota
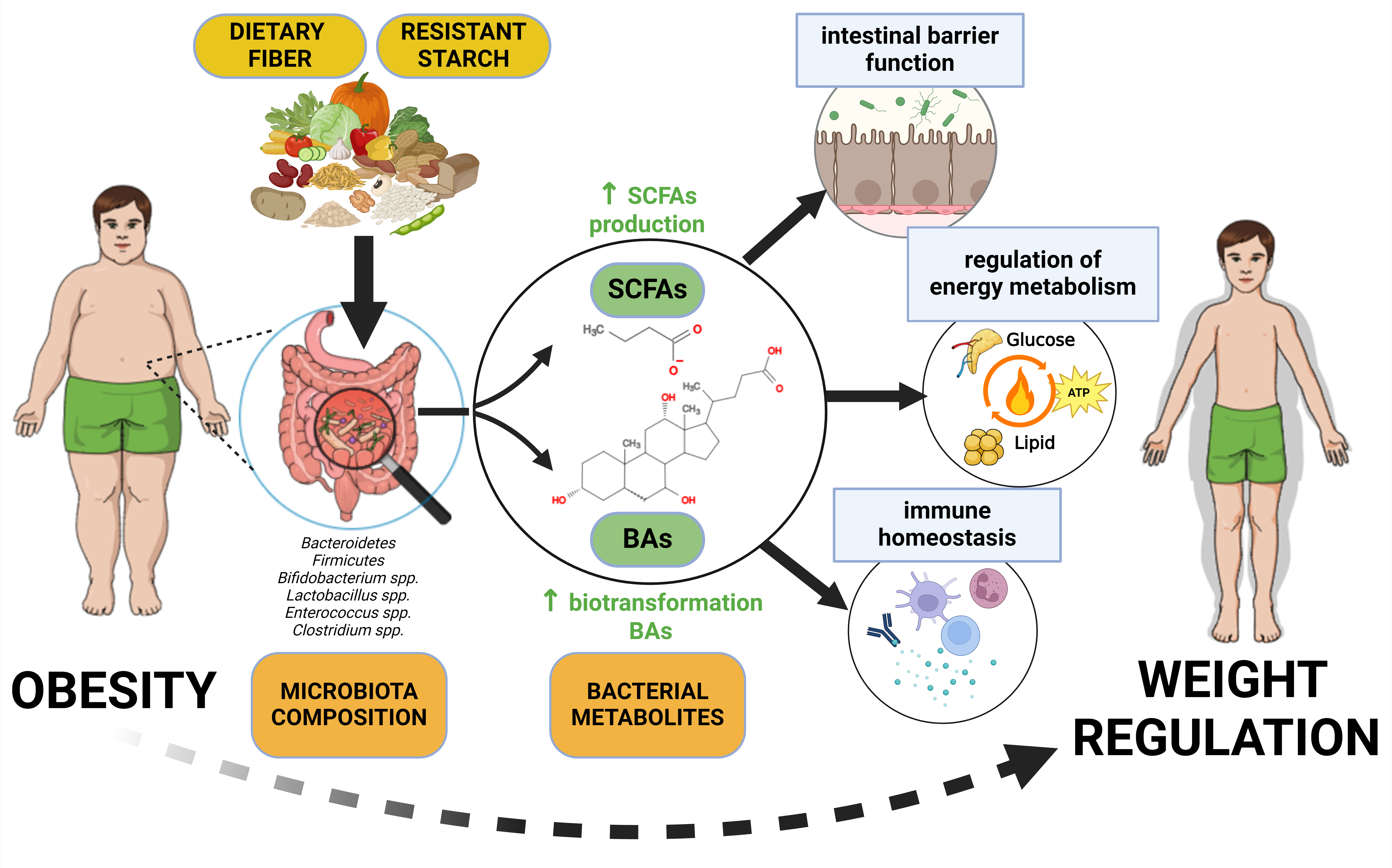
4.1.3. SCFAs and Human Obesity
4.2. Bile Acids
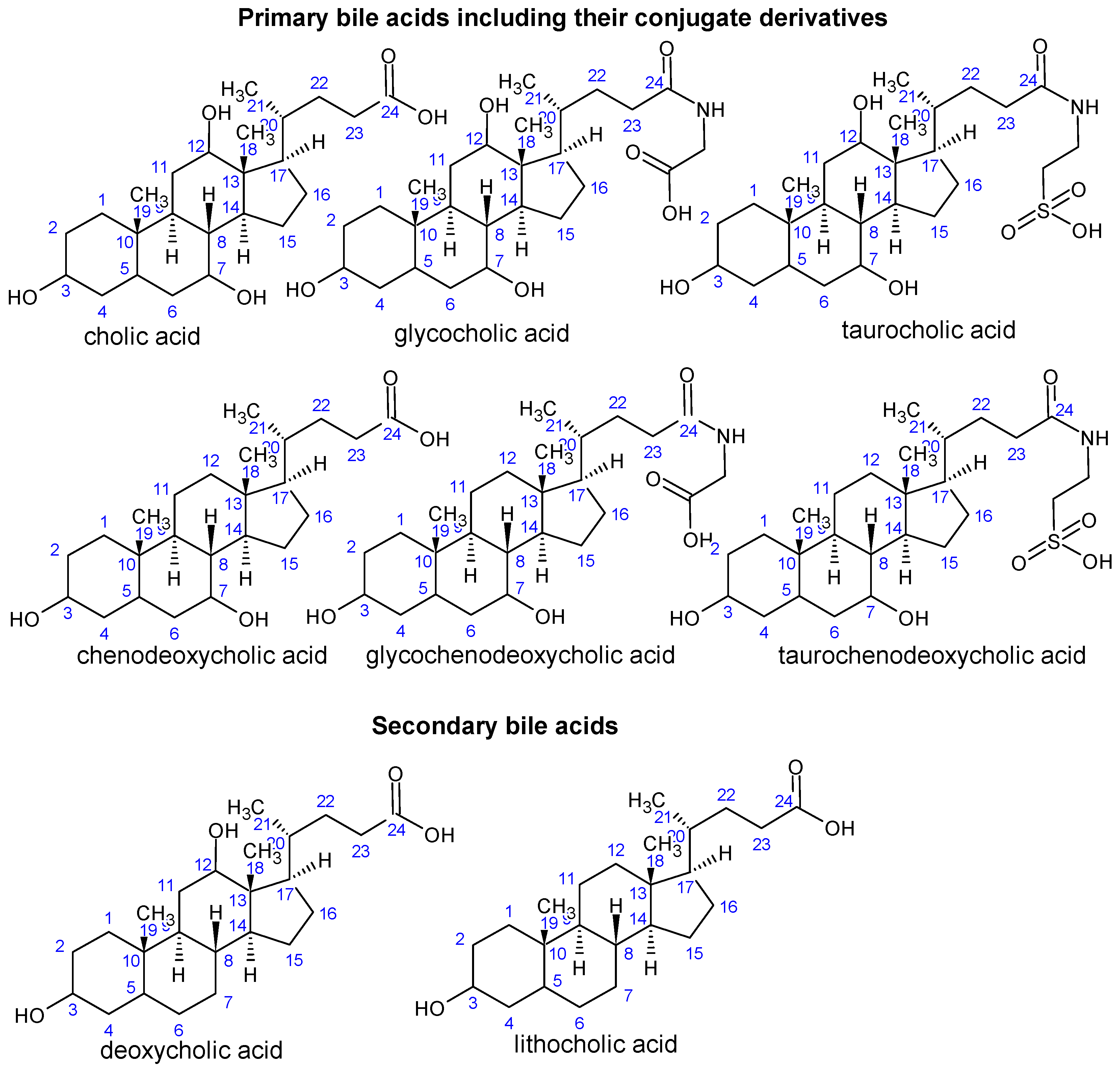
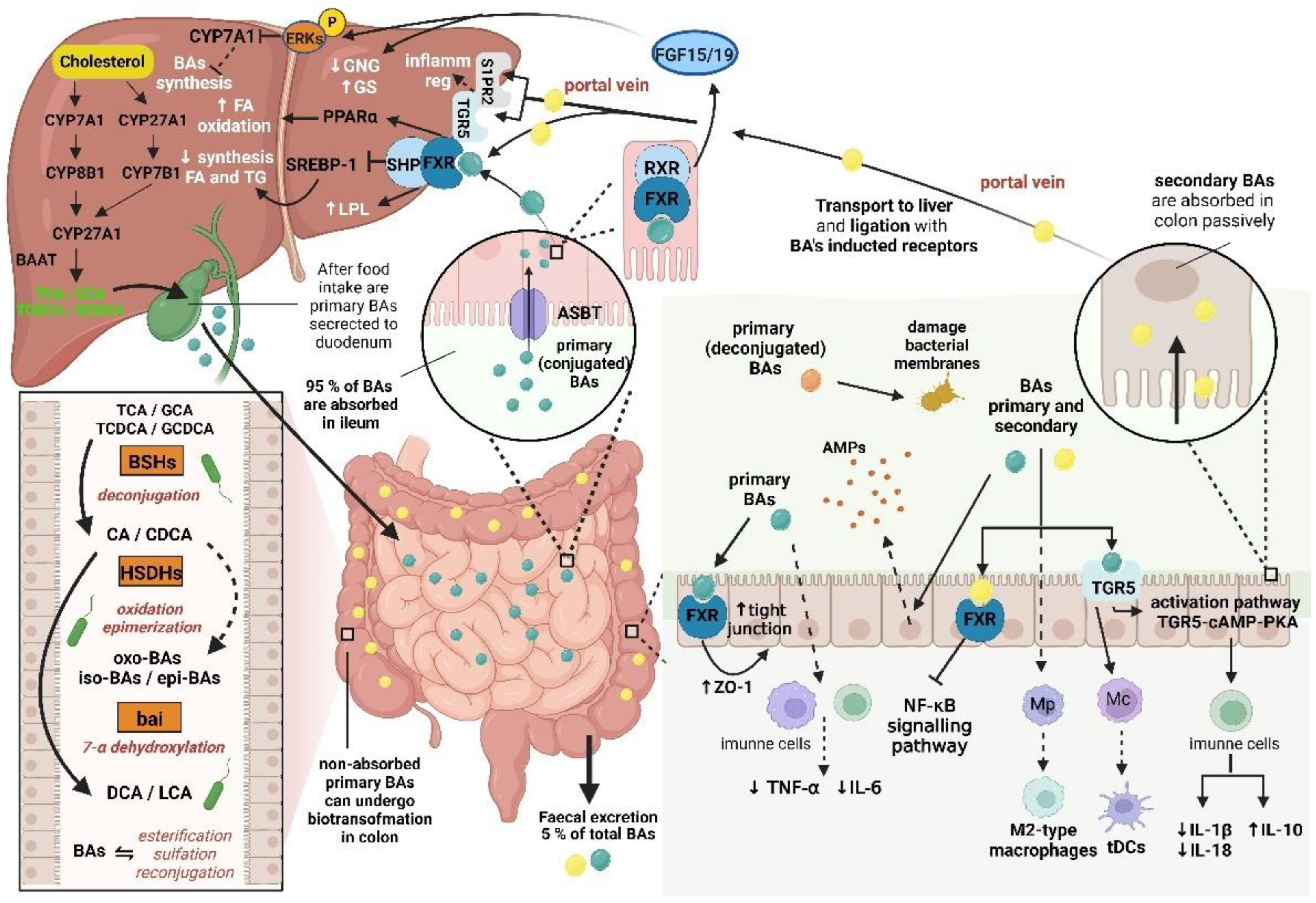
4.2.1. Structure, Synthesis and Metabolism of Primary Bile Acids
4.2.2. Production of Secondary Bile Acids
4.2.3. Effects of Bile Acids on the Gut Microbiota, Intestinal Barrier Function and Immune Homeostasis
| Biotransformation reactions | Phylum | Genera | Reference |
|---|---|---|---|
| deconjugation | Bacteroidetes | Bacteroides Parabacteroides Barnesiella Alistipes |
[45,121] |
| Firmicutes | Clostridium Eubacterium Ruminococcus Lachnospira Roseburia Lactobacillus Enterococcus |
[14,15,121,125] | |
| Actinobacteria | Bifidobacterium Eggerthella |
[15,121] | |
| 7-α dehydroxylation | Firmicutes | Clostridium spp. (C. scindens) (C. hylemonae) Peptacetobacter hiranonis |
[14,118,122] |
| oxidation / epimerization | Firmicutes | Clostridium Eubacterium Collinsella |
[14] |
| Bacteroidetes | Bacteroides | ||
| sulfation | Firmicutes | Clostridium Fusobacterium Peptococcus |
[15] |
| Proteobacteria | Pseudomonas | ||
| esterification | Firmicutes | Eubacterium Lactobacillus |
[15,123] |
| Bacteroidetes | Bacteroides |
4.2.5. BAs Analysis
5. Host Diet and Gut Microbiota
5.1. Dietary Fiber
5.2. Resistant Starch
6. Human Gut Microbiome and Obesity
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haslam, D.W; James, W.T.P. Obesity. Lancet 2005, 366, 1197-1209. [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Zeltser, L.M.; Drewnowski, A.; Ravussin, E.; Redman, L.M.; Leibel, R.L. Obesity Pathogenesis: An Endocrine Society Scientific Statement. Endocr. Rev. 2017, 38, 267-296. [CrossRef]
- Blüher, M. Obesity: Global Epidemiology And Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288-298. [CrossRef]
- Strohacker, K.; Carpenter, K.C.; McFarlin, B.K. Consequences of Weight Cycling: An Increase in Disease Risk? Int. J. Exerc. Sci. 2009, 2(3), 191-201.
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araujo-Soares, V.; Sniehotta, F.F. Long Term Maintenance Of Weight Loss With Non-Surgical Interventions In Obese Adults: Systematic Review And Meta-Analyses Of Randomised Controlled Trials. BMJ 2014, 348, g2646-g2646. [CrossRef]
- Lim, Y.Y.; Lee, Y.S.; Ooi, D.S.Q. Engineering The Gut Microbiome For Treatment Of Obesity: A Review Of Current Understanding And Progress. Biotechnol. J. 2020, 15. [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria In Obesity: An Overview Of The Metabolic Mechanisms And Therapeutic Perspectives Of Next-Generation Probiotics. Microorganisms 2022, 10. [CrossRef]
- Moser, B.; Milligan, M.A.; Dao, M.C. The Microbiota-Gut-Brain Axis: Clinical Applications In Obesity And Type 2 Diabetes. Rev. Invest. Clin. 2022, 74. [CrossRef]
- Liébana-García, R.; Olivares, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Romaní-Pérez, M.; Sanz, Y. The Gut Microbiota As A Versatile Immunomodulator In Obesity And Associated Metabolic Disorders. Best Pract. Res. Clin. Endoc. Metab. 2021, 35. [CrossRef]
- Pedroza Matute, S.; Iyavoo, S. Exploring The Gut Microbiota: Lifestyle Choices, Disease Associations, And Personal Genomics. Front. Nutr. 2023, 10. [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s Role In Human Health And The Current Progress Towards Its Clinical Application To Treat Gastrointestinal Disease. Clin. Nutr. 2023, 42, 61-75. [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota And Short Chain Fatty Acids: Implications In Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23. [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components Of The Human Gut Microbiota. Nutrients 2023, 15. [CrossRef]
- Li, R.; Andreu-Sánchez, S.; Kuipers, F.; Fu, J. Gut Microbiome And Bile Acids In Obesity-Related Diseases. Best Pract. Res. Clin. Endoc. Metab. 2021, 35. [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile Acids As Modulators Of Gut Microbiota Composition And Function. Gut Microbes 2023, 15. [CrossRef]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review On Bile Acids: Effects Of The Gut Microbiome, Interactions With Dietary Fiber, And Alterations In The Bioaccessibility Of Bioactive Compounds. J. Agric. Food Chem. 2019, 67, 9124-9138. [CrossRef]
- Beane, K.E.; Redding, M.C.; Wang, X.; Pan, J.H.; Le, B.; Cicalo, C.; Jeon, S.; Kim, Y.J.; Lee, J.H.; Shin, E.-C.; et al. Effects Of Dietary Fibers, Micronutrients, And Phytonutrients On Gut Microbiome: A Review. Appl. Biol. Chem. 2021, 64. [CrossRef]
- Kok, C.R.; Rose, D.; Hutkins, R. Predicting Personalized Responses To Dietary Fiber Interventions: Opportunities For Modulation Of The Gut Microbiome To Improve Health. Annu. Rev. Food Sci. Technol. 2023, 14, 157-182. [CrossRef]
- Chen, Z.; Liang, N.; Zhang, H.; Li, H.; Guo, J.; Zhang, Y.; Chen, Y.; Wang, Y.; Shi, N. Resistant Starch And The Gut Microbiome: Exploring Beneficial Interactions And Dietary Impacts. Food Chem. X 2024, 21. [CrossRef]
- Dagan, T.; Roettger, M.; Bryant, D.; Martin, W. Genome Networks Root The Tree Of Life Between Prokaryotic Domains. Genome Biol. Evol. 2010, 2, 379-392. [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is The Healthy Gut Microbiota Composition? A Changing Ecosystem Across Age, Environment, Diet, And Diseases. Microorganisms 2019, 7. [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The Human Gut Microbiota: Metabolism And Perspective In Obesity. Gut Microbes 2018, 1-18. [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes Of The Human Gut Microbiome. Nature 2011, 473, 174-180. [CrossRef]
- Putignani, L.; Del Chierico, F.; Petrucca, A.; Vernocchi, P.; Dallapiccola, B. The Human Gut Microbiota: A Dynamic Interplay With The Host From Birth To Senescence Settled During Childhood. Pediatr. Res. 2014, 76, 2-10. [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities And Establishment And Development Of The Infant Gut Microbiome. JAMA Pediatr. 2017, 171. [CrossRef]
- Zhang, P. Influence Of Foods And Nutrition On The Gut Microbiome And Implications For Intestinal Health. Int. J. Mol. Sci. 2022, 23. [CrossRef]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host Lifestyle Affects Human Microbiota On Daily Timescales. Genome Biol. 2014, 15. [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human Gut Microbiome Viewed Across Age And Geography. Nature 2012, 486, 222-227. [CrossRef]
- Bajinka, O.; Tan, Y.; Abdelhalim, K.A.; Özdemir, G.; Qiu, X. Extrinsic Factors Influencing Gut Microbes, The Immediate Consequences And Restoring Eubiosis. AMB Express 2020, 10. [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates Over Host Genetics In Shaping Human Gut Microbiota. Nature 2018, 555, 210-215. [CrossRef]
- Jeffery, I.B.; Claesson, M.J.; O'Toole, P.W.; Shanahan, F. Categorization Of The Gut Microbiota: Enterotypes Or Gradients? Nat. Rev. Microbiol. 2012, 10, 591-592. [CrossRef]
- Knights, D.; Ward, T.L.; McKinlay, C.E.; Miller, H.; Gonzalez, A.; McDonald, D.; Knight, R. Rethinking “Enterotypes.” Cell Host Microbe 2014, 16, 433-437. [CrossRef]
- Thursby, E.; Juge, N. Introduction To The Human Gut Microbiota. Biochem. J. 2017, 474, 1823-1836. [CrossRef]
- Patra, D.; Banerjee, D.; Ramprasad, P.; Roy, S.; Pal, D.; Dasgupta, S. Recent Insights Of Obesity-Induced Gut And Adipose Tissue Dysbiosis In Type 2 Diabetes. Front. Mol. Biosci. 2023, 10. [CrossRef]
- Vijay, A.; Valdes, A.M. Retracted Article Review: Role Of The Gut Microbiome In Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2022, 76, 489-501. [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut Microbiota In Obesity. World J. Gastroenterol. 2021, 27, 3837-3850. [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151-4160. [CrossRef]
- Kim, M.; Chun, J. 16S Rrna Gene-Based Identification Of Bacteria And Archaea Using The Eztaxon Server. In New Approaches to Prokaryotic Systematics; Methods in Microbiology; Elsevier, 2014; pp. 61-74 ISBN 9780128001769.
- Sanschagrin, S.; Yergeau, E. Next-Generation Sequencing Of 16S Ribosomal Rna Gene Amplicons. J. Vis. Exp. 2014. [CrossRef]
- Lim, M.Y.; Hong, S.; Kim, B.-M.; Ahn, Y.; Kim, H.-J.; Nam, Y.-D. Changes In Microbiome And Metabolomic Profiles Of Fecal Samples Stored With Stabilizing Solution At Room Temperature: A Pilot Study. Sci Rep 2020, 10. [CrossRef]
- Salipante, S.J.; Kawashima, T.; Rosenthal, C.; Hoogestraat, D.R.; Cummings, L.A.; Sengupta, D.J.; Harkins, T.T.; Cookson, B.T.; Hoffman, N.G.; Löffler, F.E. Performance Comparison Of Illumina And Ion Torrent Next-Generation Sequencing Platforms For 16S Rrna-Based Bacterial Community Profiling. Appl. Environ. Microbiol. 2014, 80, 7583-7591. [CrossRef]
- Byrd, D.A.; Sinha, R.; Hoffman, K.L.; Chen, J.; Hua, X.; Shi, J.; Chia, N.; Petrosino, J.; Vogtmann, E.; Rao, K. Comparison Of Methods To Collect Fecal Samples For Microbiome Studies Using Whole-Genome Shotgun Metagenomic Sequencing. mSphere 2020, 5, e00827-19. [CrossRef]
- Tessler, M.; Neumann, J.S.; Afshinnekoo, E.; Pineda, M.; Hersch, R.; Velho, L.F.M.; Segovia, B.T.; Lansac-Toha, F.A.; Lemke, M.; DeSalle, R.; et al. Large-Scale Differences In Microbial Biodiversity Discovery Between 16S Amplicon And Shotgun Sequencing. Sci Rep 2017, 7. [CrossRef]
- Souche, E.; Beltran, S.; Brosens, E.; Belmont, J.W.; Fossum, M.; Riess, O.; Gilissen, C.; Ardeshirdavani, A.; Houge, G.; van Gijn, M.; et al. Recommendations For Whole Genome Sequencing In Diagnostics For Rare Diseases. Eur. J. Hum. Genet. 2022, 30, 1017-1021. [CrossRef]
- Song, Z.; Cai, Y.; Lao, X.; Wang, X.; Lin, X.; Cui, Y.; Kalavagunta, P.K.; Liao, J.; Jin, L.; Shang, J.; et al. Taxonomic Profiling And Populational Patterns Of Bacterial Bile Salt Hydrolase (Bsh) Genes Based On Worldwide Human Gut Microbiome. Microbiome 2019, 7. [CrossRef]
- Combrink, L.; Humphreys, I.R.; Washburn, Q.; Arnold, H.K.; Stagaman, K.; Kasschau, K.D.; Jolles, A.E.; Beechler, B.R.; Sharpton, T.J. Best Practice For Wildlife Gut Microbiome Research: A Comprehensive Review Of Methodology For 16S Rrna Gene Investigations. Front. Microbiol. 2023, 14. [CrossRef]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating Taxonomic, Functional, And Strain-Level Profiling Of Diverse Microbial Communities With Biobakery 3. eLife 2021, 10. [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-Omics Of The Gut Microbial Ecosystem In Inflammatory Bowel Diseases. Nature 2019, 569, 655-662. [CrossRef]
- Karcher, N.; Pasolli, E.; Asnicar, F.; Huang, K.D.; Tett, A.; Manara, S.; Armanini, F.; Bain, D.; Duncan, S.H.; Louis, P.; et al. Analysis Of 1321 Eubacterium Rectale Genomes From Metagenomes Uncovers Complex Phylogeographic Population Structure And Subspecies Functional Adaptations. Genome Biol. 2020, 21. [CrossRef]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M.; et al. The Prevotella Copri Complex Comprises Four Distinct Clades Underrepresented In Westernized Populations. Cell Host Microbe 2019, 26, 666-679.e7. [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition In Obese And Non-Obese Persons: A Systematic Review And Meta-Analysis. Nutrients 2022, 14. [CrossRef]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best Practices For Analysing Microbiomes. Nat. Rev. Microbiol. 2018, 16, 410-422. [CrossRef]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Debelius, J.; Morton, J.T.; Hyde, E.; Robbins-Pianka, A.; Knight, R.; Arumugam, M. Correcting For Microbial Blooms In Fecal Samples During Room-Temperature Shipping. mSystems 2017, 2, e00199-16. [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established By Metagenomic Sequencing. Nature 2010, 464, 59-65. [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding The Holobiont: How Microbial Metabolites Affect Human Health And Shape The Immune System. Cell Metab. 2017, 26, 110-130. [CrossRef]
- Zhang, K.; Zhang, Q.; Qiu, H.; Ma, Y.; Hou, N.; Zhang, J.; Kan, C.; Han, F.; Sun, X.; Shi, J. The Complex Link Between The Gut Microbiome And Obesity-Associated Metabolic Disorders: Mechanisms And Therapeutic Opportunities. Heliyon 2024, 10. [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The Role Of The Gut Microbiome And Its Metabolites In Metabolic Diseases. Protein Cell 2021, 12, 360-373. [CrossRef]
- Frolova, M.S.; Suvorova, I.A.; Iablokov, S.N.; Petrov, S.N.; Rodionov, D.A. Genomic Reconstruction Of Short-Chain Fatty Acid Production By The Human Gut Microbiota. Front. Mol. Biosci. 2022, 9. [CrossRef]
- Sonnenburg, E. D.; Sonnenburg, J. L. Starving Our Microbial Self: The Deleterious Consequences Of A Diet Deficient In Microbiota-Accessible Carbohydrates. Cell Metab. 2014, 20, 779-786. [CrossRef]
- Ayakdaş, G.; Ağagündüz, D. Microbiota-Accessible Carbohydrates (Macs) As Novel Gut Microbiome Modulators In Noncommunicable Diseases. Heliyon 2023, 9. [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids And Human Colonic Function: Roles Of Resistant Starch And Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031-1064. [CrossRef]
- Topping, D.L. Short-chain fatty acids produced by intestinal bacteria. Asia. Pac. J. Clin. Nutr. 1996, 5, 15-19.
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors In Health And Disease. Physiol. Rev. 2020, 100, 171-210. [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-Chain Fatty Acid Transporters: Role in Colonic Homeostasis. Compr. Physiol. 2017, 8, 299-314. doi: 10.1002/cphy.c170014.
- Tabat, M.W.; Marques, T.M.; Markgren, M.; Löfvendahl, L.; Brummer, R.J.; Wall, R. Acute Effects Of Butyrate On Induced Hyperpermeability And Tight Junction Protein Expression In Human Colonic Tissues. Biomolecules 2020, 10. [CrossRef]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut Microbiota-Derived Butyrate Regulates Gut Mucus Barrier Repair By Activating The Macrophage/Wnt/Erk Signaling Pathway. Clin. Sci. 2022, 136, 291-307. [CrossRef]
- Blottiere, H.M.; Buecher, B.; Galmiche, J.-P.; Cherbut, C. Molecular Analysis Of The Effect Of Short-Chain Fatty Acids On Intestinal Cell Proliferation. Proc. Nutr. Soc. 2003, 62, 101-106. [CrossRef]
- Donohoe, D. R.; Garge, N.; Zhang, X.; Sun, W.; O'Connell, T. M.; Bunger, M. K.; Bultman, S. J. The Microbiome And Butyrate Regulate Energy Metabolism And Autophagy In The Mammalian Colon. Cell Metab. 2011, 13, 517-526. [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite Via A Central Homeostatic Mechanism. Nat. Commun. 2014, 5. [CrossRef]
- González Hernández, M.A.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate In Body Weight Control And Insulin Sensitivity. Nutrients 2019, 11. [CrossRef]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate Suppresses Hepatic Gluconeogenesis Via Gpr43/Ampk Signaling Pathway. Arch. Biochem. Biophys. 2019, 672. [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity Via A Pparγ-Dependent Switch From Lipogenesis To Fat Oxidation. Diabetes 2015, 64, 2398-2408. [CrossRef]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-Chain Fatty Acids Stimulate Leptin Production In Adipocytes Through The G Protein-Coupled Receptor Gpr41. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1045-1050. [CrossRef]
- Slavin, J. Fiber And Prebiotics: Mechanisms And Health Benefits. Nutrients 2013, 5, 1417-1435. [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Di Yu; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation Of Inflammatory Responses By Gut Microbiota And Chemoattractant Receptor Gpr43. Nature 2009, 461, 1282-1286. [CrossRef]
- Zapolska-Downar, D.; Naruszewicz, M. Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J. Physiol. Pharmacol. 2009, 60, 123-31.
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-Chain Fatty Acids Act As Antiinflammatory Mediatorsby Regulating Prostaglandin E2 And Cytokines. World J. of Gastroenterol. 2009, 15. [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role Of Short-Chain Fatty Acids From Gut Microbiota In Gut-Brain Communication. Front. Endocrinol. 2020, 11. [CrossRef]
- Yang, G.; Chen, S.; Deng, B.; Tan, C.; Deng, J.; Zhu, G.; Yin, Y.; Ren, W. Implication Of G Protein-Coupled Receptor 43 In Intestinal Inflammation: A Mini-Review. Front. Immunol. 2018, 9. [CrossRef]
- Tsukuda, N.; Yahagi, K.; Hara, T.; Watanabe, Y.; Matsumoto, H.; Mori, H.; Higashi, K.; Tsuji, H.; Matsumoto, S.; Kurokawa, K.; et al. Key Bacterial Taxa And Metabolic Pathways Affecting Gut Short-Chain Fatty Acid Profiles In Early Life. ISME J. 2021, 15, 2574-2590. [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-zhong; Abe, F.; Osawa, R. Age-Related Changes In Gut Microbiota Composition From Newborn To Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16. [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The Gut Microbiome As A Modulator Of Healthy Ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565-584. [CrossRef]
- Herrmann, E.; Young, W.; Reichert-Grimm, V.; Weis, S.; Riedel, C.; Rosendale, D.; Stoklosinski, H.; Hunt, M.; Egert, M. In Vivo Assessment Of Resistant Starch Degradation By The Caecal Microbiota Of Mice Using Rna-Based Stable Isotope Probing—A Proof-Of-Principle Study. Nutrients 2018, 10. [CrossRef]
- Sela, D.A.; Mills, D.A. Nursing Our Microbiota: Molecular Linkages Between Bifidobacteria And Milk Oligosaccharides. Trends Microbiol. 2010, 18, 298-307. [CrossRef]
- Conlon, M.; Bird, A. The Impact Of Diet And Lifestyle On Gut Microbiota And Human Health. Nutrients 2015, 7, 17-44. [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic Distribution Of Three Pathways For Propionate Production Within The Human Gut Microbiota. ISME J. 2014, 8, 1323-1335. [CrossRef]
- Louis, P.; Flint, H.J. Formation Of Propionate And Butyrate By The Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29-41. [CrossRef]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative In Silico Analysis Of Butyrate Production Pathways In Gut Commensals And Pathogens. Front. Microbiol. 2016, 7. [CrossRef]
- Singh, V.; Lee, G.D.; Son, H.W.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “The Sentinel Of Gut”: Their Intestinal Significance With And Beyond Butyrate, And Prospective Use As Microbial Therapeutics. Front. Microbiol. 2023, 13. [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role Of Human Gut Lachnospiraceae. Microorganisms 2020, 8. [CrossRef]
- Martín, R.; Bermúdez-Humarán, L.G.; Langella, P. Searching For The Bacterial Effector: The Example Of The Multi-Skilled Commensal Bacterium Faecalibacterium Prausnitzii. Front. Microbiol. 2018, 9. [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium Prausnitzii And Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255-261. [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte Metabolism Shapes The Gut Microbiota. Science 2018, 362. [CrossRef]
- Rivera-Chávez, F.; Zhang, L. F.; Faber, F.; Lopez, C. A.; Byndloss, M. X.; Olsan, E. E.; Xu, G.; Velazquez, E. M.; Lebrilla, C. B.; Winter, S. E.; et al. Depletion Of Butyrate-Producing Clostridia From The Gut Microbiota Drives An Aerobic Luminal Expansion Of Salmonella. Cell Host Microbe 2016, 19, 443-454. [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids In Control Of Body Weight And Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577-591. [CrossRef]
- HAMER, H.M.; JONKERS, D.; VENEMA, K.; VANHOUTVIN, S.; TROOST, F.J.; BRUMMER, R.-J. Review Article: The Role Of Butyrate On Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104-119. [CrossRef]
- Kim, K.N.; Yao, Y.; Ju, S.Y. Short Chain Fatty Acids And Fecal Microbiota Abundance In Humans With Obesity: A Systematic Review And Meta-Analysis. Nutrients 2019, 11. [CrossRef]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids—A Product Of The Microbiome And Its Participation In Two-Way Communication On The Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108-126. [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota And Scfa In Lean And Overweight Healthy Subjects. Obesity 2010, 18, 190-195. [CrossRef]
- De la Cuesta-Zuluaga, J.; Mueller, N.; Álvarez-Quintero, R.; Velásquez-Mejía, E.; Sierra, J.; Corrales-Agudelo, V.; Carmona, J.; Abad, J.; Escobar, J. Higher Fecal Short-Chain Fatty Acid Levels Are Associated With Gut Microbiome Dysbiosis, Obesity, Hypertension And Cardiometabolic Disease Risk Factors. Nutrients 2019, 11. [CrossRef]
- Wang, Y.; Wang, H.; Howard, A.G.; Meyer, K.A.; Tsilimigras, M.C.B.; Avery, C.L.; Sha, W.; Sun, S.; Zhang, J.; Su, C.; et al. Circulating Short-Chain Fatty Acids Are Positively Associated With Adiposity Measures In Chinese Adults. Nutrients 2020, 12. [CrossRef]
- Monte, M.J.; Marin, J.J.G.; Antelo, A.; Vazquez-Tato, J. Bile Acids: Chemistry, Physiology, And Pathophysiology. World J. of Gastroenterol. 2009, 15. [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial Transformations Of Human Bile Acids. Microbiome 2021, 9. [CrossRef]
- Quinn, R.A.; Melnik, A.V.; Vrbanac, A.; Fu, T.; Patras, K.A.; Christy, M.P.; Bodai, Z.; Belda-Ferre, P.; Tripathi, A.; Chung, L.K.; et al. Global Chemical Effects Of The Microbiome Include New Bile-Acid Conjugations. Nature 2020, 579, 123-129. [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4-S14. [CrossRef]
- Grüner, N.; Mattner, J. Bile Acids And Microbiota: Multifaceted And Versatile Regulators Of The Liver–Gut Axis. Int. J. Mol. Sci. 2021, 22. [CrossRef]
- Hofmann, A., F. The Enterohepatic Circulation Of Bile Acids In Mammals: Form And Functions. Front. Biosci. 2009, Volume. [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal Transport And Metabolism Of Bile Acids. J. Lipid Res. 2015, 56, 1085-1099. [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. Bile Acids As Hormones: The Fxr-Fgf15/19 Pathway. Dig. Dis. 2015, 33, 327-331. [CrossRef]
- Fiorucci, S.; Carino, A.; Baldoni, M.; Santucci, L.; Costanzi, E.; Graziosi, L.; Distrutti, E.; Biagioli, M. Bile Acid Signaling In Inflammatory Bowel Diseases. Dig. Dis. Sci. 2021, 66, 674-693. [CrossRef]
- Friedman, E.S.; Li, Y.; Shen, T.-C.D.; Jiang, J.; Chau, L.; Adorini, L.; Babakhani, F.; Edwards, J.; Shapiro, D.; Zhao, C.; et al. Fxr-Dependent Modulation Of The Human Small Intestinal Microbiome By The Bile Acid Derivative Obeticholic Acid. Gastroenterology 2018, 155, 1741-1752.e5. [CrossRef]
- Ginos, B.N.R.; Navarro, S.L.; Schwarz, Y.; Gu, H.; Wang, D.; Randolph, T.W.; Shojaie, A.; Hullar, M.A.J.; Lampe, P.D.; Kratz, M.; et al. Circulating Bile Acids In Healthy Adults Respond Differently To A Dietary Pattern Characterized By Whole Grains, Legumes And Fruits And Vegetables Compared To A Diet High In Refined Grains And Added Sugars: A Randomized, Controlled, Crossover Feeding Study. Metabolism 2018, 83, 197-204. [CrossRef]
- Vaz, F.M.; Ferdinandusse, S. Bile Acid Analysis In Human Disorders Of Bile Acid Biosynthesis. Mol. Asp. Med. 2017, 56, 10-24. [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact Of Dietary Fiber On Gut Microbiota In Host Health And Disease. Cell Host Microbe 2018, 23, 705-715. [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.M.; Marchesi, J.R. Functional And Comparative Metagenomic Analysis Of Bile Salt Hydrolase Activity In The Human Gut Microbiome. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13580-13585. [CrossRef]
- Joyce, S.A.; Gahan, C.G.M. Disease-Associated Changes In Bile Acid Profiles And Links To Altered Gut Microbiota. Dig. Dis. 2017, 35, 169-177. [CrossRef]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations By Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241-259. [CrossRef]
- Doden, H.L.; Ridlon, J.M. Microbial Hydroxysteroid Dehydrogenases: From Alpha To Omega. Microorganisms 2021, 9. [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A Metabolic Pathway For Bile Acid Dehydroxylation By The Gut Microbiome. Nature 2020, 582, 566-570. [CrossRef]
- Hofmann, A.F.; Sjövall, J.; Kurz, G.; Radominska, A.; Schteingart C.D.; Tint, G.S.; Vlachevic, Z.R.; Setchell, K.D.R. A proposed nomenclature for bile acids. J. Lipid Res. 1992, 33, 599-604. [CrossRef]
- Jia, B.; Park, D.; Chun, B.H.; Hahn, Y.; Jeon, C.O. Diet-Related Alterations Of Gut Bile Salt Hydrolases Determined Using A Metagenomic Analysis Of The Human Microbiome. Int. J. Mol. Sci. 2021, 22. [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences Of Bile Salt Biotransformations By Intestinal Bacteria. Gut Microbes 2016, 7, 22-39. [CrossRef]
- Gérard, P. Metabolism Of Cholesterol And Bile Acids By The Gut Microbiota. Pathogens 2014, 3, 14-24. [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions Between Bacteria And Bile Salts In The Gastrointestinal And Hepatobiliary Tracts. Front. Med. 2017, 4. [CrossRef]
- Sannasiddappa, T.H.; Lund, P.A.; Clarke, S.R. In Vitro Antibacterial Activity Of Unconjugated And Conjugated Bile Salts On Staphylococcus Aureus. Front. Microbiol. 2017, 8. [CrossRef]
- Kurdi, P.; Kawanishi, K.; Mizutani, K.; Yokota, A. Mechanism Of Growth Inhibition By Free Bile Acids In Lactobacilli And Bifidobacteria. J. Bacteriol. 2006, 188, 1979-1986. [CrossRef]
- Bachmann, V.; Kostiuk, B.; Unterweger, D.; Diaz-Satizabal, L.; Ogg, S.; Pukatzki, S.; Picardeau, M. Bile Salts Modulate The Mucin-Activated Type Vi Secretion System Of Pandemic Vibrio Cholerae. Plos Neglect. Trop. Dis. 2015, 9. [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium Difficile Colitis: Pathogenesis And Host Defence. Nat. Rev. Microbiol. 2016, 14, 609-620. [CrossRef]
- Guinan, J.; Villa, P.; Thangamani, S. Secondary Bile Acids Inhibit Candida Albicans Growth And Morphogenesis. Pathog. Dis. 2018, 76. [CrossRef]
- Bustos, A.Y.; Font de Valdez, G.; Fadda, S.; Taranto, M.P. New Insights Into Bacterial Bile Resistance Mechanisms: The Role Of Bile Salt Hydrolase And Its Impact On Human Health. Food Res. Int. 2018, 112, 250-262. [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, Metabolites And Host Immunity. Curr. Opin. Microbiol. 2017, 35, 8-15. [CrossRef]
- de Diego-Cabero, N.; Mereu, A.; Menoyo, D.; Holst, J.J.; Ipharraguerre, I.R. Bile Acid Mediated Effects On Gut Integrity And Performance Of Early-Weaned Piglets. BMC Vet. Res. 2015, 11. [CrossRef]
- Verbeke, L.; Farre, R.; Verbinnen, B.; Covens, K.; Vanuytsel, T.; Verhaegen, J.; Komuta, M.; Roskams, T.; Chatterjee, S.; Annaert, P.; et al. The Fxr Agonist Obeticholic Acid Prevents Gut Barrier Dysfunction And Bacterial Translocation In Cholestatic Rats. Am. J. Pathol. 2015, 185, 409-419. [CrossRef]
- Xu, M.; Cen, M.; Shen, Y.; Zhu, Y.; Cheng, F.; Tang, L.; Hu, W.; Dai, N. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation And Promotes Intestinal Inflammation. Dig. Dis. Sci. 2021, 66, 568-576. [CrossRef]
- Lajczak, N.K.; Saint-Criq, V.; O’Dwyer, A.M.; Perino, A.; Adorini, L.; Schoonjans, K.; Keely, S.J. Bile Acids Deoxycholic Acid And Ursodeoxycholic Acid Differentially Regulate Human Β-Defensin-1 And -2 Secretion By Colonic Epithelial Cells. Faseb J. 2017, 31, 3848-3857. [CrossRef]
- Cipriani, S.; Mencarelli, A.; Chini, M.G.; Distrutti, E.; Renga, B.; Bifulco, G.; Baldelli, F.; Donini, A.; Fiorucci, S.; Ryffel, B. The Bile Acid Receptor Gpbar-1 (Tgr5) Modulates Integrity Of Intestinal Barrier And Immune Response To Experimental Colitis. PLoS One 2011, 6. [CrossRef]
- Ichikawa, R.; Takayama, T.; Yoneno, K.; Kamada, N.; Kitazume, M.T.; Higuchi, H.; Matsuoka, K.; Watanabe, M.; Itoh, H.; Kanai, T.; et al. Bile Acids Induce Monocyte Differentiation Toward Interleukin-12 Hypo-Producing Dendritic Cells Via A Tgr5-Dependent Pathway. Immunology 2012, 136, 153-162. [CrossRef]
- Vavassori, P.; Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. The Bile Acid Receptor Fxr Is A Modulator Of Intestinal Innate Immunity. J. Immunol. 2009, 183, 6251-6261. [CrossRef]
- Biagioli, M.; Carino, A.; Cipriani, S.; Francisci, D.; Marchianò, S.; Scarpelli, P.; Sorcini, D.; Zampella, A.; Fiorucci, S. The Bile Acid Receptor Gpbar1 Regulates The M1/M2 Phenotype Of Intestinal Macrophages And Activation Of Gpbar1 Rescues Mice From Murine Colitis. J. Immunol. 2017, 199, 718-733. [CrossRef]
- Haselow, K.; Bode, J.G.; Wammers, M.; Ehlting, C.; Keitel, V.; Kleinebrecht, L.; Schupp, A.-K.; Häussinger, D.; Graf, D. Bile Acids Pka-Dependently Induce A Switch Of The Il-10/Il-12 Ratio And Reduce Proinflammatory Capability Of Human Macrophages. J. Leukoc. Biol. 2013, 94, 1253-1264. [CrossRef]
- Chávez-Talavera, O.; Haas, J.; Grzych, G.; Tailleux, A.; Staels, B. Bile Acid Alterations In Nonalcoholic Fatty Liver Disease, Obesity, Insulin Resistance And Type 2 Diabetes: What Do The Human Studies Tell? Curr. Opin. Lipidology 2019, 30, 244-254. [CrossRef]
- Vincent, R.P.; Omar, S.; Ghozlan, S.; Taylor, D.R.; Cross, G.; Sherwood, R.A.; Fandriks, L.; Olbers, T.; Werling, M.; Alaghband-Zadeh, J.; et al. Higher Circulating Bile Acid Concentrations In Obese Patients With Type 2 Diabetes. Ann. Clin. Biochem. 2013, 50, 360-364. [CrossRef]
- Prinz, P.; Hofmann, T.; Ahnis, A.; Elbelt, U.; Goebel-Stengel, M.; Klapp, B.F.; Rose, M.; Stengel, A. Plasma Bile Acids Show A Positive Correlation With Body Mass Index And Are Negatively Associated With Cognitive Restraint Of Eating In Obese Patients. Front. Neurosci. 2015, 9. [CrossRef]
- Chen, L.; van den Munckhof, I.C.L.; Schraa, K.; ter Horst, R.; Koehorst, M.; van Faassen, M.; van der Ley, C.; Doestzada, M.; Zhernakova, D.V.; Kurilshikov, A.; et al. Genetic And Microbial Associations To Plasma And Fecal Bile Acids In Obesity Relate To Plasma Lipids And Liver Fat Content. Cell Reports 2020, 33. [CrossRef]
- Montagnana, M.; Danese, E.; Giontella, A.; Bonafini, S.; Benati, M.; Tagetti, A.; Dalbeni, A.; Cavarzere, P.; Gaudino, R.; Pucci, M.; et al. Circulating Bile Acids Profiles In Obese Children And Adolescents: A Possible Role Of Sex, Puberty And Liver Steatosis. Diagnostics 2020, 10. [CrossRef]
- Haeusler, R.A.; Astiarraga, B.; Camastra, S.; Accili, D.; Ferrannini, E. Human Insulin Resistance Is Associated With Increased Plasma Levels Of 12A-Hydroxylated Bile Acids. Diabetes 2013, 62, 4184-4191. [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acids As Metabolic Regulators And Nutrient Sensors. Annu. Rev. Nutr. 2019, 39, 175-200. [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control Of Metabolism And Inflammation In Obesity, Type 2 Diabetes, Dyslipidemia, And Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679-1694.e3. [CrossRef]
- Preidis, G.A.; Kim, K.H.; Moore, D.D. Nutrient-Sensing Nuclear Receptors Pparα And Fxr Control Liver Energy Balance. J. Clin. Invest. 2017, 127, 1193-1201. [CrossRef]
- Sagar, N.M.; McFarlane, M.; Nwokolo, C.; Bardhan, K.D.; Arasaradnam, R.P. Mechanisms Of Triglyceride Metabolism In Patients With Bile Acid Diarrhea. World J. Gastroenterol. 2016, 22. [CrossRef]
- Ma, K. Farnesoid X Receptor Is Essential For Normal Glucose Homeostasis. J. Clin. Invest. 2006, 116, 1102-1109. [CrossRef]
- Kir, S.; Beddow, S.A.; Samuel, V.T.; Miller, P.; Previs, S.F.; Suino-Powell, K.; Xu, H.E.; Shulman, G.I.; Kliewer, S.A.; Mangelsdorf, D.J. Fgf19 As A Postprandial, Insulin-Independent Activator Of Hepatic Protein And Glycogen Synthesis. Science 2011, 331, 1621-1624. [CrossRef]
- Gowda, G.A.N.; Ijare, O.B.; Somashekar, B.S.; Sharma, A.; Kapoor, V.K.; Khetrapal, C.L. Single-Step Analysis Of Individual Conjugated Bile Acids In Human Bile Using 1 H Nmr Spectroscopy. Lipids 2006, 41, 591-603. [CrossRef]
- Yang, Y.; Griffiths, W.J.; Nazer, H.; Sjövall, J. Analysis of bile acids and bile alcohols in urine by capillary column liquid chromatography-mass spectrometry using fast atom bombardment or electrospray ionization and collision-induced dissociation. Biomed. Chromatogr. 1997, 11, 240-55.
- Griffiths, W.J.; Sjövall, J. Bile Acids: Analysis In Biological Fluids And Tissues. J. Lipid Res. 2009, 51, 23-41. [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect Of Diet On The Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11. [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly And Reproducibly Alters The Human Gut Microbiome. Nature 2014, 505, 559-563. [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns With Gut Microbial Enterotypes. Science 2011, 334, 105-108. [CrossRef]
- Xu, Z.; Knight, R. Dietary Effects On Human Gut Microbiome Diversity. Br. J. Nutr. 2015, 113, S1-S5. [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant And Diet-Responsive Groups Of Bacteria Within The Human Colonic Microbiota. ISME J. 2011, 5, 220-230. [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact Of Diet In Shaping Gut Microbiota Revealed By A Comparative Study In Children From Europe And Rural Africa. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 14691-14696. [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A Vegan Or Vegetarian Diet Substantially Alters The Human Colonic Faecal Microbiota. Eur. J. Clin. Nutr. 2012, 66, 53-60. [CrossRef]
- Sidhu, S.R.K.; Kok, C.W.; Kunasegaran, T.; Ramadas, A. Effect Of Plant-Based Diets On Gut Microbiota: A Systematic Review Of Interventional Studies. Nutrients 2023, 15. [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence Of Diet On The Gut Microbiome And Implications For Human Health. J. Transl. Med. 2017, 15. [CrossRef]
- Kase, B.E.; Liese, A.D.; Zhang, J.; Murphy, E.A.; Zhao, L.; Steck, S.E. The Development And Evaluation Of A Literature-Based Dietary Index For Gut Microbiota. Nutrients 2024, 16. [CrossRef]
- Schoonakker, M.P.; van Peet, P.G.; van den Burg, E.L.; Numans, M.E.; Ducarmon, Q.R.; Pijl, H.; Wiese, M. Impact Of Dietary Carbohydrate, Fat Or Protein Restriction On The Human Gut Microbiome: A Systematic Review. Nutr. Res. Rev. 2024, 1-18. [CrossRef]
- Dai, Z.-L. Amino Acid Metabolism In Intestinal Bacteria: Links Between Gut Ecology And Host Health. Front. Biosci. 2011, 16. [CrossRef]
- Bartlett, A.; Kleiner, M. Dietary Protein And The Intestinal Microbiota: An Understudied Relationship. iScience 2022, 25. [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects Of Dietary Fat On Gut Microbiota And Faecal Metabolites, And Their Relationship With Cardiometabolic Risk Factors: A 6-Month Randomised Controlled-Feeding Trial. Gut 2019, 68, 1417-1429. [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts Of Western Diet And Its Effects On Metabolism And Health: A Narrative Review. Nutrients 2023, 15. [CrossRef]
- Piernas, C.; Gao, M.; Jebb, S.A. Dietary Patterns Derived By Reduced Rank Regression And Non-Communicable Disease Risk. Proc. Nutr. Soc. 2022, 1-8. [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect Of Diet And Dietary Components On The Composition Of The Gut Microbiota. Nutrients 2021, 13. [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut Microbiota: A New Path To Treat Obesity. Int. J. Obes. Suppl. 2019, 9, 10-19. [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Human Gut Microbiome, Diet, And Mental Disorders. International Microbiology 2024. [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome And Short-Chain Fatty Acids In Association With Alzheimer's Disease Markers In Subjects With Mild Cognitive Impairment. EBioMedicine 2019, 47, 529-542. [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention Of Cardiovascular Disease With A Mediterranean Diet Supplemented With Extra-Virgin Olive Oil Or Nuts. N. Engl. J. Med. 2018, 378. [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence To A Mediterranean Diet Beneficially Impacts The Gut Microbiota And Associated Metabolome. Gut 2016, 65, 1812-1821. [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts On Gut Microbiota Associated To Mediterranean Diet Adherence And Specific Dietary Intakes On General Adult Population. Front. Microbiol. 2018, 9. [CrossRef]
- Pagliai, G.; Russo, E.; Niccolai, E.; Dinu, M.; Di Pilato, V.; Magrini, A.; Bartolucci, G.; Baldi, S.; Menicatti, M.; Giusti, B.; et al. Influence Of A 3-Month Low-Calorie Mediterranean Diet Compared To The Vegetarian Diet On Human Gut Microbiota And Scfa: The Cardiveg Study. Eur. J. Nutr. 2020, 59, 2011-2024. [CrossRef]
- Wang, Y.; Wymond, B.; Tandon, H.; Belobrajdic, D.P. Swapping White For High-Fibre Bread Increases Faecal Abundance Of Short-Chain Fatty Acid-Producing Bacteria And Microbiome Diversity: A Randomized, Controlled, Decentralized Trial. Nutrients 2024, 16. [CrossRef]
- Kaoutari, A.E.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The Abundance And Variety Of Carbohydrate-Active Enzymes In The Human Gut Microbiota. Nat. Rev. Microbiol. 2013, 11, 497-504. [CrossRef]
- Holscher, H.D. Dietary Fiber And Prebiotics And The Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172-184. [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation Of The Human Colonic Microbiota: Introducing The Concept Of Prebiotics. J. Nutr. 1995, 125, 1401-1412. [CrossRef]
- Roberfroid, M. Prebiotics: The Concept Revisited1. J. Nutr. 2007, 137, 830S-837S. [CrossRef]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J.; Britton, R.A.; Cani, P.D. Modulation Of The Gastrointestinal Microbiome With Nondigestible Fermentable Carbohydrates To Improve Human Health. Microbiol. Spectr. 2017, 5. [CrossRef]
- Delcour, J.A.; Aman, P.; Courtin, C.M.; Hamaker, B.R.; Verbeke, K. Prebiotics, Fermentable Dietary Fiber, And Health Claims. Adv. Nutr. 2016, 7, 1-4. [CrossRef]
- Guzman, J.R.; Conlin, V.S.; Jobin, C. Diet, Microbiome, And The Intestinal Epithelium: An Essential Triumvirate? Biomed Res. Int. 2013, 2013, 1-12. [CrossRef]
- Mogensen, T.H. Pathogen Recognition And Inflammatory Signaling In Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240-273. [CrossRef]
- Lozupone, C.A.; Knight, R. Species Divergence And The Measurement Of Microbial Diversity. Fems Microbiol. Rev. 2008, 32, 557-578. [CrossRef]
- Cadotte, M.W.; Jonathan Davies, T.; Regetz, J.; Kembel, S.W.; Cleland, E.; Oakley, T.H. Phylogenetic Diversity Metrics For Ecological Communities: Integrating Species Richness, Abundance And Evolutionary History. Ecol. Lett. 2010, 13, 96-105. [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links Between Diet, Gut Microbiota Composition And Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13-22. [CrossRef]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary Fiber Intervention On Gut Microbiota Composition In Healthy Adults: A Systematic Review And Meta-Analysis. Am. J. Clin. Nutr. 2018, 107, 965-983. [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation Of Gut Microbiota Contributes To Alleviation Of Both Genetic And Simple Obesity In Children. EBioMedicine 2015, 2, 968-984. [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review Article: Dietary Fibre-Microbiota Interactions. Aliment. Pharmacol. Ther. 2015, 42, 158-179. [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic Inulin-Type Fructans Induce Specific Changes In The Human Gut Microbiota. Gut 2017, 66, 1968-1974. [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (Fos) And Galactooligosaccharide (Gos) Increase Bifidobacterium But Reduce Butyrate Producing Bacteria With Adverse Glycemic Metabolism In Healthy Young Population. Sci Rep 2017, 7. [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A Perspective On The Complexity Of Dietary Fiber Structures And Their Potential Effect On The Gut Microbiota. J. Mol. Biol. 2014, 426, 3838-3850. [CrossRef]
- Tuncil, Y.E.; Thakkar, R.D.; Arioglu-Tuncil, S.; Hamaker, B.R.; Lindemann, S.R.; Young, V.B. Subtle Variations In Dietary-Fiber Fine Structure Differentially Influence The Composition And Metabolic Function Of Gut Microbiota. mSphere 2020, 5, e00180-20. [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. Tuning Expectations To Reality: Don’t Expect Increased Gut Microbiota Diversity With Dietary Fiber. The J. Nutr. 2023, 153, 3156-3163. [CrossRef]
- Bai, J.; Li, Y.; Li, T.; Zhang, W.; Fan, M.; Zhang, K.; Qian, H.; Zhang, H.; Qi, X.; Wang, L. Comparison Of Different Soluble Dietary Fibers During The In Vitro Fermentation Process. J. Agric. Food Chem. 2021, 69, 7446-7457. [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial Degradation Of Complex Carbohydrates In The Gut. Gut Microbes 2014, 3, 289-306. [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The Carbohydrate-Active Enzymes Database (Cazy) In 2013. Nucleic Acids Res. 2013, 42, D490-D495. [CrossRef]
- Cecchini, D.A.; Laville, E.; Laguerre, S.; Robe, P.; Leclerc, M.; Doré, J.; Henrissat, B.; Remaud-Siméon, M.; Monsan, P.; Potocki-Véronèse, G.; et al. Functional Metagenomics Reveals Novel Pathways Of Prebiotic Breakdown By Human Gut Bacteria. PLoS One 2013, 8. [CrossRef]
- Saito, Y.; Shigehisa, A.; Watanabe, Y.; Tsukuda, N.; Moriyama-Ohara, K.; Hara, T.; Matsumoto, S.; Tsuji, H.; Matsuki, T.; Zhou, N.-Y. Multiple Transporters And Glycoside Hydrolases Are Involved In Arabinoxylan-Derived Oligosaccharide Utilization In Bifidobacterium Pseudocatenulatum. Appl. Environ. Microbiol. 2020, 86, e01782-20. [CrossRef]
- Kim, C.C.; Healey, G.R.; Kelly, W.J.; Patchett, M.L.; Jordens, Z.; Tannock, G.W.; Sims, I.M.; Bell, T.J.; Hedderley, D.; Henrissat, B.; et al. Genomic Insights From Monoglobus Pectinilyticus: A Pectin-Degrading Specialist Bacterium In The Human Colon. ISME J. 2019, 13, 1437-1456. [CrossRef]
- Boger, M.C.L.; Lammerts van Bueren, A.; Dijkhuizen, L.; McBain, A.J. Cross-Feeding Among Probiotic Bacterial Strains On Prebiotic Inulin Involves The Extracellular Exo -Inulinase Of Lactobacillus Paracasei Strain W20. Appl. Environ. Microbiol. 2018, 84, e01539-18. [CrossRef]
- Delannoy-Bruno, O.; Desai, C.; Raman, A.S.; Chen, R.Y.; Hibberd, M.C.; Cheng, J.; Han, N.; Castillo, J.J.; Couture, G.; Lebrilla, C.B.; et al. Evaluating Microbiome-Directed Fibre Snacks In Gnotobiotic Mice And Humans. Nature 2021, 595, 91-95. [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria And Butyrate-Producing Colon Bacteria: Importance And Strategies For Their Stimulation In The Human Gut. Front. Microbiol. 2016, 7. [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How Glycan Metabolism Shapes The Human Gut Microbiota. Nat. Rev. Microbiol. 2012, 10, 323-335. [CrossRef]
- Sheridan, P.; Martin, J.C.; Lawley, T.D.; Browne, H.P.; Harris, H.M.B.; Bernalier-Donadille, A.; Duncan, S.H.; O'Toole, P.W.; P. Scott, K.; J. Flint, H. Polysaccharide Utilization Loci And Nutritional Specialization In A Dominant Group Of Butyrate-Producing Human Colonic Firmicutes. Microb. Genomics 2016, 2. [CrossRef]
- Rodriguez, J.; Hiel, S.; Neyrinck, A.M.; Le Roy, T.; Pötgens, S.A.; Leyrolle, Q.; Pachikian, B.D.; Gianfrancesco, M.A.; Cani, P.D.; Paquot, N.; et al. Discovery Of The Gut Microbial Signature Driving The Efficacy Of Prebiotic Intervention In Obese Patients. Gut 2020, 69, 1975-1987. [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-Utilizing Capacity Varies In Prevotella- Versus Bacteroides-Dominated Gut Microbiota. Sci Rep 2017, 7. [CrossRef]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial Enterotypes In Personalized Nutrition And Obesity Management. Am. J. Clin. Nutr. 2018, 108, 645-651. [CrossRef]
- Van den Abbeele, P.; Duysburgh, C.; Ghyselinck, J.; Goltz, S.; Berezhnaya, Y.; Boileau, T.; De Blaiser, A.; Marzorati, M. Fructans With Varying Degree Of Polymerization Enhance The Selective Growth Of Bifidobacterium Animalis Subsp. Lactis Bb-12 In The Human Gut Microbiome In Vitro. Appl. Sci. 2021, 11. [CrossRef]
- Klimenko, N.S.; Tyakht, A.V.; Popenko, A.S.; Vasiliev, A.S.; Altukhov, I.A.; Ischenko, D.S.; Shashkova, T.I.; Efimova, D.A.; Nikogosov, D.A.; Osipenko, D.A.; et al. Microbiome Responses To An Uncontrolled Short-Term Diet Intervention In The Frame Of The Citizen Science Project. Nutrients 2018, 10. [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker Of Gut Dysbiosis In Obese Patients? Nutrients 2020, 12. [CrossRef]
- Mobeen, F.; Sharma, V.; Prakash, T. Enterotype Variations Of The Healthy Human Gut Microbiome In Different Geographical Regions. Bioinformation 2018, 14, 560-573. [CrossRef]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual Dietary Fibre Intake Influences Gut Microbiota Response To An Inulin-Type Fructan Prebiotic: A Randomised, Double-Blind, Placebo-Controlled, Cross-Over, Human Intervention Study. Br. J. Nutr. 2018, 119, 176-189. [CrossRef]
- Smith, A.M. The Biosynthesis Of Starch Granules. Biomacromolecules 2001, 2, 335-341. [CrossRef]
- Sim, L.; Willemsma, C.; Mohan, S.; Naim, H.Y.; Pinto, B.M.; Rose, D.R. Structural Basis For Substrate Selectivity In Human Maltase-Glucoamylase And Sucrase-Isomaltase N-Terminal Domains. J. Biol. Chem. 2010, 285, 17763-17770. [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise For Improving Human Health. Adv. Nutr. 2013, 4, 587-601. [CrossRef]
- Jane, J.L Structural Features Of Starch Granules II. In Starch; Elsevier, 2009; pp. 193-236 ISBN 9780127462752. doi: 10.1016/B978-0-12-746275-2.00006-9.
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch–A Review. Compr. Rev. Food. Sci. Food Saf. 2006, 5, 1-17. [CrossRef]
- McCleary, B.V.; Monaghan, D.A. Measurement Of Resistant Starch. J. AOAC Int. 2002, 85, 665-675. [CrossRef]
- Englyst, H.N.; Hudson, G.J. The classification and measurement of dietary carbohydrates. Food Chem. 1996, 57, 15-21. doi.org/10.1016/0308-8146(96)00056-8.
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant Starch In Food: A Review. J. Sci. Food Agric. 2015, 95, 1968-1978. [CrossRef]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67-72. doi:10.1079/PNS2002207.
- Morrison, D.J.; Preston, T. Formation Of Short Chain Fatty Acids By The Gut Microbiota And Their Impact On Human Metabolism. Gut Microbes 2016, 7, 189-200. [CrossRef]
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity Of Bifidobacteria Within The Infant Gut Microbiota. PLoS One 2012, 7. [CrossRef]
- Suzuki, T. Regulation Of Intestinal Epithelial Permeability By Tight Junctions. Cell. Mol. Life Sci. 2013, 70, 631-659. [CrossRef]
- Segain, J.-P. Butyrate Inhibits Inflammatory Responses Through Nfkappa B Inhibition: Implications For Crohn's Disease. Gut 47, 397-403. [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces The Differentiation Of Colonic Regulatory T Cells. Nature 2013, 504, 446-450. [CrossRef]
- Krishnan, V.; Awana, M.; Samota, M.K.; Warwate, S.I.; Kulshreshtha, A.; Ray, M.; Bollinedi, H.; Singh, A.K.; Thandapilly, S.J.; Praveen, S.; et al. Pullulanase Activity: A Novel Indicator Of Inherent Resistant Starch In Rice (Oryza Sativa. L). Int. J. Biol. Macromol. 2020, 152, 1213-1223. [CrossRef]
- Barros, F.; Awika, J.; Rooney, L.W. Effect Of Molecular Weight Profile Of Sorghum Proanthocyanidins On Resistant Starch Formation. J. Sci. Food Agric. 2014, 94, 1212-1217. [CrossRef]
- Cao, R.; Liu, X.; Liu, Y.; Zhai, X.; Cao, T.; Wang, A.; Qiu, J. Applications Of Nuclear Magnetic Resonance Spectroscopy To The Evaluation Of Complex Food Constituents. Food Chem. 2021, 342. [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, And Obesity: Links With Host Genetics And Epigenetics And Potential Applications. Adv. Nutr. 2019, 10, S17-S30. [CrossRef]
- Bien, J.; Palagani, V.; Bozko, P. The Intestinal Microbiota Dysbiosis And Clostridium Difficile Infection: Is There A Relationship With Inflammatory Bowel Disease? Ther. Adv. Gastroenterol. 2013, 6, 53-68. [CrossRef]
- Shanahan, F. The Colonic Microbiota In Health And Disease. Curr. Opin. Gastroenterol. 2013, 29, 49-54. [CrossRef]
- Peterson, C.T.; Sharma, V.; Elmén, L.; Peterson, S.N. Immune Homeostasis, Dysbiosis And Therapeutic Modulation Of The Gut Microbiota. Clin. Exp. Immunol. 2015, 179, 363-377. [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding Of Dysbiosis In Disease In Human And Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137-1150. [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery In Obesity: Effects On Gut Microbiota And Micronutrient Status. Nutrients 2020, 12. [CrossRef]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation Of Energy Metabolism. J. Innate Immun. 2018, 10, 163-171. [CrossRef]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human Intestinal Microbiota Composition Is Associated With Local And Systemic Inflammation In Obesity. Obesity 2013, 21. [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison Of The Gut Microbiota Composition Between Obese And Non-Obese Individuals In A Japanese Population, As Analyzed By Terminal Restriction Fragment Length Polymorphism And Next-Generation Sequencing. BMC Gastroenterol. 2015, 15. [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association Between Body Mass Index And Firmicutes/Bacteroidetes Ratio In An Adult Ukrainian Population. BMC Microbiol. 2017, 17. [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 11070-11075. [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence Of Probiotics On The Firmicutes/Bacteroidetes Ratio In The Treatment Of Obesity And Inflammatory Bowel Disease. Microorganisms 2020, 8. [CrossRef]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota And Host Metabolism: From Proof Of Concept To Therapeutic Intervention. Microorganisms 2021, 9. [CrossRef]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A Taxonomic Signature Of Obesity In A Large Study Of American Adults. Sci Rep 2018, 8. [CrossRef]
- Hu, H.-J.; Park, S.-G.; Jang, H.B.; Choi, M.-G.; Park, K.-H.; Kang, J.H.; Park, S.I.; Lee, H.-J.; Cho, S.-H.; Zoetendal, E.G. Obesity Alters The Microbial Community Profile In Korean Adolescents. PLoS One 2015, 10. [CrossRef]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated With Diet, Obesity And Weight Loss. Int. J. Obes. 2008, 32, 1720-1724. [CrossRef]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; Granados-Silvestre, M. de los Á.; Palacios-González, B.; Menjivar, M. Altered Gut Microbiota And Compositional Changes In Firmicutes And Proteobacteria In Mexican Undernourished And Obese Children. Front. Microbiol. 2018, 9. [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Retracted Article: Obesity-Associated Gut Microbiota Is Enriched In Lactobacillus Reuteri And Depleted In Bifidobacterium Animalis And Methanobrevibacter Smithii. Int. J. Obes. 2012, 36, 817-825. [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness Of Human Gut Microbiome Correlates With Metabolic Markers. Nature 2013, 500, 541-546. [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.D.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio Of The Human Microbiota Changes With Age. BMC Microbiol. 2009, 9. [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. Scfas Strongly Stimulate Pyy Production In Human Enteroendocrine Cells. Sci Rep 2018, 8. [CrossRef]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The Short Chain Fatty Acid Propionate Stimulates Glp-1 And Pyy Secretion Via Free Fatty Acid Receptor 2 In Rodents. Int. J. Obes. 2015, 39, 424-429. [CrossRef]
- Mraz, M.; Haluzik, M. The Role Of Adipose Tissue Immune Cells In Obesity And Low-Grade Inflammation. J. Endocrinol. 2014, 222, R113-R127. [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes In Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation In High-Fat Diet–Induced Obesity And Diabetes In Mice. Diabetes 2008, 57, 1470-1481. [CrossRef]
- Trøseid, M.; Nestvold, T.K.; Rudi, K.; Thoresen, H.; Nielsen, E.W.; Lappegård, K.T. Plasma Lipopolysaccharide Is Closely Associated With Glycemic Control And Abdominal Obesity. Diabetes Care 2013, 36, 3627-3632. [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective Increases Of Bifidobacteria In Gut Microflora Improve High-Fat-Diet-Induced Diabetes In Mice Through A Mechanism Associated With Endotoxaemia. Diabetologia 2007, 50, 2374-2383. [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes In Gut Microbiota Control Inflammation In Obese Mice Through A Mechanism Involving Glp-2-Driven Improvement Of Gut Permeability. Gut 2009, 58, 1091-1103. [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth Cells, Antimicrobial Peptides And Maintenance Of Intestinal Homeostasis. Nat. Rev. Microbiol. 2011, 9, 356-368. [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome Of Prebiotic-Treated Mice Reveals Novel Targets Involved In Host Response During Obesity. ISME J. 2014, 8, 2116-2130. [CrossRef]
- Tan, T.G.; Sefik, E.; Geva-Zatorsky, N.; Kua, L.; Naskar, D.; Teng, F.; Pasman, L.; Ortiz-Lopez, A.; Jupp, R.; Wu, H.-J.J.; et al. Identifying Species Of Symbiont Bacteria From The Human Gut That, Alone, Can Induce Intestinal Th17 Cells In Mice. Proc. Natl. Acad. Sci. U. S. A. 2016, 113. [CrossRef]
- Luck, H.; Khan, S.; Kim, J.H.; Copeland, J.K.; Revelo, X.S.; Tsai, S.; Chakraborty, M.; Cheng, K.; Tao Chan, Y.; Nøhr, M.K.; et al. Gut-Associated Iga+ Immune Cells Regulate Obesity-Related Insulin Resistance. Nat. Commun. 2019, 10. [CrossRef]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.-H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T Cell–Mediated Regulation Of The Microbiota Protects Against Obesity. Science 2019, 365. [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity And Insulin Resistance. Diabetes 2007, 56, 1761-1772. [CrossRef]
- Tran, H.Q.; Ley, R.E.; Gewirtz, A.T.; Chassaing, B. Flagellin-Elicited Adaptive Immunity Suppresses Flagellated Microbiota And Vaccinates Against Chronic Inflammatory Diseases. Nat. Commun. 2019, 10. [CrossRef]
- Cavallari, J.F.; Fullerton, M.D.; Duggan, B.M.; Foley, K.P.; Denou, E.; Smith, B.K.; Desjardins, E.M.; Henriksbo, B.D.; Kim, K.J.; Tuinema, B.R.; et al. Muramyl Dipeptide-Based Postbiotics Mitigate Obesity-Induced Insulin Resistance Via Irf4. Cell Metab. 2017, 25, 1063-1074.e3. [CrossRef]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature 2008, 453, 620-625. [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein From Akkermansia Muciniphila Or The Pasteurized Bacterium Improves Metabolism In Obese And Diabetic Mice. Nat. Med. 2017, 23, 107-113. [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling Through Mtorc1. Cell 2018, 175, 947-961.e17. [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites In Health And Disease. Nat. Commun. 2018, 9. [CrossRef]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.-L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production By The Gut Microbiota Is A Key Factor In Metabolic Syndrome. Cell Metab. 2018, 28, 737-749.e4. [CrossRef]
- Laurans, L.; Venteclef, N.; Haddad, Y.; Chajadine, M.; Alzaid, F.; Metghalchi, S.; Sovran, B.; Denis, R.G.P.; Dairou, J.; Cardellini, M.; et al. Genetic Deficiency Of Indoleamine 2,3-Dioxygenase Promotes Gut Microbiota-Mediated Metabolic Health. Nat. Med. 2018, 24, 1113-1120. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





