Submitted:
23 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Gold nanoparticles (NPs) are among the most commonly employed metal NPs in biological applications, with their distinctive physicochemical features. Their extraordinary optical properties, stemming from the strong localized surface plasmon resonance (LSPR), contribute to the devel-opment of novel approaches in the areas of bioimaging, biosensing, and cancer research, especially for photothermal and photodynamic therapy. The ease of functionalization with various lig-ands provides a novel approach to the precise delivery of these molecules to targeted areas. Gold NPs ability to transfer heat and electricity positions them as valuable materials for advancing thermal management and electronic systems. Moreover, their inherent characteristics, such as in-ertness, give rise to the synthesis of novel antibacterial and antioxidant agents as they provide a biocompatible and low-toxic approach. Chemical and physical synthesis methods are utilized to produce gold NPs. The pursuit of more ecologically sustainable and economically viable large-scale technologies, such as environmentally benign biological processes referred to as green/biological synthesis, has garnered increasing interest among global researchers. Green synthesis methods are favorable among other synthesis techniques as they minimize the necessity for hazardous chemicals in the reduction process due to their simplicity, cost-effectiveness, energy efficiency, and biocompatibility. This article discusses the importance of gold NPs, their optical and conductivity properties, antibacterial, antioxidant, and anticancer properties, synthesis methods, contemporary uses, and biosafety, emphasizing the need to understand toxicology principles and green commercialization strategies.
Keywords:
1. Introduction
2. Properties of Gold Nanoparticles
2.1. Size
2.2. Shape
2.3. Surface Characteristics
2.3.1. Surface Charge
2.3.2. Surface Functionalization
2.4. Optical Properties
2.5. Electrical Conductivity
2.6. Thermal Conductivity
2.7. Delivery
Protein Delivery
Nucleic Acid Delivery
Chemotherapeutic Agent Delivery
Glycan Delivery
2.8. Anticancer Activity
2.9. Antibacterial Activity
2.10. Antioxidant Activity
| Highlighted Activity | Synthesis Method | Property | Result | Ref |
|---|---|---|---|---|
| Electrical conductivity | Chemical reduction | Size = 10 nm Shape = Spherical |
Gold NP-decorated porous carbon microspheres were developed as electrode materials for supercapacitors to enhance electrochemical performance. | [151] |
| Electrical conductivity | Electrodeposition | Size = Ranging from 20 to 30 nm Shape = - |
Gold NPs were incorporated into amperometric sensors to enhance their sensitivity and electrochemical performance. | [152] |
| Electrical conductivity | Chemical reduction | Size = Ranging from 1 to 6 nm. Shape = Spherical |
Incorporation of gold NPs into supercapacitor dielectric composites enhanced electrical conductivity and specific capacitance | [153] |
| Electrical conductivity | NPs were purchased commercially | Size = - Shape = - |
Enhancement in electrical conductivity and electrochemical properties of gelatin methacrylate hydrogels were observed following incorporation of gold NPs. | [154] |
| Electrical conductivity | Turkevich method | Size = Ranging from 9 to 46 nm Shape = Spherical |
Gold NPs (combined with copper nanowires) enhanced electrochemical conductivity in amperometric sensors, achieving up to 2.3-fold increase in performance. | [155] |
| Electrical conductivity | Laser ablation | Size = Ranging from 19.43 nm and 32.76 nm. Shape = - |
Gold NPs increased the electrical conductivity of PVP/PVA matrix, with higher concentrations leading to greater AC values. | [85] |
| Electrical conductivity | Seeded growth | Size = Ranging from 15 nm to 80 nm for spherical NPs. Length of 40.4 nm and width of 12.0 nm for rod-shaped NPs. Shape = Spherical and Rod-shaped. |
Addition of gold NPs enhanced the fluid's conductivity, with smaller spherical NPs improving electrical properties more effectively than their counterparts. |
[156] |
| Electrical conductivity | Green synthesis from plant extract Laser ablation |
Size = Ranging from 3 nm to 24 nm for green synthesized NPs; 2 nm to 30 nm for those synthesized through laser ablation. Shape = triangular, hexagonal, spherical and irregular |
Incorporation of gold NPs enhanced the electrical properties of polymer blend. Increased AC and DC conductivity, along with improved dielectric permittivity, was observed. |
[157] |
| Electrical conductivity | Electrodeposition | Size = - Shape = - |
Increase in electron transfer was observed following modification of carbon electrodes with gold NPs. |
[158] |
| Electrical conductivity | Laser ablation | Size = Average size of 77 ± 4 nm. Shape = Spherical |
Addition of Gold NPs into cement-based composites enhanced electrical conductivity, decreased electrical resistance and increased the piezoelectric response by up to 57 times | [159] |
| Thermal conductivity | Laser ablation | Size = Average diameter of 6.3 nm Shape = Crystalline structure |
Incorporation of gold NPs improved thermal conductivity of the nanofluid. Achieving 0.41 W/mK, 26% increase compared to the base fluid was observed. |
[160] |
| Thermal conductivity | Chemical reduction | Size = Ranging from 20 to 40 nm. Shape = Spherical |
Incorporation of gold NPs into silica gel composites enhanced thermal conductivity by approximately 10–15%. | [161] |
| Thermal conductivity | NPs were purchased commercially | Size = Having a diameter of 41 nm Shape = Rod-shaped |
Gold NPs improved the thermal properties of tissue-mimicking phantoms by increasing temperature response during photothermal therapy. | [162] |
| Thermal conductivity | Chemical reduction | Size = - Shape = - |
Incorporation of gold NPs improved thermal conductivity of carbon nanotube fibers by 70%. | [163] |
| Thermal conductivity | - | Size = Having approximate diameter of 4 nm Shape - |
Gold NPs enhanced heat transfer in the tree-structured polymer networks by increasing the number of thermal transfer channels | [164] |
| Optical Properties | Seeded growth | Size = Length of 45 nm and 65 nm Shape = Rod-shaped |
Highly active SERS substrates were developed using hollow gold-silver NRs. | [165] |
| Optical Properties | Chemical reduction | Size = Having a diameter of 13 nm Shape = - |
Optofluidic biosensor was developed using DNA-functionalized gold NPs for detection of mutated β-thalassemia sequence. | [166] |
| Optical Properties | NPs were purchased commercially. | Size = Having a diameter of 50 nm. Shape = Rod-shaped. |
Gold NPs were developed to enhance photothermal performance in localized tumor treatment. | [167] |
| Optical Properties | NPs were purchased commercially | Size = Average size of 95.74 nm Shape = Spherical |
Colorimetric biosensor utilizing gold NPs was developed for the enzyme-free detection of Klebsiella pneumoniae (K. pneumoniae). | [168] |
| Optical Properties | Turkevich method | Size = Having an approximate diameter around 40 nm Shape = Spherical |
Electrochemical sensor utilizing gold NPs was developed for the detection of catechol. | [169] |
| Optical Properties | Turkevich method | Size = 14 nm Shape = Spherical |
Gold NP-based lateral flow immunoassay was developed for the detection of tuberculosis antigens CFP-10 and ESAT-6. | [170] |
| Optical Properties | NPs were purchased commercially | Size = 40 nm Shape = |
Gold NP-based electrochemical immunosensors were developed for the detection of HER-1 and HER-2 biomarkers in breast cancer. | [171] |
| Optical Properties | Chemical reduction | Size = Average size of 40 nm Shape = |
Gold NP integrated plasmonic biosensors were developed for the early detection of Familial Mediterranean Fever. | [172] |
| Optical Properties | Chemical reduction | Size = Average size of 53.88 ± 1.81 nm Shape = Spherical |
Gadolinium-functionalized gold NPs were developed for dual-modal imaging and photothermal therapy in tumors. | [173] |
| Optical Properties | Chemical reduction | Size = Average diameter of 16 ± 1 nm Shape = Spherical |
Gold NPs were developed for the rapid detection of microRNAs in milk samples to assess milk quality and cattle health. | [174] |
| Delivery | Chemical reduction | Size= Ranging from 5 nm to 20 nm Shape = Spherical |
Targeted drug delivery system against SARS-CoV-2 was developed. | [175] |
| Delivery | Seeded-growth | Size = Average hydrodynamic size of 98.6 ± 0.6 nm Shape = Urchin-like |
Nasal drug delivery system utilizing gold nanourchins as a carrier for targeted brain delivery was developed. | [176] |
| Delivery | Chemical reduction | Size = 2 nm. Shape = Spherical |
Delivery system using ultra-small gold NPs to cross the BBB was developed. | [177] |
| Delivery | Chemical reduction | Size = Ranging from 13 nm to 18 nm Shape = Spherical |
Non-viral gene delivery system utilizing gold NPs as carriers for hepatocellular carcinoma treatment was developed. | [178] |
| Delivery | Turkevich method | Size = Average diameter of 12 nm Shape = Spherical |
Delivery system using gold NPs for the controlled release of dacarbazine was developed. |
[179] |
| Delivery | Chemical reduction | Size = Average diameter of 35 nm Shape = Spherical |
Resveratrol-gold NP delivery system to inhibit cataract formation was developed. | [180] |
| Delivery | Brust-Schiffrin method | Size = 4 nm. Shape = - |
Delivery system using gold NPs, functionalized with cRGD peptides, for the delivery of anticancer drug DM1 was developed. | [181] |
| Delivery | Gold NPs, with a concentration of 3000 ppm, were purchased commercially | Size = Having a diameter of 10 nm Shape = - |
Gold NP-photosensitizer conjugates were developed to enhance the efficiency of PDT in targeting lung cancer stem cells. | [182] |
| Delivery | Chemical reduction | Size = Average diameter of 24 nm Shape = - |
Gold NP-conjugated MRI contrast agents were developed to enhance the specificity and sensitivity of MRI imaging. | [183] |
| Delivery | Chemical reduction | Size = Average diameter of 13 nm Shape = - |
Gold NPs were developed for the effective delivery of miR-206 to reduce cell viability and induce apoptosis in breast cancer cells. | [184] |
| Anticancer activity | Chemical reduction | Size = Average size of 90.6 (± 9.6) nm Shape = Rod-shaped |
Anticancer agents using gold NPs decorated with bovine serum albumin were developed. | [185] |
| Anticancer activity | Green synthesis using licorice root extract |
Size = 2.647 nm to 16.25 nm size range Shape = Spherical |
At high concentrations, the gold Np mediated by licorice root demonstrated superior antiproliferative action against MCF-7. | [186] |
| Anticancer activity | Green synthesis using marine microbe Vibrio alginolyticus (V. alginolyticus) |
Size = 100 - 150 nm Shape = |
With a maximum cell death inhibition of 25 mg/mL, the biosynthesized gold NPs showed a dose-dependent inhibitory effect on colon cancer cell growth. | [187] |
| Anticancer activity | Green synthesis using Fusarium solani | Size = 40-45 nm Shape = Needle and spindle like shape |
On MCF-7 and HeLa cells, these gold NPs had strong cytotoxic effects. | [188] |
| Anticancer activity | Green synthesis using Trachyspermum ammi | Size = Average 16.63 nm Shape = Spherical and spheroidal |
HepG2 cancer cell lines were shown to respond favorably to these NPs as anticancer agents. The synthesized NPs' ability to suppress biofilm formation against pathogens, Listeria monocytogenes and Serratia marcescens (S. marcescens), at SUB-MICs. |
[189] |
| Anticancer activity | Green synthesis using Mangifera indica | Size = 20 nm Shape = round, triangle, and irregular shape |
Modest antibacterial, cytotoxic, and dose-dependent antioxidant activities were demonstrated by gold NPs. | [190] |
| Anticancer activity | Green synthesis using Vicoa indica leaf extract | Size = Average size of 13 nm Shape = Spherical |
Anticancer activity against lung cancer cell line (A549), with a IC50 value of 73.56 µg/mL, was observed . | [191] |
| Anticancer activity | Green synthesis using Gelidium pusillum | Size = Average diameter of 12 ± 4.2 nm Shape = Spherical |
Gold NPs demonstrated anticancer activity against cancerous cells ( MDA-MB-23), supported by an IC50 value of 43.09 ± 1.6 µg/mL. | [192] |
| Anticancer activity | Green synthesis using Schizophyllum commune | Size = Average size of 90 nm Shape = Spherical |
Gold NPs demonstrated dose-dependent anticancer activity against A549 lung cancer cells. Increasing the doses, from 15 μg/mL to 25 μg/mL, led to decreased cell viability. |
[193] |
| Anticancer activity | Green synthesis using Cyclopia genistoides leaf extract | Size = Average size of 37 nm Shape = Spherical and pentagonal |
Dose-dependent anticancer activity was observed against PC-3, Caco-2, and MCF-7 cells. PC-3 cell death increased by 2.5-fold compared to MCF-7 cells at a concentration of 100 µg/mL of gold NPs. |
[194] |
| Antimicrobial activity | Green synthesis using Presley leaf, Petroselinum crispum (P. crispum), extract | Size = Ranging from 20 to 80 nm Shape = Multi-shaped and spherical |
Gold NPs(A) (2.5 mL extract used) demonstrated antibacterial inhibition against two Gram-negative pathogenic bacteria and demonstrated the highest anticancer efficiency against human colon cancer cells (HCT116). | [195] |
| Antimicrobial activity | Green synthesis using Mentha longifolia (M. longifolia) leaves extracts | Size = 3.45 ± 2 nm Shape = Round oval |
The NPs markedly enhance antibacterial, antioxidant, antinociceptive, analgesic, and sedative actions | [196] |
| Antimicrobial activity | Green synthesis using Citrus macroptera (C. macroptera) | Size = 20 nm Shape = Pseudo-spherical |
The gold NPs that were manufactured demonstrate antibiofilm action against P. aeruginosa biofilm. Additionally, they primarily show cytotoxic effects on HepG2. | [197] |
| Antimicrobial activity | Green synthesis using Cynodon dactylon L. Pers (C. dactylon) | Size = 21- 33 nm Shape = Spherical and irregular |
Gold NPs exhibited significant antibacterial efficacy against pathogenic bacteria such as Enterobacter cloacae, Staphylococcus haemolyticus, Staphylococcus petrasii subsp. pragensis, and Bacillus cereus, with inhibition zones of between 12 and 13 mm. | [198] |
| Antimicrobial activity | Green synthesis using Scutellaria baicalensis | Size = 20-40 nm Shape = Spherical |
Gold NPs had strong cytotoxic, antibacterial, and antioxidant properties. They were not hazardous to RAW 264.7 or A549 cells, according to in vitro cytotoxicity data. | [199] |
| Antimicrobial activity | Green synthesis using Jatropha integerrima (J. integerrima) | Size = 38.8 nm Shape = Spherical |
Maximum and minimum antibacterial activity against B. subtilis and E. coli is demonstrated by the gold NPs. B. subtilis, S. aureus, E. coli, and K. pneumoniae were shown to have MICs of 5.0, 10, 2.5, and 2.5 lg/mL, respectively, when gold NPs were used. |
[200] |
| Antimicrobial activity | Green synthesis using Platycodon grandiflorum | Size = 15 nm Shape = Spherical |
The P. grandiflorum gold NPs that were produced demonstrated effective antibacterial action against B. subtilis (11 mm) and E. coli (16 mm). | [201] |
| Antimicrobial activity | Green synthesis using Arthrospira platensis extract | Size = Average size of 10.98 nm Shape = Rod-shaped |
Antibacterial activity against Streptococcus pneumoniae was observed with a MIC value of 12 μg/mL. | [202] |
| Antimicrobial activity | Green synthesis using Lysinibacillus odysseyi PBCW2 | Size = Average size of 31.6 ± 9.7 nm Shape = Spherical |
Antibacterial activity was observed against both Gram-positive and Gram-negative strains (S. aureus, E. coli, V. cholerae Shigella dysenteriae, Aeromonas hydrophila and Salmonella typhi) MIC and MBC values were found between 25 to 40 μg/mL and 60–85μg/mL, respectively. |
[203] |
| Antimicrobial activity | Seeded-growth | Size = 82.57 nm Shape = Rod shaped |
Gold NPs demonstrated antibacterial and antifungal activity against E. coli, S. aureus, and Candida albicans (C. albicans), at concentrations ranging from 0.25 ng/mL to 0.125 ng/mL. | [204] |
| Antioxidant activity | Green synthesis using Oak gum extract | Size = Average 10-15 nm Shape = crystalline structure |
It was found that the material demonstrated remarkable antioxidant properties through DPPH radical scavenging experiments. | [146] |
| Antioxidant activity | Green synthesis using C. pseudomontana isolated curcumin | Size = Average 20 nm Shape = Spherical |
Effective antibacterial, anti-inflammatory, and antioxidant properties were exhibited by the gold NPs. | [147] |
| Antioxidant activity | Green synthesis using Achillea bieber- steinii flower extract |
Size = Average 8 nm Shape = Spherical |
It was discovered that the Ab-gold NPs were efficient against the DPPH radicals. In addition, they showed better DPPH scavenging action than the plant extract did. | [148] |
| Antioxidant activity | Green synthesis using Paracoccus haeundaensis BC74171T | Size = Average size of 20.93 ± 3.46 Shape = Spherical |
Antioxidant activity was observed, with a DPPH radical scavenging percentage ranging from 13.04 ± 3.14% at 10 μg/ml to 73.04 ± 3.01% at 320 μg/ml. | [205] |
| Antioxidant activity | Green synthesis using Vitex negundo (V. negundo) leaf extract | Size = Ranging from 20 to 70 nm Shape = Spherical |
DPPH radical scavenging activity reached 84.64% at a concentration of 120 µg/mL, along with an IC50 value of 62.18 µg. Nitric oxide assay indicated 69.79% scavenging activity with IC50 value of 70.45 µg, for the same tested concentrations |
[206] |
| Antioxidant activity | Green synthesis using Hubertia ambavilla plant extract | Size = Average size of 50 nm Shape = Flower-shaped |
DPPH radicals were neutralized with an IC50 value of 16.5 μg/mL. Dose-dependent reduction in UV-A induced MMP-1 production in normal human dermal fibroblast cells was observed, achieving IC50 of 9.25 μg/mL. |
[207] |
| Antioxidant activity | Green synthesis using Glaucium flavum leaf extract | Size = Average size of 32 nm Shape = Spherical |
DPPH assay revealed dose-dependent antioxidant effect of gold NPs. At concentrations of 125 μg/mL, 500 μg/mL, and 1000 μg/mL, the NPs achieved reductions in DPPH radicals of 23%, 37%, and 44%, respectively. |
[208] |
| Antioxidant activity | Green synthesis using Capsicum annum fruit extract | Size = Ranging from 20 to 30 nm Shape = Spherical |
DPPH assay showed 86% efficiency of NPs, at a concentration of 100 µg/mL, in comparison to Vitamin C that displayed 69.3% efficiency for the same tested concentrations. | [209] |
| Antioxidant activity | Green synthesis using Nostoc calcicola | Size = Ranging from 20 to 140 nm Shape = Triangular, spherical and cuboidal |
DPPH radicals were effectively neutralized, with an IC50 value of 55.97 μg/mL. | [210] |
| Antioxidant activity | Green synthesis using curcumin isolated from C. pseudomontana | Size = Average diameter of 20 nm Shape = Spherical |
DPPH, hydrogen peroxide, nitric oxide, reducing power and CUPRAC assays showed dose-dependent antioxidant activity. At highest concentration of 25 μg/mL, NPs exhibited inhibition rates of 85.2%, 83.2%, 84.5%, 87.9% and 85.6%, respectively. |
[147] |
3. Synthesis of Gold Nanoparticles
3.1. Physical Synthesis
3.2. Chemical Synthesis
3.2.1. Turkevich Method
3.2.2. Electrochemical Method
3.2.3. The Brust-Schiffrin Method
3.2.4. Seeded-Growth Method
3.2.5. Digestive Ripening
3.3. Green/Biological Synthesis
3.3.1. Microorganism-Based Gold NPs Synthesis
3.3.2. Plant, Fruit, and Waste Extracts-Based Gold NPs Synthesis
3.4. Variables Influencing NPs Synthesis
pH
Temperature
Pressure
Time
Concentration of Source Extract/Biomass and Salt
3.5. Characterization of Gold NPs
3.5.1. Ultraviolet–Visible Spectroscopy (UV-vis)
3.5.2. X-ray Diffractometer (XRD)
3.5.3. Transmission Electron Microscope (TEM) and Scanning Electron Microscope (SEM)
3.5.4. Energy-Dispersive X-ray Spectroscopy (EDAX)
3.5.5. Dynamic Light Scattering (DLS)
3.5.6. Fourier Transform Infrared Spectroscopy (FT-IR)
3.5.7. X-ray Photoelectron Spectroscopy (XPS)
3.5.8. Thermogravimetric Analysis (TGA)
4. Toxicity
5. Future Trends
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Panigrahi, S.; Bhattacharyya, D.; Chakraborti, A.S. Characterization of Citrate Capped Gold Nanoparticle-Quercetin Complex: Experimental and Quantum Chemical Approach. Journal of Molecular Structure 2013, 1046, 153–163. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; De La Zerda, A. Gold Nanomaterials for Optical Biosensing and Bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; Day, E.S. Gold Nanoparticle-mediated Photothermal Therapy: Applications and Opportunities for Multimodal Cancer Treatment. WIREs Nanomed Nanobiotechnol 2017, 9, e1449. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chien, H.T.; Ding, P.P.; Chan, B.; Luh, T.Y.; Chen, P.H. Effect of Structural Character of Gold Nanoparticles in Nanofluid on Heat Pipe Thermal Performance. Materials Letters 2004, 58, 1461–1465. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.; Rotello, V. Gold Nanoparticles in Delivery Applications☆. Advanced Drug Delivery Reviews 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Venkatesh, G.; Serdaroğlu, G.; Üstün, E.; Haripriya, D.; Vennila, P.; Siva, V.; Haseena, S.; Sowmiya, V.; Pradhiksha, A. Green Synthesis, Characterization, Anti-Cancer and Antimicrobial Activity of AuNPs Extracted from Euphorbia Antiquorum Stem and Flower: Experimental and Theoretical Calculations. Journal of Drug Delivery Science and Technology 2024, 95, 105583. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Meng, F.; Wubuli, A.; Li, S.; Xiao, S.; Gu, L.; Li, J. HAuCl4-Mediated Green Synthesis of Highly Stable Au NPs from Natural Active Polysaccharides: Synthetic Mechanism and Antioxidant Property. International Journal of Biological Macromolecules 2024, 265, 130824. [Google Scholar] [CrossRef]
- Rashidipour, M.; Soroush, S.; Ashrafi, B.; Sepahvand, A.; Rasoulian, B.; Sohrabi, S.S.; Babaeenezhad, E. Green Synthesis of Gold Nanoparticles (AuNPs) Using Aqueous Extract and Essential Oils from Satureja Khuzestanica Jamzad: Evaluation of Their Antibacterial and Antifungal Activities. Biologia 2023, 79, 333–342. [Google Scholar] [CrossRef]
- Thakur, R.; Yadav, S. Smart Multifaceted Potential Microbial Inoculant Isolated from Rhizospheric Soils of Bergenia Ciliata and Possible Role in Developing Green Biosynthesized Nanoparticles. Biocatalysis and Agricultural Biotechnology 2024, 57, 103087. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M.; Jomaa, A.A.; Kamoun, M. Gold Nanoparticles: Synthesis Properties and Applications. Journal of King Saud University - Science 2021, 33, 101560. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Zarkesh Esfahani, S.H.; Razmjou, A. Gold Nanoparticles as Potent Anticancer Agent: Green Synthesis, Characterization, and in Vitro Study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ikram, S. ; Yudha S., S. Biosynthesis of Gold Nanoparticles: A Green Approach. Journal of Photochemistry and Photobiology B: Biology 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Zhang Toxicologic Effects of Gold Nanoparticles in Vivo by Different Administration Routes. IJN 2010, 771. [CrossRef]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The Forthcoming Applications of Gold Nanoparticles in Drug and Gene Delivery Systems. Journal of Controlled Release 2011, 149, 65–71. [Google Scholar] [CrossRef]
- Fröhlich, E.; Roblegg, E. Models for Oral Uptake of Nanoparticles in Consumer Products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef]
- Patra, C.R.; Bhattacharya, R.; Mukhopadhyay, D.; Mukherjee, P. Fabrication of Gold Nanoparticles for Targeted Therapy in Pancreatic Cancer. Advanced Drug Delivery Reviews 2010, 62, 346–361. [Google Scholar] [CrossRef]
- Jo, M.-R.; Bae, S.-H.; Go, M.-R.; Kim, H.-J.; Hwang, Y.-G.; Choi, S.-J. Toxicity and Biokinetics of Colloidal Gold Nanoparticles. Nanomaterials 2015, 5, 835–850. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of Gold Nanoparticles (AuNPs): A Review. Biochemistry and Biophysics Reports 2021, 26, 100991. [Google Scholar] [CrossRef]
- Document Search - Web of Science Core Collection Available online:.
- https://www.webofscience.com/wos/woscc/basic-search (accessed on 10 October 2024).
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Duman, H.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles in Therapeutics and Beyond: A Review of Mechanism Insights and Applications. Nanomaterials 2024, 14, 1618. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zhao, X.; Liu, G.; Pang, B.; Liao, N.; Li, H.; Shi, J. Diversity of Fungus-Mediated Synthesis of Gold Nanoparticles: Properties, Mechanisms, Challenges, and Solving Methods. Critical Reviews in Biotechnology 2024, 44, 924–940. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-Friendly Synthesis and Biomedical Applications of Gold Nanoparticles: A Review. Microchemical Journal 2020, 152, 104296. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic Nanoparticles: A Comprehensive Perspective in Synthesis, Characterization, Application and Its Challenges. Journal of Genetic Engineering and Biotechnology 2020, 18, 67. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Alkhursani, Sh.A.; Alqahtani, H.A.; El-damhougy, T.K.; Madani, M. Gold Nanoparticles in Microelectronics Advancements and Biomedical Applications. Materials Science and Engineering: B 2024, 301, 117191. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of Gold Nanoparticles in Advanced Biomedical Applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Shafiqa, A.R.; Abdul Aziz, A.; Mehrdel, B. Nanoparticle Optical Properties: Size Dependence of a Single Gold Spherical Nanoparticle. J. Phys.: Conf. Ser. 2018, 1083, 012040. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple Size-Controlled Synthesis of Au Nanoparticles and Their Size-Dependent Catalytic Activity. Sci Rep 2018, 8, 4589. [Google Scholar] [CrossRef]
- Lee, U.; Yoo, C.-J.; Kim, Y.-J.; Yoo, Y.-M. Cytotoxicity of Gold Nanoparticles in Human Neural Precursor Cells and Rat Cerebral Cortex. Journal of Bioscience and Bioengineering 2016, 121, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rashid, R.; Murtaza, G.; Zahra, A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm Res 2014, 13, 1169. [Google Scholar] [CrossRef]
- Jiji, S.G.; Gopchandran, K.G. Shape Dependent Catalytic Activity of Unsupported Gold Nanostructures for the Fast Reduction of 4-Nitroaniline. Colloid and Interface Science Communications 2019, 29, 9–16. [Google Scholar] [CrossRef]
- Hameed, S.; Wang, Y.; Zhao, L.; Xie, L.; Ying, Y. Shape-Dependent Significant Physical Mutilation and Antibacterial Mechanisms of Gold Nanoparticles against Foodborne Bacterial Pathogens (Escherichia Coli, Pseudomonas Aeruginosa and Staphylococcus Aureus) at Lower Concentrations. Materials Science and Engineering: C 2020, 108, 110338. [Google Scholar] [CrossRef]
- Hua, Y.; Chandra, K.; Dam, D.H.M.; Wiederrecht, G.P.; Odom, T.W. Shape-Dependent Nonlinear Optical Properties of Anisotropic Gold Nanoparticles. J. Phys. Chem. Lett. 2015, 6, 4904–4908. [Google Scholar] [CrossRef]
- Morgan, E.; Wupperfeld, D.; Morales, D.; Reich, N. Shape Matters: Gold Nanoparticle Shape Impacts the Biological Activity of siRNA Delivery. Bioconjugate Chem. 2019, 30, 853–860. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci Rep 2017, 7, 3827. [Google Scholar] [CrossRef]
- Sun, Y.-N.; Wang, C.-D.; Zhang, X.-M.; Ren, L.; Tian, X.-H. Shape Dependence of Gold Nanoparticles on In Vivo Acute Toxicological Effects and Biodistribution. j nanosci nanotechnol 2011, 11, 1210–1216. [Google Scholar] [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and Shape-Dependent Cytotoxicity Profile of Gold Nanoparticles for Biomedical Applications. J Mater Sci: Mater Med 2017, 28, 92. [Google Scholar] [CrossRef]
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The Effect of Surface Charge on in Vivo Biodistribution of PEG-Oligocholic Acid Based Micellar Nanoparticles. Biomaterials 2011, 32, 3435–3446. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, S.; He, J.; Nie, Z. Polymers and Inorganic Nanoparticles: A Winning Combination towards Assembled Nanostructures for Cancer Imaging and Therapy. Nano Today 2021, 36, 101046. [Google Scholar] [CrossRef]
- Noh, S.M.; Kim, W.-K.; Kim, S.J.; Kim, J.M.; Baek, K.-H.; Oh, Y.-K. Enhanced Cellular Delivery and Transfection Efficiency of Plasmid DNA Using Positively Charged Biocompatible Colloidal Gold Nanoparticles. Biochimica et Biophysica Acta (BBA) - General Subjects 2007, 1770, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Jeong, S.H.; Shin, W.U.; Lee, G.; Oh, C.; Son, S.W. Influence of Surface Charge of Gold Nanorods on Skin Penetration. Skin Research and Technology 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Bozich, J.S.; Lohse, S.E.; Torelli, M.D.; Murphy, C.J.; Hamers, R.J.; Klaper, R.D. Surface Chemistry, Charge and Ligand Type Impact the Toxicity of Gold Nanoparticles to Daphnia Magna. Environ. Sci.: Nano 2014, 1, 260–270. [Google Scholar] [CrossRef]
- Feng, Z.V.; Gunsolus, I.L.; Qiu, T.A.; Hurley, K.R.; Nyberg, L.H.; Frew, H.; Johnson, K.P.; Vartanian, A.M.; Jacob, L.M.; Lohse, S.E.; et al. Impacts of Gold Nanoparticle Charge and Ligand Type on Surface Binding and Toxicity to Gram-Negative and Gram-Positive Bacteria. Chem. Sci. 2015, 6, 5186–5196. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, S.P.; Arya, S.K.; Gupta, V.; Datta, M.; Singh, S.; Malhotra, B.D. Application of Thiolated Gold Nanoparticles for the Enhancement of Glucose Oxidase Activity. Langmuir 2007, 23, 3333–3337. [Google Scholar] [CrossRef]
- Zhang, S.; Moustafa, Y.; Huo, Q. Different Interaction Modes of Biomolecules with Citrate-Capped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 21184–21192. [Google Scholar] [CrossRef]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosensors and Bioelectronics 2020, 165, 112370. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current Trends in Using Polymer Coated Gold Nanoparticles for Cancer Therapy. International Journal of Pharmaceutics 2015, 484, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Encabo-Berzosa, M.M.; Sancho-Albero, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Santamaria, J.; Martín Duque, P. Polymer Functionalized Gold Nanoparticles as Nonviral Gene Delivery Reagents. J Gene Med 2017, 19, e2964. [Google Scholar] [CrossRef] [PubMed]
- Medley, C.D.; Smith, J.E.; Tang, Z.; Wu, Y.; Bamrungsap, S.; Tan, W. Gold Nanoparticle-Based Colorimetric Assay for the Direct Detection of Cancerous Cells. Anal. Chem. 2008, 80, 1067–1072. [Google Scholar] [CrossRef]
- Ozcicek, I.; Aysit, N.; Cakici, C.; Aydeger, A. The Effects of Surface Functionality and Size of Gold Nanoparticles on Neuronal Toxicity, Apoptosis, ROS Production and Cellular/Suborgan Biodistribution. Materials Science and Engineering: C 2021, 128, 112308. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold Nanoparticles: Interesting Optical Properties and Recent Applications in Cancer Diagnostics and Therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef]
- He, M.-Q.; Yu, Y.-L.; Wang, J.-H. Biomolecule-Tailored Assembly and Morphology of Gold Nanoparticles for LSPR Applications. Nano Today 2020, 35, 101005. [Google Scholar] [CrossRef]
- Cordeiro, M.; Ferreira Carlos, F.; Pedrosa, P.; Lopez, A.; Baptista, P. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics 2016, 6, 43. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent Advances in Gold Nanoparticles-Based Biosensors for Food Safety Detection. Biosensors and Bioelectronics 2021, 179, 113076. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Kandler, M.; Baran, J.; Parlinska-Wojtan, M. From Spherical to Bone-Shaped Gold Nanoparticles—Time Factor in the Formation of Au NPs, Their Optical and Photothermal Properties. Photodiagnosis and Photodynamic Therapy 2020, 30, 101670. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Casas, J.; Venkataramasubramani, M.; Tang, L. Synthesis and Characterization of Gold Nanoparticles with Plasmon Absorbance Wavelength Tunable from Visible to Near Infrared Region. ISRN Nanomaterials 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Nehl, C.L.; Hafner, J.H. Shape-Dependent Plasmon Resonances of Gold Nanoparticles. J. Mater. Chem. 2008, 18, 2415. [Google Scholar] [CrossRef]
- Njoki, P.N.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, J.; Zhong, C.-J. Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Alex, S.; Tiwari, A. Functionalized Gold Nanoparticles: Synthesis, Properties and Applications—A Review. j nanosci nanotechnol 2015, 15, 1869–1894. [Google Scholar] [CrossRef]
- Zeng, S.; Cai, M.; Liang, H.; Hao, J. Size-Dependent Colorimetric Visual Detection of Melamine in Milk at 10 Ppb Level by Citrate-Stabilized Au Nanoparticles. Anal. Methods 2012, 4, 2499. [Google Scholar] [CrossRef]
- Wilson, R. The Use of Gold Nanoparticles in Diagnostics and Detection. Chem. Soc. Rev. 2008, 37, 2028. [Google Scholar] [CrossRef]
- Porter, M.D.; Lipert, R.J.; Siperko, L.M.; Wang, G.; Narayanan, R. SERS as a Bioassay Platform: Fundamentals, Design, and Applications. Chem. Soc. Rev. 2008, 37, 1001. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous Silica Supported Orderly-Spaced Gold Nanoparticles SERS-Based Sensor for Pesticides Detection in Food. Food Chemistry 2020, 315, 126300. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med Sci 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Faid, A.H.; Shouman, S.A.; Badr, Y.A.; Sharaky, M. Enhanced Photothermal Heating and Combination Therapy of Gold Nanoparticles on a Breast Cell Model. BMC Chemistry 2022, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Wang, J.-Q.; Ashby, C.R.; Zeng, L.; Fan, Y.-F.; Chen, Z.-S. Gold Nanoparticles: Synthesis, Physiochemical Properties and Therapeutic Applications in Cancer. Drug Discovery Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Eshghi, H.; Sazgarnia, A.; Rahimizadeh, M.; Attaran, N.; Bakavoli, M.; Soudmand, S. Protoporphyrin IX–Gold Nanoparticle Conjugates as an Efficient Photosensitizer in Cervical Cancer Therapy. Photodiagnosis and Photodynamic Therapy 2013, 10, 304–312. [Google Scholar] [CrossRef]
- Madhu, B.J. Electrical Characterization of Shielding Materials. In Advanced Materials for Electromagnetic Shielding; Jaroszewski, M., Thomas, S., Rane, A.V., Eds.; Wiley, 2018; pp. 89–108 ISBN 978-1-119-12861-8.
- Li, C.; Zhang, L.; Chen, J.; Li, X.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. Recent Development and Applications of Electrical Conductive MOFs. Nanoscale 2021, 13, 485–509. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and Electrical Properties of Uniform Silver Nanoparticles for Electronic Applications. J Mater Sci 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Roshan, H.; Mosahebfard, A.; Sheikhi, M.H. Effect of Gold Nanoparticles Incorporation on Electrical Conductivity and Methane Gas Sensing Characteristics of Lead Sulfide Colloidal Nanocrystals. IEEE Sensors J. 2018, 18, 1940–1945. [Google Scholar] [CrossRef]
- Yaduvanshi, P.; Mishra, A.; Kumar, S.; Dhar, R. Enhancement in the Thermodynamic, Electrical and Optical Properties of Hexabutoxytriphenylene Due to Copper Nanoparticles. Journal of Molecular Liquids 2015, 208, 160–164. [Google Scholar] [CrossRef]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. The Chemical Record 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- Tommalieh, M.J.; Ibrahium, H.A.; Awwad, N.S.; Menazea, A.A. Gold Nanoparticles Doped Polyvinyl Alcohol/Chitosan Blend via Laser Ablation for Electrical Conductivity Enhancement. Journal of Molecular Structure 2020, 1221, 128814. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Wu, S.; Shen, J. Gold Nanoparticles Coated Polystyrene/Reduced Graphite Oxide Microspheres with Improved Dispersibility and Electrical Conductivity for Dopamine Detection. Colloids and Surfaces B: Biointerfaces 2013, 112, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Tommalieh, M.J.; Awwad, N.S.; Ibrahium, H.A.; Menazea, A.A. Characterization and Electrical Enhancement of PVP/PVA Matrix Doped by Gold Nanoparticles Prepared by Laser Ablation. Radiation Physics and Chemistry 2021, 179, 109195. [Google Scholar] [CrossRef]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically Conductive Gold Nanoparticle-Chitosan Thermosensitive Hydrogels for Cardiac Tissue Engineering. Materials Science and Engineering: C 2016, 63, 131–141. [Google Scholar] [CrossRef]
- Tan, H.W.; An, J.; Chua, C.K.; Tran, T. Metallic Nanoparticle Inks for 3D Printing of Electronics. Adv Elect Materials 2019, 5, 1800831. [Google Scholar] [CrossRef]
- R. , V.K.R.; K., V.A.; P. S., K.; Singh, S.P. Conductive Silver Inks and Their Applications in Printed and Flexible Electronics. RSC Adv. 2015, 5, 77760–77790. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Mady, M.M.; Ghannam, M.M. Rheological and Dielectric Properties of Different Gold Nanoparticle Sizes. Lipids Health Dis 2011, 10, 208. [Google Scholar] [CrossRef]
- Alkurdi, A.; Lombard, J.; Detcheverry, F.; Merabia, S. Enhanced Heat Transfer with Metal-Dielectric Core-Shell Nanoparticles. Phys. Rev. Applied 2020, 13, 034036. [Google Scholar] [CrossRef]
- Torrisi, L.; Torrisi, A. Gold Nanoparticles for Physics and Bio-Medicine Applications. Radiation Effects and Defects in Solids 2020, 175, 68–83. [Google Scholar] [CrossRef]
- Garwe, F.; Bauerschäfer, U.; Csaki, A.; Steinbrück, A.; Ritter, K.; Bochmann, A.; Bergmann, J.; Weise, A.; Akimov, D.; Maubach, G.; et al. Optically Controlled Thermal Management on the Nanometer Length Scale. Nanotechnology 2008, 19, 055207. [Google Scholar] [CrossRef]
- Jana, S.; Salehi-Khojin, A.; Zhong, W.-H. Enhancement of Fluid Thermal Conductivity by the Addition of Single and Hybrid Nano-Additives. Thermochimica Acta 2007, 462, 45–55. [Google Scholar] [CrossRef]
- Shalkevich, N.; Escher, W.; Bürgi, T.; Michel, B.; Si-Ahmed, L.; Poulikakos, D. On the Thermal Conductivity of Gold Nanoparticle Colloids. Langmuir 2010, 26, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, M.A.; Aziz, A.A.; Moradi Khaniabadi, P.; Jameel, M.S.; Oladzadabbasabadi, N.; Mohammed, S.A.; Abdullah, R.S.; Mehrdel, B. Monodisperse Gold Nanoparticles: A Review on Synthesis and Their Application in Modern Medicine. IJMS 2022, 23, 7400. [Google Scholar] [CrossRef] [PubMed]
- Graczyk, A.; Pawlowska, R.; Jedrzejczyk, D.; Chworos, A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules 2020, 25, 204. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Mekky, G.; Van Der Meer, S.B.; Seeds, M.C.; Atala, A.J.; Epple, M. Transport of Ultrasmall Gold Nanoparticles (2 Nm) across the Blood–Brain Barrier in a Six-Cell Brain Spheroid Model. Sci Rep 2020, 10, 18033. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. IJN 2020, Volume 15, 9823–9857. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. IJMS 2021, 22, 10952. [Google Scholar] [CrossRef]
- Yu, Y.-M.; Fukagawa, N.K. Protein and Amino Acids. In Present Knowledge in Nutrition; Elsevier, 2020; pp. 15–35 ISBN 978-0-323-66162-1.
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer Coated Gold Nanoparticles for Delivery Applications. Advanced Drug Delivery Reviews 2012, 64, 200–216. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. Therapeutic Platforms Based on Gold Nanoparticles and Their Covalent Conjugates with Drug Molecules. Advanced Drug Delivery Reviews 2013, 65, 663–676. [Google Scholar] [CrossRef]
- Khandelia, R.; Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. Polymer Coated Gold Nanoparticle–Protein Agglomerates as Nanocarriers for Hydrophobic Drug Delivery. J. Mater. Chem. B 2014, 2, 6472–6477. [Google Scholar] [CrossRef]
- Xu, L.; Dong, S.; Hao, J.; Cui, J.; Hoffmann, H. Surfactant-Modified Ultrafine Gold Nanoparticles with Magnetic Responsiveness for Reversible Convergence and Release of Biomacromolecules. Langmuir 2017, 33, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, S.; Karimi, E.; Khajeh, K.; Hosseinkhani, S.; Javan, M. Peptide Mediated Targeted Delivery of Gold Nanoparticles into the Demyelination Site Ameliorates Myelin Impairment and Gliosis. Nanomedicine: Nanotechnology, Biology and Medicine 2023, 47, 102609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumalsamy, H.; Kang, C.H.; Kim, S.H.; Hwang, J.-S.; Koh, S.-C.; Yi, T.-H.; Kim, Y.-J. Intracellular Synthesis of Gold Nanoparticles by Gluconacetobacter Liquefaciens for Delivery of Peptide CopA3 and Ginsenoside and Anti-Inflammatory Effect on Lipopolysaccharide-Activated Macrophages. Artificial Cells, Nanomedicine, and Biotechnology 2020, 48, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Ribeiro, A.J.; Veiga, F.; Silveira, I. Recent Advances in Nucleic Acid-Based Delivery: From Bench to Clinical Trials in Genetic Diseases. j biomed nanotechnol 2016, 12, 841–862. [Google Scholar] [CrossRef]
- Abo Dena, A.S.; El-Sherbiny, I.M. Biological Macromolecules for Nucleic Acid Delivery. In Biological Macromolecules; Elsevier, 2022; pp. 479–490 ISBN 978-0-323-85759-8.
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.-W.; Nagaraj, H.; Rotello, V.M. Delivery of Drugs, Proteins, and Nucleic Acids Using Inorganic Nanoparticles. Advanced Drug Delivery Reviews 2020, 156, 188–213. [Google Scholar] [CrossRef]
- Guo, J.; Armstrong, M.J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Positively Charged, Surfactant-Free Gold Nanoparticles for Nucleic Acid Delivery. RSC Adv. 2015, 5, 17862–17871. [Google Scholar] [CrossRef]
- Mout, R.; Ray, M.; Yesilbag Tonga, G.; Lee, Y.-W.; Tay, T.; Sasaki, K.; Rotello, V.M. Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing. ACS Nano 2017, 11, 2452–2458. [Google Scholar] [CrossRef]
- Kumar, U.S.; Afjei, R.; Ferrara, K.; Massoud, T.F.; Paulmurugan, R. Gold-Nanostar-Chitosan-Mediated Delivery of SARS-CoV-2 DNA Vaccine for Respiratory Mucosal Immunization: Development and Proof-of-Principle. ACS Nano 2021, 15, 17582–17601. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Fang, Q.; Du, Y.; Zhang, X. Gold Nanoparticle Delivery of Glut1 SiRNA Facilitates Glucose Starvation Therapy in Lung Cancer. ChemBioChem 2024, 25, e202400239. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Xue, C.; Chang, D.; Xu, H.; Salena, B.J.; Li, Y.; Wu, Z. Ribbon of DNA Lattice on Gold Nanoparticles for Selective Drug Delivery to Cancer Cells. Angew Chem Int Ed 2020, 59, 14584–14592. [Google Scholar] [CrossRef]
- Connor, D.M.; Broome, A.-M. Gold Nanoparticles for the Delivery of Cancer Therapeutics. In Advances in Cancer Research; Elsevier, 2018; Vol. 139, pp. 163–184 ISBN 978-0-12-814169-4.
- Khutale, G.V.; Casey, A. Synthesis and Characterization of a Multifunctional Gold-Doxorubicin Nanoparticle System for pH Triggered Intracellular Anticancer Drug Release. European Journal of Pharmaceutics and Biopharmaceutics 2017, 119, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Chakraborti, S.; Ramirez-Vick, J.E.; Ansari, Z.A.; Shanker, V.; Chakrabarti, P.; Singh, S.P. The Anticancer Activity of Chloroquine-Gold Nanoparticles against MCF-7 Breast Cancer Cells. Colloids and Surfaces B: Biointerfaces 2012, 95, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Niikura, K.; Iyo, N.; Matsuo, Y.; Mitomo, H.; Ijiro, K. Sub-100 Nm Gold Nanoparticle Vesicles as a Drug Delivery Carrier Enabling Rapid Drug Release upon Light Irradiation. ACS Appl. Mater. Interfaces 2013, 5, 3900–3907. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Lubin, B.-C.; Bazylevich, A.; Gellerman, G.; Shpilberg, O.; Luboshits, G.; Firer, M.A. Gold Nanoparticles Stabilize Peptide-Drug-Conjugates for Sustained Targeted Drug Delivery to Cancer Cells. J Nanobiotechnol 2018, 16, 34. [Google Scholar] [CrossRef]
- Duman, H.; Kaplan, M.; Arslan, A.; Sahutoglu, A.S.; Kayili, H.M.; Frese, S.A.; Karav, S. Potential Applications of Endo-β-N-Acetylglucosaminidases From Bifidobacterium Longum Subspecies Infantis in Designing Value-Added, Next-Generation Infant Formulas. Front. Nutr. 2021, 8, 646275. [Google Scholar] [CrossRef]
- Ju, T.; Otto, V.I.; Cummings, R.D. The Tn Antigen—Structural Simplicity and Biological Complexity. Angew Chem Int Ed 2011, 50, 1770–1791. [Google Scholar] [CrossRef]
- Parry, A.L.; Clemson, N.A.; Ellis, J.; Bernhard, S.S.R.; Davis, B.G.; Cameron, N.R. ‘Multicopy Multivalent’ Glycopolymer-Stabilized Gold Nanoparticles as Potential Synthetic Cancer Vaccines. J. Am. Chem. Soc. 2013, 135, 9362–9365. [Google Scholar] [CrossRef]
- Thomas-Moore, B.A.; Dedola, S.; Russell, D.A.; Field, R.A.; Marín, M.J. Targeted Photodynamic Therapy for Breast Cancer: The Potential of Glyconanoparticles. Nanoscale Adv. 2023, 5, 6501–6513. [Google Scholar] [CrossRef]
- Budhadev, D.; Poole, E.; Nehlmeier, I.; Liu, Y.; Hooper, J.; Kalverda, E.; Akshath, U.S.; Hondow, N.; Turnbull, W.B.; Pöhlmann, S.; et al. Glycan-Gold Nanoparticles as Multifunctional Probes for Multivalent Lectin–Carbohydrate Binding: Implications for Blocking Virus Infection and Nanoparticle Assembly. J. Am. Chem. Soc. 2020, 142, 18022–18034. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Fuller, C.; Korman, H.; Weiss, A.A.; Iyer, S.S. Glycan Encapsulated Gold Nanoparticles Selectively Inhibit Shiga Toxins 1 and 2. Bioconjugate Chem. 2010, 21, 1486–1493. [Google Scholar] [CrossRef]
- Babaei, A.; Mousavi, S.M.; Ghasemi, M.; Pirbonyeh, N.; Soleimani, M.; Moattari, A. Gold Nanoparticles Show Potential in Vitro Antiviral and Anticancer Activity. Life Sciences 2021, 284, 119652. [Google Scholar] [CrossRef] [PubMed]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold Nanoparticles Enhance 5-Fluorouracil Anticancer Efficacy against Colorectal Cancer Cells. International Journal of Pharmaceutics 2016, 513, 648–658. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Antioxidant and Anticancer Activities of Gold Nanoparticles Synthesized Using Aqueous Leaf Extract of Ziziphus Nummularia. BioNanoSci. 2021, 11, 281–294. [Google Scholar] [CrossRef]
- Babu, B.; Palanisamy, S.; Vinosha, M.; Anjali, R.; Kumar, P.; Pandi, B.; Tabarsa, M.; You, S.; Prabhu, N.M. Bioengineered Gold Nanoparticles from Marine Seaweed Acanthophora Spicifera for Pharmaceutical Uses: Antioxidant, Antibacterial, and Anticancer Activities. Bioprocess Biosyst Eng 2020, 43, 2231–2242. [Google Scholar] [CrossRef]
- Virmani, I.; Sasi, C.; Priyadarshini, E.; Kumar, R.; Sharma, S.K.; Singh, G.P.; Pachwarya, R.B.; Paulraj, R.; Barabadi, H.; Saravanan, M.; et al. Comparative Anticancer Potential of Biologically and Chemically Synthesized Gold Nanoparticles. J Clust Sci 2020, 31, 867–876. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J Nanobiotechnol 2017, 15, 65. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.G. Gold Nanoparticles Induce a Reactive Oxygen Species-Independent Apoptotic Pathway in Escherichia Coli. Colloids and Surfaces B: Biointerfaces 2018, 167, 1–7. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-Shaped Gold Nanoparticles with Nanogram Killing Efficiency as Potential Antimicrobial Surface Coatings for the Medical Devices. Sci Rep 2021, 11, 12546. [Google Scholar] [CrossRef]
- Radhi, A.B.; Khashan, K.S.; Sulaiman, G.M. Antibacterial Activity of Gold Nanoparticles Produced by One-Step Pulsed Laser Ablation in Liquid. Plasmonics 2024, 19, 1173–1185. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Al Hagbani, T.; Rizvi, S.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.; Alshammari, F.; Khafagy, E.-S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Gucchait, A.; Paul, S.; Saha, T.; Acharya, S.; Hoque, K.M.; Misra, A.K.; Chatterjee, B.K.; Chatterjee, T.; Chakrabarti, P. Virstatin-Conjugated Gold Nanoparticle with Enhanced Antimicrobial Activity against the Vibrio Cholerae El Tor Biotype. ACS Appl. Bio Mater. 2021, 4, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Elias, N.A.; Hassan, M.S.A.; Yusoff, N.A.H.; Harun, N.A.; Rahmah, S.; Sheikh, H.I.; Sung, Y.Y.; Abdullah, F.; Ishak, A.N.; Yee Ng, J.J.; et al. Antibacterial Properties of Synthesized Melaleuca Cajuputi-Leaf Gold Nanoparticles. Materials Letters 2024, 366, 136565. [Google Scholar] [CrossRef]
- Kerdtoob, S.; Chanthasena, P.; Rosyidah, A.; Limphirat, W.; Penkhrue, W.; Ganta, P.; Srisakvarangkool, W.; Yasawong, M.; Nantapong, N. Streptomyces Monashensis MSK03-Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. RSC Adv. 2024, 14, 4778–4787. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free Radicals, Oxidative Stress, and Antioxidants in Human Health and Disease. J Americ Oil Chem Soc 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef]
- Razzaq, H.; Saira, F.; Yaqub, A.; Qureshi, R.; Mumtaz, M.; Saleemi, S. Interaction of Gold Nanoparticles with Free Radicals and Their Role in Enhancing the Scavenging Activity of Ascorbic Acid. Journal of Photochemistry and Photobiology B: Biology 2016, 161, 266–272. [Google Scholar] [CrossRef]
- Zhang, Z.; Berg, A.; Levanon, H.; Fessenden, R.W.; Meisel, D. On the Interactions of Free Radicals with Gold Nanoparticles. J. Am. Chem. Soc. 2003, 125, 7959–7963. [Google Scholar] [CrossRef]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial Nanoparticle Antioxidants. In ACS Symposium Series; Andreescu, S., Hepel, M., Eds.; American Chemical Society: Washington, DC, 2011; Vol. 1083, pp. 235–253; ISBN 978-0-8412-2683-8. [Google Scholar]
- Lu, L.; Zhao, Q.; Wang, Z.; Ju, F. Oak Gum Mediated Sustainable Synthesis of Gold Nanoparticles (Au NPs): Evaluation of Its Antioxidant and Anti-Colon Cancer Effects. Journal of Experimental Nanoscience 2022, 17, 377–388. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green Synthesis of Gold Nanoparticles Using Curcuma Pseudomontana Isolated Curcumin: Its Characterization, Antimicrobial, Antioxidant and Anti- Inflammatory Activities. Environmental Chemistry and Ecotoxicology 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Taghavizadeh Yazdi, M.E.; Meshkat, Z.; Silanian Toosi, M.; Hosseini, S.M. Plant-Derived Synthesis and Characterization of Gold Nanoparticles: Investigation of Its Antioxidant and Anticancer Activity against Human Testicular Embryonic Carcinoma Stem Cells. Process Biochemistry 2021, 111, 167–177. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, F.; Chen, Y.; Alrashood, S.T.; Alharbi, S.A. Anti-Human Colon Cancer Properties of a Novel Chemotherapeutic Supplement Formulated by Gold Nanoparticles Containing Allium Sativum L. Leaf Aqueous Extract and Investigation of Its Cytotoxicity and Antioxidant Activities. Arabian Journal of Chemistry 2021, 14, 103039. [Google Scholar] [CrossRef]
- Baek, K.; Patra, J.K. Novel Green Synthesis of Gold Nanoparticles Using Citrullus Lanatus Rind and Investigation of Proteasome Inhibitory Activity, Antibacterial, and Antioxidant Potential. IJN 2015, 7253. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, Z.; Gao, X.; Liu, W.; Zhu, H. 3D Hierarchically Gold-Nanoparticle-Decorated Porous Carbon for High-Performance Supercapacitors. Sci Rep 2019, 9, 17065. [Google Scholar] [CrossRef]
- Farina, R.; Scalese, S.; Corso, D.; Capuano, G.E.; Screpis, G.A.; Coniglio, M.A.; Condorelli, G.G.; Libertino, S. Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles. Molecules 2024, 29, 3028. [Google Scholar] [CrossRef]
- Chan, K.-Y.; Yang, D.; Demir, B.; Mouritz, A.P.; Lin, H.; Jia, B.; Lau, K.-T. Boosting the Electrical and Mechanical Properties of Structural Dielectric Capacitor Composites via Gold Nanoparticle Doping. Composites Part B: Engineering 2019, 178, 107480. [Google Scholar] [CrossRef]
- Barabadi, Z.; Bahmani, A.; Jalalimonfared, M.; Ashrafizadeh, M.; Rashtbar, M.; Sharifi, E.; Tian, H. Design and Characterization of Electroactive Gelatin Methacrylate Hydrogel Incorporated with Gold Nanoparticles Empowered with Parahydroxybenzaldehyde and Curcumin for Advanced Tissue Engineering Applications. J Mater Sci: Mater Med 2024, 35, 45. [Google Scholar] [CrossRef]
- Kusnin, N.; Yusof, N.A.; Mutalib, N.A.A.; Mohammad, F.; Abdullah, J.; Sabri, S.; Mustafa, S.; Mohamad Saman, A.F.; Mohd Faudzi, F.N.; Soleiman, A.A. Enhanced Electrochemical Conductivity of Surface-Coated Gold Nanoparticles/Copper Nanowires onto Screen-Printed Gold Electrode. Coatings 2022, 12, 622. [Google Scholar] [CrossRef]
- García-Garabal, S.; Domínguez-Pérez, M.; Cabeza, O.; Arosa, Y.; Varela, L.M.; Fernández-López, C.; Pérez-Juste, J.; Pastoriza-Santos, I. Effect of Gold Nanoparticles on Transport Properties of the Protic Ionic Liquid Propylammonium Nitrate. J. Chem. Eng. Data 2021, 66, 3028–3037. [Google Scholar] [CrossRef]
- Yassin, A.Y. Synthesized Polymeric Nanocomposites with Enhanced Optical and Electrical Properties Based on Gold Nanoparticles for Optoelectronic Applications. J Mater Sci: Mater Electron 2023, 34, 46. [Google Scholar] [CrossRef]
- Rhouati, A.; Zourob, M. Development of a Multiplexed Electrochemical Aptasensor for the Detection of Cyanotoxins. Biosensors 2024, 14, 268. [Google Scholar] [CrossRef] [PubMed]
- Triana-Camacho, D.A.; Mendoza Reales, O.A.; Quintero-Orozco, J.H. Low Concentrations of Gold Nanoparticles as Electric Charge Carriers in Piezoelectric Cement-Based Materials. Materials 2024, 17, 615. [Google Scholar] [CrossRef] [PubMed]
- Mbambo, M.C.; Madito, M.J.; Khamliche, T.; Mtshali, C.B.; Khumalo, Z.M.; Madiba, I.G.; Mothudi, B.M.; Maaza, M. Thermal Conductivity Enhancement in Gold Decorated Graphene Nanosheets in Ethylene Glycol Based Nanofluid. Sci Rep 2020, 10, 14730. [Google Scholar] [CrossRef]
- Shandurkov, D.; Danchova, N.; Spassov, T.; Petrov, V.; Tsekov, R.; Gutzov, S. Silica Gels Doped with Gold Nanoparticles: Preparation, Structure and Optical Properties. Gels 2023, 9, 663. [Google Scholar] [CrossRef]
- Asadi, S.; Korganbayev, S.; Xu, W.; Mapanao, A.K.; Voliani, V.; Lehto, V.-P.; Saccomandi, P. Experimental Evaluation of Radiation Response and Thermal Properties of NPs-Loaded Tissues-Mimicking Phantoms. Nanomaterials 2022, 12, 945. [Google Scholar] [CrossRef]
- Qiu, L.; Zou, H.; Wang, X.; Feng, Y.; Zhang, X.; Zhao, J.; Zhang, X.; Li, Q. Enhancing the Interfacial Interaction of Carbon Nanotubes Fibers by Au Nanoparticles with Improved Performance of the Electrical and Thermal Conductivity. Carbon 2019, 141, 497–505. [Google Scholar] [CrossRef]
- Wei, X.; Hernandez, R. Heat Transfer Enhancement in Tree-Structured Polymer Linked Gold Nanoparticle Networks. J. Phys. Chem. Lett. 2023, 14, 9834–9841. [Google Scholar] [CrossRef]
- Michałowska, A.; Kudelski, A. Hollow Gold–Silver Nanorods—A New, Very Efficient Nanomaterial for Surface-Enhanced Raman Scattering (SERS) Measurements. Molecules 2024, 29, 4540. [Google Scholar] [CrossRef]
- Barshilia, D.; Komaram, A.C.; Chau, L.-K.; Chang, G.-E. Waveguide-Enhanced Nanoplasmonic Biosensor for Ultrasensitive and Rapid DNA Detection. Micromachines 2024, 15, 1169. [Google Scholar] [CrossRef]
- Pedrosa, T.D.L.; De Oliveira, G.M.F.; Pereira, A.C.M.V.; Crispim, M.J.B.D.S.; Da Silva, L.A.; Da Silva, M.S.; De Souza, I.A.; Melo, A.M.M.D.A.; Gomes, A.S.L.; De Araujo, R.E. Tailoring Plasmonic Nanoheaters Size for Enhanced Theranostic Agent Performance. Bioengineering 2024, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, E.; Mollasalehi, H.; Minai-Tehrani, D. A Gold Nanoparticle Conjugated Single-Legged DNA Walker Driven by Catalytic Hairpin Assembly Biosensor to Detect a Prokaryotic Pathogen. Sci Rep 2024, 14, 22980. [Google Scholar] [CrossRef] [PubMed]
- Salvo-Comino, C.; Rassas, I.; Minot, S.; Bessueille, F.; Arab, M.; Chevallier, V.; Rodriguez-Mendez, M.L.; Errachid, A.; Jaffrezic-Renault, N. Voltammetric Sensor Based on Molecularly Imprinted Chitosan-Carbon Nanotubes Decorated with Gold Nanoparticles Nanocomposite Deposited on Boron-Doped Diamond Electrodes for Catechol Detection. Materials 2020, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Seele, P.P.; Dyan, B.; Skepu, A.; Maserumule, C.; Sibuyi, N.R.S. Development of Gold-Nanoparticle-Based Lateral Flow Immunoassays for Rapid Detection of TB ESAT-6 and CFP-10. Biosensors 2023, 13, 354. [Google Scholar] [CrossRef]
- Wignarajah, S.; Chianella, I.; Tothill, I.E. Development of Electrochemical Immunosensors for HER-1 and HER-2 Analysis in Serum for Breast Cancer Patients. Biosensors 2023, 13, 355. [Google Scholar] [CrossRef]
- Karaca Acari, I.; Kurul, F.; Avci, M.B.; Yasar, S.D.; Topkaya, S.N.; Açarı, C.; Ünsal, E.; Makay, B.; Köytepe, S.; Ateş, B.; et al. A Plasmonic Biosensor Pre-Diagnostic Tool for Familial Mediterranean Fever. Nat Commun 2024, 15, 8515. [Google Scholar] [CrossRef]
- Yang, C.; Mei, T.; Fu, Q.; Zhang, Y.; Liu, Y.; Cui, R.; Li, G.; Wang, Y.; Huang, J.; Jia, J.; et al. Silk Fibroin-Induced Gadolinium-Functionalized Gold Nanoparticles for MR/CT Dual-Modal Imaging-Guided Photothermal Therapy. JFB 2022, 13, 87. [Google Scholar] [CrossRef]
- Lopez-Benitez, K.; Alcazar-Gonzalez, P.; El Qassim, L.A.; Fernandez-Argüelles, M.T.; Vicente, F.; Royo, L.J.; Menendez-Miranda, M. Development of a Gold Nanoparticle-Based Sensor for Authentication of Organic Milk Based on Differential Levels of miRNA. Nanomaterials 2024, 14, 1364. [Google Scholar] [CrossRef]
- Park, J.; Han, H.; Ahn, J.K. Development of Targeted Drug Delivery System for the Treatment of SARS-CoV-2 Using Aptamer-Conjugated Gold Nanoparticles. Pharmaceutics 2024, 16, 1288. [Google Scholar] [CrossRef]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. Gold Nanoparticles: Tunable Characteristics and Potential for Nasal Drug Delivery. Pharmaceutics 2024, 16, 669. [Google Scholar] [CrossRef]
- Kostka, K.; Sokolova, V.; El-Taibany, A.; Kruse, B.; Porada, D.; Wolff, N.; Prymak, O.; Seeds, M.C.; Epple, M.; Atala, A.J. The Application of Ultrasmall Gold Nanoparticles (2 Nm) Functionalized with Doxorubicin in Three-Dimensional Normal and Glioblastoma Organoid Models of the Blood–Brain Barrier. Molecules 2024, 29, 2469. [Google Scholar] [CrossRef] [PubMed]
- Zenze, M.; Singh, M. Receptor Targeting Using Copolymer-Modified Gold Nanoparticles for pCMV-Luc Gene Delivery to Liver Cancer Cells In Vitro. IJMS 2024, 25, 5016. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Contardo, S.; Donoso-González, O.; Lang, E.; Guerrero, A.R.; Noyong, M.; Simon, U.; Kogan, M.J.; Yutronic, N.; Sierpe, R. Optimizing Dacarbazine Therapy: Design of a Laser-Triggered Delivery System Based on β-Cyclodextrin and Plasmonic Gold Nanoparticles. Pharmaceutics 2023, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gu, P.; Liu, X.; Hu, S.; Zheng, H.; Liu, T.; Li, C. Gold Nanoparticles Encapsulated Resveratrol as an Anti-Aging Agent to Delay Cataract Development. Pharmaceuticals 2022, 16, 26. [Google Scholar] [CrossRef]
- Perrins, R.D.; McCarthy, L.-A.; Robinson, A.; Spry, K.L.; Cognet, V.; Ferreira, A.; Porter, J.; Garcίa, C.E.; Rodriguez, M.Á.; Lopez, D.; et al. Targeting Ultrasmall Gold Nanoparticles with cRGD Peptide Increases the Uptake and Efficacy of Cytotoxic Payload. Nanomaterials 2022, 12, 4013. [Google Scholar] [CrossRef]
- Crous, A.; Abrahamse, H. Effective Gold Nanoparticle-Antibody-Mediated Drug Delivery for Photodynamic Therapy of Lung Cancer Stem Cells. IJMS 2020, 21, 3742. [Google Scholar] [CrossRef]
- Queiroz, S.M.; Veriato, T.S.; Raniero, L.; Castilho, M.L. Gold Nanoparticles Conjugated with Epidermal Growth Factor and Gadolinium for Precision Delivery of Contrast Agents in Magnetic Resonance Imaging. Radiol Phys Technol 2023. [Google Scholar] [CrossRef]
- Chaudhari, R.; Nasra, S.; Meghani, N.; Kumar, A. MiR-206 Conjugated Gold Nanoparticle Based Targeted Therapy in Breast Cancer Cells. Sci Rep 2022, 12, 4713. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Salman, T.M.; Al-Dabash, S.; Abdullah, M.; Abu-Dahab, R. The Impact of Gold Nanoparticles Conjugated with Albumin on Prostate and Breast Cancer Cell Lines: Insights into Cytotoxicity, Cellular Uptake, Migration, and Adhesion Potential. J Nanopart Res 2024, 26, 101. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Facile One-Step Green Synthesis of Gold Nanoparticles (AuNp) Using Licorice Root Extract: Antimicrobial and Anticancer Study against HepG2 Cell Line. Arabian Journal of Chemistry 2021, 14, 102956. [Google Scholar] [CrossRef]
- Shunmugam, R.; Renukadevi Balusamy, S.; Kumar, V.; Menon, S.; Lakshmi, T.; Perumalsamy, H. Biosynthesis of Gold Nanoparticles Using Marine Microbe (Vibrio Alginolyticus) and Its Anticancer and Antioxidant Analysis. Journal of King Saud University - Science 2021, 33, 101260. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green Synthesis and Characterization of Gold Nanoparticles Using Endophytic Fungi Fusarium Solani and Its In-Vitro Anticancer and Biomedical Applications. Saudi Journal of Biological Sciences 2020, 27, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Perveen, K.; Husain, F.M.; Qais, F.A.; Khan, A.; Razak, S.; Afsar, T.; Alam, P.; Almajwal, A.M.; Abulmeaty, M.M.A. Microwave-Assisted Rapid Green Synthesis of Gold Nanoparticles Using Seed Extract of Trachyspermum Ammi: ROS Mediated Biofilm Inhibition and Anticancer Activity. Biomolecules 2021, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Donga, S.; Bhadu, G.R.; Chanda, S. Antimicrobial, Antioxidant and Anticancer Activities of Gold Nanoparticles Green Synthesized Using Mangifera Indica Seed Aqueous Extract. Artificial Cells, Nanomedicine, and Biotechnology 2020, 48, 1315–1325. [Google Scholar] [CrossRef]
- Deivanathan, S.K.; Prakash, J.T.J. Synthesis of Environmentally Benign of Gold Nanoparticles from Vicoa Indica Leaf Extracts and Their Physiochemical Characterization, Antimicrobial, Antioxidant and Anticancer Activity against A549 Cell Lines. Res Chem Intermed 2023, 49, 4955–4971. [Google Scholar] [CrossRef]
- Jeyarani, S.; Vinita, N.M.; Puja, P.; Senthamilselvi, S.; Devan, U.; Velangani, A.J.; Biruntha, M.; Pugazhendhi, A.; Kumar, P. Biomimetic Gold Nanoparticles for Its Cytotoxicity and Biocompatibility Evidenced by Fluorescence-Based Assays in Cancer (MDA-MB-231) and Non-Cancerous (HEK-293) Cells. Journal of Photochemistry and Photobiology B: Biology 2020, 202, 111715. [Google Scholar] [CrossRef]
- Alqurashi, Y.E.; Almalki, S.G.; Ibrahim, I.M.; Mohammed, A.O.; Abd El Hady, A.E.; Kamal, M.; Fatima, F.; Iqbal, D. Biological Synthesis, Characterization, and Therapeutic Potential of S. Commune-Mediated Gold Nanoparticles. Biomolecules 2023, 13, 1785. [Google Scholar] [CrossRef]
- Sharma, J.R.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, S.; Madiehe, A.M.; Katti, K.; Meyer, M. Anticancer and Drug-Sensitizing Activities of Gold Nanoparticles Synthesized from Cyclopia Genistoides (Honeybush) Extracts. Applied Sciences 2023, 13, 3973. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Ayat, M.S.; Shabrawy, M.A.; Millet, P. Green Synthesis of Gold Nanoparticles Using Parsley Leaves Extract and Their Applications as an Alternative Catalytic, Antioxidant, Anticancer, and Antibacterial Agents. Advanced Powder Technology 2020, 31, 4390–4400. [Google Scholar] [CrossRef]
- Rauf, A.; Ahmad, T.; Khan, A.; Maryam; Uddin, G. ; Ahmad, B.; Mabkhot, Y.N.; Bawazeer, S.; Riaz, N.; Malikovna, B.K.; et al. Green Synthesis and Biomedicinal Applications of Silver and Gold Nanoparticles Functionalized with Methanolic Extract of Mentha Longifolia. Artificial Cells, Nanomedicine, and Biotechnology 2021, 49, 194–203. [Google Scholar] [CrossRef]
- Majumdar, M.; Biswas, S.C.; Choudhury, R.; Upadhyay, P.; Adhikary, A.; Roy, D.N.; Misra, T.K. Synthesis of Gold Nanoparticles Using Citrus Macroptera Fruit Extract: Anti-Biofilm and Anticancer Activity. ChemistrySelect 2019, 4, 5714–5723. [Google Scholar] [CrossRef]
- Vinayagam, R.; Santhoshkumar, M.; Lee, K.E.; David, E.; Kang, S.G. Bioengineered Gold Nanoparticles Using Cynodon Dactylon Extract and Its Cytotoxicity and Antibacterial Activities. Bioprocess Biosyst Eng 2021, 44, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huo, Y.; Han, Y.X.; Li, J.F.; Ali, H.; Batjikh, I.; Hurh, J.; Pu, J.Y.; Yang, D.C. Biosynthesis of Gold and Silver Nanoparticles from Scutellaria Baicalensis Roots and in Vitro Applications. Appl. Phys. A 2020, 126, 424. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Babujanarthanam, R.; Alarjani, K.M.; Hussein, D.S.; Rasheed, R.A.; Kanimozhi, K. Green Synthesis of Gold Nanoparticles Using Jatropha Integerrima Jacq. Flower Extract and Their Antibacterial Activity. Journal of King Saud University - Science 2022, 34, 101830. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Jayanthi, S. Synthesis of Gold Nanoparticles Using Platycodon Grandiflorum Extract and Its Antipathogenic Activity under Optimal Conditions. Nanomaterials and Nanotechnology 2020, 10, 184798042096169. [Google Scholar] [CrossRef]
- Azmy, L.; Al-Olayan, E.; Abdelhamid, M.A.A.; Zayed, A.; Gheda, S.F.; Youssif, K.A.; Abou-Zied, H.A.; Abdelmohsen, U.R.; Ibraheem, I.B.M.; Pack, S.P.; et al. Antimicrobial Activity of Arthrospira Platensis-Mediated Gold Nanoparticles against Streptococcus Pneumoniae: A Metabolomic and Docking Study. IJMS 2024, 25, 10090. [Google Scholar] [CrossRef]
- Cherian, T.; Maity, D.; Rajendra Kumar, R.T.; Balasubramani, G.; Ragavendran, C.; Yalla, S.; Mohanraju, R.; Peijnenburg, W.J.G.M. Green Chemistry Based Gold Nanoparticles Synthesis Using the Marine Bacterium Lysinibacillus Odysseyi PBCW2 and Their Multitudinous Activities. Nanomaterials 2022, 12, 2940. [Google Scholar] [CrossRef]
- Soliman, W.E.; Elsewedy, H.S.; Younis, N.S.; Shinu, P.; Elsawy, L.E.; Ramadan, H.A. Evaluating Antimicrobial Activity and Wound Healing Effect of Rod-Shaped Nanoparticles. Polymers 2022, 14, 2637. [Google Scholar] [CrossRef]
- Patil, M.P.; Kang, M.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular Synthesis of Gold Nanoparticles Using the Marine Bacterium Paracoccus Haeundaensis BC74171T and Evaluation of Their Antioxidant Activity and Antiproliferative Effect on Normal and Cancer Cell Lines. Colloids and Surfaces B: Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef]
- Veena, S.; Devasena, T.; Sathak, S.S.M.; Yasasve, M.; Vishal, L.A. Green Synthesis of Gold Nanoparticles from Vitex Negundo Leaf Extract: Characterization and In Vitro Evaluation of Antioxidant–Antibacterial Activity. J Clust Sci 2019, 30, 1591–1597. [Google Scholar] [CrossRef]
- Ben Haddada, M.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.-L. Assessment of Antioxidant and Dermoprotective Activities of Gold Nanoparticles as Safe Cosmetic Ingredient. Colloids and Surfaces B: Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Amani, A.M. Antiviral and Antioxidant Properties of Green Synthesized Gold Nanoparticles Using Glaucium Flavum Leaf Extract. Appl Nanosci 2023, 13, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.P.; Vibhute, A.A.; Patil, S.L.; Dongale, T.D.; Tiwari, A.P. Green Synthesis of Gold Nanoparticles via Capsicum Annum Fruit Extract: Characterization, Antiangiogenic, Antioxidant and Anti-Inflammatory Activities. Applied Surface Science Advances 2023, 13, 100372. [Google Scholar] [CrossRef]
- Mandhata, C.P.; Bishoyi, A.K.; Sahoo, C.R.; Swain, S.; Bej, S.; Jali, B.R.; Meher, R.K.; Dubey, D.; Padhy, R.N. Investigation of in Vitro Antimicrobial, Antioxidant and Antiproliferative Activities of Nostoc Calcicola Biosynthesized Gold Nanoparticles. Bioprocess Biosyst Eng 2023, 46, 1341–1350. [Google Scholar] [CrossRef]
- Tangeysh, B.; Moore Tibbetts, K.; Odhner, J.H.; Wayland, B.B.; Levis, R.J. Gold Nanoparticle Synthesis Using Spatially and Temporally Shaped Femtosecond Laser Pulses: Post-Irradiation Auto-Reduction of Aqueous [AuCl 4 ] −. J. Phys. Chem. C 2013, 117, 18719–18727. [Google Scholar] [CrossRef]
- Birtcher, R.C.; Kirk, M.A.; Furuya, K.; Lumpkin, G.R.; Ruault, M.-O. In Situ Transmission Electron Microscopy Investigation of Radiation Effects. J. Mater. Res. 2005, 20, 1654–1683. [Google Scholar] [CrossRef]
- Rezende, T.S.; Andrade, G.R.S.; Barreto, L.S.; Costa, N.B.; Gimenez, I.F.; Almeida, L.E. Facile Preparation of Catalytically Active Gold Nanoparticles on a Thiolated Chitosan. Materials Letters 2010, 64, 882–884. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Sastry, T.P.; Sathish Kumar, M.; Dinesh, M.G.; Kannappan, S.; Suguna, L. Sun Light Mediated Synthesis of Gold Nanoparticles as Carrier for 6-Mercaptopurine: Preparation, Characterization and Toxicity Studies in Zebrafish Embryo Model. Materials Research Bulletin 2012, 47, 2113–2119. [Google Scholar] [CrossRef]
- Murawala, P.; Tirmale, A.; Shiras, A.; Prasad, B.L.V. In Situ Synthesized BSA Capped Gold Nanoparticles: Effective Carrier of Anticancer Drug Methotrexate to MCF-7 Breast Cancer Cells. Materials Science and Engineering: C 2014, 34, 158–167. [Google Scholar] [CrossRef]
- Guo, W.; Pi, Y.; Song, H.; Tang, W.; Sun, J. Layer-by-Layer Assembled Gold Nanoparticles Modified Anode and Its Application in Microbial Fuel Cells. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2012, 415, 105–111. [Google Scholar] [CrossRef]
- Chen, K.-S.; Hung, T.-S.; Wu, H.-M.; Wu, J.-Y.; Lin, M.-T.; Feng, C.-K. Preparation of Thermosensitive Gold Nanoparticles by Plasma Pretreatment and UV Grafted Polymerization. Thin Solid Films 2010, 518, 7557–7562. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser Ablation Synthesis in Solution and Size Manipulation of Noble Metal Nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Delaporte, Ph.; Pereira, A.; Grojo, D.; Torres, R.; Sarnet, Th.; Sentis, M. Nanofabrication with Pulsed Lasers. Nanoscale Res Lett 2010, 5, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, J.-P.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Stabilization and Size Control of Gold Nanoparticles during Laser Ablation in Aqueous Cyclodextrins. J. Am. Chem. Soc. 2004, 126, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Barcikowski, S. In Situ Bioconjugation: Single Step Approach to Tailored Nanoparticle-Bioconjugates by Ultrashort Pulsed Laser Ablation. Adv Funct Materials 2009, 19, 1167–1172. [Google Scholar] [CrossRef]
- Braguer, D.; Correard, F.; Maximova, K.; Villard, C.; Roy, M.; Al-Kattan, A.; Sentis, M.; Gingras, M.; Kabashin, A.; Esteve, M.-A. Gold Nanoparticles Prepared by Laser Ablation in Aqueous Biocompatible Solutions: Assessment of Safety and Biological Identity for Nanomedicine Applications. IJN 2014, 5415. [Google Scholar] [CrossRef]
- Wender, H.; Andreazza, M.L.; Correia, R.R.B.; Teixeira, S.R.; Dupont, J. Synthesis of Gold Nanoparticles by Laser Ablation of an Au Foil inside and Outside Ionic Liquids. Nanoscale 2011, 3, 1240. [Google Scholar] [CrossRef]
- Siegel, J.; Kvítek, O.; Ulbrich, P.; Kolská, Z.; Slepička, P.; Švorčík, V. Progressive Approach for Metal Nanoparticle Synthesis. Materials Letters 2012, 89, 47–50. [Google Scholar] [CrossRef]
- Torimoto, T.; Okazaki, K.; Kiyama, T.; Hirahara, K.; Tanaka, N.; Kuwabata, S. Sputter Deposition onto Ionic Liquids: Simple and Clean Synthesis of Highly Dispersed Ultrafine Metal Nanoparticles. Applied Physics Letters 2006, 89, 243117. [Google Scholar] [CrossRef]
- Hu, X.L.; Takai, O.; Saito, N. Synthesis of Gold Nanoparticles by Solution Plasma Sputtering in Various Solvents. J. Phys.: Conf. Ser. 2013, 417, 012030. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of Precision Gold Nanoparticles Using Turkevich Method. KONA 2020, 37, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; Helbig, W.; Quaiser, S.A.; Stimming, U.; Breuer, N.; Vogel, R. Visualization of Surfactants on Nanostructured Palladium Clusters by a Combination of STM and High-Resolution TEM. Science 1995, 267, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Helbig, W. Size-Selective Synthesis of Nanostructured Transition Metal Clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Kuge, K.; Arisawa, M.; Aoki, N.; Hasegawa, A. Preparation of Gelatin Layer Film with Gold Clusters in Using Photographic Film. Jpn. J. Appl. Phys. 2000, 39, 6550. [Google Scholar] [CrossRef]
- Kamat, P.V.; Flumiani, M.; Hartland, G.V. Picosecond Dynamics of Silver Nanoclusters. Photoejection of Electrons and Fragmentation. J. Phys. Chem. B 1998, 102, 3123–3128. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chiu, P.-H.; Wang, Y.-H.; Chen, K.-L.; Linn, J.-J.; Yang, C.-F. Electrochemically Controlling the Size of Gold Nanoparticles. J. Electrochem. Soc. 2006, 153, D193. [Google Scholar] [CrossRef]
- Song, Y.Z.; Li, X.; Song, Y.; Cheng, Z.P.; Zhong, H.; Xu, J.M.; Lu, J.S.; Wei, C.G.; Zhu, A.F.; Wu, F.Y.; et al. Electrochemical Synthesis of Gold Nanoparticles on the Surface of Multi-Walled Carbon Nanotubes with Glassy Carbon Electrode and Their Application. Russ. J. Phys. Chem. 2013, 87, 74–79. [Google Scholar] [CrossRef]
- Teimouri, M.; Khosravi-Nejad, F.; Attar, F.; Saboury, A.A.; Kostova, I.; Benelli, G.; Falahati, M. Gold Nanoparticles Fabrication by Plant Extracts: Synthesis, Characterization, Degradation of 4-Nitrophenol from Industrial Wastewater, and Insecticidal Activity – A Review. Journal of Cleaner Production 2018, 184, 740–753. [Google Scholar] [CrossRef]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold Nanoparticles: Synthesis Methods, Functionalization and Biological Applications. J Clust Sci 2023, 34, 705–725. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current Methods for Synthesis of Gold Nanoparticles. Artificial Cells, Nanomedicine, and Biotechnology 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Ward, C.J.; Tronndorf, R.; Eustes, A.S.; Auad, M.L.; Davis, E.W. Seed-Mediated Growth of Gold Nanorods: Limits of Length to Diameter Ratio Control. Journal of Nanomaterials 2014, 2014, 765618. [Google Scholar] [CrossRef]
- Sahu, P.; Prasad, B.L.V. Time and Temperature Effects on the Digestive Ripening of Gold Nanoparticles: Is There a Crossover from Digestive Ripening to Ostwald Ripening? Langmuir 2014, 30, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Choi, S.U.S.; Jang, S.P.; Lee, S.Y. Production of Aqueous Spherical Gold Nanoparticles Using Conventional Ultrasonic Bath. Nanoscale Res Lett 2012, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Peng, L.; Liang, H. A New Route to Obtain High-Yield Multiple-Shaped Gold Nanoparticles in Aqueous Solution Using Microwave Irradiation. Inorg. Chem. 2008, 47, 6344–6352. [Google Scholar] [CrossRef]
- Sidhaye, D.S.; Prasad, B.L.V. Many Manifestations of Digestive Ripening: Monodispersity, Superlattices and Nanomachining. New J. Chem. 2011, 35, 755–763. [Google Scholar] [CrossRef]
- Shimpi, J.R.; Sidhaye, D.S.; Prasad, B.L.V. Digestive Ripening: A Fine Chemical Machining Process on the Nanoscale. Langmuir 2017, 33, 9491–9507. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid Green Synthesis of Gold Nanoparticles Using Rosa Hybrida Petal Extract at Room Temperature. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2011, 79, 1461–1465. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J Nanopart Res 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Santra, T.S.; Tseng, F.-G. (Kevin); Barik, T.K. Biosynthesis of Silver and Gold Nanoparticles for Potential Biomedical Applications—A Brief Review. Journal of Nanopharmaceutics and Drug Delivery 2014, 2, 249–265. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The Molecular Mechanism of Action of Bactericidal Gold Nanoparticles on Escherichia Coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Ahmad, T.; Irfan, M.; Bustam, M.A.; Bhattacharjee, S. Effect of Reaction Time on Green Synthesis of Gold Nanoparticles by Using Aqueous Extract of Elaise Guineensis (Oil Palm Leaves). Procedia Engineering 2016, 148, 467–472. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal Activity of Gold Nanoparticles Prepared by Solvothermal Method. Materials Research Bulletin 2013, 48, 12–20. [Google Scholar] [CrossRef]
- R. , G.; D.V.L., S. Characterization and Antimicrobial Activity of Gold and Silver Nanoparticles Synthesized Using Saponin Isolated from Trianthema Decandra L. Industrial Crops and Products 2013, 51, 107–115. [Google Scholar] [CrossRef]
- Herdt, A.R.; Drawz, S.M.; Kang, Y.; Taton, T.A. DNA Dissociation and Degradation at Gold Nanoparticle Surfaces. Colloids and Surfaces B: Biointerfaces 2006, 51, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.J.; Murray, R.G. Sites of Metal Deposition in the Cell Wall of Bacillus Subtilis. J Bacteriol 1980, 141, 876–887. [Google Scholar] [CrossRef]
- Nomura, T.; Saitoh, N. Microbial Preparation of Gold Nanoparticles by Anaerobic Bacterium.
- Qiu, R.; Xiong, W.; Hua, W.; He, Y.; Sun, X.; Xing, M.; Wang, L. A Biosynthesized Gold Nanoparticle from Staphylococcus Aureus – as a Functional Factor in Muscle Tissue Engineering. Applied Materials Today 2021, 22, 100905. [Google Scholar] [CrossRef]
- Ahmad, A.; Mukherjee, P.; Senapati, S.; Mandal, D.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Oxysporum. Colloids and Surfaces B: Biointerfaces 2003, 28, 313–318. [Google Scholar] [CrossRef]
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The Use of Microorganisms for the Formation of Metal Nanoparticles and Their Application. Appl Microbiol Biotechnol 2006, 69, 485–492. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial Synthesis of Gold Nanoparticles: Current Status and Future Prospects. Advances in Colloid and Interface Science 2014, 209, 40–48. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Wang, K.; Yang, X. Different Active Biomolecules Involved in Biosynthesis of Gold Nanoparticles by Three Fungus Species. j biomed nanotechnol 2011, 7, 245–254. [Google Scholar] [CrossRef]
- Das, S.K.; Liang, J.; Schmidt, M.; Laffir, F.; Marsili, E. Biomineralization Mechanism of Gold by Zygomycete Fungi Rhizopous Oryzae. ACS Nano 2012, 6, 6165–6173. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; S. , R.; S., V.K. A Review on Biogenic Synthesis of Gold Nanoparticles, Characterization, and Its Applications. Resource-Efficient Technologies 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic Aspects in the Biogenic Synthesis of Extracellular Metal Nanoparticles by Peptides, Bacteria, Fungi, and Plants. Appl Microbiol Biotechnol 2011, 90, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular Biosynthesis of Bimetallic Au–Ag Alloy Nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. JFB 2020, 11, 84. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and Plant Derived Biomass for Removal of Heavy Metals from Wastewater. Bioresource Technology 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of Gold Using Cyanobacteria and an Eukaryotic Alga with Special Reference to Nanogold Formation – a Novel Phenomenon. J Appl Phycol 2009, 21, 145–152. [Google Scholar] [CrossRef]
- Nayak, D.; Nag, M.; Banerjee, S.; Pal, R.; Laskar, S.; Lahiri, S. Preconcentration of 198Au in a Green Alga, Rhizoclonium. J Radioanal Nucl Chem 2006, 268, 337–340. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Kannan, C.; Annadurai, G. Seaweed-Mediated Synthesis of Gold Nanoparticles Using Turbinaria Conoides and Its Characterization. J Nanostruct Chem 2013, 3, 44. [Google Scholar] [CrossRef]
- Shakibaie, M.; Forootanfar, H.; Mollazadeh-Moghaddam, K.; Bagherzadeh, Z.; Nafissi-Varcheh, N.; Shahverdi, A.R.; Faramarzi, M.A. Green Synthesis of Gold Nanoparticles by the Marine Microalga Tetraselmis Suecica. Biotech and App Biochem 2010, 57, 71–75. [Google Scholar] [CrossRef]
- Annamalai, J.; Nallamuthu, T. Characterization of Biosynthesized Gold Nanoparticles from Aqueous Extract of Chlorella Vulgaris and Their Anti-Pathogenic Properties. Appl Nanosci 2015, 5, 603–607. [Google Scholar] [CrossRef]
- Shankar, P.D.; Shobana, S.; Karuppusamy, I.; Pugazhendhi, A.; Ramkumar, V.S.; Arvindnarayan, S.; Kumar, G. A Review on the Biosynthesis of Metallic Nanoparticles (Gold and Silver) Using Bio-Components of Microalgae: Formation Mechanism and Applications. Enzyme and Microbial Technology 2016, 95, 28–44. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green Synthesis of Gold Nanoparticles Using Brown Algae Cystoseira Baccata: Its Activity in Colon Cancer Cells. Colloids and Surfaces B: Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Senapati, S.; Syed, A.; Moeez, S.; Kumar, A.; Ahmad, A. Intracellular Synthesis of Gold Nanoparticles Using Alga Tetraselmis Kochinensis. Materials Letters 2012, 79, 116–118. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Khan, T. A Review of the Green Syntheses and Anti-Microbial Applications of Gold Nanoparticles. Green Chemistry Letters and Reviews 2017, 10, 216–227. [Google Scholar] [CrossRef]
- Reddy, A.S.; Chen, C.-Y.; Chen, C.-C.; Jean, J.-S.; Chen, H.-R.; Tseng, M.-J.; Fan, C.-W.; Wang, J.-C. Biological Synthesis of Gold and Silver Nanoparticles Mediated by the Bacteria Bacillus Subtilis. j nanosci nanotechnol 2010, 10, 6567–6574. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated Synthesis of Silver and Gold Nanoparticles and Their Applications. J of Chemical Tech & Biotech 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Q.; Jiang, C.; Yang, D.; Liu, X.; Xu, S. One-Step Synthesis of Biocompatible Gold Nanoparticles Using Gallic Acid in the Presence of Poly-(N-Vinyl-2-Pyrrolidone). Colloids and Surfaces A: Physicochemical and Engineering Aspects 2007, 301, 73–79. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, A. Green Synthesis of Gold Nanoparticles from Lawsoniainermis and Its Catalytic Activities Following the Langmuir-Hinshelwood Mechanism. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2020, 606, 125447. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green Synthesis of Gold Nanoparticles from Scutellaria Barbata and Its Anticancer Activity in Pancreatic Cancer Cell (PANC-1). Artificial Cells, Nanomedicine, and Biotechnology 2019, 47, 1617–1627. [Google Scholar] [CrossRef]
- Zuorro Antonio; Iannone Annalaura; Lavecchia Roberto; Natali Stefano Green Synthesis of Gold Nanoparticles Using Kiwifruit Juice. Chemical Engineering Transactions 2020, 81, 1393–1398. [CrossRef]
- Gubitosa, J.; Rizzi, V.; Lopedota, A.; Fini, P.; Laurenzana, A.; Fibbi, G.; Fanelli, F.; Petrella, A.; Laquintana, V.; Denora, N.; et al. One Pot Environmental Friendly Synthesis of Gold Nanoparticles Using Punica Granatum Juice: A Novel Antioxidant Agent for Future Dermatological and Cosmetic Applications. Journal of Colloid and Interface Science 2018, 521, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Kala, S.M.J.; Pushparaj, T.L. Biogenic Synthesis of Gold Nanoparticles Using Jasminum Auriculatum Leaf Extract and Their Catalytic, Antimicrobial and Anticancer Activities. Journal of Drug Delivery Science and Technology 2020, 57, 101620. [Google Scholar] [CrossRef]
- Ullah, U.; Rauf, A.; El-Sharkawy, E.; Khan, F.A.; Khan, A.; Bukhari, S.M.; Bawazeer, S.; Mabkhot, Y.N.; Malikovna, B.K.; Kazhybayeva, G.; et al. Green Synthesis, in Vivo and in Vitro Pharmacological Studies of Tamarindus Indica Based Gold Nanoparticles. Bioprocess Biosyst Eng 2021, 44, 1185–1192. [Google Scholar] [CrossRef]
- Fatima, Z.; Saleem, R.; Khan, R.R.M.; Liaqat, M.; Pervaiz, M.; Saeed, Z.; Muhammad, G.; Amin, M.; Rasheed, S. Green Synthesis, Properties, and Biomedical Potential of Gold Nanoparticles: A Comprehensive Review. Biocatalysis and Agricultural Biotechnology 2024, 59, 103271. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Vijayaraghavan, K.; Yun, S.-I. Bioreduction of Trivalent Aurum to Nano-Crystalline Gold Particles by Active and Inactive Cells and Cell-Free Extract of Aspergillus Oryzae Var. Viridis. Journal of Hazardous Materials 2010, 177, 539–545. [Google Scholar] [CrossRef]
- Polyakova, N.Yu.; Polyakov, A.Yu.; Sukhorukova, I.V.; Shtansky, D.V.; Grigorieva, A.V. The Defining Role of pH in the Green Synthesis of Plasmonic Gold Nanoparticles Using Citrus Limon Extract. Gold Bull 2017, 50, 131–136. [Google Scholar] [CrossRef]
- Sheen Mers, S.V.; Umadevi, S.; Ganesh, V. Controlled Growth of Gold Nanostars: Effect of Spike Length on SERS Signal Enhancement. ChemPhysChem 2017, 18, 1358–1369. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.; Ahmad, A.; Sastry, M. Role of Halide Ions and Temperature on the Morphology of Biologically Synthesized Gold Nanotriangles. Langmuir 2006, 22, 736–741. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. Journal of Nanomaterials 2014, 2014, 417305. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Imran, M.; Khan, A.U.; Tahir, K.; Khan, Z.U.H.; Yuan, Q. Phytosynthesis and Antileishmanial Activity of Gold Nanoparticles by M Aytenus Royleanus: PHYTOSYNTHESIS AND ANTILEISHMANIAL ACTIVITY OF GOLD NANOPARTICLES. Journal of Food Biochemistry 2016, 40, 420–427. [Google Scholar] [CrossRef]
- Baer, D.R. Surface Characterization of Nanoparticles: Critical Needs and Significant Challenges. JSA 2011, 17, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-Mediated Biosynthesis of Metallic Nanoparticles: A Review of Literature, Factors Affecting Synthesis, Characterization Techniques and Applications. Journal of Environmental Chemical Engineering 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Sharma, N.; Tiwari, P.; Singh, S.R.; Sahi, S.V. Fabrication of Innocuous Gold Nanoparticles Using Plant Cells in Culture. Sci Rep 2019, 9, 12040. [Google Scholar] [CrossRef]
- Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Pavunraj, M.; Annadurai, G. Green Synthesis of Silver Nanoparticle and Silver Based Chitosan Bionanocomposite Using Stem Extract of Saccharum Officinarum and Assessment of Its Antibacterial Activity. Adv. Nat. Sci: Nanosci. Nanotechnol. 2017, 8, 035019. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Size Evaluation of Gold Nanoparticles by UV−vis Spectroscopy. J. Phys. Chem. C 2009, 113, 4277–4285. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Sýkora, D.; Kašička, V.; Mikšík, I.; Řezanka, P.; Záruba, K.; Matějka, P.; Král, V. Application of Gold Nanoparticles in Separation Sciences. J of Separation Science 2010, 33, 372–387. [Google Scholar] [CrossRef]
- Bennur, T.; Khan, Z.; Kshirsagar, R.; Javdekar, V.; Zinjarde, S. Biogenic Gold Nanoparticles from the Actinomycete Gordonia Amarae: Application in Rapid Sensing of Copper Ions. Sensors and Actuators B: Chemical 2016, 233, 684–690. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M.A. Biosynthesis of Gold Nanoparticles Using Pseudomonas Aeruginosa. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- Rajeshkumar, S. Anticancer Activity of Eco-Friendly Gold Nanoparticles against Lung and Liver Cancer Cells. Journal of Genetic Engineering and Biotechnology 2016, 14, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Esterle, A.; Sharma, N.C.; Sahi, S.V. Yucca-Derived Synthesis of Gold Nanomaterial and Their Catalytic Potential. Nanoscale Res Lett 2014, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J. Characterization of Nanomaterials Using Transmission Electron Microscopy. In Nanocharacterisation; Kirkland, A.I., Haigh, S.J., Eds.; The Royal Society of Chemistry, 2015; pp. 1–29 ISBN 978-1-84973-805-7.
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein–Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjugate Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Goldberg-Oppenheimer, P.; Regev, O. Exploring a Nanotube Dispersion Mechanism with Gold-Labeled Proteins via Cryo-TEM Imaging. Small 2007, 3, 1894–1899. [Google Scholar] [CrossRef]
- Liu, S.; Lämmerhofer, M. Functionalized Gold Nanoparticles for Sample Preparation: A Review. Electrophoresis 2019, 40, 2438–2461. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Ankamwar, B. Biosynthesis of Gold Nanoparticles (Green-gold) Using Leaf Extract of Terminalia Catappa. Journal of Chemistry 2010, 7, 1334–1339. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sciences 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Farkas, N.; Kramar, J.A. Dynamic Light Scattering Distributions by Any Means. J Nanopart Res 2021, 23, 120. [Google Scholar] [CrossRef]
- Zheng, T.; Bott, S.; Huo, Q. Techniques for Accurate Sizing of Gold Nanoparticles Using Dynamic Light Scattering with Particular Application to Chemical and Biological Sensing Based on Aggregate Formation. ACS Appl. Mater. Interfaces 2016, 8, 21585–21594. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Cruz, C.N.; Chen, M.-L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The Evolving Landscape of Drug Products Containing Nanomaterials in the United States. Nature Nanotech 2017, 12, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic Light Scattering as a Powerful Tool for Gold Nanoparticle Bioconjugation and Biomolecular Binding Studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pierre-Pierre, N.; Huo, Q. Dynamic Light Scattering for Gold Nanorod Size Characterization and Study of Nanorod–Protein Interactions. Gold Bull 2012, 45, 187–195. [Google Scholar] [CrossRef]
- Ngo, V.K.T.; Nguyen, D.G.; Huynh, T.P.; Lam, Q.V. A Low Cost Technique for Synthesis of Gold Nanoparticles Using Microwave Heating and Its Application in Signal Amplification for Detecting Escherichia Coli O157:H7 Bacteria. Adv. Nat. Sci: Nanosci. Nanotechnol. 2016, 7, 035016. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Applied Surface Science Advances 2022, 12, 100332. [Google Scholar] [CrossRef]
- Mahoney, J.; Monroe, C.; Swartley, A.M.; Ucak-Astarlioglu, M.G.; Zoto, C.A. Surface Analysis Using X-Ray Photoelectron Spectroscopy. Spectroscopy Letters 2020, 53, 726–736. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Mansfield, E.; Tyner, K.M.; Poling, C.M.; Blacklock, J.L. Determination of Nanoparticle Surface Coatings and Nanoparticle Purity Using Microscale Thermogravimetric Analysis. Anal. Chem. 2014, 86, 1478–1484. [Google Scholar] [CrossRef]
- Pang, L.S.K.; Saxby, J.D.; Chatfield, S.P. Thermogravimetric Analysis of Carbon Nanotubes and Nanoparticles. J. Phys. Chem. 1993, 97, 6941–6942. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef]
- Kohane, D.S.; Langer, R. Biocompatibility and Drug Delivery Systems. Chem. Sci. 2010, 1, 441–446. [Google Scholar] [CrossRef]
- Jia, H.Y.; Liu, Y.; Zhang, X.J.; Han, L.; Du, L.B.; Tian, Q.; Xu, Y.C. Potential Oxidative Stress of Gold Nanoparticles by Induced-NO Releasing in Serum. J. Am. Chem. Soc. 2009, 131, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Khalili Fard, J.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv Pharm Bull 2015, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in Vitro and in Vivo Toxicity of Gold Nanoparticles. Chinese Chemical Letters 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Ji, J.; Sun, J.; Zhang, Y.; Sun, X. Cell-Based Metabolomics Approach for Anticipating and Investigating Cytotoxicity of Gold Nanorods. Foods 2022, 11, 3569. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Gao, Y.; Mu, L.; Zhou, Q. Knowledge Gaps between Nanotoxicological Research and Nanomaterial Safety. Environment International 2016, 94, 8–23. [Google Scholar] [CrossRef]
- Dusinska, M.; Dusinska, M.; Fjellsb⊘, L.; Magdolenova, Z.; Rinna, A.; Runden Pran, E.; Bartonova, A.; Heimstad, E.; Harju, M.; Tran, L.; et al. Testing Strategies for The Safety of Nanoparticles Used in Medical Applications. Nanomedicine 2009, 4, 605–607. [Google Scholar] [CrossRef]
- Niżnik, Ł.; Noga, M.; Kobylarz, D.; Frydrych, A.; Krośniak, A.; Kapka-Skrzypczak, L.; Jurowski, K. Gold Nanoparticles (AuNPs)—Toxicity, Safety and Green Synthesis: A Critical Review. IJMS 2024, 25, 4057. [Google Scholar] [CrossRef]
- De Jong, W.H.; Hagens, W.I.; Krystek, P.; Burger, M.C.; Sips, A.J.A.M.; Geertsma, R.E. Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration. Biomaterials 2008, 29, 1912–1919. [Google Scholar] [CrossRef]
- Hall, J.B.; Dobrovolskaia, M.A.; Patri, A.K.; McNeil, S.E. Characterization of Nanoparticles for Therapeutics. Nanomedicine 2007, 2, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.G.; Proykova, A.; Santos, G.M.L. Dealing with Nanosafety around the Globe—Regulation vs. Innovation. International Journal of Pharmaceutics 2016, 509, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, N.; Maitra, S.S. In Vitro and in Vivo Toxicity Assessment of Nanoparticles. Int Nano Lett 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of Gold Nanoparticles Shape on Their Cytotoxicity against Human Osteoblast and Osteosarcoma in in Vitro Model. Evaluation of the Safety of Use and Anti-Cancer Potential. J Mater Sci: Mater Med 2019, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in Understanding Size and Shape Dependent Toxicity of Gold Nanomaterials in Human Skin Keratinocytes. Chemical Physics Letters 2008, 463, 145–149. [Google Scholar] [CrossRef]
- Olveira, S.; Forster, S.P.; Seeger, S. Nanocatalysis: Academic Discipline and Industrial Realities. Journal of Nanotechnology 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Hassanen, E.I.; Morsy, E.A.; Hussien, A.M.; Ibrahim, M.A.; Farroh, K.Y. The Effect of Different Concentrations of Gold Nanoparticles on Growth Performance, Toxicopathological and Immunological Parameters of Broiler Chickens. Bioscience Reports 2020, 40, BSR20194296. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Select 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding Nanoparticle Toxicity to Direct a Safe-by-Design Approach in Cancer Nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Zhou, S.; Chen, W.; Chen, H.; Liang, S.; Zheng, L.; Yu, H.; Chu, R.; Wang, M.; et al. Surface Chemistry Governs the Sub-Organ Transfer, Clearance and Toxicity of Functional Gold Nanoparticles in the Liver and Kidney. J Nanobiotechnol 2020, 18, 45. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci Rep 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Yahyaei, B.; Nouri, M.; Bakherad, S.; Hassani, M.; Pourali, P. Effects of Biologically Produced Gold Nanoparticles: Toxicity Assessment in Different Rat Organs after Intraperitoneal Injection. AMB Expr 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Megarajan, S.; Ameen, F.; Singaravelu, D.; Islam, M.A.; Veerappan, A. Synthesis of N-Myristoyltaurine Stabilized Gold and Silver Nanoparticles: Assessment of Their Catalytic Activity, Antimicrobial Effectiveness and Toxicity in Zebrafish. Environmental Research 2022, 212, 113159. [Google Scholar] [CrossRef] [PubMed]
- Laakso, S.; Mäki, M. Assessment of a Semi-automated Protocol for Multiplex Analysis of Sepsis-causing Bacteria with Spiked Whole Blood Samples. MicrobiologyOpen 2013, 2, 284–292. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; Peixoto De Almeida, M.; Araújo, A.M.; Bastos, M.D.L.; Carmo, H. Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, X.; Wang, Q.; Zou, H.; Xie, Y.; Liu, H.; Zhou, Y.; Liu, P.; Dai, H. Differential Toxicity Mechanism of Gold Nanoparticles in HK-2 Renal Proximal Tubular Cells and 786-0 Carcinoma Cells. Nanomedicine (Lond.) 2020, 15, 1079–1096. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Akila, S.; Nadar, M.S.A.M.; Narendhirakannan, R.T.; Chatterjee, S. Biosynthesized Vitis Vinifera Seed Gold Nanoparticles Induce Apoptotic Cell Death in A431 Skin Cancer Cells. RSC Adv. 2016, 6, 82205–82218. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of Biogenic Gold Nanoparticles from Terminalia Mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef]
- Priya M R, K.; Iyer, P.R. Antiproliferative Effects on Tumor Cells of the Synthesized Gold Nanoparticles against Hep2 Liver Cancer Cell Line. Egypt Liver Journal 2020, 10, 15. [Google Scholar] [CrossRef]
- Sunayana, N.; Uzma, M.; Dhanwini, R.P.; Govindappa, M.; Prakash, H.S.; Vinay Raghavendra, B. Green Synthesis of Gold Nanoparticles from Vitex Negundo Leaf Extract to Inhibit Lipopolysaccharide-Induced Inflammation Through In Vitro and In Vivo. J Clust Sci 2020, 31, 463–477. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; Costa, J.; Soares, M.E.; Dias Da Silva, D.; Bastos, M.D.L.; Carmo, H.F. Cellular Uptake and Toxicity of Gold Nanoparticles on Two Distinct Hepatic Cell Models. Toxicology in Vitro 2021, 70, 105046. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ullah, N.; Khan, M.A.; Mashwani, Z.-R.; Nadhman, A. Plant-Based Gold Nanoparticles; a Comprehensive Review of the Decade-Long Research on Synthesis, Mechanistic Aspects and Diverse Applications. Advances in Colloid and Interface Science 2019, 272, 102017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Das, N.; Rayavarapu, R.G. Role of Tunable Gold Nanostructures in Cancer Nanotheranostics: Implications on Synthesis, Toxicity, Clinical Applications and Their Associated Opportunities and Challenges. JNT 2023, 4, 1–34. [Google Scholar] [CrossRef]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C. Gold Nanoparticles as Colorimetric Probes in Food Analysis: Progress and Challenges. Food Chemistry 2023, 429, 136887. [Google Scholar] [CrossRef]
- Sarfraz, N.; Khan, I. Plasmonic Gold Nanoparticles (AuNPs): Properties, Synthesis and Their Advanced Energy, Environmental and Biomedical Applications. Chemistry An Asian Journal 2021, 16, 720–742. [Google Scholar] [CrossRef]
- Heinemann, M.G.; Rosa, C.H.; Rosa, G.R.; Dias, D. Biogenic Synthesis of Gold and Silver Nanoparticles Used in Environmental Applications: A Review. Trends in Environmental Analytical Chemistry 2021, 30, e00129. [Google Scholar] [CrossRef]
- Google Patents. Available online: https://patents.google.com/ (accessed on 10 October 2024).
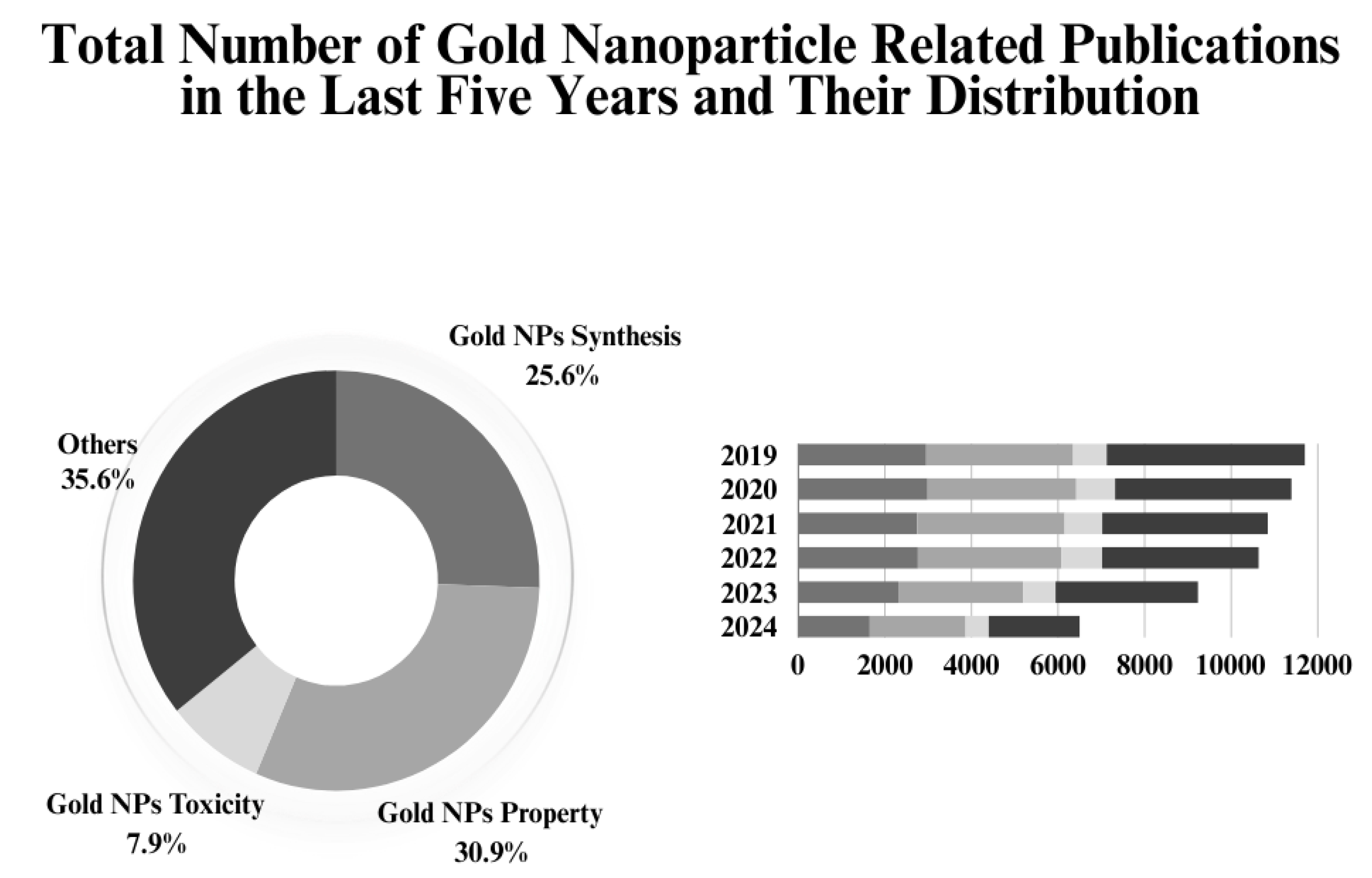
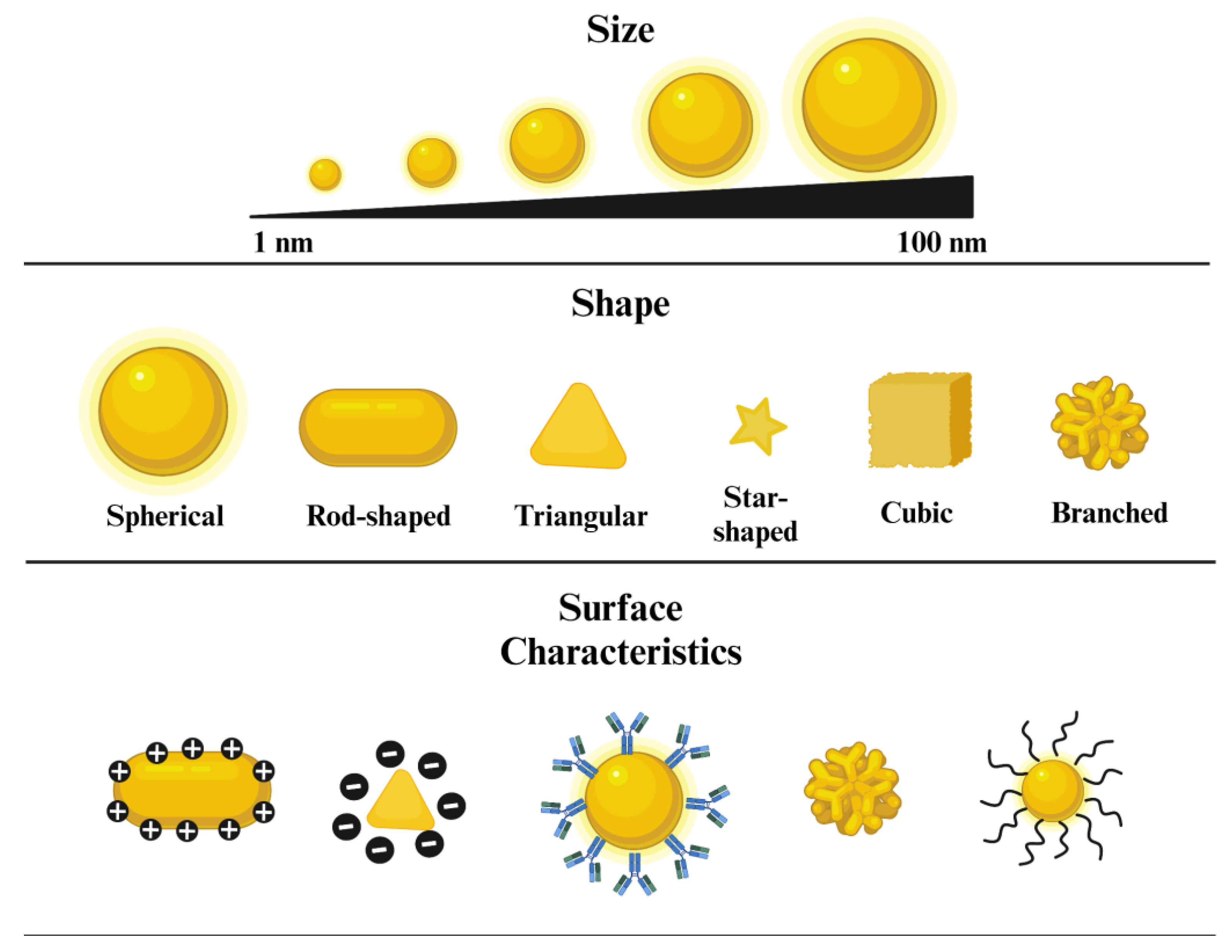
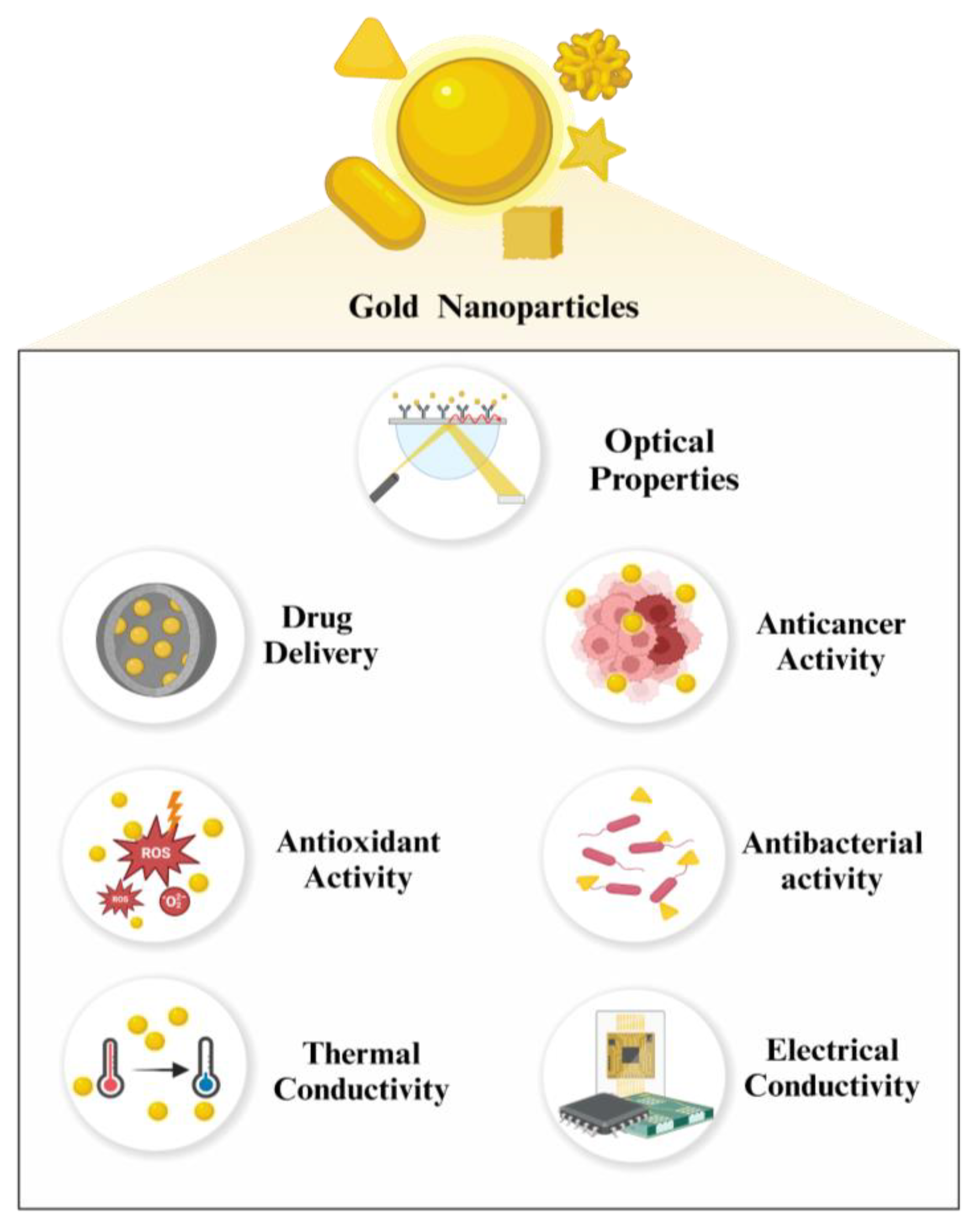
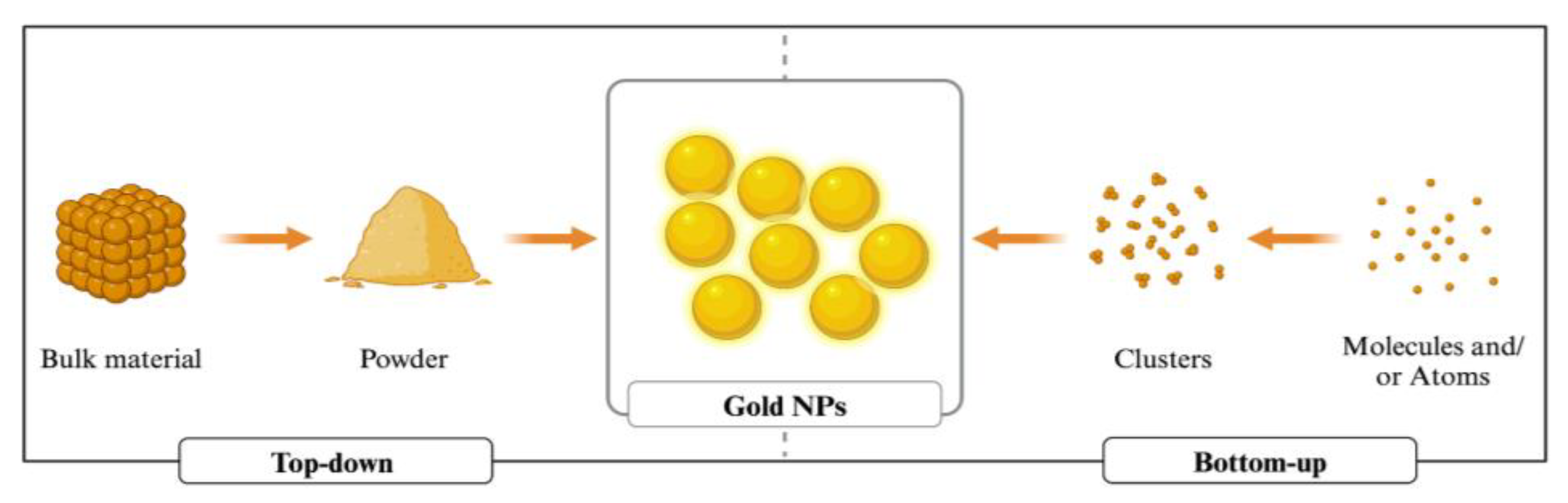
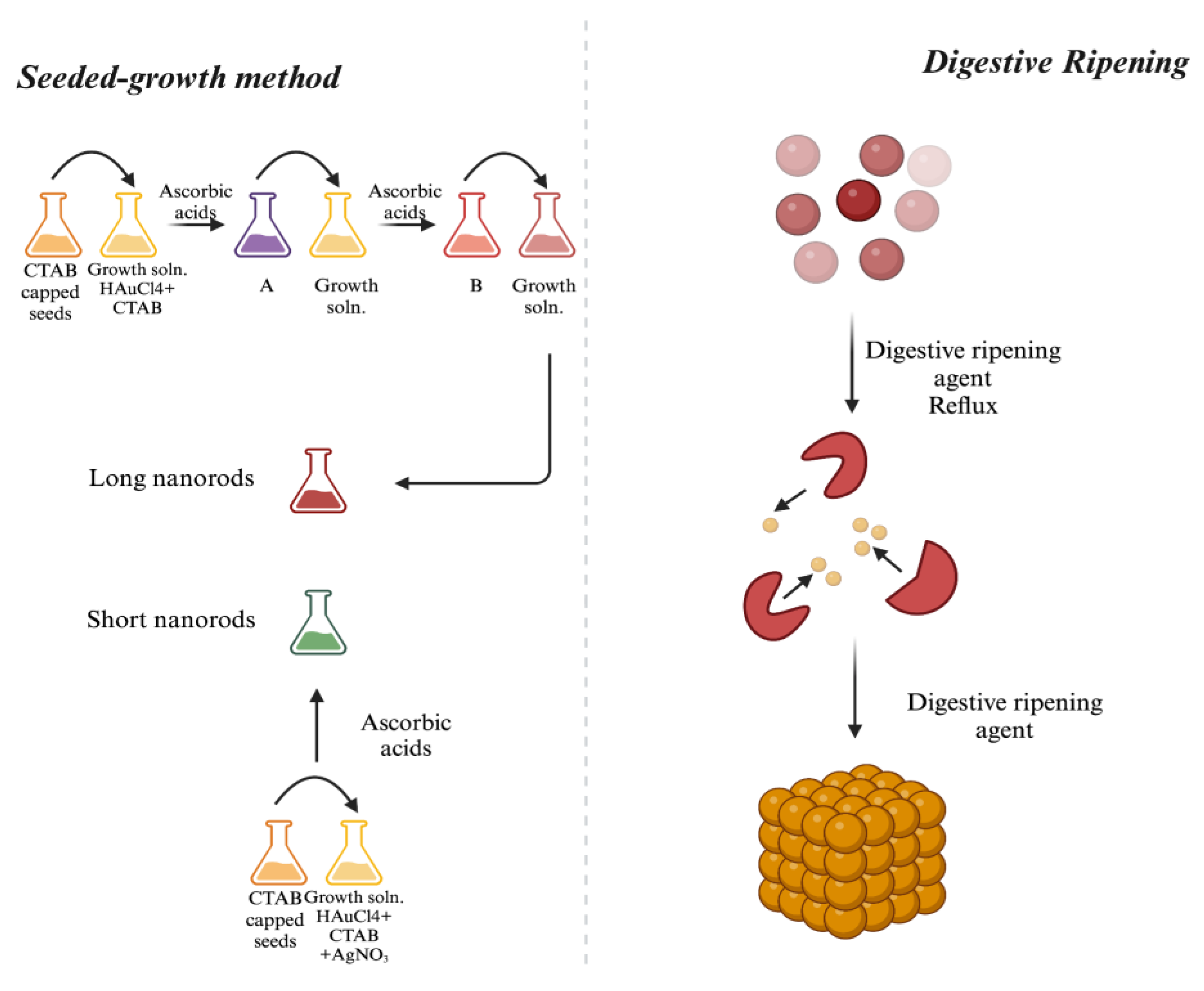
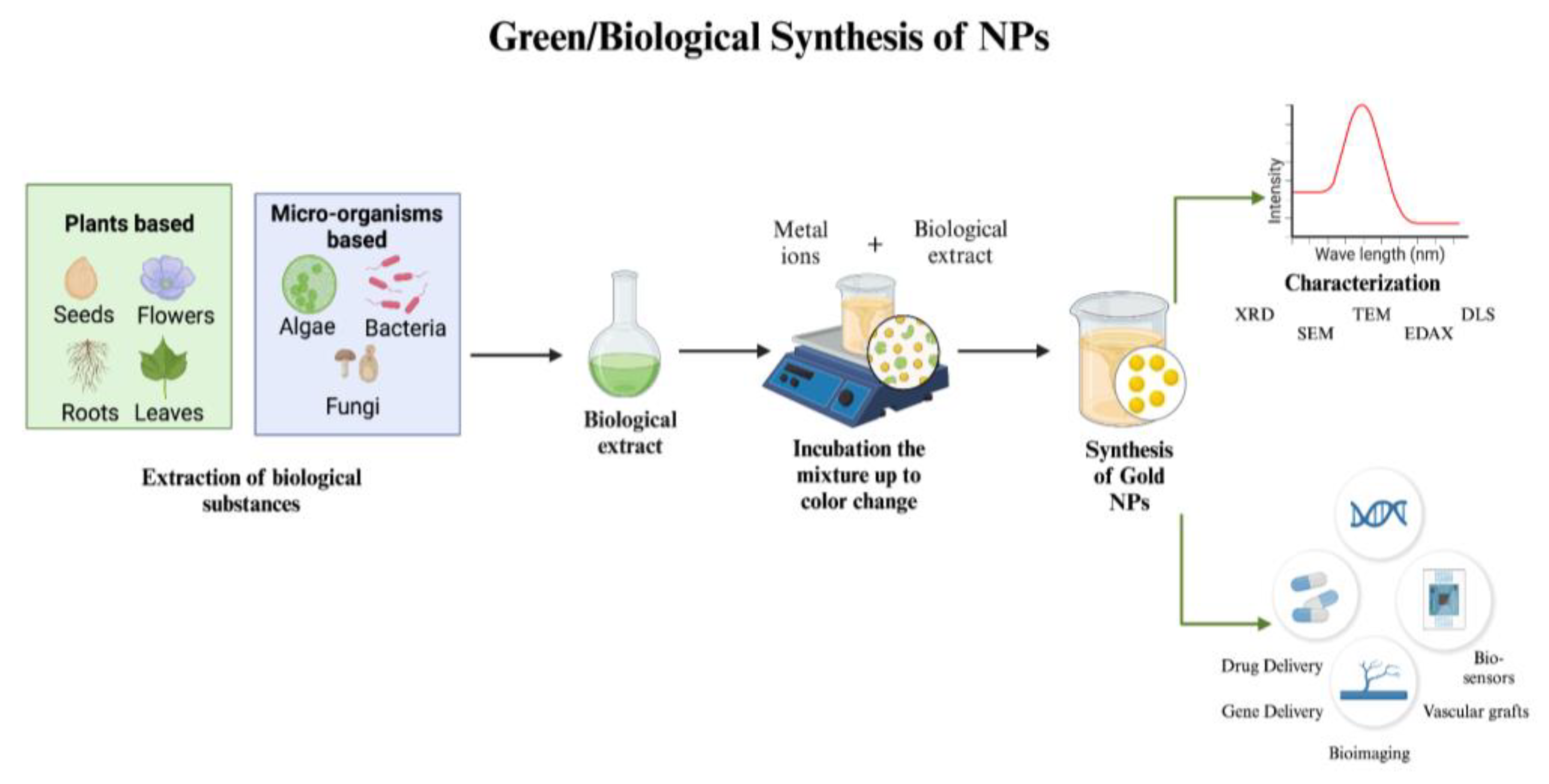
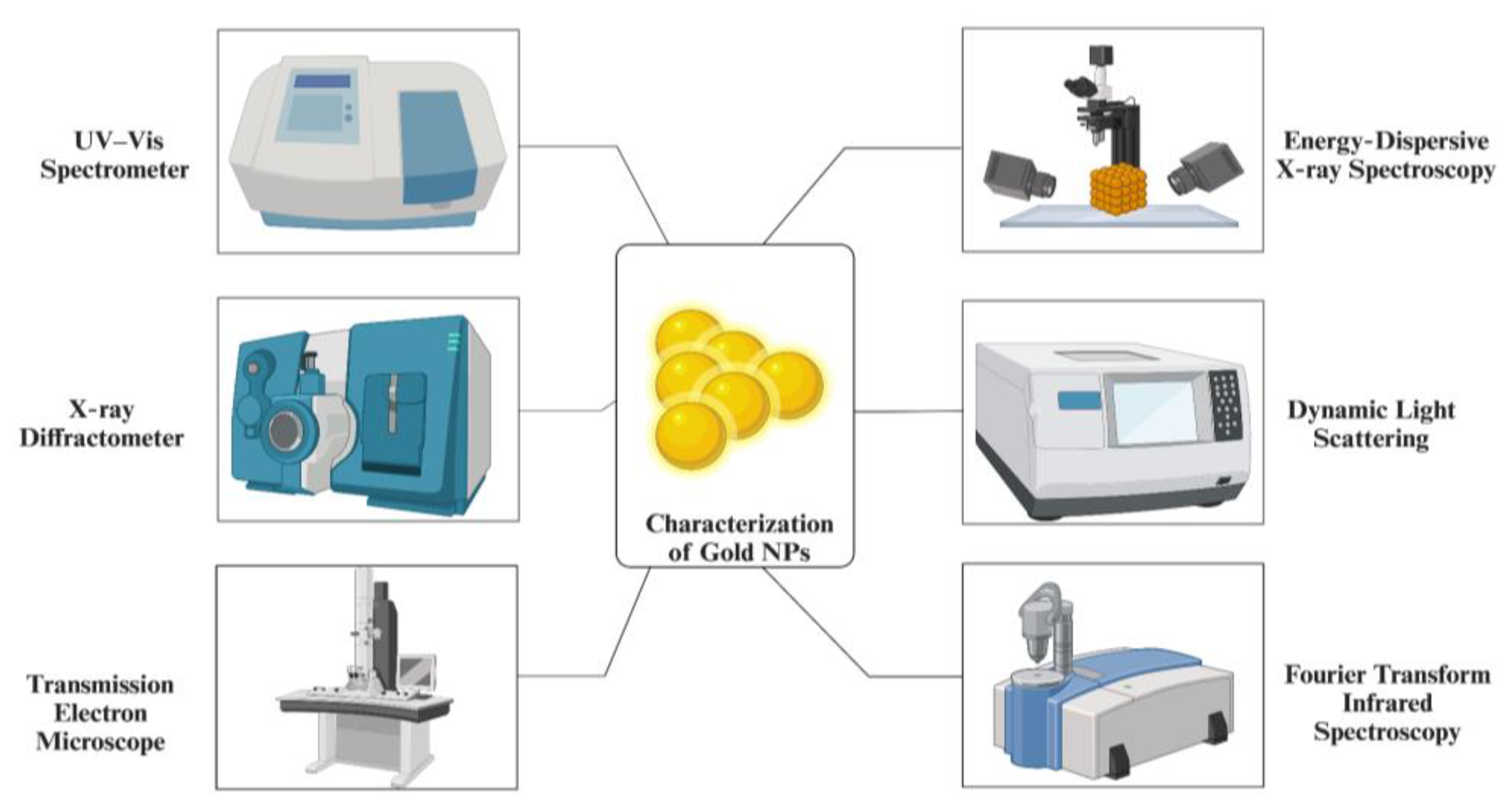
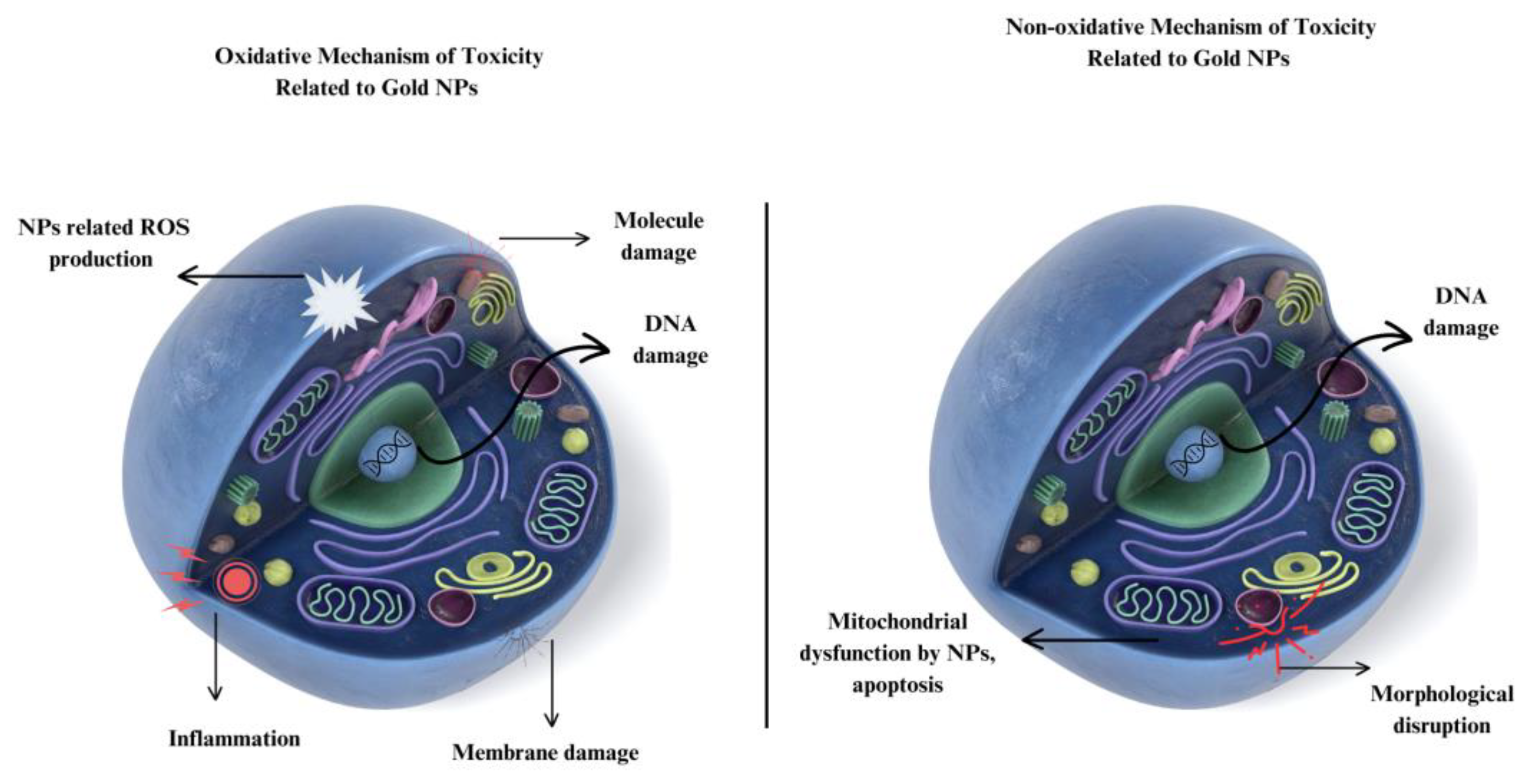
| Type of Study | Organism | Particle | Effects | Ref |
|---|---|---|---|---|
| In vivo | Mice | Laser-ablated dextran-coated gold NPs | The absence of acute and chronic toxicities and healthy animal behavior supported the safety of gold NPs, which were mostly stored in the liver and spleen and did not produce hepatic or renal toxicity. | [339] |
| In vivo | Broiler chicken | Gold NPs |
The therapy significantly damaged the blood's oxidative capacity, altered histology, elevated the expression of the IL-6 and Nrf2 genes, fragmented DNA, and reduced the antibody titer against avian influenza and Newcastle disease. | [335] |
| In vivo | Rat | Gold NPs | Different doses of gold NPs had distinct hazardous effects on different organs. Non-toxic doses, on the other hand, had no effect on the testis and only mildly affected the liver and kidney. | [340] |
| In vivo | Zebrafish animal model | N-myristoyltaurine stabilized gold NPs | According to a toxicity study conducted on an animal model of zebrafish, gold NPs are safe. | [341] |
| In vivo | Rat | Gold NPs | Despite being non-toxic, the study discovered that greater doses of gold NPs, such 2 mg/kg, were harmful to every organ examined. | [342] |
| In vitro | Human kidney-2 (HK-2) cell and proximal tubular cells | Gold NPs with different shapes (spheres and stars), capping (citrate and MUA), and diameters (13 nm and 60 nm) | For HK-2 cells, the 13 nm nanospheres were the most hazardous, damaging the mitochondria and lysosomes and increasing the generation of ROS. Severe MUA-capped gold NPs led to apoptosis. Larger 60 nm gold NPs considerably decreased cellular viability but were less hazardous. | [343] |
| In vitro | HK-2 and 786-0 cells |
Gold NPs | Gold NPs with a diameter of 5 or 200 nm have the ability to trigger autophagy in HK-2 cells to shield them from harm and apoptosis in 786-0 cells to kill tumor cells. | [344] |
| In vitro | Keratinocyte cell line (HaCaT) and human epidermoid skin cancer cell line (A431) | Vitis vinifera (V. vinifera) gold NPs | V. vinifera seed gold NPs were non-toxic to normal HaCaT cells, but they suppressed the growth of A431 skin cancer cells by cytotoxicity and death. | [345] |
| In vitro | Cancer (Caco-2, MCF-7 and HepG2) and non-cancer (KMST-6) cell lines | Terminalia mantaly (TM) extract gold NPs | Using the MTT assay, the study investigated the cytotoxic effects of TM-gold NPs on cancer and non-cancer cell lines and discovered that certain extracts were more hazardous than others. | [346] |
| In vitro | Hep2 liver cancer cell line and Vero cell line | Gold NPs | The NPs exhibited remarkable non-toxic effects on normal VERO cell line and anticancer activities in treated Hep2 liver cancer cell line. T | [347] |
| In vitro | HeLa cell lines | V. negundo extract gold NPs | At greater dosages, gold NPs are hazardous to HeLa cells. | [348] |
| In vitro | Human HepaRG cells or primary rat hepatocytes (PRH) | Gold NPs with different size (~ 15 nm and 60 nm), shape (nanospheres and nanostars) and capping [citrate- or 11-mercaptoundecanoic acid (MUA)], | In serum-free media, the 15 nm MUA-capped nanospheres exhibited considerable toxicity to PRH and HepaRG cells, indicating that their restricted application in diagnostics should be disregarded. | [349] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





