Submitted:
22 October 2024
Posted:
24 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
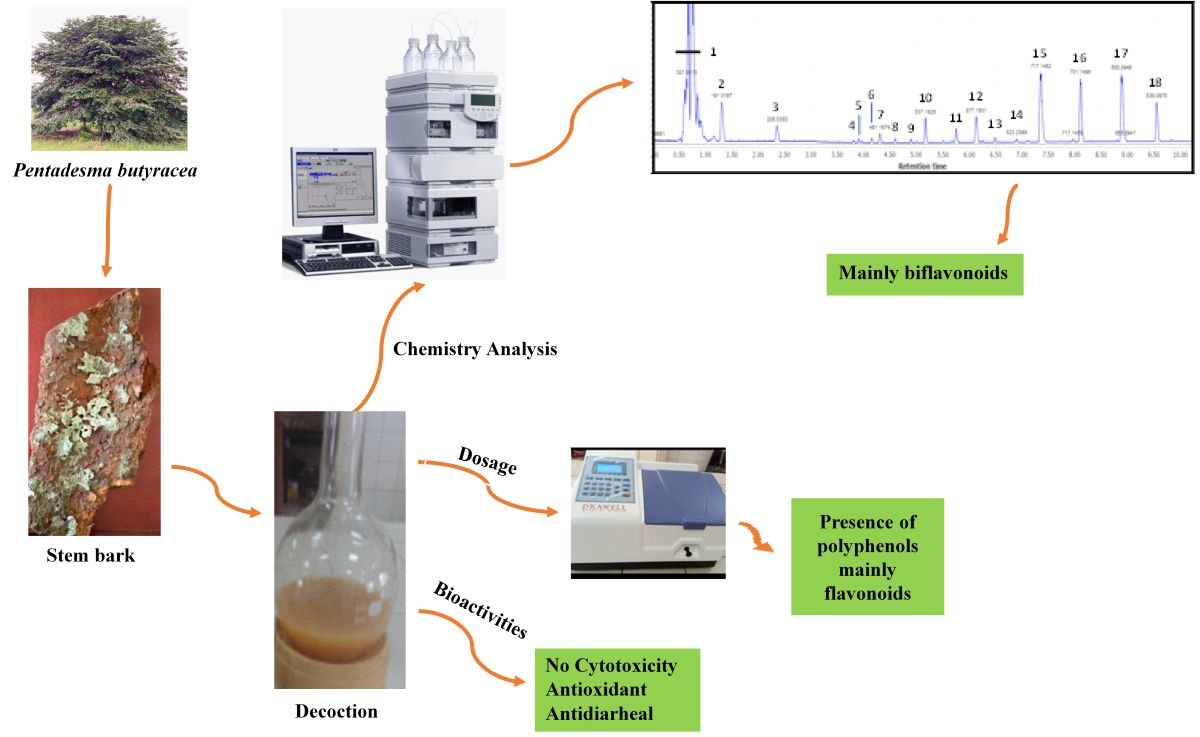
2.1. Phytochemical Analyses of Decoctions of Pentadesma butyracea Stem Barks
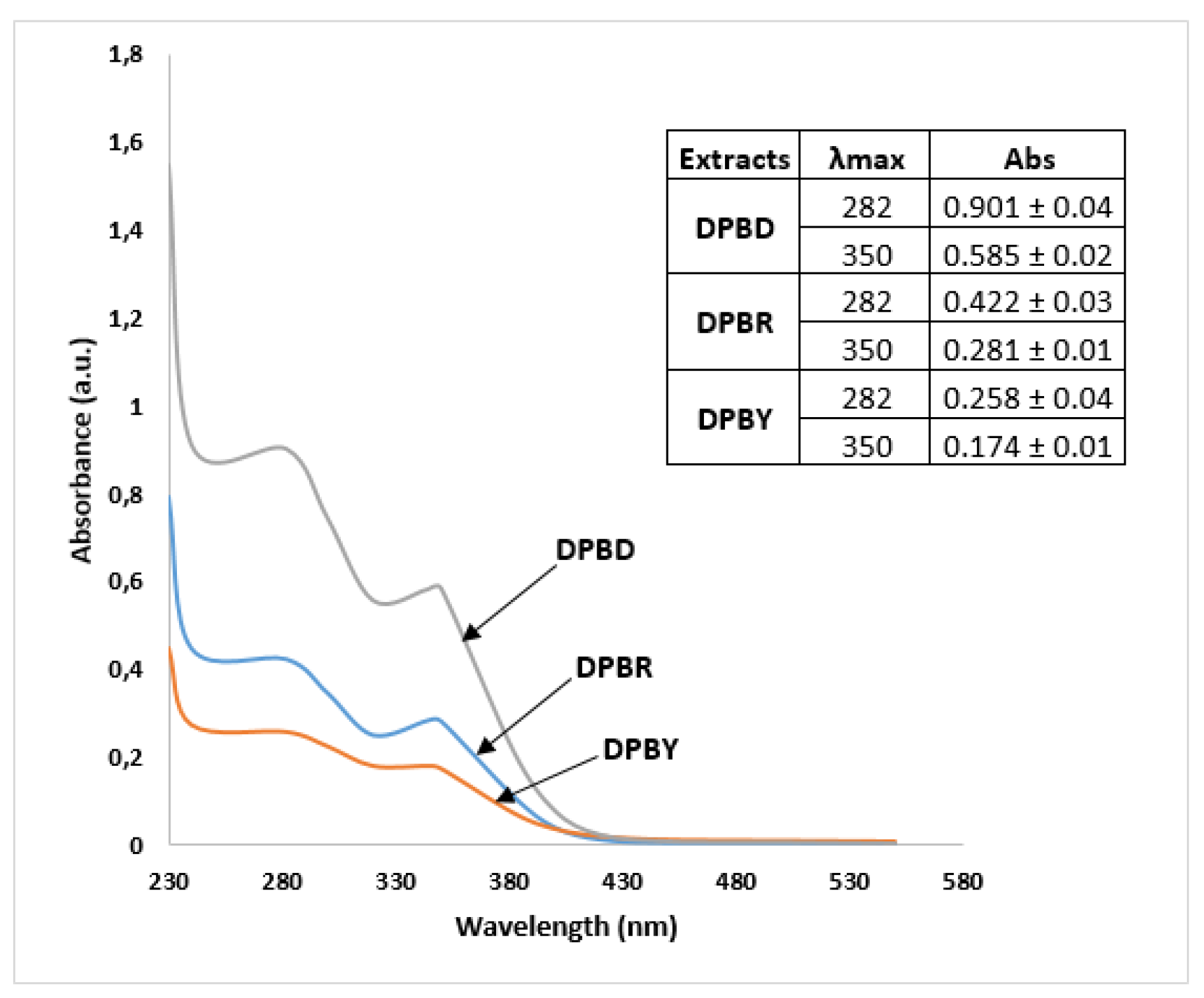
2.1.1. Total Phenolic Contents
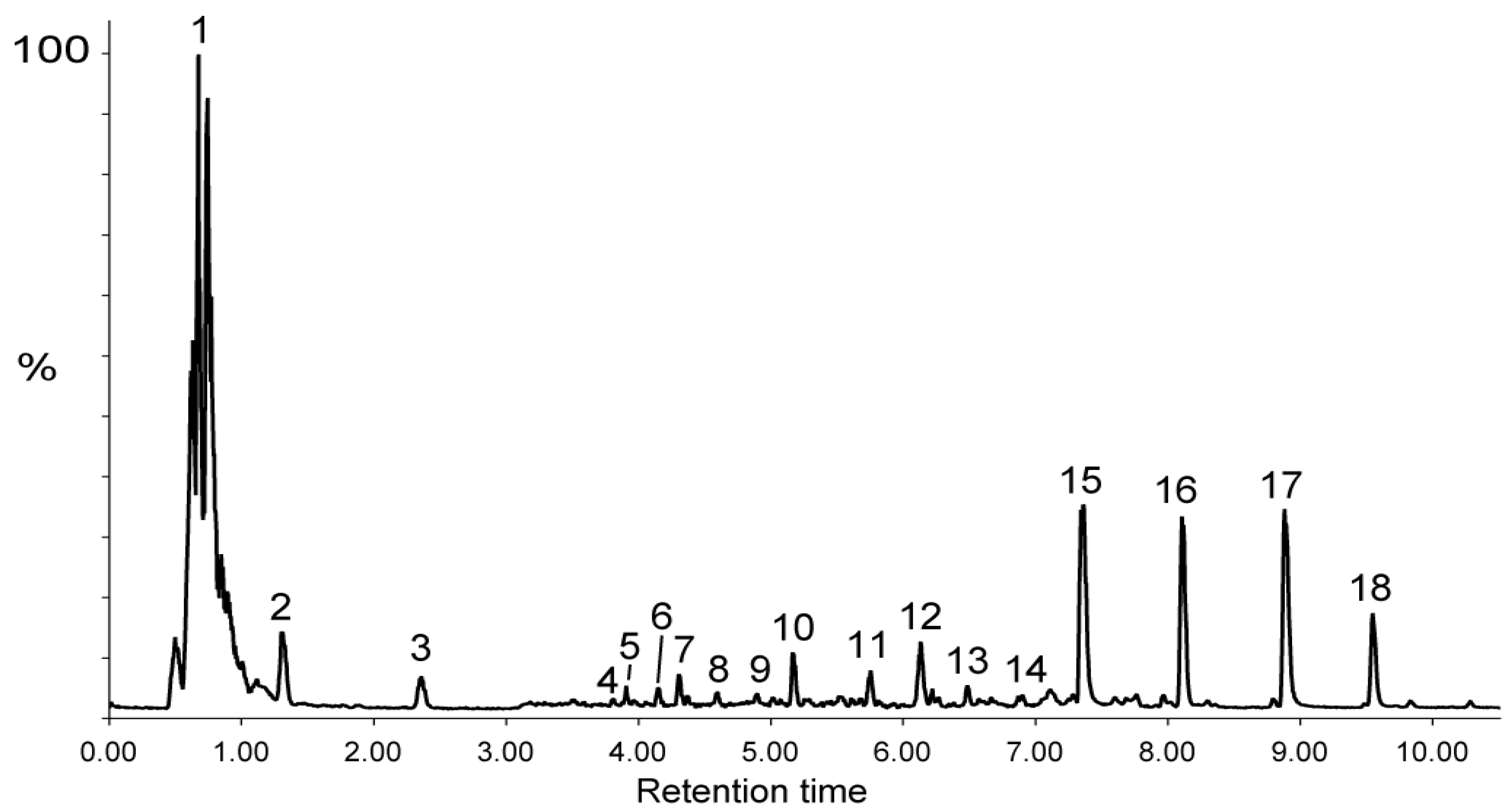
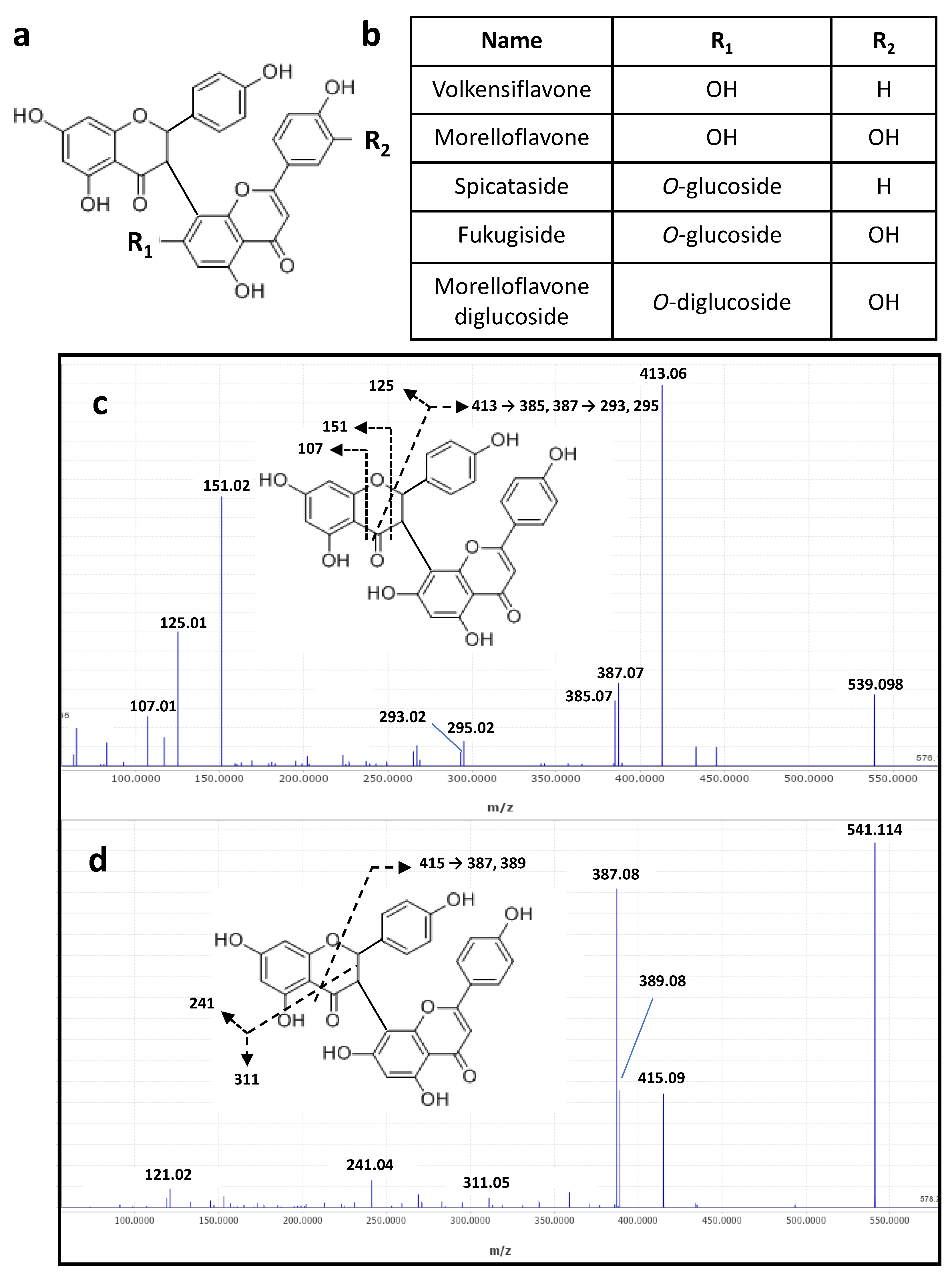
2.1.2. Mass Spectrometry Identification of Biflavonoids in the Decoction of Pentadesma butyracea Stem Barks
2.1.3. Evaluation of Biflavonoid Contents in the DPB Samples
2.2. Pharmacological Analyses of Decoction of P. butyracea Stem Barks
2.2.1. Antioxidant Activity Assay
2.2.2. Toxicity Assays
2.2.3. Activity of DPBR Sample on Smooth Muscle
2.2.4. In Vivo Antidiarrheal Activity of DPBR
3. Discussion
4. Materials and Methods
4.1. Equipment
4.2. Plant Material
4.3. Chemicals
4.4. Animal and Cell Lines Model
4.5. Preparation of P. butyracea Stem Barks Decoctions
4.6. Determination of Total Phenolic Contents
4.7. Determination of Total Flavonoid Contents
4.8. Gas Chromatography Coupled to an Electron Impact Mass Spectrometer (GC-EI-MS)
4.9. Ultra-High Performance Liquid Chromatography Coupled to a Quadrupole-Orbitrap Mass Spectrometer (UHPLC-ESI-MS/MS)
4.10. UV-VIS Spectroscopy
4.11. Antioxidant Assay
4.12. In Vitro Cytotoxicity Assay
4.13. Acute Toxicity Assay
4.14. In Vitro Antidiarrheal Assays on Excised Ileum Fragments
4.15. In Vivo Antidiarrheal Assays
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayindza-Ekaghba, E.L.; Itoudi Bignoumba, P.E.; Bourobou Bourobou, J.A.; Boukandou Mounanga, M.M. Etude épidémiologique des troubles fonctionnels intestinaux dans les structures sanitaires à Libreville (Gabon). J. Appl. Biosc. 2020, 155, 15986–15993. [Google Scholar]
- Sinsin, B.; Sinadouwirou, A.T. Valorisation socioéconomique et pérénnite du Pentadesma butyraceae Sabine en galeries forestières au Bénin. Cahier Agriculture 2003, 12, 75–79. [Google Scholar]
- Natta, A.; Sogbegnon, R.; Tchobo, F. Connaissances endogènes et importance du Pentadesma butyracea (Clusiaceae) pour les populations autochtones au Nord-Ouest Bénin. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 18–25. [Google Scholar]
- Noudogbessi, J.P.A.; Natta, A.K.; Tchobo, F.P.; Bogninou, G.S.; Bothon, F.T.D., Bossou; Figueredo, G.; Chalard, P.; Chalchat, J.C.; Sohounhloué, D.C.K. Phytochemical screening of Pentadesma butyracea Sabine (Clusiaceae) acclimated in Benin by GC/MS. ISRN Anal. Chem 2013, 172397. [Google Scholar] [CrossRef]
- Ayegnon, B.P.; Kayodé, A.P.; Tchobo, F.P.; Azokpota, P.; Soumanou, M.M.; Hounhouigan, D.J. Profiling the quality characteristics of the butter of Pentadesma butyracea with reference to shea butter. J. Sci. Food Agric. 2015, 95, 3137–3143. [Google Scholar] [CrossRef]
- Dencausse, L.; Ntsourankoua, H.; Artaud, J.; Clamou, J.L. Comparaison des compositions lipidiques des beurres de Pentadesma butyracea et de Karite. Ol. Corps Gras Lipides 1995, 2, 143–147. [Google Scholar]
- Courtin, O. Cosmetic preparation to retard the ageing skin, and application process. European Patent Applications. EP0279136A3. 1987. [Google Scholar]
- Abbiw, D.K. Useful plants of Ghana. In Intermadiate Technology Publications and the Royal Botanic Gardens Kew; London, UK, 1990. [Google Scholar]
- Adjanohoune, E.J.; Aké Assi, L.; Chibon, P.; de Vécchy, H.; Duboze, E.; Eymé, J.; Gassita, J.N.; Goudote, E.; Guinko, S.; Keita, A.; Koudogbo, B.; Le Bras, M.; Mourambou, I.; Mve-Mengome, E.; Nguéma, M.G.; Ollome, J.B.; Posso, P.; Sita, P. In Contribution aux études ethnobotaniques et floristiques au Gabon. Agence de coopération culturelle et technique: Paris, France, 9: ISBN: 92, 9028. [Google Scholar]
- Avocevou-Ayisso, C.; Avohou, T.H.; Oumorou, M.; Dossou, G.; Sinsin, B. Ethnobotany of Pentadesma butyracea in Benin: A quantitative approach. Ethnobot. Res. Applic. 2011, 9, 151–166. [Google Scholar] [CrossRef]
- Tindano, B.; Bayala, B.; Guenne, S.; Doukoure, M.; Kiendrebeogo, M.; Belemtougri, G.R. Innocuity and antioxidant activities of Pentadesma butyracea (1824) leaves for its use in hormone replacement therapy. Cameroon J. Experim. Biol. 2018, 12, 32–40. [Google Scholar] [CrossRef]
- Alitonou, G.; Avlessi, F.; Sohounhloue, D.; Bessiere, J.M.; Menut, C. Chemical and biological investigation on volatile constituents of Pentadesma butyracea Sabine (Clusiaceae) from Benin. J. Essent. Oil Res. 2010, 22, 138–140. [Google Scholar] [CrossRef]
- Zéléfack, F.; Guillet, D.; Fabre, N.; Bayet, C.; Chevalley, S.; Ngouela, S.; Ndjakou, L.B.; Valentin, A.; Diroux-Franca, M.G. Xanthones cytotoxiques et antiplasmodium de Pentadesma butyracea. J. Nat. Prod. 2009, 72, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Djoufack, N.G.L.; Valant-Vetschera, K.M.; Schinnerl, J.; Brecker, L.; Lorbeer, E.; Robien, W. Xanthones, biflavanones and triterpenes from Pentadesma grandifolia (Clusiaceae): Structural determination and bioactivity. Nat. Prod. Comm. 2010, 5, 1055–1060. [Google Scholar] [CrossRef]
- Timtey, J.A.; Alemaworn, F.; Ellis, W.O.; Pepra-Ameyaw, N.B.; Agbenorhevi, J.K. Pentadesma butyracea in Ghana – indigenous knowledge, uses, and seed characterization. Sci. Afr. 2023, 21, e01747. [Google Scholar] [CrossRef]
- Akpanika, G.A.; Winters, A.; Wilson, T.; Ayoola, G.A.; Adepoju-Bello, A.A. , Hauck, B. Polyphenols from Allanblackia floribunda seeds: identification, quantification and antioxidant activity. Food Chem. 2017, 222, 35–42. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Shao, S-Y.; Ting, Y.; Wang, J.; Sun, J.; Guo, X-F. Characterization and identification of the major flavonoids in Phyllostachys edulis leaf extract by UPLC—QTOF—MS/MS. Acta Chromatogr. 2020, 32, 228–237. [Google Scholar] [CrossRef]
- Zhu, F.X.; Wang, J.J.; Li, X.F.; Sun, E.; Jia, X.B. Qualitative and quantitative analysis of the major constituents in traditional Chinese medicine Danmu injection using LC-ESI-MS (n) and LC-DAD. Pharmacogn. Mag. 2014, 10, 254–264. [Google Scholar]
- Thai, T.H.; Hai, N.T.; Hien, N.T.; Ha, C.T.H.; Cuong, N.T.; Binh, P.T.; Dang, N.H.; Dat, N.T. Cytotoxic constituents of Mallotus Microcarpus. Nat. Prod. Comm. 2017, 12, 407–408. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Taher, M.A.; Laboni, A.A.; Shompa, S.A.; Rahman, M.M.; Hasan, M.M.; Hasnat, H.; Khan, M. Bioactive compounds extracted from leaves of G. cyanocarpa using various solvents in chromatographic separation showed anti-cancer and anti-microbial potentiality in in silico approach. Chin. J. Anal. Chem. 2023, 51, 100336. [Google Scholar] [CrossRef]
- Tindano, B.; Bayala, B.; Doukoure, M.; Belemtougri, G.R.; Tamboura, H.H.; Sawadogo, L. Phytochemical composition, acute toxicity and phytohormonal Garcinia madruno activity of hydroalcoholic extract of Pentadesma butyracea (Clusiaceae Sabine (1824)) seeds. J. Med. Plant Res. 2017, 11, 656–664. [Google Scholar] [CrossRef]
- Brusotti, G.; Papetti, A.; Serra, M.; Temporini, C.; Marini, E.; Orlandini, S.; Kada Sanda, A.; Watcho, P.; Kamtchouing, P. Allanblackia floribunda Oliv.: An aphrodisiac plant with vasorelaxant properties. J. Ethnopharmacol. 2016, 192, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Ayegnon, B.P.; Chabi, I.B.; Akogou, F.U.G.; Kayodé, A.P.P. Physicochemical characterization and microbiology quality of the Pentadesma butyracea fruit pulp collected from various parks in Benin. Sci Rep. 2021, 11, 17040. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Hormaza, L.; Ramírez, A.M.; Quintero-Ortiz, C.; Cossio, M.; Medina, S.; Ferreres, F. , Gil-Izquierdo, A.; Osorio E. Comprehensive characterization and antioxidant activities of the main biflavonoids of: A novel tropical species for developing functional products. J. Funct. Foods 2016, 27, 503–516. [Google Scholar] [CrossRef]
- Taher, M.A.; Jamal Hossain, M.; Zahan, M.S.; Hasan, M.M.; Ferdous, J.; Rahman, A.; Khan, M.; Hosain, M.K.; Rashid, M.A. Phyto-pharmacological and computational profiling of Bombax ceiba Linn. Leaves revealed pharmacological properties against oxidation, hyperglycemia, pain, and diarrhea. Heliyon, 2024; 10, e35422. [Google Scholar]
- Tantary, S.; Masood, A.; Bhat, A.H.; Dar, K.B.; Zargar, M.A.; Ganie, S.A. In vitro antioxidant and RBC membrane stabilization activity of Euphorbia wallichii. Free Radic. Antioxid. 2017, 7, 13–22. [Google Scholar] [CrossRef]
- Kim, Y.J. , Kim, E., Hahm, K.B. Oxidative stress in inflammation-based gastrointestinal tract diseases: Challenges and opportunities. J. Gastroentero.l Hepatol. 2012, 27, 1004–1010. [Google Scholar] [CrossRef]
- Miranda-Bautista, J.; Bañares, R.; Vaquero, J. The Gastrointestinal System: Anatomy and sources of oxidative stress, in: Gastrointestinal Tissue. Academic Press. 2017, pp 3-20. ISBN: 9780128053775.
- Feyisa, K.; Bisrat, D.; Tadesse, S.; Asres, K. Antidiarrhoeal activity of the 80% methanol root extract of alictrum ryhnchocarpum Dill. & A. Rich and its major constituent against castor oil-induced diarrhoea in mice. Ethiopian Pharmaceut. J. 2020, 36, 31–40. [Google Scholar]
- Diby, S.B.; Koné, M.; Yapo, A. Potentiel pharmacologique des écorces de tige de Spondias mombin L. (Anacardiaceae) sur la motricité in vitro du duodénum de lapin ; une plante médicinale utilisée dans le traitement traditionnel des troubles digestifs. Phytothérapie 2012, 10, 306–312. [Google Scholar] [CrossRef]
- Wansi, S.L.; Nguelefack-Mbuyo, E.P.; Nchouwet, M.L.; Miaffo, D.; Nyadjeu, P.; Wabo, J.P.; Mbiantcha, M.; NKeng-Efouet, P.A.; Nguelefack, T.B.; Kamanyi, A. Antidiarrheal activity of aqueous extract of the stem bark of Sapium Ellipticum (Euphorbiaceae). Trop. J. Pharmaceut. Res. 2014, 13, 929–935. [Google Scholar] [CrossRef]
- Otimenyin, O.S.; Uguru, O.M.; Akanbi, B.E. Antidiarrhea effect of aqueous extracts of Momordica balsamina and Stachytarpheta indica in Rats. J. Nat. Prod. 2008, 1, 36–45. [Google Scholar]
- Bahi, C.; N’guessan, J.D.; Guédé-Guina, F. Mise en évidence d’une action myorelaxante et cholinolytique de Bitter GG (BGG), un antidiarrhéique de source végétale. Afr. Biomed. 2000, 5, 11–18. [Google Scholar]
- Bleu, G.M.; Traoré, F.; Coulibaly, S.; Nene-Bi, S.A. Effets pharmacodynamiques d’un extrait hydroalcoolique de Curcuma longa Linné (Zingiberaceae) sur le système cardiovasculaire, la respiration et l’activité mécanique intestinale de mammifères. Phytothérapie 2011, 9, 7–17. [Google Scholar] [CrossRef]
- Mogose, B.; Bisrat, D.; Asres, K. In Vivo Antidiarrheal potential of the leaf extract of Maytenus addat (Loes.) Sebsebe and its major compound. J. Trop. Med. 2024, 5922487. [Google Scholar] [CrossRef] [PubMed]
- Gidudu, J.; Sack, D.; Pina, M.; Hudson, M.; Kohl, K.; Bishop, P.; Chatterjee, A.; Chiappini, E.; Compingbutra, A.; Da Costa, C. Diarrhea: Case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011, 29, 1053. [Google Scholar] [CrossRef]
- Rudra, S.; Tahamina, A.; Emon, N.U.; Adnan, M.; Shakil, M.; Chowdhury, M.; Uddin, H.; Barlow, J.W.; Alwahibi, M.S.; Soliman Elshikh, M. Evaluation of various solvent extracts of Tetrastigma leucostaphylum (Dennst.) Alston leaves, a Bangladeshi traditional medicine used for the treatment of Diarrhea. Molecules 2020, 25, 4994–5013. [Google Scholar] [CrossRef]
- Farack, U.M.; Kantz, U.; Loescke, K. Loperamide reduces the intestinal secretion but not the mucosal cAMP accumulation induced by cholera toxin. Naunyn Schmiedebers Arch. Pharmacol. 1981, 317, 178–179. [Google Scholar] [CrossRef]
- Karim, S.M.M.; Adaikan, P.G. The effect of the loperamide on prostaglandin-induced diarrhea in rat and man. Prostaglandins 1977, 13, 321–331. [Google Scholar] [CrossRef]
- Rawat, P.; Singh, P.K.; Kumar, V. Evidence based traditional anti-diarrheal medicinal plants and their phytocompounds. Biomed. Biomed. Pharmacother. 2017, 96, 1453–1464. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. The frequency of U-shaped dose responses in the toxicological literature. Toxicol. Sci. 2001, 62, 330–338. [Google Scholar] [CrossRef]
- Dos Santos Negreiros, P.; da Costa, D.S.; da Silva, V.G.; de Carvalho, Lima, I. B., Nunes, D.B., de Melo, Sousa, F.B., Araújo, T.L.S., Medeiros, J.V.R., dos Santos, R.F., Oliveira, R.D.C M. Antidiarrheal activity of α-terpineol in mice. Biomed. Pharmacother. 2019, 110, 631–640. [Google Scholar] [CrossRef]
- Araújo, T.S.L.; Costa, D.S.; Sousa, N.A.; Souza, L.K.M.; Araújo, S.; Oliveira, A.P.; Sousa, F.B.M.; Silva, D.A.; Barbosa, A.L.R.; Leite, J.R.S.A.; Medeiros, J.V.R. Antidiarrheal activity of cashew GUM, a complex heteropolysaccharide extracted from exudate of Anacardium occidentale L. in rodents. J Ethnopharmacol. 2015, 174, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent, Methods in Enzymology. Academic Press, 1999, 299, 152–178. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, M.; Ribéreau-Gayon, P.; Sudraud, P. In Sciences et techniques du vin. Tome 1, analyse et controle des vins. Dunod, Ed. Paris, France 1972, 671. [Google Scholar]
- Mengome, L.E. , Voxeur, A., Akue, J.P., Lerouge, P. Screening of antioxidant activities of polysaccharides extracts from endemic plants in Gabon. Bioact. Carbohydr. Diet. Fibre 2014, 3, 77–88. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). The Organization of Economic Co-Operation and Development Guidelines Test NO. 423 Acute Oral Toxicity-Acute Toxic Class Method, Guidelines for the Testing of Chemicals, Section 4. 2002, –14. 1 February.
- Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0.
- Mekonnen, B.; Asrie, A.B.; Wubneh, Z.B. Antidiarrheal activity of 80% methanolic leaf extract of Justicia schimperiana. Evid. Based Complement. Alternat. Med. 2018, 6, 3037120. [Google Scholar] [CrossRef]
| Genus, species | Family | n° NHG | Organ | Uses | Traditional indications |
|---|---|---|---|---|---|
| Aucoumea klaineana (Pierre) | Burseraceae | 599 | Stem bark | Diarrhea | Macerated stem bark is used as astringent antidiarrheal agent |
| Pentadesma butyracea (Sabine) | Clusiaceae | 14802 | Stem bark | Diarrhea | In decoction, stem bark is used as an antidiarrheal agent |
| Canarium schweinfurthii (Engl.) | Burseraceae | 1724 | Stem bark | Pain | In decoction, the stem bark is used for stomach and intestinal pains |
| Scorodophloeus zenkeri (Harms) | Fabaceae | 1418 | Stem bark | Constipation | Infusion of stem bark is used to treat constipation |
| Extract | Total phenol content as µg GAE/mg ± SD a | Total flavonoid content as µg QE/mg ± SD a |
|---|---|---|
| DPBY | 62.1 ± 1.4 | 41.9 ± 4.5 |
| DPBR | 44.1 ± 2.9 | 83.0 ± 9.3 |
| DPBD | 129.0 ± 10.3 | 150.3 ± 30.3 |
| p—value | <0.001 | <0.001 |
| R2 | 0.9916 | 0.9517 |
| n° | RT min | [M−H] ̶ a [M+HCOO] ̶ b |
[M+H]+ c [M+Na]+ d | Molecular formula | Proposed metabolite | Fragment ions in negative (−) or in positive (+) mode | |
|---|---|---|---|---|---|---|---|
| Exp m/z Calc m/z | |||||||
| 1a | 0.61 | 209.0302 a | 209.0303 a | C6H10O8 | D-Glucarate | (−) 71/85/133/191 | |
| 1b | 0.61 | 355.0514 a | 355.0518 a | C11H16O13 | Unknown glycan | (−) 59/73/87/99/115/275/337 | |
| 1c | 0.62 | 204.9989 a | 204.9992 a | C6H6O8 | Oxalomalic acid | (−) 71/99/115/143/161 | |
| 1d | 0.62 | 179.0561a | 179.0564 a | 203.0528 d | C6H12O6 | D-Glucose | (−) 59/71/89/113 |
| 1e | 0.63 | 195.0509 a | 195.0510 a | 219.0470 d | C6H12O7 | D-Gluconic acid | (−) 59/75/99/129 |
| 1f | 0.63 | 193.0353 a | 193.0353 a | 217.0320 d | C6H10O7 | D-Glucuronic acid | (−) 59/71/85/99/103/131/175 |
| 1g | 0.64 | 223.0452 a | 223.0459 a | C7H12O8 | Tetrahydroxy 2, 3, 4, 5 heptanedioic acid | (−) 59/71/73/85/103/115/133 /149/205 |
|
| 1h | 0.70 | 369.0671 a | 369.0674 a | 393.0646 d | C12H18O13 | Unknown glycan | (−) 73/99/127/189 |
| 1i | 0.70 | 105.0194 a | 105.0192 a | 129.0181 d | C3H6O4 | Glyceric acid | (−) 45/59/75 |
| 1j | 0.71 | 267.0720 a | 267.0721 a | 291.0692 d | C9H16O9 | Pentahydroxy 2, 3, 4, 6, 7 nonanedioic acid | (−) 59/71/89/113/228/249 |
| 1k | 0.71 | 351.0566 a | 351.0568 a | 353.0712 c | C12H16O12 | 4-(4-Deoxy-beta-D-gluc-4-enuronosyl)-galacturonic acid | (−) 59/71/83/99/143/171/189 |
| 1l | 0.74 | 189.0040 a | 189.0043 a | 191.0184 c | C6H6O7 | Oxalosuccinic cid | (−) 73/83/99/127/171 |
| 1m | 0.85 | 133.0143 a | 133.0142 a | 157.0110 d | C4H6O5 | Malic acid | (−) 71/89/115 |
| 1n | 0.97 | 267.0720 a | 267.0721 a | C9H16O9 | Pentahydroxy 3, 4, 5, 6, 7 nonanedioic acid | (−) 59/71/89/101/119/133/249 | |
| 2 | 1.31 | 191.0197 a | 191.0197 a | 215.0161d | C6H8O7 | Citric acid | (−) 111/173 |
| 3 | 2.36 | 205.0352 a | 205.0353 a | 229.0320 d | C7H10O7 | Methyl citric acid | (−) 71/87/101/125/187 |
| 4 | 3.79 | 153.0193 a | 153.0193 a | C7H6O4 | Dihydroxybenzoic acid | (−) 109 | |
| 5 | 3.91 | 445.1348 a | 445.1351 a | 469.1317 d | C19H26O12 | Hydroxybenzoyl rhamnosylglucose | (−) 59/93/137/289/307/417 |
| 6 | 4.15 | 461.1299 a | 461.1300 a | 463.1439 c 485.1256 d | C19H26O13 | Dihydroxybenzoyl rhamnosylglucose | (−) 109/152 |
| 7 | 4.31 | 461.1662 a | 461.1663 a | 463.1827 c 485.1640 d | C20H30O12 | Verbasoside | (−) 123/153/307 |
| 8 | 4.59 | 387.0930 a | 387.0932 a | 389.1080 c 411.0896 d | C16H20O11 | Hydroxybenzoyl pentahydroxy 2, 3, 4, 6, 7 nonanedioic acid | (−) 59/93/113/137 /211/231/249/267 |
| 9 | 4.90 | 417.1035 a | 417.1038 a | 419.1197 c | C17H22O12 | Methoxyhydroxybenzoyl pentahydroxy 2, 3, 4, 6, 7 nonanedioic acid | (−) 59/71/85/113/ 123/167/249/267 |
| 10 | 5.17 | 491.1768 a 537.1822 b | 491.179 a 537.1824 b |
493.1923 c 515.1739 d | C22H34O15 | Antiarol rutinoside | (−) 89/101/125/153/163/247/307 |
| 11 | 5.75 | 371.0980 a | 371.0983 a | C16H20O10 | Benzoyl pentahydroxy 2, 3, 4, 6, 7 nonanedioic acid | (−) 59/71/85/113/121/231/249 | |
| 12 | 6.13 | 577.1560 a | 577.1562 a | 579.1714 c | C27H30O14 | Vitexine O-rhamnoside | (−) 293/413/457 (+) 283/313/415/433 |
| 13 | 6.48 | 879.1988 a | 879.1989 a | 881.2120 c | C42H40O21 | Morelloflavone diglucoside | (−) 125/151/403/429/565/717 (+) 241/327/403/431/557/719 |
| 14 | 6.90 | 477.2335 a 523.2393 b |
477.2341 a 523.2396 b |
C23H40O13 | Dimethoxyhydroxyphenyl rhamnosylglucopyranoside | (-) 59/71/101/161/301/331 | |
| 15 | 7.36 | 717.1460 a | 717.1461 a | 719.1614 c | C36H30O16 | Fukugiside | (-) 125/151/309/403/429/565/ 591 (+) 241/327/403/431/557 |
| 16 | 8.11 | 701.1511 a | 701.1511 a | 703.1665 c | C36H30O15 | Spicataside | (-) 125/151/385/387/413/539 (+) 241/311/387/415/541 |
| 17 | 8.88 | 555.0928 a | 555.0931 a | 557.1082 c | C30H20O11 | Morelloflavone | (-) 125/151/295/401/403/429 (+) 241/327/403/431 |
| 18 | 9.55 | 539.0981 a | 539.0982 a | 541.1136 c | C30H20O10 | Volkensiflavone | (-) 107/125/151/385/387/413 (+) 241/311/387/389/415 |
| Extracts | Free radical scavenging activity - IC50 (µg/mL ± SDa) |
|---|---|
| Ascorbic acid | 6.2 ± 1.2 |
| DPBR | 23.5 ± 2.1 |
| DPBD | 8.1 ± 0.6 |
| DPBY | 11.0 ± 2.0 |
| p—value | 0.005 |
| R2 | 0.7838 |
| Extracts | Concentration (mg/mL) | % relaxation ± SDa |
|---|---|---|
| DPBR | 1 | 32 ± 5 |
| 2 | 68 ± 1.7 | |
| 4 | 100 ± 0 | |
| Loperamide | 4 | 55 ± 4 |
| Treatment | Rats with diarrhea/ group |
Protection (%) |
Number dried stools | Number wet stools | % Inhibi-tion of diarrhea | WSW (g) ± SDa |
DSW (g) ± SDa |
Humidity (%) ± SDa | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 5/5 | 0 | 3 | 13 | 0 | 2.54 ± 0.9 | 0.6 ± 0.37 | 75.5 ± 13.5 | |
| Loperamide (5 mg/kg) |
3/5 | 40 | 11 | 7 | 46.2 | 2.20 ± 0.9 | 0.96 ± 0.27 | 48.4 ± 13.5 α: ** | |
| DPBR (250 mg/kg) |
2/5 | 60 | 11 | 2 | 84.6 | 1.43 ± 0.65 | 0.57 ± 0.32 | 54.3 ± 12 α: *; β ns |
|
| DPBR (500 mg/kg) |
0/5 | 100 | 8 | 0 | 100 | 1.32 ± 1.15 | 0.84 ± 0.50 | 39 ± 7.8 α :***; β :ns |
|
| DPBR (1,000 mg/kg) |
3/5 | 40 | 9 | 9 | 30.8 | 4.12 ± 0.9 | 1.65 ± 0.27 | 54.8 ± 10.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





