1. Introduction
In recent years, significant attention has focused on advancing NO
2 sensors by employing metal oxide semiconductors, such as SnO
2, In₂O₃, ZnO, and WO
3, [
1,
2,
3,
4]. TiO₂ nanotube arrays [
5,
6], however, offer several distinct advantages over these materials for NO₂ sensing beyond their large surface-to-volume ratio. Indeed, TiO₂ nanotubes have tunable physical and electronic properties [
7,
8] that allow for more precise control over a sensor's sensitivity through modifications in the TiO
2 nanotubes’ crystallinity, diameter, length, and wall thickness. Furthermore, TiO₂ nanotubes show excellent electron mobility, which can improve sensor response times and overall sensitivity when compared to other metal oxides. Such properties make TiO₂ nanotubes very promising for various applications in a number of fields. They also have enormous potential for development compared with other nanostructure forms in fields like photocatalysis [
8] and energy storage [
9]. Moreover, TiO
2 nanotubes exhibit outstanding sensitivity and selectivity for many distinct gases, including H
2 [
10,
11], NO
2 [
12], NO
x [
13], CO [
14], NH
3 [
15], and H
2S [
16,
17], as well as Volatile Organic Compounds (VOCs). Various techniques have been applied to fabricate TiO
2 nanotubes [
18], such as electrochemical anodization [
19,
20,
21]. The production of TiO
2 nanotubes on a titanium sheet through anodization is the best process for yielding highly ordered and organized nanostructures. Toxic gases like CO, SO
2, H
2S, NO
x, and so on are harmful to human life and the environment, so there is a need for gas detectors to detect and subsequently control leaks of these unsafe gases. Extending the lifetime of sensors based on TiO
2 also provides more opportunities for developing new high-quality sensors.
To expand the use of titania nanostructures in fabricating gas sensors, some physical parameters need to be improved, namely the sensing signal, the response, and the recovery times. Several nanostructures have been used to fabricate gas detectors, one of them being vertical TiO
2 nanotube arrays prepared using electrochemical anodization, which have numerous oxygen vacancies that provide effective gas diffusion and more asset sites. They are considered an ideal platform for gas sensing due to their fast response, high sensitivity, low cost, and long-term stability [
22].
In this present study, a TiO2 nanotube array was used for NO2 gas sensing. A simple electrochemical anodization on a titanium sheet yielded layers of self-organized TiO2 nanotubes. X-ray diffraction (XRD) and other analytical methods were then used to characterize the properties of the nanomaterials. The morphology and microstructure of these layers were then determined using a scanning electron microscope (SEM) and transmission electron microscope (TEM), respectively.
Outdoor pollutants like NO and NO
2 can result in serious human harm when their concentration exceeds certain exposure limits. Above regulatory limits, these gases increase the likelihood of cardiovascular, respiratory, and cancerous diseases. Gas sensors are therefore vital for detecting dangerous pollutant levels, so developing gas monitoring systems that can sensitively and selectively track these pollutants is a priority [
23,
24,
25,
26].
This study also focuses on the sensing behavior of TiO2 nanotube array sensors toward NO2 through a homemade gas-detection cell. The aim was to develop a sensitive NO2 sensor for low-concentration detection to assure the safety, health, and wellbeing of people by limiting the presence of NO2 in the air. The results of this study will help inform future research based on using TiO2 nanotubes for gas detection, as well as other potential applications. The synthesis process for the TiO2 nanotubes in this work is simpler and less costly than that of other methods like metal oxide elaboration. Thus, a gas sensor based on TiO2 nanotubes is more suitable for daily use.
2. Preparation of the Titania Nanotubes
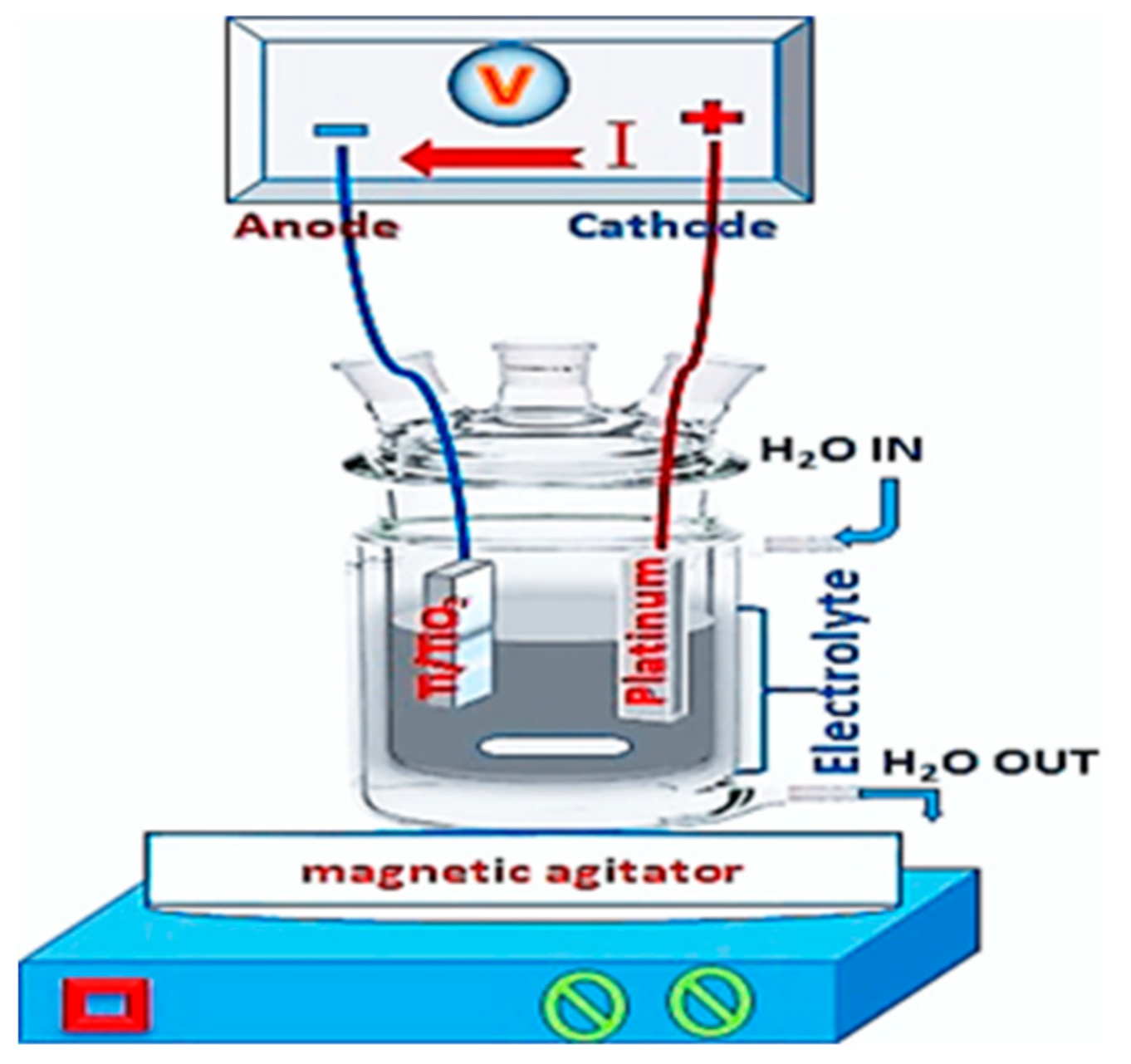
To fabricate a TiO
2 nanotube layer, we took a 1-mm-thick titanium metal sheet with 99.7% purity and subjected it to electrochemical anodization in an electrochemical unit. This process, as presented in
Figure 1, is a relatively simple and efficient way to fabricate well-aligned and highly ordered TiO
2 tubular structures. It involves two electrodes, a working electrode in titanium and the second counter electrode in platinum. The electrolyte solution in which the two electrodes are immersed is a mixture of 100 mL of ethylene glycol (EG), 1% ammonium fluoride (NH
4F), and 2% ultrapure water, as presented in
Figure 1.
The TiO2 nanotubes are prepared in two steps, the first step involves polishing titanium at 120 V for 45 min., while the second step consists of preparing the TiO2 nanotubes at 60V for 15, 30, and 60 min. for the samples used in this study. Only porous structures were prepared on the sample surface at a lower anodization voltage, but when the anodization voltage was higher, the tubes started to form on the titanium metal surface. The applications of TiO2 nanotubes are closely related to their electrical, chemical, and optical properties. The obtained anodized TiO2 nanotubes are typically amorphous, and the conductivity of native TiO2 is very low, thus hampering applications like gas sensing. In this study, we focused on modifying the TiO2 nanotubes to improve their electrical, chemical, and optical properties through thermal treatment. As such, the TiO₂ nanotubes were annealed at 400 °C for 3 hours in air to induce a phase transition from the amorphous structure to a crystalline anatase phase. X-ray diffraction scanning electron microscopy and transmission electron microscopy were then used to examine the nanotube array samples.
3. Characterization
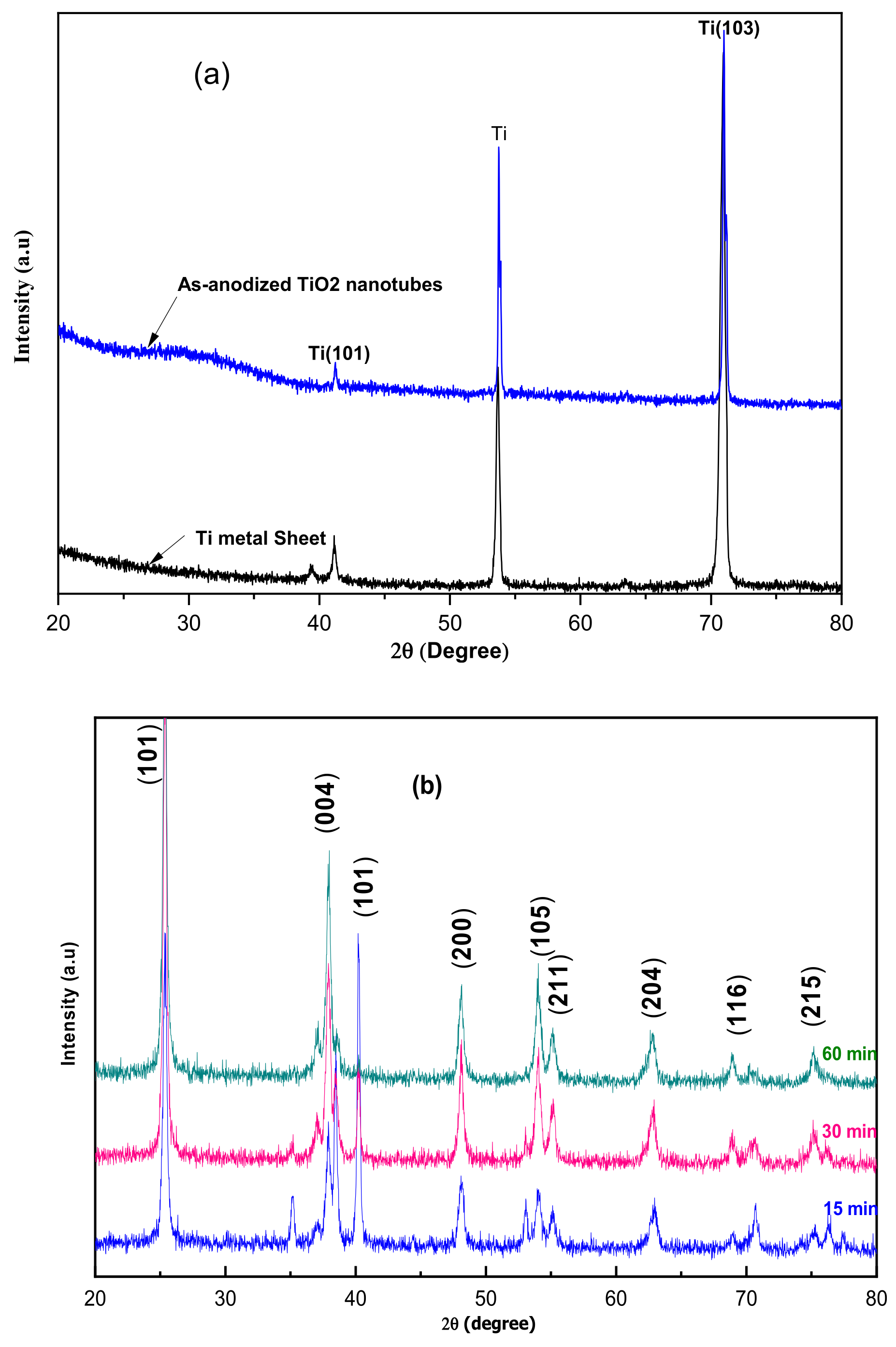
Once the TiO2 nanotubes were prepared through anodization on metallic titania, we crystallized them through thermal annealing at 400 °C for three hours in the air. The crystal structure of the produced TiO2 nanotubes was obtained through X-ray diffraction (XRD). The resulting crystal phases possessed better properties than amorphous TiO2.
Claire modifications were observed on the TiO
2 nanotubes before and after the heat treatment, as shown in
Figure 2a,b. Definite XRD peaks at 25.3°, 37.7°, 47.7°, 53.8°, 54.9°, 62.5°, 68.6, 70.5°, and 74.9° can be seen in
Figure 2, these correspond to the (101), (004), (200), (105), (211), (204), (116), (220), and (215) diffraction peaks of anatase TiO
2 (JCPDS anatase card #21-1272), thus justifying the crystallization of the TiO
2 layer through the formation of the anatase phase.
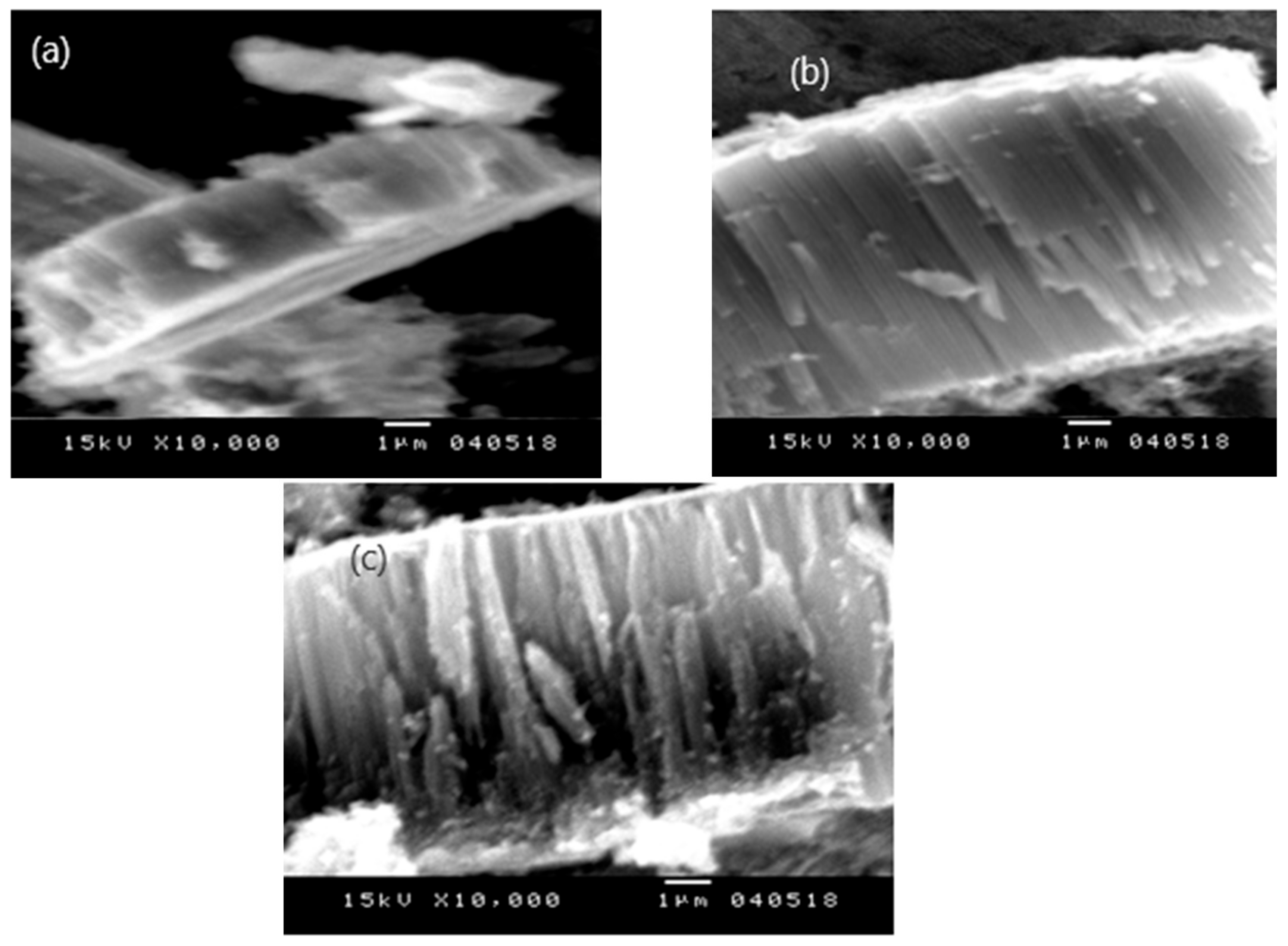
Figure 3 shows SEM images of the pure TiO
2 nanotubes formed at 15, 30, and 60 min. and annealed at 400 °C for 3 hours in air, and these show a highly ordered and organized nanostructure layer on the Ti substrate. Images of the cross-sectional SEM morphology indicate the elaboration of ordered and vertical titanium nanotubes, with the tube length varying at 3, 7, and 9 µm for 15, 30, and 60 min. anodization times, respectively. With a short anodization time, the nanotubes are short because the pore formation process has just started and the walls of the nanotubes are relatively thick. As the anodization time increases, the length of the nanotubes grows substantially. The electric field drives the oxidation of the metal at the bottom of the nanotube, while the electrolyte dissolves the oxide at the top. This process allows for the vertical growth of the nanotubes and continued etching of the walls of the tubes, causing them to thin out and the pore diameter to widen. At extremely long anodization times, the tube length reaches a saturation point where growth stops or becomes negligible and leads to minimal changes in diameter compared to the growth in length.
Transmission electron microscopy (TEM) was used to examine the morphology and uniformity of the TiO
2 nanotubes. Thus, a Philips CM30 transmission electron microscope was used to obtain accurate information about the morphology and size distribution of the pure TiO
2 nanotubes.
Figure 4 shows the sample images obtained from the transmission electron microscope, thus confirming the nanomaterial structure of the TiO
2. The TEM images (Figure 6) reveal that the followed methodology results in the fabrication and growth of highly ordered, structured nanotubes with an average inner diameter of about 100nm and a thickness in the nanotube walls of about 25nm.
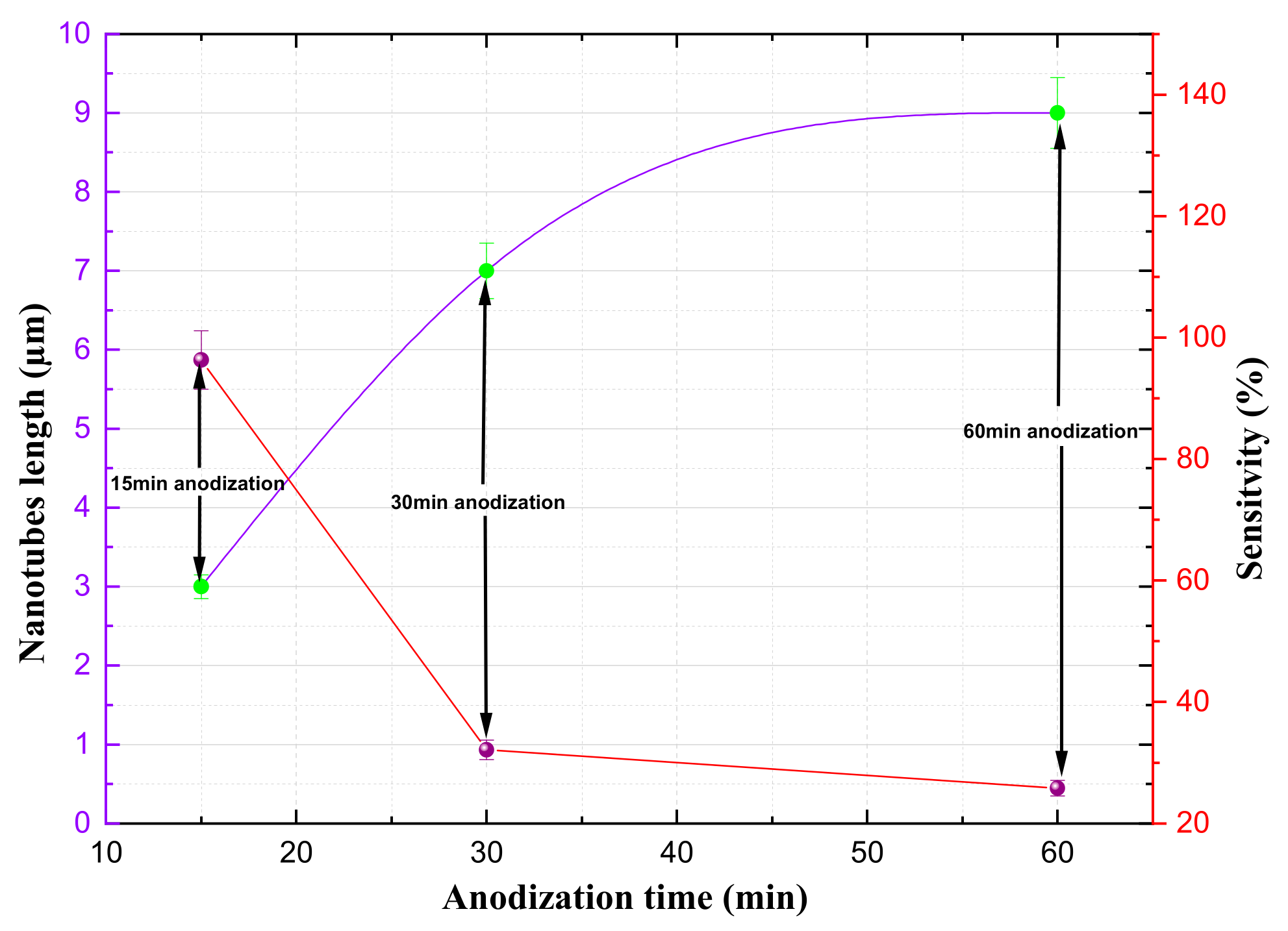
The impacts of the anodization time and nanotube length on the gas sensor’s sensitivity are shown in
Figure 5. From this, we can see that the shorter anodization cycles and shorter nanotube lengths result in enhanced sensitivity. For instance, a sensor with 3 µm nanotubes anodized for 15 minutes exhibited a sensitivity of 99.62%. On the other hand, a sensor with a longer anodization time of 60 minutes had a sensitivity of only 24.12%. Thus, this study revealed that the length of a titanium nanotube is a crucial factor affecting the sensitivity of gas sensors in that shorter nanotubes are typically associated with improved sensitivity. In summary, the various characteristics of shorter titanium nanotubes—such as their increased surface area, low mass loading, and reduced diffusion path—contribute to their enhanced sensitivity and recovery potential.
4. The Fabrication and Performance of the Sensing Device
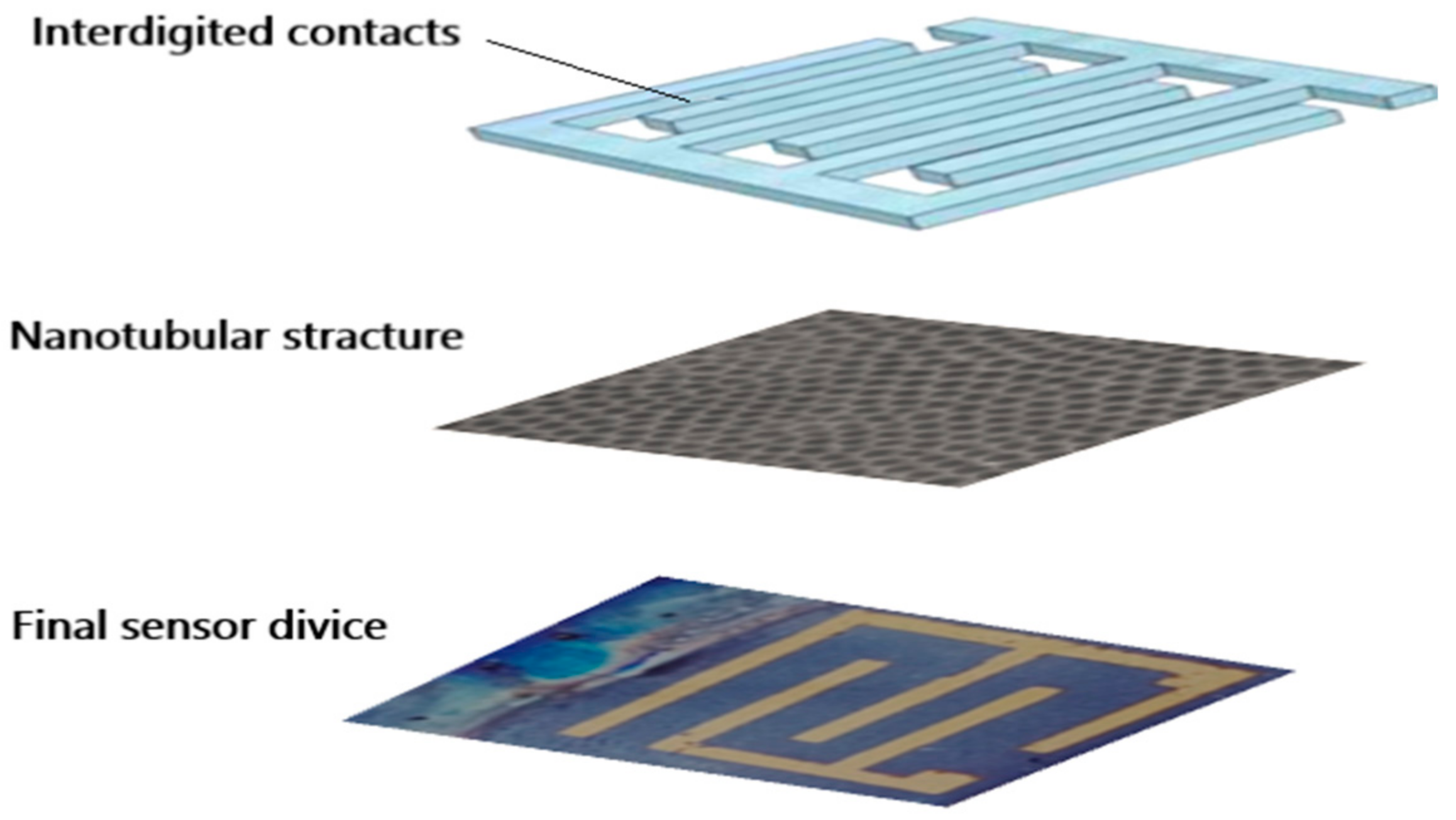
After preparing the titanium dioxide nanotubes with the anodization method, their integration into an NO2 gas-sensing device was needed to ensure their effectiveness. For this purpose, front metal contacts were needed to measure electrical properties, so the choice and design of the front grid’s contacts would affect the response of the final device in practical applications.
Figure 6 illustrates the design of the transducer on the TiO
2 substrate with a tubular structure, and it shows the metallic electrodes evaporated on the front side of the nanotubular array substrate.
Additionally, a heater was integrated onto the back of the device in order to reach and maintain the desired operating temperature on the titania nanotubes' surface.
NO
2 and TiO
2 nanotubes have numerous mechanisms that affect their electrical conductivity. NO
2 can adsorb on a TiO2 surface through either physical or chemical means. The former is triggered by the Van der Waals effect, while the latter is achieved by the formation of bonds between the two. NO
2 is a strong oxidant, meaning it readily grabs electrons from other substances. When NO
2 comes into contact with a TiO
2 surface, it snatches electrons away from the nanotube. This process is expressed in Equation (1):
At a low temperature, a reduction in conductivity is caused by the adsorption of NO2. This phenomenon is accompanied by an increase in the transfer of electrons, but the elevated temperatures caused by the chemical reaction can speed up the process and increase the likelihood of NO2 molecules separating from the TiO2 nanotubes, thus reducing the impact of the NO2 on the nanotubes' conductivity.
Temperature can have a significant impact on how certain sensors function. In addition to the NO2 molecules' attachment and detachment, it can also influence the chemical reactions occurring on the nanotube surface.
To validate the response of a TiO2 nanotube device toward NO2 gas, a homemade gas-detection cell for NO2 gas sensing was made using the TiO2 nanotube material.
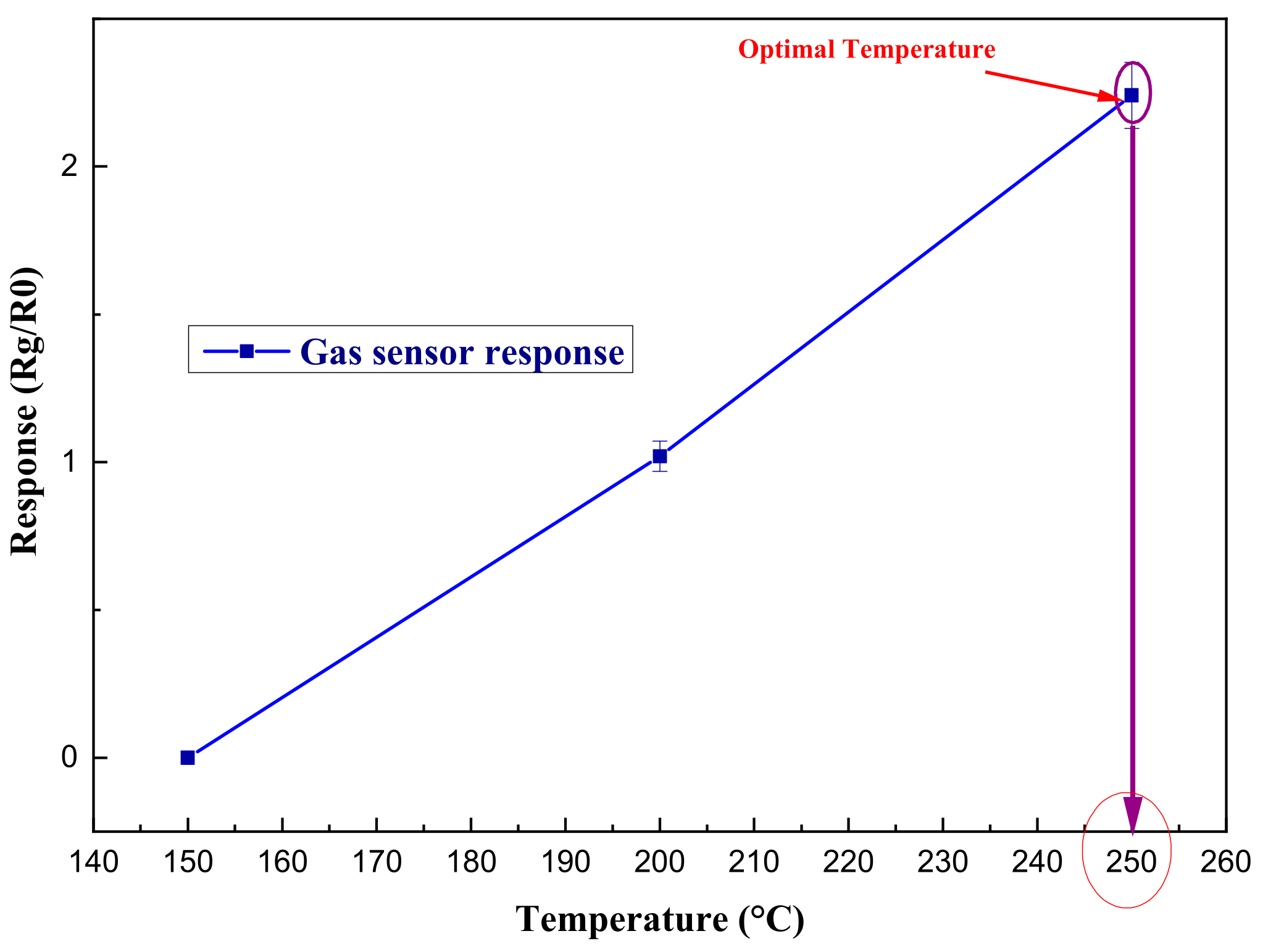
The optimization of the working temperature is illustrated in
Figure 7, such that the experiment revealed that the sensor showed the best response at 250°C.
Nevertheless, to expand the applicability of titania nanostructures as gas sensors, several parameters need to be improved, namely the conductance of TiO2 in air, the sensing signal, the response, and the recovery times [
27]. In this case, the dynamic response curve of the sample was measured in a range of 100 ppm.
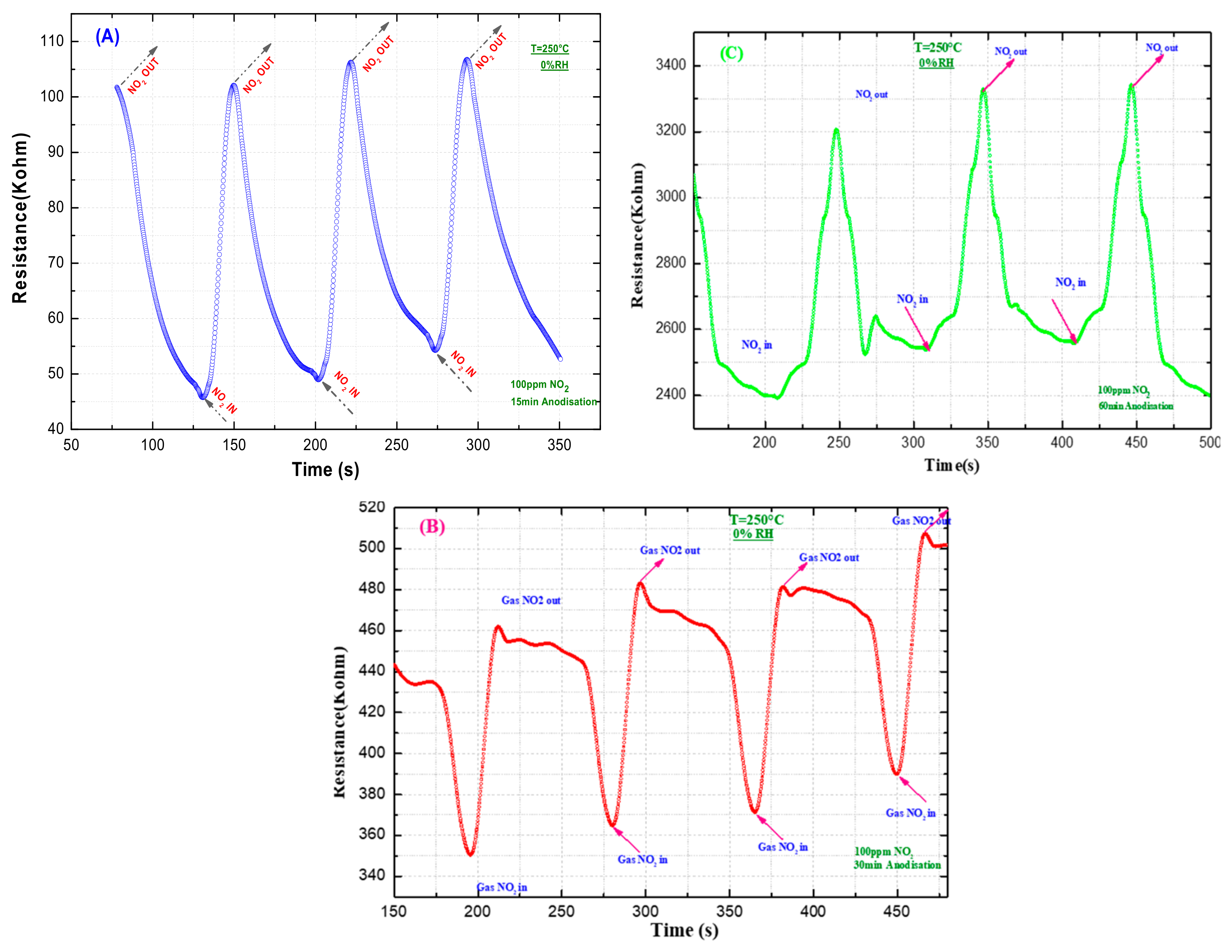
Figure 8 shows the gas-sensing measurements of TiO
2 nanotubes within a 100 ppm NO
2 gas atmosphere at 250°C.
The gas-sensing response (
R) is defined as the ratio of the resistance values of the sensor to the detected gas to the resistance in air, as follows:
Meanwhile, the gas sensitivity of the p-type sensor under NO
2 oxidizing gas is calculated through the following equation, with more details being available in Refs. [
28,
29].
Where Ra represents the resistance of the film when exposed to air, and Rg denotes the resistance of the film when exposed to the analyte gas. The response time (τr) is the time for the resistor to change from Ra to Ra+90%*(Rg−Ra). Similarly, the recovery time (τc) is defined as the time required for the resistor to decrease from Rg to Rg−90%*(Rg−Ra).
The resistance response of TiO
2 nanotubes formed through 15, 30, and 60 min. of anodization were measured in 100 ppm NO
2 gas at a 250 °C working temperature. Good sensing performance was observed for the TiO2 nanotubes anodized for 15 min, compared with anodized for 30 and 60 min., as indicated in
Figure 8 and
Table 1. In addition, the long-term stability of the TiO2 sensor (anodized for 15 min) was verified, remaining stable for 40 cycles.
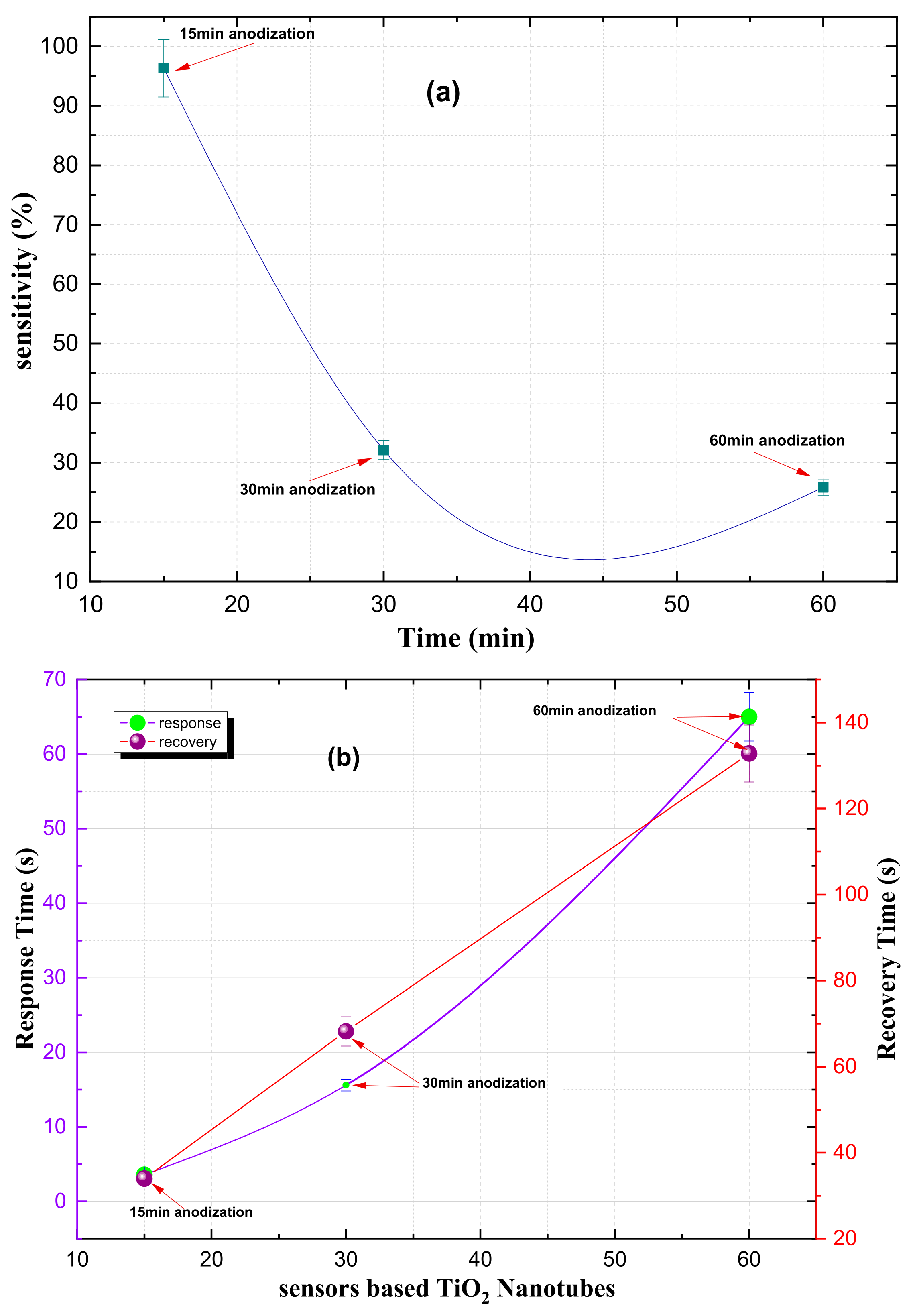
The variation in the response of TiO
2 nanotubes at different anodization times for a 100 ppm NO
2 gas concentration is shown in
Figure 9a. The NO
2 gas response was found to drop from 96% to 32% with an increase in anodization time from 15 to 30 min. before dropping further to 25.8% for a 60 min. anodization time. These lower response values are due to there being insufficient thermal energy to release the electrons from the trap defect levels and participate in the adsorption on the surface of the sensor film. In contrast, at an anodization time of 15 minutes, the maximum NO
2 gas response of 96% is obtained due to there being sufficient thermal energy to release the maximum number of electrons to overcome the trap levels below the conduction band and participate in the gas adsorption phenomenon. The response and response-recovery times of TiO
2 nanotubes operating at different anodization times for a 100 ppm NO
2 gas concentration are summarized in
Table 1 and
Figure 9b. From these, it can be seen how both the response and recovery times increase with an increasing anodization time.
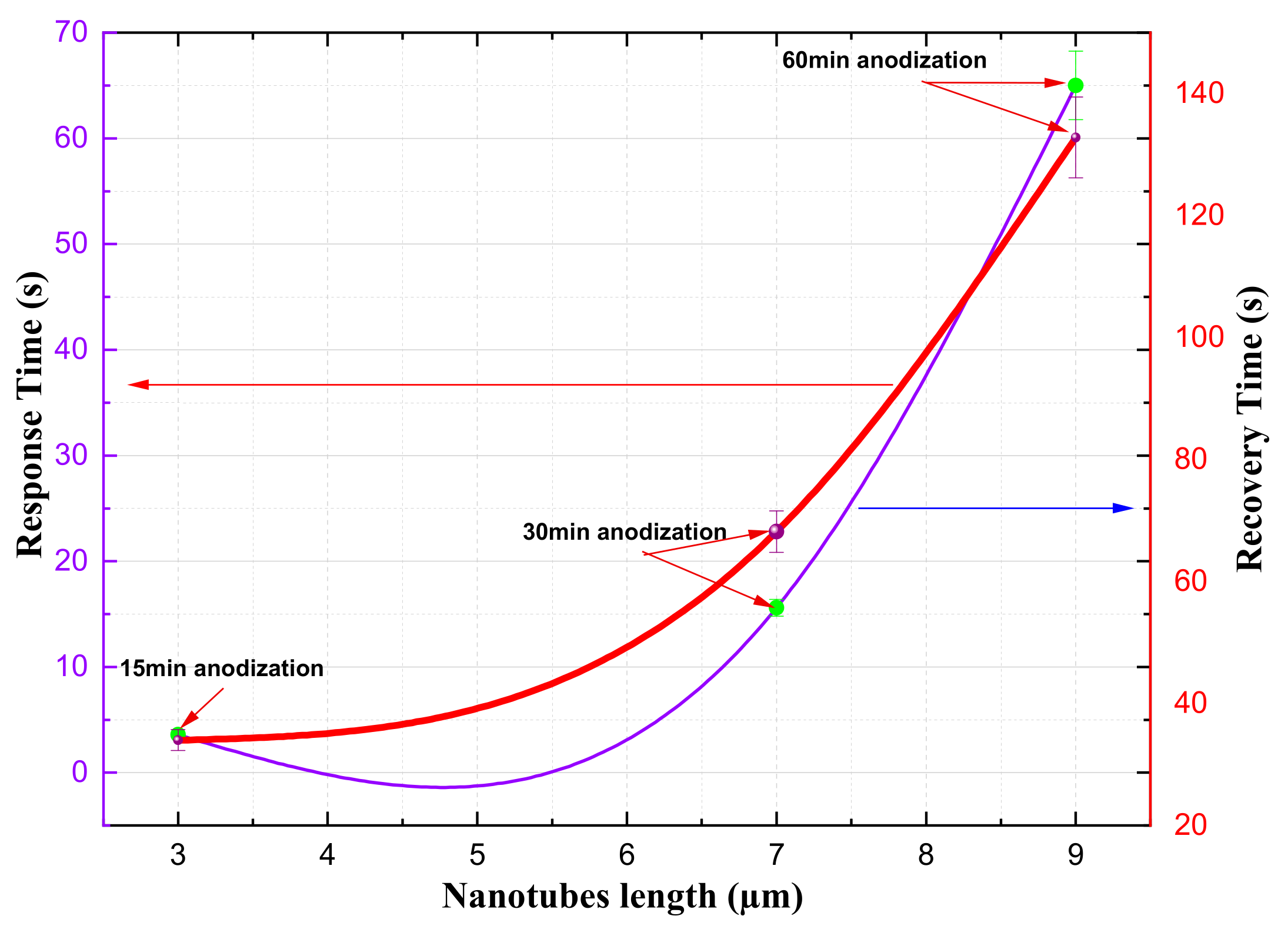
Figure 10 illustrates how the length of the titanium nanotubes affects the response time and recovery time of the gas sensor. It shows that sensors with shorter nanotubes have faster response and recovery times. For example, a sensor with 3-micrometer nanotubes has a response time of only 3.6 seconds, while a sensor with 9-micrometer nanotubes takes 65.5 seconds to respond. Additionally, the time it takes for the sensor to recover between measurements (i.e., the overlap time) also increases with nanotube length. These findings suggest that the length of the titanium nanotubes is a key factor in determining the overall performance of a gas detector.
Compared to long titanium nanotubes, they are therefore better suited for gas sensing. Investigations have also shown that doped and mixed titania structures are emerging as important materials for improving the conductometric properties of sensors [
30,
31,
32,
33].
5. Conclusions
Titania nanotube arrays were synthesized in this study through a simple and relatively inexpensive electrochemical anodization method. XRD, SEM, and TEM characterizations demonstrated that this anodization process results in self-organized arrays of highly ordered vertical nanotubes on the Ti foil. Our research results also showed that the sensitivity of TiO2 nanotubes for NO2 gas detection is in the 24–99.6% range with a response time of 3.6 s at 250° C when exposed to a flow of around 100 ppm NO2 gas. The preparation process is very simple and convenient, and the cost is relatively cheap. These advantages enhance the potential of TiO2 nanotubes as excellent candidates for use in NO2 gas detection. For instance, a sensor with 3-µm-long nanotubes after being anodized for 15 minutes exhibited a sensitivity of 99.62%.
To conclude, TiO2 nanotubes exhibited high stability, good reproducibility, and high sensitivity for sensing NO2, thus positioning them as a promising material for NO2-sensing and other applications.
References
- Öztürk, S.; Kılınç, N.; Öztürk, Z.Z. Fabrication of ZnO nanorods for NO2 sensor applications: effect of dimensions and electrode position. J. Alloys Compd. 2013, 581, 196–201. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Al Wajih, Y.A.; Al-Rammah, R.; Qamar, M.; Yamani, Z.H. Surface-engineered WO3 thin films for efficient NO2 sensing. Applied Surface Science. 2020, 517, 146235. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Guo, J.; Zhang, X.; Xu, Y.; Cheng, X.; Huo, L. Construction of highly efficient In2O3/SnO2 sensor for real-time NO2 monitoring at near room temperature. Chemical Engineering Journal 2024, 498, 155286. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Wu, Q.; Cheng, X.; Wang, Q.; Yang, Y.; Xie, E. SnO2 grains with abundant surface oxygen vacancies for the Ultra-sensitive detection of NO2 at low temperature. Applied Surface Science 2023, 614, 156223. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Cai, Y.; Zhao, J.; Gao, Z.; Song, Y.Y. The challenges and opportunities for TiO2 nanostructures in gas sensing. ACS sensors. 2024, 9, 1644–1655. [Google Scholar] [CrossRef]
- Ragab, A.H.; Al-Mhyawi, S.R.; Kamran, A.W.; Khan, I.; Khan, I. Highly selective sensing of toxic NOx gases for environmental monitoring using Ru-doped single walled TiO2 nanotube: A density functional theory study. Sensors and Actuators A: Physical. 2024, 376, 115632. [Google Scholar] [CrossRef]
- Asadpour, M.; Sadeghi, M.; Bani Asadi Bideshki, A. An Ab-Initio Study on Mechanical Properties of Titanium Dioxide Single-Wall Nanotube. Nano. 2023, 18, 2350079. [Google Scholar] [CrossRef]
- Fadlallah, M.M.; Eckern, U. Cation mono-and co-doped anatase TiO2 nanotubes: an ab initio investigation of electronic and optical properties. physica status solidi (b). 2023, 257, 1900217. [Google Scholar] [CrossRef]
- Qamar, M.; Yoon, C.R.; Oh, H.J.; Lee, N.H.; Park, K.; Kim, D.H.; Kim, S.J. Preparation and photocatalytic activity of nanotubes obtained from titanium dioxide. Catalysis Today. 2008, 131, 3–14. [Google Scholar] [CrossRef]
- Kim, J.H.; Zhu, K.; Kim, J.Y.; Frank, A.J. Tailoring oriented TiO2 nanotube morphology for improved Li storage kinetics. Electrochimica Acta. 2013, 88, 123–128. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A. Synthesis, Characterisation, and Applications of TiO and Other Black Titania Nanostructures Species (Review). Crystals 2024, 14, 647. [Google Scholar] [CrossRef]
- Kusior, A.; Radecka, M.; Zakrzewska, K.; Reszka, A.; Kowalski, B.J. Sensitization of TiO2/SnO2 nanocomposites for gas detection. Sensors and Actuators B: Chemical. 2013, 189, 251–259. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Haidry, A.A.; Saruhan, B. Nanotubular Cr-doped TiO2 for use as high-temperature NO2 gas sensor. Sensors and Actuators B: Chemical. 2015, 217, 78–87. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Li, Q.; Wu, L.; Jiang, D.; Xia, J. Application of TiO2 to amperometric NOx sensors based on NASICON. Solid State Ionics. 2016, 292, 32–37. [Google Scholar] [CrossRef]
- Shwetha, H.R.; Sharath, S.M.; Guruprasad, B.; Rudraswamy, S.B. MEMS based metal oxide semiconductor carbon dioxide gas sensor. Micro and Nano Engineering. 2022, 16, 100156. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Capitán-Vallvey, L.F.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. Chemoresistive NH3 gas sensor at room temperature based on the carbon gel-TiO2 nanocomposites. Sensors and Actuators B: Chemical. 2022, 368, 132103. [Google Scholar] [CrossRef]
- Ma, S.; Jia, J.; Tian, Y.; Cao, L.; Shi, S.; Li, X.; Wang, X. Improved H2S sensing properties of Ag/TiO2 nanofibers. Ceramics International. 2016, 42, 2041–2044. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Xie, L.; Li, X.; Lin, D.; Zhu, Z. Low-temperature and highly sensitivity H2S gas sensor based on ZnO/CuO composite derived from bimetal metal-organic frameworks. Ceramics International. 2020, 46, 15858–15866. [Google Scholar] [CrossRef]
- Arruda, L.B.; Santos, C.M.; Orland, M.O.; Schreiner, W.H.; Lisboa-Filho, P.N. Formation and evolution of TiO2 nanotubes in alkaline synthesis. Ceramics International. 2015, 41, 2884–2891. [Google Scholar] [CrossRef]
- Batool, S.A.; Salman, M.M.; Javed, M.A.; Niaz, A.; Rehman, M.A.U. A review on the fabrication and characterization of titania nanotubes obtained via electrochemical anodization. Surfaces. 2022, 5, 456–480. [Google Scholar] [CrossRef]
- Hailiang, L.; Wang, G.; Niu, J.; et al. Preparation of TiO2 Nanotube arrays with efficient photocatalytic performance and super hydrophilic properties utilizing anodized voltage method. Results Phys. 2019, 14, 0102499. [Google Scholar]
- Galstyan ,V.; Macak, J.M.; Djenizian, T. Anodic TiO2 nanotubes: a promising material for energy conversion and storage. Appl Mater Today 2022, 29, 101613. [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: sensitivity and influencing factors. Sensors. 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Hernandez, A.; Zúñiga-Islas, C.; Mendoza-Cervantes, J.C. A study of the effect of morphology on the optical and electrical properties of TiO2 nanotubes for gas sensing applications. The European Physical Journal Applied Physics. 2020, 90, 30102. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Materials Science. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Kim, W.T.; Kim, I.H.; Choi, W.Y. Fabrication of TiO2 nanotube arrays and their application to a gas sensor. Journal of nanoscience and nanotechnology. 2 0215, 15, 8161–8165. [Google Scholar] [CrossRef]
- Ragab, A.H.; Al-Mhyawi, S.R.; Kamran, A.W.; Khan, I.; Khan, I. Highly selective sensing of toxic NOx gases for environmental monitoring using Ru-doped single walled TiO2 nanotube: A density functional theory study. Sensors and Actuators A: Physical. 2024, 376, 115632. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Materials Science. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Deshmukh, S.B.; Bari, R.H. Nanostructured ZrO2 thin films deposited by spray pyrolysis techniques for ammonia gas sensing application. International Letters of Chemistry, Physics and Astronomy. 2015, 56, 120–130. [Google Scholar] [CrossRef]
- Hyodo, T.; Okusa, T.; Sakata, W.; Ueda, T.; Shimizu, Y. Impacts of Surface Modification of Pt-Sensing Electrodes with Au on Hydrogen-Sensing Properties and Mechanism of Diode-Type Gas Sensors Based on Anodized Titania. ACS sensors. 2022, 8, 61–70. [Google Scholar] [CrossRef]
- Yu, W.; Chen, D.; Li, J.; Zhang, Z. TiO2-SnS2 Nanoheterostructures for High-Performance Humidity Sensor. Crystals 2023, 13, 482. [Google Scholar] [CrossRef]
- Tong, X.; Shen, W.; Zhang, X.; Corriou, J.P.; Xi, H. Synthesis and density functional theory study of free-standing Fe-doped TiO2 nanotube array film for H2S gas sensing properties at low temperature. Journal of Alloys and Compounds. 2020, 832, 155015. [Google Scholar] [CrossRef]
- Zavatski, S.; Neilande, E.; Bandarenka, H.; Popov, A.; Piskunov, S.; Bocharov, D. Density functional theory for doped TiO2: Current research strategies and advancements. Nanotechnology. 2024, 35, 192001. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).