1. Introduction
The diversity of natural products has played and will continue to play an essential role in drug discovery and development [
1,
2]. According to Newman and collaborators, about 75 (41%) of newly approved anti-cancer drugs from 1946 to 2019 were inspired or derived from natural sources [
3]. Natural sources include microorganisms, algae, and plants; the isolated compounds may consist of tannins, stilbenes
, lignans, phenolic acids, and flavonoids. However, flavonoids are among the most abundant and bioactive compounds [
4]. The anti-cancer effects of numerous members of flavonoids have been extensively reviewed; they modulate ROS responses, arrest the cell cycle, induce cell death, and suppress the proliferation and invasiveness of cancer cells [
5,
6]. Moreover, isoflavonoids such as genistein, daidzein, biochanin A, and glycitein have been taken to the stage of clinical trials for the development of new anti-invasive chemotherapeutics [
7].
Brazilin [(6aS, 11bR) -7, 11b-dihydro-6H-indeno [2,1-c] chromene-3, 6a, 9, 10-tetrol] is a natural tetracyclic homoisoflavonoid made of a chroman skeleton fused in
cis with a 2,3-dihydro-1H indene residue [
8]. Brazilin is the most abundant compound in the heartwood of
Caesalpinia sappan L. (Leguminosae) (Southeast Asia) and was also isolated from the ethanolic extracts of the heartwood of
Haematoxylum brasiletto (Fabaceae) [
9].
H. brasiletto, regionally known as "Palo de Brasil", is commonly used in beverages, infusions, or extracts in traditional medicine in the treatment of chronic diseases [
8]. Cytotoxic effects of brazilin (
H. brasiletto) were tested against A549, LS180, HeLa, SiHa, MDA-MB-231, and NCI-H1299 cancer cell lines, showing a reduction in cell viability in all cancer cells [
9]. Previous reports showed that brazilin regulates markers of epithelial-mesenchymal transition by decreasing the expression of vimentin, Twist, and invasion in breast cancer cells [
10]. Despite the growing evidence of the anti-tumoral activities of brazilin, its effects inhibiting breast cancer progression continue to be explored.
Breast cancer is the most diagnosed and the leading cause of death in women worldwide. In 2020, in women aged 45 to 55 years, the incidence of breast cancer was more than two million new cases and mortality over a half million [
11]. Among breast cancer subtypes, triple-negative breast cancer (TNBC) is the most aggressive; patients often develop metastasis and chemoresistance [
12]. From the hallmarks of cancer, metastasis [
13] is the leading cause of death in cancer patients [
14]. Metastasis is a multi-step process in which tumor cells separate from neighboring cells by losing their cell-cell and cell-extracellular matrix (ECM) adhesions; then cells acquire migratory capacity, invade the stroma, and enter the bloodstream; finally, by extravasation, tumor cells grow in a distant organ, forming a secondary tumor [
15]. Cell migration is one of the initial and more essential steps in the metastatic cascade and involves cytoskeleton rearrangements; actin filaments polarize and form protrusions that facilitate cell movement [
16]. Besides, cell-ECM adhesions, e.g., focal adhesions (FAs), are fundamental to giving directionality and strength to the movement. Both, actin cytoskeleton dynamics and FAs, are regulated by the downstream signaling of kinases such as FAK and Src [
17]. Despite the strategies to counteract breast cancer progression with the use of mastectomy, radiation, hormonal, and targeted therapy, chemotherapy is the primary approach for treating TNBC [
18]. However, around 90% of women who have received chemotherapy have shown side effects [
19,
20]. Therefore, new therapeutic agents are needed for the treatment of invasive breast cancer.
Previous evidence suggests that brazilin prevents the migration of PDGF-stimulated vascular smooth muscle cells (VSMC); the authors proposed a mechanism by inhibiting the PDGFR-Src-ERK1/2-Akt signaling axis [
21]. Brazilin in co-treatment with DOXO inhibited cell migration through HER2-overexpressing MCF7 breast cancer cells (MCF7/HER2), possibly through downregulation of MMP2, MMP9, HER2, Rac1, and p120 protein expression [
22]. Furthermore, a recent investigation described the effects of brazilin on the invasion of breast cancer cells
in vitro and
in vivo. They found that brazilin inhibited the proliferation, migration, and invasion of 4T1 and MDA-MB-231 breast cancer cells; also, brazilin-treated mice showed increased survival, fewer metastatic nodules, and smaller tumor volume [
23]. In this regard, we aimed to evaluate the anti-migratory activity in three mammary gland-derived cell lines of brazilin and two semi-synthetic derivatives.
Semi-synthesis is a strategy to perform structural modifications to improve activity and selectivity; it usually consists of the change, addition, or removal of functional groups to the core structure of a compound [
24]. Such chemical modifications change the physicochemical and pharmacological properties of compounds, which can result in improved performance, as described before for cabazitaxel (Jevtana®, Sanofi), which is a C-7 and C-10 methoxylated derivative of docetaxel [
25]. Likewise, halichondrin B (
Halichondria okadai), initially used as a chemotherapeutic for breast cancer, was structurally simplified to eribulin mesylate with better activity and manageable secondary effects [
26,
27]. In the present study, we isolated brazilin from the heartwood of
H. brasiletto, and performed chemo-diversification by semi-synthesis; we obtained brazilin-(OMe)
3 and brazilin-(OAc)
3. We investigated the biological activity of brazilin and its methylated and acetylated derivatives on TNBC MDA-MB-231, ERα (+) MCF7 breast cancer cells, and the non-tumorigenic mammary epithelial MCF10A cell line. We evaluated their effects on cell viability, cell migration, and FAK activation; our data showed differential effects according to the cell lines and the different compounds, finding a greater impact of the three compounds in MDA-MB-231 breast cancer cells.
2. Materials and Methods
2.1 Chemistry general information
All materials and solvents were analytical grade and used as received unless otherwise noted. The reaction progress in the synthesis of all compounds was monitored on Merck aluminum plates pre-coated with silica gel 60 F254 (Merck, KGaA, Dormstadt, Germany). Silica gel column flash chromatography was performed with Silica Flash 60 230-400 mesh (Merck, KGaA, Dormstadt, Germany), and two alternative reagents were used for visualization: (a) iodine and (b) KMnO4 in water.
Proton and carbon nuclear magnetic resonance (1H-NMR and 13C-NMR) spectra were recorded in CD3OD or CDCl3 on a Bruker (500 MHz) instrument using TMS as an internal standard. Chemical shifts (δ) were determined in parts per million (ppm), and coupling constant (J) values are given in hertz (Hz). Standard abbreviations are used for peak multiplicities. Melting points were determined with a MEL-TEMP® 3.0 apparatus and are uncorrected. Optical rotation was measured on a Perkin Elmer 341 polarimeter (Perkin Elmer) at 589 nm, samples were dissolved in MeOH. High-resolution FAB+ mass spectra (HRMS) data of the new derivatives were obtained on a JEOL MStation MS-700 mass spectrometer.
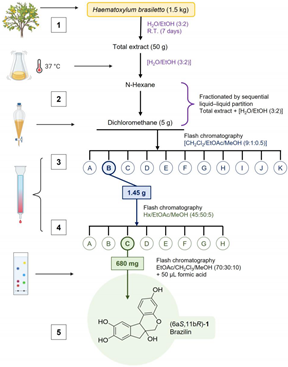
2.2. Compound Brazilin Isolation
Purification of 6a
S,11b
R-1 or (+)-brazilin: (1) The ethanolic extract from the heartwood powder of
H. brasiletto was obtained by maceration with EtOH at room temperature for seven days with regular stirring. The ethanol extracts were evaporated under reduced pressure on a rotary evaporator to yield the total extract. (2) The total extract was resuspendend in 250 mL of an aqueous mixture (3:2 of H
2O/EtOH), and the resulting suspension was fractionated by liquid-liquid separation with n-hexane, dichloromethane (CH
2Cl
2) to produce the corresponding fractions of low and low-media polarity, respectively. Fractions were stored at -4°C in amber glass vials until use. (3) Brazilin was purified on flash chromatography using gradient elution with CH
2Cl
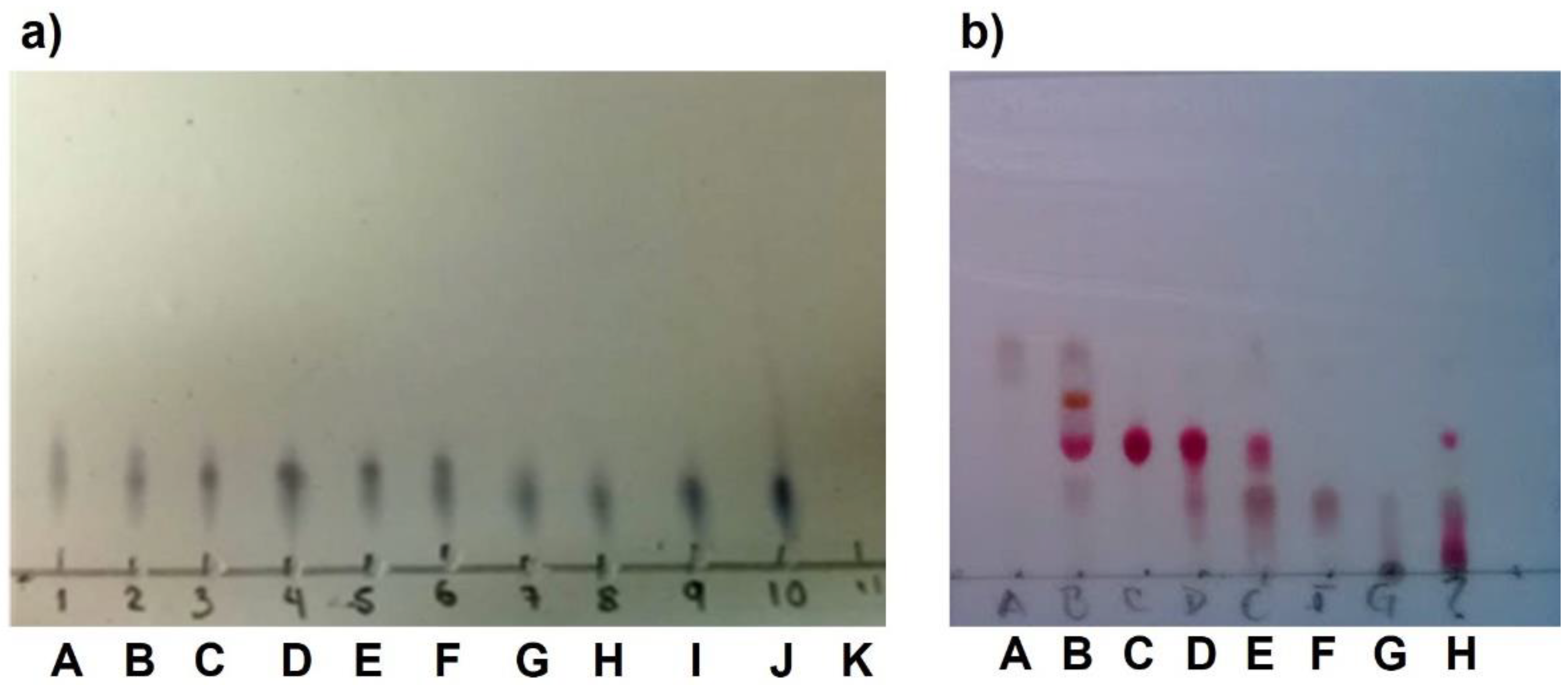
2/EtOAc/MeOH mixtures (9:1:0.5), yielding 11 main fractions (3A-3K) (
Appendix B Figure 11a). (4) Fraction 3B was purified by flash chromatography eluting with n-hexane: EtOAc: MeOH (45:50:5) to yield 8 new fractions (4A-4H) (
Appendix B Figure 11b). Final purification of fraction 4C using EtOAc: CH
2Cl
3: MeOH (70:30:10 + 50 µL formic acid) resulting in the isolation of (5) brazilin. See workflow diagram described in
Appendix A. The
1H and
13C NMR spectroscopic data for brazilin shown in
Figure 1b, supplementary
Figure S1 and described below are similar to previous reports [
28].
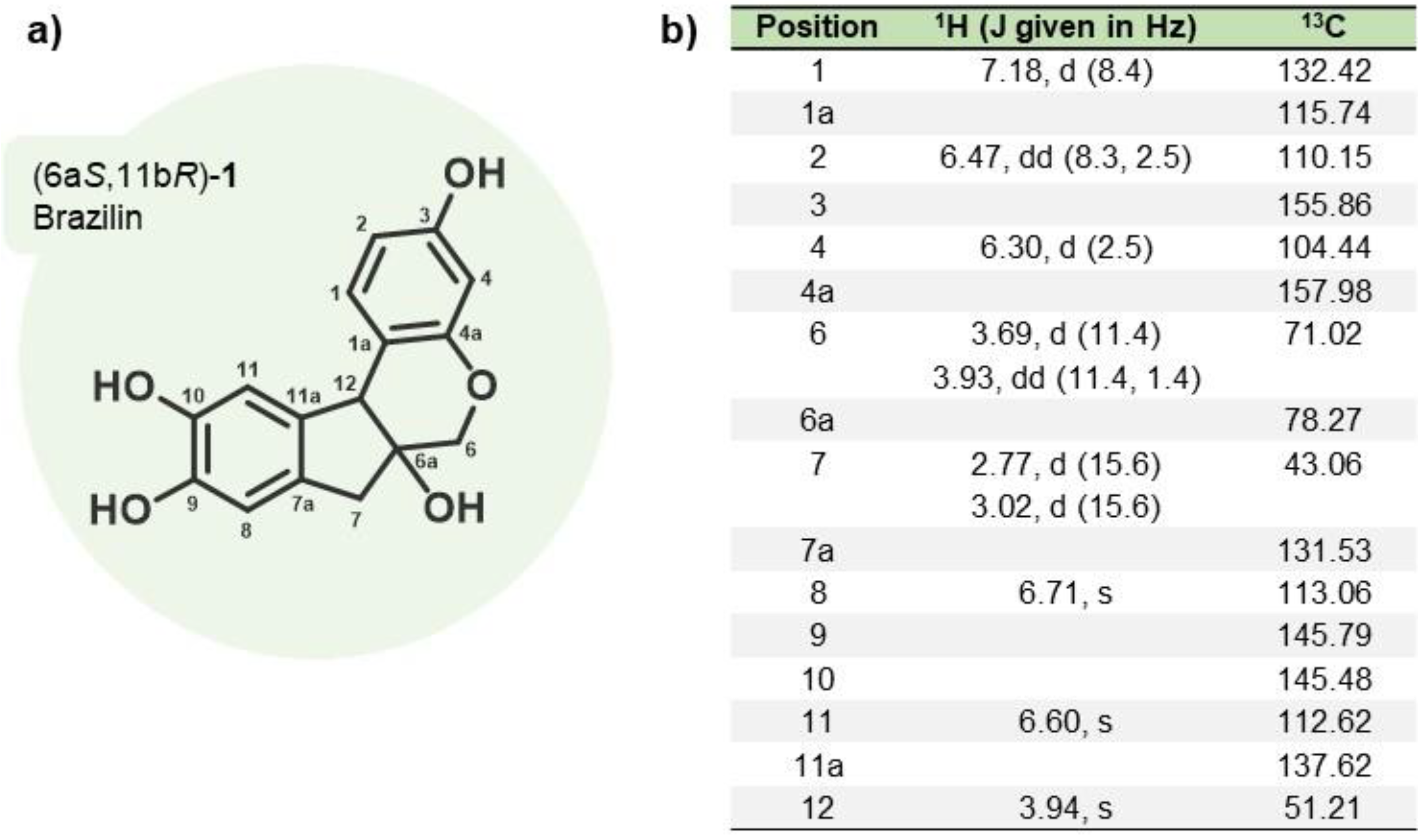
Structural elucidation of brazilin-1 (6aS,11bR)-9,10-dihydroxy-6,6a,7,11b-tetrahydroindeno[2,1-c] chromene-3,6a-diol (+)-Brazilin (C16H14O5 Wt.: 286.08) (purity of 91%).
m.p.: 119-121 °C. +69.2 (c 1.00, MeOH), 1H-NMR (500 MHz, CD3OD): δ = 7.18 (d, J = 8.4 Hz, 1H), 6.71 (s, 1H), 6.60 (s, 1H), 6.47 (dd, J = 8.3, 2.5, Hz, 1H), 6.30 (d, J = 2.5 Hz, 1H), 3.96 (s, 1H), 3.93 (dd, J = 11.3, 1.4 Hz, 1H), 3.69 (d, J = 11.3 Hz, 1H), 3.02 (d, J = 15.6 Hz, 1H), 2.77 (d, J = 15.6 Hz, 1H) ppm. 13C-NMR (125 MHz, CD3OD): δ = 157.98, 155.86, 145.79, 145.48, 137.62, 132.42, 131.53, 115.74, 113.06, 112.62, 110.15, 104.44, 78.27, 71.02, 51.21, 43.06 ppm.
HRMS (FAB+): m/z [M + H]+ Calcd. for C16H15O5: 287.0919; found: 287.0991.
2.3. Semi-Synthesis of Brazilin-(OMe)3 and Brazilin-(OAc)3
3',9',10'-
O-trimethylbrazilin or brazilin-(OMe)
3: (6aS, 11bR)-3,9,10-trimethoxy-7,11b-dihydroindeno [2,1-c] chromene-6a (6H)-ol: A solution of brazilin (40 mg, 0.14 mmol) in acetone (3.5 mL) at room temperature was treated with K
2CO
3 (57.9 mg, 0.4191 mmol) and MeI (99.16 mg, 0.6986 mmol). The reaction mixture was stirred at room temperature for 24h. The crude product was purified by column chromatography 80:20 (EtOAc-Hexane), giving the compound [28.8 mg (52%)] as a yellow oil. For reaction conditions see
Table 4 in
Appendix C.
1H NMR (500 MHz, CDCl
3):
δ =7.28 (d, J = 8.5 Hz, 1H), 6.76 (s, 1H), 6.71 (s, 1H), 6.63 (dd,
J = 8.4, 2.6, Hz, 1H), 6.46 (d,
J = 2.6 Hz, 1H), 4.10 (s, 1H), 4.01 (dd,
J = 11.3, 1.5 Hz, 1H), 3.89 (dd,
J = 13.2, 2.0 Hz, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 3.76 (s, 3H), 3.23 (d,
J = 15.8 Hz, 1H), 2.86 (d,
J = 15.8 Hz, 1H) 2.52 (br s, 1H) ppm.
13C NMR (125 MHz, CDCl
3):
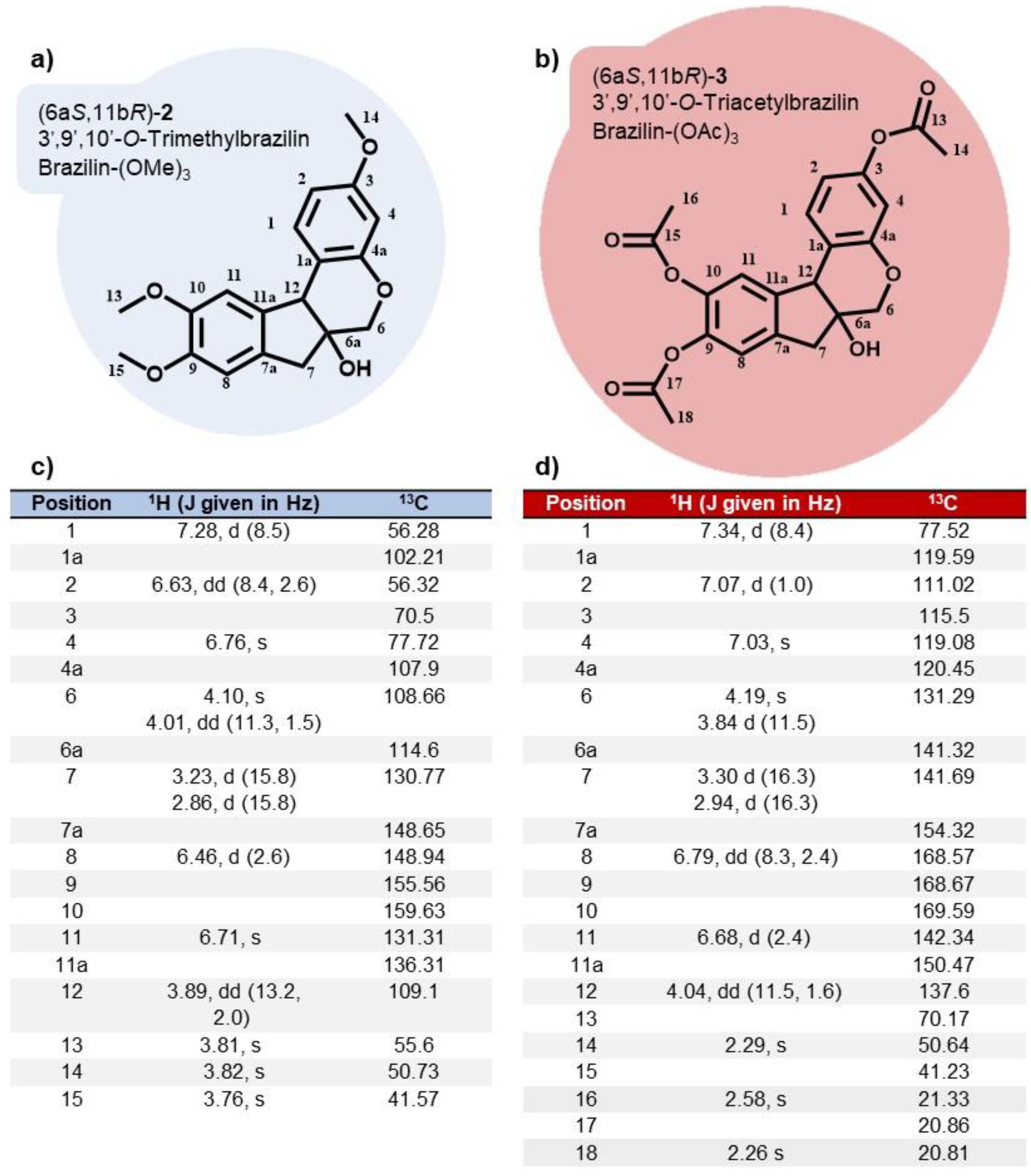
δ = 159.63, 154.59, 148.94, 148.65, 136.31, 131.31, 130.77, 114.60, 109.10, 108.66, 107.90, 102.21, 77.72, 70.50, 56.32, 56.28, 55.56, 50.73, 41.57 ppm.
Supplementary Figure S2.
3',9',10'-O-triacetylbrazilin or brazilin-(OAc)
3: (6aS, 11bR) -6a-hydroxy-6, 6a, 7,11b-tetrahydroindeno [2,1-c] chromene-3,9,10-triyl triacetate: A solution of brazilin (50 mg, 0.17 mmol) in THF (2.5 mL) at room temperature was treated with NaHO
3 (44 mg, 0.5238 mmol) and Ac
2O (89.15 mg, 0.8733 mmol). The reaction mixture was stirred at room temperature for 6 h. The crude product was purified by column chromatography (EtOAc: hexane, 65:50), yielding the compound [24 mg (48%)] as a yellow oil. For reaction conditions see
Table 5 in
Appendix C.
1H NMR (500 MHz, CDCl
3):
δ = 7.34 (d,
J = 8.4 Hz, 1H), 7.07 (d,
J = 1.0 Hz, 1H), 7.03 (s, 1H), 6.79 (dd,
J = 8.3, 2.4, Hz, 1H), 6.68 (d,
J = 2.4 Hz, 1H), 4.19 (s, 1H), 4.04 (dd,
J = 11.5, 1.6 Hz, 1H), 3.84 (d,
J = 11.5 Hz, 1H), 3.30 (d,
J = 16.3 Hz, 1H), 2.94 (d,
J = 16.3 Hz, 1H), 2.58 (br s, 1H), 2.29 (s, 3H), 2.26 (s, 6H) ppm.
13C NMR (125 MHz, CDCl
3):
δ = 169.59, 168.67, 168.57, 154.32, 150.47, 142.34, 141.69, 141.32, 137.60, 131.29, 120.45, 119.59, 119.08, 115.50, 111.02, 77.52, 70.17, 50.64, 41.23, 21.33, 20.86, 20.81 ppm.
Supplementary Figure S3.
2.4. Cell Culture and Treatments
The non-tumorigenic mammary epithelial MCF10A cell line, the ERα(+) MCF7 and TNBC MDA-MB-231 cell lines (ATCC, Manassas, VA, USA) were cultured in DMEM/F12 medium (50:50, V:V; Sigma -Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (penicillin G/streptomycin; Gibco, Waltham, MA, USA) in a humidified atmosphere containing 5% CO2 at 37 °C.
Cells cultures were FBS-starved for 4 h (MCF10A) or 24 h (MDA-MB-231 and MCF7) and rinsed with sterile PBS prior to treatments. Brazilin (1), 3',9',10'-O-Trimethylbrazilin (2) or 3',9',10'-O-Triacetylbrazilin (3) were administered in concentrations from 2.5 µM to 40 µM in DMEM/F12 media for the time indicated in each section.
2.5. Viability Assays
The cells were seeded at 105 in a 96-well plate. After adherence overnight, cells were incubated with the medium alone or with a serial dilution of brazilin, brazilin-(OMe)3 and brazilin-(OMe)3 starting with the highest concentration at 40 μM/mL for 48 h. Doxorubicin and DMSO were used as controls. Then, 20 µL of MTT solution was added to each well and mixed. After 4 h, the supernatants were removed, and 100 µl of the MTT solvent (acidified isopropanol) was added to each well to dissolve the formazan crystals. Cell viability was estimated by measuring absorbance at 630 nm using a Thermo Scientific ™ Multiskan ™ FC microplate photometer. The percentage of cell viability was calculated as a function of the absorbance ratio between the cell culture treated with brazilin and the untreated control multiplied by 100, representing the cell viability (percentage of control).
2.6. Cell Migration Assays
MDA-MB-231, MCF7, and MCF10A cells were grown to confluence in 60 mm culture plates supplemented with complete DMEM/F12. Cells were FBS-starved and treated for 2 h with 10 µM Cytosine β-D-Arabinofuranoside (AraC) to inhibit cell proliferation during the experiment. After starvation, cells were scratched using a sterile 200 μL pipet tip, suspended cells were removed by washing twice with ice-cold sterile PBS, and cultures were re-fed with DMEM/F12 in the absence (control cells or 0 condition) or presence 20 μM or 40 μM. The progress of wound healing was monitored using an EVOS FL microscope with a 10X objective. Cell cultures were photographed immediately after wounding and 48 h after the treatments; five images were analyzed per plate. The migration area was determined by measuring the total wound area using ImageJ 1.44p (NIH, USA) software.
2.7. FAK Activation by Western Blot
After treatments, cells were solubilized in ice-cold lysis buffer/proteases and phosphatases inhibitor cocktail. Total protein (20 ng) was resolved in 10% SDS-PAGE and transferred to nitrocellulose membranes. Unspecific bindings were blocked with 5% non-fat milk solution incubation for 2 h at RT. Membranes were then incubated overnight at 4°C with the primary anti-FAK (44-624G, Invitrogen) (1:1000) total FAK (sc-558, Santa Cruz Biotechnology) (1:1000) and GAPDH (AC027, ABclonal) (1:5000 dilution). Next, the membranes were washed with TBS/0.1% Tween20 and incubated with a secondary HRP-conjugated anti-rabbit antibody (1:5000 dilution) for 2 h at RT. Proteins were detected using Tanon High-sig ECL Western Blotting substrate (180-5001, ABclonal) and autoradiographic films. Images were processed using ImageJ 1.44p software (NIH, Bethesda, MD).
2.8. Statistical Analysis
The results are presented as the mean ± SE. Data were analyzed statistically by one-way ANOVA, and a Dunnett's multiple comparison test was applied afterwards. A statistical probability of P< 0.05 was considered significant.
3. Results
3.1. Brazilin Purification from H. Brasiletto And Semi-Synthesis of Brazilin-(OMe)3 and Brazilin-(OAc)3
In our study, the ground heartwood of
H. brasiletto was used to obtain the homoisoflavonoid brazilin with an efficiency of 0.04% and a purity of 91% (
Table 1). Brazilin was identified by spectroscopic
1H NMR and
13C NMR data (
Figure 1) (
Supplementary Figure S1).
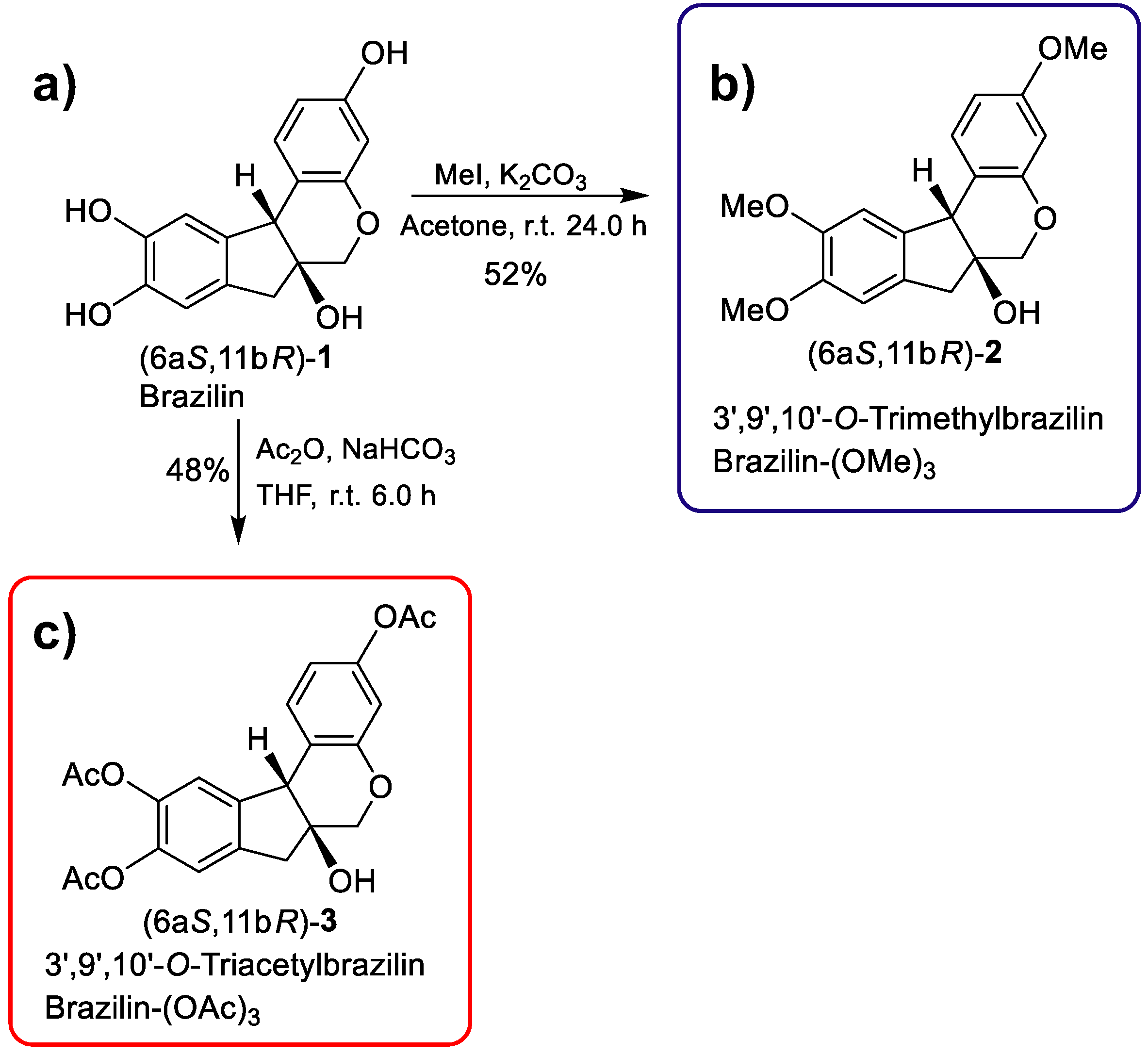
Using brazilin (purified from
H. brasiletto) as the raw material, we performed the semi-synthesis of two derivatives by addition of three methyl or acetyl groups at 3',9', and 10'-OH groups (
Figure 2). Therefore, reaction of brazilin (
Figure 2a) with methyl iodide and potassium carbonate in acetone at 25°C for 24 h generated brazilin-(OMe)
3 with a yield of 52% (
Table 2;
Figure 2b). On the other hand, the reaction of brazilin with acetic anhydride in the presence of sodium bicarbonate in tetrahydrofuran at 25°C for 6 h provided brazilin-(OAc)
3 with 48% yield (
Table 2;
Figure 2c). Brazilin-(OMe)
3 and brazilin-(OAc)
3 were identified by spectroscopic
1H NMR and
13C NMR data (
Figure 3) (
Supplementary Figure S2, S3).
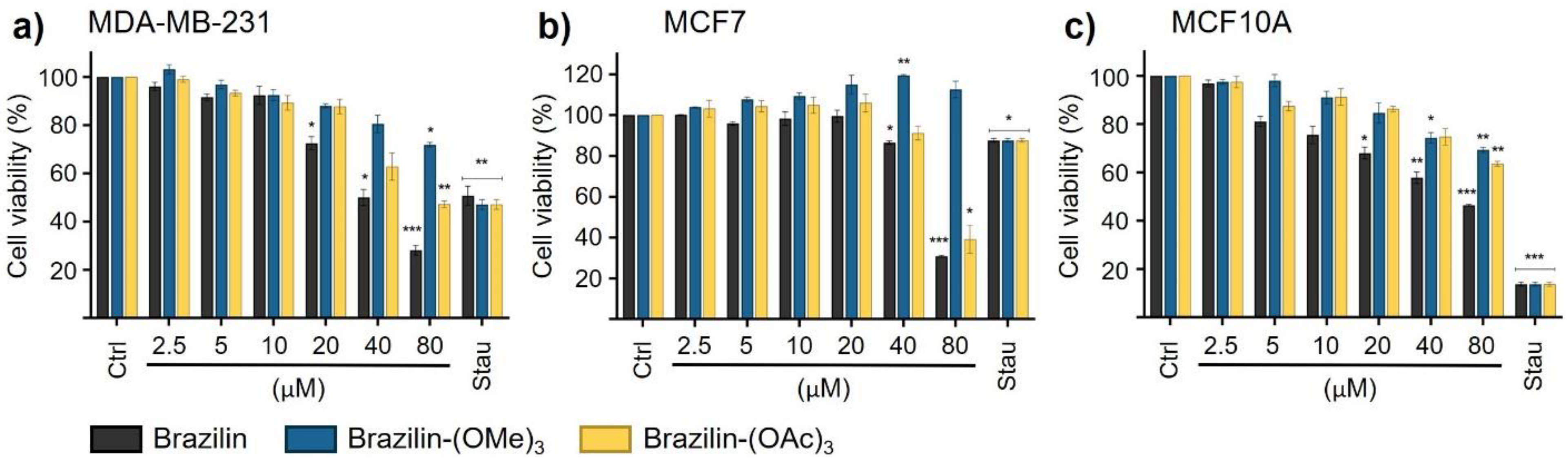
3.2. Brazilin, brazilin-(OMe)3 and brazilin-(OAc)3 effects in cell viability
The impact of brazilin, brazilin-(OMe)
3 and brazilin-(OAc)
3 on cell viability was evaluated in three mammary epithelial cell lines after 48 h. Selectivity was observed in the reduction of viability in cancer cells, particularly in the MDA-MB-231 cell line, where a lower percentage of viability was recorded at concentrations of 20 µM of brazilin and 80 µM of the chemoderivatives (
Table 3,
Figure 4a). In the MCF7 cell line, viability was significantly decreased at concentrations of 40 µM of brazilin and brazilin-(OAc)
3, compared to the control group (
Table 3,
Figure 4b). Furthermore, at 20 µM, brazilin also exerted an effect in decreasing the viability of the MCF10A cell line, while the compounds brazilin-(OMe)
3 and brazilin-(OAc)
3 showed their effect at concentrations higher than 40 µM (
Table 3,
Figure 4c).
3.3. Brazilin, Brazilin-(OMe)3 and Brazilin-(OAc)3 Inhibited Cell Migration of Breast Cancer Cell
Cell migration is an essential process during cancer cell invasion and metastasis and is an important target in cancer therapy [
29]. Once the modified brazilin products were obtained, their biological effect on cell migration of breast cancer cell lines MDA-MB-231 and MCF7, as well as non-tumorigenic breast epithelial cells MCF10A, was evaluated.
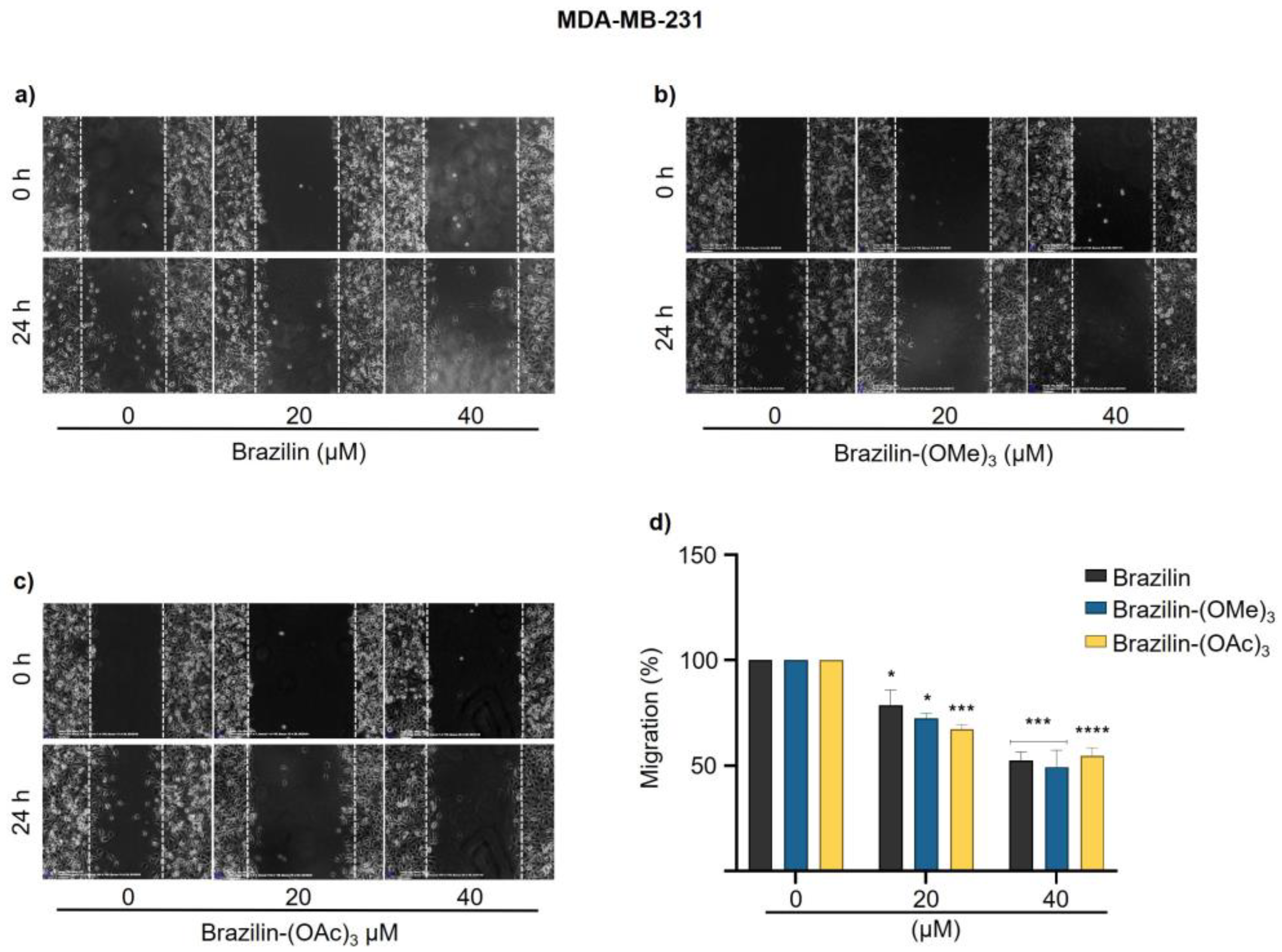
Using wound closure assays, cell migration was evaluated by measuring the wound area of cell cultures treated with different concentrations (0, 20, and 40 µM) of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 for 24 h.
In MDA-MB-231 cells, all three compounds had an inhibitory effect on cell migration in a concentration-dependent manner; as the concentration of the compounds increased, the cells decreased their migratory capacity compared to the control group (
Figure 5d). Brazilin-(OAc)
3 20 µM had a greater effect by decreasing about 30% cell migration, unlike the other two compounds (
Figure 5c); however, at 40 µM, the same effect was observed for all compounds (
Figure 5).
In MCF7 cells, brazilin-(OAc)
3 started to decrease the migration rate by about 50 % from 20 µM concentration (
Figure 6c); however, brazilin-(OMe)
3 had no activity at 20 µM, whereas, at the highest concentration, it was the compound that presented the greatest effect by decreasing 50% of cell migration (
Figure 6b).
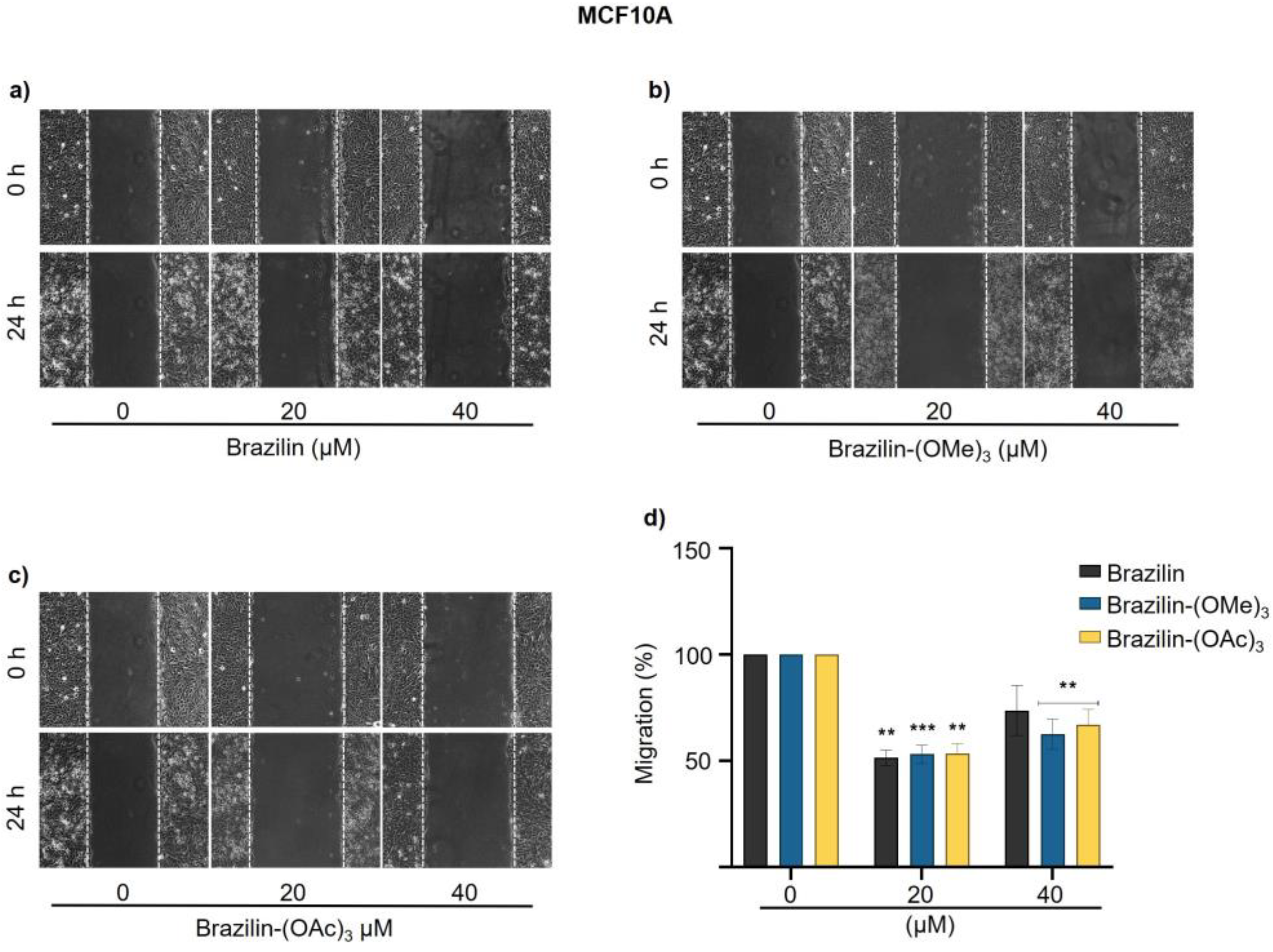
The migration rate of MCF10A cells was considerably lower than breast cancer cells. However, we observed that at 20 µM, the three compounds decreased cell migration by about 50% with respect to the control, while at 40 µM, the effect was reduced approximately 20% of cell migration (
Figure 7).
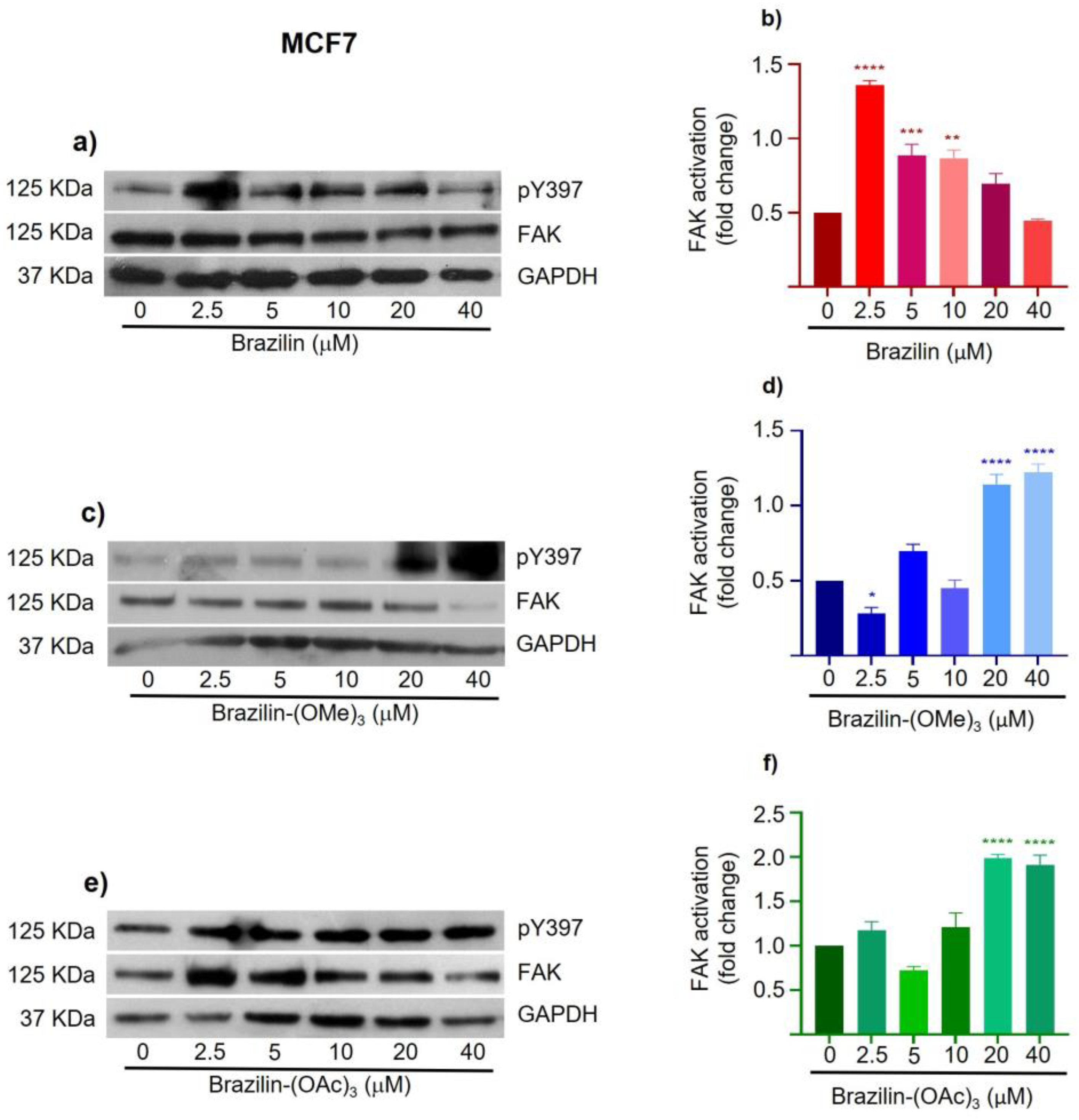
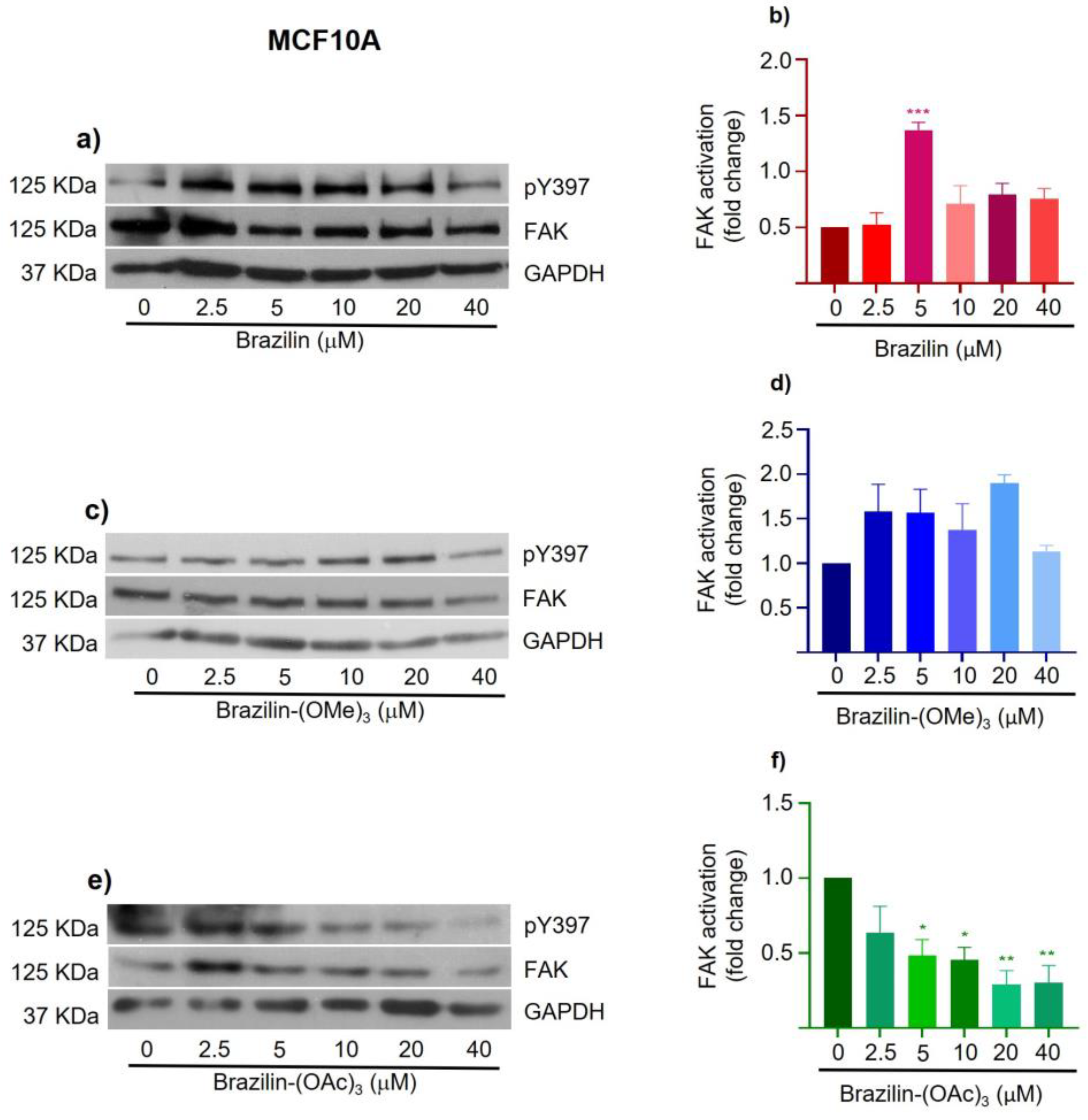
3.4. Brazilin, Brazilin-(OMe)3 and Brazilin-(OAc)3 Inhibited Activation of FAK
Focal adhesion kinase (FAK) is a kinase involved in regulating cell migration in cancer and requires the formation of focal adhesions, a process regulated by adhesion molecules such as integrins and by various intracellular proteins [
30]. FAK regulates the assembly and disassembly of adhesions during cell migration, and its activation is related to the autophosphorylation of the Y397; in addition, its expression is related to tumor progression and the clinical prognosis [
31].
To determine the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation, we performed Western blotting of total proteins obtained from MDA-MB-231, MCF7 and MCF10A cells treated with 2.5, 5, 10, 20 and 40 µM of the different compounds for 24 h.
The results obtained in MDA-MB-231 cells show a differential effect, where brazilin-(OMe)
3 and brazilin-(OAc)
3 increase FAK activation at 2.5 and 5 µM, but decrease considerably from 10 µM, showing a potential effect at 20 µM; however, in brazilin, no changes were observed at low concentrations; interestingly, we observed changes at 20 and 40 µM (
Figure 8).
In MCF7 cells, different effects are observed with the three compounds. Brazilin promotes FAK activation at low concentrations, but at 20 and 40 µM, FAK levels are reestablished with respect to the control (
Figure 9a). While brazilin-(OMe)
3 at 2.5 µM decreases FAK activation; however, as concentration increases, the two modified compounds increase FAK activation compared to the control (
Figure 9d).
The results corresponding to MCF10A cells show that brazilin and brazilin-(OMe)
3 promote FAK activation with respect to the control (
Figure 10a, b). Conversely, brazilin-(OAc)
3 decreases FAK activation concentration-dependent (
Figure 10d).
Our results show a differential effect on the three breast epithelial cell lines and the different compounds. However, all three compounds show a better effect on FAK activation in MDA-MB-231 breast cancer cells.
4. Discussion
Despite the different treatments currently available, breast cancer continues to be one of the main causes of death worldwide, so it is important to continue the search for new therapeutic agents with potential effects against breast cancer [
32,
33]. In this regard, the study of phytochemicals has increased due to their potent antitumor activity and minimal toxicity, with alkaloids, taxanes, and flavonoids the most studied molecules [
34].
Brazilin is an isoflavonoid isolated from
Haematoxilum brasiletto [
35] and
Caesalpinia sappan [
36]. It has been reported brazilin displays a wide variety of antitumor effects on various cancer cell lines, inhibiting cell proliferation and cell cycle progression, decreasing cell migration, and inducing apoptosis [
37,
38]. However, its effects on breast cancer cells are poorly known. Therefore, in this study, we evaluated the effect of brazilin, extracted from the heartwood of
H. brasiletto, and methoxylated and acetoxylated products of brazilin on cell migration and FAK activation in breast cancer cells.
The strategy for structural modification is to increase potency and selectivity, improve physicochemical, biochemical, and pharmacokinetic properties, eliminate, or reduce adverse effects, simplify the structural complexity of the candidate drug. It is described that flavonoids can act as enzyme inhibitors and receptor ligands [
39,
40]. According to our results on cell migration, a greater effect was observed with the modified compounds brazilin-(OMe)
3 and brazilin-(OAc)
3 than with the parent compound (
Figure 5,
Figure 6 and
Figure 7). Biological activity is related to aromatic rings, allowing molecular (non-covalent) interactions with proteins. Managing to develop strong dispersion interactions with non-polar amino acid residues followed by simultaneous release of water; while, in the later type, the H + junction is the most important electrostatic interaction, generating Vander wall interactions or electrostatic interactions [
41].
However, although we observed that brazilin and its derivatives have a potential effect in decreasing breast cancer cell migration, the mechanism of the inhibitory effect has not yet been elucidated; here, we decided to evaluate FAK activation in breast cancer and non-tumorigenic epithelial cell lines.
Focal adhesion kinase (FAK) is a kinase involved in regulating cell migration in cancer and requires the formation of focal adhesions, a process regulated by adhesion molecules such as integrins and by various intracellular proteins [
42]. FAK also regulates the assembly and disassembly of adhesions during cell migration, and its activation is related to the autophosphorylation of the Y397; in addition, its expression is related to tumor progression and the clinical prognosis in cancer [
43].
Interestingly, in our study, a differential effect was found, both in the cell lines and in the concentrations of the treatments, because only at high concentrations of the three compounds, FAK activation decreased by about 50% in MDA-MB-231 cells; remarkably, this result correlates with the results obtained in cell migration. Therefore, specifically in this TNBC breast cancer cell line, brazilin, brazilin-(OMe)
3, and brazilin-(OAc)
3 can decrease cell migration through FAK inhibition. However, at lower concentrations of brazilin-(OMe)
3 and brazilin-(OAc)
3, an increase in FAK activation was observed in MDA-MB-231 cells (
Figure 8). Likewise, in MCF10A cells, FAK activation was also observed at all concentrations of brazilin and brazilin-(OMe)
3 (
Figure 10). These findings may be associated with a hormetic effect, where exposure to a low dose of a cytotoxic agent can induce oxidative stress in cancer cells and thus stimulate tumor proliferation and growth [
44]. In this regard, an increase in FAK activation was also observed in MCF7 cells at most concentrations of the three compounds (
Figure 9). However, particularly in this cell line, this mechanism can be related to their estrogenic positivity because structurally, the isoflavonoids show similarities to estrogens, bind to estrogen receptors with preferential affinity for estrogen receptor beta (ERβ), compete with 17β-estradiol for the ligand-binding domain of the receptor, and exhibit both estrogenic and anti-estrogenic activities [
45,
46]. Interestingly, estrogen, through the ER receptor, has been reported to promote FAK activation in ovarian cancer cells [
47]. However, these results should be studied in depth to establish this relationship.
Furthermore, the chemical modifications to the original structure of brazilin improve the metabolic stability and allow greater absorption and permeability across cell membranes, thus enhancing its potential effect [
48].
Our results indicate that all three compounds significantly affected cell migration and FAK activation in MDA-MB-231 cells in a concentration-dependent manner. The MDA-MB-231 cell line corresponds to the triple-negative breast cancer (TNBC), characterized by the absence of hormone receptors (ER-α/PR) and negativity for HER2, and considered the most aggressive subtype, with early relapse and metastatic dissemination to several organs such as lung, liver, and brain, being responsible for more than 50 % of breast cancer deaths [
49,
50]. In this sense, brazilin and its modified products have potential effects on this cell line, they could be considered as potential new therapeutic agents in the treatment of breast cancer; however, it is important to deepen the investigation of the different mechanisms that brazilin and derivates can be used to reduce tumor progression.
Author Contributions
“Conceptualization, A.H.-M., D.A.N.-T., J.B.-M., and N.N.-T.; methodology, A.H.-M., D.A.N.-T., M.D.Z.-E., M.O.-F., T.H.-M., M.O.; validation, M.D.Z.-E., M.O.-F., T.H.-M., M.O., A.E.Z.-G.; formal analysis, A.H.-M., D.A.N.-T., M.D.Z.-E. J.B.-M., M.O.-F., T.H.-M., A.E.Z.-G., M.A.M.-C., and N.N.-T.; investigation, M.O.-F., T.H.-M., A.E.Z.-G., and M.A.M.-C.; resources, J.B.-M. and N.N.-T.; writing—original draft preparation, A.H.-M., D.A.N.-T., M.D.Z.-E., and N.N.-T.; writing—review and editing, A.H.-M., D.A.N.-T., J.B.-M. and N.N.-T.; visualization, M.A.M.-C.; supervision, M.O., and N.N.-T.; project administration, N.N.-T.; funding acquisition, J.B.-M., M.O., and N.N.-T. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Brazilin isolated from H. brasiletto. a) chemical structure and b) spectroscopic data (1H NMR and 13C NMR) of brazilin.
Figure 1.
Brazilin isolated from H. brasiletto. a) chemical structure and b) spectroscopic data (1H NMR and 13C NMR) of brazilin.
Figure 2.
Semisynthetic route and with the experimental conditions used for the chemodiversification of a) brazilin to b) brazilin-(OMe)3 and c) brazilin-(OAc)3.
Figure 2.
Semisynthetic route and with the experimental conditions used for the chemodiversification of a) brazilin to b) brazilin-(OMe)3 and c) brazilin-(OAc)3.
Figure 3.
Chemoderivatives of brazilin. Chemical structure and spectroscopic data (1H NMR and 13C NMR) of brazilin-(OMe)3 (a, c) and brazilin-(OAc)3 (b,d).
Figure 3.
Chemoderivatives of brazilin. Chemical structure and spectroscopic data (1H NMR and 13C NMR) of brazilin-(OMe)3 (a, c) and brazilin-(OAc)3 (b,d).
Figure 4.
Cell viability of breast cancer cells and MCF10A cells after treatment with brazilin for 24 h. Differences from the control were determined with one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05), **P < 0.01), ***P < 0.001).
Figure 4.
Cell viability of breast cancer cells and MCF10A cells after treatment with brazilin for 24 h. Differences from the control were determined with one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05), **P < 0.01), ***P < 0.001).
Figure 5.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MDA-MB-231 cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation, and treated with 20 µM and 40 µM of a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 for 24 h. d) Graphs represent the quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, ***P < 0.001, ****P < 0.0001. .
Figure 5.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MDA-MB-231 cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation, and treated with 20 µM and 40 µM of a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 for 24 h. d) Graphs represent the quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, ***P < 0.001, ****P < 0.0001. .
Figure 6.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MCF7 cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation, and treated with 20 µM and 40 µM of a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 for 24 h. d) Graphs represent the quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences respect to control were determined by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: **P < 0.01), ***P < 0.001).
Figure 6.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MCF7 cells. Cell cultures were exposed for 2 h with Ara-C to inhibit proliferation, and treated with 20 µM and 40 µM of a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 for 24 h. d) Graphs represent the quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences respect to control were determined by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: **P < 0.01), ***P < 0.001).
Figure 7.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MCF10A cells. Cell cultures were exposed for 2 h with Ara-C to inhibit prolifera-tion, and treated with 20 µM and 40 µM of a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 for 24 h. d) Graphs represent quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: **P < 0.01, ***P < 0.001.
Figure 7.
Wound closure assays of the effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on cell migration of MCF10A cells. Cell cultures were exposed for 2 h with Ara-C to inhibit prolifera-tion, and treated with 20 µM and 40 µM of a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 for 24 h. d) Graphs represent quantification of percent cell migration for each condition. The image is representative of three independent experiments. Differences with respect to control were determined by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: **P < 0.01, ***P < 0.001.
Figure 8.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MDA-MB-231 cells. Cell cultures were treated with the different compounds (2.5 μM - 40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 in MDA-MB-231 cells. d) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, ***P < 0.001, ****P < 0.0001. .
Figure 8.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MDA-MB-231 cells. Cell cultures were treated with the different compounds (2.5 μM - 40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 in MDA-MB-231 cells. d) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, ***P < 0.001, ****P < 0.0001. .
Figure 9.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MCF7 cells. Cell cultures were treated with the different compounds (2.5 μM - 40 μM) for 24 h. Western blot of FAK activation corre-sponding to treatments with a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 in MDA-MB-231 cells. d) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, **P < 0,01, ***P < 0.001, ****P < 0.0001. .
Figure 9.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MCF7 cells. Cell cultures were treated with the different compounds (2.5 μM - 40 μM) for 24 h. Western blot of FAK activation corre-sponding to treatments with a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 in MDA-MB-231 cells. d) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, **P < 0,01, ***P < 0.001, ****P < 0.0001. .
Figure 10.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MCF10A cells. Cell cultures were treated with the different compounds (2.5 μM - 40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 in MDA-MB-231 cells. d) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001. .
Figure 10.
Effect of brazilin, brazilin-(OMe)3, and brazilin-(OAc)3 on FAK activation in MCF10A cells. Cell cultures were treated with the different compounds (2.5 μM - 40 μM) for 24 h. Western blot of FAK activation corresponding to treatments with a) brazilin, b) brazilin-(OMe)3, and c) brazilin-(OAc)3 in MDA-MB-231 cells. d) Graphs corresponding to the densitometric and statistical analysis of the bands obtained by WB. Statistical analysis was performed by one-way ANOVA and Dunnett's multiple comparison test. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001. .
Table 1.
Efficiency and purity of brazilin isolated from H. brasiletto heartwood.
Table 1.
Efficiency and purity of brazilin isolated from H. brasiletto heartwood.
| Raw material (g) |
Yield (g) |
Efficiency (%) |
Purity (%) |
| 1,500 |
0.680 |
0.047 |
91 |
Table 2.
Efficiency of semi-synthesis of brazilin-(OMe)3 and brazilin-(OAc)3
Table 2.
Efficiency of semi-synthesis of brazilin-(OMe)3 and brazilin-(OAc)3
| |
Raw material (mg)
(Brazilin) |
Sample obtained (mg) |
Efficiency (%) |
| Brazilin-(OMe)3
|
40 |
20.8 |
52 |
| Brazilin-(OAc)3
|
50 |
24 |
48 |
Table 3.
*IC50 of brazilin and derivatives in breast cancer and epithelial non-tumorigenic cells.
Table 3.
*IC50 of brazilin and derivatives in breast cancer and epithelial non-tumorigenic cells.
| CELL LINE |
BRAZILIN |
BRAZILIN-(OME)3
|
BRAZILIN-(OAC)3
|
| MDA-MB-231 |
49.92±1.26 |
>80 µM |
70.75±3.48 |
| MCF7 |
67.59±0.40 |
>80 µM |
49.97±3.91 |
| MCF10A |
63.64±3.02 |
>80 µM |
104.09±4.34 |
| |
*CALCULATED BY NON-LINEAR REGRESSION |