1. Introduction
The rising global demand for sustainable and nutritious food sources has positioned pulses, including beans, as key contributors to enhancing food security and dietary quality. Pulses are rich in protein, fiber, and bioactive compounds such as polyphenols and flavonoids, which are known for their antioxidant and anti-inflammatory properties. Thai pulses like mung bean (
Vigna radiata), black bean (
Vigna mungo), red bean (
Phaseolus vulgaris), and soybean (
Glycine max) have garnered attention for their nutritional benefits and potential as functional ingredients in food products [
1,
2]. These beans are particularly valued for their ability to improve the nutritional profile of food products and contribute to food sustainability [
2]. Additionally, the growing interest in functional foods highlights the need to explore variations in the physico-chemical properties and bioactive compounds of pulse flours, which offer health benefits beyond basic nutrition [
3,
4].
Despite the recognized importance of pulses, comprehensive studies on the variations in the physico-chemical properties and bioactive compounds of Thai pulse flours remain limited [
5]. Grown under diverse agro-climatic conditions, Thai pulses hold significant potential as functional ingredients, yet their characteristics are underexplored. This study addresses this gap by performing a comparative evaluation of the physico-chemical properties and bioactive compound variations in flours derived from four key Thai beans. The findings contribute to the development of more nutritious and functional food products, advancing efforts to enhance food system resilience and sustainability.
This study aims to address significant knowledge deficiencies by performing a comparative evaluation of the physico-chemical properties and bioactive compound variations in flours derived from four key Thai beans: mung bean, black bean, red bean, and white bean. The novelty of this research lies in its comprehensive approach, which examines multiple bean varieties under uniform conditions while exploring the effects of different processing methods on their nutritional and functional attributes. By bridging these critical shortcomings, the study aims to contribute to the development of more nutritious and functional food products. Furthermore, it advances the understanding of how Thai pulse flours can be optimized for various food applications, aligning with global efforts to enhance food system resilience and promote the development of sustainable, health-oriented food solutions for international markets.
3. Results
3.1. The Chemical Compositions of the Pulse Flours
The chemical composition of four different pulse flours, including mung bean, red bean, white bean, and black bean, revealed significant variations in protein, fiber, fat, and ash content (
Table 1). Mung bean flour had the highest protein content (25.965±0.065%), significantly higher than red bean (19.630±0.151%), white bean (19.478±0.203%), and black bean (21.321±0.206%). In terms of fiber, mung bean flour also exhibited the highest amount (17.460±0.760%), significantly greater than the other flours, with red bean (6.190±0.334%) and white bean (6.420±0.456%) showing similar levels, and black bean flour having the lowest fiber content (3.622±0.205%). Fat content was highest in white bean flour (2.918±0.024%), followed by red bean (1.618±0.077%) and black bean (1.662±0.334%), with mung bean flour having the lowest fat content (0.905±0.072%). Regarding ash content, red bean flour had the highest level (4.063±0.049%), though it did not differ significantly from mung bean (3.998±0.371%) and black bean (3.622±0.205%) flours, while white bean flour had the lowest ash content (2.977±0.216%). These results highlight the distinct nutritional profiles of each pulse flour, with mung bean standing out for its high protein and fiber content, while white bean is notable for its higher fat content.
3.2. Mineral Contents Analysis of Pulse Flours
The mineral contents of four different pulse flours—mung bean, red bean, white bean, and black bean—was analyzed, revealing distinct variations in both macro and micro minerals. Among the macro minerals, black beans had the highest calcium (4.9899 g/100 g) and sodium (0.1182 g/100 g) levels, making them a rich source of these nutrients. Mung beans, on the other hand, showed the highest phosphorus (5.4621 g/100 g), potassium (7.4901 g/100 g), and magnesium (1.1583 g/100 g), emphasizing their importance for daily mineral intake. In contrast, white beans had the lowest potassium (4.8615 g/100 g), and red beans contained the lowest sodium (0.0247 g/100 g), indicating their moderate mineral content. For micro minerals, red and white beans had the highest selenium (0.0006 mg/100 g), while black beans were rich in zinc (0.0326 mg/100 g), iron (0.0587 mg/100 g), and manganese (0.0486 mg/100 g). Mung beans had the highest copper levels (0.0105 mg/100 g). These findings highlighted the unique nutritional profiles of different pulse flours, offering diverse health benefits. The study suggested that incorporating a variety of pulse flours into the diet could help meet essential mineral needs, promoting bone health, cardiovascular function, and immune support, making these pulses a valuable part of a balanced diet, particularly in plant-based nutrition.
3.3. Amino Acid Profiles of Pulse Flours
The amino acid contents of four different pulse flours, including mung bean, red bean, white bean, and black bean, showed significant variations in both essential and non-essential amino acids (
Table 3). Among the essential amino acids, mung bean flour had the highest levels of histidine (0.954±0.055 g/100g protein), isoleucine (1.436±0.042 g/100g protein), methionine (0.403±0.182 g/100g protein), and threonine (19.929±3.702 g/100g protein), while black bean flour had the highest leucine (1.803±0.064 g/100g protein), valine (0.528±0.042 g/100g protein), phenylalanine (1.004±0.057 g/100g protein), and arginine (1.415±0.033 g/100g protein) contents. White bean flour was the richest in lysine (1.214±0.021 g/100g protein). Tryptophan was not detected in any of the flours. Overall, mung bean flour had the highest total essential amino acid content (26.766 g/100g protein), followed by red bean (22.389 g/100g protein), black bean (21.994 g/100g protein), and white bean (21.083 g/100g protein). For non-essential amino acids, white bean flour had the highest glycine (21.729±0.071 g/100g protein) and serine (1.308±0.150 g/100g protein), while mung bean had the highest tyrosine (0.902±0.033 g/100g protein) and glutamic acid (3.591±0.075 g/100g protein). Cysteine content was highest in black bean flour (0.870±0.062 g/100g protein). Glutamine, alanine, asparagine, and aspartic acid were not detected in any of the flours. The total non-essential amino acid content was highest in white bean flour (26.527 g/100g protein), followed by mung bean (21.900 g/100g protein), red bean (14.578 g/100g protein), and black bean (13.864 g/100g protein). These findings highlight the unique amino acid profiles of each pulse flour, with mung bean excelling in essential amino acids and white bean being particularly rich in non-essential amino acids.
3.4. Bioactive Compounds in Four Types of Pulse Flours
The antioxidant content of four different pulse flours, including mung bean, red bean, white bean, and black bean, was evaluated in terms of flavonoids, phenolics, DPPH, ABTS, FRAP, and total anthocyanin (
Table 4). Significant differences (p ≤ 0.05) were observed across these antioxidant parameters, indicating varied antioxidant capacities among the different types of flour. Flavonoid content was found to be highest in mung bean (mg QE/g db), followed by black bean, red bean (23.869 ± 0.068 mg QE/g db), and white bean (16.671 ± 0.050 mg QE/g db). Phenolic content was highest in black bean (19.518 ± 0.089 mg GAE/g db), followed by red bean (16.319 ± 0.015 mg GAE/g db), white bean (10.886 ± 0.037 mg GAE/g db), and mung bean (9.226 ± 0.045 mg GAE/g db). For DPPH radical scavenging activity, black bean exhibited the highest activity (17.291 ± 0.009 mg TE/g db), which was significantly higher than that of red bean (14.946 ± 0.020 mg TE/g db), white bean (5.891 ± 0.007 mg TE/g db), and mung bean (5.046 ± 0.004 mg TE/g db). ABTS activity was also strongest in red bean (25.804 ± 0.040 mg TE/g db) and black bean (25.792 ± 0.092 mg TE/g db), with lower values observed in mung bean (5.828 ± 0.396 mg TE/g db) and white bean (5.061 ± 0.030 mg TE/g db). Similarly, FRAP activity followed the same trend, with black bean showing the highest value (15.229 ± 0.415 mg TE/g db), followed by red bean (11.283 ± 0.188 mg TE /g db), white bean (5.469 ± 0.068 mg TE/g db), and mung bean (2.662 ± 0.125 mg TE/g db). Total anthocyanin was detected only in red bean (8.2144 ± 0.4640 mg CyE/g) and black bean (7.4864 ± 0.2474 mg CyE/g), while it was not detected (ND) in either mung bean or white bean.
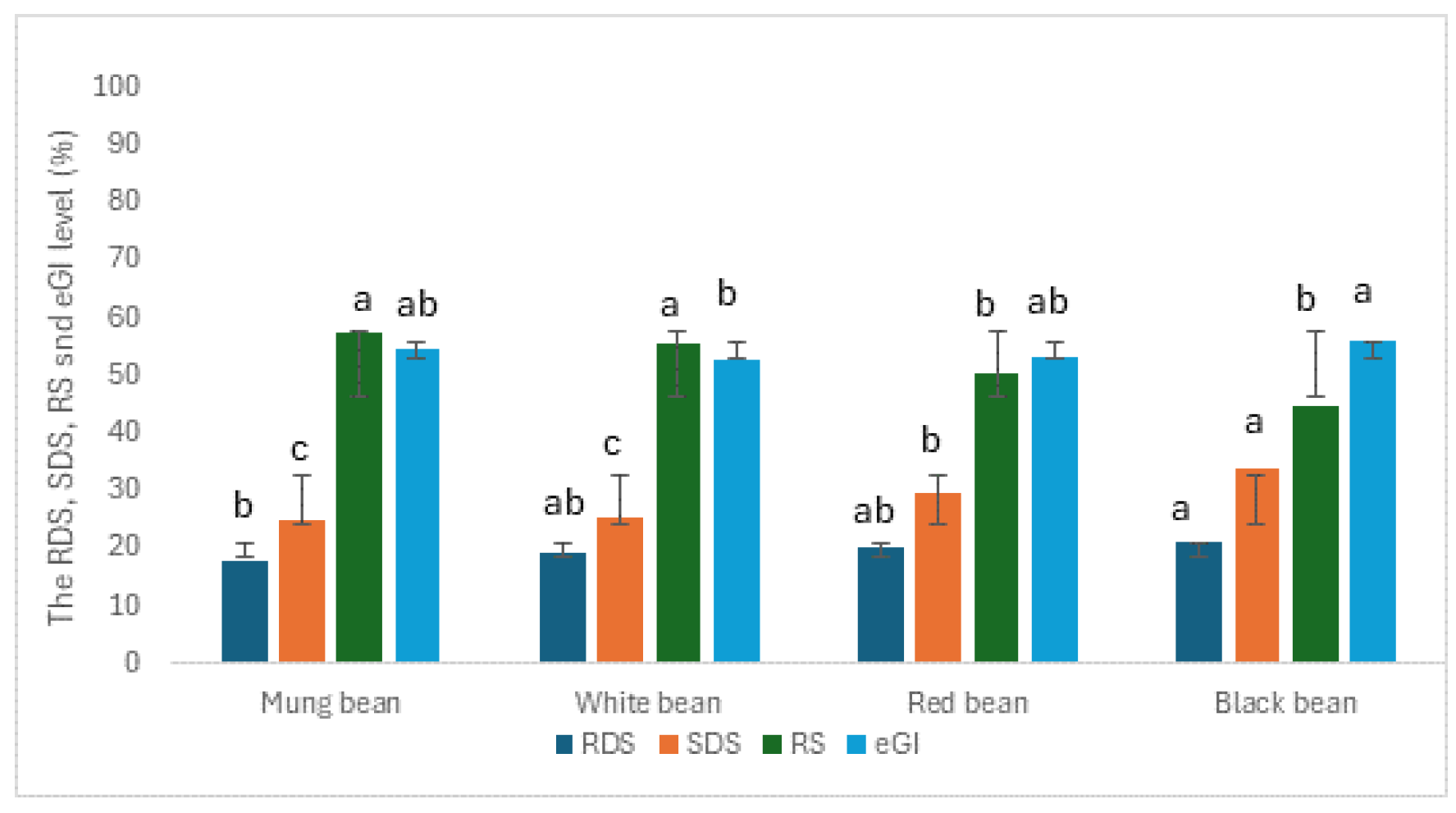
3.5. In Vitro Rapidly Available Glucose
The in vitro starch digestibility and estimated glycemic index (eGI) of four pulse flours—mung bean, white bean, red bean, and black bean—were analyzed, and the findings are presented in
Figure 1. Significant differences were observed across the flours in terms of Rapidly Digestible Starch (RDS), Slowly Digestible Starch (SDS), Resistant Starch (RS), and eGI. Among the four pulse flours, black bean flour exhibited the highest level of rapidly digestible starch (RDS), followed by white bean and red bean flours, which did not differ significantly from each other. In contrast, mung bean flour had a noticeably lower RDS level. Regarding slowly digestible starch (SDS), black bean flour again had the highest content, followed by red bean flour with a moderately high SDS value. Mung bean and white bean flours showed significantly lower SDS levels, with mung bean flour having the lowest SDS content. In terms of resistant starch (RS), mung bean and white bean flours contained the highest amounts, with no significant difference between them, while red bean and black bean flours had considerably lower RS levels. As for the estimated glycemic index (eGI), black bean flour had the highest value, followed by red bean and white bean flours, which were statistically similar, while pulse flours had the lowest eGI. These findings suggested that flours with higher levels of rapidly digestible starch (RDS) and lower levels of resistant starch (RS), such as black bean flour, tended to have a higher eGI, indicating faster carbohydrate digestion and a greater potential to raise blood glucose levels. In contrast, flours with higher contents of slowly digestible starch (SDS) and RS, like white bean flour, generally had lower eGI values. Overall, all types of beans studied were classified as low GI foods, reflecting slower starch digestion and a more moderate impact on blood glucose levels.
3.6. Functional Properties
3.6.1. WAI, WSI and OAI
The water absorption index (WAI), water solubility index (WSI), and oil absorption index (OAI) of mung bean, red bean, white bean, and black bean flours showed significant variations (p ≤ 0.05) in WAI and WSI, while OAI remained consistent across all samples (
Table 5). Red bean flour exhibited the highest WAI (2.381±0.001 g/g), followed by white bean (1.854±0.071 g/g) and black bean (1.827±0.050 g/g), with mung bean having the lowest WAI (1.729±0.049 g/g). For WSI, mung bean flour showed the highest solubility (27.699±0.400%), followed by black bean (25.744±0.153%), red bean (20.635±0.105%), and white bean (17.677±0.034%). However, no significant differences were observed in OAI among the flours, with values ranging from 2.225±0.040 g/g to 2.271±0.030 g/g. These findings suggest that red bean flour, with its high water absorption, is better suited for applications requiring moisture retention, while mung bean flour, with its high solubility, may be more appropriate for formulations needing rapid solubility.
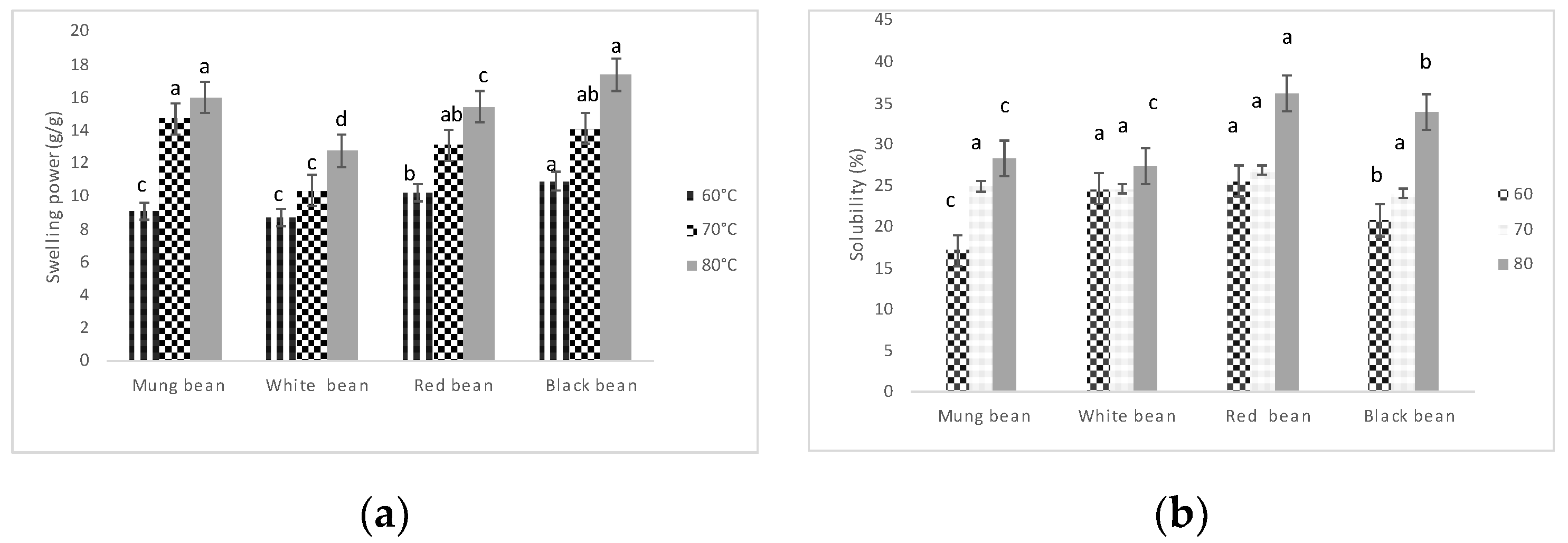
3.6.2. Swelling Power and Solubility
The swelling power and solubility of mung bean, white bean, red bean, and black bean flours were evaluated at three different temperatures (60°C, 70°C, and 80°C), showing significant differences (p ≤ 0.05) between the flours and across temperatures (
Figure 2). Swelling power increased with temperature for all flours, with black bean and mung bean reaching the highest values at 80°C (approximately 16 g/g and 17 g/g, respectively), while white bean had the lowest swelling power, peaking at around 13 g/g. For solubility, red bean flour exhibited the highest value (~35%) at 80°C, followed by black bean (~33%), with mung bean and white bean showing lower solubility (around 30% and 25%, respectively). Solubility increased significantly with temperature for all flours, with the lowest values observed at 60°C. Overall, these results demonstrate that both temperature and flour type significantly affect swelling and solubility properties, with black bean and mung bean excelling in swelling power, and red bean showing the highest solubility at elevated temperatures.
3.6.3. Protein Solubility
The protein solubility of four different pulse flours, including mung bean, red bean, white bean, and black bean, showed significant variations (p ≤ 0.05) (
Table 6). Red bean exhibited the highest protein solubility (2.112 ± 0.005%), followed by mung bean (1.870 ± 0.017%) and black bean (1.694 ± 0.158%), while white bean had the lowest solubility (1.455 ± 0.005%). These findings indicated that red bean flour had better protein solubility compared to the other flours, with white bean showing the least solubility.
3.6.4. Emulsion Capacity (EC) and Stability (ES)
The emulsifying capacity and emulsion stability of four different pulse flours—mung bean, red bean, white bean, and black bean —are presented in
Table 7, showing significant differences (p ≤ 0.05). Red bean flour exhibited the highest emulsifying capacity (71.000±1.414%) and emulsion stability (87.143±2.020%), significantly outperforming the other flours. White bean and black bean flours showed similar emulsifying abilities (49.000±1.414%), though their emulsion stability differed, with white bean having the lowest stability (66.000±2.828%) compared to black bean (68.000±5.657%). Mung bean flour had the lowest emulsifying capacity (41.000±1.414%) but moderate emulsion stability (77.500±3.536%), comparable to that of red bean. These findings suggest that red bean flour has superior emulsifying properties and stability, making it the most effective for applications requiring high emulsion capacity, while mung bean, despite its lower emulsifying capacity, maintains relatively good emulsion stability.
3.6.5. Foaming Capacity (FC) and Stability (FS)
The foaming capacity and foaming stability of four different pulse flours—mung bean, red bean, white bean, and black bean —are presented in
Table 8, showing significant differences (p ≤ 0.05). Black bean flour exhibited the highest foaming capacity (70.000±0.673%) and foaming stability (62.381±0.673%), closely followed by red bean, which had slightly lower foaming capacity (67.143±0.673%) and stability (60.476±0.673%). Mung bean flour showed moderate foaming capacity (43.333±0.673%) and stability (35.714±0.673%), while white bean had the lowest foaming capacity (42.183±0.953%) and stability (13.810±0.673%). These results indicated that black bean and red bean flours were more effective in foam formation and stability, making them suitable for applications where foaming properties were essential, while white bean flour showed poor foaming performance.
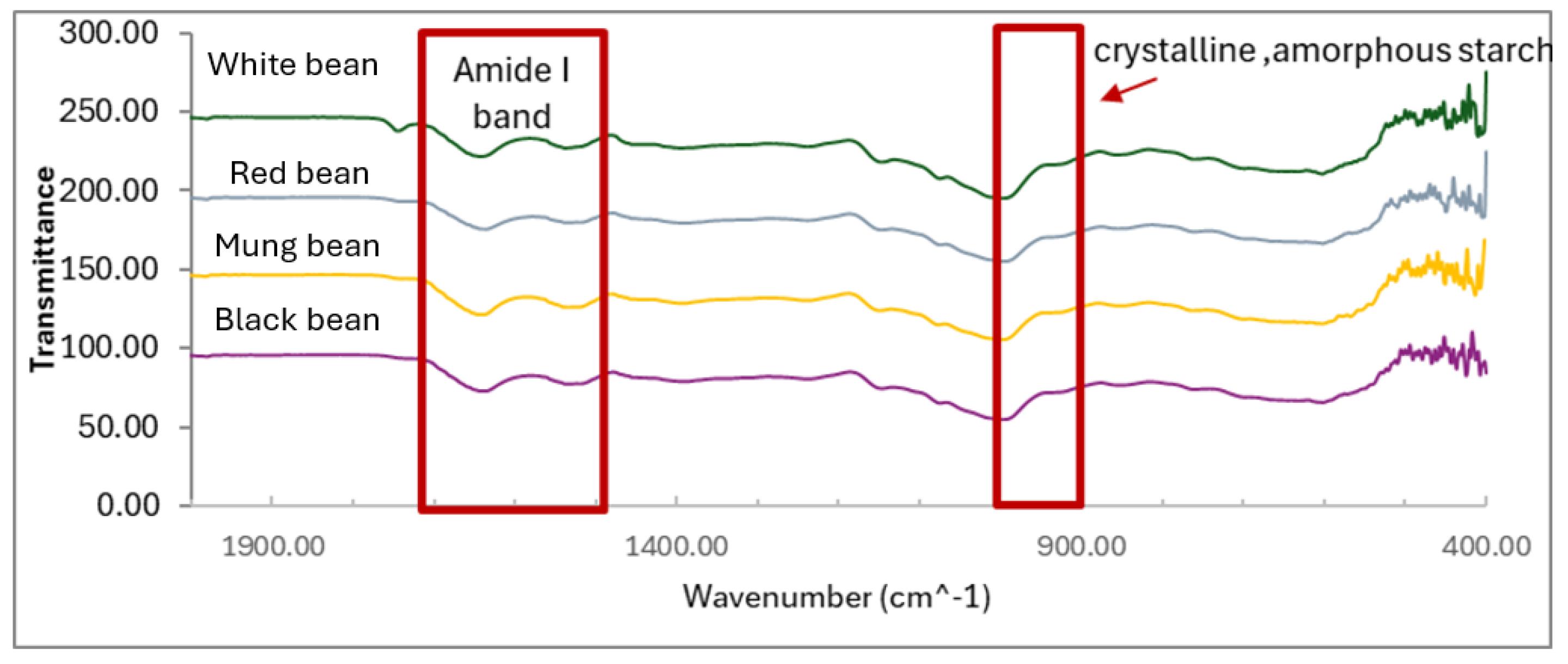
3.7. Structure Properties
The structural properties of the Amide I band in various pulse flours, as shown in
Figure 3, revealed three primary protein structural elements: β-sheet (1610–1640 cm⁻¹), α-helix (1650–1658 cm⁻¹), and β-turn (1660–1670 cm⁻¹), with the β-sheet being the predominant structure across all pulse types. These β-sheets, formed through intermolecular hydrogen bonding, provided rigidity, while the β-turn contributed a more flexible, helical structure. Additionally, the starch distribution in the absorption range identified distinct categories, including crystalline starch (994–995 cm⁻¹), amorphous starch (1022–1023 cm⁻¹), and ordered starch (1044–1045 cm⁻¹), indicating that pulse flours exhibited a semi-crystalline structure. This semi-crystallinity, closely linked to the prevalence of β-sheet structures, contributed to both the strength and flexibility of the flours. Overall, the structural composition of pulse flours showed a high degree of similarity, with proteins, particularly β-sheets, being the primary component, while the semi-crystalline starch provided additional functional properties.
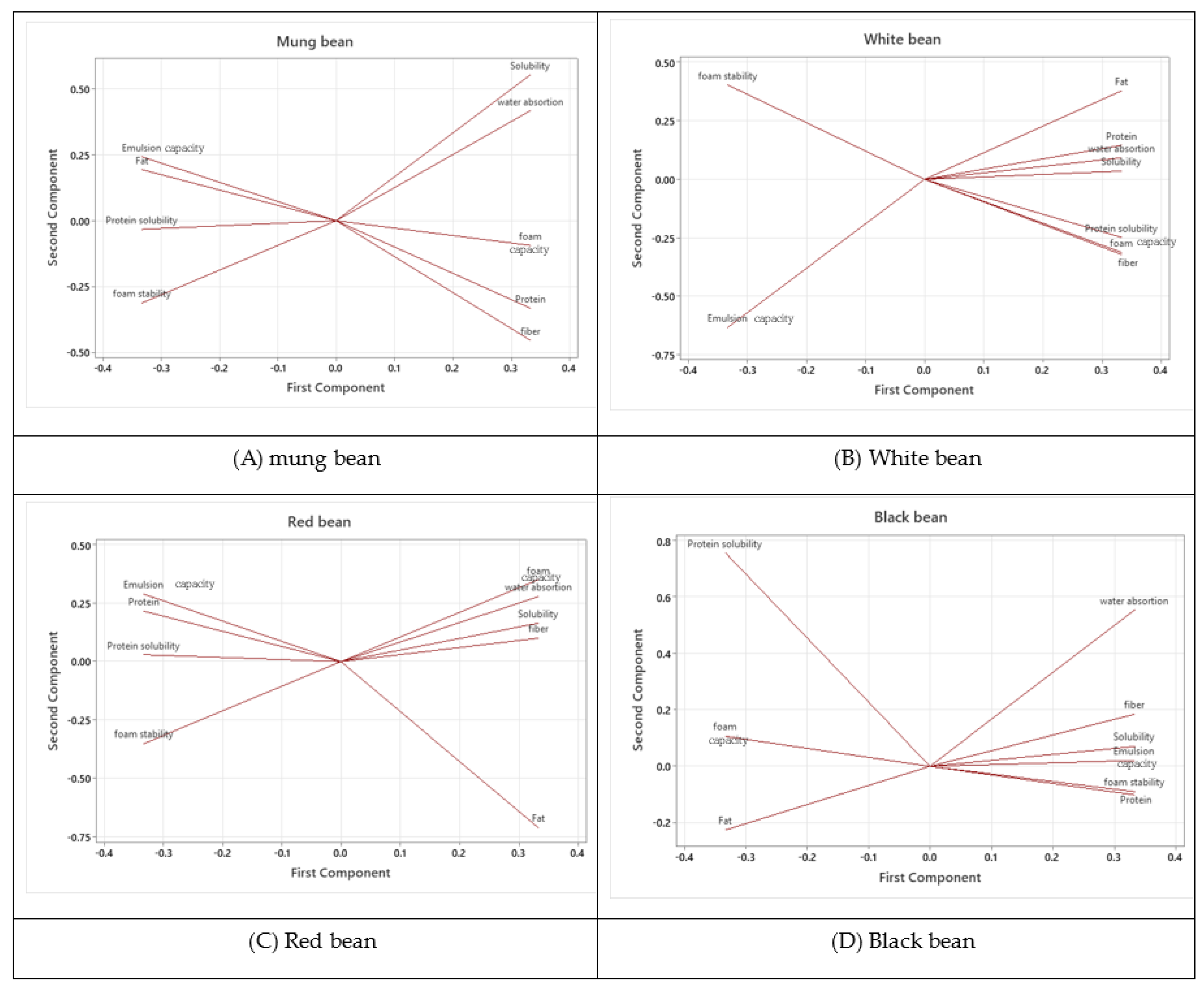
3.8. Relationship Between Various Properties of PULSE flours
The Principal Component Analysis (PCA) results for the functional properties of the four pulse flours—mung bean, white bean, red bean, and black bean—revealed distinct patterns in how their functional components were related. In mung bean flour, solubility and water absorption were highly correlated with the first component, indicating a strong relationship between these properties, while protein solubility, foam stability, and emulsion capacity were negatively associated, suggesting an inverse relationship with solubility and water absorption. White bean flour showed the strongest correlation between fat content and the first component, with solubility and water absorption also positively contributing, but foam stability and emulsion capacity exhibited a strong negative correlation, implying these properties might inversely impact fat and solubility. For red bean flour, foam, water absorption, and solubility were positively correlated with the first component, while fat was negatively correlated, and protein solubility and foam stability showed weaker, negative contributions. In black bean flour, protein solubility was uniquely correlated with the second component, setting it apart from other flours, while water absorption, solubility, and fiber were highly correlated with the first component. Foam stability and emulsion capacity contributed minimally to either component in comparison to other flours. Overall, the PCA results suggested that solubility and water absorption were key factors across all flours, with variations in fat, protein solubility, and foam stability influencing the distinct functional profiles of each flours.
Figure 4.
PCA in of four types of pulse flours (A) mung bean, (B) White bean, (C) Red bean, (D) Black bean.
Figure 4.
PCA in of four types of pulse flours (A) mung bean, (B) White bean, (C) Red bean, (D) Black bean.
4. Discussion
The chemical analysis of pulse flours derived from mung bean, red bean, white bean, and black bean revealed distinct nutritional profiles that positioned them for specialized applications in the food industry. Mung bean flour, which had the highest protein content (25.97%), was enriched with globulin and albumin proteins. These proteins not only enhanced emulsification due to their amphiphilic nature, promoting interactions between water and oil to stabilize emulsions, but also played a role in water retention and structural stability in food matrices. The relationship between protein content and emulsification observed in mung bean flour was similarly reflected in other high-protein pulses like chickpeas, widely used in emulsified products [
18,
19]. Furthermore, the high protein content supported its compatibility with products requiring protein enrichment, such as protein drinks or food formulations aimed at muscle recovery.
On the other hand, black bean flour, although lower in protein content, stood out due to its high calcium (4.99 mg/g) and magnesium content, essential for bone health and enzymatic functions. This result was connected with the broader functional role of these flours, highlighting their dual capability of supporting both structural food systems (through protein functionality) and health-related benefits (through mineral enrichment). The correlation between calcium and magnesium levels in black bean flour and its potential in bone health products provided opportunities for functional food development in the nutraceutical sector [
20].
When comparing these pulse flours to other pulses like chickpeas and lentils, the protein fractions differed significantly. Chickpeas, which had a high albumin and globulin content, offered strong emulsifying properties, while lentils, with their lysine-rich profile, were ideal for muscle recovery and growth in protein-enriched formulations. This comparison highlighted that while all pulse flours had protein functionality, their specific application in food products depended on the protein fractions and related bioactivity. For example, lentils, with their lysine-rich protein content, provided a complementary role to mung bean flour in protein-rich formulations, making them valuable in combined applications [
21,
22]. Additionally, lupin beans, another protein-rich pulses, demonstrated lipid-binding properties, contributing to cholesterol-lowering effects and offering cardiovascular benefits. This emphasized the versatility of pulse flours in targeting specific health conditions through careful selection based on nutritional profiles [
23].
The key functional attribute of these pulse flours was their starch composition. The high resistant starch (RS) content in mung and white bean flours provided significant glycemic control, aligning with the broader health benefits of pulses such as navy beans and lentils. RS, which resisted digestion in the small intestine, slowed glucose absorption and promoted the growth of beneficial gut bacteria. This glycemic control property was particularly valuable given the rising demand for functional foods aimed at managing diabetes. Interestingly, the co-existence of high protein and high RS content in mung bean flour suggested a synergistic role in both structural food properties and health benefits. The RS contributed to prolonged satiety and stable postprandial glucose levels, while the protein supported muscle repair and growth, further enhancing its appeal for health-conscious consumers [
24]. Additionally, the presence of slowly digestible starch (SDS) in black bean flours contributed to a gradual release of glucose, complementing the RS function by providing sustained energy over time, making them ideal candidates for glycemic control products aimed at athletes and individuals managing diabetes [
25,
27].
The bioactive compounds in red bean and black bean flours, particularly phenolics and flavonoids, played a significant role in promoting health through their antioxidant and anti-inflammatory properties. These compounds were able to scavenge reactive oxygen species (ROS), which were factors contributing to oxidative stress and could lead to the development of chronic diseases such as heart disease, diabetes, and cancer. Moreover, these compounds inhibited enzymes involved in inflammatory processes, such as COX and LOX, effectively reducing inflammation. While mung bean and white bean flours contained higher levels of resistant starch (RS) compared to red and black beans, which helped regulate blood sugar levels and promoted gut health, red and black beans contained higher amounts of antioxidants, making them more effective in reducing the risk of chronic diseases. These antioxidants also induced apoptosis (cell death) in cancer cells by triggering the destruction of abnormal cells, which was beneficial in preventing cancers such as breast and colon cancer. The combined effects of blood sugar regulation and oxidative stress reduction enhanced the potential for preventing metabolic diseases like type 2 diabetes and heart disease, as well as degenerative diseases related to oxidative stress, such as Alzheimer's. Therefore, while mung bean and white bean had higher RS content, red bean and black bean stood out for their strong antioxidant properties, making them ideal for development into functional foods that could play a key role in preventing chronic and degenerative diseases in the future [
28,
29,
30].
From an industrial perspective, the technological properties of these pulse flours, particularly their water absorption index (WAI) and emulsifying capacity, made them highly versatile ingredients for a variety of food applications. Red bean flour, with its high WAI, was particularly suitable for products that required water retention, such as soups and sauces. This property not only enhanced the texture of such products but also aligned with the broader functional role of pulses in improving moisture retention in gluten-free and reduced-calorie food formulations. The strong emulsifying properties of red bean flour, attributed to its amphiphilic proteins, further extended its application to salad dressings and creams, where water and oil phase stability was critical [
31]. These technological properties were similarly observed in chickpea and lentil flours, known for their role in gluten-free baking where emulsification and water retention were crucial for maintaining texture and sensory qualities [
32].
FT-IR spectroscopy on pulse flours in the regions 994-995 cm⁻¹, 1022-1023 cm⁻¹, 1044-1045 cm⁻¹, and the amide I region (1600–1700 cm⁻¹) demonstrated molecular mechanisms that influenced the functional properties of starches and proteins in pulse flours. The protein structures in the amide I region corresponded to the C=O (carbonyl stretch) vibrations, covering secondary structures such as α-helices, β-sheets, and β-turns [
33]. Each of these structures played a significant role in the stability and function of the proteins. α-helices arose from hydrogen bonding within the helix, providing flexibility and enabling the protein to withstand stress and bending. In contrast, β-sheets, formed by parallel or anti-parallel arrangements of polypeptide chains, created strong, stable structures via hydrogen bonding between chains. β-turns allowed polypeptide chains to reverse direction, contributing to protein flexibility and diverse functions, such as water retention and emulsification. In the 994-995 cm⁻¹ range, the spectral vibrations corresponded to crystalline starch, where the ordered arrangement of glucose molecules created hydrogen bonds, resulting in strong, durable starch resistant to degradation. In the 1022-1023 cm⁻¹ range, the vibrations indicated amorphous starch, which exhibited a less ordered structure, making it more flexible and capable of retaining water. This amorphous structure played a crucial role in moisture retention within food products. The 1044-1045 cm⁻¹ range signified semi-crystalline starch, a hybrid structure that balanced the strength of crystalline starch with the flexibility of amorphous starch, making it suitable for food applications where both strength and water retention were needed. The semi-crystalline structure of starch contributed significantly to functional properties, with crystalline regions providing strength and stability, while amorphous regions enhanced flexibility and water retention. This made pulse flour starches suitable for industrial processing, such as baking or heat treatment. The semi-crystalline nature of these starches also enabled slow and consistent carbohydrate release, which benefited blood sugar regulation and promoted gut health through the presence of slowly digestible starches and resistant starches [
34]. Additionally, distinct peaks were observed in the 1000–1200 cm⁻¹ range, corresponding to resistant starch (RS) and slowly digestible starch (SDS), confirming their significance in glycemic control. This finding aligned with previous reports on the health benefits of these pulse flours, particularly in regulating blood sugar levels and promoting gut health [
34]. In conclusion, the secondary protein structures found in the amide I region (1600–1700 cm⁻¹), including α-helices, β-sheets, and β-turns, as well as the starch structures found in the 994-995 cm⁻¹, 1022-1023 cm⁻¹, and 1044-1045 cm⁻¹ regions, all contributed to the functional properties of pulse flours. These structures played essential roles in emulsification, water retention, and health benefits, making pulse flours highly suitable for use in food industry applications [
35].
In conclusion, this study demonstrated how the distinct nutritional profiles, bioactive compounds, and functional properties of pulse flours made them versatile and valuable ingredients in food product development. By linking protein content to emulsification, RS and SDS to glycemic control, and bioactive compounds to antioxidant activity, the study highlighted how these flours served multiple roles in health-focused food formulations.
5. Conclusions
This study addressed the critical need for comprehensive data on the physico-chemical, functional, and bioactive properties of Thai pulse flours—specifically black bean, red bean, mung bean, and white bean. The lack of such data had been a significant barrier to optimizing these flours for use in nutritionally enhanced food products. Our findings revealed substantial differences in the nutritional composition and bioactive compounds of these flours, which were essential for developing targeted health food applications. Black bean flour demonstrated the highest levels of phenolic and flavonoid compounds, resulting in superior antioxidant activity, while red bean flour was richest in anthocyanins and exhibited the highest water absorption capacity. Mung bean flour was distinguished by its high protein and fiber content, making it suitable for high-protein, high-fiber dietary products. The study contributed valuable insights into the potential of Thai pulse flours to serve as key ingredients in functional foods designed to address specific health concerns, such as oxidative stress and metabolic disorders. For future applications, it is recommended that further research explore how various cultivation practices, processing methods, and storage conditions influence the stability and bioavailability of these bioactive compounds. Additionally, studies on consumer preferences and sensory evaluations will be vital in understanding market potential and facilitating the integration of these flours into food industry formulations. This research lays the foundation for developing functional, health-oriented foods by leveraging local agricultural resources to create innovative, sustainable food solutions. Moreover, gaining insights into consumer preferences will be essential for driving the adoption of these pulse flours in the food industry, emphasizing the importance of utilizing local agricultural products to develop health-promoting food solutions.