1. Introduction
Gastric carcinoma (GC) is rare in the general dog population, corresponding to less than 1% of all neoplastic changes identified [
1]. It is diagnosed at a median age of 8.5 to 10 years through endoscopy confirmed by histological evaluation of tissue biopsies [
2,
3,
4]. No specific hematological abnormalities or biomarkers have yet been identified to diagnose GC in dogs [
5]. Surgery, with or without adjuvant chemotherapy, is the only potential curative treatment. However, prognosis even with surgery is very poor, with a median survival time of 33-178 days [
6,
7,
8]. The diagnosis is often delayed by the non-specific, and initially mild clinical signs. Gastric carcinoma has even been diagnosed without overt clinical signs in Belgian shepherds [
9].
The etiology of GC in dogs is unknown. However, breed predispositions to GC in the dog have been reported, suggesting a genetic component to the etiology [
1,
2,
3,
10]. Especially, in the long-haired Belgian Shepherd dog varieties Tervueren and Groenendael a strong breed predisposition to GC has been reported [
11,
12,
13,
14].
Although the breed predisposition for GC in the Belgian shepherds indicates a genetic cause for the disease, currently no strong information about heritability of the disorder is available. Globally, Belgian Shepherd breeders are seeking tools to decrease the frequency of GC in their breed. In order to predict the effectiveness of selection against GC in breeding strategies, knowledge on age distribution, heritability and incidence of the disease in the population is necessary. Also, risk assessment for GC based on disease status of one or both of the parents may help in decision-making for breeding strategies.
Therefore, the aim of the current study was to determine age, sex distribution and incidence, to calculate the odds ratios for developing GC in offspring from affected parents, and to determine heritability of GC in the Belgian Shepherd Tervueren and Groenendael population.
2. Materials and Methods
2.1. Case and Control Definition and Recruitment
A database of GC cases was built by including all cases of GC, regardless of breed variety or country of residence, seen by referral for gastroscopy to one of the authors (PM) and subsequent confirmed by histology. Additionally, all cases of GC in Dutch pure-bred Belgian Shepherds reported to the primary investigator (SH) by dog owners directly, cooperating pathology laboratories or via the GC contact point of the Dutch Belgian Shepherd breed association (Nederlandse Vereniging voor Belgische Herdershonden, NVBH) between 2013 and 2024 were included. For these cases the medical records were requested and evaluated if available to assess the GC diagnosis.
A tier 1 diagnosis of GC was defined as GC confirmed by endoscopy or post-mortem. A tier 2 diagnosis was assigned to cases with incomplete or unavailable records and those based on ultrasonography or clinical presentation.
Belgian shepherds without gastro-intestinal signs above the age of 10 years were actively recruited via the breed organisation and the primary author and included in a database of possible control dogs. Health status was followed-up by phone calls, e-mail and follow-up questionnaires via the breed association and the primary investigator until death. Dogs that were followed until death and died without gastrointestinal signs above the age of 13 years were used as control dog for this study.
The full pedigree database of Belgian Shepherds in the Netherlands was obtained via the Dutch Belgian Shepherd Club (NVBH). Including only the long-haired varieties and Malinois with long-haired offspring.
2.2. Age and Sex Distribution of Gastric Carcinoma
Median and range of age of GC death were calculated for all cases with a reported date or age of death, per breed variety and per sex. The students t-test was used to test the difference in age of death by breed variety or sex and the Chi-squared test for a difference in sex distribution.
2.3. Incidence
Belgian Shepherds with gastric carcinoma living in the Netherlands and born between 2000 and 2010 were selected from the GC database. The incidence was defined as the percentage of GC cases per birthyear born in the Netherlands. And the cumulative incidence as the average incidence over these years. Incidence was calculated by dividing the total number of positive cases per birthyear by the total number of Tervueren and Groenendael born and registered in the Netherlands per year.
2.4. Calculation of Odds Ratios for Affected Parents
A univariable logistic regression model was used to calculate the odds ratio for an individual being affected depending on disease status of the parents. For this analysis, only the dogs were included from the case and control database for which at least one parent was also reported to be either case or control. The disease status of the other parent could be unknown. Odds ratio and 95% confidence interval were reported.
2.5. Heritability
All Dutch GC cases in Tervueren and Groenendael dogs that were bred with a Fédération Cynologique Internationale (FCI) pedigree were included in heritability analysis. Dogs with a non-Dutch pedigree living outside of the Netherlands were only included in the heritability calculation if they were a first line relative of a Dutch GC case.
Narrow-sense heritability was calculated for the GC as a binary trait (affected and free). Using R core commands (R version 4.1.2), the dogs registered in the dataset with a phenotype and their parental data were filtered from the dataset. Using the package optiSel the additive relationship matrix of the dogs was calculated [
15]. Using the package Sommer a linear mixed models approach was used with random effects based on the additive relationship matrix to calculate the genetic and residual variances [
16]. Narrow-sense heritability was then calculated with the following formula:
3. Results
3.1. Cases and Controls
In total, 383 cases of GC in Belgian Shepherds were identified and entered into the database. Of these dogs, 343 Tervueren or Groenendael dogs were born in or living in the Netherlands or were first line relative to a Dutch dog. Only 3 Malinois and no Lakenois cases were present in the GC database.
Five of the Tervueren and Groenendael dogs had no reliable pedigree data and were excluded from analysis, leaving a study dataset of 338 GC cases. Two hundred sixteen cases had tier 1 evidence, 122 had tier 2 evidence of GC.
Three hundred twenty potential healthy control dogs were entered into the database of the GC research. Out of these 159 (112 Tervueren, 47 Groenendael) qualified the criteria of being Dutch and having no gastrointestinal signs before death at 13 years or older. The mean age at death of this group was 14.7 years (range 13.0-17.4).
3.2. Age and Sex Distribution of Gastric Carcinoma
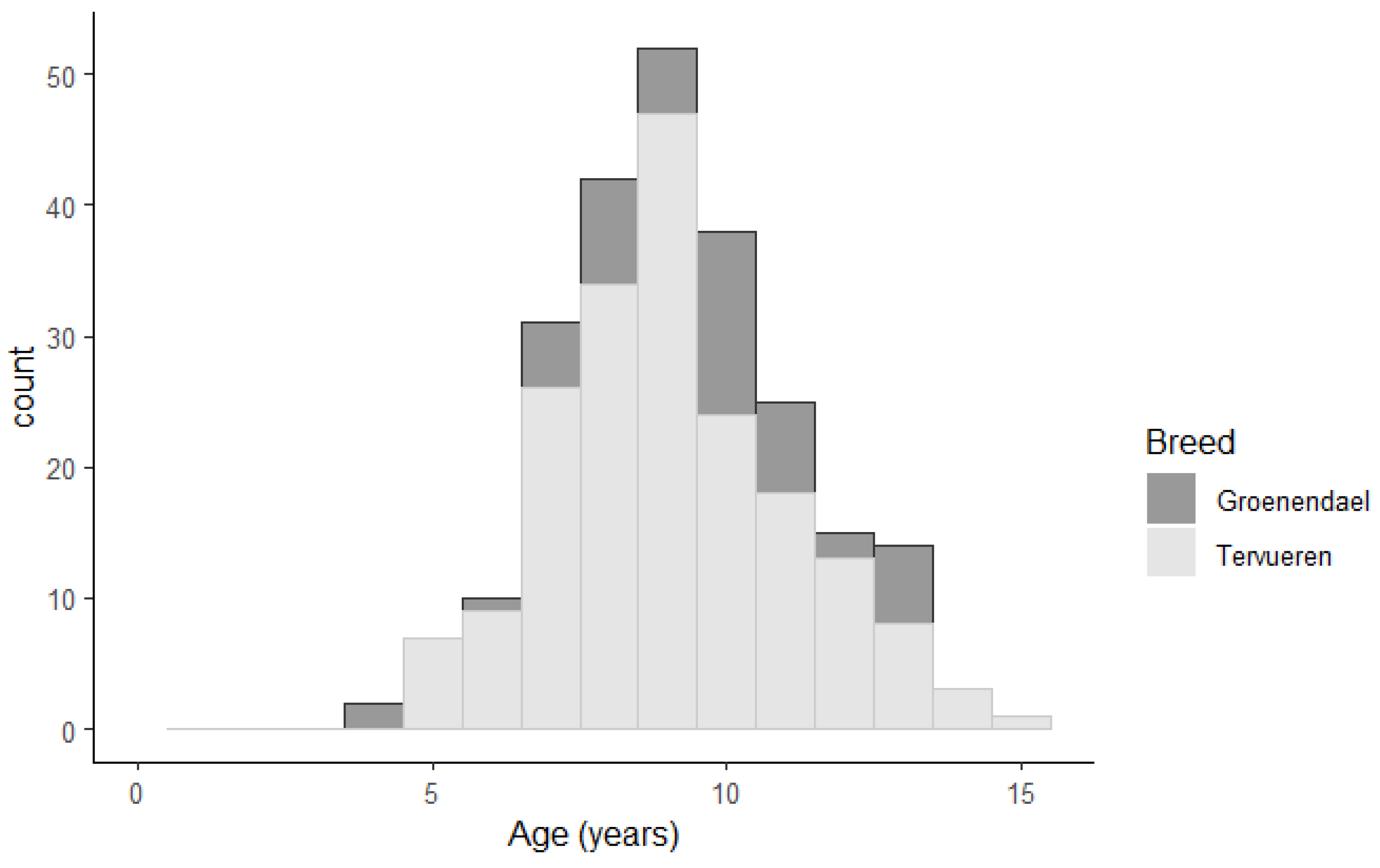
Age of death from GC was available from 240 cases. The median age of death was 9.0 years (4.4-15.5). Age of death related to GC was not significantly different for males and females or tier 1 and tier 2 GC evidence and not significantly higher for the Groenendael cases (9.7 years) compared to the Tervueren (9.0 years, p= 0.1,
Figure 1). Eleven cases were older than 13 years at death due to GC (3% of tier 1 cases, 7% of tier 2 cases).
There was no sex predilection to GC in the study dataset with 169 male cases and 169 female cases.
3.3. Incidence
Two hundred twenty-four long-haired Belgian shepherds in the research dataset were born between the years 2000 and 2010. In the same period, 5929 pups were born and registered, which means that the cumulative incidence of GC in the Belgian Shepherd long-haired varieties is 3.8%. For Tervueren the 10-year cumulative incidence is 4.7%, for Groenendael 2.1%. The incidence per year varies between 0 and 5.6% for the Groenendael and 2.4% and 8.4% for the Tervueren (
Table 1).
3.4. Calculation of ORs for Affected Relatives
Of all dogs with GC in the study cohort, 30% had at least one litter in the Netherlands. For 137 dogs from the case or control group, the GC status of either dam or sire or both was known. The dam phenotype was known for all of these, the father phenotype for 67 dogs.
The odds of getting GC are significantly higher if at least one parent is affected versus no (known) parents are affected (OR 4.98, 95% CI 2.41 – 11.0). (
Table 2).
3.5. Heritability
The entire pedigree of Tervueren and Groenendael available consisted of 19,494 dogs, bred between 1949 and 2024, spanning 23 generations. The heritability of GC in the narrow sense was calculated as 0.53 with a standard error of 0.15.
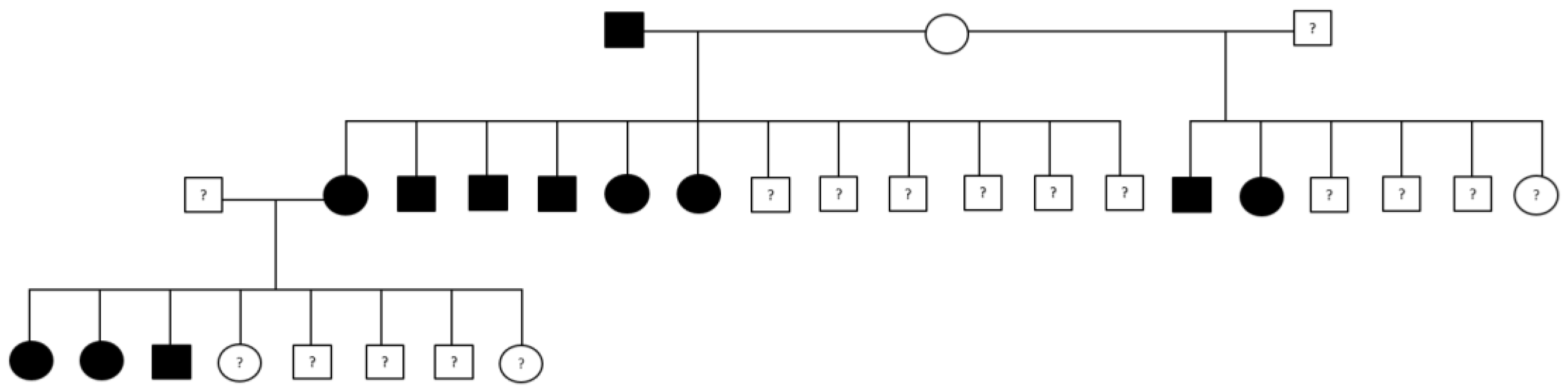
Figure 2.
Example of a family tree with dogs affected by gastric carcinoma. Males are represented by squares, females by circles. Affected dogs are black, control dogs white. Unknown phenotype is indicated by a question mark.
Figure 2.
Example of a family tree with dogs affected by gastric carcinoma. Males are represented by squares, females by circles. Affected dogs are black, control dogs white. Unknown phenotype is indicated by a question mark.
4. Discussion
Gastric carcinoma is very common in the Belgian Shepherd Tervueren and Groenendael varieties and carries a grave prognosis [
4,
6,
17]. The median age of death from GC reported here is similar to previous reports, but with a larger range [
17]. Possibly because older dogs with suspected GC do not seek diagnosis confirmation and are therefore not included in studies based on histopathology or endoscopy only. This is also reflected in the higher percentage of cases older than 13 years in the tier 2 evidence group. In this study we used age of death instead of age at diagnosis as age at diagnosis was not available for all cases. However, given the short median survival time for GC in the Belgian Shepherd, age of death can be used to infer age of occurrence [
17].
In contrast to smaller studies in the past suggesting a sex predisposition of males, this study confirms our previous results that there is no sex predisposition to GC in the Belgian Shepherd [
1,
4,
14,
17].
The increased prevalence for GC in the Belgian Shepherd has been described in several countries including the Netherlands, Italy, Finland, and Norway [
1,
11,
13,
14]. The first GC incidence inventory in Dutch Tervueren was performed previously over the years 1991-2002. This study only incorporated 92 dogs with 50 known pedigrees. The reported incidence per year based on this study varied between 0.31 and 2.2% [
14]. The higher incidence reported in this more comprehensive study (2.4% - 8.4%) could be explained by the more pro-active and better organization for the collection of GC cases by the investigators in close cooperation with the breed association. A true increase in incidence cannot be excluded however. An underestimation of the true incidence remains possible, as cases can still be missed.
The incidence of GC varied during the 10-year study period, but was overall high for both Tervueren and Groenendael varieties. One reason for the ongoing high incidence of GC in the Tervueren and Groenendael, is because age of occurrence is much higher than the breeding age. This is reflected in the high percentage of GC cases that have produced offspring. Nine dogs affected with GC in this dataset even had two affected parents. The odds of being affected with GC are almost five times higher with at least one parent affected compared to at least one parent with a control dog status.
The high heritability of 0.53 is an additional strong indication for a genetic component to GC in this breed.
Not all family relations can be taken into account in the heritability calculation, as only animals with known phenotype were used to construct the genetic additive matrix. We chose to only include controls if above the age of 13, and without any GI signs. Other limitations of this study are that not all GC cases had a tier 1 diagnosis definitively confirming GC and not all patient files were available on the older cases. Abdominal ultrasound can be highly indicative of GC, especially loss of wall layering [
19,
20]. However, ultrasound is not very sensitive for GC missing up to 50% of cases [
21]. An additional limitation of this study for calculation of the heritability and OR of parental affect was that not all parental status was known. Including more data would make it possible to calculate the additive effect of multiple parents affected. And if grandparent status is available for more animals, it could perhaps be possible to select based on these.
A striking point and unique feature of the Belgian Shepherd breed is that only two of the four breed varieties are highly affected by GC. This is reflected in the fact that over a 15-year period only 3 Malinois and no Lakenois were identified with GC, even though the Malinois is the far more popular variety with annual registrations of nearly three times the number of pups per year compared to the Tervueren and five times for the Groenendael. This fact holds both promise for finding genetically protective factors for GC and implies the possibility of reducing disease prevalence using Tervueren born from Malinois or pure Malinois or Lakenois in breeding programs. In absence of genetic screening possibilities, selection for reduction of GC in the Tervueren and Groenendael is at the moment nearly impossible. To decrease the incidence in the population, breeding from affected parents should be avoided. One possibility would be to only use semen from older males and freezing sperm to ensure genetic availability of dogs that are unaffected above 13 years. As selection based on first line relatives affected does not appear possible at the moment, there is a strong need for the identification of causal mutations or associated variants that can be used in selection of breeding stock. Future research should therefor focus on the genetic factors underlying GC risk. Additionally, ongoing screening and inventory of all breeding dogs is necessary for the Tervueren and Groenendael to build more multigenerational data to strengthen future analysis and breeding advice. A veterinary practice disease monitoring tool such as the PetScan system in the Netherlands may be a good addition to avoid underreporting by lack of referral [
22].
Author Contributions
Sanne Hugen, data acquisition, analysis, conceptualization and writing of manuscript. Citlalli limpens, data analysis, manuscript writing. Joris Robben; manuscript revision. Paul Mandigers, data acquisition, conceptualization, manuscript revision. Hille Fieten; manuscript revision.
Acknowledgments
We would like to thank Anne-Marie Smolders, for her contacts with the breed association and all Belgian Shepherd owners. This was invaluable for all data gathering. And Jacquelyn Evans for critically reviewing the manuscript.
Conflicts of Interest
None.
References
- Seim-Wikse T, Jörundsson E, Nødtvedt A, et al. Breed predisposition to canine gastric carcinoma--a study based on the norwegian canine cancer register. Acta Vet Scand. 2013;55(25):1–6. [CrossRef]
- von Babo V, Eberle N, Mischke R, et al. Canine non-hematopoietic gastric neoplasia. Tierartzliche Praxis Kleintiere. 2012:243–249. [CrossRef]
- Sullivan M, Lee R, Fisher EW, Nash AS, McCandlish IAP. A study of 31 cases of gastric carcinoma in dogs. Vet Rec. 1987;120:79–83. [CrossRef]
- Gualtieri M, Monzeglio MG, Scanziani E. Gastric neoplasia. Veterinary Clinics of North America - Small Animal Practice. 1999;29(2):415–440.
- Seim-Wikse T, Skancke E, Nodtvedt A, et al. Comparison of body condition score and other minimally invasive biomarkers between dogs with gastric carcinoma and dogs with chronic gastritis. J Am Vet Med Assoc. 2019;254(2):226–235. [CrossRef]
- Abrams B, Wavreille VA, Husbands BD, et al. Perioperative complications and outcome after surgery for treatment of gastric carcinoma in dogs: A veterinary society of surgical oncology retrospective study of 40 cases (2004–2018). Veterinary Surgery. 2019;48(6):923. [CrossRef]
- Swann HM, Holt DE. Canine gastric adenocarcinoma and leiomyosarcoma: A retrospective study of 21 cases (1986-1999) and literature review. J Am Anim Hosp Assoc. 2002;38(2):157–164.
- Eisele J, Mcclaran JK, Runge JJ, et al. Evaluation of risk factors for morbidity and mortality after pylorectomy and gastroduodenostomy in dogs. Veterinary Surgery. 2010;39(2):261–267. [CrossRef]
- Cândido MV, Syrjä P, Hanifeh M, et al. Gastric mucosal pathology in belgian shepherd dogs with and without clinical signs of gastric disease. Acta Vet Scand. 2021;63(1):1–15. [CrossRef]
- Bilek A, Hirt RA. Breed-associated increased occurrence of gastric carcinoma in chow-chows. Wien Tierarztl Monatsschr. 2007;94(3-4):71–79.
- Candido MV, Syrjä P, Kilpinen S, Spillmann T. Canine breeds associated with gastric carcinoma, metaplasia and dysplasia diagnosed by histopathology of endoscopic biopsy samples. Acta Vet Scand. 2018;60(1):1–9. [CrossRef]
- Fonda D, Gualtieri M, Scanziani E. Gastric carcinoma in the dog: A clinicopathological study of 11 cases. J Small Anim Pract. 1989;30(6):353–360. [CrossRef]
- Scanziani E, Giusti AM, Gualtieri M, Fonda D. Gastric carcinoma in the belgian shepherd dog. J Small Anim Pract. 1991;32:465–469. [CrossRef]
- Lubbes D, Mandigers PJJ, Heuven HCM, Teske E. Incidentie van maagcarcinomen bij de nederlandse tervuerense herders geboren tussen 1991 en 2002. Tijdschr Diergeneeskd. 2009;134(14-15):606–610.
- Wellmann R. Optimum contribution selection for animal breeding and conservation: The R package optiSel. BMC Bioinformatics. 2019;20(1):25–5. [CrossRef]
- Covarrubias-Pazaran G. Genome-assisted prediction of quantitative traits using the R package sommer. PLoS One. 2016;11(6):e0156744. [CrossRef]
- Kijan C, Hugen S, Thomas RE, et al. The histopathological characteristic of gastric carcinoma in the belgian tervueren and groenendael dog: A comparison of two classification methods. Animals (Basel). 2023;13(9):1532. [CrossRef]
- Cândido MV, Syrjä P, Hanifeh M, et al. Gastric mucosal pathology in belgian shepherd dogs with and without clinical signs of gastric disease. Acta Vet Scand. 2021;63(1):7–6. [CrossRef]
- Penninck DG, Moore AS, Gliatto J. Ultrasonography of canine gastric epithelial neoplasia. Veterinary Radiology and Ultrasound. 1998;39(4):342–348. [CrossRef]
- Simeoni F, Del Signore F, Terragni R, Tamburro R, Aste G, Vignoli M. Diagnostic imaging of gastrointestinal tumours in dogs and cats: A review. American Journal of Animal and Veterinary Sciences. 2020;15(2):89–101. [CrossRef]
- Marolf AJ, Bachand AM, Sharber J, Twedt DC. Comparison of endoscopy and sonography findings in dogs and cats with histologically confirmed gastric neoplasia. J Small Anim Pract. 2015;56(5):339–344. [CrossRef]
- Keijser SFA, Vernooij JCM, Rothuizen J, et al. PETscan: Measuring incidence of disease phenotypes to prioritize genetic studies in companion animals. Anim Genet. 2018;49(5):492–495. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).