1. Introduction
The musculoskeletal system plays a vital role in the human body, serving essential functions that involve both structural and metabolic. It supports structure, facilitates movement, protects internal organs, and contributes to metabolic processes related to mineral storage and blood cell production [
1,
2]. Musculoskeletal injury, the most common cause of disability and daily life limitations, presents a significant socioeconomic challenge [
3,
4,
5].
Regeneration of musculoskeletal injuries poses challenges in patients with extensive tissue damage, disease, and aging with reduced tissue regeneration capacity. Mesenchymal stem cells (MSCs) based therapy could be the option for repairing and regenerating musculoskeletal tissues. For example, osteoblasts produce a substantial volume of macromolecules in the bone matrix, including collagenous and noncollagenous proteins that provide a scaffold for matrix mineralization through the deposition of calcium phosphate in the form of hydroxyapatite. Moreover, osteoblasts specialize in matrix production and mineralization for the formation of strong bones. In a mouse model, the origins of osteoblasts include chondrocytes in the growth plate, quiescent bone-lining cells on the bone surface, specialized fibroblasts in the craniofacial structures, and MSCs in the bone marrow. Especially, MSCs in the bone marrow have been shown to be associated with bone development, regeneration, bone anabolism and pathological ossification [
6].
Bone marrow aspiration concentrate (BMAC) has become one of orthopedics’s most common cell-based musculoskeletal injury therapies. BMAC has the characteristic of a rich source of pluripotent MSCs and growth factors. Moreover, BMAC has anti-inflammatory and regenerative effects for treating musculoskeletal diseases. Many reports have highlighted using BMAC in various orthopedic fields, including osteonecrosis, nonunion of bone fracture, bone defects, and cartilage repair [
7,
8,
9,
10,
11,
12,
13,
14].
In biological MSCs-based therapy, the primary goal is to improve the methods for obtaining high concentrations of MSCs. Autologous bone marrow obtained from iliac crest bone marrow aspiration has been one of the most utilized sources for cell-based therapies in orthopedics. BMAC contains MSCs, red blood cells, white blood cells, platelets, hematopoietic cells, and nonhematopoietic precursors. The MSC population in BMAC is very few, typically estimated to be 0.01-0.02% of the total cell volume [
15,
16]. However, the aspiration technique for offering the highest yield is controversial, and the prevalence of MSCs remains obscure. There are various methods for obtaining bone marrow, but consensus still needs to be reached. Because of the aspiration volume can affect the numbers of MSCs, the method of aspiration at a single or multiple level may affect the concentration and quality of MSCs.
In this study, it was hypothesized that if bone marrow were aspirated from at multiple levels, more MSCs could be obtained due to each new aspirated bone marrows compared to bone marrow aspirated in a single level. The purpose of this study was to compare the concentration of MSCs and their potency of BMAC in two different techniques of bone marrow aspiration over the anterior iliac crest from the single level versus the multiple level using Fluorescence-activated Cell Sorting (FACS) analysis and osteogenic differentiation.
2. Materials and Methods
2.1. Antibodies and Reagents
PE-CyTM7 Mouse Anti-Human CD73, APC Anti-Human CD90, FITC Mouse Anti-Human CD105, and PE Mouse Anti-Human CD34 were purchased from BD Biosciences. Fetal bovine serum and Type IV collagenase was purchased from Gibco (Grand Island, NY, USA), ascorbic acid, β-glycerophosphate, and Alizarin Red was purchased from Sigma-Aldrich (St. Louis, MO, USA), and Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Lonza (Rockland, ME, USA).
2.2. Human Subjects
This study enrolled patients undergoing orthopedic surgery between 30 and 60 years old. The Institutional Review Board of Kyungpook National University Hospital approved using human bone marrow biopsies (IRB File No of KNUH 2021-12-028). We obtained written informed consent from all patients before the surgical procedure. The human sample list is described in
Table 1.
2.3. Bone Marrow Aspiration
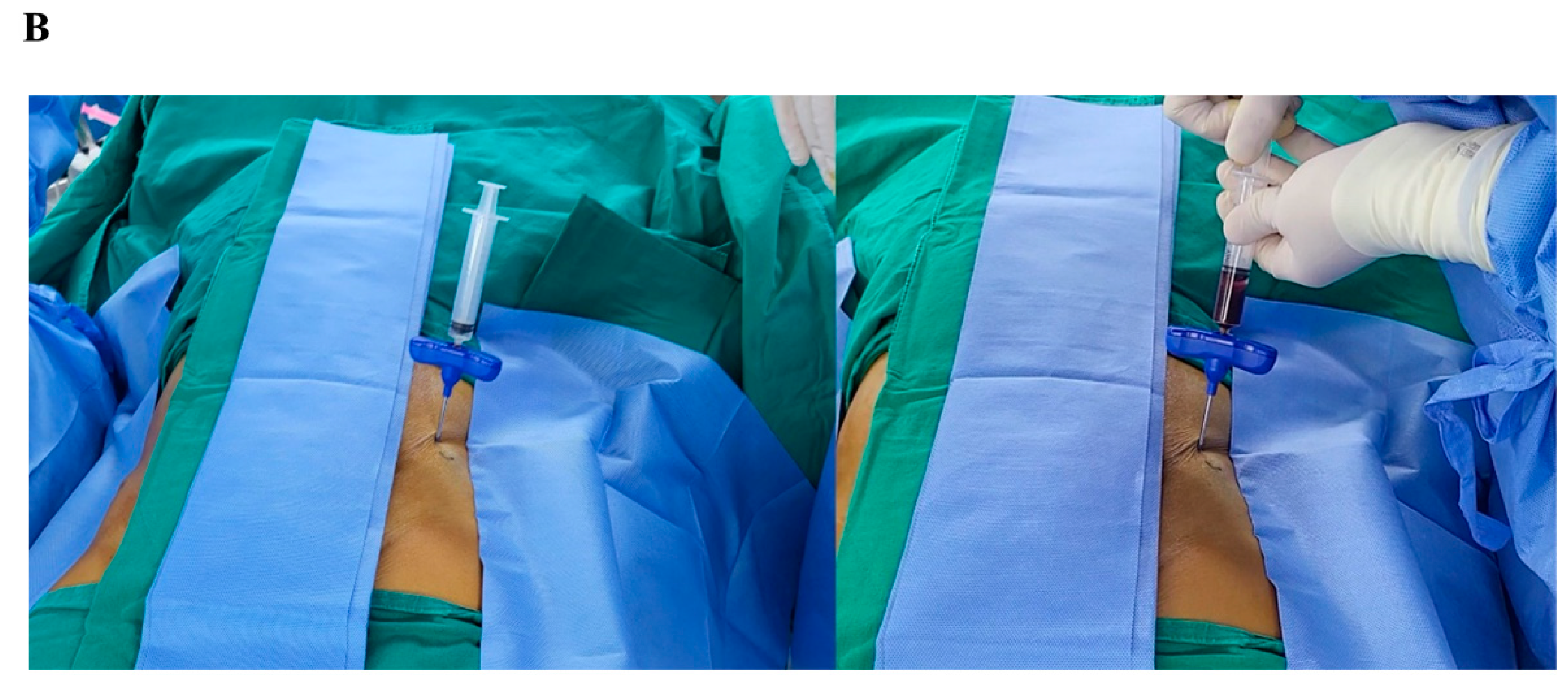
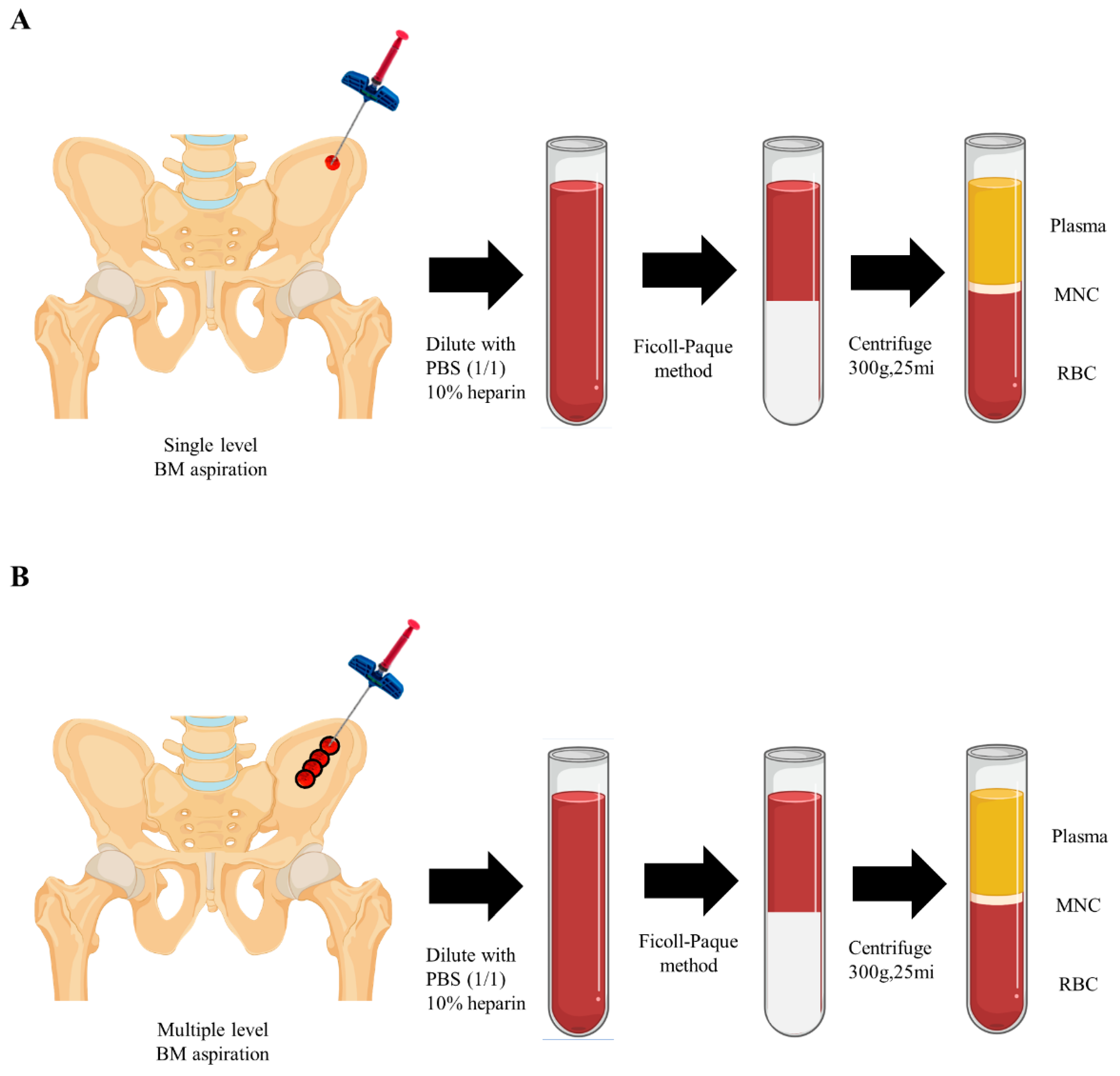
Bone marrow cells were isolated from the anterior iliac crest using single or multiple level needle puncture aspiration from 9 male patients (mean age 46.7 ±11.8 years, range 31 to 59 years) after receiving written informed consent. Briefly, bone marrow aspirates (4mL) were collected with the syringe with anticoagulant (10 U/mL heparin), and Ficoll-Paque (1.078 g/mL) method was used for cell separation.Anterior iliac crests were selected in 7 adult patients for marrow aspiration sites. Aspiration was achieved with an 11-gauge needle (length, 100 mm; diameter, 2.3 mm) specifically manufactured for bone marrow collection (BD, Becton, USA) connected to a 10-mL syringe (
Figure 1).
In single level (SL) samples, 4mL of bone marrow was aspirated at a single level of 7cm depth without changing the needle direction. In multiple level (ML) samples, on the other side over the same portion of the iliac crest, each 1mL of bone marrow was gained at multiple depths while retrieving the needle at a distance of 1cm and changing the tip direction with 180 degrees. A total of 4mL of aspirate was obtained (
Figure 2). The samples were blindly sent to the laboratory without the notice of methods.
2.4. Fluorescence-Activated Cell Sorting
We characterized bone marrow cells obtained from single or multiple level isolation methods by their mesenchymal phenotypes. MSC positive and negative markers were used to characterize the MSC phenotyping. For Fluorescence-activated Cell Sorting (FACS), the bone marrow cells were washed with FACS buffer containing 2.5% BSA and 0.25% sodium azide (Sigma A) in PBS and incubated with positive markers of CD73 (PE-CyTM7-conjugated), CD90 (APC-conjugated), CD105 (FITC-conjugated), and negative markers of CD34 (PE-conjugated) for 1 hour at 4 ℃ then washed briefly in FACS buffer. FACS data were obtained using a FACS Calibur II (BD Biosciences).
2.5. Cell Seeding and Osteogenic Differentiation
For osteogenic differentiation, bone marrow-derived mononuclear cells (MNCs) were seeded in the 24-well plate at a density of 1x10
4/cm2 and incubated in an osteogenic medium containing alpha-minimum essential medium (α-MEM, Gicbo BRL, Gaithersburg. MD) supplemented with 10% fetal bovine serum (FBS, Gibco BR, Gaithersburg. MD L), ascorbic acid (50 μg/mL) and beta-glycerophosphate (10 mM) for osteoblast differentiation [
17]. MSCs was cultured in an osteogenic medium for 28 days with medium change three times a week.
2.6. Alizarin Red Staining
Osteoblast mineralization was detected by Alizarin Red staining. After 28 days, cells were washed three times with PBS, fixed with 4% PFA (paraformaldehyde) for 10 min at room temperature, and stained with 2% alizarin red (pH 4.2) to evaluate calcium deposition in differentiated osteoblast. Samples were washed in filtered deionized water and analyzed with a Leica microscope.
2.7. Statistical Analysis
Statistical analyses were performed by GraphPad Prism 9 (GraphPad, San Diego), and data were expressed as mean ± standard deviation. Statistical significance was analyzed using a student’s t-test and indicated as follows: *p < 0.05, ***p < 0.01.
3. Results
3.1. Advanced MSC Population in a Single Level Bone Marrow Aspiration
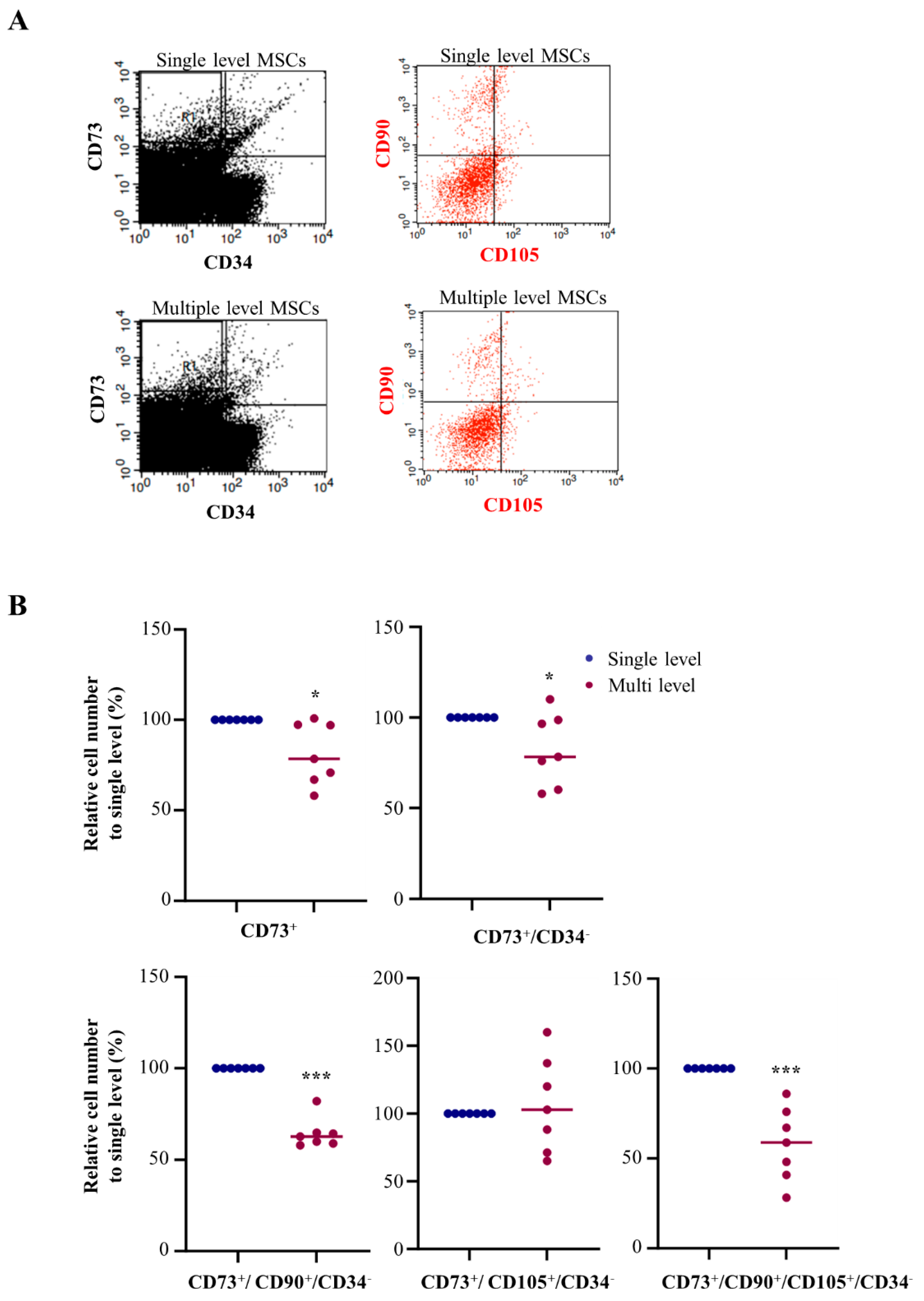
Separate MSCs were analyzed using FACS assay to assess the MSC population in single and multiple level using bone marrow aspiration methods. For quantification, high purity of MSC population CD73 (PE-CyTM7-conjugated), CD90 (APC-conjugated), and CD105 (FITC-conjugated) as a positive marker, while CD34 (PE-conjugated) was used as negative markers in FACS analysis (
Figure 3A). First, we checked the CD73+ cell numbers from the single and multiple level of bone marrow aspiration using FACS analysis. FACS analysis of the bone marrow cells obtained from each group revealed that multiple level bone marrow aspiration significantly decreased CD73 to 81.3% compared to single level bone marrow aspiration. To exclude the hematopoietic stem cells and adipose tissue derived-MSCs, we determined the cells CD73+ while CD34- using FACS analysis. Compared with single level bone marrow aspiration, multiple level bone marrow aspiration significantly decreased CD73+ and CD34- to 82.6%. In addition, to determine the high purify bone marrow derived-MSCs quantification, we determined the cells both CD90+ and CD105+ among the CD73+ while CD34- gated cells using FACS analysis. Compared with single level bone marrow aspiration, multiple level bone marrow aspiration significantly decreased CD90+ while CD73+ and CD34- to 64.3%; however, there was no significance in the CD105+ while CD73+ and CD34- cells. Finally, the number of CD90+ and CD105+ while CD73+ and CD34- cells in multiple level bone marrow aspiration was significantly decreased to 57.8% compared with single level bone marrow aspiration (
Figure 3B,
Table 2).
3.2. Advanced MSC Population in a Single Level Bone Marrow Aspiration
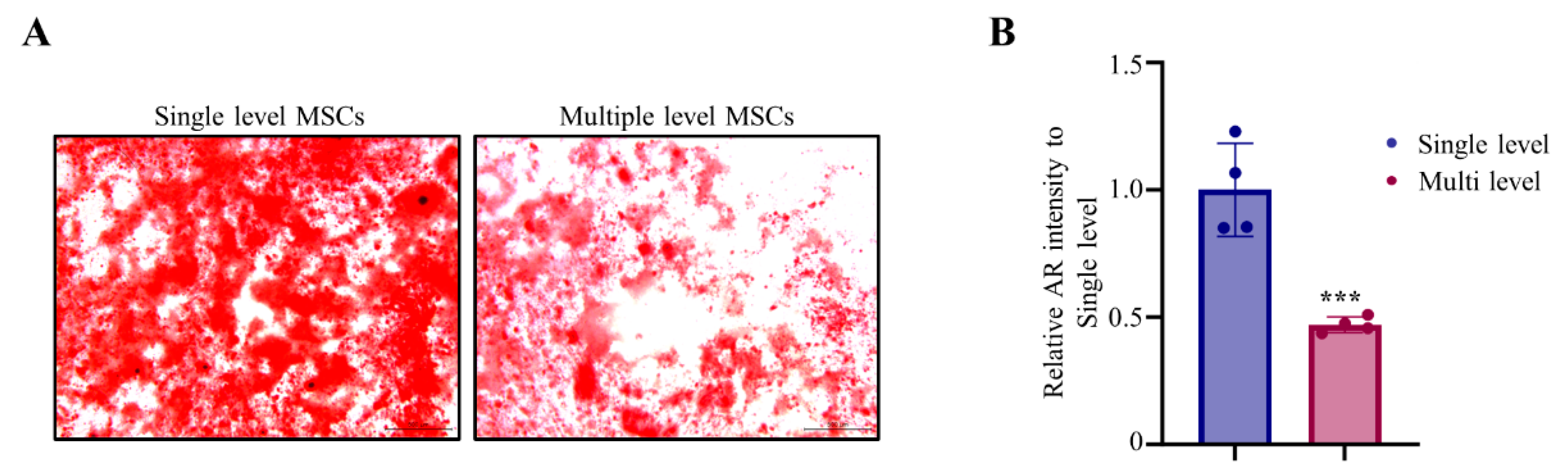
Next, we performed in vitro osteogenic differentiation using MNCs derived single and multiple level aspiration. Alizarin Red analysis of the bone marrow cells obtained from each group revealed that multiple level bone marrow aspiration significantly decreased bone mineralization to 47% compared to single level bone marrow aspiration. Mesenchymal stem cells of single level bone marrow aspiration were more rapidly differentiated into osteoblasts and promoted mineralization effects than those of multiple level bone marrow aspiration in Alizarin Red staining (
Figure 4A). The quantification of osteoblast mineralization areas was also showed significantly enhanced in SL compared with ML (
Figure 4B,
Table 3). These results indicate that single level bone marrow aspiration has promoted ability in osteogenic differentiation.
4. Discussion
This study found that bone marrow aspiration from a single level showed a higher population of MSCs than those of multiple level one. In osteoblast differentiation, MSCs exhibited more advanced features of osteoblastic abilities in the single level rather than the multiple level method. These results indicate that the single level bone marrow aspiration showed higher yield and prevalence of MSCs and advanced osteoblastic differentiation than the multiple level technique. The changes in depth during aspiration could affect the concentration and quality of MSCs and it is thought that the concentration of MSCs varies depending on the depth and is lower when performing aspiration BM at a shallower depth.
In orthopedic surgery, MSCs could be the options for regenerative treatment in various problems, and several studies are ongoing. MSCs enable the regeneration of musculoskeletal tissues, especially bone marrow stromal cells (BMSCs), which can differentiate into several lineages, including chondrocytes, osteoblasts, and adipocytes. BMSCs were commonly used sources of MSCs because of the easy collection method. Although BMSCs can be used as a cell suspension after being expanded by culture or as a concentrate, the clinical practice may be limited, considering the problems related to cell manipulation and expansion. However, BMAC can overcome the need for culture expansion, and minimal manipulation can be applied in a one-step treatment. It has been widely utilized in the clinical practice of orthopedic areas. It also could prevent the risk of allogenic disease and infection. Therefore, the clinical use of BMAC will expand further.
MSCs from BMAC could be applied in various areas of orthopedics. First, it could be used in the treatment of osteonecrosis. Hernigou et al [
16] suggested that ONFH may be the disease of bone cells and/or MSCs. The numbers and activity of MSCs decreased in bone marrow in patients with ONFH. Moreover, the number of progenitor cells in patients who underwent corticosteroid treatment was lower than in patients with a different underlying etiology. In this situation, BMAC could be the treatment option. Autologous MSCs exhibit anti-inflammatory, immunomodulatory, and tissue-protective “trophic” effects [
18]. BMAC also applies to osteoarthritis (OA) patients, especially in the knee. The treatment options were various in patients with knee OA. From non-pharmacological treatment, including weight loss and physical treatment, to pharmacological treatment, including oral medication and intraarticular injection, the various options can be applied by non-surgical treatment. Recently, the treatment using MSCs has been considered the new treatment option for OA due to its structural contributions to tissue repair, immunomodulatory and anti-inflammatory actions through direct cell-to-cell interaction, or secretion of bioactive factors. Finally, BMAC could be used in the treatment of fractures and nonunion. In the musculoskeletal system, bone is a dynamic self-healing tissue that continuously remodels in response to mechanical loadings throughout life. However, bone remodeling cannot repair between 5 and 10% of all bone fractures, resulting in delayed or failed healing in extensive traumatic injuries [
19,
20]. In clinical practice, the preferred approach for treating fractures is autologous iliac crest bone graft replacement, considered the gold standard for treatment [
21]. However, the application limitation was in the significant bone defect, and the donor site morbidity could be a problem after autologous bone graft. The other methods for nonunion, including allograft, bone substitutes, demineralized bone matrix (DBM), and ultrasound and pulsed electromagnetic field, could be used for bone healing. Osteogenic stem cells in bone marrow have the biological efficacy of cancellous bone [
22,
23]. Therefore, they would be applied to the treatment of fracture or nonunion [
24,
25].
In biological MSCs-based therapy, the primary goal is to improve the methods for obtaining high concentrations of MSCs. Autologous bone marrow obtained from iliac crest bone marrow aspiration has emerged as one of the most utilized sources for cell-based therapies in orthopedics. BMAC contains MSCs, red blood cells, white blood cells, platelets, hematopoietic cells, and nonhematopoietic precursors. The MSC population in BMAC is rare and typically estimated to be 0.01-0.02% of the total cell volume.15,16 The osteogenic capacity correlated with the cell concentration [
26], and the efficacy could be related to the number of progenitors.16 Therefore, efforts to increase the progenitor cells and maximize the MSC content at the time of the aspiration are essential, and accurate methods for bone marrow aspiration are very important for promising therapeutic options for orthopedic treatments. There still needs to be a consensus on the BMAC aspiration method. Numerous research studies have concentrated on enhancing bone regeneration by applying MSCs based therapy. Oliver et al [
27] reported no differences in cell concentration between the single site and multiple sites harvesting systems, but the pain was decreased in the single site technique. In this study, the single level bone marrow aspiration technique obtained a higher population of MSCs with MSC positive markers of CD73, CD90, and CD105, while negative MSC markers of CD34 compared with multiple level bone marrow aspiration technique. This is thought that changes in position, depth and direction during aspiration affect the concentration and quality of MSCs. Obtaining a high-quality bone marrow aspirate for clinical application could be helpful.
The standardized measurement and reporting on the yield and quality of bone marrow aspirate samples, the colony-forming unit, and the combination of different markers have been suggested to characterize MSCs present within BMAC. The International Society for Cellular Therapy (ISCT) has established standardized terminology and minimal criteria for characterized MSCs features involved, has plastic-adherent ability in standard culture system in vitro, expressed surface markers CD73, CD90, and CD105 and lack expression of hematopoietic markers CD34, CD45, CD14, CD19, and HLA-DR (human leukocyte antigen-antigen D-related), and can differentiate to osteoblasts, adipocytes, and chondroblasts in vitro culture system [
28,
29,
30,
31]. Our data determined that single level bone marrow aspiration technique obtained a higher population of MSCs with MSC positive markers of CD73, CD90, and CD105 while negative MSCs markers of CD34 compared with multiple level bone marrow aspiration technique.
The limitation of this study was a small number of patients. The volume of BM aspirate, the size of the syringe, and the pressure during aspiration could change the results. However, this study evaluated only the aspiration method between single and multiple level. Further larger-scale studies considering various methods of aspiration should be evaluated.
5. Conclusions
A high-quality bone marrow aspirate could be obtained through a single level aspiration technique at the anterior iliac crest rather than a multiple level aspiration technique. This approach is known for its effectiveness in obtaining specific populations of mesenchymal stem cells with the desired characteristics, making them valuable for regenerative therapies for musculoskeletal injuries.
Author Contributions
Conceptualization, X.-G.C., H.-J.K., C.-W.O. and J.-Y.C.; methodology, X.-G.C., H.-J.K., C.-W.O., and J.-Y.C.; software, X.-G.C. and H.-J.K.; validation, X.-G.C., H.-J.K., C.-W.O. and J.-Y.C.; formal analysis, X.-G.C., H.-J.K., K.-H, P., J.-W.K. and H.-S.K.; investigation, X-G.C. and H.-J.K.,; provided human samples, H.J.K., J.-W.K., K.-H, P., and H.S.K.; data curation, X-G.C., H.-J.K., C.-W.O. and J.Y.C.; writing—original draft preparation, X-G.C., H.-J.K., C.-W.O. and J.-Y.C.; writing—review and editing, X.J., J.-W.K., J.-O.L., K.-H, P. and H.-S.K..; visualization, X.-G.C. and H.-J.K.,; supervision, J.-O.L., and H.-S.K..; project administration, C.-W.O.; funding acquisition, C.-W.O.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korean Fund for Regenerative Medicine(KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare) (21C0705L1).
Institutional Review Board Statement
The study received approval from the Institutional Review Board (IRB) of Kyungpook National University Hospital under protocol number KNUH 2021-12-028. All methodologies adhered to pertinent guidelines and regulations. All patients signed informed consent before participating in this study.
Informed Consent Statement
The study received approval from the Institutional Review Board (IRB) of Kyungpook National University Hospital under protocol number KNUH 2021-12-028. All methodologies adhered to pertinent guidelines and regulations. All patients signed informed consent before participating in this study.
Data Availability Statement
Data is provided within the manuscript.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10:3815-[.
- Maestroni L, Read P, Bishop C, Papadopoulos K, Suchomel TJ, Comfort P, Turner A. The benefits of strength training on musculoskeletal system health: Practical applications for interdisciplinary care. Sports Med. 2020;50:1431-50.
- Briggs AM, Woolf AD, Dreinhofer K, Homb N, Hoy DG, Kopansky-Giles D, Akesson K, March L. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ. 2018;96:366-8.
- Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, Horst C, Kaldjian A, Matyasz T, Scott KW, Bui AL, Campbell M, Duber HC, Dunn AC, Flaxman AD, Fitzmaurice C, Naghavi M, Sadat N, Shieh P, Squires E, Yeung K, Murray CJL. Us health care spending by payer and health condition, 1996-2016. JAMA. 2020;323:863-84.
- Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, Chapin A, Hamavid H, Horst C, Johnson EK, Joseph J, Lavado R, Lomsadze L, Reynolds A, Squires E, Campbell M, DeCenso B, Dicker D, Flaxman AD, Gabert R, Highfill T, Naghavi M, Nightingale N, Templin T, Tobias MI, Vos T, Murray CJ. Us spending on personal health care and public health, 1996-2013. JAMA. 2016;316:2627-46.
- Mizoguchi T, Ono N. The diverse origin of bone-forming osteoblasts. J Bone Miner Res. 2021;36:1432-47.
- Haeusner S, Jaukovic A, Kupczyk E, Herrmann M. Review: Cellularity in bone marrow autografts for bone and fracture healing. Am J Physiol Cell Physiol. 2023;324:C517-C31.
- Modest JM, Lemme NJ, Testa EJ, Evans AR, Reid DBC. Successful fracture healing for femoral neck nonunion with bone marrow aspirate concentrate. R I Med J (2013). 2022;105:13-6.
- Chahla J, Alland JA, Verma NN. Bone marrow aspirate concentrate for orthopaedic use. Orthop Nurs. 2018;37:379-81.
- Yoshioka T, Mishima H, Akaogi H, Sakai S, Li M, Ochiai N. Concentrated autologous bone marrow aspirate transplantation treatment for corticosteroid-induced osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2011;35:823-9.
- Piuzzi NS, Chahla J, Schrock JB, LaPrade RF, Pascual-Garrido C, Mont MA, Muschler GF. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: A systematic review of the literature. J Arthroplasty. 2017;32:1698-708.
- Kim GB, Seo MS, Park WT, Lee GW. Bone marrow aspirate concentrate: Its uses in osteoarthritis. Int J Mol Sci. 2020;21:3224.
- Ha CW, Park YB. Editorial commentary: Considering clinical application of bone marrow aspirate concentrate for restoration of cartilage defects in the knee? Is it a kind of stem cell therapy? Arthroscopy. 2019;35:1878-9.
- Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT, Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77-90.
- Schafer R, DeBaun MR, Fleck E, Centeno CJ, Kraft D, Leibacher J, Bieback K, Seifried E, Dragoo JL. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J Transl Med. 2019;17:115.
- Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430-7.
- Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, Jo JS, Ryoo HM. Expression patterns of bone-related proteins during osteoblastic differentiation in mc3t3-e1 cells. J Cell Biochem. 1996;61:609-18.
- Caplan AI. Why are mscs therapeutic? New data: New insight. J Pathol. 2009;217:318-24.
- Ekegren CL, Edwards ER, de Steiger R, Gabbe BJ. Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int J Environ Res Public Health. 2018;15:2845.
- Marolt Presen D, Traweger A, Gimona M, Redl H. Mesenchymal stromal cell-based bone regeneration therapies: From cell transplantation and tissue engineering to therapeutic secretomes and extracellular vesicles. Front Bioeng Biotechnol. 2019;7:352.
- Rodriguez-Merchan EC. A review of recent developments in the molecular mechanisms of bone healing. Int J Mol Sci. 2021;22:1815-26.
- Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989:270-80.
- Burwell RG. The function of bone marrow in the incorporation of a bone graft. Clin Orthop Relat Res. 1985:125-41.
- Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991:259-70.
- Healey JH, Zimmerman PA, McDonnell JM, Lane JM. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990:280-5.
- Connolly J, Guse R, Lippiello L, Dehne R. Development of an osteogenic bone-marrow preparation. J Bone Joint Surg Am. 1989;71:684-91.
- Oliver K, Awan T, Bayes M. Single- versus multiple-site harvesting techniques for bone marrow concentrate: Evaluation of aspirate quality and pain. Orthop J Sports Med. 2017;5:2325967117724398.
- Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schafer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146.
- Piuzzi NS, Mantripragada VP, Sumski A, Selvam S, Boehm C, Muschler GF. Bone marrow-derived cellular therapies in orthopaedics: Part i: Recommendations for bone marrow aspiration technique and safety. JBJS Rev. 2018;6:e4.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315-7.
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells--the international society for cellular therapy (isct) working proposal. Cytotherapy. 2013;15:1054-61.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).