1. Introduction
It was in 1888 that Hofmeister reported that ions influenced the solubility of proteins and that some ions enhanced protein solubility, known as chaotropes, and others induced precipitation, known as kosmotropes . He observed that anions have a greater effect than cations [
1]. Later it has been observed that the trend identified by Hofmeister is broadly ubiquitous for the effect of ions on macromolecules and similar water dispersible materials and even that similar effects are observed in other polar solvents and not just water [
2,
3,
4,
5].
Figure 1 presents a simplified version of the Hofmeister series.
The origin of this effect has proven to be hard to tie down. Initially it was thought that the ions affect the bulk structure of water disrupts the hydrogen bonding. However recent work has shown that this is not the case.
To investigate the influence of anions on aqueous solution structure, Bakker and co-workers measured the orientational correlation time for water molecules in various salt solutions [
5]. The results showed that correlation times for first hydration shell water molecules of Cl
−, Br
−, I
−, ClO
4− or SO
42− were much slower than those for bulk water, as might be expected. However, the experiments clearly showed that these anions had no influence on the dynamics of bulk water, even at very high concentrations (up to 6 M) of both kosmotropic ions e.g. (SO
42−) and chaotropic ions e.g. (ClO
4−) [
5].
Synthetic polymers such as poly-N-isopropyl acrylamide, (polyNIPAM) respond in an analogous way to ions as proteins. Such polymers have a lower consolute solution temperature (LCST), which is the temperature when the polymer comes out of solution [
6,
7]. The sodium salts of several anions were studied and the ability of a particular anion to lower the LCST followed the Hofmeister series. Significantly, the effect of the anions could be explained based on three direct interactions of the anions with the macromolecule and its immediately adjacent hydration shell. Firstly, kosmotropic anions were found to polarize water molecules that were directly hydrogen bonded to the polymer; secondly, both chaotropes and kosmotropes could increase the cost of hydrophobic hydration; and finally, chaotropic anions could bind directly to the polymer. The first two effects lead to the lowering of the LCST, whilst the third increased the LCST.
Similar effects have also been seen for the polymer polyethylene oxide (also known as polyethylene glycol). In a series of papers Boucher et al [
8,
9,
10,
11] have reported on the effect of various electrolytes on the theta temperature for polyethylene oxide solutions. (Note strictly they report on the LCST rather than the theta temperature as the latter is the LCST for an infinite molecular weight polymer).
High molecular weight polyethylene oxide in the presence of certain silica-based minerals such as silica nanoparticles [
12,
13,
14,
15,
16,
17,
18,
19], or the synthetic clay laponite, [
20,
21,
22] has some interesting and rather strange behavior. Such mixtures are shear thickening, but not in the normal shear thickening way where at high particle volume fractions the particles are jammed together at high shear, such as is observed with corn flour or indeed sand on a beach [
18,
19,
23]. In these systems the particle volume fraction is typically around 20%, not high enough for the particles to be jammed. Moreover, the time for the system to revert to its original state is much longer, and ranges from seconds to days. Such systems have become known as shake gels, as the simplest way to form them is by simply shaking a mixture of the polymer and nanoparticles. It is believed that in these systems the particles, which are smaller than the size of the polymer coils, bridge between polyethylene oxide. In the liquid state the polymer is in its random coil state and the particles bridge intramolecularly, on shaking (or experiencing extensional shear), the random coils stretch and some of the intramolecular “bonds” are broken, leaving bare sites on the silica such that polyethylene oxide from another molecule adsorbs on these sites, effectively crosslinking the polymer and hence forming a gel. Over time when the system is no longer being stretched, the polymer reverts to its random coil configuration, which is its lowest energy state, by the inter molecular bonds between the polymer and silica being replaced by intramolecular ones.
It is not straightforward to characterize the nature of the shake gels. It is relatively simple to create a “phase diagram” of which formulations form a gel and which do not, as the formulation can simply be shaken, but to characterize how easily the gel forms a breakup is not so straightforward. In recent papers of ours we have used rheological techniques for this purpose by shearing the sample at a high shear rate until its forms a gel and then dropping the shear rate to a low value and then monitoring the drop in viscosity with time. In this way the gelation time and relaxation time of the system can be determined. In our earlier papers we determined how the polymer and silica concentration, polymer molecular weight and temperature affect these parameters [
24,
25]. Also, we investigated the effect of adding an electrolyte, namely sodium and calcium chloride and found that for these electrolytes their effect was to aggregate the silica, such that above a critical electrolyte concentration the system aggregated without any shear being applied and this effect was irreversible [
26].
Since the polymer used in making these shake gels was polyethylene oxide, whose stability is affected by ions in the Hofmeister series, in this paper we investigate the effect of both kosmotropes and chaotropes, has on the formation of shake gels and find that trends with the Hofmeister series are observed.
2. Materials and Methods
2.1. Materials
The shake-gels in this study were created and characterized using polyethylene oxide (PEO) (Sigma Aldrich, 900,000 g.mol-1; Lot # MKBS9423V), silica LUDOX- TM50 (Sigma Aldrich - Merck; average diameter 22nm; Batch#: MKCP8331) at a weight concentration of 50% silica particles, as well as salts that include Sodium chloride (NaCl, Sigma Aldrich - Merck), Calcium chloride (CaCl2, Sigma Aldrich - Merck), Ammonium Chloride ( NH4Cl , Sigma Aldrich – Merck), Sodium Sulphate (Na2SO4 , Sigma Aldrich – Merck) , Sodium Iodide (NaI , Sigma Aldrich – Merck), Sodium perchlorate (NaClO4, Sigma Aldrich – Merck).
2.2. Shake Gel Preparation
Initially, deionized water was used to prepare a 2 wt% stock solution of PEO. Subsequently, the necessary proportions of the silica suspension, PEO solution (2 wt%) and deionized water were combined to achieve the necessary shaking gel concentrations. In this work, the concentrations of silica and PEO were maintained at 25 wt% and 3500 ppm (0.35 wt%), respectively, whereas constant 0.02 M concentration of various salts were added according to our investigation. These concentrations were selected because they easily created shake gels in the rheometer. The pH range of the samples was approximately pH 9-9.5. Initially, the samples were shaken forcefully until they formed a gel, they were then left for a full day (24 hours) before undergoing rheological characterization.
It is important to keep in mind that to produce consistent results, this process has to be strictly followed. When this was not done, or when other batches of silica or PEO were employed, the absolute gelation and relaxation times varied, but the overall patterns stayed the same.
2.3. Rheology Experimental Procedure
The overall protocol that was followed was similar to that of our earlier paper [
26], where a full description of protocol is written and here only a brief description is written.
The flow curves were generated using a stress-controlled Haake Mars Rheometer model 60, equipped with a range from a minimum torque rotation of 10-5 mNm to a maximum torque rotation of 200 mNm. This rheometer employed a double gap concentric cylinder geometry, with dimensions measuring 40.00 mm in length, 21.2 mm in diameter, and 27.2 mm in cup diameter. Data analysis was conducted using RheoWin software. The temperature was kept constant at 25 °C.
To understand the viscosity-time flow curve, the procedure began by filling the rheometer cup with a 3 ml sample of shake gel using a measuring cylinder. Initially, the sample underwent shearing for 60 seconds at a velocity of 1 s-1. Following this, the shear rate was rapidly increased to a high value (4000 s-1) for a specific duration, during which gel formation occurred, leading to a sudden surge in viscosity.
Subsequently, the shear rate was reduced back to 1 s-1, and the relaxation process was observed over time.
3. Results
3.1. Effect of Cations
3.1.1. Gelation Time
The study aims to explore the impact of varying the cations according to the Hofmeister series in cations while maintaining a constant anion (Cl-). All salt concentrations were maintained at 0.02M, with constant concentrations of silica at 25 wt% and PEO at 3500 ppm (0.35 wt%). The focus was specifically on observing the time required for the sample to initiate gel formation and the duration needed for the gel to revert to its original liquid state.
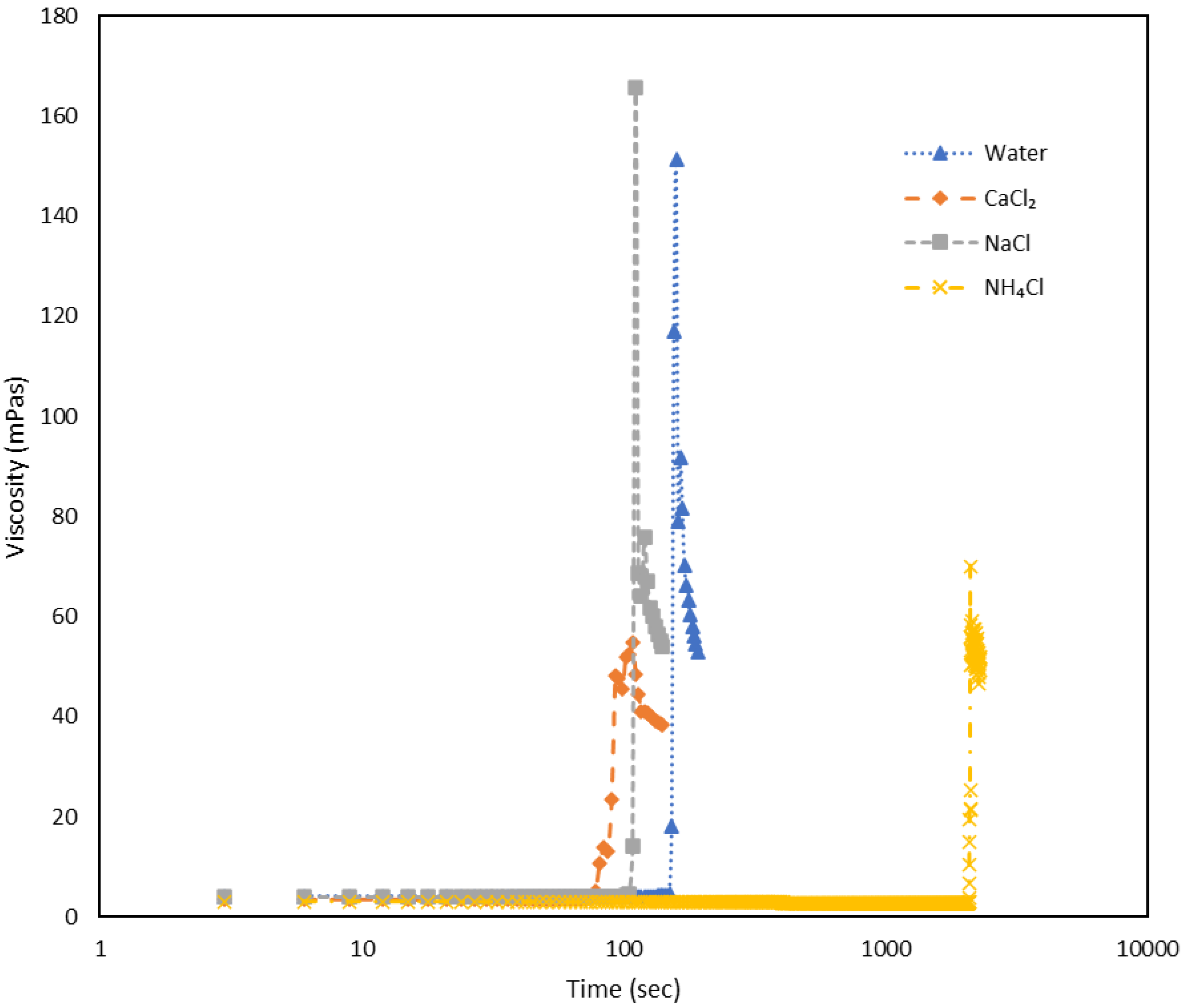
Figure 2 presents the viscosity against time following the application of a shear rate of 4000 s
-1. A noticeable trend emerges. High shear application in each case induced gelation of the solution, evident from the upsurge in viscosity, from which the gelation time can be determined.
Table 1 shows the gelation time of the different salts, and for comparison the gelation time in water is also given.
As it can been seen that NH4Cl takes much more time to form the gel and consecutively NaCl and followed by that CaCl2 quickly forms a gel.
3.1.2. Relaxation Time
In the experimental setup, after the high shear phase, the shear rate was decreased to 1 s⁻¹. The findings are detailed in
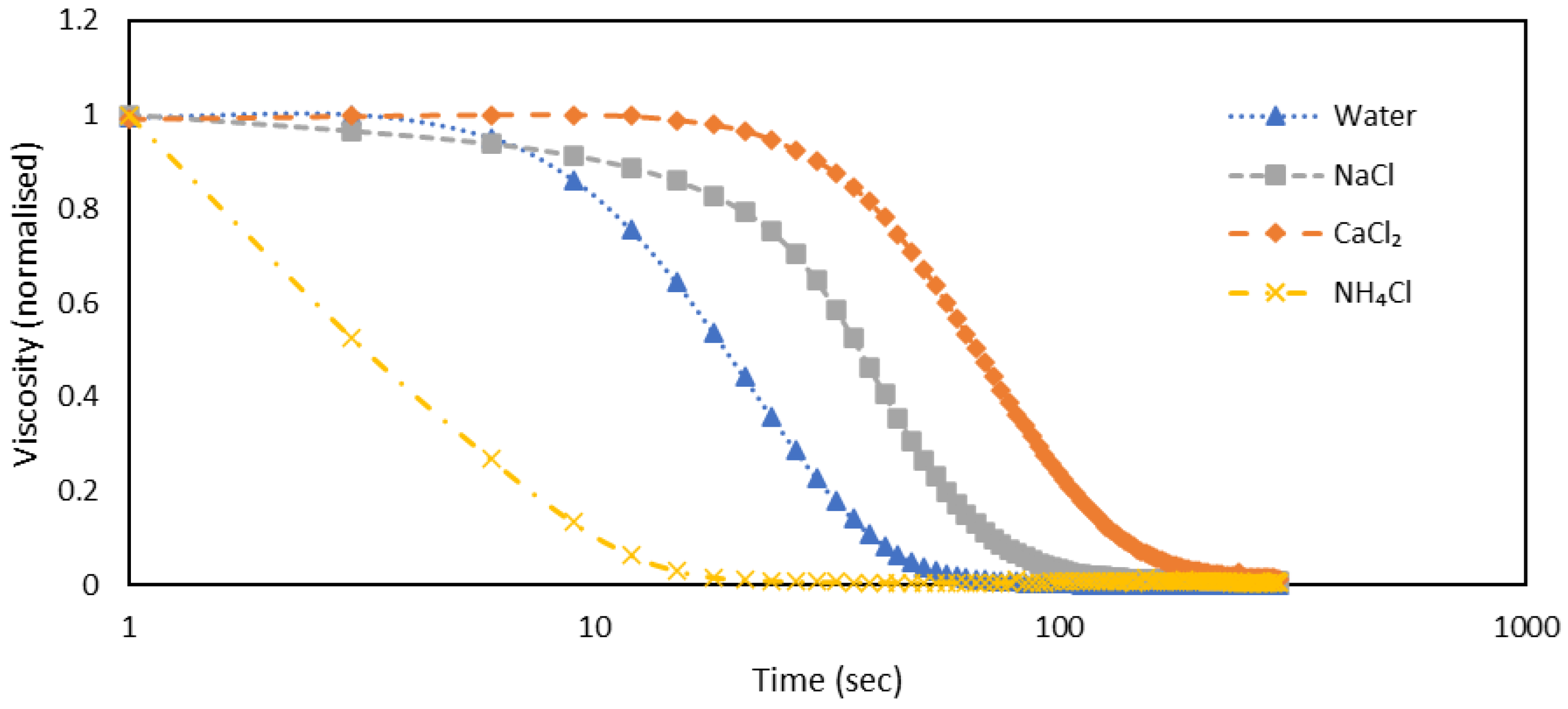
Figure 3, where the viscosity is normalized by dividing all viscosity measurements by the highest value of viscosity. This normalization was necessary because initial values varied among samples, making it challenging to discern trends without this adjustment. The absolute values for each parameter utilized in the relaxation process can be found in
Figure S1 of the supplemental information.
In this plot, we observe that CaCl₂ takes around 200 seconds to return to a liquid state, remaining in a gelled state for approximately 24 seconds before starting to drop. In the case of NaCl, it takes about 125 seconds to relax, with the gelled state lasting around 15 seconds. For NH₄Cl, it takes around 24 seconds to relax, but the viscosity drops almost instantly once the shear is stopped. This suggests that the gel formed by NH₄Cl is not as strong, whereas CaCl₂ forms a much stronger gel. Also, the relaxation process' half-life, or the amount of time it takes for the rheological parameter to be reduced in half from its starting value, is shown in
Table 2.
It is evident by looking at the half lifetime that CaCl2 takes much more time to relax followed by that NaCl and NH4Cl quickly relaxes.
3.2. Effect of Anions
3.2.1. Gelation Time
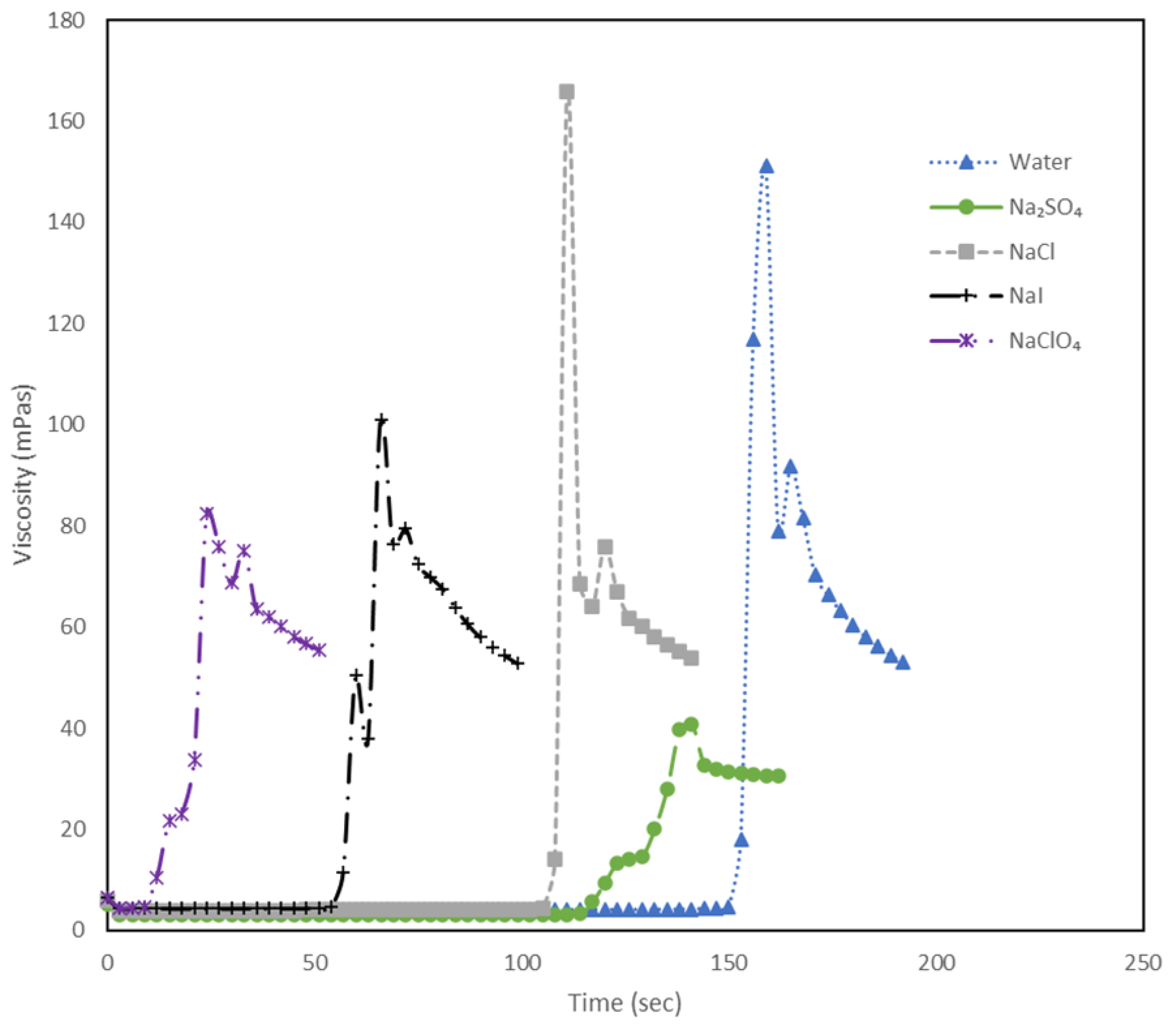
Similar experiments were then performed to investigate the effect of anions on the shake gel by maintaining the cation constant (Na
+). In
Figure 4, depicting viscosity against time for the initial 250 seconds after applying a shear rate of 4000 s
-1 , a consistent pattern emerges.
Here, it can be observed that NaClO
4 quickly gels , whereas the Na
2SO
4 takes much longer to form a gel.
Table 3 illustrates the gelation time or the time when a sudden spike of viscosity takes place.
3.2.2. Relaxation Time
In the experimental sequence, following the high shear phase, the shear rate was decreased to 1 s⁻¹. The outcomes are presented in
Figure 5, where the viscosity is again normalized by dividing all the viscosity values by the highest value of viscosity. This normalization was necessary because of variations in the initial viscosity values among the samples, making it difficult to discern trends without this adjustment. Detailed absolute values for each parameter involved in the relaxation process can be found in
Figure S2 of the supplemental data.
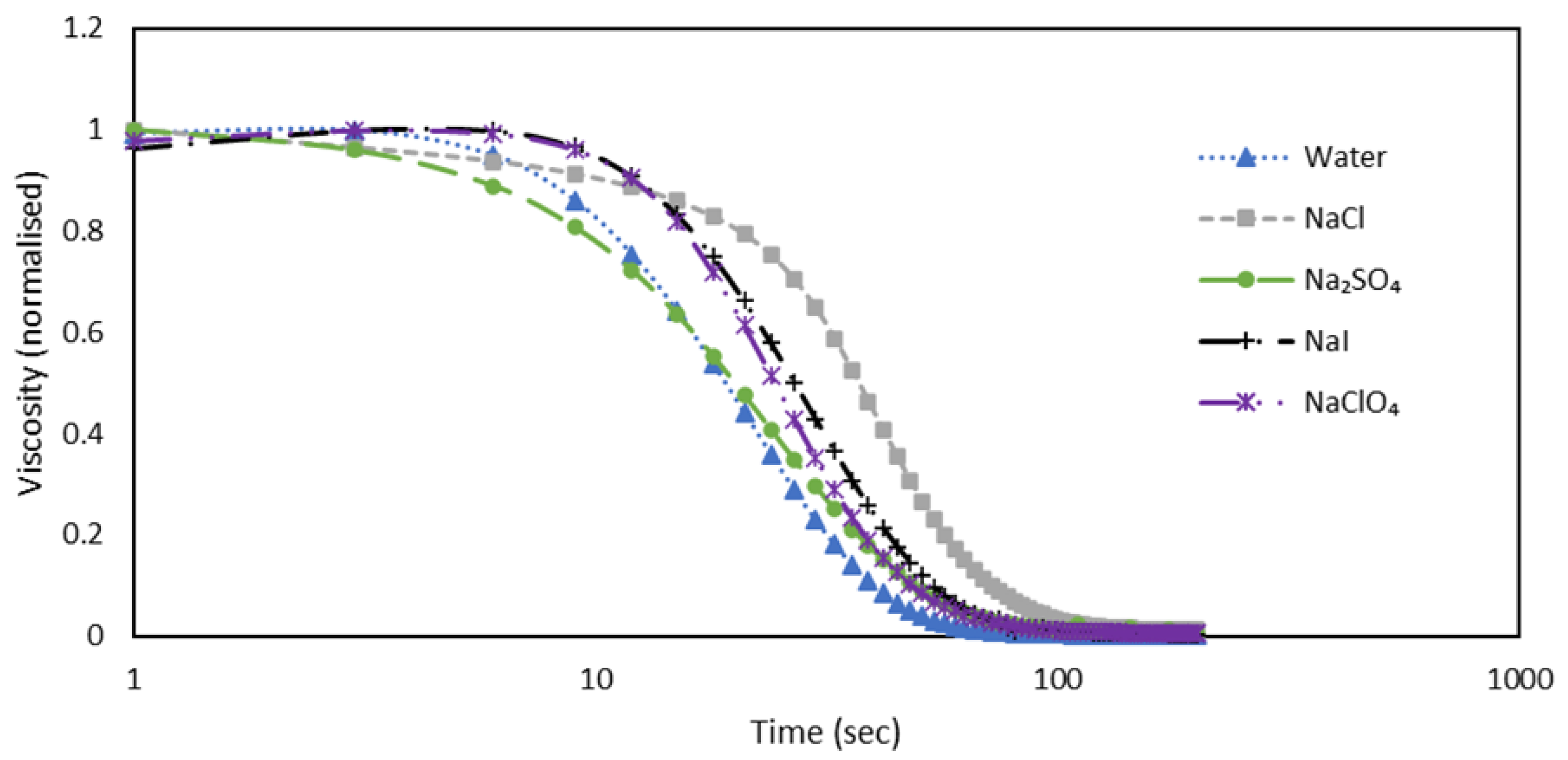
In this plot, we see that NaClO₄ takes around 90 seconds to return to a liquid state, remaining in a gelled state for approximately 9 seconds before starting to drop. For NaI, it takes about 75 seconds to relax, with the gelled state lasting around 6 seconds. In the case of NaCl, it takes about 125 seconds to relax, with the gelled state lasting around 15 seconds. For Na₂SO₄, it takes about 90 seconds to relax, but the viscosity drops almost instantly once the shear is stopped, suggesting that the gel formed by Na₂SO₄ is not strong. The half-life of the relaxation process, or the time it takes for the viscosity to reduce by half from its initial value, is shown in
Table 4.
It is hard to determine any trends in the half-life and discern based on this data, as the values are quite similar and fall within the margin of error of error of the experiment.
4. Discussion
Previous research has extensively investigated the formation of shake gels, revealing that in a quiescent state, silica particles interact with polyethylene oxide (PEO) in a manner similar to currents in a bun, yet they fail to bridge between polymer molecules for reasons that remain unclear. Under shear, the polymer's random coil configuration expands, disrupting some of the silica-polymer connections and enhancing the potential for crosslinking. Once crosslinking occurs, the system's rheological properties increase, leading to gel formation. On the removal of the shear. the polymer gradually reverts to its original random coil configuration. In our previous work we have shown that increasing the electrolyte concentration accelerates gelation and decreases relaxation time in the presence of NaCl and CaCl2. Interestingly, at specific concentrations (0.02M), CaCl2 exhibits faster gelation and significantly slower relaxation compared to NaCl.
In this work, our focus is on understanding how the rheological behavior within our system is affected by the presence of salts that spread the range of salting in and salting out ion in the Hofmeister series.
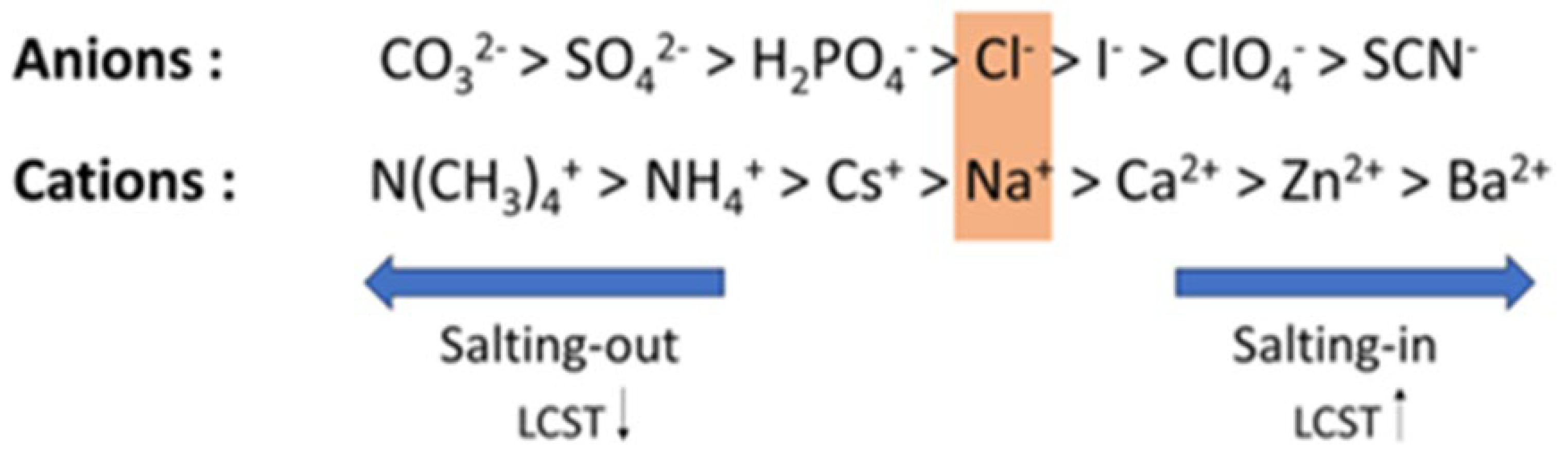
Firstly, let us examine the time taken to form the shake gel under a constant shear rate in the case of cations and anions. According to
Figure 2, in the case of cations, CaCl
2 forms a gel the quickest, at 78 seconds, followed by NaCl at 105 seconds, and finally NH
4Cl, which takes much longer at 2094 seconds to form a gel, significantly longer than the others. Similarly, according to
Figure 4, in the case of anions, NaClO
4 forms a gel the quickest, at 9 seconds, followed by NaI at 54 seconds, NaCl at 105 seconds, and lastly Na
2SO
4 at 117 seconds. Thus, referring to the Hofmeister series from
Figure 1, we observe that salts positioned towards the right-hand side (salting-in side) lead to quicker gelation, while those on the left-hand side (salting-out side) result in slower gelation. This relationship highlights the impact of ion-specific effects on the gelation process.
Before explaining this observation, it is essential to understand the Hofmeister series, which ranks ions based on their ability to stabilize or destabilize macromolecules in solution. The ions located on the right side of the series (chaotropes) (e.g., ClO4-) enhance polymer stability and increase the phase separation temperature (LCST), a phenomenon known as the salting-in effect. Conversely, ions located on the left side of the series (kosmotropes) (e.g.,SO42- ) exhibit a stronger destabilizing effect, decreasing the collapse temperature and producing a salting-out effect. Below the phase transition (collapse) temperature, the polymer chain is hydrated, swollen, expanded, and considered hydrophilic. When heated to the collapse temperature, which is concentration-dependent, the polymer chain shrinks and dehydrates, becoming a hydrophobic collapsed globule.
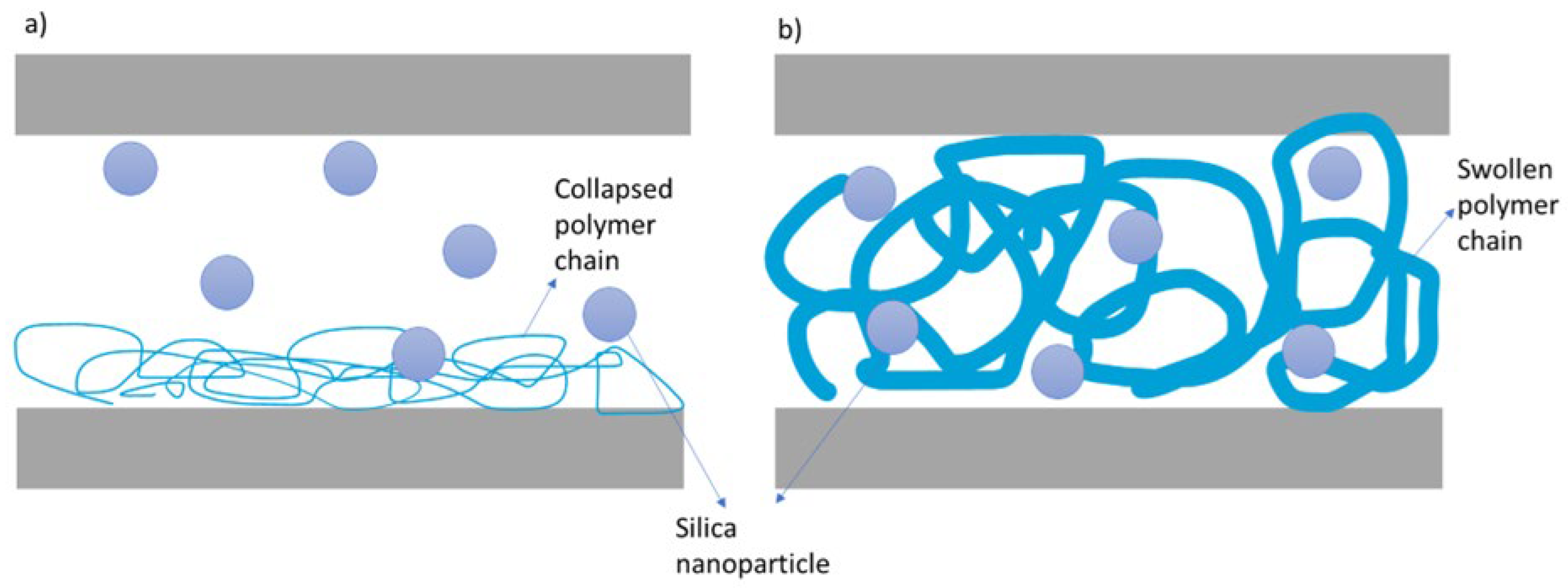
The viscosity of a collapsed polymer generally decreases. In this collapsed state, the polymer chains lose their hydration, adopting a more compact conformation. This reduction in volume and compact arrangement leads to fewer interactions and entanglements between the polymer chains, allowing the solvent molecules to move more freely. As a result, during shear, it becomes difficult to expand and break the polymer, taking more time for the polymer to bond with silica nanoparticles, resulting in slower gelation as shown in
Figure 6a.
On the other hand, the viscosity of a swollen and expanded polymer increases due to the fully hydrated, extended polymer chains occupying more volume in the solution. This extended conformation leads to greater interactions and entanglements between the polymer chains. As the polymer absorbs water and swells, it expands, forming a network that restricts the movement of solvent molecules. Hence, applying a high shear quickly expands the swollen polymer and also it is already expanded, breaking sooner, and bridging with silica nanoparticles to convert to a gel state as shown in
Figure 6b. This phenomenon is similar to the salts located on the right side of the Hofmeister series (chaotropes), where quicker gelation is observed. The degree of this duration depends on the positioning of the salt in the Hofmeister series and the affinity of the polymer-salt interaction.
Secondly, we analysed the time taken for the gel to relax back to its liquid state under a constant shear rate. According to
Figure 3, in the case of cations, the half-life times for relaxation are as follows: NH
4Cl relaxes very quickly at 3 seconds, NaCl takes 24 seconds, and CaCl
2 takes the longest at 45 seconds. Similarly,
Figure 4 shows the half-life times for anions: Na
2SO
4 takes around 21 seconds, NaCl takes 24 seconds, NaI also takes 24 seconds, and NaClO
4 takes around 18 seconds. Although the trend for anions is not as clear, a pattern emerges when considering the Hofmeister series.
Referring to the Hofmeister series, salts located on the right-hand side lead to quicker gelation and form a stronger gel, resulting in slower relaxation. Conversely, salts on the left-hand side result in slower gelation and quicker relaxation.
Explaining the relaxation mechanism with reference to LCST (Lower Critical Solution Temperature), salts on the right-hand side of the Hofmeister series stabilize the polymer and lead to a more swollen configuration. In this state, the polymer is hydrated and bonded with silica nanoparticles, forming stable bonds that take longer to break. The swollen condition restricts polymer movement, prolonging the time required to relax back to the liquid state. On the other hand, salts on the left-hand side lead to a collapsed state where the polymers are very unstable. As soon as the shear is stopped, the polymer tries to return to its random coil configuration (its lowest free energy state), which it achieves by the intermolecular bonds between the polymer and silica particles being replaced by intermolecular bond.
5. Conclusions
This study investigates a shear-induced transition from fluid to solid and back to fluid in mixtures of colloids and polymers, focusing on the interaction between silica nanoparticles and polyethylene oxide (PEO). In these mixtures, PEO adsorbs onto the silica nanoparticles, and when the adsorption is not fully saturated, PEO can bridge the silica particles, leading to the formation of aggregates. Under strong shear, some PEO bonds desorb and reabsorb onto other silica nanoparticles, causing the aggregates to grow significantly and form a gel. After gelation, the PEO detaches from the nanoparticles and returns to its random coil conformation, a process referred to as relaxation.
The transition to gelation is marked by a substantial change in viscosity, spanning several orders of magnitude. Observing the time taken for the material to revert to a liquid state at a consistent shear rate provides insight into the relaxation process. The gelation time varied significantly across experiments, ranging from seconds to many minutes, with some systems taking even longer.
This work specifically examines the impact of salts within the framework of the Hofmeister series, which ranks ions based on their ability to stabilize or destabilize macromolecules in solution. The study found that salts on the right-hand side of the Hofmeister series (salting-in agents or chaotropes) promote quicker gelation and slower relaxation, while those on the left-hand side (salting-out agents or kosmotropes) result in slower gelation and faster relaxation. This distinct behavior is attributed to the effect of the salts on the polymer: salting-in agents cause the polymer to have more hydrogen bonded water and so swell, making it more stable and allowing it to bond quickly with the nanoparticles, leading to faster gelation and a more prolonged relaxation. In contrast, salting-out agents cause the polymer to have less hydrogen bonded water associated with them and so collapse, slowing down the bonding process with silica and leading to slower gelation and quicker relaxation as the unstable polymer rapidly loses its bond with silica and returns to its random coil conformation.
Author Contributions
Devajyoti Banerjee – Methodology, Software, Formal Analysis, Investigation, Data curation, Writing – original draft preparation, Writing – Reviewing and editing, Visualization. Paul F. Luckham – Conceptualization, Methodology, Software, Validation, Resources, Writing – Reviewing and editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and also in supplementary information.
Acknowledgments
We would like to thank Jin Hau Lew and Adrielle Sousa Santos for their support and assistance in conducting some of the experiments, as well as for the engaging discussions.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- F. Hofmeister, Zur Lehre Von Der Wirkung der Salz "About the science of the effect of salts"., Naunyn-Schmiedeberg's Arch. Pharmacol., , 24 (1888) 247-260.
- K.P. Gregory, E.J. Wanless, G.B. Webber, V.S.J. Craig, A.J. Page, The electrostatic origins of specific ion effects: quantifying the Hofmeister series for anions, Chemical Science, 12 (2021) 15007-15015.10.1039/D1SC03568A. [CrossRef]
- K.P. Gregory, G.B. Webber, E.J. Wanless, A.J. Page, Lewis Strength Determines Specific-Ion Effects in Aqueous and Nonaqueous Solvents, The Journal of Physical Chemistry A, 123 (2019) 6420-6429.10.1021/acs.jpca.9b04004. [CrossRef]
- V. Mazzini, V.S.J. Craig, Specific-ion effects in non-aqueous systems, Current Opinion in Colloid & Interface Science, 23 (2016) 82-93. [CrossRef]
- A.W. Omta, M.F. Kropman, S. Woutersen, H.J. Bakker, Negligible Effect of Ions on the Hydrogen-Bond Structure in Liquid Water, Science, 301 (2003) 347-349. [CrossRef]
- R. Freitag, F. Garret-Flaudy, Salt Effects on the Thermoprecipitation of Poly-(N-isopropylacrylamide) Oligomers from Aqueous Solution, Langmuir, 18 (2002) 3434-3440.10.1021/la0106440.
- H.G. Schild, D.A. Tirrell, Microcalorimetric detection of lower critical solution temperatures in aqueous polymer solutions, The Journal of Physical Chemistry, 94 (1990) 4352-4356.10.1021/j100373a088.
- M. Ataman, Properties of aqueous salt solutions of poly(ethylene oxide). Cloud points, θ temperatures, Colloid and Polymer Science, 265 (1987) 19-25.10.1007/BF01422658.
- M. Ataman, E.A. Boucher, Properties of aqueous salt solutions of poly(ethylene oxide), Journal of Polymer Science: Polymer Physics Edition, 20 (1982) 1585-1592. [CrossRef]
- E.A. Boucher, P.M. Hines, Effects of inorganic salts on the properties of aqueous poly(ethylene oxide) solutions, Journal of Polymer Science: Polymer Physics Edition, 14 (1976) 2241-2251. [CrossRef]
- E.A. Boucher, P.M. Hines, Properties of aqueous salt solutions of poly(ethylene oxide): Thermodynamic quantities based on viscosity and other measurements, Journal of Polymer Science: Polymer Physics Edition, 16 (1978) 501-511. [CrossRef]
- B. Cabane, K. Wong, P. Lindner, F. Lafuma, Shear induced gelation of colloidal dispersions, Journal of Rheology, 41 (1997) 531-547.10.1122/1.550874.
- H. Collini, M. Mohr, P. Luckham, J. Shan, A. Russell, The effects of polymer concentration, shear rate and temperature on the gelation time of aqueous Silica-Poly(ethylene-oxide) "Shake-gels", Journal of Colloid and Interface Science, 517 (2018) 1-8.10.1016/j.jcis.2018.01.
- Y. Huang, M. Kobayashi, Direct Observation of Relaxation of Aqueous Shake-Gel Consisting of Silica Nanoparticles and Polyethylene Oxide, Polymers, 12 (2020).10.3390/polym12051141.
- Y. Huang, S. Sato, M. Kobayashi, Conditions for Shake-Gel Formation: The Relationship between the Size of Poly(Ethylene Oxide) and the Distance between Silica Particles, Molecules, 27 (2022).10.3390/molecules27227770.
- S. Kawasaki, M. Kobayashi, Affirmation of the effect of pH on shake-gel and shear thickening of a mixed suspension of polyethylene oxide and silica nanoparticles, Colloids and Surfaces a-Physicochemical and Engineering Aspects, 537 (2018) 236-242.10.1016/j.colsurfa.2017.10.033.
- M. Mar Ramos-Tejada, P.F. Luckham, Shaken but not stirred: The formation of reversible particle - polymer gels under shear, Colloids and Surfaces a-Physicochemical and Engineering Aspects, 471 (2015) 164-169.10.1016/j.colsurfa.2015.02.021.
- M. van Hecke, Running on corn flour, Nature, 487 (2012) 174-175.10.1038/487174a.
- N.J. Wagner, J.F. Brady, Shear thickening in colloidal dispersions, Physics Today, 62 (2009) 27-32.10.1063/1.3248476.
- V. Can, O. Okay, Shake gels based on Laponite-PEO mixtures: effect of polymer molecular weight, Designed Monomers and Polymers, 8 (2005) 453-462.10.1163/1568555054937917.
- D.C. Pozzo, L.M. Walker, Reversible shear gelation of polymer-clay dispersions, Colloids and Surfaces a-Physicochemical and Engineering Aspects, 240 (2004) 187-198.10.1016/j.colsurfa.2004.04.040.
- J. Zebrowski, V. Prasad, W. Zhang, L.M. Walker, D.A. Weitz, Shake-gels: shear-induced gelation of laponite–PEO mixtures, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 213 (2003) 189-197. [CrossRef]
- J. Comtet, G. Chatté, A. Niguès, L. Bocquet, A. Siria, A. Colin, Pairwise frictional profile between particles determines discontinuous shear thickening transition in non-colloidal suspensions, Nature Communications, 8 (2017) 15633.10.1038/ncomms15633.
- D. Banerjee, J.H. Lew, P.F. Luckham, On the rheological properties of silica – Polyethylene oxide dispersions: Shake gels I the effect of polymer concentration and temperature, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 703 (2024) 135194. [CrossRef]
- D. Banerjee, J.H. Lew, P.F. Luckham, On the rheological properties of Silica – Poly(ethylene-oxide) dispersions: "Shake-gels" II The effect of silica concentration and molecular weight of polymer, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 703 (2024) 135195. [CrossRef]
- D. Banerjee, P.F. Luckham, On the rheological properties of Silica – Poly(ethylene-oxide) dispersions: "Shake gels" III The effect of salt concentration, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 703 (2024) 135196. [CrossRef]
Figure 1.
The Hofmeister series for some common ions. The series categorizes ions based on their ability to stabilize or destabilize polymers. Ions on the right side of the series produce a salting-in (stabilizing) effect on the polymer, while ions on the left side lead to a salting-out (destabilizing) effect. Cl⁻ and Na⁺ ions are typically considered as the borderline between the strongly and weakly hydrated anions and cations, respectively.
Figure 1.
The Hofmeister series for some common ions. The series categorizes ions based on their ability to stabilize or destabilize polymers. Ions on the right side of the series produce a salting-in (stabilizing) effect on the polymer, while ions on the left side lead to a salting-out (destabilizing) effect. Cl⁻ and Na⁺ ions are typically considered as the borderline between the strongly and weakly hydrated anions and cations, respectively.
Figure 2.
The plot illustrates viscosity (mPas) against time (sec) at 4000 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in cations but maintaining a constant anion (Cl-) at a concentration of 0.02 mol.dm-3. The large vertical spikes in viscosity highlight the moments of gel formation.
Figure 2.
The plot illustrates viscosity (mPas) against time (sec) at 4000 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in cations but maintaining a constant anion (Cl-) at a concentration of 0.02 mol.dm-3. The large vertical spikes in viscosity highlight the moments of gel formation.
Figure 3.
The plot illustrates viscosity (normalized) against time (sec) at 1 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in cations but maintaining a constant anion (Cl-) at a concentration of 0.02 mol.dm-3.
Figure 3.
The plot illustrates viscosity (normalized) against time (sec) at 1 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in cations but maintaining a constant anion (Cl-) at a concentration of 0.02 mol.dm-3.
Figure 4.
The plot illustrates viscosity (mPas) against time (sec) at 4000 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in anions but maintaining a constant cation (Na+) at a concentration of 0.02 mol.dm-3. The large vertical spikes in viscosity highlight the moments of gel formation.
Figure 4.
The plot illustrates viscosity (mPas) against time (sec) at 4000 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in anions but maintaining a constant cation (Na+) at a concentration of 0.02 mol.dm-3. The large vertical spikes in viscosity highlight the moments of gel formation.
Figure 5.
The plot illustrates viscosity (normalized) against time (sec) at 1 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in anions but maintaining a constant cation (Na+) at a concentration of 0.02 mol.dm-3.
Figure 5.
The plot illustrates viscosity (normalized) against time (sec) at 1 s-1 for a shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in anions but maintaining a constant cation (Na+) at a concentration of 0.02 mol.dm-3.
Figure 6.
Hofmeister Effect of Salts on Polymer a) Ions on the left-hand side of the Hofmeister series cause the polymer to collapse, taking more time to bond with silica nanoparticles. b) Ions on the right-hand side of the Hofmeister series cause the polymer to swell, expand and quickly bond with silica nanoparticles once shear is applied.
Figure 6.
Hofmeister Effect of Salts on Polymer a) Ions on the left-hand side of the Hofmeister series cause the polymer to collapse, taking more time to bond with silica nanoparticles. b) Ions on the right-hand side of the Hofmeister series cause the polymer to swell, expand and quickly bond with silica nanoparticles once shear is applied.
Table 1.
Gelation time of shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in cations but maintaining a constant anion (Cl-) at a concentration of 0.02 mol.dm-3.
Table 1.
Gelation time of shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in cations but maintaining a constant anion (Cl-) at a concentration of 0.02 mol.dm-3.
| |
Gelation time (sec) |
| Water |
150 |
| NH4Cl |
2094 |
| NaCl |
105 |
| CaCl2
|
78 |
Table 2.
Time takes to attain half-life for cations at a concentration of 0.02 mol.dm-3.
Table 2.
Time takes to attain half-life for cations at a concentration of 0.02 mol.dm-3.
| |
Half - Life time (sec) |
| Water |
18 |
| NH4Cl |
3 |
| NaCl |
24 |
| CaCl2
|
45 |
Table 3.
Gelation time of shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in anions but maintaining a constant cation (Na+) at a concentration of 0.02 mol.dm-3.
Table 3.
Gelation time of shake-gel sample containing 3500 ppm PEO and 25 wt% silica, with different salts varying in anions but maintaining a constant cation (Na+) at a concentration of 0.02 mol.dm-3.
| |
Gelation time (sec) |
| Water |
150 |
| Na2SO4
|
117 |
| NaCl |
105 |
| NaI |
54 |
| NaClO4
|
9 |
Table 4.
Time taken for the shake gel to attain half-life for various anions at a concentration of 0.02 mol.dm-3.
Table 4.
Time taken for the shake gel to attain half-life for various anions at a concentration of 0.02 mol.dm-3.
| |
Half – Lifetime (sec) |
| Water |
18 |
| Na2SO4
|
21 |
| NaCl |
24 |
| NaI |
24 |
| NaClO4
|
18 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).