Submitted:
23 October 2024
Posted:
25 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study sites
2.2. Sampling and data collection
2.3. Insect rearing and identifications
2.4. DNA extraction
2.5. Polymerase Chain Reaction (PCR), DNA sequencing and phylogenetic analyses
2.6. Statistical analysis
3. Results
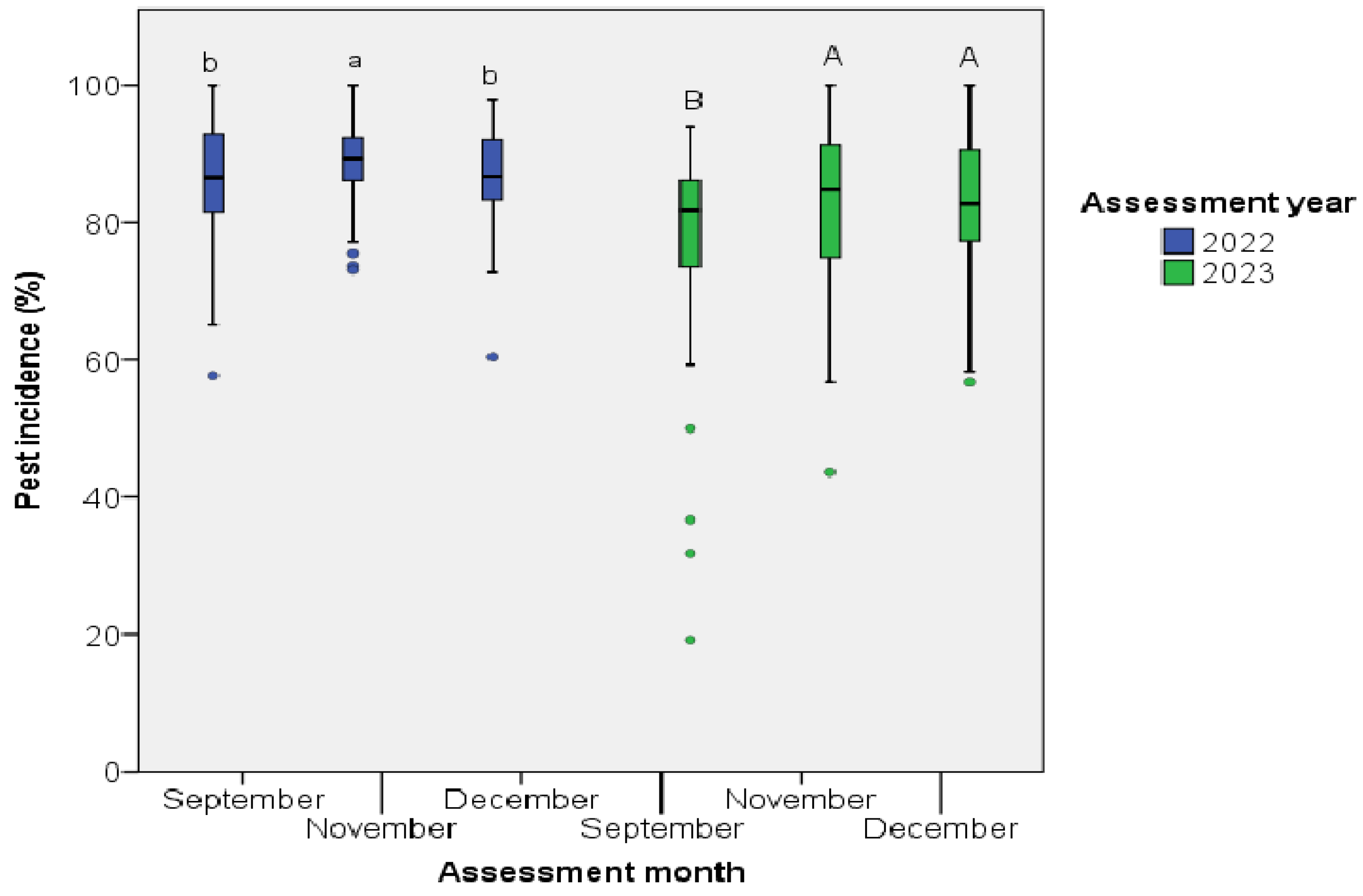
3.1. Factors influencing Ziziphus fruit and leaves insect pest incidences
3.2. Ziziphus tree fruits and leaves insect pests infestation levels
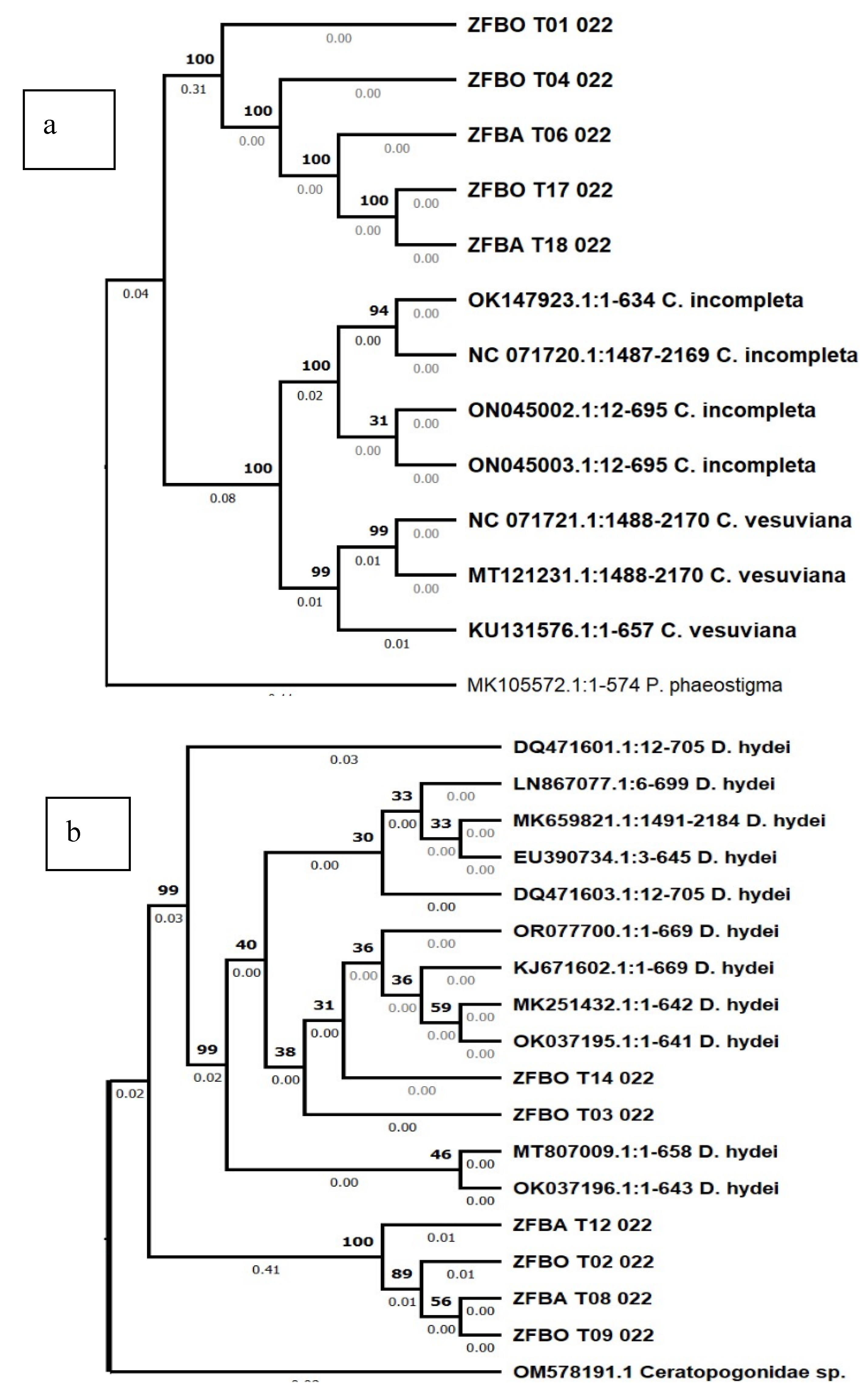
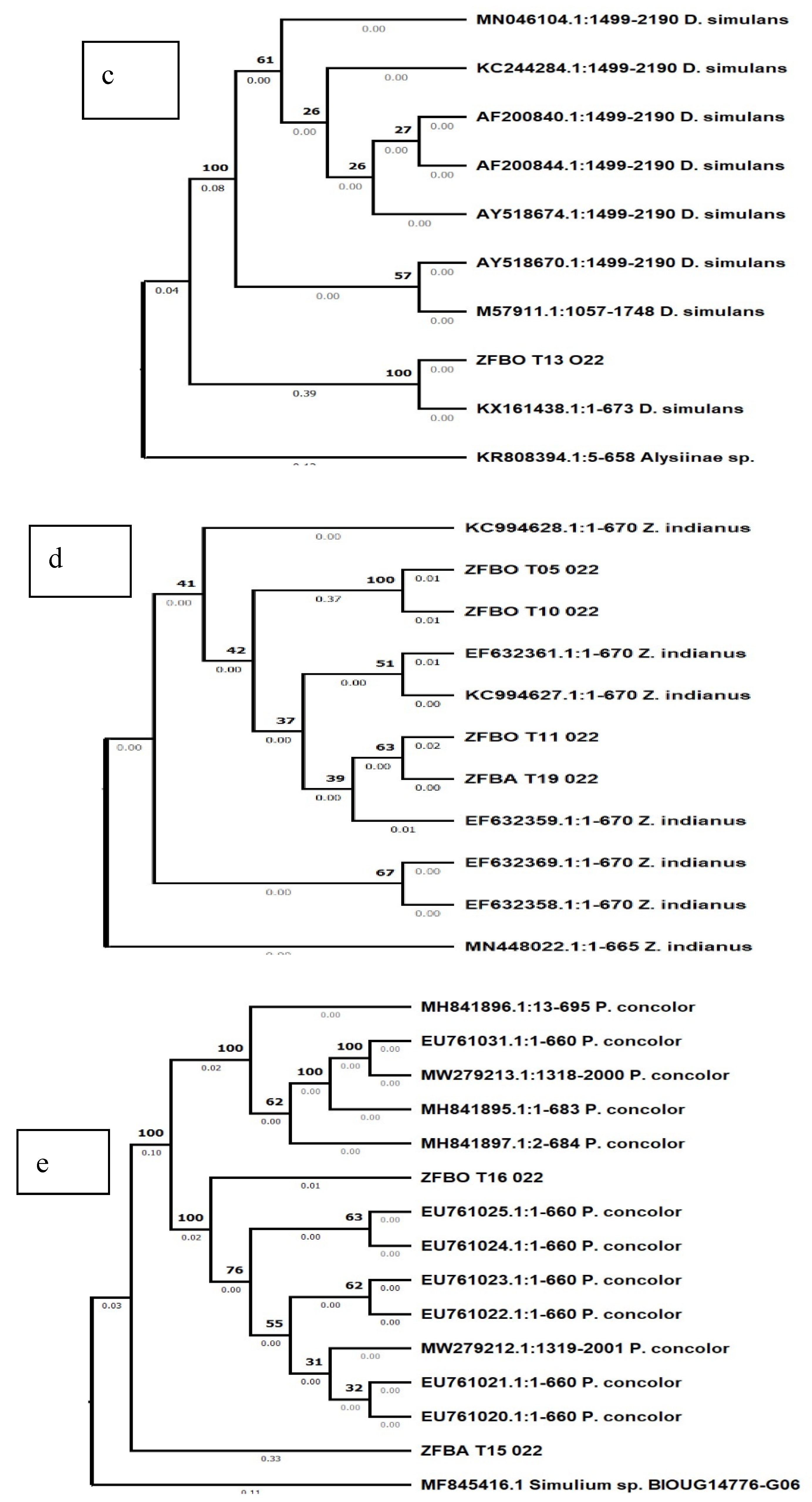
3.3. Morphological characterization and molecular identification
3.4. Phylogenetic characterization
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mongalo, N.I.; Mashele, S.S.; Makhafola, T.J. Ziziphus mucronata Willd. (Rhamnaceae): its botany, toxicity, phytochemistry and pharmacological activities. Heliyon, 2020, 6, e03708. [Google Scholar] [CrossRef] [PubMed]
- Leakey, R.R.B.; Tientcheu, A.M.L.; Awazi, N.P.; Assogbadjo, A.E.; Mabhaudhi, T.; Hendre, P.S.; Degrande, A.; Hlahla, S.; Manda, L. The future of food: domestication and commercialization of indigenous food crops in Africa over the third decade (2012-2021). Sustainability, 2022, 14, 2355. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Weber, J.C.; Page, T.; Cornelius, J.P.; Akinnifesi, F.K.; Roshetko, J.M.; Tchoundjeu, Z.; Jamnadass, R. Tree Domestication in Agroforestry: Progress in the Second Decade (2003–2012). In Advances in Agroforestry—The Future of Global Land Use; Springer: New York, NY, USA, 2012; pp. 145–173. [Google Scholar]
- Kalaba, F.K.; Chirwa, P.W.; Prozesky, H. The contribution of indigenous fruit trees in sustaining rural livelihoods and conservation of natural resources. African Journal of Wood Science and Forestry, 2019, 7, 1–6. [Google Scholar]
- Karuppaiah, V. Biology and management of ber fruit fly, Carpomyia vesuviana Costa (Diptera : Tephritidae): A review. African Journal of Agricultural Research, 2014, 9, 1310–1317. [Google Scholar]
- Yadav, J.; Gaur, R.K.; Kumar, Y. Evaluation of antixenotic and allelochemical traits of ber (Ziziphus mauritiana Lamk) fruits as a source of host plant resistance against fruit fly (Carpomyia vesuviana Costa) (Diptera : Tephritidae) in a semi-arid region of India. Phytoparasitica, 2020, 48, 607–620. [Google Scholar] [CrossRef]
- Dhaliwal, G.S.; Singh, R. Host Plant Resistance to Insects: Concepts and applications. New Delhi, Panama. Publication, 2014, pp:578.
- Ibrahim, A.S.; Samira, A.M.; Mahmoud, M.E.E.; Salih, A.I.S.; Saqib, A.; Ali, A. Monitoring of Tephritidae of Fruit Trees and Their Level of Infestation in South Kordofan State, Sudan. International Journal of Agriculture Institute. Canadian Journal of Plant Protection, 2014, 2, 1–7. [Google Scholar]
- Haldhar, S.M.; Deshwal, H.L.; Jat, G.C.; Berwal, M.K. Pest scenario of ber (Ziziphus mauritiana Lam. ) in arid regions of Rajasthan: a review. Journal of Agriculture and Ecology, 2016, 1, 10–21. [Google Scholar]
- Meghwal, P.R.; Kumar, P.; Singh, D. Climate variability during flowering and fruiting reduces fruit yield of ber (Ziziphus mauritiana ) in Western Rajasthan. Journal of Agriculture and Ecology, 2018, 6, 31–38. [Google Scholar] [CrossRef]
- Karuppaiah, V. Seasonality and management of stone weevil, Aubeus himalayanus Voss (Curculionidae : Coleoptera): An emerging pest in Indian Jujube (Ziziphus mauritiana L. ). Africa Journal of Agricultural Research, 2015, 10, 871–876. [Google Scholar]
- Sharma, V.P.; Lal, O.P.; Rohidas, S.B.; Pramanick, P.K. Varietal resistance in ber (Ziziphus mauritiana Lamk. ) against the fruit fly, Carpomyia vesuviana Costa (Diptera : Tephritidae) under the field conditions. Journal of Entomological Research, 1998, 22, 61–67. [Google Scholar]
- Karuppaiah, V.; More, T.A. , Sivalingam, P. N.; Hanif, K.; Bagle, B.G. Prevailing insect pests of ber (Zizipus mauritiana Lamk) and their natural enemies in a hot arid ecosystem. Haryana Journal of Horticultural Sciences, 2010, 39, 214–216. [Google Scholar]
- Singh, M.P. Managing menace of insect pests on ber. Indian horticulture, 2018, 53, 31–32. [Google Scholar]
- Gaur, R.K.; Kumar, M.; Sharma, S.; Singh, B. Survey studies on insects and non-insect pests associated with ber crop in South West Haryana. Journal of Entomology and Zoology Studies, 2020, 8, 856–863. [Google Scholar]
- Girma N, Zinabu N, Mubarek E. Assessment of Diseases and Insect Pests on Ziziphus Species Fruit in Waghimra Administrative Zone, Amhara National Regional State, Ethiopia. In Editors, Menale Wondie, Abraham Abiyu and Mulugeta Alemayehu (2017) Proceedings of the 10th and 11th Annual Regional Conference on Completed Research Activities of Forestry, March 6-13, Amhara Agricultural Research Institute (ARARI), Bahir Dar, Ethiopia, 2017.
- Bagle, B. Incidence and control of fruitfly (Carpomyia vesuviana Costa) of ber (Ziziphus mauritiana Lamk. ). Indian Journal of Plant Protection, 1992, 20, 205–207. [Google Scholar]
- Sonawane, B.R. Study of pest of tropical ber with special reference to fruit borer Meridarchis scyrodes Meyr. MSc Thesis, Poona University, Poona.
- Mayhew, P.J. Explaining global insect species richness: lessons from a decade of macro-evolutionary entomology. Entomologia experimentalists et applicate, 2018, 166, 225–250. [Google Scholar] [CrossRef]
- Whiting, D. Key to insect orders. CMG GardenNotes. 2017, pp:314–316.
- Tahir, H.; Noor, M.; Mehmood, A.; Sherawat, S.M.; Qazi, M.A. Evaluating the accuracy of morphological identification of insect pests of rice crops using DNA barcoding. Mitochondrial DNA Part B, 2018, 3, 1220–1224. [Google Scholar] [CrossRef]
- Smith, M.A. , Eveleigh, E. S.; Mccann, K.S. Barcoding a quantified food web: crypsis, concepts, ecology and hypotheses. PLoS One, 2011, 6, e14424. [Google Scholar]
- Wallace, L.J.; Boilard, S.M.; Eagle, S.H. DNA barcodes for everyday life: routine authentication of natural health products, Food Research International, 2012, 49, 446–452.
- Bihon, W.; Burgess, T.; Slippers, B.; Wingfield, M.J.; Wingfield, B.D. Distribution of Diplodia pinea and its genotypic diversity within asymptomatic Pinus patula trees. Australasian Plant Pathology, 2011, 40, 540–548. [Google Scholar] [CrossRef]
- Chacón, I.A.; Janzen, D.H.; Hallwachs, W. Cryptic species within cryptic moths: new species of Dunama Schaus (Notodontidae, Nystaleinae) in Costa Rica. ZooKeys, 2013, 264, 11–45. [Google Scholar] [CrossRef]
- NMSA. Addis Ababa National Meteorology Service Agency (NMSA),2020, Addis Ababa, Ethiopia.
- FAO (Food and Agriculture Organization of the United Nations), success stories. climate-smart agriculture (CSA) on the ground understanding, 2015.
- Madden, L.V.; Hughes, G.; van den Bosch, F. The Study of Plant Disease Epidemics APS Press, St. Paul, MN, 2007.
- Nagrare, V.S.; Kranthi, S.; Biradar, V.K.; Zade, N.N.; Sangode, V.; Kakde, G.; Shukla, R.M.; Shivare, D.; Khadi, B.M.; Kranthi, K.R. Widespread infestation of the exotic mealybug species Phenacoccus solenopsis Tinsley (Hemiptera: Pseudococcidae) on cotton in India. Bull. Entomology Research, 2011, 99, 537–541. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal genes. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, Editors. PCR Protocols; Academic Press; New York, 1990.
- Costa, F.O.; Dewaard, J.R.; Boutillier, J. Biological identifications through DNA barcodes: the case of the Crustacea. Canadian Journal of Fishery and Aquatic Sciences, 2007, 62, 272–295.
- Saiki, R.K.; Gelfand, D.H.; Stoffel, S.; Scharf, S.J.; Higuchi, R.; Horn, G.T.; Mullis, K.B.; Erlich, H.A. Primer directed enzymatic ampliÞcation of DNA with a thermostable DNA polymerase. Science, 1988, 239, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Marion, C.; Jade, L.; Laury-Ann, D.; Ariane, T.; Grégoire, C.; Cloé, L.; Eric, P.; Geneviève, J.; Parent. Optimized QIAGEN DNeasy Blood & Tissue kit Protocol for Environmental DNA Extraction. 2023, protocols.io. [CrossRef]
- Borghuis, A.; Pinto, J.D.; Platner, G.R.; Stouthamer, R. Partial cytochrome oxidase II sequences distinguish the sibling species Trichograma minutum Riley and T. platneri Nagarkatti. Biol. Con.. 2004, 30, 90–94. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular cloning: a laboratory manual (2nd Ed). Cold Spring Harbor, NY. Cold Spring Harbor Laboratory Press, Selection of the Formosan subterranean termite. J. Beh.Eco., 1989, 19, 764–773. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Marine Bio. and Biot., 1994, 3, 294–299. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences, 1977, 74, 5413–5467. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: a user-friendly biological sequence alignment [ed. ], and analysis program for Windows 95/98/NT. Nucleic Acids Symposiums Serous, 1999, 41, 95–98. [Google Scholar]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: architecture and applications. BMC Bioinformatics, 2009, 10, 421. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics. Oxford University Press, New York.
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Molecular Biology and Evolution, 2011. [CrossRef]
- Team, R.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2023, URL https://www.R-project.org/.
- Kavitha Z, Savithri P (2002) New record of some natural enemies on ber pests in Tirupati Region. South Indian Horti., 2002, 50(4-6):513-514.
- Lakra, R.K.; Singh, Z. Seasonal fluctuations in the incidence of ber fruitfly Carpomyia vesuviana Costa (Diptera: Tephritidae) under agroclimatic conditions of Hisar. Haryana Agricultural University J. Res., 1985, 15, 42–50. [Google Scholar]
- Coissac, E.; Hollingsworth, P.M.; Lavergne, S. From barcodes to genomes: extending the concept of DNA barcoding, Molecular Ecology, 2016, 25, 1423–1428.
- Nandihalli, B.S.; Patil, D.R.; Jagginavar, S.B.; Biradar, A.P.; Guled, M.B.; Surkod, V.S. Incidence of fruit borer (Meridarchis scyrodes Meyr.), and fruit fly (Carpomyia vesuviana Costa) on different varieties of ber. Advances in Agri. Res. in India, 1996, 6, 13–18. [Google Scholar]
- Gopali, J.B.; Sharanabasappa Yelshetty, S. Incidence of ber fruit borer, Meridarchi scyrodes Meyrick (Lepidoptera: Carposinidae) in relation to weather parameters. Insect Envi., 2003, 9, 165–166. [Google Scholar]
- Grewal, J.S.; Kapoor. Relative incidence of infestation by two species of fruit flies Carpomyia vesuviana and Dacuszonatus (Diptera: Tephritidae) on ber in the Punjab. Indian J. of Eco., 1986, 8, 123–125. [Google Scholar]
- Farrar, N.; Golestaneh, R.; Askari, H.; Assareh, M.H. Studies on parasitism of Fopius carpomyie (Silvestri) (Hymenoptera: Braconidae), an egg-pupal parasitoid of ber fruit fly, Carpomyia vesuviana Costa (Diptera: Tephritidae). Bushehr-Iranian Acta Horti 2009, 840, 431–438. [Google Scholar] [CrossRef]
- Sander, N.L.; da Silva, C.J.; Duarte, A.V.M.; Zago, B.W.; Galbiati, C. The Influence of Environmental Features on the Morphological Variation in Mauritia flexuosa L.f. Fruits and Seeds. Plants, 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Arora, P.K.; Kaur, N.; Thind, S.K.; Aulakh, P.S. Screening of some ber varieties for resistance against fruit fly. Horti. J., 2001, 14, 117–120. [Google Scholar]
- Mitra, S.K.; Dhaliwal, S.S. Effect of potassium on fruit quality and their storage life. Proceedings IPI-OUAT-IPNI International symposium, 2009.
- Karar, H.; Bashir, M.A.; Khan, K.A.; Farooq, A.B.; Aziz, I.; Ali, H.; Ghramh, H.A.; Abbas, G.; Alghanem, S.M. Response of leading ber (Zizyphus jujuba) varieties against fruit flies (Tephritidae : Diptera) and estimation of their losses. Fre. Envi. Bulletin, 2020, 29, 10311–10319. [Google Scholar]
- Muhammad S (2006) Incidence of Insect Pests on Ber (Zizyphus jujube) Tree. Journal of Zoology, 38, 261-263.
- Sarwar, M. Incidence of insect pests on Ber (Zizyphus jujube) tree. Pakistan J. of Zoo., 2006, 38, 261–263. [Google Scholar]
- Tembo, L.; Chiteka, Z.; Kadzere, I.; Akinnifesi, F.K.; Tagwira, F. Ripening stage and drying method affecting colour and quality attributes of Ziziphus mauritiana fruits in Zimbabwe. Afri.. J. of Biotec., 2008, 7, 2509–2513. [Google Scholar]
- Nizamani IA, Rustamani MA, Nizamani SM, Nizamani SA, Khaskheli MI (2015) Population Density of Foliage Insect Pest on Jujube, Ziziphus mauritiana Lam. Ecosystem. Journal of Basic and Applied Sciences, 11, 304–313.
- Al-Masudey, A.D.; Al-Yousuf, A.A. Effect of jujube fruit cultivars on chemical control of jujabe fruit fly Carpomyia incompleta. Kufa J. of Agri. Sci., 2013, 5, 111–124. [Google Scholar]
- Hoddle, M.S.; Mound, L.A. The genus Scirtothrips in Australia (Thysanoptera, Thripidae). Zootaxa, 2003, 268, 1–40. [Google Scholar] [CrossRef]
- Korneyev, V.A.; Mishustin, R.I.; Korneyev, S.V. The Carpomyini fruit flies (Diptera: Tephritidae) of Europe, Caucasus, and Middle East: new records of pests, with improved keys. Vestn Zoo., 2017, 51, 453–470. [Google Scholar] [CrossRef]
- CABI (2022) Invasive species compendium. Available online: https://www. cabi.org/isc/datasheet/11408.
- Zavitha Z, Savithri P, Vijayaragavan C (2002) Insect pests of ber, Ziziphusjujuba in Tirupati Region. Ins. Envi., 2002, 7, 157–158.
- Balikai, R.A.; Kotikal, Y.K.; Prasanna, P.M. Global scenario of insect and non-insect pests of jujube and their management options. Acta Horti., 2013, 993, 253–277. [Google Scholar] [CrossRef]
- Inmaculada, G.J.; Enrique, Q.M.; Meelad, Y.Y. Zizyphus fruit fly (Carpomya incompleta (Becker), Diptera: Tephritidae) is expanding its range in Europe. Spa. J. of Agri. Res., 2022, 20 (4): e10SC02.
- Cini, A.; Ioriatti, C.; Anfora, A. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bulletin of Insectology, 2012, 65, 149–160. [Google Scholar]
- Sasaki, M.; Sato, R. Bionomics of the cherry drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae) in Futeushima prefecture. Annual Report of the Society of Plant Protection of North Japan, 1995, 46, 164–172. [Google Scholar]
- Rota-Stabelli O, Blaxter M, Anfora G (2013) Drosophila suzukii. Current Biology, 23, R8–R9.
- Commar, L.S.; Conceição, L.G.; Carlos, C.R.; Claudia, M.A.C. Taxonomic and evolutionary analysis of Zaprionus indianus and its colonization of Palearctic and Neotropical regions, Gen. and Mol. Bio., 2012, 35, 395–406. [Google Scholar]
- Szépligeti, G. In: Marchal P. (Ed.), Sur un Braconide [Hym.] nouveau, parasite du Dacus oleae. Bulletin de la Société entomologique de France, 1910, 13, 243–244.
- Daniell, R.R.F.; Elton, L.A. Occurrence of Zaprionus indianus Gupta (Diptera: Drosophilidae) In “Juazeiro” Fruits Ziziphus Joazeiro Mart. (Rhamnaceae). In The State of Rio Grande Do Norte, Brazil. Revista Brasileira de Fruticultura, Jaboticabal-SP, 2011, 33, 1356–1358. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques, M.J.A.; Justesen, A.F.; Magnusson, C.S.; Milonas, P.; Navas-Cortes, J.A.; Parnell, S.; Potting, R.; Reignault, P.L.; Stefani, E.; Thulke, H.H.; Van der Werf, W.; Vicent Civera, A.; Yuen, J.; Zappala, L.; Gregoire, J.C.; Malumphy, C.; Kertesz, V.; Maiorano, A.; MacLeod, A. Pest categorisation of Zaprionus indianus. EFSA, 2022, 20, 7144. [CrossRef]
- Brunner, P.C.; Flemming, C.; Frey, J.E. A molecular identification key for economically important thrips species (Thysanoptera: Thripidae) using direct sequencing and a PCR-RFLP-based approach. Agri. and For.Ent., 2002, 4, 127–136. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Robert, W.; Tom van, N.; Richard, S. Molecular differentiation of the Psyttalia concolor (Szépligeti) species complex (Hymenoptera: Braconidae) associated with olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), in Africa. Bi. Con., 2009, 49, 17–26. [Google Scholar] [CrossRef]
- Amiri, A.; Talebi, A.A.; Zamani, A.A.; Kamali, K. Effect of temperature on demographic parameters of the hawthorn red midget moth, Phyllonorycter corylifoliella, on apple. J. Ins. Sci., 2010, 10, 134. [Google Scholar] [CrossRef] [PubMed]
| Fruits | |||||
|---|---|---|---|---|---|
| Variables | Coefficients (B) | S.E | Exponential of B | t-value | P-value |
| Agro-ecological zone | -2.3 | 2.23 | -0.11 | -1.03 | 0.303 |
| Land use types | -0.26 | 0.65 | 0.04 | 0.40 | 0.687 |
| Assessment year | -10.51 | 1.98 | -0.50 | -5.31 | 0.001*** |
| Adjusted R square | 0.096 | ||||
| Standard error | 10.11 | ||||
| p > F | 10.55 | ||||
| Leaves | |||||
| Agro-ecological zone | -2.54 | 2.78 | -0.09 | -0.92 | 0.36 |
| Land use types | 0.60 | 0.81 | 0.07 | 0.74 | 0.46 |
| Assessment months | 0.17 | 0.11 | 0.15 | 1.57 | 0.12 |
| Adjusted R square | 0.069 | ||||
| Standard error | 13.45 | ||||
| P > F | 7..64 | ||||
| Fruits | ||||
|---|---|---|---|---|
| Determinant factors | Insect pest incidence | AEZ | Land use types | Assessment year |
| AEZ | 0.071 | |||
| Land use types | 0.053 | 0.88* | ||
| Assessment year | 0.289 | 0.001 | 0.00 | |
| Assessment months | 0.173 | 0.001 | 0.00 | 0.84* |
| Leaves | ||||
| AEZ | 0.02 | |||
| Land use types | 0.01 | 0.86* | ||
| Assessment year | 0.27 | -0.02 | ||
| Assessment months | 0.18 | 0.004 | 0.004 | 0.85* |
| Fruits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LUT | Mean (± SE) fruits examined/ tree | Mean infestation level per tree | ||||||||
| Very low | Low | Medium | Severe | Very severe | ||||||
| Farmland | 58±1.4 | 6±0.4c | 20.8±0.8 | 21±0.9 | 9 ±0.7 | 5.3±0.5 | ||||
| home garden | 56.9±1.1 | 7.1±0.5b | 19.9±0.7 | 20.7±0.9 | 8.6±0.5 | 5±0.5 | ||||
| Roadside | 59.4±1.2 | 8±0.5a | 19.7±0.7 | 21.7±0.7 | 9±0.6 | 5.1±0.6 | ||||
| AEZ | ||||||||||
| Lowland | 59±0.9 | 9.8±0.5 | 20.4±0.5 | 21.6±0.6 | 8.2±0.4a | 5.9±0.4b | ||||
| Midland | 57.1±0.8 | 9.0±0.5 | 19.8±0.5 | 20.6±0.5 | 9.3±0.4b | 4.2±0.2a | ||||
| Year | ||||||||||
| 2022 | 55.6±0.6 | 7.1±0.3b | 20.1±0.4 | 20.8±0.4 | 7.9±0.3b | 4.9±0.3 | ||||
| 2023 | 60.6±1 | 11.7±0.6a | 20.2±0.6 | 21.4±0.6 | 9.8±0.5a | 5±0.3 | ||||
| Leaves | ||||||||||
| LUT | Mean (± SE) number of leaves examined/ tree | Mean infestation level per tree | ||||||||
| Nil | Low | Medium | Severe | |||||||
| Farmland | 52.6±1.1 | 34.5±1.1 | 13.1±0.5 | 6.6±0.4 | 4.5±0.4 | |||||
| home garden | 49.2±1.1 | 32.7±1.1 | 12.5±0.4 | 6.5±0.4 | 3.7±0.4 | |||||
| Roadside | 53±1.2 | 33.8±1.2 | 13.4±0.5 | 7.3±0.5 | 4.4±0.2 | |||||
| AEZ | ||||||||||
| Lowland | 51.1±0.8 | 32.9±0.8a | 12.8±0.4 | 7.3±0.3b | 4.2±0.3 | |||||
| Midland | 52.7±1.2 | 35.2±1.1b | 13.6±0.4 | 6±0.4a | 4.1±0.3 | |||||
| Year | ||||||||||
| 2022 | 50±0.8 | 30.5±0.6a | 14.2±0.4b | 7.7±0.4b | 4.1±0.3 | |||||
| 2023 | 53.2±1.1 | 36.9±1.1b | 11.8±0.4a | 6.0±0.4a | 4.2±0.2 | |||||
| Species Name | Isolate number | host | Origin | Collector | Per cent identity | Accession |
|---|---|---|---|---|---|---|
| C. incompleta | AHL2 | Jujube | Iraq | Tahir,H.M. | 99.85 | ON045003 |
| C. incompleta | AHL1 | Jujube | Iraq | Tahir,H.M. | 99.85 | ON045002 |
| C. incompleta | Italy 01 | Jujube | Italy | Zhang Y. | 99.71 | NC_071720 |
| C. vesuviana | I1 | Jujube | Spain | Garrido JI. | 99.68 | OK147923 |
| C. vesuviana | China, Xinjiang 01 | Jujube | China | Zhang,Y. | 95.31 | MT121231 |
| C. vesuviana | Iran 01 | Jujube | Iran | Zhang,Y. | 95.31 | NC071721 |
| C. vesuviana | FUN12 | Jujube | China | Jing,L. | 95.43 | KU131576 |
| C. vesuviana | ZFBO T01 022 | Ziziphus | Ethiopia | Tigabu R. | QU5908887 | |
| C. vesuviana | ZFBO T04 022 | Ziziphus | Ethiopia | Tigabu R. | QU8343087 | |
| C. vesuviana | ZFBO T17 022 | Ziziphus | Ethiopia | Tigabu R. | QU1834357 | |
| C. vesuviana | ZFBA T06 022 | Ziziphus | Ethiopia | Tigabu R. | QU6288145 | |
| C. vesuviana | ZFBA T18 022 | Ziziphus | Ethiopia | Tigabu R. | QU1912175 | |
| D. hydei | CRX36794.1 | Melon | Italy | Patrizia T. | 99.43 | LN867077 |
| D. hydei | DHYDE20161106 | Berry | China | Qian Z.Q. | 99.14 | MK659821 |
| D. hydei | Africa | Berry | China | Wang B.C | 98.85 | DQ471603 |
| D. hydei | CH55 | Melon | Iran | Oshaghi,MA | 99.7 | OR077700 |
| D. hydei | DQ37 | Melon | New Zealand | Simon Hodge | 99.55 | KJ671602 |
| D. hydei | Duke.Bio203L | Berry | USA | Eric Spana | 99.39 | MT807009 |
| D. hydei | TEN104-102 | Melon | Spain | Vilchez R.I. | 99.84 | OK037195 |
| D. hydei | QDE57910.1 | Melon | South Africa | Liana I. A. | 99.53 | MK251432 |
| D. hydei | ABH5 | Melon | Spain | Vilchez R.I. | 99.38 | OK037196 |
| D. hydei | 15085-1641.58 | Melon | Spain | Evans A.L. | 99.38 | EU390734 |
| D. hydei | AQ49 | Berry | China | Wang B.C. | 93.97 | DQ471601 |
| D. hydei | ZFBO T03 022 | Ziziphus | Ethiopia | Tigabu R. | QU3000047 | |
| D. hydei | ZFBO T02 022 | Ziziphus | Ethiopia | Tigabu R. | QU2682351 | |
| D. hydei | ZFBO T09 022 | Ziziphus | Ethiopia | Tigabu R. | QU3295235 | |
| D. hydei | ZFBO T14 022 | Ziziphus | Ethiopia | Tigabu R. | QU7962979 | |
| D. hydei | ZFBA T08 022 | Ziziphus | Ethiopia | Tigabu R. | QU7664841 | |
| D. hydei | ZFBA T12 022 | Ziziphus | Ethiopia | Tigabu R. | QU7906397 | |
| D. simulans | UKG21278.1 | Melon | China | Li,T. | 99.86 | MN046104 |
| D. simulans | sm21 | peach | Brazil | Montooth KL. | 99.86 | KC244283 |
| D. simulans | AU023 | Ziziphus | Kenya | Ballard J.W. | 99.86 | AY518674 |
| D. simulans | Sc00 | melon | Seychelles | Ballard J.W. | 99.86 | AF200844 |
| D. simulans | DSR | Apple | Madagascar | Ballard J.W. | 99.86 | AF200841 |
| D. simulans | DSW | Apple | USA | Ballard J.W. | 99.86 | AF200840 |
| D. simulans | C167 | Banana | Kenya | Ballard J.W. | 99.86 | AF200839 |
| D. simulans | KY215 | Banana | Kenya | Ballard J.W. | 99.71 | AY518672 |
| D. simulans | KY007 | Apple | USA | Ballard J.W. | 99.71 | AY518670 |
| D. simulans | SL3 | Melon | Spain | Satta,Y. | 99.71 | M57911.1 |
| D. simulans | simw501 | Apple | Brazil | Montooth KL. | KC244284 | |
| D. simulans | ZFBO T13 022 | Ziziphus | Ethiopia | Tigabu R. | QU2055377 | |
| Z. indianus | haplotype 6 | Fig, | Brazil | Mendonca MP | 98.96 | KC994628 |
| Z. indianus | Duke.Bio203L | Fig | USA | Mohamed N. | 99.1 | MN448022 |
| Z. indianus | haplotype 5 | Fig | DRC | Mendonca MP | 98.81 | KC994627 |
| Z. indianus | ABR08559.1 | Fig | Brazil | Amir,Y. | 98.81 | EF632369 |
| Z. indianus | ABR08548.1 | Fig | Brazil | Amir,Y. | 98.66 | EF632358 |
| Z. indianus | ABR08551.1 | Fig | Madagascar | Amir,Y. | 98.51 | EF632361 |
| Z. indianus | ABR08549.1 | Fig | Madeira | Amir,Y. | 98.51 | EF632359 |
| Z. indianus | ZFBO T05 022 | Ziziphus | Ethiopia | Tigabu R. | QU2155377 | |
| Z. indianus | ZFBO T10 022 | Ziziphus | Ethiopia | Tigabu R. | QU2212817 | |
| Z. indianus | ZFBO T11 022 | Ziziphus | Ethiopia | Tigabu R. | QU2280917 | |
| Z. indianus | ZFBA T19 022 | Ziziphus | Ethiopia | Tigabu R. | QU2348287 | |
| P. concolor | PRJ076 | Ziziphus | Morocco | Rugman-JP.F. | 99.09 | EU761024 |
| P. concolor | TN0216 | Ziziphus | Italy | Rugman-JP.F. | 99.09 | EU761022 |
| P. concolor | TN0222 | Ziziphus | USA | Rugman-JP.F. | 99.09 | EU761021 |
| P. concolor | TN0227 | Ziziphus | Morocco | Rugman JP.F. | 98.94 | EU761025 |
| P. concolor | TN0223 | Ziziphus | Italy | Rugman-JP.F. | 98.94 | EU761023 |
| P. humilis | Ps29 | Ziziphus | South Africa | Barbara VA. | 95.61 | MH841897 |
| P. humilis | Ps24 | Ziziphus | South Africa | Barbara VA. | 95.61 | MH841896 |
| P. humilis | Ps25 | Ziziphus | South Africa | Barbara VA. | 95.61 | MH841895 |
| P. humilis | QTC30726.1 | Ziziphus | Portugal | Powell,C. | 95.61 | MW279213 |
| P. humilis | TN0220 | Ziziphus | South Africa | Rugman-JP.F. | 95.45 | EU761031 |
| P. humilis | TN0223 | Ziziphus | Namibia | Rugman-JP.F. | 95 | EU761030 |
| P. humilis | ZFBA T15 022 | Ziziphus | Ethiopia | Tigabu R. | QU2497311 | |
| P. humilis | ZFBO T16 022 | Ziziphus | Ethiopia | Tigabu R. | QU2608411 |
| Type of insect pest | Percentage of insect pests recorded across the different | |||||||
| Land use types | Assessment Months | AEZ | ||||||
| Farm land |
Home garden | Roadsides | September | October | November | Low land |
Mid land |
|
| C. incompleta | 51.4 | 47.8 | 39.0 | 43.6 | 41.2 | 36.3 | 48.3 | 42.8 |
| D. hydei | 18.6 | 23.9 | 22.9 | 15.7 | 15.9 | 17.0 | 22.9 | 20.2 |
| D. simulans | 19.9 | 17.6 | 31.1 | 31.6 | 33.0 | 31.0 | 21.8 | 27.4 |
| Z. indianus | 6.3 | 8.8 | 4.6 | 6.7 | 7.4 | 10.8 | 5.6 | 6.0 |
| P. concolor | 3.7 | 1.9 | 2.5 | 2.4 | 2.5 | 5.0 | 1.3 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





