1. Introduction
Short noncoding RNAs, known as miRNAs, consist of 21–23 nucleotides and are found in plants, animals, and certain viruses [
1,
2]. Since their identification in the early 1990s, miRNAs have revolutionized our comprehension of cellular mechanisms, especially regarding gene regulation in diverse biological situations, including development, differentiation, cell proliferation, and illness. These small RNA molecules work by attaching to complementary sequences on target messenger RNA (mRNA) transcripts. This stops the translation or breaks down the mRNA. This technique enables miRNAs to precisely regulate gene expression across several biological pathways, ensuring the proper execution of cellular functions.
MicroRNAs (miRNAs) participate in RNA silencing and the post-transcriptional control of gene expression [
3,
4]. MiRNAs are important because they play a big role in gene regulation networks if they can pair up with complementary sequences in mRNA molecules. In contrast to protein-coding genes, miRNAs do not undergo translation into proteins. Their function relies on their capacity to bind with protein-coding mRNAs, thereby influencing the translation or stability of these transcripts. The seed region of miRNA is what makes it work. It is a short sequence of 2–8 nucleotides that binds to the complementary segment in the target mRNA. This segment is usually found in the 3′ untranslated region (3′ UTR). When miRNAs bind to their targets, they can either destroy the target mRNA or stop it from being translated into proteins, depending on how well they match. A very exact match generally results in mRNA destruction, whereas partial complementarity leads to translational repression [
5].
MicroRNAs are essential regulators in almost all biological processes, encompassing embryogenesis, cell cycle regulation, apoptosis, and immunological responses. People commonly refer to these diminutive RNA molecules as "fine-tuners" of gene expression because they can attenuate the production of certain target genes without completely inhibiting their function. This fine-tuning is crucial for preserving cellular homeostasis and adapting to fluctuating external conditions. For example, miRNAs can swiftly modulate gene expression in reaction to stress or damage, rendering them essential contributors to tissue repair and regeneration [
6].
The identification of miRNAs has broadened the understanding of gene regulation beyond the limitations established by protein-coding genes. Before their discovery, we believed that transcription factors primarily managed gene regulation, activating or inhibiting the transcription of genes from DNA to mRNA. The discovery of miRNAs revealed the possibility of post-transcriptional regulation of gene expression, providing an extra layer of control and flexibility over cellular activities. This revelation was transformative, offering a novel perspective on cellular management of the intricacies inside their gene regulatory networks [
7].
Evolutionary conservation among species demonstrates the extensive influence of miRNAs on gene expression. MicroRNAs are present in nearly all multicellular creatures, ranging from plants to humans, indicating that their regulatory functions are essential to life. For instance, Drosophila, nematodes, and humans all conserve miR-1, one of the earliest identified miRNAs in mammals, which is essential for regulating muscle development. This discussion underscores the significance of miRNAs in regulating vital cellular activities and preserving evolutionary stability [
8].
In addition to their role in standard biological functions, miRNAs have been linked to several illnesses. Cancer, cardiovascular illnesses, neurological disorders, and metabolic syndromes frequently link to dysregulation of miRNA expression. For instance, several malignancies, including breast, lung, and colon tumors, frequently overexpress miR-21, which acts as an oncogene by promoting cell proliferation and suppressing apoptosis. Also, the downregulation of miR-34, an miRNA that stops tumors from growing, is clear in many types of cancer, leading to uncontrolled cell growth and tumor formation. The participation of miRNAs in numerous diseases has rendered them appealing targets for treatment strategies. Researchers are currently investigating methods to restore normal miRNA function or block detrimental miRNAs for illness treatment [
9].
2. miRNA Nobel Prize
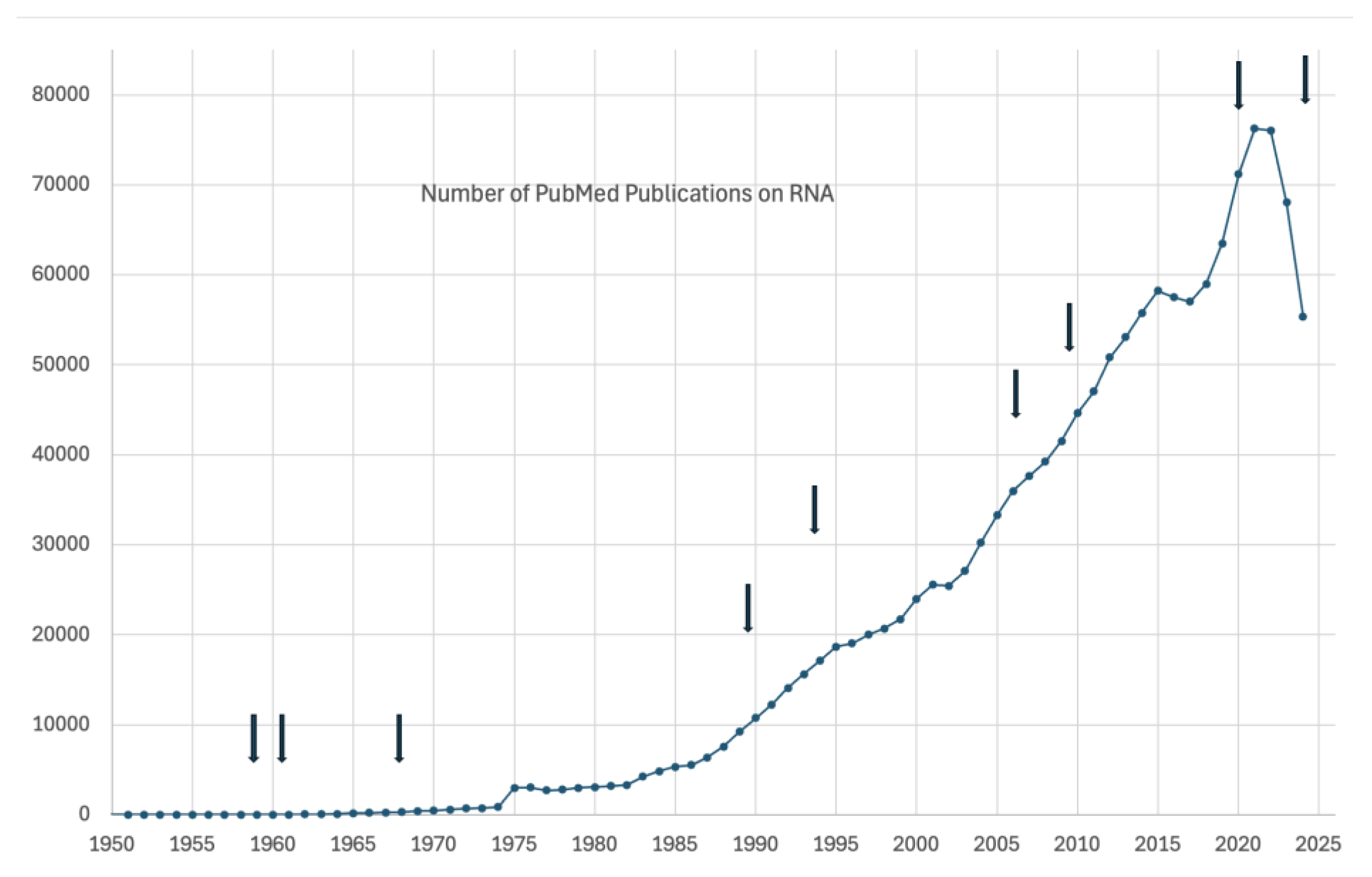
Nobel prizes bring instant recognition and add awareness of technologies, as we anticipate the 2024 award will do to the field of microRNA. To bring the state of the art of research of miRNA, we are summarizing its properties as well as projecting its future applications. The interest in RNAs is well demonstrated by the number of publications listed in PubMed on the topic. The field of RNA has been very productive in bringing many remarkable contributions to molecular biology.
Table 1 shows the types of RNAs, their prevalence, where applicable and other RNA inventions that have received Nobel Prizes. (
Figure 1).
Table 1 lists the recognitions of the various types of RNAs.
Victor Ambros and Gary Ruvkun have received the 2024 Nobel Prize in Physiology or Medicine for their groundbreaking research in the discovery and characterization of microRNAs (miRNAs), which marks the culmination of decades of investigation into small, non-coding RNA molecules that modulate gene expression. The quest for the Nobel Prize commenced in 1993 when Victor Ambros and his team at Harvard University, in collaboration with Gary Ruvkun at Massachusetts General Hospital, identified lin-4, the inaugural known miRNA, in the nematode Caenorhabditis elegans. This was among the initial revelations about the mechanism by which miRNAs inhibit gene expression post-transcriptionally [
6]. This discovery challenged the widely held notion that proteins alone regulate gene expression and unveiled a new realm of genetic regulation. We discovered that Lin-4 modulates the time of C. elegans development by inhibiting the expression of the lin-14 gene through a method that does not engage protein-coding processes. The lin-4 RNA attached to matching sequences in the 3′ untranslated region (3′ UTR) of the lin-14 mRNA and stopped it from being translated into protein. This constituted the initial unequivocal proof that short, non-coding RNAs can modulate gene expression post-transcriptionally [
8]. This discovery revealed, for the first time, that short non-coding RNAs may regulate gene expression at the post-transcriptional level, a thought that was groundbreaking at the time [
5,
9].
Although the discovery of lin-4 was revolutionary, it was previously considered an anomaly—an occurrence unique to nematodes. In 2000, Ruvkun's team made a significant discovery by identifying let-7, a second miRNA in C. elegans that likewise affected developmental time. Let-7 is particularly significant due to its evolutionary conservation across species, ranging from nematodes to humans. This discovery indicated that miRNAs were not simply an anomaly of worm biology but rather a ubiquitous mechanism of gene control. Once let-7 was found, a lot of research into miRNAs began because scientists realized how important these short RNAs are for controlling genes in many different types of organisms [
10,
11]. This discovery expanded the domain of miRNA research and indicated that these tiny RNAs are not an evolutionary anomaly but a crucial component of gene regulation in complex organisms.
Thomas Tuschl and associates pioneered the initial cloning and sequencing technique for the systematic identification of miRNAs, resulting in the discovery of several novel miRNAs in Drosophila, mice, and humans [
12]. This technological advancement enabled researchers to catalog miRNAs across several species and tissues, demonstrating that the miRNAs participate in nearly all biological processes, including cell division, differentiation, death, and metabolism.
3. Nomenclature
Under a standard nomenclature system, names are assigned to experimentally confirmed miRNAs before publication [
13,
14]. The prefix "miR" is followed by a dash and a number, the latter often indicating order of naming. For example, miR-124 was named and likely discovered prior to miR-456. A capitalized "miR-" refers to the mature form of the miRNA, while the uncapitalized "mir-" refers to the pre-miRNA and the pri-miRNA [
15]. The genes encoding miRNAs are also named using the same three-letter prefix according to the conventions of the organism gene nomenclature. For examples, the official miRNAs gene names in some organisms are "
mir-1 in
C. elegans and
Drosophila, Mir1 in
Rattus norvegicus and
MIR25 in human.
miRNAs with nearly identical sequences except for one or two nucleotides are annotated with an additional lower-case letter. For example, miR-124a is closely related to miR-124b. For example:
hsa-miR-181a: aacauucaACgcugucggugAgu
hsa-miR-181b: aacauucaUUgcugucggugGgu
Pre-miRNAs, pri-miRNAs and genes that lead to 100% identical mature miRNAs but that are located at different places in the genome are indicated with an additional dash-number suffix. For example, the pre-miRNAs hsa-mir-194-1 and hsa-mir-194-2 lead to an identical mature miRNA (hsa-miR-194) but are from genes located in different genome regions.
Species of origin is designated with a three-letter prefix, e.g., hsa-miR-124 is a human (Homo sapiens) miRNA and oar-miR-124 is a sheep (Ovis aries) miRNA. Other common prefixes include "v" for viral (miRNA encoded by a viral genome) and "d" for Drosophila miRNA (a fruit fly commonly studied in genetic research).
When two mature microRNAs originate from opposite arms of the same pre-miRNA and are found in roughly similar amounts, they are denoted with a -3p or -5p suffix. (In the past, this distinction was also made with "s" (sense) and "as" (antisense)). However, the mature microRNA found from one arm of the hairpin is usually much more abundant than that found from the other arm [
3], in which case, an asterisk following the name indicates the mature species found at low levels from the opposite arm of a hairpin. For example, miR-124 and miR-124* share a pre-miRNA hairpin, but much more miR-124 is found in the cell.
Table 2 lists the miRNA database that lists thousands these identified miRNAs.
4. Biogenesis
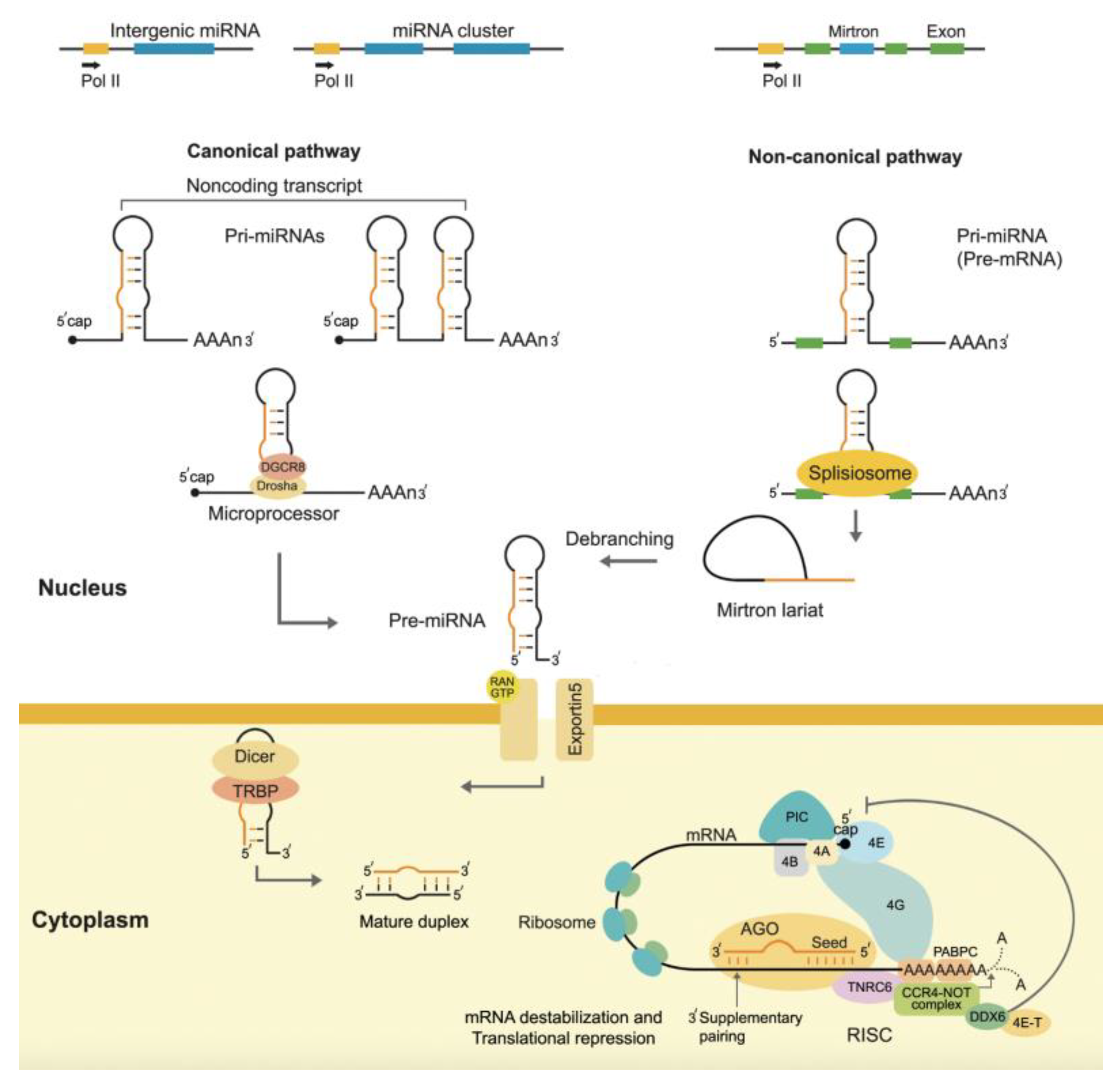
The biogenesis—the synthesis of miRNA by living organisms—involves several distinct steps: transcription, nuclear processing, export, and cytoplasmic maturation.
The first step in miRNA biogenesis is for RNA polymerase II to turn miRNA genes into long primary transcripts called pri-miRNAs [
16]. RNA polymerase II caps and polyadenylates these pri-miRNAs, making them resemble typical mRNA transcripts. A microprocessor complex, consisting of Drosha, an RNase III enzyme, and its cofactor DGCR8 (DiGeorge syndrome critical region 8), then processes the pri-miRNA, cleaving it into a shorter precursor miRNA (pre-miRNA), approximately 70 nucleotides long. This pre-miRNA has a characteristic hairpin structure that is essential for its recognition and further processing. Exportin-5, a transporter protein that recognizes the hairpin structure, exports the produced pre-miRNA from the nucleus to the cytoplasm [
6].
The Dicer enzyme, another RNase III protein, further processes the pre-miRNA in the cytoplasm by cleaving its hairpin loop, resulting in a double-stranded RNA molecule approximately 20–25 nucleotides in length. The duplex comprises two strands: the guide strand, representing the mature miRNA, and the passenger strand, also known as miRNA*, which usually undergoes degradation. We then incorporate the guide strand into the RNA-induced silencing complex (RISC), a multiprotein complex that mediates gene silencing. One of the key proteins in RISC is argonaute (AGO), which plays a critical role in miRNA-mediated gene silencing. AGO binds the miRNA and helps facilitate its interaction with target mRNA molecules [
17].
RNA polymerase II (Pol II) transcribes most miRNAs as primary miRNAs (pri-miRNAs), which are several kilobases long, capped, polyadenylated, and structured with stem-loop formations. However, RNA polymerase III transcribes some miRNAs. These pri-miRNAs can come from separate miRNA genes, the introns of genes that code for proteins, or polycistronic clusters that hold more than one miRNA sequence [
18].
Nuclear Processing of pri-miRNA: Once transcribed, pri-miRNAs undergo processing within the nucleus. The RNase III enzyme Drosha and its cofactor DiGeorge syndrome critical region gene 8 (DGCR8) form a microprocessor complex that cleaves the pri-miRNA at the stem-loop region, releasing a shorter precursor miRNA (pre-miRNA) of approximately 70 nucleotides. This cleavage step is crucial for defining the 5' and 3' ends of the miRNA [
19].
Nuclear Export of pre-miRNA: The pre-miRNA is then exported from the nucleus to the cytoplasm. Exportin-5 is a nuclear transport receptor that depends on Ran-GTP. It finds the pre-miRNA's double-stranded stem structure and helps it move across the nuclear membrane. Only properly processed pre-miRNAs leave the nucleus due to the high-affinity interaction between Exportin-5 and pre-miRNA [
20].
Cytoplasmic Processing of pre-miRNA: Once in the cytoplasm, the RNase III enzyme Dicer further processes the pre-miRNA by cleaving its loop structure, resulting in a miRNA duplex of approximately 22 nucleotides. The trans-activator RNA-binding protein (TRBP) and argonaute (AGO) proteins work together with Dicer to make the RNA-induced silencing complex (RISC). In RISC, AGO proteins preferentially load the guide strand of the miRNA duplex, while degrading the complementary passenger strand [
21].
Functional Maturation and Targeting: The mature miRNA-RISC complex now functions in gene silencing. The miRNA points RISC to specific mRNAs, where it usually attaches to the 3' UTRs of those transcripts by making an imperfect base pair. This binding leads to either translational repression or, if the complementarity is high, mRNA cleavage. The extent of complementarity between the miRNA and its target determines the mode of gene silencing [
6].
To sum up, miRNAs are made by transcription by Pol II, nuclear processing by Drosha, export by Exportin-5, cytoplasmic maturation by Dicer, and finally loading into RISC, where they silence genes. This regulatory pathway is crucial for various biological processes, including development, cell differentiation, and disease mechanisms (
Figure 2).
4.1. Biogenesis in Plants
miRNA biogenesis in plants differs from animal biogenesis mainly in the MiRNA biogenesis in plants differs from animal biogenesis mainly in the steps of nuclear processing and export. Instead of being cleaved by two different enzymes, once inside and once outside the nucleus, both cleavages of the plant miRNA are performed by a Dicer homolog, called Dicer-like 1 (DL1). DL1 is expressed only in the nucleus of plant cells, which indicates that both reactions take place inside the nucleus. Before plant miRNA:miRNA* duplexes are transported out of the nucleus, its 3' overhangs are methylated by a RNA methyltransferase protein called Hua-Enhancer1 (HEN1). The duplex is then transported out of the nucleus to the cytoplasm by a protein called Hasty [
22], an Exportin 5 homolog, where they disassemble, and the mature miRNA is incorporated into the RISC [
23].
5. Mechanism of miRNA Action
In human and animal cells, miRNAs predominantly function by destabilizing mRNA; hence, they regulate gene expression at the posttranscriptional level. We acknowledge that miRNA dysregulation indicates the condition and functionality of cells and tissues, potentially leading to their malfunction. The discovery of many extracellular miRNAs in bodily fluids has highlighted their potential in biomarker research.
miRNA works by changing gene expression by attaching to specific parts on target messenger RNA (mRNA). This can either break down mRNA or stop translation. Post-transcriptional gene regulation is essential for governing numerous biological processes, including cellular development and disease progression. Our comprehension of miRNA production and its regulatory functions has greatly enhanced our understanding of gene regulation, uncovering a complex network in which miRNAs can precisely modulate gene expression.
The mechanism via which miRNAs modulate gene expression is mostly contingent upon the extent of complementarity between the miRNA and its target mRNA. In animals, complete base matching between miRNA and its target mRNA is infrequent. Some miRNAs have partial complementarity, especially in the "seed region" (nucleotides 2–8), which is important for recognizing the target. MiRNAs usually stop translation when they bind to the 3′ untranslated region (3′ UTR) of their target mRNA through partial complementarity. The miRNA-RISC complex binds to the mRNA and messes up the translation machinery. This stops the mRNA from being turned into a protein. In certain instances, this interaction induces mRNA deadenylation and decapping, culminating in mRNA breakdown [
7].
Conversely, in certain plant and animal instances, miRNAs may demonstrate a near-perfect match to their target mRNAs. In these cases, miRNAs promote mRNA cleavage instead of translational repression. This cleavage results in the swift destruction of the target mRNA, thereby silencing the gene. Irrespective of the precise mechanism—be it mRNA degradation or translation inhibition—the ultimate outcome is less expression of the target protein [
26].
MiRNAs modulate various cellular activities by regulating the expression of numerous target genes. Multiple miRNAs can regulate a single mRNA, and each miRNA can modulate numerous distinct mRNAs. This enables miRNAs to engage in intricate gene regulation networks that precisely modulate cellular responses. For instance, miR-1 and miR-133, two microRNAs implicated in muscle development, exhibit unique yet complimentary roles: miR-1 facilitates muscle differentiation, whereas miR-133 augments the proliferation of muscle progenitor cells. These miRNAs collaborate to regulate muscle growth and development. In the immune system, miR-155 is essential for modulating immunological responses, especially during inflammation and immune cell activation [
27].
MiRNAs influence not only fundamental gene regulation but also intricate processes such as cellular differentiation, development, and disease progression. In the realm of oncogenesis, dysregulation of miRNAs can activate oncogenes or inhibit tumor suppressors, thereby facilitating tumor formation and progression [
28]. One or more of the following mechanisms allow mRNA molecules to function: A cohesive mathematical model delineates and integrates the mechanisms of miRNA action [
30]:
Cap-40S initiation inhibition.
60S Ribosomal unit joining inhibition.
Elongation inhibition.
Ribosome drop-off (premature termination).
Co-translational nascent protein degradation.
Sequestration in P-bodies [
31].
mRNA decay (destabilization) by shortening its poly(A) tail.
mRNA cleavage; the mRNA strand into two pieces.
Transcriptional inhibition through microRNA-mediated chromatin reorganization followed by gene silencing.
Histone modification and DNA methylation of promoter sites, which affects the expression of target genes [
32,
33].
It is often impossible to discern these mechanisms using experimental data about stationary reaction rates. Nevertheless, they are differentiated in dynamics and have different
kinetic signatures [
30]
5.1. RNA-Induced Silencing Complex (RISC)
Dicer is an enzyme belonging to the RNase III family that is essential to produce short RNAs, such as miRNAs and siRNAs. It cleaves double-stranded RNA precursors into shorter double-stranded fragments, subsequently processed into functional small RNAs. Pre-miRNAs are changed by Dicer into mature miRNA duplexes, which are made up of the guide strand (mature miRNA) and the passenger strand. The RNA-induced silencing complex (RISC) then integrates the mature miRNA, directing gene silencing by binding to target mRNAs.
The microRNA that is fully grown joins the RNA-induced silencing complex (RISC), which is a key part of the process of turning off genes. RISC consists of several proteins, with the central component being an argonaute (AGO) protein that directly interacts with the mature miRNA. Dicer is essential for miRNA biogenesis; however, its function inside the mature RISC is temporary. During the early stages, Dicer changes precursor miRNAs (pre-miRNAs) into mature miRNA duplexes, but it doesn't stay as a stable part of the active RISC. Argonaute, specifically AGO2, remains the primary protein in the active complex, facilitating the subsequent gene silencing actions [
34].
Once the miRNA duplex forms, AGO2 incorporates the guide strand, while the passenger strand typically undergoes degradation. The guide strand, now associated with AGO2, guides RISC to target mRNAs. The RISC complex is guided to specific mRNAs by the complementarity between miRNA and target mRNA. This is where AGO2 silences genes. In instances of perfect or near-perfect complementarity, AGO2 can directly cut the target mRNA, resulting in its destruction. If the complementarity is only partial, RISC can stop translation or cause mRNA to be deadened and broken down by bringing in other regulatory proteins [
35]. GW182 and other related proteins are very important for these processes because they connect the RISC complex to the machinery for breaking down mRNA, which includes deadenylases and decapping enzymes [
35].
5.2. Mechanisms of Silencing and Regulatory Feedback Loops
Gene silencing can occur through mRNA degradation or by inhibiting mRNA translation. As an example, miR16 has a sequence that matches the AU-rich region [
36] found in the 3'UTR of many unstable mRNAs, such as TNF alpha and GM-CSF [
37]. Full complementarity between the miRNA and target mRNA sequence enables Ago2 to cleave the mRNA, leading to direct mRNA destruction. When complementarity is absent, translation inhibition achieves silencing [
38]. There may be simple negative regulation between miRNA and its target mRNA, but more often than not, there are also mechanisms like the "coherent feed-forward loop," "mutual negative feedback loop" (also called "double negative loop"), and "positive feedback/feed-forward loop." Certain miRNAs function as buffers against arbitrary fluctuations in gene expression resulting from stochastic occurrences in transcription, translation, and protein stability. Negative feedback loops or incoherent feed-forward loops generally accomplish this control by decoupling protein output from mRNA transcription.
5.3. Turnover
The turnover of mature miRNA is essential for rapid alterations in miRNA expression profiles. The Argonaute protein is believed to incorporate the guide strand during miRNA maturation in the cytoplasm, providing stability, while preferentially degrading the complementary ("passenger") strand. Argonaute may employ a "use it or lose it" strategy, favoring retaining miRNAs with numerous targets while degrading those with few or no targets [
39].
The 5'-to-3' exoribonuclease XRN2, also known as Rat1p, facilitates the degradation of mature miRNAs in Caenorhabditis elegans [
40]. In plants, members of the SDN (short RNA degrading nuclease) family degrade miRNAs in the reverse (3'-to-5') direction. Animal genomes encode similar enzymes, but their functions are still unknown [
39].
Various miRNA alterations influence miRNA stability. Studies on the model organism Arabidopsis thaliana (thale cress) suggest that the addition of methyl groups at the 3' end stabilizes mature plant miRNAs. URIDYL TRANSFERASE ENZYMES can't add uracil (U) residues because of the 2'-O-conjugated methyl groups. This may be related to miRNA degradation. However, uridylation may also protect certain miRNAs, although we don't fully understand the implications of this modification. We have documented uridylation of several animal miRNAs. The insertion of adenine (A) residues to the 3' terminus of miRNAs can modify both plant and animal miRNAs. An extra adenine at the end of human miR-122, a liver-enriched miRNA important in hepatitis C, makes the molecule more stable, and plant miRNAs that end with an adenine residue have slower decay rates [
39].
5.4. Cellular Functions
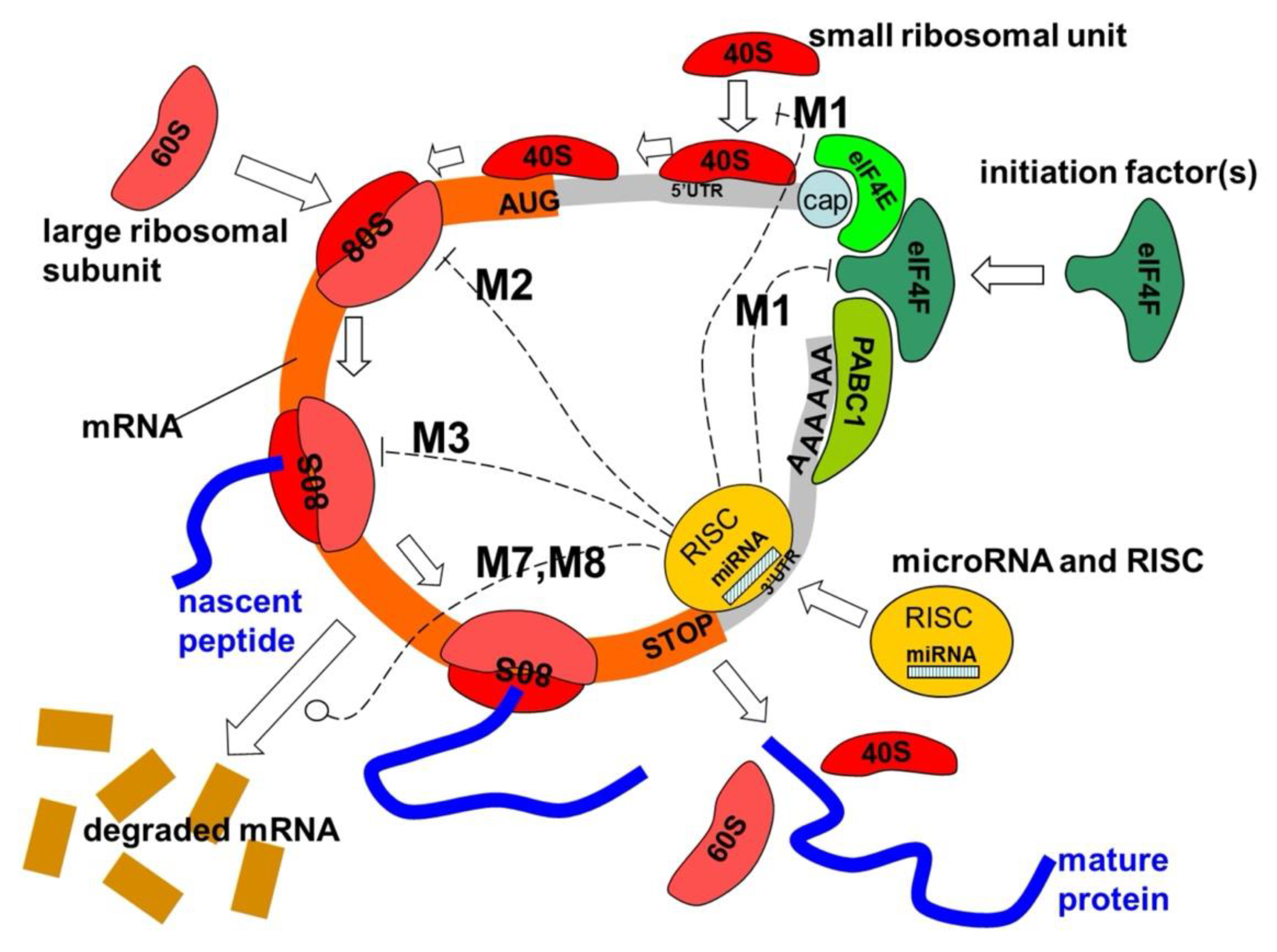
Figure 3.
miRNA interacts with the protein translation process. We illustrate multiple mechanisms of translation repression: M1) during the initiation phase, which obstructs the construction of the initiation complex or the recruitment of the 40S ribosomal subunit; M2), which affects ribosome assembly; M3), which hinders the translation process; and M7 and M8), which influence mRNA degradation [
30]. 40S and 60S represent the light and heavy subunits of the ribosome, respectively, while 80S denotes the assembled ribosome associated with mRNA. eIF4F functions as a translation initiation factor, PABC1 serves as the Poly-A binding protein, and "cap" refers to the mRNA cap structure essential for mRNA circularization, which may be the standard m7G-cap or a modified A-cap. The beginning of mRNA can happen without the cap being present by bringing the 40S ribosomeal subunit to the Internal Ribosome Entry Site (IRES) in the 5' untranslated region (5'UTR). RISC executes RNA silencing, using an Argonaute protein (AGO) as the primary catalytic subunit, while miRNA serves as a template to identify specific mRNA sequences.
https://commons.wikimedia.org/wiki/File:MiRNA_mechanisms.jpg.
Figure 3.
miRNA interacts with the protein translation process. We illustrate multiple mechanisms of translation repression: M1) during the initiation phase, which obstructs the construction of the initiation complex or the recruitment of the 40S ribosomal subunit; M2), which affects ribosome assembly; M3), which hinders the translation process; and M7 and M8), which influence mRNA degradation [
30]. 40S and 60S represent the light and heavy subunits of the ribosome, respectively, while 80S denotes the assembled ribosome associated with mRNA. eIF4F functions as a translation initiation factor, PABC1 serves as the Poly-A binding protein, and "cap" refers to the mRNA cap structure essential for mRNA circularization, which may be the standard m7G-cap or a modified A-cap. The beginning of mRNA can happen without the cap being present by bringing the 40S ribosomeal subunit to the Internal Ribosome Entry Site (IRES) in the 5' untranslated region (5'UTR). RISC executes RNA silencing, using an Argonaute protein (AGO) as the primary catalytic subunit, while miRNA serves as a template to identify specific mRNA sequences.
https://commons.wikimedia.org/wiki/File:MiRNA_mechanisms.jpg.
MicroRNAs (miRNAs) seem to play a role in gene regulation. To achieve this, a miRNA is complementary to a segment of one or more messenger RNAs (mRNAs). Animal miRNAs typically exhibit complementarity to a location in the 3' UTR, whereas plant miRNAs generally demonstrate complementarity to coding regions of mRNAs [
41]. Optimal or almost optimal base pairing with the target RNA facilitates its cleavage [
42]. This constitutes the principal mechanism of plant miRNAs [
43]. The pairings in animals are flawed.
For partially complementary microRNAs to identify their targets, nucleotides 2–7 of the miRNA, known as the 'seed region,' must exhibit complete complementarity. Animal miRNAs suppress the protein translation of target mRNA, a phenomenon that is present but less prevalent in plants. Partially complementary microRNAs can accelerate deadenylation, resulting in the earlier degradation of mRNAs [
47].
In contrast to plant microRNAs, animal microRNAs target a variety of genes. Even so, genes involved in basic cellular processes like gene expression have fewer microRNA target sites and seem to be able to be chosen to avoid being targeted by miRNAs [
48]. A robust link exists between the regulation of the ITPR gene and mir-92 and mir-19 [
49].
MicroRNAs interact with complementary sequences on genes and pseudogenes with sequence homology. This is thought to be a secondary way for genes that have structural similarities to each other but have diverged from a common ancestral gene to talk to each other and control expression levels. "Competing endogenous RNAs" (ceRNAs) are microRNAs that interact with "microRNA response elements" on genes and pseudogenes. This could help us understand how non-coding DNA stays around.
According to research, the mRNA cargo of exosomes may help with implantation by making it easier for trophoblasts to stick to the endometrium. This can be done by either increasing or decreasing the expression of genes related to adhesion or invasion [
51].
5.5. Evolution
Both plants and animals highly conserve miRNAs, which are considered a crucial and evolutionarily ancient element of gene control. Plants and animals retain fundamental elements of the microRNA pathway, but their miRNA repertoires appear to have developed separately, exhibiting distinct principal mechanisms of action [
52].
MicroRNAs serve as valuable phylogenetic markers due to their seemingly low evolutionary rate [
53]. Originally derived from earlier RNA interference machinery, microRNAs served as a regulatory mechanism to defend against external genetic material, including viruses [
54]. Their emergence may have made it easier for morphological innovation to happen. This, in turn, made it possible for more specific and finely tuned gene expression, which led to the development of complex organs and maybe even complex life. An elevated rate of microRNA accumulation typically links to rapid surges of morphological innovation.
Many mechanisms generate new microRNAs. There are two ways that new microRNAs can form: randomly building hairpins in "non-coding" parts of DNA (like introns or intergenic areas) or copying and changing microRNAs that already exist [
56]. MicroRNAs may also come from copies of protein-coding regions that are turned around, which makes it easier for a foldback hairpin structure to form [
57]. The rate of evolution (i.e., nucleotide substitution) in new microRNAs is similar to that in non-coding DNA, which suggests evolution through neutral drift. On the other hand, the rate of change in older microRNAs is much slower (often less than one substitution per hundred million years), which suggests that once a microRNA gets a function, it goes through purifying selection [
56]. Different parts of a miRNA gene are subject to different levels of evolutionary pressure. Parts that are needed for processing and function are more likely to stay the same [
58]. An animal's genome rarely lacks a microRNA today, but it frequently loses more recent, likely non-functional microRNAs. Researchers estimate the net flux of miRNA genes in Arabidopsis thaliana to range from 1.2 to 3.3 genes per million years [
60]. This makes them a significant evolutionary marker, and researchers view them as a potential solution to unresolved phylogenetic issues, such as the relationships of arthropods [
61]. Conversely, in numerous instances, microRNAs have a weak correlation with phylogeny, suggesting that their phylogenetic concordance may predominantly stem from restricted microRNA sampling [
62].
MicroRNAs are present in the genomes of the majority of eukaryotic species, ranging from brown algae to mammals. The disparity in the functionality and processing of these microRNAs indicates that they originated independently in plants and mammals [
64].
6. Empirical Techniques
Currently, the miRTarBase library has identified and cataloged over 3,000 distinct human miRNAs, with an estimated capacity of around five million possible miRNA-target interactions [
65]. Nonetheless, there are concerns regarding the quality of miRNAs. The identification of miRNAs as a unique category of regulatory molecules has impacted molecular biology. For decades, the prevailing paradigm in gene regulation emphasized transcription factors and promoter regions as the primary control points for gene expression. The discovery of miRNAs unveiled an extra dimension of post-transcriptional control, enabling cells to refine gene expression with greater precision. MiRNAs function by binding to target mRNAs, typically within their 3′ UTR, leading to either mRNA destruction or translational inhibition. This process is highly specialized, with each miRNA controlling several genes; hence, it increases the complexity of the gene regulatory networks that govern cellular processes [
66].
The identification of miRNAs has also illuminated the evolutionary conservation of gene regulation systems. The conservation of miRNAs, such as Let-7, from worms to humans implies that miRNA-mediated regulation is a fundamental biological mechanism. Moreover, researchers identified miRNAs as participating in various cellular functions, including development, differentiation, cell cycle regulation, and death. Researchers have demonstrated that these tiny compounds govern essential developmental processes, ensuring that cells differentiate and proliferate at the correct times and locations. Alterations in miRNA regulation have been associated with several developmental abnormalities and illnesses [
5].
Annotations in publicly accessible databases contribute to the reproducibility of microRNA research [
9]. However, the meticulously managed miRNA gene database MirGeneDB recognizes around 500 human miRNAs as authentic [
67]. The precise quantity continues to rise as novel sequencing technology and computational techniques uncover supplementary miRNAs. Recent investigations have revealed numerous novel miRNAs implicated in the human brain and other tissues, thereby increasing the known miRNA repertoire [
68,
69].
As researchers concentrated on miRNA expression in physiological and pathological processes, several technological variables concerning microRNA isolation arose. Researchers have scrutinized the integrity of preserved miRNA samples [
70]. Due to their shorter length and the ubiquitous presence of RNases, microRNAs degrade more readily than mRNAs. It is essential to refrigerate samples on ice and utilize RNase-free apparatus [
71].
A two-step polymerase chain reaction procedure, consisting of modified reverse transcription polymerase chain reaction and quantitative polymerase chain reaction, can evaluate microRNA expression. This method's variations attain absolute or relative quantification [
72]. Microarrays, slides, or chips containing probes for numerous miRNA targets can hybridize MiRNAs, enabling the determination of relative miRNA levels across various samples [
73]. High-throughput sequencing techniques (microRNA sequencing) can identify and characterize microRNAs [
74]. We can experimentally suppress the function of a miRNA using a locked nucleic acid (LNA) oligonucleotide, a Morpholino oligonucleotide, or a 2'-O-methyl RNA oligonucleotide. A corresponding antagomir can inhibit a particular miRNA. Steric blocking oligonucleotides can obstruct microRNA maturation at multiple stages [
77]. A steric-blocking oligonucleotide can obstruct the miRNA target site of an mRNA transcript [
78]. You can use LNA or Morpholino probes for "in situ" detection of miRNA [
79]. The locked shape of LNA improves its ability to hybridize and makes it more sensitive and selective, making it perfect for finding short miRNA [
80].
The high-throughput quantification of miRNAs is susceptible to errors due to greater variance associated with methodological issues compared to mRNAs. Therefore, researchers frequently examine mRNA expression to evaluate the influence of miRNAs on their levels [
81]. Databases can facilitate the pairing of mRNA and miRNA data to determine miRNA targets based on their nucleotide sequences [
82]. Typically, this happens after identifying miRNAs of interest, often because of their elevated expression levels; however, researchers have proposed analytical techniques that combine mRNA and miRNA expression data [
83].
MiRNA plays a similar role in the regular operation of eukaryotic cells, and its dysregulation has been associated with illness. Manually maintained and publicly accessible, the miR2Disease database documents the established associations between miRNA dysregulation and clinical diseases [
84].
7. Clinical Applications
Utilizing miRNA to inhibit specific proteins often involves employing synthetic miRNA mimics that replicate the action of endogenous miRNAs, thereby restoring the activity of under-expressed miRNAs and facilitating the destruction of pathogenic proteins. They prove particularly advantageous when the downregulation of the endogenous miRNA, which normally inhibits a detrimental protein, occurs. Extracellular circulating miRNAs also exist [
85].
Additionally, anti-miRs (antagomirs) are chemically modified oligonucleotides that bind to natural miRNAs, thereby inhibiting their action. We use these when an overexpressed miRNA increases the production of harmful proteins. By blocking these miRNAs, anti-miRs obstruct the downregulation of tumor suppressor genes and other advantageous proteins.
Investigators commenced examining the function of miRNAs in cardiovascular illnesses, neurological disorders, and immunological control. For instance, miR-126 is essential for angiogenesis, the formation of new blood vessels, and its dysregulation correlates with atherosclerosis and other cardiovascular disorders [
86].
7.1. Biomarker
An exciting application of miRNAs is their use in developing synthetic miRNA circuits that can function as biosensors within cells. These circuits would change miRNA activity in response to changes in cells, like the presence of disease markers. This would allow for a controlled therapeutic response. This approach may be particularly advantageous in cancer therapy, as miRNA circuits could selectively target tumor cells while preserving healthy tissues.
Blood, saliva, and urine contain stable circulating miRNAs that are excellent candidates for non-invasive diagnostics. MicroRNAs (miRNAs) reflect the physiological state of tissues and are often dysregulated in diseases such as cancer, cardiovascular disorders, and neurodegenerative diseases. Researchers have recognized miR-155 as a biomarker for various diseases, including lymphoma and breast cancer, with elevated levels related to tumor progression. Reduced levels of miR-126 have been associated with atherosclerosis, highlighting its potential in cardiovascular diagnostics.
The potential of miRNAs as biomarkers includes early disease detection and monitoring. MiRNA-based liquid biopsies are a non-invasive alternative to traditional diagnostic methods, which sometimes need invasive procedures or imaging techniques, for finding diseases early, checking how well treatments are working, and keeping an eye out for recurrences. Oncology may use liquid biopsies to track the growth of tumors by looking at changes in miRNAs (like miR-21) that are linked to different types of cancer. Blood and CSF fluid release circulating miRNAs, which may serve as biomarkers in many illnesses. [
85,
87]
Advancements in miRNA biomarker research may enable the development of disease-specific diagnostic panels. By identifying the unique miRNA expression profiles associated with different diseases, physicians can provide tailored diagnostic and prognostic information, improving patient outcomes through prompt interventions and individualized treatment approaches.
The development of synthetic miRNA circuits functioning as molecular biosensors within cells is another compelling area of research. Researchers can design these circuits to identify specific indicators of sickness and subsequently adjust miRNAs, providing a meticulously controlled method for gene regulation. This approach may be particularly advantageous in diseases like cancer, as miRNA circuits can selectively target tumor cells while preserving healthy tissues [
88].
One of the most intriguing prospective applications of miRNAs is their use as biomarkers for disease diagnosis and monitoring. MicroRNAs, which remain stable in circulation and are detectable in biological fluids like blood, saliva, and urine, serve as ideal candidates for non-invasive diagnostic techniques. Given their tissue specificity and common dysregulation in diseases, miRNA expression patterns can provide valuable insights into the onset and progression of conditions such as cancer, cardiovascular disease, and neurodegenerative disorders. Researchers have connected elevated levels of miR-155 in blood samples to adverse outcomes in tumors like lymphoma, and reduced levels of miR-126 to cardiovascular diseases like atherosclerosis.
The growth of miRNA-based diagnostics may enable the development of liquid biopsies, allowing clinicians to detect diseases at an early stage and monitor their course with simple blood tests. This non-invasive technology offers significant advantages over traditional biopsy methods, which can be painful, expensive, and often perilous for patients. When it comes to oncology, liquid biopsies may be especially helpful because they can check miRNA levels, which could help find tumor recurrence or metastasis early, before normal imaging methods find visible cancers. Integrating supplementary molecular markers, such as circulating tumor DNA (ctDNA), with miRNA biomarkers may improve the specificity and sensitivity of these diagnostic procedures, enabling more accurate disease monitoring.
7.2. Oncology
The first human disease recognized as associated with miRNA dysregulation was chronic lymphocytic leukemia. Numerous supplementary miRNAs have been associated with cancer, leading to their designation as "oncomirs" [
91]. In cancerous B cells, miRNAs play a key role in many important aspects of cell growth. These include B-cell receptor (BCR) signaling, B-cell motility and adhesion, intercellular interactions within immunological niches, and the production and switching of immunoglobulin classes. MicroRNAs have an effect on how B cells mature and how many pre-, marginal zone, follicular, B1, plasma, and memory B cells there are [
89].
Another use of miRNA in cancer is to employ their expression levels for prognostic applications. Diminished levels of miR-324a in NSCLC samples may signify unfavorable survival outcomes. In colorectal cancer, increased levels of miR-185 or decreased levels of miR-133b may correlate with metastases and poor survival results.
Furthermore, specific miRNAs may be associated with different histological subtypes of colorectal cancer. Colorectal malignancies that produce mucin and colon cancers associated with ulcerative colitis exhibit elevated levels of miR-205 and miR-373, while colonic adenocarcinomas that do not produce mucus do not exhibit these levels. In vitro studies showed that miR-205 and miR-373 may effectively cause a number of features of mucinous-associated neoplastic growth in intestinal epithelial cells.
The interaction between miR-21 and MAP2K3, a tumor suppressor gene, may contribute to the proliferation of hepatocellular carcinoma cells [
91]. Effective cancer treatment requires the accurate identification of patients for risk-stratified therapy. Individuals with a rapid reaction to their first medication may benefit from shortened treatment regimens, underscoring the significance of accurate sickness response evaluations. Diagnostic laboratories can quantify cell-free circulating miRNAs, also known as cimiRNAs, which exhibit remarkable stability in blood and significantly overexpress in cancer. In classical Hodgkin lymphoma, plasma miR-21, miR-494, and miR-1973 function as potential biomarkers for disease response [
95]. The circulation of miRNAs can assist in clinical decision-making and enhance the interpretation of positron emission tomography in conjunction with computed tomography. Each visit could include them to evaluate the disease response and identify any relapses.
A variety of miRNAs can directly target and inhibit cell cycle genes to modulate cell growth. An innovative strategy for tumor treatment is suppressing tumor cell proliferation by restoring the dysfunctional miRNA system in cancers [
96]. Cancer arises from the accumulation of mutations caused by DNA damage or uncorrected errors during DNA replication. Deficiencies in DNA repair mechanisms contribute to the buildup of mutations, which may ultimately result in cancer. MicroRNAs regulate a variety of genes related to DNA repair [
98].
Germline mutations in DNA repair genes constitute around 2–5% of colon cancer patients [
92]. Malignancies are frequently associated with altered microRNA expression, resulting in DNA repair deficiencies, and they may constitute a substantial causative component.
MicroRNAs regulate the expression of HMGA proteins (HMGA1a, HMGA1b, and HMGA2) and link them to cancer. HMGA expression is almost undetectable in differentiated adult tissues but is markedly elevated in various cancers. HMGA proteins are polypeptides consisting of around 100 amino acid residues, characterized by a modular sequence architecture. These proteins have three very charged parts called AT hooks that attach to the minor groove of AT-rich DNA sequences in certain places. Human neoplasms, including thyroid, prostatic, cervical, colorectal, pancreatic, and ovarian carcinomas, have a substantial increase in HMGA1a and HMGA1b protein levels [
100].
Variations in single nucleotide polymorphisms (SNPs) can influence the binding of miRNAs to 3' UTRs. For instance, hsa-mir181a and hsa-mir181b engage with the CDON tumor suppressor gene [
93].
The role of miRNAs in cancer is one of the most thoroughly investigated areas in miRNA research. Depending on their targets, miRNAs can either facilitate cancer progression (oncomiRs) or inhibit tumor growth (tumor suppressor miRNAs). Breast, lung, and colorectal cancers frequently overexpress them. By targeting tumor suppressor genes such as PTEN, PDCD4, and TP53, which are crucial for regulating cell cycle progression and apoptosis, miR-21 promotes tumor proliferation. By inhibiting these crucial tumor suppressors, miR-21 enables cancer cells to evade apoptosis and proliferate indiscriminately, promoting tumorigenesis and metastasis.
A primary strategy for miRNA-based therapeutics is the application of miRNA mimics. These synthetic RNA molecules aim to restore the function of downregulated tumor-suppressive or protective miRNAs in diseases such as cancer. For instance, several malignancies, such as lung, colon, and pancreatic cancers, frequently downregulate miR-34, a significant tumor suppressor microRNA. MRX34, a miR-34 mimic, was the first miRNA-based therapeutic to initiate clinical trials. It demonstrated the ability to restore tumor-suppressive functions by facilitating apoptosis and inhibiting cell proliferation in cancer cells. Despite the trial's cessation due to adverse effects on the immune system, MRX34 represented a significant advancement in the evolution of miRNA cancer therapies.
On the other hand, malignant situations often downregulate tumor-suppressor miRNAs like let-7. Let-7 targets numerous oncogenes, including RAS, MYC, and HMGA2, which promote cell proliferation. The lack of let-7 expression leads to the overexpression of oncogenes, resulting in uncontrolled cell proliferation and tumor formation. Reduced levels of let-7 have been associated with an unfavorable prognosis in multiple malignancies, including lung cancer. MicroRNAs have two critical functions in cancer: they can either promote tumor proliferation or inhibit it. This renders them significant targets for therapy strategies.
MicroRNAs may serve as potential biomarkers for cancer diagnosis and prognosis. Researchers have linked increased concentrations of miR-155 in the bloodstream to adverse outcomes in lymphoma and other cancers, making it a significant biomarker for assessing disease progression. The detection of miRNAs in bodily fluids such as blood and urine facilitates cancer screening and monitoring without causing harm to the individual. Clinicians are increasingly exploring this approach [
102].
MRX34 exhibited promise in preclinical studies and was the first miRNA mimic to progress to Phase I clinical trials. The trial focused on individuals with advanced solid tumors, including hepatocellular carcinoma, melanoma, and renal cell carcinoma. However, unexpected immune-related adverse effects in several patients abruptly terminated the project despite its initial success. This outcome demonstrated the difficulty of delivering miRNA treatments throughout the entire body. It also demonstrated the significance of improving delivery systems and enhancing methods to attenuate immune responses.
7.3. Cardiovascular Disorders
The Centers for Disease Control and Prevention identify stroke as a primary cause of mortality and long-term disability in the United States. Ischemic strokes account for 87% of instances, resulting from an obstruction in the artery supplying oxygenated blood to the brain. The impediment of blood circulation prevents the brain from obtaining essential nutrients, including oxygen and glucose, and from eliminating waste products, such as carbon dioxide. MicroRNAs (miRNAs) help to silence genes after they have been translated by targeting genes that are involved in the harmful effects of cerebral ischemia, such as those that control inflammation, angiogenesis, and apoptosis [
96].
MicroRNAs (miRNAs) play a key role in controlling processes like angiogenesis, cardiomyocyte proliferation, and the heart's response to damage in heart diseases. MiR-126 plays a crucial role in maintaining the health of endothelial cells and promoting angiogenesis. Heart diseases, like atherosclerosis and coronary artery disease, are linked to lower levels of miR-126. These diseases hurt tissues by preventing blood flow. Approaches to therapy that restore miR-126 expression have shown promise in improving vascular healing and lowering the number of ischemic events, such as heart attacks and spinal cord injuries [
11].
Likewise, miR-1 and miR-133 are essential for the growth and functionality of cardiac muscle. The development of cardiomyocytes, the cells responsible for cardiac muscle contraction, is facilitated by miR-1, while their proliferation is enhanced by miR-133. The dysregulation of these miRNAs has been associated with heart failure and cardiac hypertrophy, diseases marked by compromised cardiac function and muscular enlargement. Getting the balance of miR-1 and miR-133 back to normal in animal models has shown to improve heart function and reduce scarring after a myocardial infarction. This suggests that restoring this balance could be a way to treat heart disease because these two genes control important processes like blood vessel growth, heart muscle cell proliferation, and the response to injury. As an example, miR-126 is a key regulator of endothelial cell activity and helps blood vessels grow by targeting the PI3K-Akt signaling pathway. The dysregulation of miR-126 is associated with atherosclerosis and coronary artery disease, wherein compromised angiogenesis leads to ischemia occurrences. Therapeutic approaches that elevate miR-126 levels may enhance vascular health and facilitate tissue regeneration post-myocardial infarction.
Moreover, miR-1 and miR-133 are essential for the regulation of cardiomyocyte differentiation and cardiac muscle regeneration. These miRNAs facilitate the appropriate development of heart tissue and the response to damage. Restoring miR-133 expression has been shown to help cardiac muscle regeneration and reduce the growth of scar tissue in models of heart failure and myocardial infarction. This could be a potential therapeutic strategy for preventing heart failure after a cardiac injury.
Researchers are investigating the role of miRNAs in angiogenesis, cardiac remodeling, and damage response in cardiovascular disease research. Researchers are studying miR-126, a prominent miRNA, for its role in regulating endothelial cell function and its critical importance in preserving vascular integrity. Decreased levels of miR-126 have been associated with atherosclerosis and coronary artery disease. Researchers are investigating if reinstating miR-126 expression can enhance angiogenesis and improve results in patients with ischemic heart disease.
Another miRNA pertinent to cardiovascular disease is miR-133, which is crucial to cardiomyocyte proliferation and cardiac muscle regeneration. Situations such as cardiac hypertrophy and heart failure diminish miR-133, and preclinical models have demonstrated the potential of treatment approaches aimed at reinstating miR-133 levels. Numerous active clinical trials are examining the efficacy of miR-133-based treatments in avoiding heart failure and facilitating cardiac healing after myocardial infarction.
7.4. Neurodegenerative Diseases
In the nervous system, miRNAs regulate neuronal differentiation, synaptic plasticity, and neuronal survival. Dysregulation of miRNAs has been linked to neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. miR-124 is one of the most abundant miRNAs in the brain and plays a critical role in maintaining neuronal identity and promoting synaptic function. In neurodegenerative diseases, miR-124 is often downregulated, leading to neuronal dysfunction and synaptic loss. Restoring miR-124 expression in animal models of neurodegeneration has shown promise in protecting neurons from degeneration and improving cognitive function.
In Alzheimer’s disease, miRNAs such as miR-29 and miR-146a regulate the production of amyloid-beta, a toxic protein that accumulates in the brains of Alzheimer’s patients. miR-29 suppresses the expression of beta-secretase, an enzyme involved in amyloid-beta production. Reduced levels of miR-29 in Alzheimer’s patients are associated with increased amyloid-beta accumulation, suggesting that restoring miR-29 function could help slow disease progression. Similarly, miR-146a modulates the brain’s inflammatory response, which is often dysregulated in neurodegenerative diseases. Targeting miRNAs involved in inflammation and amyloid-beta production offers a promising therapeutic approach for treating Alzheimer’s disease.
Neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, and Amyotrophic Lateral Sclerosis (ALS) increasingly recognize the role of microRNAs. MicroRNAs in the brain govern neuronal differentiation and function while modulating synaptic plasticity. For instance, neurons abundantly express miR-124, which is crucial for sustaining neuronal identity and enhancing synaptic function. Dysregulation of miR-124 and other miRNAs contributes to neuronal death and synaptic dysfunction in neurodegenerative disorders.
In Alzheimer's disease, miRNAs like miR-29 and miR-146a have been associated with the regulation of amyloid beta formation, a critical element in the illness's progression. Beta-secretase is an enzyme that causes amyloid-beta plaques to form in the brains of people with Alzheimer's disease. MiR-29 reduces its expression. Restoring miR-29 functionality may diminish amyloid-beta concentrations and decelerate illness advancement. Similarly, Alzheimer's disease disrupts the brain's inflammatory response, which miR-146a modulates. Targeting these miRNAs presents a viable therapeutic strategy for the treatment of neurodegenerative illnesses by tackling both the fundamental causes and manifestations.
7.5. Autoimmune Disorders
In the immune system, miRNAs modulate the synthesis of cytokines and other immune-associated proteins, thereby affecting inflammation and immunological responses. In this scenario, miR-146a and miR-155 play a significant role. MiR-146a reduces inflammation by targeting parts of the NF-B signaling pathway activated during immune responses. This miRNA inhibits excessive inflammation, safeguarding tissues from chronic inflammatory injury. Conversely, miR-155 facilitates the development and activation of T cells and macrophages, thereby augmenting the immune response when required. Autoimmune disorders have linked miR-155 dysregulation, underscoring its significance in maintaining immune system equilibrium [
7].
In autoimmune illnesses, miRNAs are pivotal in modulating immune cell activity and the synthesis of inflammatory mediators. MiR-146a plays a crucial role in regulating inflammation, acting as a negative feedback mechanism within the NF-B pathway to reduce excessive inflammatory reactions. The dysregulation of miR-146a correlates with chronic inflammatory illnesses, including rheumatoid arthritis and lupus, in which uncontrolled inflammation results in tissue destruction. Animal models of autoimmune diseases have shown that increasing the expression of miR-146a reduces inflammation, suggesting that these approaches could potentially treat these disorders [
97].
A lot of autoimmune diseases also raise miR-155, which helps immune cells become active and makes the overactive immune response that comes with conditions like inflammatory bowel disease and multiple sclerosis worse. Blocking miR-155 in animal models of autoimmune diseases resulted in less severe diseases, indicating its potential as a therapeutic target to alter the immune response.
7.6. Viral Infections
MicroRNAs have demonstrated potential as therapeutic agents for viral infections, especially for viruses that utilize host microRNAs for replication. For instance, miR-122, a liver-specific microRNA, is essential for the replication of the hepatitis C virus (HCV). Miravirsen, an antagomir that targets miR-122, was one of the first miRNA-based medicines for viral infections to go through clinical trials. Miravirsen diminishes viral replication and decreases the viral load in HCV-infected patients by suppressing miR-122. This miRNA-based strategy provides an innovative method for addressing viral infections without directly attacking the virus, hence minimizing the potential for treatment resistance.
Likewise, miRNAs are under investigation for their capacity to impede HIV replication. Specific host miRNAs, including miR-29, can target and degrade HIV transcripts, thereby diminishing viral replication. Scientists are working on new antiviral drugs to help people who have HIV and to stop drug-resistant strains from spreading. One way they plan to do this is by increasing the expression of certain host miRNAs or giving people synthetic miRNAs that copy their activity.
Even though there are issues with miRNA-based treatments, such as delivery and stability, new developments in delivery technologies are expected to make miRNA therapeutics more useful.
Viral microRNAs significantly influence the regulation of gene expression in both viral and host genes to the advantage of the virus. Consequently, miRNAs are pivotal in host-virus interactions and the development of viral illnesses [
98]. It is thought that viral miRNA changes how transcription activators are made by human herpesvirus-6 DNA [
99].
7.7. Alcohol Dependence
MicroRNAs play a crucial function in gene expression pertinent to addiction, particularly alcoholism. Chronic alcohol misuse leads to enduring modifications in brain function, partially driven by changes in gene expression [
100]. Globally, MiRNA regulates numerous downstream genes, playing a crucial role in reorganizing synaptic connections and bringing about enduring alterations in the brain associated with alcohol consumption, cessation, or dependence [
101]. Researchers have found changes in up to 35 different miRNAs in the brains of alcoholics who have died. These miRNAs all target genes that control the cell cycle, apoptosis, cell adhesion, nervous system development, and cell signaling [
100]. Mice that were dependent on alcohol had different levels of miRNA in their medial prefrontal cortex. This suggests that miRNA controls translational imbalances and makes proteins that are expressed differently in a part of the brain that is linked to complex thinking and making decisions [
102].
Persistent alcohol consumption can either increase or downregulate miRNAs. The prefrontal cortex of rats that were dependent on alcohol had higher levels of miR-206. This miR-206 targeted the transcription factor brain-derived neurotrophic factor (BDNF) and decreased its expression. BDNF is essential for the development and maturation of new neurons and synapses, indicating its potential involvement in synaptic growth and plasticity among alcohol abusers [
103]. Seeing that miR-155 levels are high, which is important for controlling neuroinflammatory responses caused by alcohol, suggests that microglia and inflammatory cytokines play a role in alcohol pathophysiology [
104]. miR-382 levels went down in the nucleus accumbens, a part of the basal forebrain that controls emotions related to rewards that drive motivated behaviors. MiR-382 targets the dopamine receptor D1 (DRD1) and increases its levels. This increases the levels of DRD1 and delta fosB, a transcription factor that sets off a series of transcriptional events in the nucleus accumbens, which makes addictive behaviors more likely. On the other hand, increasing miR-382 decreased alcohol consumption and stopped the upregulation of DRD1 and delta fosB in rat models of alcoholism. This suggests the potential use of miRNA-targeted therapeutics in treatment.
7.8. Senescence
Defective proteins accumulate during the aging process and disrupt normal cellular functions. Therapeutics based on miRNA may target many proteins that help with aging processes like inflammation, cellular senescence, and slower tissue repair. Cells often enter a state known as cellular senescence as they age, characterized by the release of senescence-associated secretory phenotype (SASP), a group of pro-inflammatory cytokines, growth factors, and proteases. These proteins facilitate tissue failure and inflammation in aged tissues.
• miR-146a: This microRNA can suppress IL-6 and IL-1β, two principal inflammatory cytokines that are elevated in old, senescent cells. Utilizing miR-146a mimics to target these cytokines may diminish inflammation and impede tissue deterioration.
• miR-29: This microRNA can stop collagen-degrading enzymes, such as matrix metalloproteinases (MMPs), from doing their job. MMPs help break down the extracellular matrix in old tissues. We may utilize miR-29 mimics to preserve tissue architecture and mitigate fibrosis, a condition that becomes increasingly prevalent with aging.
A lot of proteins that affect how we age, like the insulin-like growth factor (IGF) pathway and the oxidative stress response, may be downregulated by miRNAs.
• miR-375: This microRNA inhibits IGF-1R, a receptor in the IGF signaling pathway that promotes growth and could potentially contribute to age-related diseases like cancer. The overexpression of miR-375 may diminish IGF-1R activity and alleviate these concerns.
• miR-34a: Studies have shown that this microRNA inhibits p53 and Bcl-2, proteins linked to apoptosis and stress responses, respectively. Changing the amount of miR-34a may help control apoptosis in old cells, which could stop unwanted cell death in important places like the brain or heart.
The aging process frequently correlates with metabolic dysfunctions, including insulin resistance and mitochondrial deterioration, resulting in conditions such as type 2 diabetes. MiRNAs can target proteins associated with metabolism to postpone these age-related diseases. MiR-103/107 specifically targets caveolin-1, which is a modulator of insulin signaling. Inhibiting these miRNAs may improve insulin sensitivity, which often diminishes with age and thus aids in the prevention or management of metabolic disorders.
7.9. Obesity
MiRNAs are essential in regulating the differentiation of stem cell progenitors into adipocytes [
107]. Researchers conducted research on the role of pluripotent stem cells in adipogenesis using the immortalized human bone marrow-derived stromal cell line hMSC-Tert20 [
108]. It has been shown that miR-155, miR-221, and miR-222 are downregulated during the adipogenic programming in both immortalized and primary human mesenchymal stem cells. This suggests that they play a negative regulatory role in differentiation. Expression of miRNAs 155, 221, and 222 outside of their normal locations significantly slowed down adipogenesis and prevented the activation of the main regulators PPAR and CEBPA [
109]. This facilitates potential genetic interventions for obesity.
The let-7 family is another class of miRNAs that modulate insulin resistance, obesity, and diabetes. Let-7 accumulates in human tissues throughout the aging process [
110]. Upon aberrantly overexpressing let-7 to mimic accelerated aging, mice displayed insulin resistance, making them more vulnerable to obesity and diabetes resulting from a high-fat diet [
111]. On the other hand, when Let-7 was blocked by giving the mice Let-7-specific antagomirs, they became more sensitive to insulin and were much less likely to get diabetes or become overweight when they ate a lot of fat. Inhibition of let-7 could not only prevent obesity and diabetes but also reverse and cure the conditions. The experimental results indicate that let-7 inhibition may serve as a novel treatment for obesity and type 2 diabetes.
7.10. Hemostasis
Under normal physiological conditions, miRNAs preserve cellular homeostasis by modulating the expression of genes implicated in essential processes such as development and cell differentiation. The essential regulators of muscle growth are miR-1 and miR-133. While miR-1 facilitates the differentiation of muscle progenitor cells into myocytes, miR-133 stimulates the proliferation of muscle progenitor cells, thereby maintaining an equilibrium between muscle development and differentiation. These miRNAs are crucial for appropriate muscle development and the regeneration of muscle tissue post-injury.
MicroRNAs (miRNAs) play a big role in controlling complex enzyme chains, such as the system that stops bleeding [
113]. Recent extensive research on functional miRNA targeting has identified rational therapeutic targets within the hemostatic system [
114]. They are directly associated with calcium homeostasis in the endoplasmic reticulum, which is essential for cell differentiation throughout early development [
115].
Metabolic control is another domain in which miRNAs are essential. The liver significantly expresses miR-122, which modulates the metabolism of cholesterol and fatty acids. The role of miR-122 in lipid homeostasis highlights its significance in preserving metabolic health. Improper function of miR-122 may lead to metabolic diseases such as non-alcoholic fatty liver disease (NAFLD) and hyperlipidemia. This shows how important miRNAs are for normal body functions.
7.11. Additional Applications
The increasing data connecting miRNAs to human diseases has stimulated interest in the development of miRNA-based diagnostics and therapies. The advancement of miRNA technology is leading to a multitude of innovative applications, one of which is the integration of miRNA therapies with established treatments like chemotherapy, immunotherapy, or gene therapy, an emerging trend. MiRNA modification can augment the effectiveness of conventional therapies by sensitizing neoplastic cells to chemotherapy or diminishing drug resistance. Each of these databases fulfills distinct functions, encompassing miRNA sequence data, target prediction and validation, disease connections, and further applications.
A mutation in the seed region of miR-96 results in hereditary progressive hearing loss [
117]. Hereditary keratoconus and anterior polar cataract result from a mutation in the seed area of miR-184 [
118]. The elimination of the miR-17~92 cluster results in skeletal and growth abnormalities [
119].
Furthermore, miRNA miR-183/96/182 appears to be crucial in circadian rhythm regulation [
120].
7.12. Flora
MiRNAs play a crucial role in regulating various developmental, homeostatic, and immunological processes in plants. Their functions in plant development encompass shoot apical meristem development, leaf growth, flower formation, seed generation, and root expansion. Furthermore, they assume a multifaceted function in reactions to many abiotic stimuli, including thermal stress, low-temperature stress, drought stress, light stress, and gamma radiation exposure [
121].
8. Potential Opportunities
8.1. MicroRNAs in Precision Medicine
Personalized or precision medicine seeks to customize medical treatments for individual patients according to their genetic, environmental, and lifestyle determinants. Precision medicine ideally incorporates microRNAs because of their crucial role in regulating gene expression and their capacity to provide insights into an individual's unique genetic background. Advancements in miRNA sequencing technologies facilitate the identification of miRNA expression patterns associated with certain diseases or therapeutic responses. Patients exhibiting distinct miRNA signatures may demonstrate enhanced responsiveness to particular cancer therapies, whereas others could necessitate alternate strategies informed by their miRNA profiles.
Incorporating miRNA data into precision medicine frameworks may facilitate customized therapy targeting the specific molecular aberrations underlying a patient's condition. In oncology, this may involve finding oncomiRs that are elevated in a specific tumor and employing antagomirs to suppress these miRNAs, thus impeding cancer progression. Targeting miRNAs that modulate vascular health or inflammation in cardiovascular disease may inhibit disease progression or improve outcomes after incidents like myocardial infarctions. Customized miRNA therapies could reduce the likelihood of adverse medication reactions by tailoring treatments to the patient's unique miRNA profile, leading to more effective and safer interventions.
8.2. MicroRNAs in Regenerative Medicine
Researchers anticipate a significant expansion in the use of miRNAs in regenerative medicine as their research advances. MiRNAs have demonstrated their ability to facilitate the differentiation into specific cell types, making them valuable instruments for tissue regeneration and repair. For instance, researchers have utilized miR-375 to encourage stem cells to transform into insulin-secreting beta cells, offering potential diabetes treatments. Likewise, miR-1 and miR-133 play a role in cardiac muscle regeneration, and the modulation of these miRNAs may facilitate the repair of heart tissue following injury.
In the future, miRNA-based medicines may facilitate the engineering of tissues and organs for transplantation, providing remedies for individuals with organ failure or significant damage. Researchers can make tissues that are genetically compatible with patients by using miRNAs to guide the development of pluripotent stem cells into functional tissues. This lowers the risk of rejection and improves the success of transplants. This method possesses considerable promise for addressing conditions such as heart failure, diabetes, and neurodegenerative diseases, where organ damage is presently irreversible.
8.3. Synthetic Biology and miRNA-Targeted Therapeutics
We anticipate that synthetic biology will play a crucial role in the future of miRNA-based treatments, specifically in the development of bespoke miRNA circuits. These circuits may serve as biosensors to identify specific alterations within cells, such as variations in the expression of disease-related genes. Upon activation, miRNA circuits may elicit specific therapeutic responses, rendering them suitable for targeted cancer treatments or regenerative medicine. By using miRNAs as molecular switches, these man-made structures would allow precisely controlled interventions, which would lower the risk of side effects that often get in the way of current therapeutic strategies. A group of researchers is looking into making synthetic miRNA circuits that can work as biosensors inside cells, changing gene expression in response to changes in the cell environment. These circuits may deliver precisely regulated therapeutic responses, activating only in the presence of specific illness indicators and deactivating upon attainment of the therapeutic objective. This synthetic biology technique signifies a promising advancement in miRNA research, with potential applications in regenerative medicine, cancer treatment, and chronic illness management.
We may engineer synthetic miRNA circuits in cancer therapy to identify the expression of oncogenes or certain tumor markers. Upon identifying these indicators, the circuit might activate miRNAs that inhibit tumor proliferation or trigger death in cancer cells while preserving healthy tissues. This degree of specificity could significantly enhance the results of miRNA-based cancer therapeutics, minimizing adverse effects and improving treatment efficacy.
8.4. MicroRNAs in Gene Editing
The identification of miRNAs and their function in modulating gene expression has created new opportunities for therapeutic uses. By focusing on miRNAs, we can either get gene expression back to normal in diseases where miRNAs are out of whack or stop miRNAs from doing harmful things. Since miRNAs are involved in various physiological processes like differentiation, proliferation, apoptosis, and metabolism, we can use therapeutic regulation of these tiny RNA molecules to treat a wide range of disorders. These encompass malignancies, cardiovascular ailments, neurological conditions, viral infections, and inflammatory disorders. Two primary strategies for miRNA-based therapeutics are miRNA mimics and antagomirs (anti-miRNA oligonucleotides). This section outlines the exploration of various methodologies for the development of innovative therapeutics, taking into account the existing obstacles and breakthroughs in miRNA delivery systems.
MiRNAs are essential regulators of gene expression, significantly influencing normal physiological functions and contributing to the etiology of several disorders when dysregulated. Their role in cancer, cardiovascular disease, neurological illnesses, and autoimmune problems renders them significant targets for therapeutic intervention and effective biomarkers for disease detection. Ongoing research reveals the extensive regulatory networks controlled by miRNAs, highlighting their significant potential to transform the diagnosis and treatment of various complicated disorders.
As technologies such as CRISPR-Cas9 transform gene editing, miRNAs may function as essential instruments in refining gene editing results. While CRISPR allows for direct DNA alteration, miRNAs act as a post-transcriptional mechanism to control the expression of modified genes, ensuring the appropriate expression of genetic modifications. By selectively silencing unwanted genetic alterations, MiRNAs can work in tandem with CRISPR to mitigate off-target consequences of gene editing.
Ensuring that the introduced genes express in the appropriate tissues and at optimal amounts is a primary hurdle in gene therapy. Combining CRISPR technology with miRNA regulation could help scientists make medicines that not only change a person's DNA but also carefully control gene expression to match normal physiological levels. This would lower the risk of overexpression and its effects.
Researchers are developing lipid nanoparticles, viral vectors, and exosome-based delivery systems to enhance the selectivity and efficacy of miRNA-based therapeutics. These delivery platforms safeguard miRNAs from degradation in the circulation and guarantee their delivery to the designated target regions. For instance, oncological treatments use lipid nanoparticles (LNPs) to administer miR-34 mimics, while exosome-based approaches show potential in targeting specific cell types for miRNA delivery without causing off-target effects. Along with improvements in delivery, new miRNA therapies may also benefit from changes in design and chemistry. Currently, researchers employ locked nucleic acids [
123] and 2′-O-methyl modifications to enhance the stability and binding affinity of miRNA mimics and antagomirs. As miRNA design gets better, it may become more selective, reducing effects that aren't supposed to happen, and making gene expression changes more precisely controlled by miRNA.
Chemical modifications such as 2′-O-methylation and locked nucleic acids, in addition to enhanced delivery mechanisms, enhance the stability and binding affinity of miRNA mimics and antagomirs. These alterations diminish the probability of off-target effects, thereby augmenting the therapeutic efficacy of miRNA-based interventions. As delivery technologies and chemical changes progress, the safety and efficacy of miRNA treatments will improve, thereby enabling their widespread clinical application.
We anticipate that other additional domains of RNA study need investigation, potentially resulting in further Nobel Prizes in this discipline. Forecasting future Nobel Prizes for RNA-related discoveries is conjectural; however, recent scientific research highlights numerous prospective domains within RNA BIOLOGY THAT MAY yield substantial advancements and potential Nobel acknowledgment.
• miRNA Revitalization of Therapeutic Protein Expression: It is important to control the stable and long-lasting expression of therapeutic proteins when they are being made, especially in gene therapies, so that they have a long-lasting therapeutic effect. We can utilize MiRNAs to renew or modulate protein expression, especially when aging or disease naturally diminishes their synthesis.
• Augmenting Protein Expression in Senescent Tissues: Aging frequently results in a reduction of essential protein synthesis, potentially contributing to conditions such as sarcopenia, neurodegeneration, and metabolic disorders. We can formulate MiRNA therapy to restore the expression of these therapeutic proteins by blocking protein production inhibitors or activating necessary transcription factors. We can use the downregulation of miR-34a, associated with aging, to boost the production of sirtuins, proteins involved in lifespan and metabolism, or substances that promote muscle regeneration, like IGF-1 (insulin-like growth factor).
• Regulating Protein Synthesis in Gene Therapy: It's crucial to ensure that the therapeutic gene expresses at the right time and in the right quantity in gene therapies. By making synthetic miRNA circuits that respond to signals from the environment or cells, miRNA-based systems may be able to precisely control protein expression. For example, miRNA switches may regulate the expression of therapeutic proteins as required, minimizing side effects and enhancing the overall safety profile of gene therapies. This may be especially advantageous in scenarios necessitating intermittent treatment, such as intermittent hormone shortages or enzyme replacement regimens [
125].
• miRNAs play a crucial role in enhancing protein stability and folding, as they can influence chaperone proteins that aid in the folding of therapeutic proteins. In conditions such as cystic fibrosis, proper protein folding is essential, and mutations may result in misfolding. MiRNA-based therapeutics have the potential to target pathways that enhance the synthesis of molecular chaperones, thereby improving the folding and functionality of therapeutic proteins [
126].
• miRNA-Based Vaccines: Conventionally, vaccines trigger immune responses against specific infections by introducing an antigen, such as an attenuated virus or protein components, into the body. Nonetheless, miRNA technology may unlock new possibilities in vaccine production, providing numerous benefits such as improved specificity, prolonged immune responses, and the capacity to construct tailored vaccinations. One of the most compelling applications of miRNA technology in vaccines is the development of vaccinations that prompt host cells to internally generate antigens. By creating a vaccine with miRNAs that target viral RNA or mRNA that codes for viral proteins, host cells can make specific viral antigens that trigger a more natural immune response. This method may be especially beneficial for quickly evolving viruses, such as influenza or SARS-CoV-2. We may develop a synthetic miRNA-based vaccination to target conserved sections of the viral genome, thereby lessening the impact of viral changes. This would confer prolonged protection and eliminate the necessity for regular revisions to vaccine compositions. The design of cancer vaccines involves activating the immune system to identify and target tumor-specific antigens. By targeting tumor-specific mutations, miRNA technology may facilitate the development of tailored cancer vaccinations. Tumors frequently downregulate or alter proteins that inhibit tumor proliferation, such as p53, or proteins that typically signal the immune system to the existence of malignant cells. MiRNA-based vaccines may augment the expression of these proteins in tumor cells, thereby elevating the probability of immune system detection and eradication. A further advanced application involves utilizing miRNAs to enhance antigen presentation in conventional vaccinations. Antigen-presenting cells (APCs) play a crucial role in the immune response by presenting antigens to T cells. By upregulating essential pathways implicated in antigen processing and presentation, MiRNAs can enhance the functionality of these cells. For example, miR-155 participates in the activation of immune cells, and its overexpression may augment the efficacy of vaccine-induced immunological responses. Adding miRNAs that improve antigen presentation to regular vaccines could help scientists make vaccines that give stronger and longer-lasting immunity [
128].
• miRNA in Plant-Derived Vaccines: miRNAs may play a significant role in the development of plant-derived vaccines, which utilize plants to generate antigens for oral or conventional injectable administration. Researchers could augment the yield and stability of antigen synthesis in plants by modifying miRNAs inside plant systems. For instance, researchers could target miRNAs that modulate protein synthesis or stress responses in plants to enhance the efficacy of vaccine antigen production in consumable crops like lettuce or tomatoes. This methodology may facilitate the development of cost-effective and readily scalable vaccinations appropriate for worldwide application, especially in resource-constrained environments [
129].
8.5. MiRNA Specific to Tissue
Various tissues possess unique collections of miRNAs that govern tissue-specific functions, including cell differentiation, proliferation, metabolism, and homeostasis. Tissue-specific miRNAs are essential for preserving the distinct functions of each tissue. The table below lists some significant tissue-specific miRNAs, their principal functions, and the corresponding tissues they relate to. Referred to as "myo-miRs," miR-1, miR-133, and miR-206 play a crucial role in regulating muscle formation (myogenesis), differentiation, and repair. They inhibit genes associated with muscle atrophy and facilitate the development of new muscle fibers following injury or exercise. miR-1 and miR-133 are essential for regulating cardiac muscle differentiation in the heart. Specific roles for miR-208a and miR-499 include cardiac contractility and protecting the heart from hypertrophy. MiR-122 is specific to the liver and plays a crucial role in the metabolism of cholesterol and fatty acids. It is crucial for hepatic growth and functionality. MiR-21 and miR-199a are involved in liver fibrosis and hepatocyte proliferation. miR-124 is among the most prevalent miRNAs in the brain, playing a role in neuronal development and neuroprotection. miR-9 facilitates the regulation of neurogenesis, whereas miR-132 and miR-128 are crucial for synaptic plasticity and cognitive function. MiR-143 and miR-103 modulate adipocyte development and insulin sensitivity, making them crucial in lipid metabolism and energy regulation. MiR-155 contributes to the regulation of inflammation in adipose tissue. MiR-375 plays a crucial role in regulating insulin release and ensuring the proper functioning of pancreatic beta cells. It governs glucose homeostasis and pancreatic cell differentiation, thereby enhancing overall metabolic health. miR-192 and miR-194 are crucial for renal development and the preservation of nephron integrity. Renal pathologies implicate miR-29 and miR-21 in kidney fibrosis and damage response, serving essential functions. MiR-21 and miR-126 play a crucial role in lung development, inflammation, and repair mechanisms, particularly in the context of fibrosis. miR-29b modulates collagen accumulation and is crucial in pulmonary fibrotic conditions. MiR-26a, miR-29b, and miR-214 play a crucial role in bone development by regulating osteoblast differentiation and bone production. These miRNAs serve as targets for enhancing bone repair and regeneration. MiR-203 and miR-205 modulate keratinocyte differentiation, which contributes to skin integrity and facilitates wound healing. miR-31 facilitates epidermal growth and healing following damage. miR-150, miR-155, and miR-223 are pivotal regulators in hematopoietic cells, governing the development of immune cells and contributing to inflammation and immune response. MiR-210 and miR-141 regulate the development of the placenta and its response to hypoxia, while miR-517 plays a role in trophoblast function and placental growth. MiR-34c and miR-449a play a role in spermatogenesis and regulate Sertoli and Leydig cells. Male fertility and testicular development are associated with miR-202.
8.7. Nanoparticles Conjugated with Ligands for Receptor-Mediated Targeting
Due to their ability to encapsulate nucleic acids and protect them from degradation, researchers extensively use nanoparticles for the delivery of miRNAs. By modifying the surface of nanoparticles with ligands, we enable receptor-mediated endocytosis, which exclusively allows cells that express the matching receptors to internalize the nanoparticles.
We can functionalize nanoparticles with ligands that specifically interact with receptors expressed solely on myocytes to target miRNAs in muscle cells. Using ligands that attach to the IGF-1 receptor, abundantly expressed on muscle cells, is a possible strategy. Folic acid conjugates or peptides that target the insulin-like growth factor receptor can make it easier for miRNA-loaded nanoparticles to get to muscle tissues. This lets muscle growth, repair, or metabolism be precisely controlled.
Nanoparticles may conjugate ligands bound to natriuretic peptide receptors, abundantly expressed in the heart, or integrin receptors to target cardiomyocytes. We have utilized aptamers, small, single-stranded DNA or RNA molecules that specifically bind to proteins, to deliver medicinal agents directly to the heart by targeting cardiac-specific integrins. This method enables the targeted delivery of miRNA products to the heart, circumventing other tissues.
The blood-brain barrier (BBB) is a significant obstacle for interventions aimed at the brain. Nanoparticles coupled with ligands that specifically target receptors present on the blood-brain barrier may administer miRNA products to address this issue. It is possible for nanoparticles that have been modified with transferrin or lactoferrin to get miRNAs to the brain through receptor-mediated transcytosis. Similarly, rabies virus glycoprotein (RVG)-linked nanoparticles can bind to the acetylcholine receptor, which lets miRNA get to neurons.
8.8. Cell-Penetrating Peptides (CPPs)
Cell-penetrating peptides (CPPs) are short peptides that enable the translocation of miRNAs across cellular membranes. Designers can incorporate homing sequences into these peptides to selectively target specific tissues or cells, providing a diverse mechanism for tissue-specific delivery.
We can use peptides from muscle-specific proteins to guide miRNAs to muscle cells. Peptides generated by myostatin, a muscle development regulator, can route miRNA-loaded CPPs to muscle tissues, thereby affecting muscle regeneration or metabolism.
We can engineer heart-specific cell-penetrating peptides by integrating peptides originating from natriuretic peptides or angiotensin receptors. These receptors are prevalent on cardiomyocytes and endothelial cells throughout the heart. We could tailor miRNA therapies to the heart by designing CPPs that specifically target these receptors, thereby reducing off-target effects in other organs.
Brain-targeting sequences like TAT (trans-activator of transcription) or RVG (rabies virus glycoprotein) peptides can couple with CPPs for brain-targeted administration, enhancing the translocation of miRNAs across the blood-brain barrier (BBB). These CPPs can be associated with miRNA-loaded nanoparticles or oligonucleotides that provide therapeutic miRNAs directly to neurons or glial cells within the brain.
8.9. Aptamers for Targeting Specific Cell Types
Aptamers are concise RNA or DNA sequences that can selectively attach to cell surface proteins. They can serve as targeting agents to deliver miRNA products to tissues or cell types.
Using aptamers that bind to myostatin receptors or fibroblast growth factor receptors (FGFR) can make it easier for miRNA treatments to reach muscle cells. Myostatin functions as a negative regulator of muscle growth, and we can use aptamers that bind to myostatin-expressing cells to target miRNA therapies aimed at promoting muscle regeneration or addressing muscular dystrophy.
Heart-targeted therapeutics can engineer aptamers to bind to angiotensin II receptors or cardiac-specific integrins. These aptamers can guide miRNA products to cardiomyocytes, which makes it easier to change gene expression to improve heart regeneration, protect against ischemia injury, or stop heart failure from happening.
We can employ aptamers that bind to receptors expressed on the blood-brain barrier, such as the low-density lipoprotein receptor-related protein (LRP) or the nicotinic acetylcholine receptor, to target the brain. When these aptamers are linked to miRNA-encased nanoparticles or oligonucleotides, the miRNA product can get through the blood-brain barrier and target specific brain cells.
8.10. Virus-Like Particles (VLPs) for Targeting Specific Tissues
Virus-like particles (VLPs) are synthetic entities that replicate the architecture of viruses but are devoid of viral genetic material. VLPs can encapsulate and distribute miRNAs to targeted tissues. MiRNA therapies can be targeted to specific cells or organs by adding tissue-specific targeting ligands to the surface of VLPs.
Designers can design VLPs with surface proteins that attach to receptors on muscle cells, such as the insulin-like growth factor-I receptor. These VLPs can administer miRNA treatments that enhance muscle growth or repair by targeting muscle-specific pathways.
We can engineer VLPs to incorporate cardiac-tropic proteins, which specifically bind to cardiomyocytes for targeted delivery to the heart. These VLPs can transport miRNA products to the heart, where they can regulate gene expression to address cardiac conditions such as heart failure or myocardial infarction.
Proteins like transferrin or apolipoprotein E (ApoE) can adorn VLPs to help them cross the blood-brain barrier and reach the brain. Upon entering the brain, these VLPs can transport miRNA products to neurons or glial cells, presenting a promising approach for addressing neurodegenerative disorders such as Alzheimer’s and Parkinson’s.
To make a tissue-specific viral vector, you have to change the vector's parts so that it only targets and delivers its genetic payload to the cells you want it to reach. There are numerous methods to enhance the vector's ability to recognize and infiltrate particular cell types.
The viral capsid (in non-enveloped viruses) or envelope (in enveloped viruses) constitutes the external structure of the virus that engages with the surface receptors of the target cell. The viral vector can selectively bind to receptors abundantly expressed in the target tissue by altering the viral capsid or envelope, thereby achieving tissue-specific tropism.
The incorporation of ligands that selectively bind to receptors strongly expressed in the target tissue but not in other locations is a prevalent method for achieving tissue specificity in viral vectors. These ligands may consist of peptides, antibodies, or receptor-binding proteins that are unique to a cell surface marker on the target tissue. The viral capsid can integrate ligands that attach to insulin-like growth factor (IGF-1) receptors, abundantly present in muscle tissue, to target muscle cells. This would guarantee that the viral vector selectively infiltrates muscle cells. Targeting cardiomyocytes (heart muscle cells) may involve targeting natriuretic peptide receptors or integrin receptors. We can design viral vectors to provide aptamers or antibodies that attach to these cardiac-specific surface proteins. We can engineer viral vectors to interact with transferrin or lactoferrin receptors, enabling transcytosis through the blood-brain barrier (BBB) for precise brain delivery. Alternatively, we can employ rabies virus glycoprotein (RVG) to selectively target neurons through its binding to acetylcholine receptors.
Pseudotyping entails substituting the viral envelope or capsid proteins of one virus with those from another virus that inherently targets the intended tissue. Enclosed viral vectors, such as lentiviral and retroviral vectors, frequently employ this technique. The glycoprotein from VSV-G (vesicular stomatitis virus) can pseudotype a lentiviral vector to target the brain, thereby enhancing its entrance into neurons. Likewise, pseudotyping with RVG may enable the viral vector to more precisely target neurons.
An effective technique for achieving tissue specificity is to regulate gene expression using tissue-specific promoters. The viral vector may infiltrate various tissues, but only the target tissue where the promoter is operational will express the therapeutic gene (or miRNA). To achieve muscle-specific targeting, one can utilize promoters that are only active in muscle cells, such as the Muscle Creatine Kinase (MCK) and Myosin Heavy Chain (MHC) promoters. Utilizing these promoters, the viral vector will exclusively express the therapeutic gene (or miRNA) in muscle cells, regardless of the vector's presence in other tissues.
For cardiac targeting, heart cell-specific promoters, such as alpha-myosin heavy chain (α-MHC) and cardiac troponin T, can be utilized. These promoters ensure that cardiomyocytes exclusively produce the transgene, thereby preventing off-target effects in other organs.
The neuron-specific For astrocytes, the glial fibrillary acidic protein (GFAP) promoter selectively targets neurons or glial cells in the brain, ensuring that the viral vector exclusively expresses its cargo in the central nervous system, thereby minimizing the possibility of off-target gene expression.
Certain viral vectors inherently demonstrate tissue tropism, indicating a preference for infecting specific tissues. Choosing the appropriate viral vector with a defined tropism can enhance the probability of hitting the intended tissue. Despite the somewhat broad tropism of AAV vectors, one can choose various AAV serotypes based on their inherent affinity for specific tissues. AAV1 and AAV9 exhibit a natural predilection for skeletal and cardiac muscle. AAV9 is proficient at traversing the blood-brain barrier, rendering it appropriate for targeting the brain. AAV8 has a strong preference for the liver, but surface modifications can modify it to target other organs.
Choosing the right AAV serotype can help with targeting specific tissues better, but more targeting methods, like changing the capsid or adding tissue-specific promoters, may be needed for even better specificity.
8.11. Lenivirus and Retrovirus
Lentiviral and retroviral vectors naturally join cells that are dividing, which makes them good for going after tissues like hematopoietic cells, hepatic cells, or other organs that are growing. In non-dividing tissues, pseudotyping or capsid alteration can improve targeting. A straightforward method for attaining tissue-specific distribution is via direct injection or administration into the target tissue. This method does not necessitate intricate viral alterations but restricts its use to localized delivery. You can administer viral vectors directly into muscle tissue, ensuring that the majority of viral particles infiltrate the target cells. Gene therapy frequently utilizes this for treating muscular dystrophy and other myopathic disorders. It is easier to get the viral vector to the heart tissue when an intracoronary injection or direct injection into the myocardium is used after surgery. This approach is especially advantageous in situations when systemic administration could result in off-target consequences. By effectively circumventing the blood-brain barrier, intracerebroventricular (ICV) or intrathecal injection can send the viral vector directly into the cerebrospinal fluid, ensuring its delivery to neurons or glial cells.
8.12. CRISPR/Cas9 with Aptamers for Improved Targeting
Viral vectors can integrate with CRISPR/Cas9 systems or aptamers, tiny molecules that specifically connect to cell surface proteins, to achieve precise tissue-specific editing and improve tissue targeting. Viral vectors can administer CRISPR/Cas9, while tissue-specific promoters can regulate the expression of the Cas9 enzyme and guide RNA (gRNA). This ensures the restriction of gene-editing activity to the targeted tissue, thereby mitigating off-target effects in other tissues. Aptamers are short oligonucleotides that precisely attach to proteins on the surfaces of target cells. We can couple viral vectors with aptamers that specifically attach to tissue receptors, ensuring selective uptake by target cells. This technique provides precise targeting, particularly for tissues exhibiting distinct surface markers.
8.13. CRISPR-Enhanced miRNA Activation Mechanisms
Using CRISPR-based gene activation tools to selectively increase or decrease miRNA expression in certain tissues could be part of a progressive and well-targeted strategy. CRISPR-based systems may be able to control miRNA levels only in certain types of cells by creating tissue-specific guide RNAs (gRNAs) that target specific miRNA loci.
We may engineer a CRISPR system to selectively activate miR-206, a key player in muscle regeneration, only in skeletal muscle cells. Muscle-specific gRNAs and tissue-specific promoters would be used in this method to keep miRNA activity in the muscle and reduce effects on other parts of the body.
We may employ CRISPR-based technologies to upregulate miRNAs like miR-1 and miR-133 in the heart, thereby enhancing cardiomyocyte survival and regeneration. Cardiomyocytes would exclusively activate these mechanisms, ensuring that therapeutic miRNA expression remains restricted to the heart.
9. Ex Vivo vs In Vivo
When considering the use of miRNA technology for therapeutic purposes, two major Strategies utilized include ex vivo modification and in vivo delivery. Both methodologies seek to attain accurate gene control while reducing off-target effects; however, each possesses unique advantages and constraints.
Analogous in vitro transcription (IVT) techniques employed for mRNA generation can synthesize ex vivo miRNAs. There are extra steps in the miRNA synthesis process that make sure the miRNA precursor (pre-miRNA) is folded and processed correctly so that it can become active.
9.1. Ex-Vivo
Ex vivo manipulation entails the extraction of cells from a patient's body, followed by genetic or epigenetic modification in a controlled laboratory environment, and subsequently reinfusing them into the patient. For miRNA therapeutics, this means adding or changing the amount of miRNA in these cells outside of living things while making sure they work as a therapy before transplantation.
Ex vivo modification gives a lot of accuracy and control over the environment of cells, which lets cells be carefully watched and changed to get the best miRNA expression. Researchers can ensure the operation of only the targeted miRNAs and identify any undesirable side effects before reintroducing the cells into the patient. Ex vivo alteration allows for the customization of cells to express specific miRNAs in precise quantities, thereby reducing the risk of off-target effects during transplantation.
Consequently, ex vivo manipulation provides enhanced precision control and minimizes the likelihood of off-target effects due to the regulated laboratory environment. In vivo administration, albeit more direct, is more vulnerable to off-target delivery issues.
We alter cells externally and evaluate them for unintended consequences, which diminishes the risk of off-target gene expression. We reintroduce only cells exhibiting the appropriate miRNA activity into the patient, thereby reducing the likelihood of inadvertent gene regulation. The utilization of the patient's own cells (autologous) significantly reduces the chance of immunological rejection. Nonetheless, the utilization of donor cells (allogeneic) may result in immunological responses.
In cancer treatments like CAR-T cell therapy, changing miRNA outside of living cells can make immune cells more effective before they are reintroduced into the body.
Ex vivo methods are better for tissue regeneration tasks that need to carefully control miRNA expression to help stem cells differentiate into specific cell types, such as neurons, muscle cells, or hepatocytes.
Ex vivo manipulation is nonetheless labor-intensive and necessitates specialized facilities for cell culture, gene editing, and reintroduction. The process entails several stages, including the extraction, modification, and transplanting of cells, which can be labor-intensive and costly. Ex vivo therapies are exceptionally individualized, as they depend on the extraction and modification of the patient's cells. While this may be beneficial for individual patients, its broad implementation becomes increasingly challenging.
9.2. In vivo
Putting miRNA molecules (either miRNA mimics or inhibitors) directly into a patient's body using different delivery methods, such as liposomes, viral vectors, or nanoparticles, is what "in vivo administration" means. This method aims to selectively target particular tissues or cells in the body to modify miRNA expression and attain the intended therapeutic outcome. A primary worry for in vivo miRNA treatment is the potential for miRNAs to unintentionally influence non-target genes in different tissues, resulting in off-target effects. Notwithstanding advancements in delivery technologies, including tissue-specific nanoparticles, miRNAs may still arrive at unexpected sites and inhibit or activate non-target genes. The direct delivery of miRNA treatments may elicit an immunological response, especially when utilizing viral vectors. Furthermore, systemic administration of the therapy may elicit widespread immunological responses, potentially resulting in inflammation or damage.
In vivo delivery frequently encounters difficulties in ensuring that miRNA therapy targets just the intended cells or tissues. Targeting methods, like ligand-modified nanoparticles, are used a lot, but miRNAs may still have unwanted effects if they get into the wrong places. In contrast to ex vivo modification, precisely controlling miRNA delivery in vivo is harder because of the complex nature of the immune system, the way blood flows, and the way organs interact with each other.
Less invasive techniques, such as injections or oral administration, can deliver in vivo miRNA therapies, making them more feasible for treating extensive populations. This method involves fewer steps than ex vivo manipulation and is typically more scalable. Direct application of in vivo administration to patients, without the need for cell extraction and re-infusion, speeds up clinical procedures.
In vivo delivery is more appropriate for addressing systemic diseases that require modulation of miRNA expression throughout the body, such as metabolic disorders (e.g., diabetes) or cardiovascular ailments. We can administer MiRNA therapeutics in vivo to directly modify gene expression in tumors or infected tissues, targeting cancer or viral infections. In vivo miRNA vaccines present a promising strategy for enduring immune protection against infections.
Both ex vivo manipulation and in vivo injection have their benefits. However, ex vivo manipulation is better because it is easier to control and avoid side effects. This makes it ideal for situations where safety and accuracy are very important. On the other hand, administering drugs in living organisms is easier and more scalable when it comes to treating common illnesses. However, better delivery methods (such as tissue-specific nanoparticles) are needed to reduce side effects and improve therapeutic accuracy.
The future of miRNA therapy will likely see a combination of these approaches, with improvements in delivery methods and gene-editing technologies helping to bridge the gap between the safety of ex vivo manipulation and the practicality of in vivo administration.
Table 3 presents comparison of ex vivo and in vivo development
9.3. Plan of Action
Many things affect how long a miRNA stays in a tissue and how well it can be permanently integrated. These include the delivery method, how stable the miRNA is, how it breaks down in the tissue, and whether it is introduced ex vivo or in vivo.
Nucleases and cellular recycling mechanisms affect the stability of miRNA in tissues. The administration of miRNA mimics or inhibitors in vivo typically results in a temporary presence within the tissue, generally persisting for a duration of hours to days. The length is contingent upon several critical factors:
• Delivery System: Using nanoparticles, liposomes, or viral vectors to deliver the miRNA may protect it from breaking down quickly, which would make it work longer. However, nucleases in the circulation or tissues swiftly destroy unprotected miRNAs.
• Tissue Type: Distinct tissues exhibit differing rates of miRNA turnover. For instance, the liver's elevated metabolic activity rapidly destroys miRNAs, while distinct enzymatic environments in the brain or muscle may allow them to persist longer.
• Endosomal Escape: The miRNA must extricate itself from the endosome following cellular internalization to become active. If the delivery method fails to efficiently promote endosomal escape, the miRNA may undergo intracellular destruction before exerting its effect on the target mRNA.
• The half-life of endogenous miRNAs generally ranges from 12 to 24 hours; however, this may fluctuate depending on the cellular context. You can adjust synthetic miRNA mimics to improve stability, but if you administer them constantly, they often show temporary effects.
In vivo delivery of miRNA is typically transient, necessitating repeated administration for prolonged effects. On the other hand, incorporating miRNAs into a gene-editing or steady expression framework through ex vivo manipulation can offer a more durable solution.
• Stable Expression Vectors: Viral vectors (e.g., lentiviruses or AAVs) that integrate into the host cell's genome can deliver miRNAs in ex vivo manipulation, enabling sustained expression of the miRNA after transplanting the cells back into the patient. In this scenario, the host cells would continuously synthesize the miRNA, thereby providing a lasting solution.
We can use lentiviral vectors, which integrate into the genome, to permanently introduce miRNAs into dividing cells like the liver or blood. If we don't use extremely specific targeting tactics, this approach runs the risk of insertional mutagenesis, which disrupts essential genes, making it suboptimal for permanent in vivo miRNA therapy.
CRISPR-Mediated miRNA Activation: Another ex vivo method utilizes CRISPR-based gene editing to permanently activate or inhibit certain miRNA genes within a cell's genome. This would guarantee that the miRNA remains perpetually active in the cells reintroduced in the patient. CRISPR/Cas9-mediated gene editing provides a potential approach for the permanent incorporation of miRNA in vivo. It may be possible to permanently change the expression of miRNAs in certain tissues by using CRISPR/Cas9 to directly turn on or off the expression of endogenous miRNAs. CRISPRa (CRISPR activation) can upregulate the expression of miRNAs that confer advantageous effects, including those associated with muscle regeneration or cardioprotection. This method entails administering both the CRISPR components and the guide RNA to targeted tissues. Similarly, we can use CRISPRi (CRISPR interference) to permanently suppress miRNAs that cause harmful effects like fibrosis or inflammation. Lipid nanoparticles, plasmids, or alternative delivery vehicles can administer CRISPR in vivo, facilitating the targeting of specific cells by the Cas9 protein and guide RNA (gRNA), leading to a permanent alteration of the genome. This method offers a prospective, enduring remedy for genetic disorders or tissue-specific alterations.
Synthetic mRNA therapy entails the administration of messenger RNA (mRNA) that encodes therapeutic proteins. Although conventional mRNA therapies are ephemeral, recent advancements have enabled the prolongation of mRNA expression duration. The advanced mRNA technique called self-amplifying RNA lets the delivered RNA copy itself inside the cell, which means that protein expression can continue without the need for viral vectors. SaRNA has the genetic instructions for making the therapeutic protein and the tools for self-replication in the cytoplasm of the target cell, which makes it easier for the therapeutic protein to be made for a longer time. In cancer immunotherapy, saRNA can generate immune-stimulating proteins for an extended duration, resulting in prolonged anti-tumor actions. SaRNA encoding muscle growth hormones such as IGF-1 may facilitate prolonged muscle regeneration and hypertrophy.
Non-viral gene therapy techniques, like plasmid DNA or synthetic mRNA, can facilitate the prolonged expression of miRNAs; nonetheless, they typically do not result in permanent expression. New developments in synthetic RNA circuits that copy natural transcriptional networks may make it easier to control miRNA expression in a way that lasts and is specific to each tissue. This could mean that viral vectors aren't needed for long-lasting miRNA therapies. Notwithstanding the potential of these methods, numerous obstacles hinder the permanent integration of miRNAs in targeted tissues:
• Immunological Response: One significant issue with sustained in vivo miRNA expression is its potential to trigger an immune response. Both viral and non-viral delivery mechanisms can stimulate the immune system, resulting in the elimination of the vector or possible harm to the host tissue. This is especially pertinent for viral vectors that remain in the body for extended durations.
Using lentiviruses or other viral vectors to deliver miRNA constructs comes with the risk of insertional mutagenesis, which happens when the viral genome merges into an important part of the host genome. This can mess up important genes and cause bad effects like cancer.
The permanent installation of miRNA in a tissue may result in off-target gene regulation, wherein the miRNA influences genes outside the designated target. Although miRNAs are typically specific, they can target numerous genes, which introduces the potential for unwanted long-term repercussions.
Nanoparticles can help deliver miRNA mimics or inhibitors directly to muscle tissues over time, giving you more control when you do this over and over again. We can engineer these nanoparticles with ligands that specifically connect to muscle cells, like the IGF-1 receptor, to ensure precise delivery.
Designing nanoparticles to gradually release miRNA mimics or inhibitors can ensure prolonged effects without the need for regular injections. This method might be advantageous for sportsmen or persons rehabilitating from injury.
Lipid nanoparticles (LNPs) frequently administer mRNA treatment, protecting the mRNA from degradation and boosting cellular uptake. Designing LNPs with slow-release characteristics can prolong the time of mRNA expression. In some cases, a series of injections can deliver mRNA over time to achieve a prolonged therapeutic effect.
We can engineer nanoparticle-based delivery systems to gradually release their therapeutic payload, resulting in prolonged effects. Designers can incorporate miRNAs, siRNAs, or other therapeutic agents into polymeric nanoparticles. These nanoparticles undergo gradual degradation within the body, delivering their payload over the course of weeks or even months. This method is effective for attaining enduring therapeutic results without the necessity for repeated administration. Polymeric nanoparticles may facilitate the delivery of miRNAs that enhance muscle regeneration, ensuring a steady release of miRNAs to enable sustained muscle growth.
Hydrogels represent an additional slow-release mechanism for encapsulating therapeutic proteins, miRNAs, or growth factors. When implanted in or near the target area, hydrogels gradually dissolve, releasing therapeutic chemicals over an extended duration. You can place an IGF-1-loaded hydrogel in muscle tissue to promote prolonged muscle regeneration after injury.
9.4. Cellular Therapies
Cell-based therapies entail the transplanting of genetically engineered cells capable of producing therapeutic proteins or miRNAs for prolonged durations. This method is especially effective for attaining enduring outcomes in gene therapy or regenerative medicine.
We can extract cells from the patient, genetically alter them ex vivo, and then reintroduce them into the patient. These cells, upon modification to express a therapeutic gene, can synthesize the requisite proteins or miRNAs for an extended duration. We can engineer muscle progenitor cells to overexpress muscle-specific miRNAs (e.g., miR-206) or growth factors (e.g., IGF-1), leading to sustained muscle repair and hypertrophy upon reintegration into the organism. We could engineer pancreatic cells to synthesize insulin or insulin-regulating miRNAs, ensuring sustained regulation of glucose levels in diabetes.
We can genetically engineer induced pluripotent stem cells (iPSCs) to express therapeutic genes or microRNAs. Upon reintroduction in the patient, iPSCs can develop into the targeted tissue type and persistently synthesize the therapeutic protein for an extended duration. Induced pluripotent stem cells (iPSCs) engineered to produce dopamine-generating enzymes may serve as a long-term therapeutic approach for Parkinson's disease.
10. Discovery
Identifying novel miRNAs necessitates a synthesis of high-throughput sequencing, bioinformatics evaluation, and experimental confirmation. Utilizing these tools, researchers can discover novel miRNAs in particular tissues or under specific conditions, enhancing their understanding of their functions in gene regulation and illness. Finding new miRNAs will become easier and more common as sequencing technologies and computer programs get better. This will help us learn more about miRNA biology in a wider range of animals and tissues.
High-throughput sequencing technologies, such as RNA sequencing (RNA-Seq), represent some of the most potent instruments for miRNA identification. Next-generation sequencing (NGS) makes it possible to sequence all small RNAs (18–25 nucleotides long) in a sample. This includes microRNAs (miRNAs) and gives a detailed picture of the small RNA population.
Essential Procedure:
Extract total RNA from the relevant tissue or cells.
Utilize specific approaches to enrich tiny RNAs, including miRNAs, often employing size-selection techniques.
Prepare a small RNA library by ligating particular adapters to the 5' and 3' termini of isolated short RNAs.
Conduct high-throughput sequencing on the small RNA collection. This produces millions of brief sequences corresponding to tiny RNAs in the sample.
Align the sequenced reads to the reference genome to detect both known and potentially novel miRNAs. By recognizing sequences that conform to the criteria for miRNA precursor structures, including the creation of a hairpin secondary structure, we can find novel miRNAs.
Once sequencing identifies candidate miRNAs, researchers can use Northern blotting to confirm their expression. This technique identifies tiny RNA molecules by size, enabling researchers to verify the existence and quantity of a novel miRNA in a particular tissue or developmental phase.
We can use quantitative PCR (qPCR) to validate the expression levels of newly identified miRNAs.
Researchers have developed numerous computational techniques and algorithms to forecast new miRNAs from sequencing data or genomic sequences. These methods generally employ the following criteria for miRNA prediction:
• Hairpin configurations exist in precursor miRNAs, also known as pre-miRNAs.
• Preservation of sequences among species.
• Determine the minimum free energy (MFE) of the anticipated secondary structure to evaluate its potential to form a stable hairpin.
Prevalent miRNA prediction tools:
• miRDeep: A prevalent method that detects novel miRNAs by matching short RNA sequences to the genome and forecasting precursor structures.
• miRBase: An extensive miRNA database that encompasses data on established miRNAs and facilitates the verification of potential novel miRNAs.
RNAfold is a program that can guess the secondary structure of RNA sequences and help figure out if a possible sequence has the stable hairpin shape that miRNA precursors have.
10.1. Comparative Genomics
Comparative genomics facilitates the identification of new miRNAs through the analysis of short RNA sequence conservation across several species. Conserved miRNAs across species are more likely to possess significant biological activities, and their identification may reveal previously unrecognized miRNAs in underresearched species.
Steps:
• Align sequences from closely similar species to identify conserved areas of short RNAs.
• Examine the conservation of the anticipated secondary structures of the miRNA precursors.
10.2. Models of Machine Learning
Recently, researchers have employed machine learning methodologies for miRNA identification. These approaches utilize characteristics such as nucleotide composition, sequence motifs, and the secondary structure of miRNA precursors to predict novel miRNAs. We can train machine learning algorithms on established miRNA datasets and then use them to predict novel miRNAs in unexplored genomes or tissues.
Upon identification of a novel miRNA, it is essential to functionally validate its role and ascertain the target genes it modulates. We can overexpress the new miRNA in cellular or animal models to investigate its effects on gene expression and cellular phenotypes. We can employ antagomirs or small interfering RNAs (siRNAs) to suppress or inhibit the activity of the new miRNA. We then evaluate the impact of miRNA depletion on target gene expression and cellular function.
By attaching to matching sequences in the 3' untranslated regions (3' UTR) of target mRNAs, miRNAs change how genes are expressed. Forecasting the target genes of new miRNAs helps elucidate their biological roles. TargetScan and miRanda are two bioinformatics methods that predict likely mRNA targets by looking at seed sequence complementarity and evolutionary conservation.
We can use luciferase reporter experiments to confirm that a new miRNA modulates a putative target gene. We insert the 3' UTR of the target mRNA downstream of a luciferase gene into a reporter vector. Binding the new miRNA to the 3' UTR will inhibit luciferase expression, thereby diminishing luminosity.
Certain tissues or developmental phases express certain miRNAs in minimal quantities, complicating their identification. In such instances, it may be essential to employ extremely sensitive sequencing techniques or conduct deep sequencing to detect these uncommon miRNAs.
Many non-canonical miRNAs don't follow the usual biogenesis routes for miRNAs (for example, those that are processed without Drosha or Dicer) and may not have the typical precursor hairpin structure. Detecting these miRNAs necessitates specialized algorithms and experimental methodologies.
Due to the intricacy of the small RNA transcriptome, sequencing frequently identifies numerous short RNA fragments that do not represent authentic miRNAs. To make sure that the identification of new miRNAs is correct, it is important to use strict criteria in bioinformatics analysis and experimental validation methods such as Northern blot and qPCR.
11. Regulatory
We must address significant ethical considerations as miRNA-based therapeutics advance. A key concern is the possibility of off-target effects that may result in inadvertent gene silencing or overexpression. Being able to change miRNA pathways and gene expression with such accuracy makes people wonder what the long-term effects of these interventions will be, especially when it comes to changes that can be passed down through generations.
Before integrating miRNA-based medicines and diagnostics into standard clinical practice, we must resolve numerous hurdles and ethical considerations, despite their great promise. A key difficulty is the lack of complete understanding of miRNA off-target effects. MiRNAs often regulate multiple genes, and targeting a single miRNA may inadvertently impact unrelated biological pathways. Future research must concentrate on enhancing the specificity of miRNA-based therapeutics, guaranteeing that they exclusively target the intended pathways without eliciting unexpected side effects.
The regulatory framework for miRNA-based therapeutics remains in development. As these therapies advance and proliferate, regulatory bodies such as the FDA and EMA must formulate guidelines to ensure the safety and efficacy of miRNA-based therapeutics. Before miRNA therapeutics could be used by everyone, they would probably have to go through a lot of tests in clinical studies to make sure they don't have any side effects or are safe in the long term.
The future of miRNAs in medicine has numerous promising opportunities. MiRNAs serve as non-invasive biomarkers and hold promise for tailored treatments, significantly impacting precision medicine, cancer treatment, cardiovascular disease management, and regenerative medicine. As delivery technologies progress and our comprehension of miRNA biology expands, the capacity to modify miRNAs safely and efficiently will become more attainable.
We expect miRNAs to play a fundamental role in personalized medicine in the future, enabling more precise and targeted therapies tailored to the genetic profiles of individual patients. Nonetheless, the effective incorporation of miRNAs into clinical practice necessitates ongoing advancements in delivery systems, safety protocols, and regulatory supervision. These improvements position miRNA-based medicines to potentially transform the treatment of various diseases, providing optimism for more effective and safer therapeutic options in the future.
12. Obstacles and Constraints
Notwithstanding the significant advancements in comprehending microRNAs (miRNAs) and their therapeutic potential, numerous hurdles and restrictions remain. We must resolve these issues before we can widely implement miRNA-based therapeutics in clinical practice. The intricacies of miRNA biology, challenges associated with delivery systems, and the potential for off-target effects and immune responses represent some obstacles encountered by researchers. This section examines the principal problems that restrict the utilization of miRNA therapeutics and diagnostics while also addressing the novel strategies being devised to surmount these impediments.
A major hurdle in miRNA research is the intrinsic complexity of miRNA control. MicroRNAs can target several genes, and a single microRNA may affect hundreds of distinct mRNAs. This trait renders miRNAs exceedingly versatile; however, it complicates the prediction of their precise functions in biological processes. The interactions between miRNAs and their targets are frequently context-dependent, indicating that the same miRNA may exert varying effects across different cell types or physiological situations.
This intricacy complicates the design of targeted miRNA therapeutics, as predicting the impact of modifying a single miRNA on other pathways is often difficult. Furthermore, the tissue-specific expression of target mRNAs, the presence of competing endogenous RNAs (ceRNAs), and RNA-binding proteins that can alter miRNA activity all influence the regulation of miRNAs. These aspects hamper therapy development due to the necessity for a comprehensive understanding of the miRNA's function within the unique disease environment [
134].
Moreover, whereas bioinformatics techniques have enhanced our capacity to anticipate miRNA targets, these predictions are not consistently dependable. It is often necessary to do experiments to confirm how miRNA and mRNA interact, which adds to the complexity and delays the development of miRNA-based therapeutics [
135].
12.1. Off-Target Effects and Immune Responses
MicroRNAs frequently regulate numerous genes, and this multi-targeted characteristic raises concerns over off-target consequences. When miRNA mimics or inhibitors are given, they might change the expression of genes that aren't connected to the target disease pathway, which could have effects that were not expected. Off-target effects are especially worrisome in cancer treatment when the objective is to precisely target tumor cells while sparing healthy cells. Unintentional gene silence in normal cells may interfere with critical biological processes and result in toxicity [
136].
Furthermore, the application of synthetic miRNA therapies, including miRNA mimics or antagomirs, can elicit immunological responses. The immune system identifies certain synthesized RNA molecules as foreign, triggering an immunological response that may lead to inflammation and other detrimental effects. This is especially pertinent when employing viral vectors, which exhibit significant immunogenicity. Some ways to lower these immune responses include making miRNAs that are chemically changed, like ones that contain 2′-O-methyl or locked nucleic acids, which make the immune system less likely to react.
12.2. Stability and Degradation of miRNA
The instability of miRNAs within the body presents an additional constraint that hinders their therapeutic application. Nucleases swiftly destroy miRNAs in the bloodstream, complicating the maintenance of therapeutic quantities at the target region. MiRNA mimics and antagomirs need to be kept from breaking down. This can be done by changing them chemically or putting them in protective carriers like liposomes or nanoparticles. Nonetheless, these procedures introduce complexity to medication development and may elevate production costs [
137].
Stability concerns can impact the utilization of circulating miRNAs as biomarkers. Although miRNAs exhibit greater stability than mRNAs, they remain susceptible to degradation, complicating their detection and measurement in biological fluids such as blood, saliva, or urine. This affects the dependability of miRNA-based diagnostic procedures, particularly in the detection of early-stage diseases.
13. Intellectual Property
Recent miRNA discoveries, encompassing their sequences, functions, and therapeutic uses, are eligible for patenting if they satisfy the requirements of patentability: novelty, non-obviousness, and utility. Although naturally existing miRNAs may encounter obstacles to patentability in certain jurisdictions, their novel applications, alterations, and delivery methods are patentable. Researchers and firms must meticulously traverse existing patents and previous art to guarantee that their miRNA-related inventions are protected and financially viable.
Recent discoveries about miRNAs, encompassing their sequences, functions, and prospective therapeutic applications, are eligible for patenting if they satisfy the requisite patentability criteria. However, similar to other forms of intellectual property, the patenting of miRNAs is subject to certain legal and regulatory factors, and certain inventions related to miRNAs may already have patents.
To patent a new miRNA discovery or associated application, it must satisfy the broad requirements of novelty, non-obviousness, and utility, akin to those required for patenting any biological innovation. Various facets of miRNA discoveries are eligible for patenting. Unidentified or functionally uncharacterized novel miRNA sequences are eligible for patenting. This pertains to both the mature miRNA sequence and the precursor miRNA sequence (pre-miRNA). Patents for naturally occurring sequences may encounter obstacles, especially in certain jurisdictions, where legislation may limit the patenting of naturally occurring biological molecules without substantial alteration.
MiRNA-based therapeutic applications, such as the use of miRNA mimics or inhibitors (antagomirs) in illness treatment, are eligible for patent awards. Additionally, delivery mechanisms for miRNA treatments, such as nanoparticles or viral vectors, and methodologies for employing miRNAs to modulate gene expression in targeted tissues for therapeutic objectives, such as enhancing muscle hypertrophy or suppressing tumor proliferation, are also subject to patent awards.
Patents may encompass the application of miRNAs as biomarkers for disease diagnosis, monitoring disease development, or forecasting patient responses to treatments. For instance, copyright protection may apply to miRNA expression profiles used in non-invasive cancer screening or neurodegenerative illness diagnosis.
Novel target genes controlled by miRNAs, especially those with previously unknown interactions that significantly influence gene regulation or illness, are eligible for patenting. This is especially pertinent if the target gene is associated with a particular therapeutic strategy or diagnostic method.
Patents can be given for new ways to deliver miRNA therapies, like modified nanoparticles, lipid carriers, or tissue-targeted vectors, as long as they have unique and not-obvious ways of doing so.
Since they are considered non-naturally occurring and involve human involvement, modifications of natural miRNAs to improve their stability, specificity, or therapeutic efficacy—such as chemically altered nucleotides, engineered stem-loop configurations, or synthetic miRNAs—are patentable.
Biotechnology firms and academic organizations have patented a multitude of miRNAs, along with their activities and therapeutic applications. For instance, firms such as miRagen therapies, Regulus Therapeutics, and Alnylam Pharmaceuticals have secured patents for diverse miRNA therapies, diagnostic applications, and delivery mechanisms. Regulus Therapeutics has submitted patents for the application of specific miRNAs as biomarkers for diseases, despite the fact that these miRNA sequences occur naturally. These patents pertain to particular applications in the diagnosis of diseases, such as hepatitis C or cardiovascular disorders, utilizing miRNA expression profiles.
Legislation and legal precedents may restrict the patenting of naturally occurring biological sequences in many jurisdictions, including the United States. In the pivotal case of Association for Molecular Pathology v. Myriad Genetics, Inc. (2013), the U.S. Supreme Court determined that naturally occurring DNA sequences are not patentable, whereas modified or synthetic sequences may still qualify for patent protection. Comparable issues may pertain to naturally occurring miRNA sequences, indicating that patent protection may be challenging if the miRNA remains unaltered.
Even if a miRNA is original, patenting may be challenging if comparable sequences, uses, or applications have been documented in the art. Prior art encompasses any publicly accessible information regarding the miRNA, its sequence, or prospective applications that existed prior to the submission of the patent application. The patent office meticulously examines prior art to confirm that the invention is indeed novel.
13.1. Instances of miRNA Intellectual Property Rights:
MiRNA mimics and inhibitors targeting cancer (e.g., miR-21 or miR-155) and cardiovascular disorders (e.g., targeting miR-208a for heart failure) have been the subject of patent applications.
Regulus Therapeutics has submitted patents for the utilization of specific miRNAs as biomarkers for illnesses, despite the fact that these miRNA sequences occur naturally. These patents pertain to particular applications in the diagnosis of diseases, such as hepatitis C or cardiovascular disorders, utilizing miRNA expression profiles. We have awarded patents for using miRNAs as biomarkers for conditions like liver cancer (e.g., miR-122 as a diagnostic indicator) and for assessing cardiac injury after myocardial infarction (e.g., miR-1 and miR-133).
There are patents for delivery systems made just for miRNA-based medicines. These include biodegradable polymers and lipid nanoparticles that can be targeted to certain tissues like the liver or muscle.
Patents can also be obtained for combinations of miRNAs or in conjunction with other medicinal medicines. These mixes can create new, not-obvious ways to treat diseases that use the controlling roles of several miRNAs in different biological pathways. A patent could propose a treatment strategy that combines miR-1 for cardiac repair and miR-206 for muscle regeneration, aiming to target multiple organ systems after a traumatic injury. The delivery mechanism for miRNA-based therapeutics is patentable, despite the miRNA being naturally occurring. MiRNA therapeutics work best when they are delivered in a way that targets specific tissues, makes them more stable, and controls their release. Patents for delivery systems may include nanoparticles that selectively transport miRNAs to the heart, brain, or muscles, as well as viral vectors or other carriers specifically formulated to enhance the absorption and effectiveness of miRNA-based therapeutics in the targeted organs.
Organizations such as Dicerna Pharmaceuticals have created and patented specialized lipid nanoparticle delivery methods for miRNA therapies. These nanoparticles enhance the stability and bioavailability of miRNA in vivo, rendering them patented even though the miRNA sequence itself is not patentable.
Creating synthetic miRNA analogues, which mimic naturally existing miRNAs but undergo modifications in sequence, structure, or function, is one method to patent miRNAs. These synthetic miRNAs may include enhanced attributes, like increased selectivity for target genes, superior stability, or augmented efficiency.
miRNA sponges, or miRNA decoys, are synthetic entities that attach to and block certain miRNAs, thereby obstructing their interaction with target mRNAs. If we conceive these constructs in an innovative and non-obvious manner, we may patent them.
The patentability of miRNAs, particularly those that occur naturally, can differ markedly by jurisdiction. Natural miRNA sequences encounter obstacles in the U.S. and the European Union; however, there are notable differences in patent legislation worldwide:
United States: Subsequent to the Myriad ruling, naturally existing biological sequences are typically not patentable unless they are altered or utilized in a novel and non-obvious manner. Nonetheless, therapeutic uses and delivery systems continue to be patentable.
Under the Biotechnology Directive, the European Union, like the United States, restricts patents on naturally occurring biological sequences unless they substantiate an industrial application. Nevertheless, synthesized or modified miRNAs, their delivery techniques, and therapeutic applications are eligible for patent protection.
India: The country's patent legislation is more stringent when it comes to biological substances. Section 3(j) of the Indian Patent Act prohibits the patentability of "plants and animals in their entirety or in any part, excluding microorganisms, but including seeds, varieties, and species, as well as essentially biological processes for the production or propagation of plants and animals." Nonetheless, modified or synthesized miRNAs, together with their innovative applications, may remain eligible for patent protection.
China's patent legislation permits the patenting of changed biological sequences and the applications of miRNAs in diagnostics and therapies, rendering it more lenient in certain aspects relative to other jurisdictions.
14. Commercial Products
Several companies are actively developing miRNA-based products (
Table 4)
15. Conclusions
Regenerative medicine provides prospective therapeutic approaches for numerous diseases and tissue repair situations.
These domains exemplify the avant-garde potential of RNA research, and forthcoming findings may significantly influence medicine, therapeutics, and molecular biology.
In conclusion, whereas miRNAs possess considerable potential as therapeutic agents and diagnostic instruments, substantial obstacles persist. MiRNA biology is complicated, and there are problems with targeted delivery, immune system responses, and problems with stability and degradation. These are all things that make it hard to use miRNA-based therapeutics effectively.
The prospects of miRNA research and its medical uses are promising. MiRNAs serve as non-invasive indicators for early illness diagnosis and facilitate personalized treatment interventions, hence enhancing human health. As delivery technology and molecular design methods improve, miRNA-based therapeutics are likely to become very important in treating many diseases, such as cancer, heart disease, neurological disorders, and viral infections. The incorporation of miRNA data into precision medicine, alongside advancements in synthetic biology and regenerative medicine, will enhance the potential applications of miRNA. Addressing the problems of specificity, delivery, and ethical considerations is essential to ensuring that miRNA-based therapeutics are safe and successful as they progress from research to clinical practice.
Multiple miRNA-based therapies are currently undergoing clinical trials, demonstrating the increasing recognition of microRNAs as potent instruments in combating various diseases. MicroRNAs provide an innovative strategy for addressing the fundamental genetic underpinnings of diseases, ranging from cancer and cardiovascular ailments to viral infections and fibrotic conditions. However, before integrating miRNA therapies into routine clinical practice, we must resolve challenges concerning delivery, immunological responses, and off-target effects. The potential for miRNA-based therapeutics to transform personalized medicine and enhance patient outcomes is increasingly evident as research advances and more sophisticated delivery mechanisms emerge [
88,
94,
138].
The prospects for miRNA-based therapeutics are quite optimistic, with customized medicine anticipated to significantly influence their advancement. With advancements in miRNA profiling technologies, clinicians will be capable of identifying the precise miRNAs implicated in a patient's condition and developing customized medicines that target those miRNAs. This methodology has the potential to transform the treatment of intricate diseases such as cancer, where distinct tumors frequently have distinctive miRNA expression profiles.
Furthermore, we anticipate the rise of combination therapies, which combine miRNA therapeutics with alternative treatments like small-molecule inhibitors or immunotherapies. By simultaneously targeting numerous pathways, these combination strategies may improve therapy efficacy and diminish the risk of drug resistance, especially in conditions such as cancer and viral infections [
138].
Despite the significant potential of miRNA-based therapeutics, their clinical advancement encounters numerous obstacles. A key challenge is the delivery of miRNA mimics and antagomirs to target tissues while minimizing off-target effects. Systemic delivery of miRNA therapeutics can lead to widespread distribution throughout the body, increasing the risk of off-target gene silencing and potential toxicity. So, researchers are working on making more precise delivery technologies, like lipid nanoparticles (LNPs) and exosome-based carriers, that can send miRNAs directly to the tissues they need to reach.
Another problem involves regulating the immunological responses elicited by synthetic miRNA molecules. The immune-related adverse effects observed in clinical studies for MRX34 represented a significant setback.
The advancement of miRNA-based therapeutics encounters numerous regulatory obstacles. As miRNAs are relatively novel in the therapeutic domain, regulatory bodies such as the FDA and EMA are currently formulating standards for the approval and application of these medicines. Guaranteeing that miRNA-based therapies adhere to safety and efficacy requirements necessitates extensive preclinical and clinical evaluations, which can be both time-intensive and expensive.
Ethically, issues exist regarding the long-term implications of changing gene expression by miRNA manipulation. Modifying miRNA levels may result in unwanted effects on off-target genes, potentially causing unforeseen health complications, especially in germline editing or therapies for inherited diseases. As miRNA therapies approach clinical deployment, it is essential to address these ethical concerns through suitable regulatory frameworks and informed consent procedures.
References
- Salloway, S., et al., Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol, 2022. 79(1): p. 13-21. [CrossRef]
- Bartel, D.P., Metazoan MicroRNAs. Cell, 2018. 173(1): p. 20-51.
- Bartel, D.P., MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell, 2004. 116(2): p. 281-297.
- Qureshi, A., et al., VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database (Oxford), 2014. 2014. [CrossRef]
- Ambros, V., The functions of animal microRNAs. Nature, 2004. 431(7006): p. 350-5. [CrossRef]
- Bartel, D.P., MicroRNAs: target recognition and regulatory functions. Cell, 2009. 136(2): p. 215-33. [CrossRef]
- He, L. and G.J. Hannon, MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet, 2004. 5(7): p. 522-31.
- Lee, R.C., R.L. Feinbaum, and V. Ambros, The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell, 1993. 75(5): p. 843-54. [CrossRef]
- Lagos-Quintana, M., et al., Identification of novel genes coding for small expressed RNAs. Science, 2001. 294(5543): p. 853-8. [CrossRef]
- Ruvkun, G., Molecular biology. Glimpses of a tiny RNA world. Science, 2001. 294(5543): p. 797-9. [CrossRef]
- van Rooij, E. and E.N. Olson, MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest, 2007. 117(9): p. 2369-76.
- Lagos-Quintana, M., et al., New microRNAs from mouse and human. Rna, 2003. 9(2): p. 175-9. [CrossRef]
- Ambros, V., et al., A uniform system for microRNA annotation. Rna, 2003. 9(3): p. 277-9. [CrossRef]
- Griffiths-Jones, S., et al., miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Research, 2006. 34(suppl_1): p. D140-D144. [CrossRef]
- Wright, M.W. and E.A. Bruford, Naming 'junk': Human non-protein coding RNA (ncRNA) gene nomenclature. Human Genomics, 2011. 5(2): p. 90. [CrossRef]
- O'Brien, J., et al., Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne), 2018. 9: p. 402. [CrossRef]
- Kim, V.N. and J.W. Nam, Genomics of microRNA. Trends Genet, 2006. 22(3): p. 165-73.
- Ha, M. and N. Kim, Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology, 2014. 15: p. 509-524.
- Lee, Y., et al., The nuclear RNase III Drosha initiates microRNA processing. Nature, 2003. 425(6956): p. 415-9.
- Yi, R., et al., Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev, 2003. 17(24): p. 3011-6.
- MacRae, I.J., K. Zhou, and J.A. Doudna, Structural determinants of RNA recognition and cleavage by Dicer. Nat Struct Mol Biol, 2007. 14(10): p. 934-40. [CrossRef]
- Miller, T.M., et al., An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol, 2013. 12(5): p. 435-42. [CrossRef]
- Lelandais-Brière, C., et al., Small RNA diversity in plants and its impact in development. Curr Genomics, 2010. 11(1): p. 14-23. [CrossRef]
- Jonas, S. and E. Izaurralde, Towards a molecular understanding of microRNA-mediated gene silencing. Nature Reviews Genetics, 2015. 16(7): p. 421-433.
- Guo, H., et al., Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature, 2010. 466(7308): p. 835-840. [CrossRef]
- Ha, M. and V.N. Kim, Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol, 2014. 15(8): p. 509-24. [CrossRef]
- Pillai, R.S., S.N. Bhattacharyya, and W. Filipowicz, Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol, 2007. 17(3): p. 118-26.
- Calin, G.A. and C.M. Croce, MicroRNA signatures in human cancers. Nat Rev Cancer, 2006. 6(11): p. 857-66. [CrossRef]
- Krol, J., I. Loedige, and W. Filipowicz, The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet, 2010. 11(9): p. 597-610. [CrossRef]
- Morozova, N., et al., Kinetic signatures of microRNA modes of action. Rna, 2012. 18(9): p. 1635-55. [CrossRef]
- Luo, Y., Z. Na, and S.A. Slavoff, P-Bodies: Composition, Properties, and Functions. Biochemistry, 2018. 57(17): p. 2424-2431. [CrossRef]
- Tan, Y., et al., Transcriptional inhibiton of Hoxd4 expression by miRNA-10a in human breast cancer cells. BMC Molecular Biology, 2009. 10(1): p. 12. [CrossRef]
- Hawkins, P.G. and K.V. Morris, RNA and transcriptional modulation of gene expression. Cell Cycle, 2008. 7(5): p. 602-607. [CrossRef]
- Meister, G., Argonaute proteins: functional insights and emerging roles. Nat Rev Genet, 2013. 14(7): p. 447-59.
- Hammond, S.M., An overview of microRNAs. Adv Drug Deliv Rev, 2015. 87: p. 3-14. [CrossRef]
- Shaw, G. and R. Kamen, A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell, 1986. 46(5): p. 659-667. [CrossRef]
- Jing, Q., et al., Involvement of MicroRNA in AU-Rich Element-Mediated mRNA Instability. Cell, 2005. 120(5): p. 623-634. [CrossRef]
- Lim, L.P., et al., Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature, 2005. 433(7027): p. 769-773. [CrossRef]
- Kai, Z.S. and A.E. Pasquinelli, MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nature Structural & Molecular Biology, 2010. 17(1): p. 5-10. [CrossRef]
- Chatterjee, S. and H. Großhans, Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature, 2009. 461(7263): p. 546-549. [CrossRef]
- Wang, X.-J., et al., Prediction and identification of Arabidopsis thaliana microRNAs and their mRNA targets. Genome Biology, 2004. 5(9): p. R65. [CrossRef]
- Kawasaki, H. and K. Taira, MicroRNA-196 inhibits HOXB8 expression in myeloid differentiation of HL60 cells. Nucleic Acids Symposium Series, 2004. 48(1): p. 211-212. [CrossRef]
- Moxon, S., et al., Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening. Genome Res, 2008. 18(10): p. 1602-9. [CrossRef]
- Lewis, B.P., C.B. Burge, and D.P. Bartel, Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell, 2005. 120(1): p. 15-20. [CrossRef]
- Mazière, P. and A.J. Enright, Prediction of microRNA targets. Drug Discovery Today, 2007. 12(11): p. 452-458. [CrossRef]
- Williams, A.E., Functional aspects of animal microRNAs. Cellular and Molecular Life Sciences, 2008. 65(4): p. 545-562. [CrossRef]
- Eulalio, A., et al., Deadenylation is a widespread effect of miRNA regulation. Rna, 2009. 15(1): p. 21-32. [CrossRef]
- Stark, A., et al., Animal MicroRNAs Confer Robustness to Gene Expression and Have a Significant Impact on 3′UTR Evolution. Cell, 2005. 123(6): p. 1133-1146. [CrossRef]
- He, L. and G.J. Hannon, MicroRNAs: small RNAs with a big role in gene regulation. Nature Reviews Genetics, 2004. 5(7): p. 522-531.
- Salmena, L., et al., A <em>ceRNA</em> Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell, 2011. 146(3): p. 353-358.
- Cuman, C., et al., Human Blastocyst Secreted microRNA Regulate Endometrial Epithelial Cell Adhesion. eBioMedicine, 2015. 2(10): p. 1528-1535. [CrossRef]
- Axtell, M.J., J.O. Westholm, and E.C. Lai, Vive la différence: biogenesis and evolution of microRNAs in plants and animals. Genome Biology, 2011. 12(4): p. 221.
- Wheeler, B.M., et al., The deep evolution of metazoan microRNAs. Evolution & Development, 2009. 11(1): p. 50-68. [CrossRef]
- Pashkovskiy, P.P. and S.S. Ryazansky, Biogenesis, evolution, and functions of plant microRNAs. Biochemistry (Moscow), 2013. 78(6): p. 627-637. [CrossRef]
- Heimberg, A.M., et al., MicroRNAs and the advent of vertebrate morphological complexity. Proceedings of the National Academy of Sciences, 2008. 105(8): p. 2946-2950. [CrossRef]
- Nozawa, M., S. Miura, and M. Nei, Origins and Evolution of MicroRNA Genes in Drosophila Species. Genome Biology and Evolution, 2010. 2: p. 180-189. [CrossRef]
- Allen, E., et al., Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nature Genetics, 2004. 36(12): p. 1282-1290. [CrossRef]
- Warthmann, N., et al., Comparative Analysis of the MIR319a MicroRNA Locus in Arabidopsis and Related Brassicaceae. Molecular Biology and Evolution, 2008. 25(5): p. 892-902. [CrossRef]
- Peterson, K.J., M.R. Dietrich, and M.A. McPeek, MicroRNAs and metazoan macroevolution: insights into canalization, complexity, and the Cambrian explosion. Bioessays, 2009. 31(7): p. 736-47. [CrossRef]
- Fahlgren, N., et al., MicroRNA gene evolution in Arabidopsis lyrata and Arabidopsis thaliana. Plant Cell, 2010. 22(4): p. 1074-89. [CrossRef]
- Caravas, J. and M. Friedrich, Of mites and millipedes: recent progress in resolving the base of the arthropod tree. Bioessays, 2010. 32(6): p. 488-95. [CrossRef]
- Kenny, N.J., et al., Draft genome assemblies and predicted microRNA complements of the intertidal lophotrochozoans Patella vulgata (Mollusca, Patellogastropoda) and Spirobranchus (Pomatoceros) lamarcki (Annelida, Serpulida). Marine Genomics, 2015. 24: p. 139-146. [CrossRef]
- Cock, J.M., et al., The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature, 2010. 465(7298): p. 617-621. [CrossRef]
- Cuperus, J.T., N. Fahlgren, and J.C. Carrington, Evolution and Functional Diversification of MIRNA Genes. The Plant Cell, 2011. 23(2): p. 431-442. [CrossRef]
- MIRTBASE. miRTarBase: The experimentally validated microRNA-target interactions database. 2024.
- Pasquinelli, A.E., et al., Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature, 2000. 408(6808): p. 86-9.
- Fromm, B., et al., MirGeneDB 2.0: the metazoan microRNA complement. Nucleic Acids Research, 2019. 48(D1): p. D132-D141.
- Wang, H., A Review of Nanotechnology in microRNA Detection and Drug Delivery. Cells, 2024. 13(15): p. 1277. [CrossRef]
- Friedländer, M.R., et al., Evidence for the biogenesis of more than 1,000 novel human microRNAs. Genome Biology, 2014. 15(4): p. R57. [CrossRef]
- Boeckel, J.-N., et al., From heart to toe: Heart's contribution on peripheral microRNA levels. International Journal of Cardiology, 2014. 172(3): p. 616-617.
- Liu, C.-G., et al., MicroRNA expression profiling using microarrays. Nature Protocols, 2008. 3(4): p. 563-578. [CrossRef]
- Chen, C., et al., Real-time quantification of microRNAs by stem–loop RT–PCR. Nucleic Acids Research, 2005. 33(20): p. e179-e179. [CrossRef]
- Shingara, J., et al., An optimized isolation and labeling platform for accurate microRNA expression profiling. Rna, 2005. 11(9): p. 1461-70. [CrossRef]
- Buermans, H.P.J., et al., New methods for next generation sequencing based microRNA expression profiling. BMC Genomics, 2010. 11(1): p. 716. [CrossRef]
- Flynt, A.S., et al., Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nature Genetics, 2007. 39(2): p. 259-263. [CrossRef]
- Meister, G., et al., Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. Rna, 2004. 10(3): p. 544-50.
- Gebert, L.F.R., et al., Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Research, 2013. 42(1): p. 609-621. [CrossRef]
- Choi, W.-Y., A.J. Giraldez, and A.F. Schier, Target Protectors Reveal Dampening and Balancing of Nodal Agonist and Antagonist by miR-430. Science, 2007. 318(5848): p. 271-274. [CrossRef]
- Lagendijk, A.K., J.D. Moulton, and J. Bakkers, Revealing details: whole mount microRNA in situ hybridization protocol for zebrafish embryos and adult tissues. Biology Open, 2012. 1(6): p. 566-569. [CrossRef]
- Kaur, H., et al., Thermodynamic, Counterion, and Hydration Effects for the Incorporation of Locked Nucleic Acid Nucleotides into DNA Duplexes. Biochemistry, 2006. 45(23): p. 7347-7355. [CrossRef]
- Nielsen, J.A., et al., Integrating microRNA and mRNA expression profiles of neuronal progenitors to identify regulatory networks underlying the onset of cortical neurogenesis. BMC Neuroscience, 2009. 10(1): p. 98. [CrossRef]
- Griffiths-Jones, S., et al., miRBase: tools for microRNA genomics. Nucleic Acids Research, 2007. 36(suppl_1): p. D154-D158. [CrossRef]
- Artmann, S., et al., Detection of simultaneous group effects in microRNA expression and related target gene sets. PLoS One, 2012. 7(6): p. e38365. [CrossRef]
- Jiang, Q., et al., miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Research, 2008. 37(suppl_1): p. D98-D104. [CrossRef]
- Kumar, S. and P.H. Reddy, Are circulating microRNAs peripheral biomarkers for Alzheimer's disease? Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 2016. 1862(9): p. 1617-1627.
- Yu, B., et al., An integrated hypothesis for miR-126 in vascular disease. Med Res Arch, 2020. 8(5). [CrossRef]
- van den Berg, M.M.J., et al., Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Progress in Neurobiology, 2020. 185: p. 101732.
- FDA. Nucleic Acid Based Tests. 2024.
- Kyriakidis, I., K. Kyriakidis, and A. Tsezou, MicroRNAs and the Diagnosis of Childhood Acute Lymphoblastic Leukemia: Systematic Review, Meta-Analysis and Re-Analysis with Novel Small RNA-Seq Tools. Cancers, 2022. 14(16): p. 3976. [CrossRef]
- Akçakaya, P., et al., miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. International journal of oncology, 2011. 39(2): p. 311-318.
- Xu, G., et al., MicroRNA-21 promotes hepatocellular carcinoma HepG2 cell proliferation through repression of mitogen-activated protein kinase-kinase 3. BMC Cancer, 2013. 13: p. 469. [CrossRef]
- Jasperson, K.W., et al., Hereditary and Familial Colon Cancer. Gastroenterology, 2010. 138(6): p. 2044-2058. [CrossRef]
- Gibert, B., et al., Regulation by miR181 Family of the Dependence Receptor CDON Tumor Suppressive Activity in Neuroblastoma. JNCI: Journal of the National Cancer Institute, 2014. 106(11). [CrossRef]
- Thakur, S., et al., A perspective on oligonucleotide therapy: Approaches to patient customization. Frontiers in Pharmacology, 2022. 13. [CrossRef]
- Rink, C. and S. Khanna, MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics, 2011. 43(10): p. 521-8. [CrossRef]
- Neag, M.A., et al., miRNA Involvement in Cerebral Ischemia-Reperfusion Injury. Front Neurosci, 2022. 16: p. 901360. [CrossRef]
- Baltimore, D., et al., MicroRNAs: new regulators of immune cell development and function. Nat Immunol, 2008. 9(8): p. 839-45. [CrossRef]
- Qureshi, A., et al., VIRmiRNA: a comprehensive resource for experimentally validated viral miRNAs and their targets. Database, 2014. 2014. [CrossRef]
- Tuddenham, L., et al., Small RNA Deep Sequencing Identifies MicroRNAs and Other Small Noncoding RNAs from Human Herpesvirus 6B. Journal of Virology, 2012. 86(3): p. 1638-1649. [CrossRef]
- Lewohl, J.M., et al., Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res, 2011. 35(11): p. 1928-37. [CrossRef]
- Tapocik, J.D., et al., Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. Pharmacogenomics J, 2013. 13(3): p. 286-96. [CrossRef]
- Gorini, G., Y.O. Nunez, and R.D. Mayfield, Integration of miRNA and protein profiling reveals coordinated neuroadaptations in the alcohol-dependent mouse brain. PLoS One, 2013. 8(12): p. e82565. [CrossRef]
- Tapocik, J.D., et al., microRNA-206 in Rat Medial Prefrontal Cortex Regulates BDNF Expression and Alcohol Drinking. The Journal of Neuroscience, 2014. 34(13): p. 4581-4588. [CrossRef]
- Lippai, D., et al., Chronic alcohol-induced microRNA-155 contributes to neuroinflammation in a TLR4-dependent manner in mice. PLoS One, 2013. 8(8): p. e70945. [CrossRef]
- Li, J., et al., MicroRNA expression profile and functional analysis reveal that miR‐382 is a critical novel gene of alcohol addiction. EMBO Molecular Medicine, 2013. 5(9): p. 1402-1414.
- Li, J., et al., MicroRNA expression profile and functional analysis reveal that miR‐382 is a critical novel gene of alcohol addiction. EMBO Molecular Medicine, 2013. 5(9): p. 1402-1414-1414.
- Romao, J.M., et al., MicroRNA regulation in mammalian adipogenesis. Experimental Biology and Medicine, 2011. 236(9): p. 997-1004. [CrossRef]
- Skårn, M., et al., Adipocyte Differentiation of Human Bone Marrow-Derived Stromal Cells Is Modulated by MicroRNA-155, MicroRNA-221, and MicroRNA-222. Stem Cells and Development, 2011. 21(6): p. 873-883. [CrossRef]
- Zuo, Y., L. Qiang, and S.R. Farmer, Activation of CCAAT/Enhancer-binding Protein (C/EBP) α Expression by C/EBPβ during Adipogenesis Requires a Peroxisome Proliferator-activated Receptor-γ-associated Repression of HDAC1 at the <em>C/ebp</em>α Gene Promoter *. Journal of Biological Chemistry, 2006. 281(12): p. 7960-7967.
- Jun-Hao, E.T., R.R. Gupta, and N. Shyh-Chang, Lin28 and <em>let-7</em> in the Metabolic Physiology of Aging. Trends in Endocrinology & Metabolism, 2016. 27(3): p. 132-141.
- Zhu, H., et al., The <em>Lin28/let-7</em> Axis Regulates Glucose Metabolism. Cell, 2011. 147(1): p. 81-94.
- Frost, R.J.A. and E.N. Olson, Control of glucose homeostasis and insulin sensitivity by the <i>Let-7</i> family of microRNAs. Proceedings of the National Academy of Sciences, 2011. 108(52): p. 21075-21080.
- Teruel-Montoya, R., F.R. Rosendaal, and C. Martínez, MicroRNAs in hemostasis. Journal of Thrombosis and Haemostasis, 2015. 13(2): p. 170-181.
- Nourse, J. and S. Danckwardt, A novel rationale for targeting FXI: Insights from the hemostatic microRNA targetome for emerging anticoagulant strategies. Pharmacology & Therapeutics, 2021. 218: p. 107676. [CrossRef]
- Berardi, E., et al., miRNAs in ESC differentiation. American Journal of Physiology-Heart and Circulatory Physiology, 2012. 303(8): p. H931-H939.
- Seyhan, A.A., Trials and Tribulations of MicroRNA Therapeutics. International Journal of Molecular Sciences, 2024. 25(3): p. 1469. [CrossRef]
- Mencía, Á., et al., Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nature Genetics, 2009. 41(5): p. 609-613. [CrossRef]
- Hughes, Anne E., et al., Mutation Altering the miR-184 Seed Region Causes Familial Keratoconus with Cataract. The American Journal of Human Genetics, 2011. 89(5): p. 628-633. [CrossRef]
- de Pontual, L., et al., Germline deletion of the miR-17∼92 cluster causes skeletal and growth defects in humans. Nature Genetics, 2011. 43(10): p. 1026-1030.
- Zhou, L., et al., A genome-wide microRNA screen identifies the microRNA-183/96/182 cluster as a modulator of circadian rhythms. Proceedings of the National Academy of Sciences, 2021. 118(1): p. e2020454118.
- Volná, A., et al., What Do We Know about Barley miRNAs? International Journal of Molecular Sciences, 2022. 23(23): p. 14755.
- Curaba, J., et al., miRNA regulation in the early development of barley seed. BMC Plant Biology, 2012. 12(1): p. 120. [CrossRef]
- Elnashar, A.M., et al., Female sexual dysfunction in Lower Egypt. BJOG, 2007. 114(2): p. 201-6. [CrossRef]
- Fedorczak, A., A. Lewiński, and R. Stawerska, Involvement of Sirtuin 1 in the Growth Hormone/Insulin-like Growth Factor 1 Signal Transduction and Its Impact on Growth Processes in Children. Int J Mol Sci, 2023. 24(20). [CrossRef]
- Kitada, T., et al., Programming gene and engineered-cell therapies with synthetic biology. Science, 2018. 359(6376). [CrossRef]
- De Palma, F.D.E., et al., The Multifaceted Roles of MicroRNAs in Cystic Fibrosis. Diagnostics (Basel), 2020. 10(12). [CrossRef]
- Cañas, J.A., et al., MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Frontiers in Immunology, 2021. 11. [CrossRef]
- Gaudino, S.J. and P. Kumar, Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Frontiers in Immunology, 2019. 10. [CrossRef]
- Monreal-Escalante, E., et al., Plant-Based Vaccines: Antigen Design, Diversity, and Strategies for High Level Production. Vaccines (Basel), 2022. 10(1). [CrossRef]
- Zheng, H., et al., Advances in the Techniques for the Prediction of microRNA Targets. International Journal of Molecular Sciences, 2013. 14(4): p. 8179-8187. [CrossRef]
- Agarwal, V., et al., Predicting effective microRNA target sites in mammalian mRNAs. eLife, 2015. 4: p. e05005. [CrossRef]
- Nourse, J., et al., Large‐scale identification of functional microRNA targeting reveals cooperative regulation of the hemostatic system. Journal of Thrombosis and Haemostasis, 2018. 16(11): p. 2233-2245. [CrossRef]
- Diener, C., A. Keller, and E. Meese, The miRNA-target interactions: An underestimated intricacy. Nucleic Acids Res, 2024. 52(4): p. 1544-1557.
- Wilczynska, A. and M. Bushell, The complexity of miRNA-mediated repression. Cell Death & Differentiation, 2015. 22(1): p. 22-33.
- Kuhn, D.E., et al., Experimental validation of miRNA targets. Methods, 2008. 44(1): p. 47-54. [CrossRef]
- Lin, A., et al., Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci Transl Med, 2019. 11(509).
- Zare, M., et al., Encapsulation of miRNA and siRNA into Nanomaterials for Cancer Therapeutics. Pharmaceutics, 2022. 14(8). [CrossRef]
- Bajan, S. and G. Hutvagner, RNA-Based Therapeutics: From Antisense Oligonucleotides to miRNAs. Cells, 2020. 9(1): p. 137. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).