Submitted:
24 October 2024
Posted:
25 October 2024
You are already at the latest version
Abstract
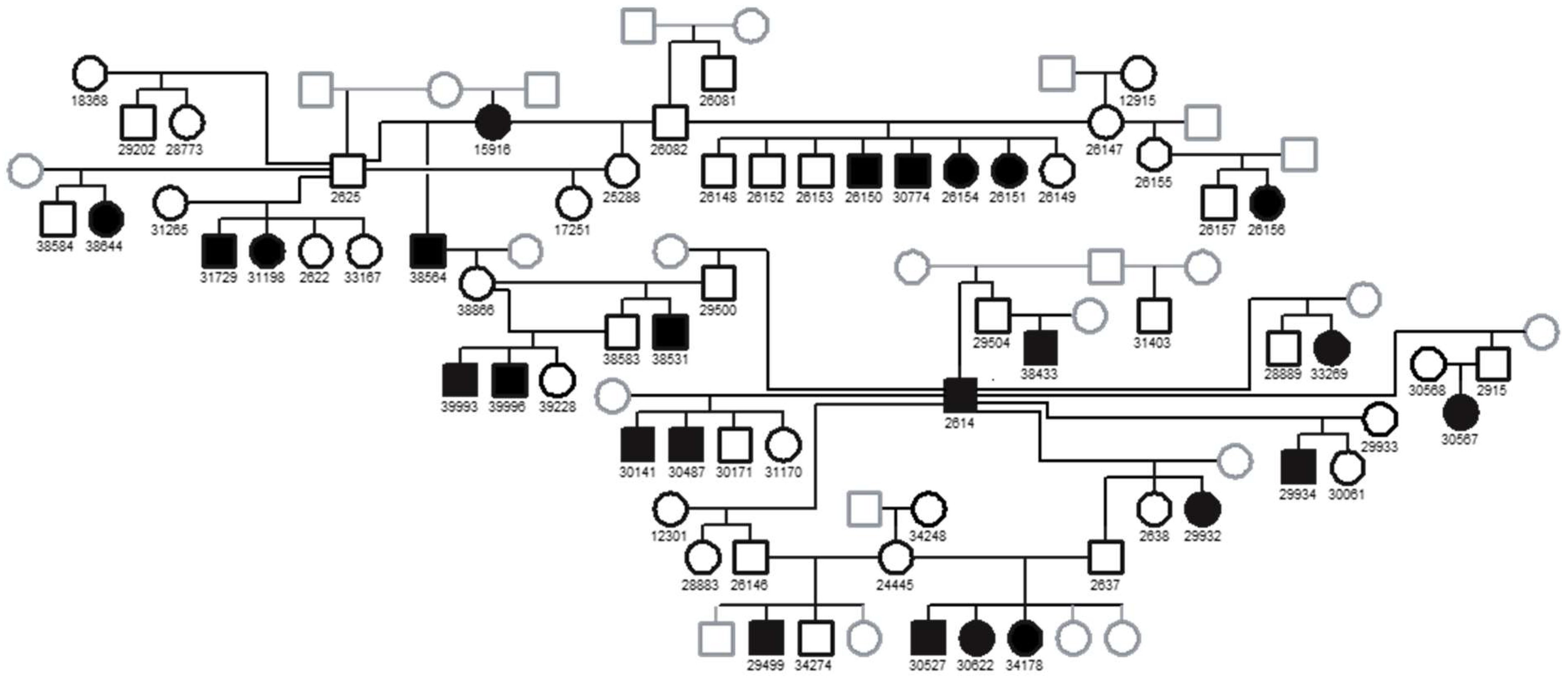
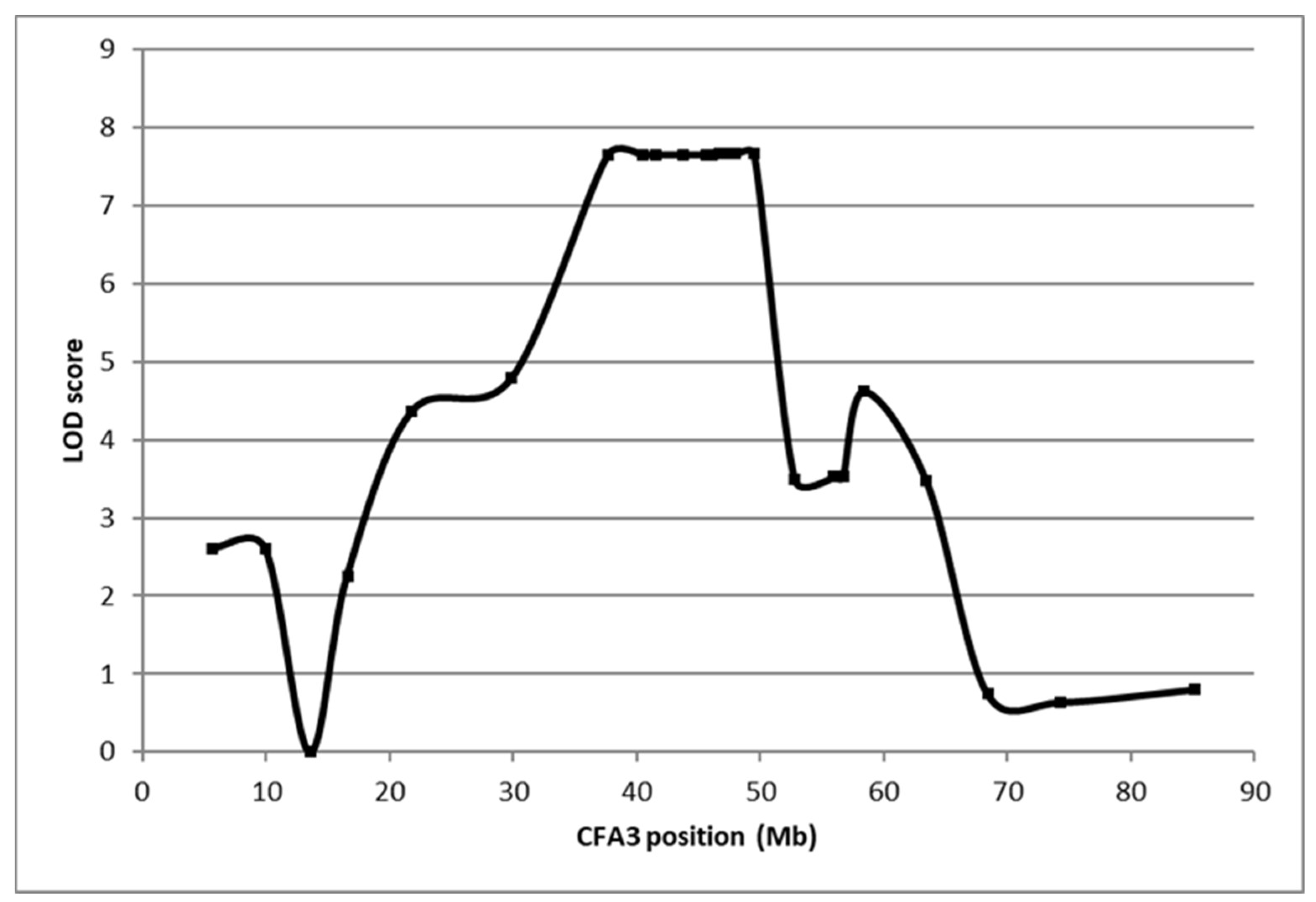
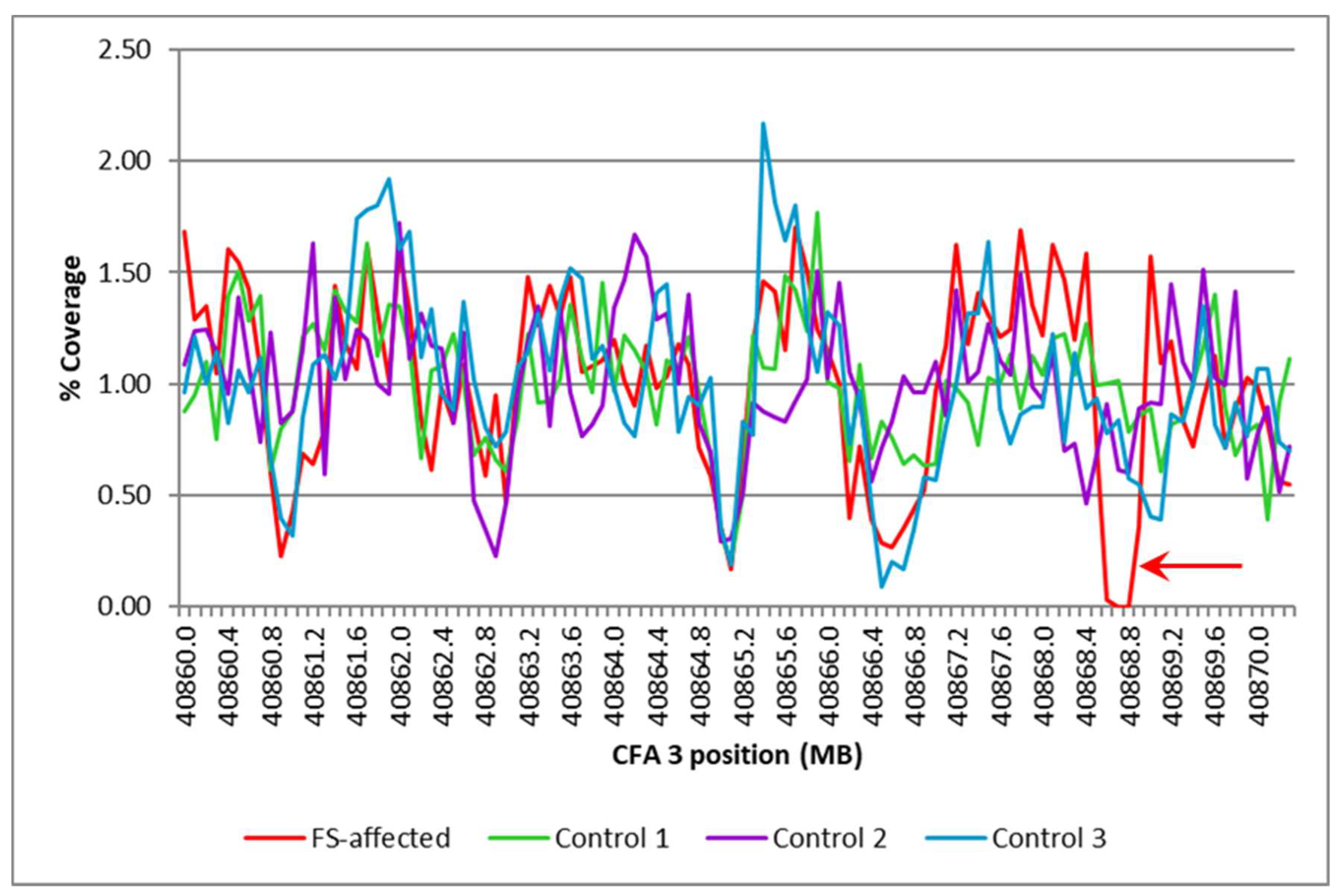
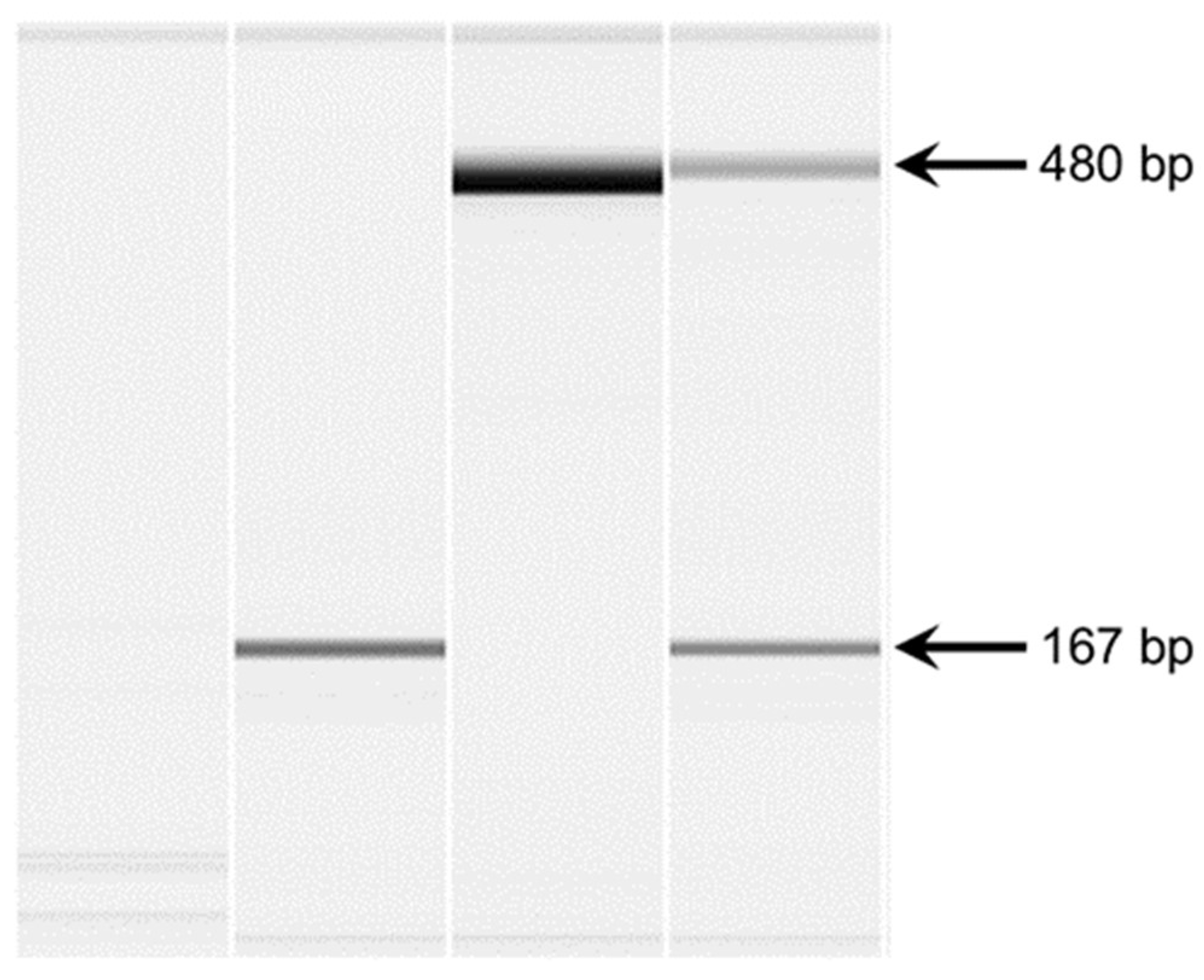
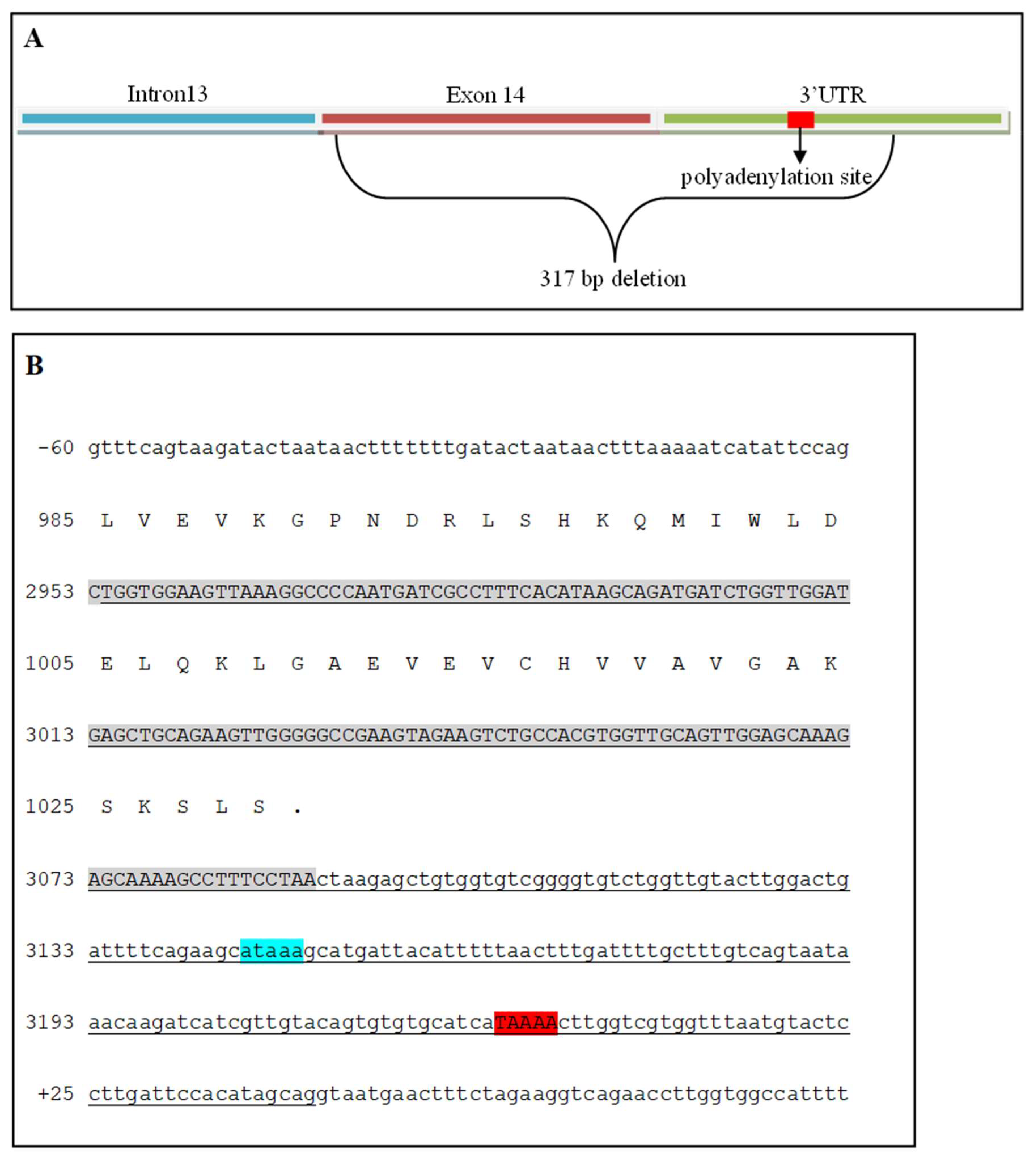
Fanconi syndrome is a disorder of renal proximal tubule transport characterized by metabolic acidosis, amino aciduria, glucosuria, and phosphaturia. There are acquired and hereditary forms of this disorder. Fanconi syndrome in Basenjis was first described in 1976 and is now recognized as an inherited disease in these dogs. Linkage analysis within a large family of Basenjis that included both affected and unaffected individuals was performed to localize the causative variant within the genome. Significant linkage was identified between chromosome 3 (CFA3) makers and the disease phenotype. Fine mapping restricted the region to a 2.7 Mb section of CFA3. A whole genome sequence of a Basenji affected with Fanconi syndrome was generated, and the sequence data were examined for the presence of potentially deleterious homozygous variants within the mapped region. A homozygous 317 bp deletion was identified in the last exon of FAN1 of the proband. 78 Basenjis of known disease status were genotyped for the deletion variant. Among these dogs, there was almost complete concordance between genotype and phenotype. The only exception was one dog that was homozygous for the deletion variant but did not exhibit signs of Fanconi syndrome. These data indicate that the disorder is very likely the result of FAN1 deficiency. The mechanism by which this deficiency causes the disease signs remains to be elucidated. FAN1 has endonuclease and exonuclease activity that catalyzes incisions in regions of double-stranded DNA containing interstrand crosslinks. FAN1 inactivation may cause Fanconi syndrome in Basenjis by sensitization of kidney proximal tubule cells to toxin-mediated DNA crosslinking resulting in accumulation of genomic and mitochondrial DNA damage in the kidney. Differential exposure to environmental toxins that promote DNA crosslink formation may explain the wide age-at-onset variability for the disorder in Basenjis.
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreman, J.W. Fanconi Syndrome. Pediatr Clin North Am 2019, 66, 159–167. [CrossRef]
- Albuquerque, A.L.B.; Dos Santos Borges, R.; Conegundes, A.F.; Dos Santos, E.E.; Fu, F.M.M.; Araujo, C.T.; Vaz de Castro, P.A.S.; Simoes E Silva, A.C. Inherited Fanconi Syndrome. World J Pediatr 2023, 19, 619–634. [CrossRef]
- Kunchur, M.G.; Mauch, T.J.; Parkanzky, M.; Rahilly, L.J. A Review of Renal Tubular Acidosis. J Vet Emerg Crit Care (San Antonio) 2024, 34, 325–355. [CrossRef]
- Lobitz, S.; Velleuer, E. Guido Fanconi (1892-1979): A Jack of All Trades. Nat Rev Cancer 2006, 6, 893–898. [CrossRef]
- Baum, M. The Cellular Basis of Fanconi Syndrome. Hosp Pract (Off Ed) 1993, 28, 137–138. [CrossRef]
- Magen, D.; Berger, L.; Coady, M.J.; Ilivitzki, A.; Militianu, D.; Tieder, M.; Selig, S.; Lapointe, J.Y.; Zelikovic, I.; Skorecki, K. A Loss-of-Function Mutation in NaPi-IIa and Renal Fanconi’s Syndrome. N Engl J Med 2010, 362, 1102–1109. [CrossRef]
- Lichter-Konecki, U.; Broman, K.W.; Blau, E.B.; Konecki, D.S. Genetic and Physical Mapping of the Locus for Autosomal Dominant Renal Fanconi Syndrome, on Chromosome 15q15.3. Am J Hum Genet 2001, 68, 264–268. [CrossRef]
- Emma, F.; Bertini, E.; Salviati, L.; Montini, G. Renal Involvement in Mitochondrial Cytopathies. Pediatr Nephrol 2012, 27, 539–550. [CrossRef]
- Lloyd, S.E.; Pearce, S.H.; Fisher, S.E.; Steinmeyer, K.; Schwappach, B.; Scheinman, S.J.; Harding, B.; Bolino, A.; Devoto, M.; Goodyer, P.; et al. A Common Molecular Basis for Three Inherited Kidney Stone Diseases. Nature 1996, 379, 445–449. [CrossRef]
- Lowe, M. Structure and Function of the Lowe Syndrome Protein OCRL1. Traffic 2005, 6, 711–719. [CrossRef]
- Baum, M. The Fanconi Syndrome of Cystinosis: Insights into the Pathophysiology. Pediatr Nephrol 1998, 12, 492–497. [CrossRef]
- Kintzel, P.E. Anticancer Drug-Induced Kidney Disorders. Drug Saf 2001, 24, 19–38. [CrossRef]
- Gonick, H.; Indraprasit, S.; Neustein, H.; Rosen, V. Cadmium-Induced Experimental Fanconi Syndrome. Curr Probl Clin Biochem 1975, 4, 111–118.
- Yui, J.C.; Geara, A.; Sayani, F. Deferasirox-Associated Fanconi Syndrome in Adult Patients with Transfusional Iron Overload. Vox Sang 2021, 116, 793–797. [CrossRef]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug Toxicity in the Proximal Tubule: New Models, Methods and Mechanisms. Pediatr Nephrol 2022, 37, 973–982. [CrossRef]
- Anguissola, G.; Leu, D.; Simonetti, G.D.; Simonetti, B.G.; Lava, S.A.G.; Milani, G.P.; Bianchetti, M.G.; Scoglio, M. Kidney Tubular Injury Induced by Valproic Acid: Systematic Literature Review. Pediatr Nephrol 2023, 38, 1725–1731. [CrossRef]
- Easley, J.R.; Breitschwerdt, D.B. Glucosuria Associated with Renal Tubular Dysfunction in Three Basenji Dogs. J Am Vet Med Assoc 1976, 168, 938–943.
- Bovee, K.C.; Joyce, T.; Reynolds, R.; Segal, S. Spontaneous Fanconi Syndrome in the Dog. Metabolism 1978, 27, 45–52. [CrossRef]
- Bovee, K.C.; Joyce, T.; Reynolds, R.; Segal, S. The Fanconi Syndrome in Basenji Dogs: A New Model for Renal Transport Defects. Science 1978, 201, 1129–1131. [CrossRef]
- Bovee, K.C.; Joyce, T.; Blazer-Yost, B.; Goldschmidt, M.S.; Segal, S. Characterization of Renal Defects in Dogs with a Syndrome Similar to the Fanconi Syndrome in Man. J Am Vet Med Assoc 1979, 174, 1094–1099.
- Bovee, K.C.; Anderson, T.; Brown, S.; Goldschmidt, M.H.; Segal, S. Renal Tubular Defects of Spontaneous Fanconi Syndrome in Dogs. Prog Clin Biol Res 1982, 94, 435–447.
- Medow, M.S.; Reynolds, R.; Bovee, K.C.; Segal, S. Proline and Glucose Transport by Renal Membranes from Dogs with Spontaneous Idiopathic Fanconi Syndrome. Proc Natl Acad Sci U S A 1981, 78, 7769–7772. [CrossRef]
- Mainka, S.A. Fanconi Syndrome in a Basenji. Can Vet J 1985, 26, 303–305.
- Noonan, C.H.; Kay, J.M. Prevalence and Geographic Distribution of Fanconi Syndrome in Basenjis in the United States. J Am Vet Med Assoc 1990, 197, 345–349.
- Yearley, J.H.; Hancock, D.D.; Mealey, K.L. Survival Time, Lifespan, and Quality of Life in Dogs with Idiopathic Fanconi Syndrome. J Am Vet Med Assoc 2004, 225, 377–383. [CrossRef]
- Freeman, L.M.; Breitschwerdt, E.B.; Keene, B.W.; Hansen, B. Fanconi’s Syndrome in a Dog with Primary Hypoparathyroidism. J Vet Intern Med 1994, 8, 349–354. [CrossRef]
- Settles, E.L.; Schmidt, D. Fanconi Syndrome in a Labrador Retriever. J Vet Intern Med 1994, 8, 390–393. [CrossRef]
- Appleman, E.H.; Cianciolo, R.; Mosenco, A.S.; Bounds, M.E.; Al-Ghazlat, S. Transient Acquired Fanconi Syndrome Associated with Copper Storage Hepatopathy in 3 Dogs. J Vet Intern Med 2008, 22, 1038–1042. [CrossRef]
- King, J.B. Proximal Tubular Nephropathy in Two Dogs Diagnosed with Lead Toxicity. Aust Vet J 2016, 94, 280–284. [CrossRef]
- Yabuki, A.; Iwanaga, T.; Giger, U.; Sawa, M.; Kohyama, M.; Yamato, O. Acquired Fanconi Syndrome in Two Dogs Following Long-Term Consumption of Pet Jerky Treats in Japan: Case Report. J Vet Med Sci 2017, 79, 818–821. [CrossRef]
- Bommer, N.X.; Brownlie, S.E.; Morrison, L.R.; Chandler, M.L.; Simpson, J.W. Fanconi Syndrome in Irish Wolfhound Siblings. J Am Anim Hosp Assoc 2018, 54, 173–178. [CrossRef]
- Ahn, J.-O.; Kim, S.-M.; Song, W.-J.; Ryu, M.-O.; Li, Q.; Chung, J.-Y.; Youn, H.-Y. Transient Fanconi Syndrome After Treatment with Firocoxib, Cefadroxil, Tramadol, and Famotidine in a Maltese. J Am Anim Hosp Assoc 2019, 55, 323–327. [CrossRef]
- Takashima, S.; Nasu, T.; Ohata, K.; Oikawa, T.; Sugaya, T.; Kobatake, Y.; Shibata, S.; Nishii, N. Urinary Liver-Type Fatty Acid-Binding Protein in Two Dogs with Acquired Fanconi Syndrome: A Case Report. Open Vet J 2022, 12, 864–867. [CrossRef]
- Nybroe, S.; Bjornvad, C.R.; Hansen, C.F.H.; Andersen, T.S.L.; Kieler, I.N. Outcome of Acquired Fanconi Syndrome Associated with Ingestion of Jerky Treats in 30 Dogs. Animals (Basel) 2022, 12. [CrossRef]
- Hooper, A.N.; Roberts, B.K. Fanconi Syndrome in Four Non-Basenji Dogs Exposed to Chicken Jerky Treats. J Am Anim Hosp Assoc 2011, 47, e178-87. [CrossRef]
- Major, A.; Schweighauser, A.; Hinden, S.E.; Francey, T. Transient Fanconi Syndrome with Severe Polyuria and Polydipsia in a 4-Year Old Shih Tzu Fed Chicken Jerky Treats. Schweiz Arch Tierheilkd 2014, 156, 593–598. [CrossRef]
- Hill, T.L.; Breitschwerdt, E.B.; Cecere, T.; Vaden, S. Concurrent Hepatic Copper Toxicosis and Fanconi’s Syndrome in a Dog. J Vet Intern Med 2008, 22, 219–222. [CrossRef]
- Preston, R.; Naylor, R.W.; Stewart, G.; Bierzynska, A.; Saleem, M.A.; Lowe, M.; Lennon, R. A Role for OCRL in Glomerular Function and Disease. Pediatr Nephrol 2020, 35, 641–648. [CrossRef]
- Sharari, S.; Abou-Alloul, M.; Hussain, K.; Ahmad Khan, F. Fanconi-Bickel Syndrome: A Review of the Mechanisms That Lead to Dysglycaemia. Int J Mol Sci 2020, 21. [CrossRef]
- Govers, L.P.; Toka, H.R.; Hariri, A.; Walsh, S.B.; Bockenhauer, D. Mitochondrial DNA Mutations in Renal Disease: An Overview. Pediatr Nephrol 2021, 36, 9–17. [CrossRef]
- Khandelwal, P.; Mahesh, V.; Mathur, V.P.; Raut, S.; Geetha, T.S.; Nair, S.; Hari, P.; Sinha, A.; Bagga, A. Phenotypic Variability in Distal Acidification Defects Associated with WDR72 Mutations. Pediatr Nephrol 2021, 36, 881–887. [CrossRef]
- Sheppard, S.E.; Barrett, B.; Muraresku, C.; McKnight, H.; De Leon, D.D.; Lord, K.; Ganetzky, R. Heterozygous Recurrent HNF4A Variant p.Arg85Trp Causes Fanconi Renotubular Syndrome 4 with Maturity Onset Diabetes of the Young, an Autosomal Dominant Phenocopy of Fanconi Bickel Syndrome with Colobomas. Am J Med Genet A 2021, 185, 566–570. [CrossRef]
- Duan, N.; Huang, C.; Pang, L.; Jiang, S.; Yang, W.; Li, H. Clinical Manifestation and Genetic Findings in Three Boys with Low Molecular Weight Proteinuria - Three Case Reports for Exploring Dent Disease and Fanconi Syndrome. BMC Nephrol 2021, 22, 24. [CrossRef]
- Elsayed, A.K.; Al-Khawaga, S.; Hussain, K.; Abdelalim, E.M. An Induced Pluripotent Stem Cell Line Derived from a Patient with Neonatal Diabetes and Fanconi-Bickel Syndrome Caused by a Homozygous Mutation in the SLC2A2 Gene. Stem Cell Res 2021, 54, 102433. [CrossRef]
- Grunert, S.C.; Schumann, A.; Baronio, F.; Tsiakas, K.; Murko, S.; Spiekerkoetter, U.; Santer, R. Evidence for a Genotype-Phenotype Correlation in Patients with Pathogenic GLUT2 (SLC2A2) Variants. Genes (Basel) 2021, 12. [CrossRef]
- Kanako, K.-I.; Sakakibara, N.; Murayama, K.; Nagatani, K.; Murata, S.; Otake, A.; Koga, Y.; Suzuki, H.; Uehara, T.; Kosaki, K.; et al. BCS1L Mutations Produce Fanconi Syndrome with Developmental Disability. J Hum Genet 2022, 67, 143–148. [CrossRef]
- Sharari, S.; Aouida, M.; Mohammed, I.; Haris, B.; Bhat, A.A.; Hawari, I.; Nisar, S.; Pavlovski, I.; Biswas, K.H.; Syed, N.; et al. Understanding the Mechanism of Dysglycemia in a Fanconi-Bickel Syndrome Patient. Front Endocrinol (Lausanne) 2022, 13, 841788. [CrossRef]
- Agakidou, E.; Agakidis, C.; Kambouris, M.; Printza, N.; Farini, M.; Vourda, E.; Gerou, S.; Sarafidis, K. A Novel Mutation of VPS33B Gene Associated with Incomplete Arthrogryposis-Renal Dysfunction-Cholestasis Phenotype. Case Rep Genet 2020, 2020, 8872294. [CrossRef]
- Forst, A.-L.; Reichold, M.; Kleta, R.; Warth, R. Distinct Mitochondrial Pathologies Caused by Mutations of the Proximal Tubular Enzymes EHHADH and GATM. Front Physiol 2021, 12, 715485. [CrossRef]
- Guan, Y.-J.; Guo, Y.-N.; Peng, W.-T.; Liu, L.-L. Case Report: Cystinosis in a Chinese Child With a Novel CTNS Pathogenic Variant. Front Pediatr 2022, 10, 860990. [CrossRef]
- Kudo, H.; Suzuki, R.; Kondo, A.; Nozu, K.; Nakamura, Y.; Mikami, H.; Soma, J.; Nakaya, I. Association of Familial Fanconi Syndrome with a Novel GATM Variant. Tohoku J Exp Med 2023, 260, 337–340. [CrossRef]
- Sivaji, V.; Raju, P.; Marimuthu, S.; Sundar, S. Familial Renal Glycosuria Identified in an Indian Family. BMJ Case Rep 2024, 17. [CrossRef]
- Saiteja, P.; Krishnamurthy, S.; Deepthi, B.; Krishnasamy, S.; Sravani, M. Distal Renal Tubular Acidosis as Presenting Manifestation of Wilson Disease in a 11-Year-Old Girl. CEN Case Rep 2024, 13, 93–97. [CrossRef]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A Mutation in the CLN8 Gene in English Setter Dogs with Neuronal Ceroid-Lipofuscinosis. Biochem Biophys Res Commun 2005, 327. [CrossRef]
- Nagase, T.; Ishikawa, K.; Suyama, M.; Kikuno, R.; Hirosawa, M.; Miyajima, N.; Tanaka, A.; Kotani, H.; Nomura, N.; Ohara, O. Prediction of the Coding Sequences of Unidentified Human Genes. XIII. The Complete Sequences of 100 New CDNA Clones from Brain Which Code for Large Proteins in Vitro. DNA Res 1999, 6, 63–70. [CrossRef]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein Tyrosine Phosphatases in the Human Genome. Cell 2004, 117, 699–711. [CrossRef]
- Azzedine, H.; Bolino, A.; Taieb, T.; Birouk, N.; Di Duca, M.; Bouhouche, A.; Benamou, S.; Mrabet, A.; Hammadouche, T.; Chkili, T.; et al. Mutations in MTMR13, a New Pseudophosphatase Homologue of MTMR2 and Sbf1, in Two Families with an Autosomal Recessive Demyelinating Form of Charcot-Marie-Tooth Disease Associated with Early-Onset Glaucoma. Am J Hum Genet 2003, 72, 1141–1153. [CrossRef]
- Yoshikiyo, K.; Kratz, K.; Hirota, K.; Nishihara, K.; Takata, M.; Kurumizaka, H.; Horimoto, S.; Takeda, S.; Jiricny, J. KIAA1018/FAN1 Nuclease Protects Cells against Genomic Instability Induced by Interstrand Cross-Linking Agents. Proc Natl Acad Sci U S A 2010, 107, 21553–21557. [CrossRef]
- Liu, T.; Ghosal, G.; Yuan, J.; Chen, J.; Huang, J. FAN1 Acts with FANCI-FANCD2 to Promote DNA Interstrand Cross-Link Repair. Science 2010, 329, 693–696. [CrossRef]
- MacKay, C.; Declais, A.-C.; Lundin, C.; Agostinho, A.; Deans, A.J.; MacArtney, T.J.; Hofmann, K.; Gartner, A.; West, S.C.; Helleday, T.; et al. Identification of KIAA1018/FAN1, a DNA Repair Nuclease Recruited to DNA Damage by Monoubiquitinated FANCD2. Cell 2010, 142, 65–76. [CrossRef]
- Smogorzewska, A.; Desetty, R.; Saito, T.T.; Schlabach, M.; Lach, F.P.; Sowa, M.E.; Clark, A.B.; Kunkel, T.A.; Harper, J.W.; Colaiacovo, M.P.; et al. A Genetic Screen Identifies FAN1, a Fanconi Anemia-Associated Nuclease Necessary for DNA Interstrand Crosslink Repair. Mol Cell 2010, 39, 36–47. [CrossRef]
- Kratz, K.; Schopf, B.; Kaden, S.; Sendoel, A.; Eberhard, R.; Lademann, C.; Cannavo, E.; Sartori, A.A.; Hengartner, M.O.; Jiricny, J. Deficiency of FANCD2-Associated Nuclease KIAA1018/FAN1 Sensitizes Cells to Interstrand Crosslinking Agents. Cell 2010, 142, 77–88. [CrossRef]
- Jin, H.; Cho, Y. Structural and Functional Relationships of FAN1. DNA Repair (Amst) 2017, 56, 135–143. [CrossRef]
- Thongthip, S.; Bellani, M.; Gregg, S.Q.; Sridhar, S.; Conti, B.A.; Chen, Y.; Seidman, M.M.; Smogorzewska, A. Fan1 Deficiency Results in DNA Interstrand Cross-Link Repair Defects, Enhanced Tissue Karyomegaly, and Organ Dysfunction. Genes Dev 2016, 30, 645–659. [CrossRef]
- Hoover, A.; Turcotte, L.M.; Phelan, R.; Barbus, C.; Rayannavar, A.; Miller, B.S.; Reardon, E.E.; Theis-Mahon, N.; MacMillan, M.L. Longitudinal Clinical Manifestations of Fanconi Anemia: A Systematized Review. Blood Rev 2024, 101225. [CrossRef]
- Woo, A.Y.-H.; Jia, L. ALDH2 Mutations and Defense against Genotoxic Aldehydes in Cancer and Inherited Bone Marrow Failure Syndromes. Mutat Res 2024, 829, 111870. [CrossRef]
- Parsa, F.G.; Nobili, S.; Karimpour, M.; Aghdaei, H.A.; Nazemalhosseini-Mojarad, E.; Mini, E. Fanconi Anemia Pathway in Colorectal Cancer: A Novel Opportunity for Diagnosis, Prognosis and Therapy. J Pers Med 2022, 12. [CrossRef]
- Mehta, P.A.; Ebens, C. Fanconi Anemia. 1993.
- Gwon, G.H.; Kim, Y.; Liu, Y.; Watson, A.T.; Jo, A.; Etheridge, T.J.; Yuan, F.; Zhang, Y.; Kim, Y.; Carr, A.M.; et al. Crystal Structure of a Fanconi Anemia-Associated Nuclease Homolog Bound to 5’ Flap DNA: Basis of Interstrand Cross-Link Repair by FAN1. Genes Dev 2014, 28, 2276–2290. [CrossRef]
- Zhang, J.; Walter, J.C. Mechanism and Regulation of Incisions during DNA Interstrand Cross-Link Repair. DNA Repair (Amst) 2014, 19, 135–142. [CrossRef]
- Jin, H.; Roy, U.; Lee, G.; Scharer, O.D.; Cho, Y. Structural Mechanism of DNA Interstrand Cross-Link Unhooking by the Bacterial FAN1 Nuclease. J Biol Chem 2018, 293, 6482–6496. [CrossRef]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy Metal Poisoning: The Effects of Cadmium on the Kidney. Biometals 2010, 23, 783–792. [CrossRef]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy Metal Poisoning: The Effects of Cadmium on the Kidney. Biometals 2010, 23, 783–792. [CrossRef]
- Chakraborty, S.; Dutta, A.R.; Sural, S.; Gupta, D.; Sen, S. Ailing Bones and Failing Kidneys: A Case of Chronic Cadmium Toxicity. Ann Clin Biochem 2013, 50, 492–495. [CrossRef]
- Senthilnathan, S.; Nallusamy, G.; Varadaraj, P. An Interesting Case of Acquired Renal Fanconi Syndrome. Cureus 2024, 16, e65208. [CrossRef]
- Sheridan, R.; Mirabile, J.; Hafler, K. Determination of Six Illegal Antibiotics in Chicken Jerky Dog Treats. J Agric Food Chem 2014, 62, 3690–3696. [CrossRef]
- Pal, S.; Kim, J.Y.; Park, S.H.; Lim, H. Bin; Lee, K.-H.; Song, J.M. Quantitative Classification of DNA Damages Induced by Submicromolar Cadmium Using Oligonucleotide Chip Coupled with Lesion-Specific Endonuclease Digestion. Environ Sci Technol 2011, 45, 4460–4467. [CrossRef]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M.K. Cadmium Inhibits the Electron Transfer Chain and Induces Reactive Oxygen Species. Free Radic Biol Med 2004, 36, 1434–1443. [CrossRef]
- Thevenod, F.; Friedmann, J.M.; Katsen, A.D.; Hauser, I.A. Up-Regulation of Multidrug Resistance P-Glycoprotein via Nuclear Factor-KappaB Activation Protects Kidney Proximal Tubule Cells from Cadmium- and Reactive Oxygen Species-Induced Apoptosis. J Biol Chem 2000, 275, 1887–1896. [CrossRef]
- Tzirogiannis, K.N.; Panoutsopoulos, G.I.; Demonakou, M.D.; Hereti, R.I.; Alexandropoulou, K.N.; Basayannis, A.C.; Mykoniatis, M.G. Time-Course of Cadmium-Induced Acute Hepatotoxicity in the Rat Liver: The Role of Apoptosis. Arch Toxicol 2003, 77, 694–701. [CrossRef]
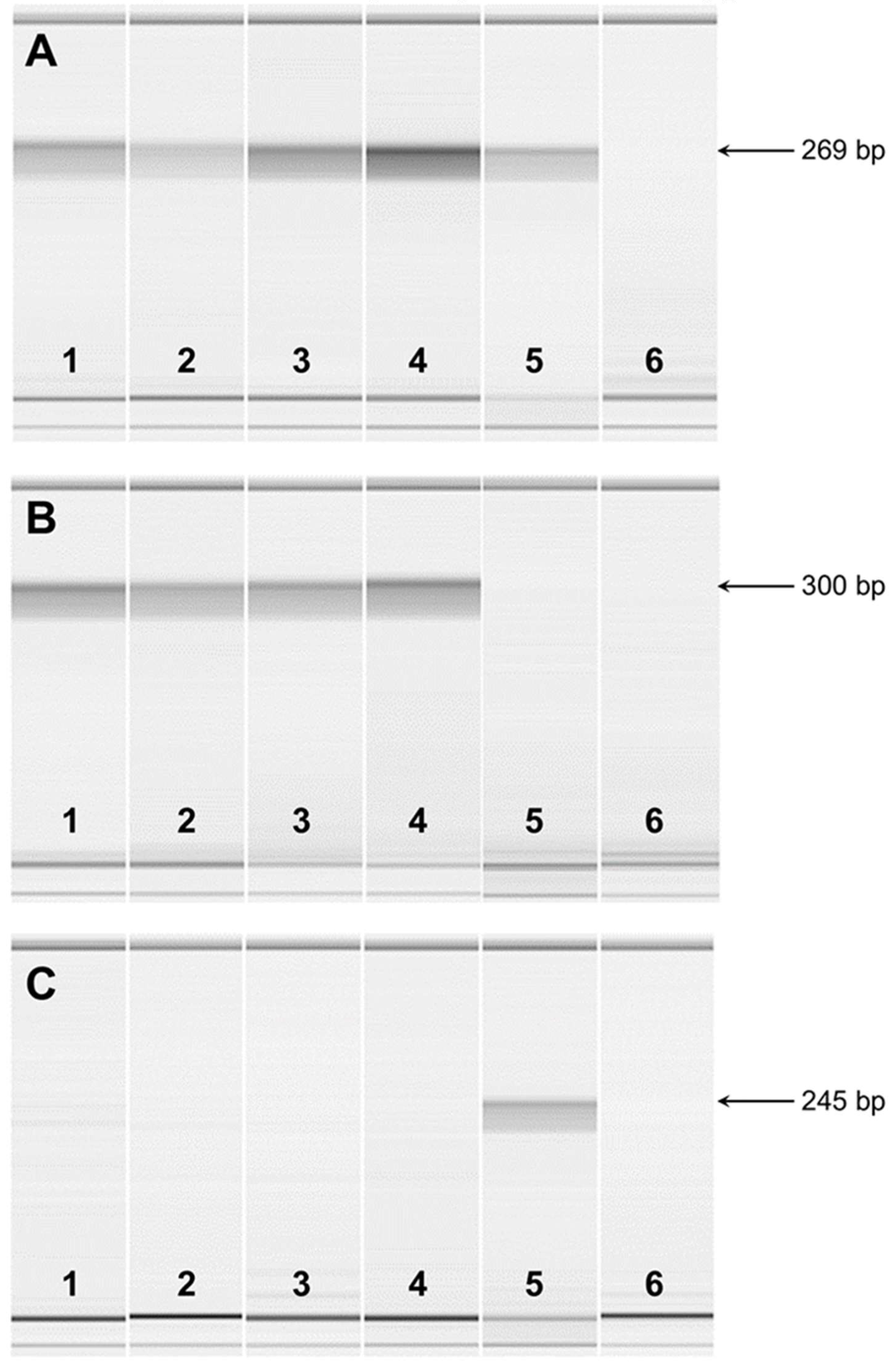
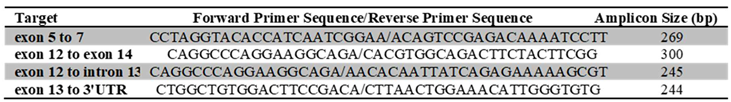 |
| Phenotype | Del/Del | Genotypes Del/Wt |
Wt/Wt | Total |
|---|---|---|---|---|
| FS affected | 32 | 0 | 0 | 32 |
| FS unaffected | 1 | 33 | 12 | 46 |
| Total | 33 | 33 | 12 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





