Introduction
Salivary gland tumors are relatively rare neoplasms, accounting for approximately 3-6% of all head and neck tumors [
1]. Among these, carcinoma of the parotid gland represents the most common malignant subtype, comprising approximately 70-80% of all salivary gland malignancies. While most cases of parotid carcinoma occur sporadically, certain risk factors have been identified, including exposure to ionizing radiation, viral infections (such as Epstein-Barr virus), and genetic predisposition [
1].
Histologically, parotid carcinomas are heterogeneous, encompassing various subtypes with distinct clinical behaviors and prognoses. The most common histological types include mucoepidermoid carcinoma, adenoid cystic carcinoma, acinic cell carcinoma, and carcinoma ex pleomorphic adenoma [
2].
Parotid gland tumors, though rare, require a tailored approach depending on the stage and extent of disease, whether localized or metastatic. Surgery remains the cornerstone for localized parotid malignancies. The primary objective is complete tumor resection with negative margins while preserving facial nerve function, although radical resection with nerve sacrifice may be necessary in advanced cases. For high-grade tumors or cases with positive margins, adjuvant radiotherapy (RT) significantly reduces local recurrence rates. The role of RT in treating locally advanced disease, particularly when lymph nodes are involved, is well supported, with intensity-modulated radiotherapy (IMRT) offering superior precision and sparing of adjacent critical structures [
1].
In the metastatic setting, treatment options are more limited, and the focus shifts to systemic therapies. For salivary duct carcinomas (SDC) or high-grade adenocarcinomas, platinum-based chemotherapy (e.g., cisplatin with 5-fluorouracil or paclitaxel) is commonly employed, although response rates remain modest. Hormonal therapies such as androgen receptor blockade (e.g., bicalutamide) have shown promise in SDCs expressing androgen receptors (AR) with reported meaningful clinical responses [
3]. In the era of Immunotherapy, Immune Checkpoint Inhibitors (ICI) are also being explored, with early-phase clinical trials evaluating the efficacy of PD-1/PD-L1 inhibitors, such as pembrolizumab, in advanced salivary gland tumors showing some activity in tumors with high PD-L1 expression.
In recent years, advances in molecular profiling have revealed actionable mutations in parotid tumors, opening avenues for targeted therapies. For example, the use of HER2-targeted therapies like trastuzumab and pertuzumab in HER2-positive SDCs has shown clinical activity [
4].
In this case report we present a patient with HER2 positive metastatic PGC treated with dual HER2 Blockade achieving a complete response.
Case Presentation
Mrs. Maria is a 60-year-old previously healthy patient. On physical examination, a palpable mass is noted in the region of the parotid gland. There are no apparent signs of other metastases on physical examination.
The patients underwent parotid gland biopsy which showed a high-grade adenocarcinoma (ki67 > 45%) solid-cystic apocrine cells, with neuroinvasive tubulo-trabecular pattern, arising in a sclerohyalinotic nodule including marginal residual foci of a plasmacytoid myoepithelial mesenchymal component (vimentin +++, GFAP +++, S100 +++, p63 +++), to an alcyanophilic basophilic myxoid stroma corresponding to an old pleomorphic adenoma of the right parotid gland. IIC: HER2 score 3+ in 100% of neoplastic cells, Androgen Receptors clone AR441-Dako= 3+ in 40% of neoplastic cells, CK34betaE12 +++, Estrogen Receptor=absent, Progesterone Receptor=absent.
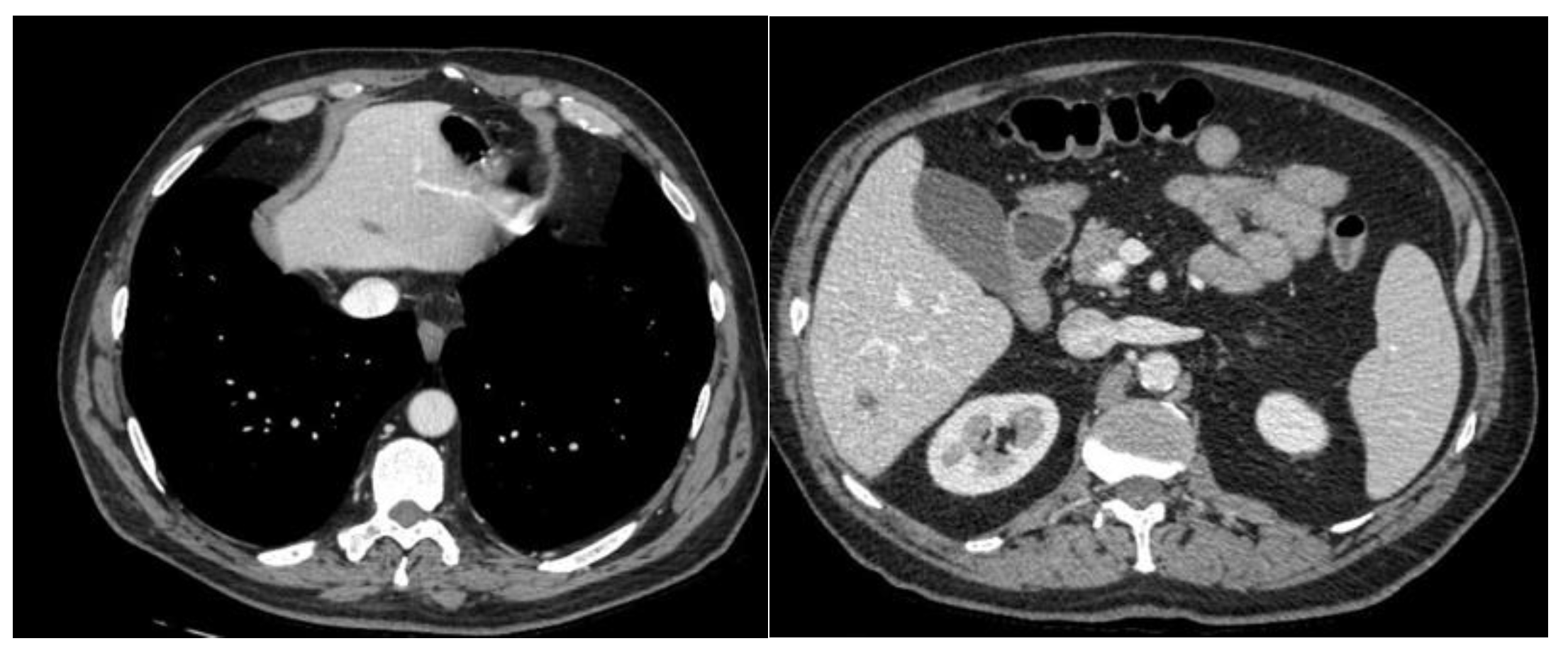
A CT scan was performed with evidence of Multiple hepatic and bilateral nodal metastases [
Figure 1].
She started a chemotherapy regimen combined with docetaxel, trastuzumab, and pertuzumab. She received nominal therapy with the following schedule:
- Docetaxel: 75 mg/m^2, administered intravenously every 3 weeks.
- Trastuzumab: 8 mg/kg loading dose, followed by 6 mg/kg every 3 weeks, administered intravenously.
- Pertuzumab: 840 mg loading dose, followed by 420 mg every 3 weeks, administered intravenously.
After four cycles of treatment, the patient experienced a near-complete radiological response of the disease, with a significant reduction in the size of hepatic and nodal metastases. [
Figure 2]
The oncology team will consider subsequent therapeutic options, which may include surgery, radiotherapy, or additional targeted therapies based on the patient's response to current treatment and overall condition.
We decided to undergo patient a lymph node dissection (level I-IV) with primitive tumor resection, meanwhile we performed radiosurgery on the residual liver metastases. Currently the patient continues treatment as maintenance, although well tolerated (PFS 12 mo).
Discussion
The management of metastatic parotid gland cancer, particularly involving hepatic and lymph node metastases, is complex and often relies on a multidisciplinary approach that integrates surgery and radiotherapy. Surgical resection remains a primary treatment option when metastases are isolated and resectable, especially in cases of oligometastatic disease, where complete surgical removal can lead to long-term disease control.
Actually, lateral neck lymph node dissection (LND) is a key surgical strategy of parathyroid carcinoma with lymph node metastases, although data on its safety, efficacy, recurrence, and systemic disease control remain limited due to the rarity of the disease. The main matter is potential risks of injury to the recurrent laryngeal nerve, hypoparathyroidism, and postoperative hematoma. Several case series, [
5,
6] have demonstrated that complication rates are relatively low when performed by experienced surgeons. The risk of postoperative hypocalcemia is present due to manipulation or removal of remaining parathyroid tissue, but this is often managed effectively with calcium and vitamin D supplementation.
However, LND can provide significant locoregional control, and in many patients, parathyroid hormone levels decrease following surgery.
Recurrence is common in parathyroid carcinoma, even after aggressive surgical intervention. In the largest retrospective series, recurrence rates after LND have been reported between 30-60%. According to a study by Obara et al. [
7], patients with nodal metastases had a significantly higher risk of recurrence, suggesting that LND may delay but not completely prevent recurrence. Recurrences often occur in the neck or mediastinum and may require further surgical intervention.
Despite aggressive surgery, systemic recurrence occurs in a significant proportion of patients and the objective response rate (ORR) after LND, in terms of complete or partial responses, is generally high for achieving local control in the neck. Various small studies suggest that locoregional control rates can exceed 70-80%, depending on the extent of metastasis and the surgical margins achieved. But the way, the long-term survival rates are poor, particularly for patients with distant metastases. The overall 5-year survival for patients undergoing LND has been reported to be around 50-70% but drops significantly when systemic metastases are present (1,7).
Moreover, hepatic metastases from parathyroid carcinoma are extremely rare, and the optimal treatment approach remains under investigation. Traditionally, surgical resection (hepatic metastasectomy) was considered the mainstay of treatment for isolated liver metastases, aiming for local control and potentially improved survival. However, recent advances in stereotactic body radiotherapy (SBRT) and radiosurgery have emerged as superior locoregional treatment options, offering effective control with fewer complications compared to surgery. Studies suggest that SBRT provides excellent local control rates in hepatic metastases from parathyroid carcinoma, with control rates exceeding 85-90% at 2 years, as documented by case series involving patients with liver metastases from rare endocrine malignancies, including parathyroid carcinoma [
8]. This high precision radiotherapy targets the metastatic lesion while sparing the surrounding liver tissue, reducing the risks associated with major liver surgery.
Hepatic metastases from parathyroid carcinoma are rare but pose significant therapeutic challenges. Traditionally, surgical resection has been considered for eligible patients with isolated liver metastases; however, emerging evidence supports the superiority of stereotactic body radiotherapy (SBRT) or radiosurgery (SRS) over surgery in terms of outcomes, particularly for patients who are not candidates for surgery due to tumor location or medical comorbidities.
Several studies have demonstrated that SBRT provides excellent local control with fewer complications compared to surgery. SBRT allows for high precision in targeting liver metastases while sparing surrounding healthy tissue, making it an attractive option for these patients. A case series by Silva et al. [
9] showed that SBRT achieved local control rates of over 80% for parathyroid carcinoma liver metastases, with minimal toxicity. This contrasts with surgical series, where recurrence rates following liver metastasectomy are typically higher (30-50%) due to the aggressive nature of the disease and potential incomplete resection.
In terms of outcomes, SBRT has been associated with improved progression-free survival (PFS) and overall survival (OS). Reports suggest that PFS can reach up to 18-24 months in patients treated with SBRT for liver metastases from parathyroid carcinoma, compared to approximately 12-18 months for patients undergoing surgery. The time to recurrence following SBRT is also generally longer, with recurrence-free intervals exceeding 12 months in many cases. Overall survival (OS) in patients treated with SBRT can vary, but several studies report median OS times of around 36-48 months depending on the extent of systemic disease at the time of treatment.
The dosages used in SBRT typically range from 30 to 50 Gy delivered in 3-5 fractions, which has been found to balance efficacy and safety, with minimal hepatic toxicity. A trial by Herfarth et al. demonstrated that fractionated doses of 40 Gy in 5 fractions achieved durable local control in patients with hepatic metastases while maintaining a low risk of toxicity. Moreover, SBRT can be repeated in some cases of local recurrence, offering additional control options without the risks associated with repeat surgeries. [
10]
In conclusion, the use of SBRT for hepatic metastases in parathyroid carcinoma offers superior outcomes in terms of local control, PFS, and OS compared to surgery. It is especially beneficial for patients ineligible for surgery and those with multiple or inoperable lesions [
7].
About the systemic treatment, the combination of docetaxel, pertuzumab, and trastuzumab has emerged as a promising therapeutic option for patients with HER2-positive metastatic parotid gland cancer, particularly salivary duct carcinoma (SDC), a histological subtype known for its aggressive behavior and frequent HER2 overexpression. This regimen, widely used in HER2-positive breast cancer, is being explored in salivary gland malignancies due to molecular similarities, with HER2 amplification found in up to 30% of SDC cases. Our patient had this mutational alteration so we used this promising combination therapy extensively studied in breast cancer. [
1]
Several case reports and small case series suggest that this combination can be used before surgery to downstage the tumor, improve resectability, and potentially delay progression. For instance, case reports from Takahashi et al. [
4] demonstrate that patients with HER2-positive metastatic parotid carcinoma responded well to the combination, showing significant tumor shrinkage that allowed for a more conservative surgical approach or even the avoidance of surgery in some cases. This strategy aims to reduce tumor burden, enhance surgical outcomes, and prolong disease-free intervals post-operatively.
ORR with this combination have been reported as high as 60-70% in small studies, with some patients achieving a partial or complete response.
PFS has been reported to range between 10 to 18 months depending on the extent of disease at the start of therapy. [
11]
OS for patients treated with this regimen can extend beyond 24 months, particularly in those with good initial responses. In contrast, those with poor responses to the combination tend to progress more rapidly and may have an OS closer to 12 months. [
11]
PFS2 (the time from the start of the second line of therapy until progression) is a newer concept being explored in these patients, though robust data on PFS2 for this regimen in parotid carcinoma is limited. Current reports suggest that patients who initially respond to trastuzumab and pertuzumab may maintain control with subsequent HER2-targeted therapies in later lines.
The efficacy of this regimen is being evaluated in several ongoing clinical trials. For example, NCT03666268 is a phase II trial assessing HER2-directed therapies in HER2-positive salivary gland tumors, including the use of docetaxel, trastuzumab, and pertuzumab. Future studies aim to evaluate novel combinations with immunotherapies (e.g., PD-1/PD-L1 inhibitors) to further enhance response rates and delay recurrence.s
The table below summarizes case reports and case series from the literature:
| STUDY/AUTHORS |
YEAR |
PATIENTS |
OUTCOME |
PFS |
PURPOSE |
| Li et al. 2021 |
2021 |
10 |
ORR 60%, SD IN 4/9 pts |
9 months |
Metastatic |
| Takahashi et al. |
2020 |
5 |
Tumor reduction in all patients |
12 months |
neoadjuvant |
| Ahn et al. |
2022 |
8 |
50% ORR, with 3 achieving CR |
10 months |
Metastatic |
| Chen et al. |
2023 |
6 |
Improved symptoms and tumor size reduction |
8 months |
Adjuvant |
| Hwang et al. |
2022 |
4 |
Significant shrinkage observed |
6 months |
Neoadjuvant |
| Park et al. |
2021 |
12 |
ORR of 75%, 6 patients with PR |
14 months |
Metastatic |
| Kato et al. |
2022 |
7 |
Prolonged disease control |
11 months |
metastatic |
In summary, the docetaxel, trastuzumab, and pertuzumab regimen holds promise for patients with HER2-positive metastatic parotid cancer, particularly in the neoadjuvant setting to improve surgical outcomes. Ongoing trials and case reports highlight the efficacy of this approach, with significant responses and prolonged survival outcomes. Further research is needed to optimize sequencing and explore combination therapies in this rare but aggressive cancer.
Conclusion
This case report highlights the clinical course and management of metastatic carcinoma of the parotid gland. Despite the challenges posed by advanced disease, multimodal treatment approaches incorporating chemotherapy and targeted therapy can achieve favorable outcomes in selected patients.
The range of action of these drugs is similar to that of the most studied HER 2 positive tumors, such as breast cancer.
Interestingly, in cases of progression, patients who initially receive docetaxel, pertuzumab, and trastuzumab may be considered for alternative HER2-targeted therapies such as trastuzumab emtansine (T-DM1) or lapatinib. The exact sequence of therapy remains under investigation, but these options have shown activity in small cohorts of patients with HER2-positive metastatic SDC.
As the understanding of HER2-positive metastatic parotid gland tumors, particularly salivary duct carcinoma (SDC), evolves, the use of novel HER2-targeted therapies like trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan is gaining traction. T-DM1, an antibody-drug conjugate (ADC) combining trastuzumab with the cytotoxic agent emtansine, has shown promising results in breast cancer and is being evaluated for efficacy in HER2-positive salivary gland malignancies. Early clinical data suggest that T-DM1 may lead to substantial tumor reduction and prolonged progression-free survival (PFS) in patients with metastatic parotid tumors who have previously received trastuzumab and pertuzumab. A case series reported by Li et al. [2021] demonstrated an ORR of 60% in patients treated with T-DM1, with a median PFS of 9 months and overall survival (OS) extending to 18 months in responders.
The future landscape of treatment for HER2-positive metastatic parotid tumors will likely involve combinations of these novel agents with existing therapies, with ongoing trials exploring optimal sequencing and combination strategies. For instance, NCT04566374 is a phase II trial evaluating the efficacy of trastuzumab deruxtecan in patients with advanced HER2-positive solid tumors, including salivary gland cancers. As clinical evidence accumulates, T-DM1 and trastuzumab deruxtecan may become integral components of a comprehensive treatment plan, providing new hope for patients facing the challenges of metastatic parotid tumors.
Ongoing clinical trials continue to investigate novel therapies. Notably, the NCT03366116 trial is evaluating androgen receptor-targeting agents in recurrent/metastatic AR-positive salivary gland cancers, while NCT03666268 is assessing the efficacy of pembrolizumab in metastatic salivary gland carcinomas. Future directions include combination approaches using targeted therapies, immunotherapy, and chemotherapy, aiming to improve outcomes in this challenging malignancy.
References
- Salivary gland cancer: ESMO–European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up†van Herpen, C. et al. ESMO Open, Volume 7, Issue 6, 100602.
- Steuer, C.E.; Hanna, G.J.; Viswanathan, K.; Bates, J.E.; Kaka, A.S.; Schmitt, N.C.; Ho, A.L.; Saba, N.F. The evolving landscape of salivary gland tumors. CA: A Cancer J. Clin. 2023, 73, 597–619. [CrossRef]
- Dalin M, Watson P, Ho A and Morris L: Androgen Receptor Signaling in Salivary Gland Cancer. Cancers 9: 17, 2017.
- Takahashi H, Tada Y, Saotome T, et al. "Trastuzumab and Pertuzumab in HER2-positive salivary duct carcinoma." Annals of Oncology, 2019.
- Sawhney S, Vaish R, Jain S, Mittal N, Ankathi SK, Thiagarajan S and Chaukar D: Parathyroid Carcinoma: a Review. Indian Journal of Surgical Oncology 13: 133, 2021.
- Alberti, A.; Smussi, D.; Zamparini, M.; Turla, A.; Laini, L.; Marchiselli, C.; Grisanti, S.; Bossi, P.; Berruti, A. Treatment and outcome of metastatic parathyroid carcinoma: A systematic review and pooled analysis of published cases. Front. Oncol. 2022, 12, 997009. [CrossRef]
- Obara, T.; Fujimoto, Y. Diagnosis and treatment of patients with parathyroid carcinoma: An update and review. World J. Surg. 1991, 15, 738–744. [CrossRef]
- Goodman KA and Kavanagh BD: Stereotactic Body Radiotherapy for Liver Metastases. Semin Radiat Oncol 27: 240–246, 2017.
- da Silva, L.P.; Serpa, M.S.; Viveiros, S.K.; Sena, D.A.C.; Pinho, R.F.d.C.; Guimarães, L.D.d.A.; Andrade, E.S.d.S.; Pereira, J.R.D.; da Silveira, M.M.F.; Sobral, A.P.V.; et al. Salivary gland tumors in a Brazilian population: A 20-year retrospective and multicentric study of 2292 cases. J. Cranio-Maxillofacial Surg. 2018, 46, 2227–2233. [CrossRef]
- Mahadevan, A.; Blanck, O.; Lanciano, R.; Peddada, A.; Sundararaman, S.; D’ambrosio, D.; Sharma, S.; Perry, D.; Kolker, J.; Davis, J. Stereotactic Body Radiotherapy (SBRT) for liver metastasis – clinical outcomes from the international multi-institutional RSSearch® Patient Registry. Radiat. Oncol. 2018, 13, 1–11. [CrossRef]
- Kurzrock R, Bowles DW, Kang H, et al.: Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: results from MyPathway, a phase IIa multiple basket study. Ann Oncol 31: 412–421, 2020.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).