Submitted:
25 October 2024
Posted:
25 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Results and Discussion
3.1. Association of Adverse Childhood Experiences with Central Sensitisation
3.2. Association of Adverse Childhood Experiences with Connectome
3.3. Parts of the Connectome Implicated in Prenatal and Childhood Trauma
3.3.1. The Cerebrum
3.3.2. The Limbic System
3.4. Mechanisms of Connectome Alteration
3.5. Sexual dimorphism
3.6. Sex Assigned at Birth and the Limbic System
3.7. Sex and Adverse Childhood Experiences
5. Limitations
6. Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Achenbach, J. , Rhein, M., Gombert, S., Meyer-Bockenkamp, F., Buhck, M., Eberhardt, M., Leffler, A., Frieling, H., & Karst, M. (2019). Childhood traumatization is associated with differences in TRPA1 promoter methylation in female patients with multisomatoform disorder with pain as the leading bodily symptom. Clinical epigenetics, 11(1), 126. [CrossRef]
- Antoniou, G. , Lambourg, E., Steele, J. D., & Colvin, L. A. (2023). The effect of adverse childhood experiences on chronic pain and major depression in adulthood: a systematic review and meta-analysis. British journal of anaesthesia, 130(6), 729–746. [CrossRef]
- Arout, C. A. , Sofuoglu, M., Bastian, L. A., & Rosenheck, R. A. (2018). Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. Journal o’ women's health (2002), 27(8), 1035–1044. [CrossRef]
- Banihashemi, L. , Peng, C. W., Verstynen, T., Wallace, M. L., Lamont, D. N., Alkhars, H. M., Yeh, F. C., Beeney, J. E., Aizenstein, H. J., & Germain, A. (2021). Opposing relationships of childhood threat and deprivation with stria terminalis white matter. Human brain mapping, 42(8), 2445–2460. [CrossRef]
- Bath K., G. (2020). Synthesizing Views to Understand Sex Differences in Response to Early Life Adversity. Trends in neurosciences, 43(5), 300–310. [CrossRef]
- Blasi, V. , Pirastru, A., Cabinio, M., Di Tella, S., Laganà, M. M., Giangiacomo, A., Baglio, G., Zanette, M., Canevini, M. P., Walder, M., Clerici, M., & Baglio, F. (2020). Early Life Adversities and Borderline Intellectual Functioning Negatively Impact Limbic System Connectivity in Childhood: A Connectomics-Based Study. Frontiers in psychiatry, 11, 497116. [CrossRef]
- Cabañero, D. , Villalba-Riquelme, E., Fernández-Ballester, G., Fernández-Carvajal, A., & Ferrer-Montiel, A. (2022). ThermoTRP channels in pain sexual dimorphism: new insights for drug intervention. Pharmacology & therapeutics, 240, 108297. [CrossRef]
- Camilleri, M. (2020). Sex as a biological variable in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society, 32(7), e13802. [CrossRef]
- Čeko, M. , Frangos, E., Gracely, J., Richards, E., Wang, B., Schweinhardt, P., & Catherine Bushnell, M. (2020). Default mode network changes in fibromyalgia patients are largely dependent on current clinical pain. NeuroImage, 216, 1168. [CrossRef]
- Chandan, J. S. , Keerthy, D., Zemedikun, D. T., Okoth, K., Gokhale, K. M., Raza, K., Bandyopadhyay, S., Taylor, J., & Nirantharakumar, K. (2020). The association between exposure to childhood maltreatment and the subsequent development of functional somatic and visceral pain syndromes. EClinicalMedicine, 23, 100392. [CrossRef]
- Conversano, C. , Ciacchini, R., Orrù, G., Bazzichi, M. L., Gemignani, A., & Miniati, M. (2021). Gender differences on psychological factors in fibromyalgia: a systematic review on the male experience. Clinical and experimental rheumatology, 39 Suppl 130(3), 174–185. [CrossRef]
- Davis, E. P. , & Pfaff, D. (2014). Sexually dimorphic responses to early adversity: implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology, 49, 11–25. [CrossRef]
- de Kruijf, M. , Bos, D., Huygen, F. J., Niessen, W. J., Tiemeier, H., Hofman, A., Uitterlinden, A. G., Vernooij, M. W., Ikram, M. A., & van Meurs, J. B. (2016). Structural Brain Alterations in Community Dwelling Individuals with Chronic Joint Pain. AJNR. American journal of neuroradiology, 37(3), 430–438. [CrossRef]
- Di Pietro, F. , Lee, B. A. ( 41(13), 3781–3793. [CrossRef]
- Dionisio, S. , Mayoglou, L., Cho, S. M., Prime, D., Flanigan, P. M., Lega, B., Mosher, J., Leahy, R., Gonzalez-Martinez, J., & Nair, D. (2019). Connectivity of the human insula: A cortico-cortical evoked potential (CCEP) study. Cortex; a journal devoted to the study of the nervous system and behavior, 120, 419–442. [CrossRef]
- Doménech-García, V. , Peirotén, A. R., Imaz, M. L., Palsson, T. S., Herrero, P., & Bellosta-López, P. (2022). Not just sensitization: sympathetic mechanisms contribute to expand experimental referred pain. The Korean journal of pain, 35(3), 240–249. [CrossRef]
- Ehrlich, K. B. , Miller, G. E., Rogosch, F. A., & Cicchetti, D. (2021). Maltreatment exposure across childhood and low-grade inflammation: Considerations of exposure type, timing, and sex differences. Developmental psychobiology, 63(3), 529–537. [CrossRef]
- Enokido, M. , Suzuki, A. ( 14, 277. [CrossRef] [PubMed]
- Erpelding, N. , Sava, S., Simons, L. E., Lebel, A., Serrano, P., Becerra, L., & Borsook, D. (2014). Habenula functional resting-state connectivity in pediatric CRPS. Journal of neurophysiology, 111(2), 239–247. [CrossRef]
- Erpelding, N. , Simons, L., Lebel, A., Serrano, P., Pielech, M., Prabhu, S., Becerra, L., & Borsook, D. (2016). Rapid treatment-induced brain changes in pediatric CRPS. Brain structure & function, 221(2), 1095–1111. [CrossRef]
- Fischer, S. , Markert, C., Strahler, J., Doerr, J. M., Skoluda, N., Kappert, M., & Nater, U. M. (2018). Thyroid Functioning and Fatigue in Women With Functional Somatic Syn–romes - Role of Early Life Adversity. Frontiers in physiology, 9, 564. [CrossRef]
- Gallo, E. A. G. , De Mola, C. L., Wehrmeister, F., Gonçalves, H., Kieling, C., & Murray, J. (2017). Childhood maltreatment preceding depressive disorder at age 18 years: A prospective Brazilian birth cohort study. Journal of affective disorders, 217, 218–224. [CrossRef]
- Ganguly, P. , & Brenhouse, H. C. (2015). Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental cognitive neuroscience, 11, 18–30. [CrossRef]
- Hanson, J. L. , Knodt, A. R., Brigidi, B. D., & Hariri, A. R. (2018). Heightened connectivity between the ventral striatum and medial prefrontal cortex as a biomarker for stress-related psychopathology: understanding interactive effects of early and more recent stress. Psychological medicine, 48(11), 1835–1843. [CrossRef]
- Häuser, W. , Hoffmann, E. M., Wolfe, F., Worthing, A. B., Stahl, N., Rothenberg, R., & Walitt, B. (2015). Self-reported childhood maltreatment, lifelong traumatic events and mental disorders in fibromyalgia syndrome: a comparison of US and German outpatients. Clinical and experimental rheumatology, 33(1 Suppl 88), S86–S92.
- Hellou, R. , Häuser, W., Brenner, I., Buskila, D., Jacob, G., Elkayam, O., Aloush, V., & Ablin, J. N. (2017). Self-Reported Childhood Maltreatment and Traumatic Events among Israeli Patients Suffering from Fibromyalgia and Rheumatoid Arthritis. Pain research & management, 2017, 3865249. [CrossRef]
- Henao-Pérez, M. , López-Medina, D. C., Arboleda, A., Bedoya Monsalve, S., & Zea, J. A. (2022). Patients With Fibromyalgia, Depression, and/or Anxiety and Sex Differences. American journal’of men's health, 16(4), 15579883221110351. [CrossRef]
- Herringa, R. J. , Birn, R. J. ( 110(47), 19119–19124. [CrossRef]
- Ho, T. C. , Dennis, E. L., Thompson, P. M., & Gotlib, I. H. (2018). Network-based approaches to examining stress in the adolescent brain. Neurobiology of stress, 8, 147–157. [CrossRef]
- Hruschak, V. , Flowers, K. M., Azizoddin, D. R., Jamison, R. N., Edwards, R. R., & Schreiber, K. L. (2021). Cross-sectional study of psychosocial and pain-related variables among patients with chronic pain during a time of social distancing imposed by the coronavirus disease 2019 pandemic. Pain, 162(2), 619–9. [CrossRef]
- Jedd, K. , Hunt, R. H., Cicchetti, D., Hunt, E., Cowell, R. A., Rogosch, F. A., Toth, S. L., & Thomas, K. M. (2015). Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Development and psychopathology, 27(4 Pt 2), 1577–1589. [CrossRef]
- Jiang’, L. , D'Souza, R. S., Oh, T., Vincent, A., Mohabbat, A. B., Ashmore, Z., Mauck, W. D., Ge, L., Whipple, M. O., McAllister, S. J., Wang, Z., & Qu, W. (2020). Sex-Related Differences in Symptoms and Psychosocial Outcomes in Patients With Fibromyalgia: A Prospective Questionnaire Study. Mayo Clinic proceedings. Innovations, quality & outcomes, 4(6), 767–774. [CrossRef]
- Kodila, Z. N. , Shultz, S. R., Yamakawa, G. R., & Mychasiuk, R. (2023). Critical Windows: Exploring the Association Between Perinatal Trauma, Epigenetics, and Chronic Pain. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry, 10738584231176233. Advance online publication. [CrossRef]
- Larson, A. A. , Pardo, J. D. ( 30(6), 544–555. [CrossRef]
- Li, L. , Di, X., Zhang, H., Huang, G., Zhang, L., Liang, Z., & Zhang, Z. (2022). Characterization of whole-brain task-modulated functional connectivity in response to nociceptive pain: A multisensory comparison study. Human brain mapping, 43(3), 1061–1075. [CrossRef]
- Liu, J. , Liu, H., Mu, J., Xu, Q., Chen, T., Dun, W., Yang, J., Tian, J., Hu, L., & Zhang, M. (2017). Altered white matter microarchitecture in the cingulum bundle in women with primary dysmenorrhea: A tract-based analysis study. Human brain mapping, 38(9), 4430–4443. [CrossRef]
- Lu, X. W. , Guo, H., Sun, J. R., Dong, Q. L., Zhao, F. T., Liao, X. H., Zhang, L., Zhang, Y., Li, W. H., Li, Z. X., Liu, T. B., He, Y., Xia, M. R., & Li, L. J. (2018). A shared effect of paroxetine treatment on gray matter volume in depressive patients with and without childhood maltreatment: A voxel-based morphometry study. CNS neuroscience & therapeutics, 24(11), 1073–1083. [CrossRef]
- Lurie D., I. (2018). An Integrative Approach to Neuroinflammation in Psychiatric disorders and Neuropathic Pain. Journal of experimental neuroscience, 12, 1179069518793639. [CrossRef]
- Malfliet, A. , De Pauw, R., Kregel, J., Coppieters, I., Meeus, M., Roussel, N., Danneels, L., Cagnie, B., & Nijs, J. (2019). Gender Differences in the Association of Brain Gray Matter and Pain-Related Psychosocial Characteristics. Pain physician, 22(3), E191–E203.
- Mansour, A. , Baria, A. T., Tetreault, P., Vachon-Presseau, E., Chang, P. C., Huang, L., Apkarian, A. V., & Baliki, M. N. (2016). Global disruption of degree rank order: a hallmark of chronic pain. Scientific reports, 6, 34853. [CrossRef]
- Martinez-Torteya, C. , Muzik, M. L. ( 57(3), 356–364. [CrossRef]
- McQuaid, R. J. , Gabrys, R. L., McInnis, O. A., Anisman, H., & Matheson, K. (2019). Understanding the Relation Between Early-Life Adversity and Depression Symptoms: The Moderating Role of Sex and an Interleukin-1β Gene Variant. Frontiers in psychiatry, 10, 151. [CrossRef]
- Mejía-Terrazas, G. E. , López-Muñoz, E., Hidalgo-Bravo, A., Santamaria-Olmedo, M. G., & Valdés-Flores, M. (2022). Association between CACNG2 polymorphisms (rs4820242, rs2284015 and rs2284017) and chronic peripheral neuropathic pain risk in a Mexican population. European review for medical and pharmacological sciences, 26(12), 4354–4366. [CrossRef]
- Melikoglu, M. A. , & Celik, A. (2017). Does Neuropathic Pain Affect the Quality of Sleep?. The Eurasian journal of medicine, 49(1), 40–43. [CrossRef]
- Merrick, M. T. , Ford, D. C., Ports, K. A., & Guinn, A. S. (2018). Prevalence of Adverse Childhood Experiences From the 2011-2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA pediatrics, 172(11), 1038–1044. [CrossRef]
- Meyers, J. L. , Lowe, S. R., Eaton, N. R., Krueger, R., Grant, B. F., & Hasin, D. (2015). Childhood maltreatment, 9/11 exposure, and latent dimensions of psychopathology: A test of stress sensitization. Journal of psychiatric research, 68, 337–345. [CrossRef]
- Morton, P. M. , & Ferraro, K. F. ( 61(4), 503–522. [CrossRef]
- Nakua, E. K. , Otupiri, E., Dzomeku, V. M., Owusu-Dabo, E., Agyei-Baffour, P., Yawson, A. E., Folson, G., & Hewlett, S. (2015). Gender disparities of chronic musculoskeletal disorder burden in the elderly Ghanaian population: study on global ageing and adult health (SAGE WAVE 1). BMC musculoskeletal disorders, 16, 204. [CrossRef]
- Negrón-Blanco, L. , de Pedro-Cuesta, J., Almazán, J., Rodríguez-Blázquez, C., Franco, E., Damián, J., & DISCAP-ARAGON Research Group (2016). Prevalence of and factors associated with homebound status among adults in urban and rural Spanish populations. BMC public health, 16, 574. [CrossRef]
- Neville, S. J. , Clauw, A. E. ( 34(10), 909–917. [CrossRef]
- Nugent, A. C. , Farmer, C., Evans, J. W., Snider, S. L., Banerjee, D., & Zarate, C. A., Jr (2019). Multimodal imaging reveals a complex pattern of dysfunction in corticolimbic pathways in major depressive disorder. Human brain mapping, 40(13), 3940–3950. [CrossRef]
- Osborne, N. R. , Anastakis, D. J., Kim, J. A., El-Sayed, R., Cheng, J. C., Rogachov, A., Hemington, K. S., Bosma, R. L., Fauchon, C., & Davis, K. D. (2021). Sex-Specific Abnormalities and Treatment-Related Plasticity of Subgenual Anterior Cingulate Cortex Functional Connectivity in Chronic Pain. Frontiers in pain research (Lausanne, Switzerland), 2, 673538. [CrossRef]
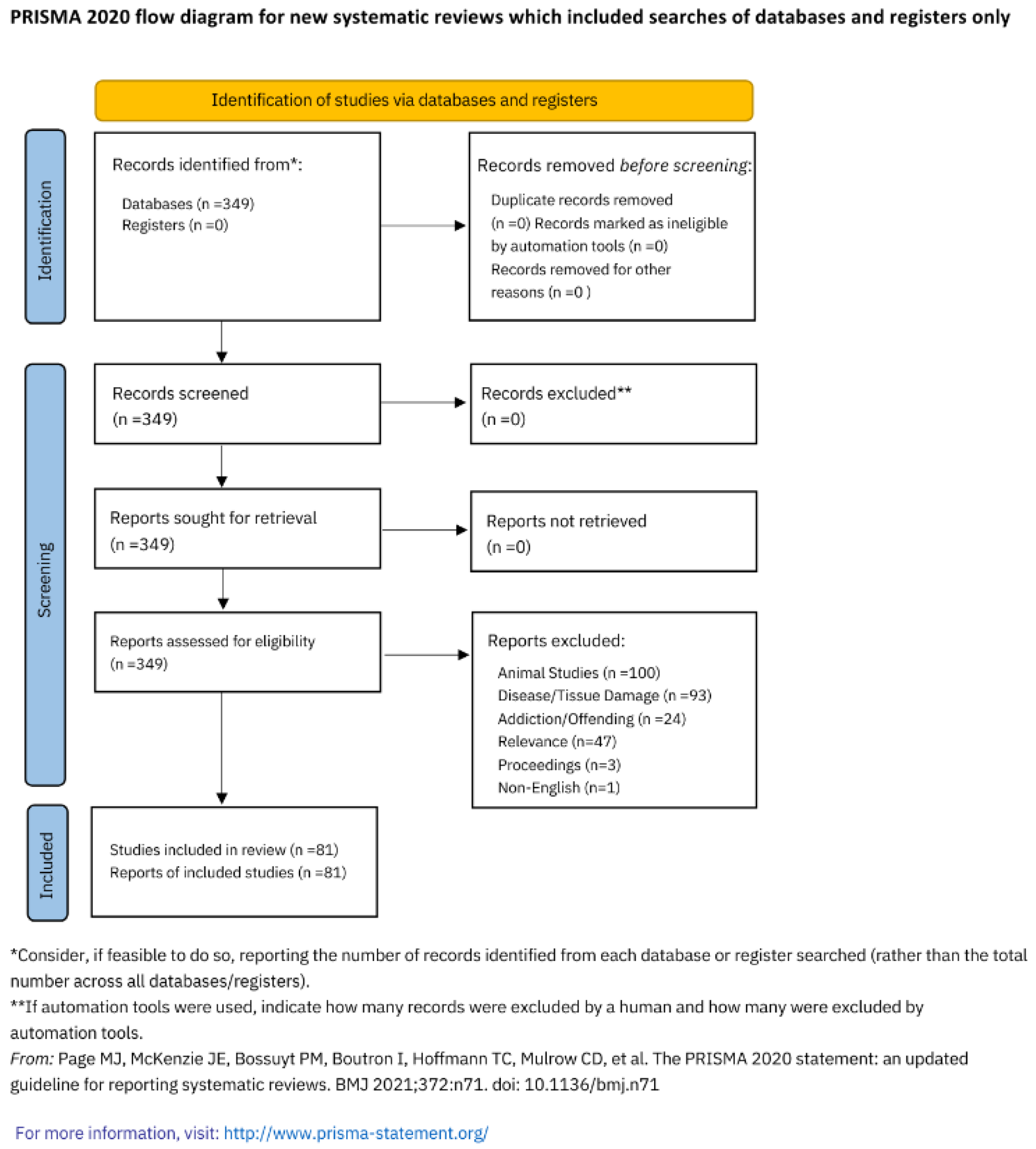
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [CrossRef]
- Paredes-Echeverri, S. , Guthrie, A. J., & Perez, D. L. (2022). Toward a possible trauma subtype of functional neurological disorder: Impact on symptom severity and physical health. Frontiers in psychiatry, 13, 1040911. [CrossRef]
- Petersen, M. W. , Schröder, A., Jørgensen, T., Ørnbøl, E., Meinertz Dantoft, T., Eliasen, M., Benros, M. E., & Fink, P. (2020). Irritable bowel, chronic widespread pain, chronic fatigue and related syndromes are prevalent and highly overlapping in the general population: DanFunD. Scientific reports, 10(1), 3273. [CrossRef]
- Puetz, V. B. , Parker, D., Kohn, N., Dahmen, B., Verma, R., & Konrad, K. (2017). Altered brain network integrity after childhood maltreatment: A structural connectomic DTI-study. Human brain mapping, 38(2), 855–868. [CrossRef]
- Radcliff, E. , Crouch, E., & Strompolis, M. (2018). Rural-urban differences in exposure to adverse childhood experiences among South Carolina adults. Rural and remote health, 18(1), 4434. [CrossRef]
- Ruschak, I. , Montesó-Curto, P., Rosselló, L., Aguilar Martín, C., Sánchez-Montesó, L., & Toussaint, L. (2023). Fibromyalgia Syndrome Pain in Men and Women: A Scoping Review. Healthcare (Basel, Switzerland), 11(2), 223. [CrossRef]
- Samplin, E. , Ikuta, T., Malhotra, A. K., Szeszko, P. R., & Derosse, P. (2013). Sex differences in resilience to childhood maltreatment: effects of trauma history on hippocampal volume, general cognition and subclinical psychosis in healthy adults. Journal of psychiatric research, 47(9), 1174–1179. [CrossRef]
- Sandman, C. A. , Glynn, L. M., & Davis, E. P. (2013). Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. Journal of psychosomatic research, 75(4), 327–335. [CrossRef]
- Segura-Jiménez, V. , Estévez-López, F., Soriano-Maldonado, A., Álvarez-Gallardo, I. C., Delgado-Fernández, M., Ruiz, J. R., & Aparicio, V. A. (2016). Gender Differences in Symptoms, Health-Related Quality of Life, Sleep Quality, Mental Health, Cognitive Performance, Pain-Cognition, and Positive Health in Spanish Fibromyalgia Individuals: The Al-Ándalus Project. Pain research & management, 2016, 5135176. [CrossRef]
- Shalev, I. , Moffitt, T. E., Braithwaite, A. W., Danese, A., Fleming, N. I., Goldman-Mellor, S., Harrington, H. L., Houts, R. M., Israel, S., Poulton, R., Robertson, S. P., Sugden, K., Williams, B., & Caspi, A. (2014). Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Molecular psychiatry, 19(11), 1163–1170. [CrossRef]
- Slapšinskaitė, A. , Hristovski, R., Razon, S., Balagué, N., & Tenenbaum, G. (2017). Metastable Pain-Attention Dynamics during Incremental Exhaustive Exercise. Frontiers in psychology, 7, 2054. [CrossRef]
- Slavich, G. M. , & Sacher, J. (2019). Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology, 236(10), 3063–3079. [CrossRef]
- Smith, M. T. , Jr, Remeniuk, B., Finan, P. H., Speed, T. J., Tompkins, D. A., Robinson, M., Gonzalez, K., Bjurstrom, M. F., & Irwin, M. R. (2019). Sex differences in measures of central sensitization and pain sensitivity to experimental sleep disruption: implications for sex differences in chronic pain. Sleep, 42(2), zsy209. [CrossRef]
- Sokołowski, A. , Folkierska-Żukowska, M., Jednoróg, K., Moodie, C. A., & Dragan, W. Ł. (2020). The relationship between early and recent life stress and emotional expression processing: A functional connectivity study. Cognitive, affective & behavioral neuroscience, 20(3), 588–603. [CrossRef]
- Söreskog, E. , Jacobson, T., Kirketeig, T., Fritzell, P., Karlsten, R., Zethraeus, N., & Borgström, F. (2023). Impact of spinal cord stimulation on sick leave and disability pension in patients with chronic neuropathic pain: a real-world evidence study in Sweden. Pain, 164(3), 666–673. [CrossRef]
- Staud, R. , Boissoneault, J., Lai, S., Mejia, M. S., Ramanlal, R., Godfrey, M. M., & Stroman, P. W. (2021). Spinal cord neural activity of patients with fibromyalgia and healthy controls during temporal summation of pain: an fMRI study. Journal of neurophysiology, 126(3), 946–956. [CrossRef]
- Tan, A. C. , Jaaniste, T., & Champion, D. (2019). Chronic Widespread Pain and Fibromyalgia Syndrome: Life-Course Risk Markers in Young People. Pain research & management, 2019, 6584753. [CrossRef]
- Taylor, R. L. , Cooper, S. M. ( 3(11), e2023774. [CrossRef]
- Toriyama, T. , Horiuchi, T., & Hongo, K. (2017). Characterization of migraineurs presenting interictal widespread pressure hyperalgesia identified using a tender point count: a cross-sectional study. The journal of headache and pain, 18(1), 117. [CrossRef]
- Ujhelyi Nagy, A. , Kuritár Szabó, I. ( 16(6), 1048. [CrossRef]
- Vagaska, E. , Litavcova, A., Srotova, I., Vlckova, E., Kerkovsky, M., Jarkovsky, J., Bednarik, J., & Adamova, B. (2019). Do lumbar magnetic resonance imaging changes predict neuropathic pain in patients with chronic non-specific low back pain?. Medicine, 98(17), e15377. [CrossRef]
- Wang, L. , Dai, Z., Peng, H., Tan, L., Ding, Y., He, Z., Zhang, Y., Xia, M., Li, Z., Li, W., Cai, Y., Lu, S., Liao, M., Zhang, L., Wu, W., He, Y., & Li, L. (2014). Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Human brain mapping, 35(4), 1154–1166. [CrossRef]
- Watanabe, K. , Watanabe, M. ( 12, 744561. [CrossRef] [PubMed]
- Weimer, M. B. , Macey, T. A., Nicolaidis, C., Dobscha, S. K., Duckart, J. P., & Morasco, B. J. (2013). Sex differences in the medical care of VA patients with chronic non-cancer pain. Pain medicine (Malden, Mass.), 14(12), 1839–1847. [CrossRef]
- Yeung, E. W. , Davis, M. C., & Ciaramitaro, M. C. (2016). Cortisol Profile Mediates the Relation Between Childhood Neglect and Pain and Emotional Symptoms among Patients with Fibromyalgia. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine, 50(1), 87–97. [CrossRef]
- Younis, S. , Hougaard, A., Noseda, R., & Ashina, M. (2019). Current understanding of thalamic structure and function in migraine. Cephalalgia : an international journal of headache, 39(13), 1675–1682. [CrossRef]
- Zhang, H. , Lian, Y., Xie, N., Cheng, X., Chen, C., Xu, H., & Zheng, Y. (2019). Factors affecting the therapeutic effect of botulinum toxin A on trigeminal neuralgia: A follow-up retrospective study of 152 patients. Experimental and therapeutic medicine, 18(5), 3375–3382. [CrossRef]
- Zhang, J. , Zhao, T., Zhang, J., Zhang, Z., Li, H., Cheng, B., Pang, Y., Wu, H., & Wang, J. (2022). Prediction of childhood maltreatment and subtypes with personalized functional connectome of large-scale brain networks. Human brain mapping, 43(15), 4710–4721. [CrossRef]
- Zhou, Q. , Li, M., Fan, Q., Chen, F., Jiang, G., Wang, T., He, Q., Fu, S., Yin, Y., Lin, J., & Yan, J. (2022). Cerebral perfusion alterations in patients with trigeminal neuralgia as measured by pseudo-continuous arterial spin labeling. Frontiers in neuroscience, 16, 1065411. [CrossRef]
| Authors | Facts |
|---|---|
| Arout et al., 2018 (3) | 77,087 of 2,216,621 veterans with pain diagnoses were diagnosed with fibromyalgia and that they were three times more likely to be female (25.5% compared to 7.7%) and more likely to have multiple psychiatric comorbidities. In addition, females diagnosed with fibromyalgia were likely to be younger and more likely to have headaches, connective tissue diseases, and psychiatric comorbidities, while older males had more comorbid medical conditions. This study showed that there is a gender discrepancy between the types of chronic pain associated with females and males, with females having a higher probability of chronic pain secondary to conditions such as fibromyalgia. |
| Camilleri, 2020 (8) | A review looking for differences between sexes genders in IBS, the brain-gut axis and sex hormones, epidemiology, pain perception, colonic transit, abdominal distension, overlapped with urogynecological conditions, psychological issues, anorexia, fibromyalgia, serotonin, and responsiveness to treatment of IBS. The authors found that in Western countries, the female to male ratio of IBS is 2:1 with the prevalence of 14.5% of females and 7.7% of males in the U.S. householder Survey. |
| Conversano et al., 2021 (11) | A systemic review found that females with fibromyalgia tend to seek out medical care earlier than males with fibromyalgia. |
| Henao-Pérez et al., 2022 | 1,106 cases of fibromyalgia were reviewed with 295 females and 811 males, but 42.6% of males suffered from depression and anxiety. The results also showed a relationship between sex (female PR = 0.5 [0.28–0.86]) and low socioeconomic strata (PR = 0.53 [0.33–0.70]) remained constant. This study concluded that there was a significant relationship between sex, whereby females were less likely to experience depression and/or anxiety than males with fibromyalgia. |
| Jiang et al., 2020 (32) | A questionnaire study with 668 patients with fibromyalgia (606 females), found a significant correlation between being of female sex and having a greater trigger point count. However, there were no sex-related differences in demographic characteristics, depression, anxiety, sleep problems, fibromyalgia symptom severity, cognitive dysfunction, and quality of life. |
| Melikoglu & Celik, 2017 (44) | 80% of 70 individuals suffering from neuropathic pain presented poor quality of sleep (significantly higher scores of sleep latency, sleep duration, sleep efficiency, and daytime dysfunction). The Pittsburg Sleep Quality Index total was observed in patients with neuropathic pain compared to controls with factors including female sex, pain intensity, being factors related to having poor quality of sleep in patients with neuropathic pain. These findings suggest that females suffering from neuropathic pain will have a higher probability of impaired sleep. |
| Nakua et al., 2015 (48) | A sampling study stratified by population and age with a structured questionnaire found that the prevalence of both chronic back pain and chronic joint pain from arthritis was significantly higher in females compared to males. Females with primary education had a prevalence of chronic back pain of 36.2% (95% CI; 29.2, 43.3) and chronic arthritis/joint pain a prevalence of 15.8% (95% CI; 11.1, 20.6), while males had prevalence rates of 29.0% (95% CI; 23.4, 34.5) and 9.8% (95% CI; 6.4, 13.2) respectively. These findings support the hypothesis that there is the existence of sex differences between females and males in the prevalence of chronic pain. |
| Petersen et al., 2020 (55); | A stratified sample of 1590 participants with 943 females and 647 males found that there was a prevalence of having at least one functional somatic syndrome, including irritable bowel syndrome, fibromyalgia/chronic widespread pain, chronic fatigue syndrome, whiplash associated disorders, and multiple chemical sensitivity was 9.3% (95% CI: 8.1–10.6), with all functional somatic syndromes more prevalent in females. |
| Ruschak et al., 2023 (58) | A scoping review concluded that the subjective perception and widespread pain are higher in females compared to males but that males would typically have more significant pathology, more painful experiences, and more catastrophic thoughts regarding their pain. Their findings support the hypothesis that there is a significant difference in pain among the sexes and that females and males differ in their responses to pain, with both a greater sensitivity to pain and a higher risk of clinical pain more often observed among females. |
| Söreskog et al., 2023 (67) | A large retrospective observational cohort study with 480 males and 602 females treated with spinal cord stimulation found that the number of disability days varied considerably depending on age, sex, socioeconomic variables, and comorbidities. Male sex was associated with less net disability days compared with female sex. |
| Toriyama et al., 2017 (71) | A study of 176 episodic migraineurs compared to 132 age- and sex-matched controls found that risk factors associated with interictal widespread pressure hyperalgesia were female gender, younger age at migraine onset, higher frequency of migraine attacks, severe headache impact, cutaneous allodynia, and depression. |
| Vagaska et al., 2019 (73) | MRIs of the lumbar spine in 21 females and 21 males with chronic non-specific lower back pain were examined to evaluate for correlation with pain intensity and a predictor to neuropathic pain. While the study found no correlation between the severity of degenerative changes and pain, there were 2 independent predictors of neuropathic pain, including being female with an odds ratio of 11.9 and having a pain intensity of ≥4.5 in the previous 4 weeks with an odds ratio of 13.1. These findings support that females with chronic pain have a higher susceptibility to developing neuropathic pain regardless of tissue injury. |
| Weimer et al., 2013 (76) | 17,583 veteran patients (1,945 female and 15,638 male) with moderate to severe chronic non-cancer pain were retrospectively. Females were more often diagnosed with two or more chronic pain conditions, including fibromyalgia, low back pain, inflammatory bowel disease, migraine headache, neck or joint pain, and arthritis (67% females vs 56% males). This study concludes that there is significant evidence that there is a sex difference present in chronic pain in female veterans compared to male veterans, regardless of diagnostic factors. |
| Mechanism | |
| Larson et al., 2014 (34) | A systemic review showed that females are less likely than males to recruit brown adipose tissue adaptations in response to chronic stress, correlating with a reduced body temperature, lower metabolic rates, and reduced circulating cortisol and corticosterone in response to stress; all hallmarks of fibromyalgia. |
| de Kruijf et al., 2016 (13) | Brain volumes measured in 3892 subjects found decreased total GMV in females with chronic pain specifically in the temporal lobe, frontal lobe, and hippocampus in females with no statistical differences observed in males. |
| Neville et al., 2018 (50) | Males endure pain longer and have higher rates of anxiety and depression. In a study of 129 patients (68 females and 61 males) with osteoarthritis of the knee, 3.8% met the criteria of fibromyalgia, whereby females and males differed significantly in nearly every outcome, including fibromyalgia severity, clinical pain, anxiety, depression, and pressure pain sensitivity. In females, fibromyalgia scores significantly correlated with pressure pain sensitivity but not conditioned pain modulation or temporal summation, such that increased sensitivity was associated with greater fibromyalgia severity at all body sites examined. Additionally, as fibromyalgia scores increased, the association between pain sensitivity at the surgical knee and that at remote body sites also increased. No relationship between fibromyalgia score and quantitative sensory testing was observed in males. |
| Malfliet et al. 2019 (39) | An MRI based study correlating brain grey matter morphology with self-reported psychosocial characteristics in 32 males and 62 females suffering from chronic spinal pain with perceived consequences, emotional representations, chronicity, and pain catastrophising. Males showed larger associations of the precuneus cortex, the precentral gyrus, and the insula with perceived personal control and kinesiophobia. This study supports the findings that different grey matter morphological changes relate differently to psychosocial characteristics in females compared to males. |
| Doménech-García et al., 2022 (16) | A RCT-based study found that there was a greater sympathetic vasomotor response contributing to expanding pressure-induced referral pain in females at multiple locations in the upper extremities, including the shoulder, arm, and forearm. These findings support the hypothesis that females are more susceptible contributors of central sensitisation compared to males. |
| Mejía-Terrazas et al., 2022 (43) | Mutations in the voltage-dependent calcium channel gamma-2 subunit gene (CACNG2) were associated with neuronal hyperexcitability, including neuropathic pain. The authors concluded that certain alleles and genotypes could constitute severity markers in chronic peripheral neuropathic pain with a sex-biased effect. |
| Author | Observations / Findings |
|---|---|
| Bath, 2020 (5), | This review highlights the importance of studying sex as a biological variable and understanding sex differences in response to ELA. It examines the historical and unfounded exclusion of female subjects from studies, chromosomal and hormonal effects on gene expression that contribute to sex selective effects on neurodevelopment and sex disparities in early postnatal care, where females may receive more significant levels of abuse. In contrast, males receive higher levels of maternal contact. |
| Davis & Pfaff, 2014 (12) | A review of 12 papers illustrating the neurodevelopmental consequences of foetal exposure to stress and stress hormones for males and females concluded that males have higher mortality, increased ASD rates, and females exhibit increased affective disorders, often unmasked during hormonal events. |
| Enokido et al., 2014 (18) | 581 unrelated Japanese healthy subjects. Perceived parental care was assessed together with the leukocyte relative telomere length to determine the ratio of telomere/single copy gene.???A multiple regression analyses showed shorter telomere length in males was related to lower scores of paternal care, while that in females was related to lower scores of maternal care. |
| Erlich et al., 2021 (17) | Examined links between child maltreatment and low-grade inflammation in adulthood in a sample of 155 low-income children (ages 8-12), half of whom had been exposed to maltreatment. Blood samples from children assessed C-reactive protein and cytokines, which were used to form a composite of low-grade inflammation. Analyses suggested that maltreatment exposure was associated with higher inflammation for females but not males. Females with exposures before the age of five had the highest low-grade inflammation and females who were exposed to two or more forms of maltreatment had higher inflammation compared to females who were not maltreated and had higher inflammation compared to girls who experienced one form of maltreatment. Males’ inflammation scores did not significantly differ as a function of the number of types of maltreatment they experienced. |
| Gallo et al., 2017 (22) | Participants in a population-based, birth cohort study in Pelotas, Brazil (N=3715) self-reported exposure to maltreatment (emotional abuse, physical neglect, physical abuse, sexual abuse, domestic violence) in confidential questionnaires at age 15 years, were assessed for major depression in interviews at age 18 years. Females exposed to emotional abuse and domestic violence were at increased risk for depression after adjustment for confounders and other types of maltreatment. Females exposed to two or more forms of maltreatment were at particularly high risk for depression compared with females not exposed to maltreatment. In adjusted analyses, maltreatment was not associated with depression for males. However, for both sexes, exposure to multiple forms of maltreatment (two or more types of maltreatment) increased risk of major depression. |
| Ganguly & Brenhouse, 2015 (23) | A review examines how early life adversity (ELA) has been associated with various psychopathologies and how sex differences contribute. A history of ELA was shown to increase the risk of developing a psychiatric disorder in adulthood. Female adolescents exposed to ELA expressed higher levels of IL-6 that forecasted higher cortisol and depression 6 months later. In females but not in males, increased HPA activity during childhood was shown to predict lower functional connectivity between the amygdala and PFC and the amygdala and hippocampus. Microglial colonization of the brain occurs much earlier in males than in females in the parietal cortex, hippocampus, and amygdala, which may contribute to distinct windows of neuroimmune vulnerability between males and females. Therefore, the sexes might be impacted by ELA in a gender specific manner, with females more vulnerable to early neuroendocrine-induced changes in corticolimbic circuitry and males more vulnerable to later neuroinflammation, possibly through microglial sensitization. |
| Martinez-Torteya et al., 2015 (41) | Changes in infant cortisol levels from 7 to 16 months of age and the effects of known correlates of HPA axis activity, including sex, temperament, maternal psychopathology, maternal parenting, and demographic factors were examined. The authors used validated, age-appropriate stress induction tasks with a sample of infants whose mothers were (74%) or were not (26%) victims of maltreatment during their childhood. Participants were 167 mother–infant dyads (56% male infants) drawn from a larger longitudinal study of stress during childbearing years (n = 269). Infants did not show a cortisol response to psychosocial stress at 7 months but displayed reactivity at 16 months. Infant sex at 7 months significantly moderated the changes in cortisol secretion patterns. Females baseline cortisol levels declined more than males from 7 to 16 months, possibly reflecting differential maturation of the HPA axis and is consistent with previous findings of enhanced behavioral regulation in toddler females relative to males. |
| McQuaid et al., 2019 (42) | Examined the moderating role of three independent cytokine single nucleotide polymorphisms (SNPs; IL-1β rs16944, IL-6 rs1800795 SNP, TNF-α rs1800629) in the relationship between ELA and depressive symptoms, and whether these relationships were influenced by sex. The original sample comprised 925 Carleton University first year students, 343 females and 132 males (age range 17–35 yrs). The relation between childhood adversity and depressive symptoms was moderated by the IL-1β SNP. Among females, ELA was accompanied by elevated depressive symptoms irrespective of the IL-1β SNP, but among males, this relationship was pronounced for those carrying the GG genotype of the IL-1β SNP. Genetic variations of IL-1β functioning are related to depressive symptomatology associated with ELA and this may vary among males and females. |
| Merrick et al., 2018 (45) | Data were collected through the Behavioral Risk Factor Surveillance System. Of the 214 157 respondents included in the sample (51.51% female), 61.55% had at least 1 and 24.64% reported 3 or more Adverse Childhood Experiences (ACEs). Compared with male respondents, female respondents reported a greater prevalence of child sexual abuse (16.33% vs 6.70%), household substance abuse (28.72% vs 26.33%), and household mental illness (19.19% vs 13.71%). |
| Morton & Ferraro, 2020 (47); | This longitudinal study investigates whether childhood exposures influence adult chronic inflammation and mortality risk via adult health characteristics and socioeconomic status and whether gender moderates these relationships. A sample of 9,310 males and females over age 50 was analyzed and found that childhood socioeconomic status, parental behaviors, and adolescent behaviors were associated with adult chronic inflammation via health characteristics and socioeconomic status in adulthood which subsequently raised mortality risk. Gender moderated the mediating influence of childhood socioeconomic status via unhealthy behaviors and parental behaviors via adult socioeconomic status. Notably, females generally had higher levels of inflammation but lower mortality risk. |
| Slavich & Sacher, 2019 (64) | This review found depression is strongly predicted by early life stress and comorbid with anxiety disorders and certain physical disease conditions, including chronic pain. Excesses in maternal glucocorticoids and abnormalities in immunologic activity have been found to have sex-dependent effects on foetal brain circuits that regulate mood, autonomic activity, blood pressure, and metabolism, resulting in recurrent major depressive disorder across the lifespan characterised by autonomic dysfunction, dysregulated immunologic stress reactivity, and cardiometabolic dysregulation later in life. Research suggests that ovarian hormone fluctuations modulate female susceptibility to stress, brain structure and function, and inflammatory activity and reactivity. |
| Samplin et al., 2013 (59) | The study investigated the association of childhood maltreatment to hippocampal volumes in 67 Caucasian healthy adults (30 males, 37 females, 36.94±14.77 yrs) were assessed for a history of childhood emotional abuse, emotional neglect and physical abuse and received high resolution structural MR imaging scans. Total hippocampal volume, general cognitive ability and subclinical psychopathology were measure and compared. A significant correlational exists between overall childhood trauma score and hippocampus volumes. A positive history of emotional abuse was significantly associated with total hippocampal volume in males but not in females. When the left and right hippocampus were separately assessed, the interaction between emotional abuse and sex was only significant for the left hippocampus.???The study suggests that while females may be more resilient to the neurological effects of childhood maltreatment, they are not more resilient to the psychiatric symptoms associated with childhood maltreatment. |
| Sandman et al., 2013 (60) | A longitudinal prospective study evaluated the evidence for sex differences in foetal programming, assessed by summarising previously published sex difference findings (6 studies) and new analyses of previously published findings in which sex differences were not reported (6 studies). Maternal cortisol was evaluated in 125 mother-infant pairs, and infant mental and motor development was assessed at 12 months of age using the Bayley Scales of Infant Development. Data revealed that negative association between prenatal maternal cortisol and 12 month mental development was significant only among male infants, suggesting that males are more susceptible to the effects of early maternal cortisol on developmental delays during infancy. However, females also are influenced by exposure to early adversity. Female foetal exposure to psychobiological stress selectively influences fear/anxiety which persists into preadolescence. Male exposure to early adversity is associated with increased mortality, effectively culling the weak and creating a surviving cohort of the fittest. Females adjust to early adversity but with increased risk for anxiety and affective problems. |
| Shalev et al., 2014 (62) | A longitudinal study that tested the association between the persistence of internalizing disorders and leukocyte telomere length (LTL) in the prospective-longitudinal Dunedin Study (N=1037). Analyses showed that the persistence of internalizing disorders across repeated assessments from ages 11 to 38 years predicted shorter LTL at age 38 years in a dose-response manner, specifically in males. Additional analyses using DNA from blood collected at ages 26 and 38 years showed that LTL erosion was accelerated among males who were diagnosed with internalizing disorder in the interim. No significant associations were found among females in any analysis, highlighting potential sex differences in internalizing-related telomere biology. |
| Ujhelyi et al., 2021 (72) | Carried out a representative survey research in Hungary and in Central–Eastern Europe to assess the prevalence of adverse childhood experiences (ACEs) among adults. 1200 persons aged from 18 years to 112 years (37.65% of the respondents were male). 25% (n = 293) of adults reported any childhood adversity; 5% (n = 59) of them had four or more ACEs. There were no significant gender differences regarding the co-occurrence of ACEs, however among females, emotional abuse and physical abuse were more prevalent (7% (n = 51) for emotional abuse and 6% (n = 44) for physical abuse) than among males (4% (n = 18) for physical abuse and 3% (n = 13) for emotional abuse). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





