1. Introduction
Over the last two decades, mortality related to obstetric complications, namely haemorrhage and infection has steadily declined whereas deaths from non-obstetric pathologies, particularly cardiac diseases has remained stable despite surgical advances in correcting congenital heart disease, antenatal diagnosis of cardiac abnormalities and optimization of medical treatments.[
1]
Overall, cardiac diseases complicate 1 to 4% of pregnancies, with higher rates in low and middle-income countries.[
2] In Western countries, up to 33% of maternal death are attributed to cardiovascular disorders, namely decompensation of preexisting cardiac disorders, uncontrolled hypertension, pulmonary embolism, stroke, aortic dissection or peripartum cardiomyopathy (PPCM).[
2] Among women of Afro-Caribbean descent, the burden of sickle cell anaemia (SCA), - an autosomal recessive blood disorder -, increases the risk of maternal and foetal death as a result of anaemia, thrombosis and acute vaso-occlusive crisis that develop in various organs, including the heart and the lungs.[
3]
Cardiogenic shock (CS) is the most extreme cardiovascular condition in the peripartum period. Occasionally, mechanical circulatory support (MCS) with an intra-aortic balloon pump, ventricular assist device, or veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is required as a bridge to functional recovery or cardiac transplantation.[
4]
Herein, we describe the case of a young woman with SCA who presented acute cardiorespiratory distress following caesarean section that required VA-ECMO support and transfer by helicopter to a referral cardiac intensive care unit (ICU).
2. Case Report
A 24-old year Caribbean woman with a history of SCA and current pregnancy (G2P1; 36 weeks) was admitted to the University Hospital of Guadeloupe in Pointe à Pitre for acute pain on her back and legs. At the age of five, she was diagnosed with sickle cell trait (SCT) that was clinically expressed by recurrent acute chest syndromes and vaso-occlusive crisis requiring blood exchange transfusion. Shortly after her admission, she developed fever with normal vital parameters and no signs of fetal distress. Laboratory testing revealed elevated cardiac troponin-T ([cTp-T], 1.450 ng/ml), low hemoglobin ([Hb] 9.0 g/dl with a mean corpuscular volume 92 fL), and 9% of reticulocytes), 30% of the variant Hb S and low haptoglobin level (52 mcg/ml); the peripheral blood smear demonstrated severe anisopoikilocytes with large numbers of elongated sickle cells. The transthoracic echocardiogram (TTE) showed a normal functioning heart. Given clinical and biological evidence of acute red cell sickling, the patient underwent an emergent caesarean section under general anaesthesia with minimal blood loss (400 ml) and delivery of a healthy boy (Apgar score of 8 at 1 min and 9 at 5 min).
Shortly after surgery, she presented shortness of breath associated with diffuse bilateral pulmonary crackles and oxygen desaturation (oxygen pulsed saturation [SpO2] of 92% on room air) that required admission in the ICU and initiation of non-invasive ventilation with high levels of fractional inspired oxygen (FIO2). Fluorescence-based quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) of a throat swab was tested positive for coronavirus disease 19 (COVID-19) while a multiplex PCR respiratory panel (FilmArray®) was negative for other infectious agents. Chest computed tomography scan demonstrated bilateral areas of ground-glass opacities with no signs of thromboembolism, that supported the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-Cov 2). Due to severe hypoxemia (arterial oxygen partial pressure [PaO2] of 55 mmHg with 50% FIO2), orotracheal intubation was carried out and mechanical ventilation was initiated under full sedation and muscle paralysis. Empiric antibiotic therapy was started (ceftriaxone and amikacin) and exchange red blood cell transfusion was conducted with three units of packed red blood cells. Although oxygenation improved (PaO2/FIO2 ratio 150), the hemodynamic condition deteriorated progressively (mean arterial pressure of 55 mmHg, heart rate 120 beats/min), cTp-T further increased (8450 ng/ml) and the ECG showed peaking T wave with slight ST segment depression. The TTE demonstrated severe global hypokinesia with a left ventricular ejection fraction (LVEF) of 15%, tricuspid annular peak systolic excursion (TAPSE) of 8 mm, velocity time integral in the LV outflow tract of 7 cm and slight enlargement of cardiac cavities. Administration of dobutamine (5-10 mcg/kg/min) and norepinephrine (0.3-0.6 mcg/kg/min) did not produce favorable clinical and echocardiographic changes while serum creatinine and arterial lactate levels were rising (151 mg/dl and 3.5 mmole/L, respectively), central venous oxygen saturation (ScvO2) dropped from 70% to 61% and veno-arterial difference in partial pressure of carbon dioxide (CO2 gap) widened from 6 to 12 mmHg. This case of cardiogenic shock (stage D of the Society of Cardiovascular Angiographic and Interventions [SCAI] classification) was discussed with surgical and ICU colleagues at the University Hospital of Martinique (UHM) and the decision was taken to provide urgent extracorporeal cardiopulmonary support. Two hours later, the ECMO mobile team took-off from the UHM, arrived at the ICU in Pointe à Pitre and inserted heparin-bounded cannulas in the right femoral artery and vein using the Seldinger technique in this critically-ill woman. A catheter was placed in the right femoral superficial artery to preserve distal limb perfusion and unfractionated heparin infusion was started to target an activated clotting time between 150-180 sec. The Cardiohelp® pump (Maquet, Rastatt, Germany) was set at a flow rate of 3.5 L/min with a sweep gas flow of 3.0 L/min (FIO2 of 1.0) while tidal volume on the ventilator was decreased from 6 to 4 ml/kg ideal body weight and positive end-expiratory pressure (PEEP) was increased from 6 to 9 cm H20.
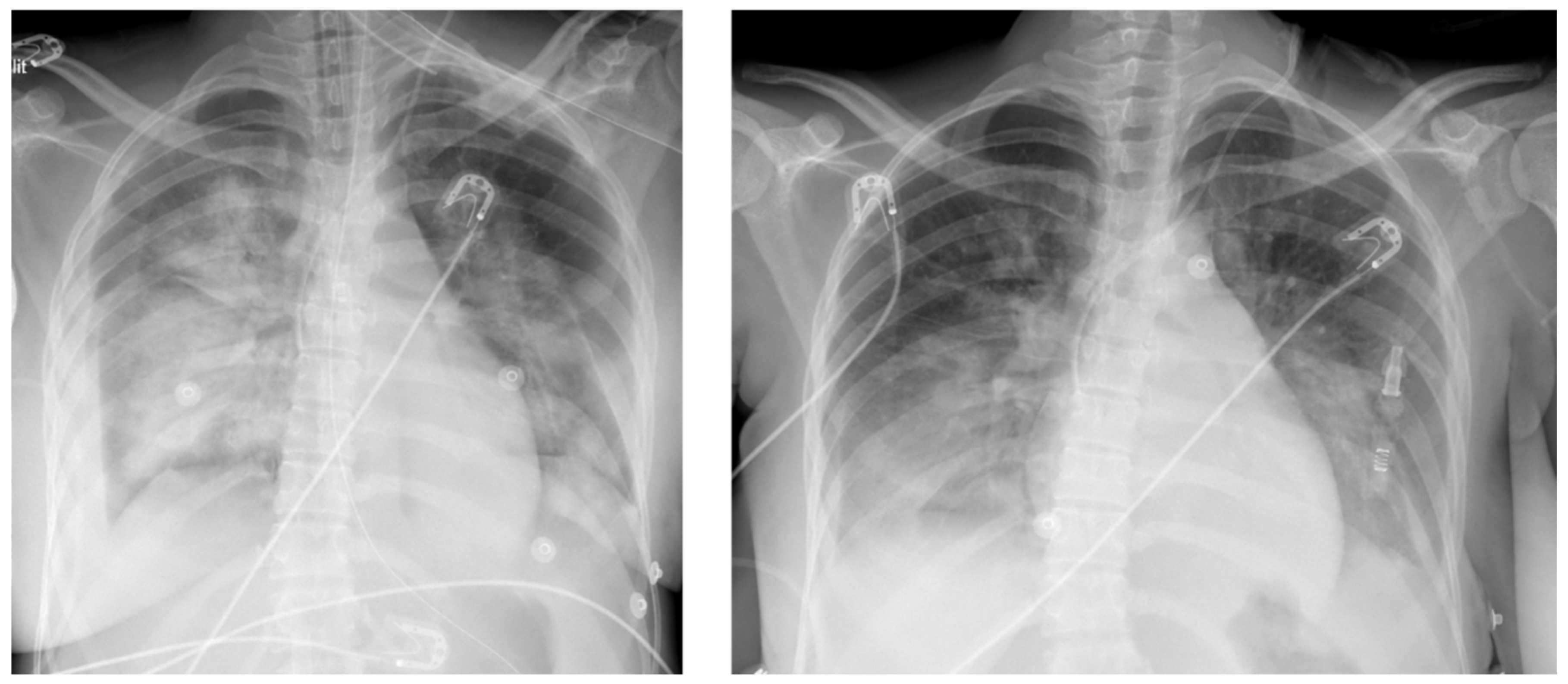
After 1-hour cardiopulmonary stabilization, the patient was transferred by helicopter to the cardiac ICU in Fort-de-France (195 kms, 48 min) under protective mechanical ventilation and VA-ECMO support. After her admission, chest X Rays showed persistent bilateral infiltrates with air bronchogram (
Figure 1A), dobutamine infusion was stopped and norepinephrine was reduced to 0.2 mcg/kg/min while the ECG ischemic changes disappeared with no indication to vent the left cavities given appropriate arterial differential pressure (>35 mmHg) and no signs of pulmonary congestion. Two days after ECMO implantation, TTE showed partial recovery of both right and left ventricular function and the flow rate of the ECMO was reduced to 2 L/min to promote left ventricular contraction and forward blood flow through the aortic valve. Biological signs of inflammation had regressed and cultures from both blood and broncho-alveolar lavage fluids were all negative that allowed discontinuation of antibiotics. There were no signs of ongoing hemolysis. On the 3
d day after implantation, pump flow was gradually reduced and after a successful ECMO weaning trial, the femoral cannulas were removed. On the 4th day, the trachea was extubated as the patient was able to breath spontaneously with low levels of inspiratory pressure support and 30% FIO
2. Two days later, chest X-Rays showed partial resolution of lung edema with residual areas of consolidation (
Figure 1B) while TTE demonstrated improvement in LVEF and TAPSE (55% and 15 mm, respectively) with normalization of the LV end-diastolic dimension (40 mm). Hence, the patient was discharged from the cardiac ICU in Martinique and transferred back to the obstetric department in Guadeloupe on oral medical treatment (aspirin 75 mg, bisoprolol 2.5 mg, spironolactone 25 mg, bromocryptine 2.5 mg and rivaroxaban 10 mg).
3. Discussion
This is the first report of a patient with SCT who develops acute cardiorespiratory failure following caesarean section requiring emergent mechanical ventilation, VA-ECMO support and airflight transportation. We hypothesize that SARS-Cov-2 infection in late pregnancy was associated with severe sickle cell crisis involving the coronary microvasculature that led to acute heart failure.
3.1. CS in Pregnant Woman and Treatment with VA-ECMO
Owing to major physiological changes associated with pregnancy (i.e., increased blood volume and cardiac output, prothrombotic status), acute heart failure with CS is more likely to occur in women with preexisting heart diseases, preeclampsia, various forms of cardiomyopathies (i.e., familial, arrhythmogenic, left ventricular noncompaction) or, as a result of amniotic fluid embolism or spontaneous coronary dissection.[
5] When the aforementioned heart disorders are excluded, the diagnosis of peripartum cardiomyopathy (PPCM) is retained with its multifactorial aetiologies including genetic mutations, viral myocarditis, nutritional deficiencies and autoimmune disorders.[
6] During pregnancy, PPCM is the most common cause of acute heart failure with a higher incidence in sub-Saharian Africa and Caribbean islands than in Western countries (1 in 300 births versus 1 in 1000-4000).[
7]
Using the SCAI classification of the severity of CS, in-hospital mortality rate increases from 16% to 62% across stages C, D and E.[
8] Analysis of the US Nationwide Readmission database from 2010 to 2020 (N=39’790’772 deliveries), showed that CS occurred in 776 parturients (40% PPCM), with 18.8% mortality (compared with 0.02% without CS).[
8,
9] Over the last decades, a three-fold increase in CS incidence was associated with a 25% increased incidence of preexisting heart diseases among pregnant women (e.g., congenital cardiac abnormalities and acquired valvular diseases).
In the 2022 guidelines from the American Heart Association/American College of Cardiology/Heart Failure Society of America, treatment with any MCS devices (intra-aortic balloon pump [IABP], ventricular assist device [VAD] or VA ECMO) is advocated in patients with advanced heart failure and hemodynamic compromise (class IIa).[
10] These recommendations are largely based on the analysis of patients with heart failure following cardiac surgery, acute myocardial infarct, cardiac arrest, myocarditis or sepsis. Despite improvement of circulatory flow with MCS, short-term outcome is relatively poor. A meta-analysis of 32 cohort studies including patients with CS (N=12’756), reported 62% in-hospital mortality along with high incidences of major complications, namely acute renal failure (50%), bleeding (48.5%), multiple organ dysfunction (24.4%), or stroke (12.5%).[
11]
Data regarding pregnant women with CS are relatively scarce. However, better outcome has been reported after MCS application that could be attributed to their younger age, fewer comorbidities and less severe associated organ dysfunction. [
12,
13,
14] In the last analysis of the US National Inpatient Sample from 2016 to 2020 (N=19’524’846), VA-ECMO was applied in 4’455 women with CS, including 125 (2.8%) pregnant women who demonstrated better survival (71%) and a lower incidence of acute renal failure and sepsis (6.5%).[
15] Likewise, in the last summary of the international registry of the Extracorporeal Life Support Organization (ELSO; N=56’210), 277 pregnant women have been treated with VA-ECMO (N=128), VV ECMO (N=113) or a hybrid mode (N=36) for refractory CS, thromboembolism, amniotic fluid embolism and respiratory failure.[
14] The survival rate of 70% in pregnant woman (65.6% for VA ECMO, 74.1% for VV-ECMO) was higher than in non-pregnant adults with respiratory or cardiac failure (59% and 42%, respectively).[
16] In our patient, the SAVE (Survival After Veno-arterial ECMO) score that was developed from the ELSO registry (N=3’846), provided a mortality risk of 25%.[
17]
So far, given the limited number of pregnant women with CS, the optimal use of MCS for this sub-population cannot be properly addressed. Therefore, dedicated goal-directed medical treatment protocols and techniques using the new generation of canula and pumps should be applied to reverse circulatory failure and reduce the occurrence of adverse events in women with peripartum CS.
3.2. Sickle Cell Anaemia
SCA arises from a missense mutation in the
HBB gene encoding the β-globin subunit of haemoglobin. Accordingly, substitution of glutamic acid (hydrophilic) for valine (hydrophobic) in the 6th position of the β-chain produces a less soluble form of haemoglobin (HbS) than the normal adult form of Hb (HbA). Under regional hypoxic conditions, red blood cells undergo morphological changes due to HbS polymerization resulting in shortened life span, increased blood viscosity, arteriolar vasoconstriction and endothelial damage that promote low microvascular flow and vascular occlusion.[
18]
The homozygous (Hb-SS) and the heterozygotous forms (Hb-SS and HbSA or SCT, respectively) of SCA affect 1 in 400 to 600 and 1 in 12 to 14 Afro-American and Caribbean people, respectively.[
19] In most cases, SCT disease is clinically milder than Hb-SS disease but is also associated with reduced survival and poor quality of life owing to a higher prevalence of congestive heart failure, stroke, thromboembolism, kidney disease, leg ulcers and pulmonary lesions.[
20] Contrasting with the general asymptomatic presentation of SCT carriers, recent clinical studies and case series suggest that these SCT carriers also suffer from severe vaso-occlusive crisis triggered by stressful events that required hospitalizations and blood exchange therapy as witnessed in the present case. From a population-based cohort of 8.8 million births, 1210 pregnant women exhibited acute crisis, 89.8% of them were diagnosed with Hb-SS and 3.14% with SCT.[
21] The occurrence of vaso-occlusive events was associated with a higher risk of maternal mortality (odds ratio [OR] of 15.8 and 95% confidence interval [CI] of 1.8 to 135.6) and cardiomyopathy (OR of 13.8 and 95% CI of 1.6 to 122.9).[
21] To manage these pregnant women with SCA, the British Society for Haematology guidelines recommend the following measures: multidisciplinary antenatal care team involving obstetricians, haematologists and cardiologists (grade 1C), discontinuation of hydroxycarbamide (2C) and iron chelators (grade 1B), antibiotic prophylaxis (grade 1A), updated vaccinations (i.e., flu, COVID; grade 1B), supplementation with folic acid daily (5mg; grade 1A) and iron if laboratory evidence of deficiency (grade 1A), aspirin daily starting from 12 weeks of gestation (75-150 mg; grade 1B), exchange blood transfusion if worsening anaemia or complications (grade 1B).[
22]
In the current case, the pregnant woman failed to followed these recommendations, except for blood transfusion. At hospital admission, the clinical expression of vaso-occlusive crisis was confirmed by laboratory markers of hemolysis [
23] and, CS developed shortly after the onset of SARV-Cov-2. Hypoxia-induced red blood cell sickling within the coronary microvasculature likely promoted diffuse myocardial ischemia as reflected by concomitant elevation of cTp blood levels and appearance of T wave at the ECG. Two cohort studies have confirmed that, the elevation of cTp correlates with the burden of hemolysis and suggests that myocardial ischemia and cardiac stunning may result from the obstructed flow within the coronary microvasculature and endothelial lesions during sickle cell crisis.[
24,
25]
To date, a single publication has described the progression of an acute chest syndrome to ARDS in a 25-week pregnant woman with SCA and successful management with red blood cell exchange transfusion, mechanical ventilation and, VV-ECMO support.[
26]
3.3. Airflight Transportation of High-Risk Cardiac Patients in French West Indies
The department of cardiothoracic surgery at the UHM in Fort de France provides all types of cardiac interventions, - except transplantations-, for approximately 1,4 million people living in the French West Indies territories (Martinique, Guadeloupe, St Maarten) as well as the French Guiana and some neighboring islands (Dominica and Saint Lucia). As these territories are hundreds of kilometers apart, aeromedical transport for emergent cases needs to be rapidly organized by helicopter or by military airplane, with close communication between healthcare providers from these remote locations and the referral cardiac centre in Martinique. Since 2010, the department of cardiac surgery has set up an ECMO mobile team to perform on site VA ECMO implantations and repatriation of these critically-ill patients to the cardiac ICU at the UHM. All cases are discussed with cardiac surgeons and ICU physicians who provide advices for optimization of pharmacological treatment and to validate the decision of VA-ECMO implantation.[
27,
28] Early referral of high-risk pregnant women with severe heart diseases may prevent delays and adverse outcomes. Whenever possible, these high-risk women are transferred to the UHM where delivery is planned under advanced hemodynamic monitoring and eventual MCS. Over the last 5 years, 35 VA-ECMO have been implanted in patients with heart failure in Pointe à Pitre (Guadeloupe) and in Cayenne (French Guiana). After hemodynamic and respiratory stabilization, these patients have been transferred with airborne mobile ICU to the cardiac ICU in Fort de France.
Such a “flying bridge” allows critically-ill patients with CS to be transferred from a remote centre to a specialized cardiac centre with/without MCS until functional recovery or further evaluation for heart transplantation or long-term VAD. Given the limited access to MCS in many overseas territories and the long distances between hospital centres, there is a need to develop regional networks with dedicated ECMO centres and to implement standardized protocols for safe transportation.[
29]
4. Conclusions
The current literature concerning pregnant patients with CS and treated with VA ECMO is limited to anecdotal case reports and few cohorts (ELSO registry, US databases). Given their younger age and lesser comorbid condition, prognosis of peripartum CS is better than in non-pregnant individuals. To date, this is the first case of a pregnant woman with SCT who developed SARS Cov-2 with refractory CS and who fully recovered thanks to a three days support with VA-ECMO. Analysis of large database including such pregnant patients is needed to determine the outcome after VA-ECMO support and to develop dedicated guidelines in this special subgroup of patients with CS.
Author Contributions
Conceptualization, M.L. and A.P.; methodology, A.P. and M.L.; software, A.P. and M.L.; validation, A.P., M.L., S.B. and A.K.; formal analysis, A,P.; investigation, A.P. and M.L.; resources, M.L.; data curation, A.P. and M.L.; writing—original draft preparation, A.P. and M.L.; writing—review and editing, M.L., D.E.M., S.B., M.S., S.M., and A.K.; visualization, A.P., M.L., D.E.M., S.S., P.B., C.S.-L. and A.K.; supervision, M.L.; project administration, A.P. and M.L. All authors have read and agreed to the published version of the manuscript supervision, M.L.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Souza, J.P.; Day, L.T.; Rezende-Gomes, A.C.; Zhang, J.; Mori, R.; Baguiya, A.; Jayaratne, K.; Osoti, A.; Vogel, J.P.; Campbell, O.; et al. A Global Analysis of the Determinants of Maternal Health and Transitions in Maternal Mortality. The Lancet Global Health 2024, 12, e306–e316. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.G.; Altendahl, M.; Martinez, G.; Ng, A.; Lin, J.P.; Benharash, P.; Afshar, Y. Cardiovascular Disease in Pregnancy. JACC: Advances 2024, 3, 101071. [Google Scholar] [CrossRef] [PubMed]
- Knight-Madden, J.; Asnani, M.; King, L.; Hardy-Dessources, M.-D. Sickle Cell Disease in the Caribbean: Progress in Newborn Screening, Clinical Care, and Research through Collaboration. The Lancet Haematology 2023, 10, e581–e582. [Google Scholar] [CrossRef] [PubMed]
- Greer, O.Y.O.; Anandanadesan, R.; Shah, N.M.; Price, S.; Johnson, M.R. Cardiogenic Shock in Pregnancy. BJOG 2024, 131, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Giorgione, V.; Cauldwell, M.; Thilaganathan, B. Pre-Eclampsia and Cardiovascular Disease: From Pregnancy to Postpartum. Eur Cardiol 2023, 18, e42. [Google Scholar] [CrossRef]
- Safira, A.; Tjahjadi, A.K.; Adytia, G.J.; Waitupu, A.; Sutanto, H. Peripartum Cardiomyopathy Unveiled: Etiology, Diagnosis, and Therapeutic Insights. Current Problems in Cardiology 2024, 49, 102474. [Google Scholar] [CrossRef]
- Arany, Z. Peripartum Cardiomyopathy. N Engl J Med 2024, 390, 154–164. [Google Scholar] [CrossRef]
- Lawler, P.R.; Berg, D.D.; Park, J.-G.; Katz, J.N.; Baird-Zars, V.M.; Barsness, G.W.; Bohula, E.A.; Carnicelli, A.P.; Chaudhry, S.-P.; Jentzer, J.C.; et al. The Range of Cardiogenic Shock Survival by Clinical Stage: Data From the Critical Care Cardiology Trials Network Registry. Critical Care Medicine 2021, 49, 1293–1302. [Google Scholar] [CrossRef]
- Azad, H.; Wen, T.; Bello, N.A.; Booker, W.A.; Purisch, S.; D’Alton, M.E.; Friedman, A.M. Peripartum Cardiomyopathy Delivery Hospitalization and Postpartum Readmission Trends, Risk Factors, and Outcomes. Pregnancy Hypertension 2023, 34, 116–123. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.-J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure: Executive Summary. Journal of Cardiac Failure 2022, 28, 810–830. [Google Scholar] [CrossRef]
- Rajsic, S.; Treml, B.; Jadzic, D.; Breitkopf, R.; Oberleitner, C.; Popovic Krneta, M.; Bukumiric, Z. Extracorporeal Membrane Oxygenation for Cardiogenic Shock: A Meta-Analysis of Mortality and Complications. Ann. Intensive Care 2022, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, C.; Li, F.; Yan, Z.; Ye, S.; Ma, J.; Wen, J. Five Critically Ill Pregnant Women/Parturients Treated with Extracorporeal Membrane Oxygenation. J Cardiothorac Surg 2022, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Hong, Y.S.; Rim, S.J.; Yu, S.H. Extracorporeal Membrane Oxygenation in a Patient With Peripartum Cardiomyopathy. The Annals of Thoracic Surgery 2007, 84, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Tan, C.S.; Rycus, P.; Anders, M.; Lorusso, R.; Zhang, J.J.Y.; MacLaren, G. Extracorporeal Membrane Oxygenation in Pregnancy: An Analysis of the Extracorporeal Life Support Organization Registry. Critical Care Medicine 2020, 48, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, M.; Poudel, S.; Ghimire, K.; Rajak, K.; Khanal, R.; Hodo, F.; Chaudhary, S.; Guru, P.; Kadariya, D. VENO-ARTERIAL EXTRACORPOREAL MEMBRANE OXYGENATION (VA- ECMO) OUTCOMES IN PREGNANT PATIENTS WITH CARDIOGENIC SHOCK - A NATIONAL INPATIENT STUDY. Journal of the American College of Cardiology 2024, 83, 2536. [Google Scholar] [CrossRef]
- Olson, T.L.; O’Neil, E.R.; Ramanathan, K.; Lorusso, R.; MacLaren, G.; Anders, M.M. Extracorporeal Membrane Oxygenation in Peripartum Cardiomyopathy: A Review of the ELSO Registry. International Journal of Cardiology 2020, 311, 71–76. [Google Scholar] [CrossRef]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Hodgson, C.; Scheinkestel, C.; Cooper, D.J.; Thiagarajan, R.R.; et al. Predicting Survival after ECMO for Refractory Cardiogenic Shock: The Survival after Veno-Arterial-ECMO (SAVE)-Score. Eur Heart J 2015, 36, 2246–2256. [Google Scholar] [CrossRef]
- Hakami, F.; Alhazmi, E.; Busayli, W.M.; Althurwi, S.; Darraj, A.M.; Alamir, M.A.; Hakami, A.; Othman, R.A.; Moafa, A.I.; Mahasi, H.A.; et al. Overview of the Association Between the Pathophysiology, Types, and Management of Sickle Cell Disease and Stroke. Cureus 2023. [Google Scholar] [CrossRef]
- Thomson, A.M.; McHugh, T.A.; Oron, A.P.; Teply, C.; Lonberg, N.; Vilchis Tella, V.; Wilner, L.B.; Fuller, K.; Hagins, H.; Aboagye, R.G.; et al. Global, Regional, and National Prevalence and Mortality Burden of Sickle Cell Disease, 2000–2021: A Systematic Analysis from the Global Burden of Disease Study 2021. The Lancet Haematology 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- Gladwin, M.T. Cardiovascular Complications and Risk of Death in Sickle-Cell Disease. The Lancet 2016, 387, 2565–2574. [Google Scholar] [CrossRef]
- Alayed, N.; Kezouh, A.; Oddy, L.; Abenhaim, H.A. Sickle Cell Disease and Pregnancy Outcomes: Population-Based Study on 8.8 Million Births. Journal of Perinatal Medicine 2014, 42, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Oteng-Ntim, E.; Pavord, S.; Howard, R.; Robinson, S.; Oakley, L.; Mackillop, L.; Pancham, S.; Howard, J.; the British Society for Haematology Guidelines Committee Management of Sickle Cell Disease in Pregnancy. A British Society for Haematology Guideline. Br J Haematol 2021, 194, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Arishi, W.A.; Alhadrami, H.A.; Zourob, M. Techniques for the Detection of Sickle Cell Disease: A Review. Micromachines 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Huang, Y.; Raman, S.V.; Kraut, E.; Desai, P. Myocardial Injury and Coronary Microvascular Disease in Sickle Cell Disease. haematol 2021, 106, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Akkus, N.I.; Rajpal, S.; Hilbun, J.; Dwary, A.; Smith, T.R.; Mina, G.; Reddy, P.C. Troponin Elevation in Sickle Cell Disease. Med Princ Pract 2021, 30, 437–442. [Google Scholar] [CrossRef]
- Chambers, J.; Smith, N.; Sehring, M.; Chittivelu, S. Acute Chest Syndrome Progressing to ARDS in a Patient of 25-Week Gestation. Case Reports in Critical Care 2018, 2018, 1–3. [Google Scholar] [CrossRef]
- Lebreton, G.; Sanchez, B.; Hennequin, J.-L.; Resiere, D.; Hommel, D.; Leonard, C.; Mehdaoui, H.; Roques, F. The French Airbridge for Circulatory Support in the Carribean. Interactive CardioVascular and Thoracic Surgery 2012, 15, 420–425. [Google Scholar] [CrossRef]
- Lebreton, G.; Sanchez, B.; Isetta, C.; Hennequin, J.-L.; Mnif, M.-A.; Pécout, F.; Villain-Coquet, L.; Clerel, M.; Combes, A.; Leprince, P.; et al. Transportation of Patients under Extracorporeal Membrane Oxygenation Support on an Airliner: Flying Bridge to Transplantation. Archives of Cardiovascular Diseases 2023, 116, 335–341. [Google Scholar] [CrossRef]
- Sams, V.G.; Anderson, J.; Hunninghake, J.; Gonzales, M. Adult ECMO in the En Route Care Environment: Overview and Practical Considerations of Managing ECMO Patients During Transport. Curr Trauma Rep 2022, 8, 246–258. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).