Introduction
Invasive fungal infections (IFIs) are a growing global health concern as rates of fungal infections rise while fungal drug and vaccine development stagnate. It is estimated that approximately 1.6 million people die from IFIs worldwide annually; the number of cases is expected to rise as risk factors like climate change, immunocompromised population, and anti-fungal drug resistance increase1. Due to the concern of increased IFIs and associated co-morbidities, the World Health Organization organized the first Fungal Priority Pathogens List in 2022 to address research needs of prevalent fungal pathogens2. Listed at the top of the critical priority group is Cryptococcus neoformans. C. neoformans is an encapsulated yeast that is the causative agent of cryptococcal meningoencephalitis and the fungal pathogen responsible for the largest percentage of fungal meningitis cases worldwide. Recently, Cryptococcus species complex has been divided into seven different species3.To simplify the writing, here we will use traditional C. neoformans and C. gattii to describe the species complex. While C. neoformans is responsible for ~19% of HIV/AIDS related deaths annually and immunocompromised populations are at the highest risk, sibling species C. gattii can also infect immunocompetent hosts4. Thus, C. gattii is a high-risk primary pathogen. A key point highlighted on the fungal pathogen priority list is the lack of a vaccine in the global anti-fungal arsenal.
As highlighted by Rivera and colleagues 2022 review, the rationale of slow fungal vaccine development is due to a complexity of issues including socioeconomic considerations, similarity of fungal and mammalian cellular machinery, lack of fungal immunological understanding, and difficulty in mass commercialization of fungal vaccine research for limited populations5. Particularly, the targeting of fungal wall antigens that may induce a desired protective immune response while minimizing off target effects in human hosts remains to be carefully defined and elucidated. The polysaccharide encapsulation of the Cryptococcus cell is unique in its anti-phagocytic properties and is considered a major immunogen masking component that contributes to immune evasion and ultimate dissemination throughout its host. Establishing methods to inhibit cryptococcal dissemination either by prophylaxis treatment or vaccination approaches prior to infection has proven difficult and is an area of active research in the field. In this review we focus on highlighting known cryptococcal fungal cell components, (namely the capsule, α/β-glucans, chitin/chitosan, mannoproteins, and extracellular vesicles) and how the immunogenicity of these antigens, or lack thereof, can be harnessed to shape and modulate the host immune response in novel cryptococcal vaccine approaches.
Cryptococcal Capsule
Pathogenic microorganisms containing a polysaccharide capsule have predominantly included bacteria (i.e.,
Streptococcus pneumoniae,
Haemophilus influenzae,
Neisseria meningitis)
6, but are present on some fungi, most notably
Cryptococcus. The
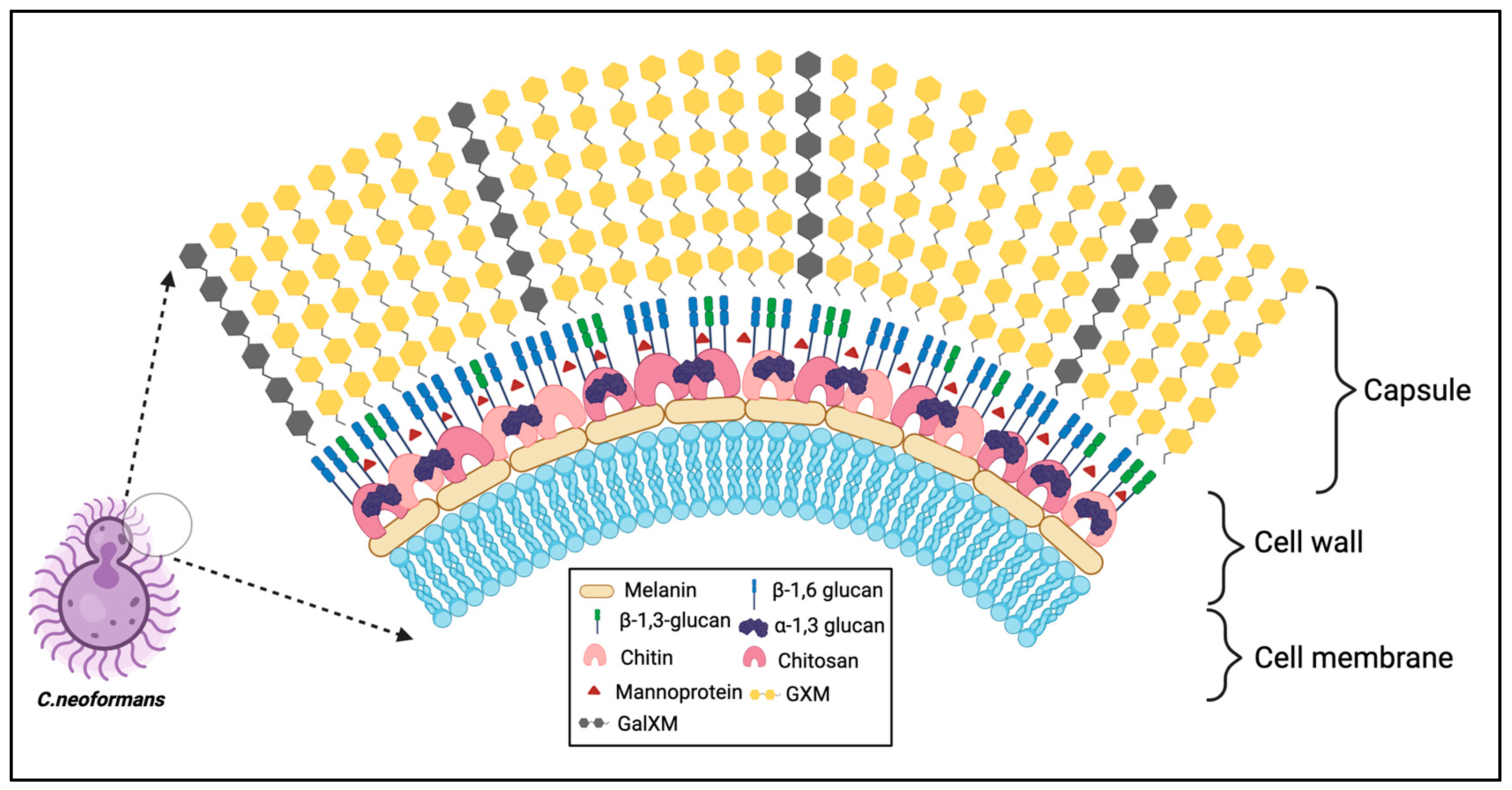
Cryptococcus species complex has been historically well defined and identifiable by its unique encapsulation by a polysaccharide capsule that serves as a key virulence factor for survival in the host. The polysaccharide capsule of
C. neoformans is connected to the cell wall as capsular polysaccharide or in a shed form identified as extracellular polysaccharide. These polysaccharides are comprised predominantly of glucuronoxylomannan (GXM) and galactoxylomannan (GalXM) at ratios of 90% to 10% respectively (
Figure 1)
7. GXM is structured by a mannan backbone with xylose and glucuronic acid substitutions while GalXM possesses a galactan backbone with mannose and galactose side chain substitutions which can furthermore by substituted with xylose and glucuronic acid residues
8,9. In clinical settings cryptococcal meningitis is often diagnosed by the presence of cryptococcal capsule shedding in the spinal fluid or serum of patients
10,11. The ability of the capsule to be continuously shed throughout the
Cryptococcus cell lifespan and alter its size based on its environment greatly contributes to
Cryptococcus survival and hijacking of host effector cells to migrate toward the central nervous system
12-17.
Understanding the structural basis of the capsule is key to further elucidating how we can modulate immunogenicity of the Cryptococcus cell. Namely, the GXM mannose backbone structural differences of capsule polysaccharide makeup have been utilized to classify serotypes of cryptococcal strains (serotypes A, B, C, D, and AD,) based on their antigenic differences18. In the past few decades studies have shown that highly immunogenic mannoproteins (MPs) constitute as a small fraction of the capsule makeup (~1-2% by mass) and reside in spatially different regions of the capsule18. MPs have not been shown experimentally to be covalently bound to the cryptococcal cell wall potentially due in part to their ability to be secreted outside of the cell and possession of GPI anchors to keep them bound to the cell wall18. Recently one predicted mannoprotein Krp1 in C. gattii was found to contribute to capsule structure as well as GXM shedding into the supernatant19. Whether there is a physical interaction between Krp1 and capsule remains to be determined. Additionally, loss of Krp1 resulted in diminished beta glucan synthesis. However, whether other mannoproteins contribute to altering capsule structure or synthesis remains to be seen and is discussed later in this review.
Studies on capsule synthesis and modification of capsule structure in nutrient limited host conditions have been key to elucidating the role of capsule in Cryptococcus virulence and insightful in progression of potential targets for vaccine development. Early studies by Kwon-Chung and Yang identified key capsule synthesis genes required for capsule synthesis (i.e., CAP59, CAP60, CAP64, and CAP10). Loss of any of these single genes results in acapsular strains of Cryptococcus20-24,7. Defects in capsule synthesis will result in attenuation of virulence and loss of immune evasion from responding phagocytes25. Cryptococcal vaccine studies focused on role of capsule on a glycolipid sterylglucosidase deficient strain (sgl1Δ), showed that sterylglucoside accumulation in the sgl1Δ mutant alters the structural and physical properties of GXM. Consequentially, capsular GXM was required for complete host protection as the acapsular cap59Δ sgl1Δ strain failed to induce a TH1 protective inflammatory response. Similarly, the cryptococcal vaccine candidate (Znf2OE) that overexpresses a zinc finger transcription factor Znf2 also requires the capsule for vaccine protection27. This study utilized an acapsular strain deficient in both GalXM and GXM in the Znf2OE background. While the exact impact of Znf2 overexpression on GXM or GalXM was not defined, it was observed that sera from mice immunized with heat killed ZNf2OE contained IgG and IgM antibodies that bound to antigens highly abundant in the center of the Znf2OE capsule as compared to H99. Studies with various whole cell vaccine candidates that affect different molecular pathways, and subsequent cryptococcal cell antigens, also suggests that these mutants require the capsule to act as the carrier or as a prop to expose their immunogens and thus induce protective immunological responses26,27. However, the observations that there is no protection with cap59Δ vaccination strategies and that acapsular strains are rapidly cleared in the host upon challenge, suggest that exposure of immunogens in the cell wall alone are not sufficient for eliciting a protective immune response against cryptococcal infection.
The Cryptococcus capsule is known to be anti-phagocytic and to elicit T cell independent TH2 responses. The inability of GXM to induce memory T cell activation by the host or induce antibody affinity maturation and immunoglobulin class switching contributes to the poor immunogenic potential of GXM28,29. Nevertheless, antibodies against GalXM and GXM capsular components have been explored for fungal vaccine and therapeutic treatment uses. Casadevall and colleagues generated monoclonal antibody 18B7 as a neutralizing antibody for cryptococcosis treatment and showed its ability to bind to four serotypes of C. neoformans with specificity to GXM in lung tissue retrieved from murine infection models 30. Phase 1 human clinical trials were conducted for monoclonal antibody 18B7 as a therapeutic treatment for cryptococcosis, however treatment only showed moderate amelioration of symptoms31. GXM-based vaccine approaches have focused on conjugating GXM to carrier proteins such as tetanus toxoid (GXM-TT) that induces antibody mediated protection and showed prolonged survival in vaccinated mice after C. neoformans challenge.32,33 Another GXM-based vaccine is the GXM mimicking peptide, P13 conjugated to tetanus toxoid (P13-TT)34. In human immunoglobulin transgenic mice P13-TT immunization studies, subcutaneously vaccinated cohorts showed prolonged survival compared to controls34. This P13-TT protection was antibody mediated as vaccinated mice produced IgG2 and IgG4 antibodies against P13-TT and immunogenic idiotype-positive antibodies to GXM. Recently, semisynthetic glycoconjugate vaccines containing an identical synthetic decasaccharide M2 motif antigen bound to anthrax and CRM197 were found to induce protective antibodies against GXM but showed modest protection in murine models compared to controls35.
Another antibody-based vaccine candidate is based on the predominant component of the capsule, namely GalXM, conjugated to antigenic carrier bovine serum albumin (GalXM-BSA)36. In GalXM-BSA vaccine studies, the BSA conjugated GalGXM complex was able to induce passive IgM and IgG antibodies but saw no induction of host defense against Cryptococcus infection between vaccinated and unvaccinated cohorts36. Modifications need to be implemented to prolong protection and induce CD4+ T cell protective immunity. Cryptococcal capsule based vaccine candidates are an active area of research. A better understanding of how capsule synthesis is regulated and altered while in different host conditions is important to move capsule-conjugated vaccine research forward. Moreover, identifying epitopes or synthetic modifications modelled off the capsule may chart a path forward for multivalent subunit-based pan-fungal vaccines.
Cryptococcal Glucans
The fungal cell wall is a critical surface structure that maintains cell integrity against biological, physical, and chemical stressors and decides the fate of the pathogen37. This rigid structure is also dynamic and flexible in nature to undergo morphologic changes during mating, budding or cellular interactions including the one with host cells38.
The cryptococcal cell wall consists of glucans, chitin, chitosan, glycoproteins, melanin and lipids39. These components are major fungal pathogen-associated molecular patterns (PAMPs) and help fungi to sense their surroundings and contribute to survival inside the host. The synthesis of precursors of cell wall glucans involves the coordinated action of glycosyltransferase with donor sugar molecules, enzyme activities and the availability of acceptor substrates40. Unlike Saccharomyces cerevisiae, C. neoformans has abundant α-glucans and chitosan polymers followed by more β-1,6 linkages with minor levels of β-1,3 linkages40-42.
The cryptococcal cell wall primarily consists of α-1,3-glucan linkages derived from membrane bound α-glucan synthase, Ags1p, primarily associated with the outer cell wall41. Loss of AGS1 results in loss of α-1,3-glucan and capsule followed by redistribution of β-glucans and chitin making the cells more fragile43. These findings show support of the association of α-1,3-glucan in binding of capsular polysaccharides. Although C. neoformans has a significantly lower percentage of β-1,3-glucan, the gene FKS1 encoding for β-1,3 glucan is essential42. The phenotypic defects observed in loss of FKS1 supports that β-1,3 glucan plays a critical role in cell viability and capsule organization. Inhibitors of β-1,3-glucan synthesis (i.e., Echinocandins) have no effect on cryptococcal β-1,3-glucan production possibly suggesting the presence of transporters to pump out the compounds or the exopolysaccharide capsule42,44. In comparison, the mechanism of synthesis for β-1,6-glucan is complex since no synthase enzyme has been identified. The synthesis depends on multiple genes with a dominant role played by KRE5.45 While there are 7 KRE genes in C. neoformans, deletion of KRE5 alone or KRE6 with SKN1 led to complete loss of β-1,6-glucan from the cell wall, resulting in compromised cell integrity rendering it avirulent in an animal inhalation model of infection as the yeast cells were unable to survive at host temperature45.
Cell wall glucans not only play an important role in cell integrity but also induce immunomodulatory effects in the host. Basso et al evaluated the immunostimulatory activity of β-1,3-glucan containing exopolysaccharide (EPS) isolated from the edible mushroom Auricularia auricula to phagocytes and to mice infected with C. neoformans46. Treatment with EPS resulted in the activation of innate cells like macrophages and dendritic cells after engagement of Dectin-1 receptor culminating in pro-inflammatory cytokine production and cell maturation via Syk-dependent pathway signaling. EPS treatment resulted in the upregulation of genes associated with host protection against C. neoformans, Dectin-1-mediated signaling in macrophages and enhanced the survival of C. neoformans infected mice46.
Glucan particles (GP) can be used for dual purpose as a combined delivery system and an adjuvant for cryptococcal vaccine due to its ability to elicit protective immune responses. Glucan particles are recognized by complement and Dectin-1 receptors present on innate immune cells47. Mice immunized with GPs containing trapped ovalbumin resulted in TH1/TH17 CD4+T cell responses followed by robust antigen-specific antibody responses48,49. Based on these protective responses, Specht et al recombinantly expressed six purified cryptococcal proteins (Cda1, Cda2, Cda3, Fpd1, Sod1, and MP88) in Escherichia coli, and loaded these antigens into GPs as a potential vaccine candidate50. Different mouse strains were vaccinated with these antigen-laden GPs and challenged with C. neoformans and C. gattii. The results showed varied protection depending upon the antigen, mouse strain and cryptococcal species50. Furthermore, vaccination with GP containing C. neoformans Cda1 and Cda2 induced robust protective TH1 and TH17 responses. In these recent GP-Cda1 and GP-Cda2 vaccination studies, murine models deficient in pro-inflammatory cytokines IFNγ, IL-1b, IL-6 or IL- 23 were not protected upon live C. neoformans challenge51. These studies emphasize the idea of employing cell wall protein antigens as novel vaccine adjuvant or delivery system against cryptococcosis.
Cryptococcal Chitin and Chitosan
Chitin is virtually present in all fungi; it is arranged into microfibrils to provide strength and rigidity. C. neoformans is chitin rich and produces 3-6 times more chitin which increases as the density of the culture progresses as compared to the model yeast S. cerevisiae. Membrane protein chitin synthase (CHS) encodes eight CHSs in different classes based on the protein sequence of the catalytic domain 52. The enzymes in class I-III have seven transmembrane domains while class IV-VI has six predicted transmembrane domains53. Chs1 and Chs3 (class IV); Chs2 and Chs7 (class II); Chs4 and Chs5 (class V) and Chs6 and Chs8 (class I and II). Chitosan, the deacetylated form of chitin, is formed through enzymatic conversion of N-acetylglucosamine to glucosamine by chitin deacetylases (CDAs). Three chitin deacetylases genes are essential for chitosan production in C. neoformans. Chitosan is mainly produced during the vegetative phase of growth and increases with culture density. The coordinated activities of Cda1 and Cda2 are essential for cryptococcal virulence54. Deletion of genes associated with chitin and chitosan result in an array of phenotypes, including temperature sensitivity, lack of chitosan, altered cell wall integrity, budding defects, leaky melanin; and enlarged capsule thus rendering the cells avirulent52,55.
Innate recognition of PAMPs induces a strong adaptive response 56. Chitin is one of the PAMPs present in the cell wall which is required for virulence and chitosan deficiency alters the Th1-based protective host responses57-59. Upadhya et al emphasized how different culture media and pH affects the amount of chitin and chitosan in the cell wall, in turn these changes alter the cell wall architecture and host response60. Vaccination with a chitosan-deficient cda1Δ2Δ3Δ (lacking all three chitin deacetylases) strain conferred protective immunity in mice to a subsequent challenge with a virulent wild-type C. neoformans infection. In contrast, mice infected with a chitosan deficient chs3Δ strain died within 36 hours post infection due to an aberrant hyperinflammatory response, thus highlighting the critical immunomodulatory role of chitosan61. Vaccination with a single Cda protein induced cross-reactive antibody and IFN-γ immune responses to other Cda protein family members62. In summary, the complex structure of chitin is buried in the cell wall, shielded by mannoproteins and glucans. The strong elicitation of host protective immune responses by chitosan suggests that this is an attractive vaccine candidate with adjuvant and antigenic properties56,63,64.
Mannoproteins
Mannoproteins are glycoproteins with heavily glycosylated mannose sidechains that have been identified as potential fungal antigens that can be targeted for vaccine development based on their ability to activate both innate and adaptive arms of the immune response. Their structure has been characterized as relatively conserved, having a signal peptide on the N terminus and a Glycophosphoinositol (GPI) anchor toward the C terminus with multiple N-linked and O-linked glycosylation sites throughout65-67. Mannoproteins either end up being secreted into the external environment or lodged in the cell wall due to their GPI anchors. Furthermore, mannoprotein structure is characterized by having a Serine/Threonine rich region for extensive O-linked mannosylation.
Mannoproteins have been reported to be involved in fungal virulence and cell wall structural integrity in multiple fungal species68-70. For example, C. albicans mannoprotein MP-58 is located on the cell surface and has been found to elicit a strong IgG antibody response68. Monoclonal antibody treatment blocking the C terminal antibody binding region of MP-58 in C. albicans-infected mice reduced mortality as compared to the non-treated cohort. The secreted mannoprotein Mp1p was shown to be a key virulence factor in the thermal dimorphic fungus Talaromyces marneffei69,70. Loss of Mp1p attenuated T. marneffei virulence seen and resulted in reduced survival and proliferation in macrophages. As previously mentioned, predicted Cryptococcus mannoprotein Krp1 was found to alter some virulence factors including capsule thickness, cell wall integrity, and phagocytosis in vitro in C. gattii, but had no effect in murine cryptococcosis models19. In studies focused on determining GPI anchor containing mannoprotein MP-98, monoclonal antibodies against MP-98 were not able to bind to cryptococcal capsule in serotype A compared to serotype D suggesting mannoproteins are antigenically diverse throughout the serotypes of Cryptococci18. Most of the research on mannoproteins has focused on MP-84, MP-88, and MP-98. Over 40 potential mannoproteins in C. neoformans have yet to be characterized for function and contribution to cell physiology and virulence71,65.
Fungal cell glycosylation differs from mammalian glycosylation in that mannose is used to extend the branched glycosylation sites. Mammalian cells use monosaccharaides and a multitude of glucotransferases to alter the N-glycan branching. A major difference between O-linked and N-linked glycosylation is the specificity of branching glycan sidechains added onto the amino acid Asparagine (N-linked) versus a linear addition of glycan sidechains to the hydroxyl group of Serine and Threonine amino acid saturated regions of the protein.
Glycosylation of mannoproteins is a key contributor to their enhanced immunogenicity in C. neoformans66. Mansour and Levitz characterized the initial findings of mannoproteins as immunogenic antigens of C. neoformans that can trigger protective host immunity72. This and subsequent studies found that mannoproteins MP-88 and MP-98 (Cda2) can stimulate TH1 protective cytokine production by CD4+ T cells67,66,72. Subsequent studies found that glycosylation of mannoproteins is key to the activation of dendritic cells triggered through danger associated molecular pattern receptors DC-SIGN that can subsequently induce T cell response71. Additionally, MP-84 (Cda3) and MP-115 were identified as important targets of antibodies as they react strongly with sera of cryptococcal meningoencephalitis AIDS patients18,73. De-glycosylated recombinant versions of these MPs in E. coli induced a significantly weaker response in AIDS patients sera as compared to the naturally heavily glycosylated versions73. Furthermore, the differences in O-linked versus N-linked glycosylation have been identified to play an important role in the immunogenicity of cryptococcal mannoproteins. Recently, Su-Bin Lee and colleagues found that when core N-glycan structures were truncated in C. neoformans MP-84 (Cda3) and to some extent MP-98 (Cda2) the capacity to induce the immune response of bone-marrow derived dendritic cells was reduced74. Interestingly, complete ablation of N-glycosylation on MP-84 enhanced adhesion to host epithelial cells and increased cytokine production compared to the wildtype N-glycans74. This study emphasized the importance of structure-dependent effects of N-glycans on the function of mannoproteins and lung cell interactions. In aggregate, these findings highlight the importance of glycosylation in mannoprotein immunogenicity.
Cryptococcal vaccination studies involving MP-98 (Cda2) and MP-84 (Cda3) are a prime example of mannoproteins being identified as novel targets for fungal vaccination51,75,60. As previously referenced, Upadhya et al showed that the triple chitin deacetylase-deficient strain cda1Δ2Δ3Δ, contains cell wall integrity defects that contribute to its attenuated virulence and ability to induce protective cytokines in murine vaccination models54. Indeed, recent work aimed at developing multi-epitope subunit vaccines based on the chitin deacetylase (Cda1, Cda2, and Cda3) and MP-88, predicted that utilizing a combination of T cell and B cell epitopes together with adjuvants and linkers induced protective cytokine responses in silico76. While these findings would need to be validated and confirmed in both in vitro and in vivo models of C. neoformans infection, it highlights the novelty of “reverse vaccinology” utilizing an immunoinformatic approach to identify immunogenically favorable epitopes and test them in hypothetical models that reduce financial limitations and speed up vaccine screening approaches. Furthermore, recent work by Wang et al has shown that a quadrivalent cryptococcal subunit vaccine (Cda1+Cda2+Blp4+cpd1Δ) combined with Cationic Adjuvant Formulation 01 (CAF01) can induce a robust TH1 and TH17 CD4+ T cell response for long term protection against Cryptococcus77. Modulation of these cryptococcal cell antigens or other fungal species are critical to creating novel cryptococcal vaccines and for the identification of new anti-fungal drug targets. While the chitin deacetylase mannoproteins have been elucidated to be key functional mannoproteins that are themselves immunogenic, it emphasizes the need to further expound other mannoproteins in Cryptococcus pathogenesis that may hold the potential for vaccine use.
Cytokine Inducing Glycoprotein 1 (Cig1) is another mannoprotein whose function is important for Cryptococcus survival in host and holds immunogenic potential for vaccination studies. Cig1 was named for its ability to induce protective cytokines from immune cells and to interact with antibodies from serum obtained from AIDS patients with cryptococcosis78. Additionally, Cig1 has been found shed in media during C. neoformans growth. The mannoprotein Cig1 mediates iron uptake from heme as a hemophore under iron starved host like conditions and contributes to virulence79. This impact on virulence is attenuated only when other proteins including Cfo1 that allot functional redundancy in the iron uptake regulatory system of C. neoformans is also deleted. Like other mannoproteins, Cig1 contains a GPI anchor as identified by Levitz and colleagues65. Cadieux et al show that Cig1 is found excreted into the supernatant as previously described and that it is found towards the outside of the cryptococcal cell wall79.
Interestingly, CIG1 transcripts are highly abundant in cryptococcus retrieved from cerebral spinal fluid of cryptococcosis patients in clinical settings further suggesting that Cig1 plays an important role in survival in harsh host conditions80. O’Meara et al showed that the PKA pathway regulates pH dependent transcription factor RIM101, which is known to regulate CIG1 gene expression81,10. The protein kinase A (PKA) signaling pathway is also involved in regulating CIG1 expression via RIM101. Further studies showed capsule structure is altered where the rim101Δ mutant is hypo capsular yet propagates a hypervirulent phenotype82. Meanwhile Geddes et al showed the cAMP/PKA pathway also regulates extracellular secretion of Cig1 in a PKA1 dependent manner observed in the pka1Δ mutant compared to the wildtype83. Follow-up studies from Geddes and colleagues observed a connection between the PKA pathway and the Ubiquitin Proteolysis pathway where the PKA expression alters proteostasis of virulence-related genes and endoplasmic reticulum control in capsule production84. Recently, the SCF (Skp1, Cullins, F-box proteins) E3 ligase ubiquitin complex was identified to regulate Crk1, a CDK-related kinase in C. neoformans85. This study found that Crk1 is a substrate of F-box protein 1 and a downstream regulator of the cAMP/PKA pathway via phosphorylation of Gpa185. In the fbp1Δ mutant proper ubiquitin tagging and degradation is lost. This resulted in Crk1 accumulation and induction of titan cell formation. Furthermore, overaccumulation of Crk1 attenuated virulence and increase induction of TH1/TH17 cytokines by CD4+ T cells. The connection between Cig1 and the cAMP/PKA pathway via Rim101 combined with recent findings identifying Crk1 as a novel regulator of cAMP/PKA, suggest there could be a potential role of Cig1 as an immunogen in the whole cell heat-killed F-box protein deficient (HK-fbp1) vaccine candidate. While studies with Cig1 deletion mutants have focused on functionality in C. neoformans in iron uptake, the immunogenicity of Cig1 in the context of vaccination and ability to prime protective CD4+ T cells remain to be seen.
Immunological Responses of Current Whole Cell Cryptococcal Vaccine Candidates
Immunocompromised populations lacking either the adaptive or innate arm of the host immune response are highly susceptible to an array of fungal species (i.e. Cryptococcus, Aspergillus, Candida, Coccidioides, Histoplasma, and Blastomyces). This susceptibility will continue to rise with expanded use of immunosuppressant treatments to remedy other diseases. One of the defining risk factors for Cryptococcus infection is lack of CD4 T cell populations as observed in the high percentage (~19%) of Cryptococcus related deaths in HIV/AIDS patients4. Thus, an ideal vaccine candidate needs be able to circumnavigate the loss of this immune cell population and be able to induce long term protection93. Exciting developments in understanding both the innate and adaptive immunological mechanisms of protection in response to several whole cell-based vaccine candidates has greatly contributed to identifying how we can manipulate cryptococcal antigens to induce desired immune responses in vaccine development.
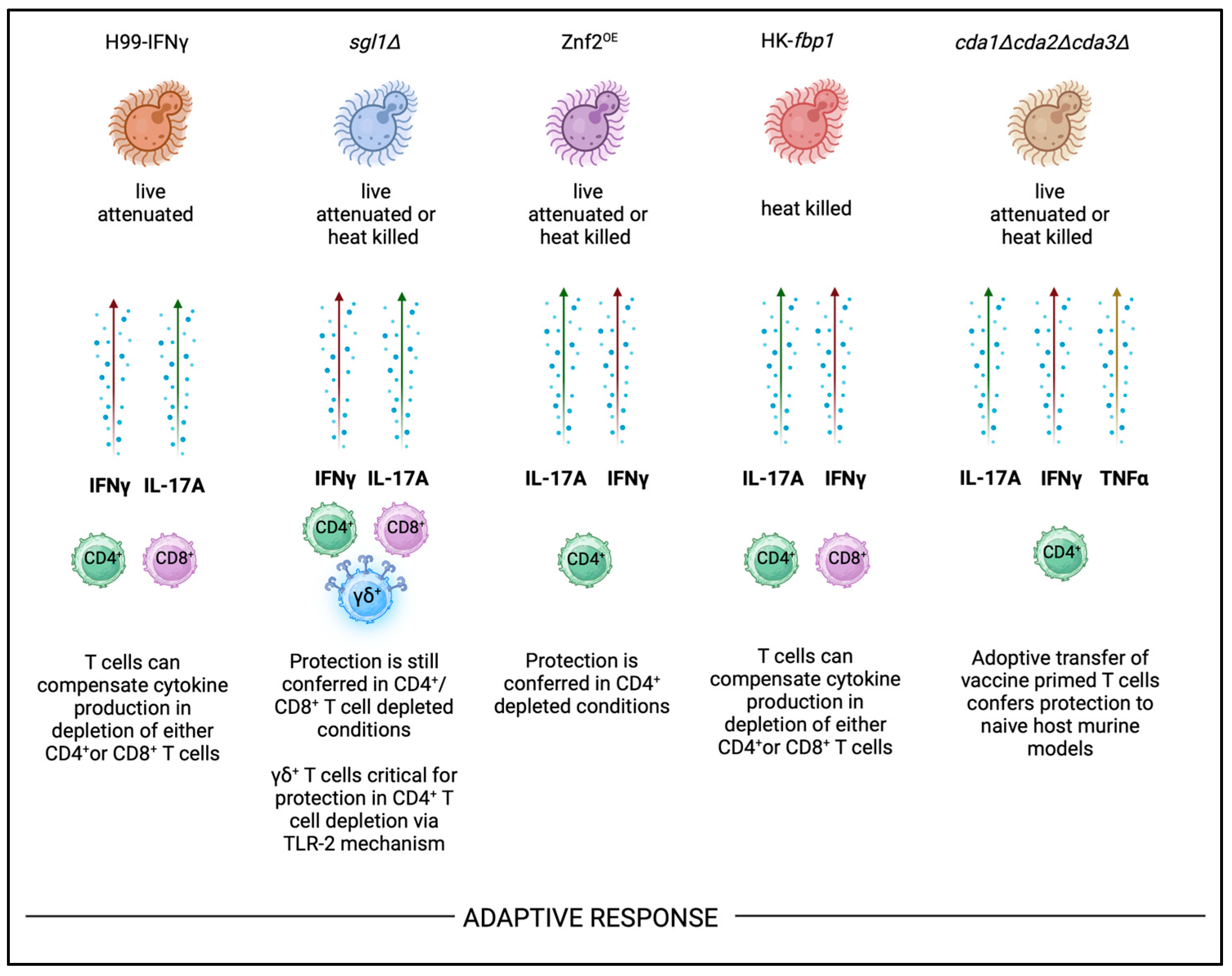
Currently reported whole cell based cryptococcal vaccine candidates include a human interferon γ producing genetically modified strain (H99γ)
94-97; a sterylglucoside deficient strain (
sgl1Δ); an overexpressed mating specific zinc finger transcription factor strain that restrains cryptococcal cells in a pseudo hyphal morphological stage (Znf2
OE)
98,26,99; an F-box protein deletion strain that alters SCF E3 ligase protein proteolysis pathway (
fbp1Δ)
100-104; and the chitin deacetylase triple mutant (
cda1Δcda2Δcda3Δ)
54,75,58,59,105,61 (
Table 1). Each of these strains have shown successful protection against
C. neoformans challenge following vaccination in different mouse strain backgrounds. Furthermore, these strains appear to share somewhat conserved immunological mechanisms of action in protective anti-fungal responses.
Adaptive Immunity
The adaptive immune response plays a critical role in host defense against
Cryptococcus species. Protection against
C. neoformans infection is primarily mediated via IFNγ and IL-17A cytokine producing CD4
+ T cells responses in immunocompetent settings
106. Expansion of these CD4
+ T cell populations while balancing the influx of T
H1 and T
H17 cytokines responses are also important for the host survival following vaccination and subsequent challenge. Importantly, the production of these cytokines must still be preserved in CD4
+ T cell deficient settings in vaccination applications for
C. neoformans. Protection against
C. neoformans challenge by these T
H1 and T
H17 responses are similarly induced by all current cryptococcal vaccine candidates highlighted in this review (
Figure 2). The live attenuated H99γ strain has been shown to confer protection in CD4
+ and CD8
+ T cell depletion models, but protection is lost when the mutant is in heat killed form
107,94,108. However, many of the listed candidates can continue to confer protection in heat killed forms in CD4
+ deficient host models so long as other T cell compartments are intact (
Figure 2). For instance, the
sgl1Δ vaccine can confer protection in either CD4
+ or CD8
+ deficient backgrounds, so long as one or the other is intact. In double CD4
+/CD8
+ T cell antibody depletion murine models,
sgl1Δ vaccine protection is lost and mice succumb to infection following H99 challenge
109. CD4
+ T cells were found to be required for vaccine mediated protection against other cryptococcal strains including
C. gattii, but it is unknown whether this vaccination can provide cross protection against other fungal pathogens. Interestingly, protection conferred by
sgl1Δ vaccination is dependent on γδ T cells, that can produce key protective cytokines IFNγ and IL-17A
27. In the HK-
fbp1 vaccine candidate, RAGKO mice genetically lacking the entire adaptive immune response (CD4
+, CD8
+, and B cells) also succumb to infection following challenge
100,110. Wang and colleagues identified that when CD4
+ T cell compartment was depleted in the HK-
fbp1 vaccination model, the CD8
+ T cell compartment would expand and produce key protective cytokines to compensate for the other population’s loss
100. Furthermore, the HK-
fbp1 vaccine is the only cryptococcal vaccine candidate that has been shown to provide cross protection against other fungi such as
Aspergillus and
Candida. This protection against
A. fumigatus infection was also surprisingly conferred in neutropenic murine model. In the context of
Aspergillus infection where monocytes and neutrophils are critical innate immune cells that orchestrate anti-fungal immune responses
111-113, it was exciting to see vaccination with HK-
fbp1 C. neoformans was able to provide heterologous protection against other fungal species
100. In summary, these findings emphasize that while IFNγ and IL-17A production by both CD4
+ or CD8
+ T cells can confer protection in these models there may be a role for other sources of these protective cytokines (i.e γδ T cells) in some vaccine candidates. Furthermore, vaccination with these strains may be able to induce cross-protection against other medically relevant fungal pathogens.
The role of B cell-mediated immunity in defense against Cryptococcus infection has been found to be moderate impact in both immunocompetent and compromised settings. Moreover, the impact of antibody-mediated protection has been found to vary based on Cryptococcus strain and murine models used to investigate humoral responses114. B cell-mediated immunity was dispensable for protection in live H99γ−vaccinated mice since B-cell deficient mice survived H99 challenge108. B cells are also found dispensable in the cda1∆cda2∆cda3∆ vaccination115. Studies on the impact of humoral response in sgl1Δ, vaccination models employed CD19-depleted murine models and found a minimal impact for B cells in this model109. In the Znf2OE vaccination model, IgG and IgM antibodies from Znf2OE vaccinated mice showed high intensity binding to Znf2OE C. neoformans cells compared to the H99 strain, indicating that antibody titers of vaccinated host are increased. However, the role of B cells in cryptococcal vaccination candidates Znf2OE and fbp1Δ, have not been explicitly investigated in either live or heat killed vaccination forms.
Innate Immunity and Trained Innate Immunity Responses
While the adaptive response is key to preventing dissemination in
Cryptococcus infection, the role of the innate immune system cannot be underestimated. The innate immune arm comprised of first responder phagocytes (macrophages, monocytes, neutrophils) and professional antigen presenting dendritic cells are required for priming CD4
+ T cell responses. Specifically, C-type lectin receptors (i.e. Dectin-1, Dectin-2, DC-SIGN, and Mincle) and Toll-like receptor (TLR2, TLR4, and TLR9) are established Pattern recognition receptors (PRRs) that play key roles in recognition of fungal antigens (i.e. mannans, β-glucans) that are required for priming and expanding T
H1 and T
H17 CD4
+ T cells
116-121. Furthermore, this priming of effector CD4
+ and activation of cytolytic CD8
+ T cells is required in vaccine mediated protection in multiple models for combating infectious disease
122. Excitingly, the role of “trained immunity” where innate immune cells have the capacity to retain immunological memory originally thought to be retained only by T and B lymphocytes has been shown to play a role in anti-fungal vaccination
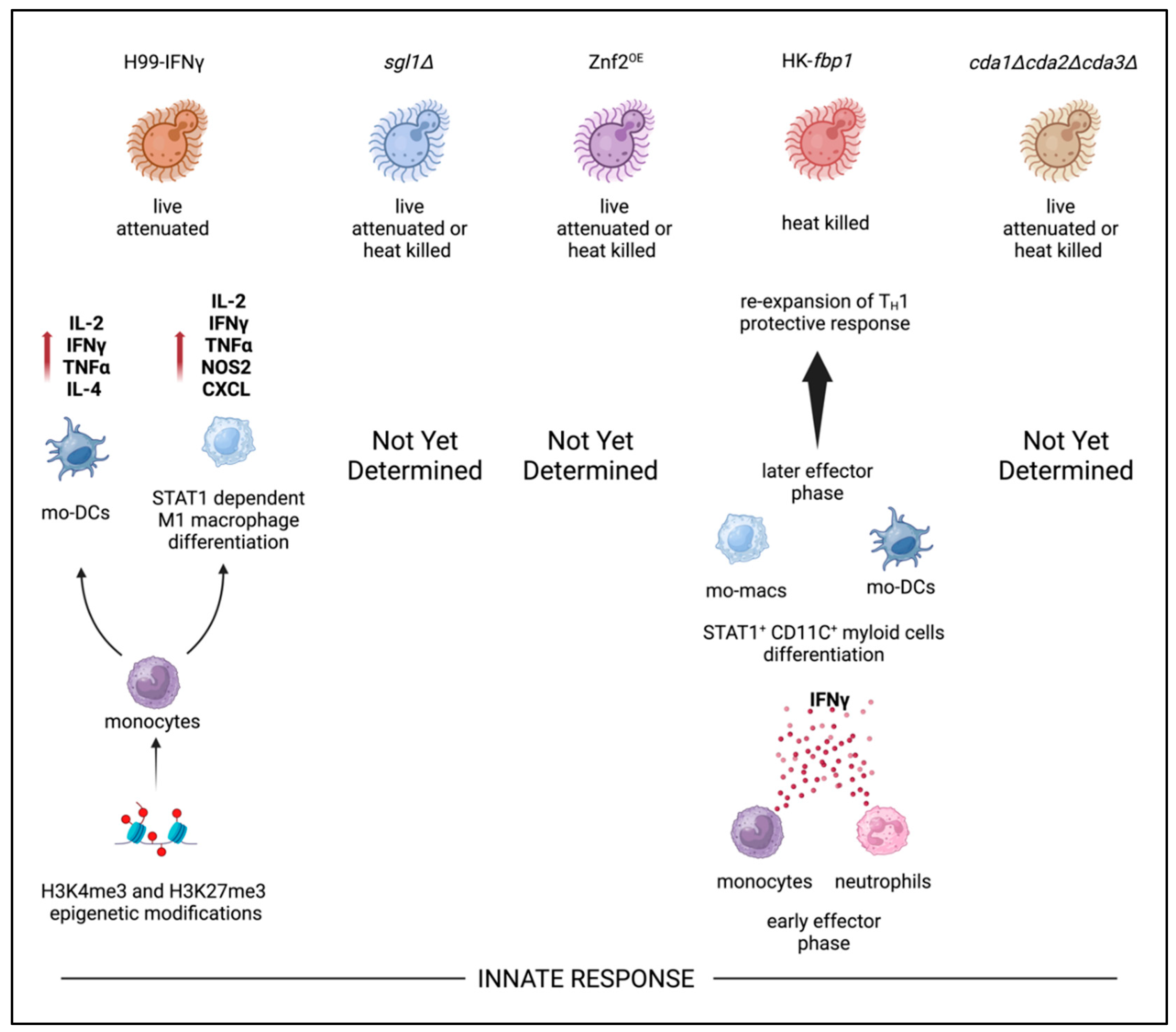
123-125. For example, trained innate immunity of monocytes in transition to macrophages undergo STAT1-dependent epigenetic reprogramming driven by H99γ strain IFNγ production
95 (
Figure 3). In this model, protection continued up to 70 days post H99 challenge in the absence of B cells, CD4
+ T cells, neutrophils, and NK cells
95. These findings emphasized the impact of trained innate immune memory against specific cryptococcal antigen independent of canonical antigen presentation by APCs to CD4
+ T cells. However, these methods of protection are apparent only in live attenuated versions of this strain and protection is lost in the heat killed format. In recent studies, Wang and colleagues identified that monocytes and neutrophils are important producers of IFNγ after HK-
fbp1 vaccination
102. Furthermore, STAT1 expression in CD11c
+ cells (alveolar macrophages, monocyte derived macrophages, and monocyte derived dendritic cells) is required for vaccine-induced protection
102. Currently, other whole cell
C. neoformans based vaccine candidates have shown correlations of increased inflammatory cytokine production in the lung milieu along with increased leukocytes following vaccination and subsequent live challenge
51,109,54,60,61.However, evidence of epigenetic reprogramming of leukocytes attributed to innate immunity training in the remaining vaccine candidates has yet to be elucidated. In aggregate, current studies from several groups suggest trained innate immunity plays a critical role in cryptococcal vaccine candidate that future studies need to focus on as research pivots eventually to multi-valent subunit vaccine approaches.
Acknowledgments
All figures were created in BioRender. Avina, S. (2024)
https://BioRender.com/t55r359. We thank Jeisac Guzman Rivera for technical support. This work is supported by NIH grants R01AI141368-01A and R01AI123315-06A1 and R01AI155647.
Disclosure Statement
The authors have no assets, memberships, or affiliations that disrupt the objectivity of this written review.
References
- Dao A, Kim HY, Garnham K, et al. Cryptococcosis—a systematic review to inform the world health organization fungal priority pathogens list. Medical Mycology. 2024;62(6).
- WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; 2022. License: CC BY-NC-SA 3.0 IGO.
- Hagen F, Khayhan K, Theelen B, et al. Recognition of seven species in the cryptococcus gattii/cryptococcus neoformans species complex. Fungal genetics and biology. 2015;78:16–48.
- Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. The Lancet infectious diseases. 2022;22(12):1748–1755.
- Rivera A, Lodge J, Xue C. Harnessing the immune response to fungal pathogens for vaccine development. Annu Rev Microbiol. 2022;76:703–726.
- An H, Liu Y, Qian C, et al. Chapter 5 - bacterial capsules. In: Tang Y, Hindiyeh MY, Liu D, Sails A, Spearman P, Zhang J, eds. Molecular medical microbiology (third edition). Academic Press; 2024:69–96. https://www.sciencedirect.com/science/article/pii/B9780128186190001507. [CrossRef]
- Boodwa-Ko D, Doering TL. A quick reCAP: Discovering cryptococcus neoformans capsule mutants. Journal of Fungi. 2024;10(2). [CrossRef]
- Cherniak R, Reiss E, Slodki ME, Plattner RD, Blumer SO. Structure and antigenic activity of the capsular polysaccharide of cryptococcus neoformans serotype A. Mol Immunol. 1980;17(8):1025–1032.
- Merrifield EH, Stephen AM. Structural investigations of two capsular polysaccharides from cryptococcus neoformans. Carbohydr Res. 1980;86(1):69–76.
- O'Meara T,R., Alspaugh JA. The cryptococcus neoformans capsule: A sword and a shield. Clin Microbiol Rev. 2012;25(3):387–408.
- Fonseca FL, Reis FCG, Sena BAG, Jozefowicz LJ, Kmetzsch L, Rodrigues ML. The overlooked glycan components of the cryptococcus capsule. Fungal Physiology and Immunopathogenesis. 2019:31–43.
- Bouklas T, Jain N, Fries BC. Modulation of replicative lifespan in cryptococcus neoformans: Implications for virulence. Frontiers in microbiology. 2017;8:98.
- Tejas B, Ximo P, Goldman David L, Batya E, Aviv B, Fries Bettina C. Old cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio. 2013;4(4):10.1128/mbio.00455–13. [CrossRef]
- Al-Huthaifi AM, Radman BA, Al-Alawi AA, Mahmood F, Liu T. Mechanisms and virulence factors of cryptococcus neoformans dissemination to the central nervous system. Journal of Fungi. 2024;10(8):586.
- Carolina C, Emma C, Antonio S, Alexandre A, Arturo C. Intranasal inoculation of cryptococcus neoformans in mice produces nasal infection with rapid brain dissemination. mSphere. 2019;4(4):10.1128/msphere.00483–19. [CrossRef]
- Liu Y, Zhang Y, Zhao X, Lu W, Zhong Y, Fu YV. Antifungal peptide SP1 damages polysaccharide capsule of cryptococcus neoformans and enhances phagocytosis of macrophages. Microbiology Spectrum. 2023;11(2):4562.
- Zaragoza O, Chrisman CJ, Castelli MV, et al. Capsule enlargement in cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008;10(10):2043–2057.
- De Jesus M, Moraes Nicola A, Chow S, et al. Glucuronoxylomannan, galactoxylomannan, and mannoprotein occupy spatially separate and discrete regions in the capsule of cryptococcus neoformans. Virulence. 2010;1(6):500–508.
- Reuwsaat JCV, Motta H, Garcia AWA, et al. A predicted mannoprotein participates in cryptococcus gattii capsular structure. Msphere. 2018;3(2):10.1128/msphere. 00023–18.
- Chang YC, Cherniak R, Kozel TR, et al. Structure and biological activities of acapsular cryptococcus neoformans 602 complemented with the CAP64 gene. Infect Immun. 1997;65(5):1584–1592.
- Chang YC, Kwon-Chung KJ. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of cryptococcus neoformans. J Bacteriol. 1999;181(18):5636–5643.
- Chang YC, Kwon-Chung KJ. Isolation of the third capsule-associated gene, CAP60, required for virulence in cryptococcus neoformans. Infect Immun. 1998;66(5):2230–2236.
- Chang YC, Penoyer LA, Kwon-Chung KJ. The second capsule gene of cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun. 1996;64(6):1977–1983.
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14(7):4912–4919.
- Kozel TR, Gotschlich EC. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. Journal of immunology (Baltimore, Md.: 1950). 1982;129(4):1675–1680.
- Jianfeng L, Tuyetnhu P, Kenton H, Nathan G, Yumeng F, Xiaorong L. Immunoprotection against cryptococcosis offered by Znf2 depends on capsule and the hyphal morphology. mBio. 2022;13(1):2785. [CrossRef]
- Normile TG, Chu TH, Sheridan BS, Del Poeta M. Vaccine protection by cryptococcus neoformans Δsgl1 is mediated by γδ T cells via TLR2 signaling. Mucosal Immunology. 2022;15(6):1416–1430. [CrossRef]
- Datta K, Pirofski L. Towards a vaccine for cryptococcus neoformans: Principles and caveats. FEMS yeast research. 2006;6(4):525–536.
- Maitta Robert W, Kausik D, Qing C, et al. Protective and nonprotective human immunoglobulin M monoclonal antibodies to cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect Immun. 2004;72(8):4810–4818. [CrossRef]
- Casadevall A, Cleare W, Feldmesser M, et al. Characterization of a murine monoclonal antibody to cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42(6):1437–1446.
- Larsen RA, Pappas PG, Perfect J, et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother. 2005;49(3):952–958. [CrossRef]
- Casadevall A, Mukherjee J, Devi SJ, Schneerson R, Robbins JB, Scharff MD. Antibodies elicited by a cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165(6):1086–1093.
- Devi SJ. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of cryptococcus neoformans in a murine model. Vaccine. 1996;14(9):841–844.
- Fleuridor R, Lees A, Pirofski L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with cryptococcus neoformans Infection1. J Immunol. 2001;166(2):1087–1096. https://doi.org/10.4049/jimmunol.166.2.1087. Accessed 8/25/2024. [CrossRef]
- Crawford CJ, Liporagi-Lopes L, Coelho C, et al. Semisynthetic glycoconjugate vaccine candidates against cryptococcus neoformans. ACS Infect Dis. 2024;10(6):2089–2100. [CrossRef]
- Chow S, Casadevall A. Evaluation of cryptococcus neoformans galactoxylomannan–protein conjugate as vaccine candidate against murine cryptococcosis. Vaccine. 2011;29(10):1891–1898. [CrossRef]
- Doering TL. How sweet it is! cell wall biogenesis and polysaccharide capsule formation in cryptococcus neoformans. Annu Rev Microbiol. 2009;63:223–247. [CrossRef]
- Mukaremera L. The cryptococcus wall: A different wall for a unique lifestyle. PLOS Pathogens. 2023;19(2):e1011141. [CrossRef]
- Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: Mechanism of action. Infect Immun. 1995;63(8):3131–3136. [CrossRef]
- James PG, Cherniak R, Jones RG, Stortz CA, Reiss E. Cell-wall glucans of cryptococcus neoformans cap 67. Carbohydr Res. 1990;198(1):23–38. [CrossRef]
- Reese AJ, Doering TL. Cell wall alpha-1,3-glucan is required to anchor the cryptococcus neoformans capsule. Mol Microbiol. 2003;50(4):1401–1409. [CrossRef]
- Thompson JR, Douglas CM, Li W, et al. A glucan synthase FKS1 homolog in cryptococcus neoformans is single copy and encodes an essential function. J Bacteriol. 1999;181(2):444–453. [CrossRef]
- Reese AJ, Yoneda A, Breger JA, et al. Loss of cell wall alpha(1-3) glucan affects cryptococcus neoformans from ultrastructure to virulence. Mol Microbiol. 2007;63(5):1385–1398. [CrossRef]
- Cannon RD, Lamping E, Holmes AR, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22(2):291–321.
- Gilbert NM, Donlin MJ, Gerik KJ, et al. KRE genes are required for β-1, 6-glucan synthesis, maintenance of capsule architecture and cell wall protein anchoring in cryptococcus neoformans. Mol Microbiol. 2010;76(2):517–534.
- Basso AMM, De Castro RJA, de Castro TB, et al. Immunomodulatory activity of β-glucan-containing exopolysaccharides from auricularia auricular in phagocytes and mice infected with cryptococcus neoformans. Medical Mycology. 2019;58(2):227–239. [CrossRef]
- Huang H, Ostroff GR, Lee CK, et al. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J Immunol. 2012;189(1):312–317. [CrossRef]
- Specht CA, Homan EJ, Lee CK, et al. Protection of mice against experimental cryptococcosis by synthesized peptides delivered in glucan particles. mBio. 2021;13(1):e0336721. [CrossRef]
- Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. mBio. 2010;1(3). [CrossRef]
- Specht CA, Lee CK, Huang H, et al. Vaccination with recombinant cryptococcus proteins in glucan particles protects mice against cryptococcosis in a manner dependent upon mouse strain and cryptococcal species. mBio. 2017;8(6). [CrossRef]
- Wang R, Oliveira LVN, Lourenco D, et al. Immunological correlates of protection following vaccination with glucan particles containing cryptococcus neoformans chitin deacetylases. npj Vaccines. 2023;8(1):6. [CrossRef]
- Banks IR, Specht CA, Donlin MJ, Gerik KJ, Levitz SM, Lodge JK. A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen cryptococcus neoformans. Eukaryot Cell. 2005;4(11):1902–1912. [CrossRef]
- Bacon J, Jones D, Farmer VC, Webley DM. The occurrence of α (1–3) glucan in cryptococcus, schizosaccharomyces and polyporus species, and its hydrolysis by a streptomyces culture filtrate lysing cell walls of cryptococcus. Biochimica et Biophysica Acta (BBA)-General Subjects. 1968;158(2):313–315.
- Upadhya R, Lam WC, Maybruck B, Specht CA, Levitz SM, Lodge JK. Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of cryptococcus neoformans. mBio. 2016;7(3). [CrossRef]
- Baker LG, Specht CA, Donlin MJ, Lodge JK. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in cryptococcus neoformans. Eukaryot Cell. 2007;6(5):855–867. [CrossRef]
- Levitz SM, Huang H, Ostroff GR, Specht CA. Exploiting fungal cell wall components in vaccines. Seminars in Immunopathology. 2015;37(2):199–207. [CrossRef]
- Baker Lorina G, Specht Charles A, Lodge Jennifer K. Cell wall chitosan is necessary for virulence in the opportunistic pathogen cryptococcus neoformans. Eukaryotic Cell. 2011;10(9):1264–1268. [CrossRef]
- Upadhya R, Baker LG, Lam WC, Specht CA, Donlin MJ, Lodge JK. Cryptococcus neoformans Cda1 and its chitin deacetylase activity are required for fungal pathogenesis. mBio. 2018;9(6). [CrossRef]
- Lam WC, Upadhya R, Specht CA, et al. Chitosan biosynthesis and virulence in the human fungal pathogen cryptococcus gattii. mSphere. 2019;4(5). [CrossRef]
- Upadhya R, Lam WC, Hole CR, Vasselli JG, Lodge JK. Cell wall composition in cryptococcus neoformans is media dependent and alters host response, inducing protective immunity. Frontiers in Fungal Biology. 2023;4:1183291.
- Hole CR, Lam WC, Upadhya R, Lodge JK. Cryptococcus neoformans chitin synthase 3 plays a critical role in dampening host inflammatory responses. MBio. 2020;11(1):10.1128/mbio. 03373–19.
- Hester MM, Oliveira LVN, Wang R, et al. Cross-reactivity between vaccine antigens from the chitin deacetylase protein family improves survival in a mouse model of cryptococcosis. Front Immunol. 2022;13:1015586. [CrossRef]
- Zaman M, Chandrudu S, Toth I. Strategies for intranasal delivery of vaccines. Drug Deliv Transl Res. 2013;3(1):100–109. [CrossRef]
- Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62(1):59–82. [CrossRef]
- Levitz SM, Specht CA. The molecular basis for the immunogenicity of cryptococcus neoformans mannoproteins. FEMS yeast research. 2006;6(4):513–524.
- Specht CA, Nong S, Dan JM, Lee CK, Levitz SM. Contribution of glycosylation to T cell responses stimulated by recombinant cryptococcus neoformans mannoprotein. J Infect Dis. 2007;196(5):796–800.
- Levitz SM, Nong S, Mansour MK, Huang C, Specht CA. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to cryptococcus neoformans. Proceedings of the National Academy of Sciences. 2001;98(18):10422–10427. [CrossRef]
- Viudes A, Lazzell A, Perea S, et al. The C-terminal antibody binding domain of candida albicans mp58 represents a protective epitope during candidiasis. FEMS Microbiol Lett. 2004;232(2):133–138.
- Woo PC, Lau SK, Lau CC, et al. Mp1p is a virulence factor in talaromyces (penicillium) marneffei. PLoS neglected tropical diseases. 2016;10(8):e0004907.
- Cao L, Chan C, Lee C, Sai-yin Wong S, Yuen K. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus penicillium marneffei. Infect Immun. 1998;66(3):966–973.
- Pietrella D, Corbucci C, Perito S, Bistoni G, Vecchiarelli A. Mannoproteins from cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 2005;73(2):820–827.
- Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. The Journal of Immunology. 2002;168(6):2872–2879.
- Biondo C, Messina L, Bombaci M, et al. Characterization of two novel cryptococcal mannoproteins recognized by immune sera. Infect Immun. 2005;73(11):7348–7355.
- Lee S, Mota C, Thak EJ, et al. Effects of altered N-glycan structures of cryptococcus neoformans mannoproteins, MP98 (Cda2) and MP84 (Cda3), on interaction with host cells. Scientific Reports. 2023;13(1):1175.
- Upadhya R, Lam WC, Hole CR, et al. Cryptococcus neoformans Cda1 and Cda2 coordinate deacetylation of chitin during infection to control fungal virulence. The Cell Surface. 2021;7:100066. https://www.sciencedirect.com/science/article/pii/S2468233021000190. [CrossRef]
- Khan MAS, Miah MI, Rahman SR. A comprehensive immunoinformatic analysis of chitin deacetylase’s and MP88 for designing multi-epitope vaccines against cryptococcus neoformans. Journal of Biomolecular Structure and Dynamics. 2023:1–16.
- Wang R, Oliveira LV, Hester MM, et al. Protection against experimental cryptococcosis elicited by cationic adjuvant formulation 01-adjuvanted subunit vaccines. bioRxiv. 2024.
- Biondo C, Mancuso G, Midiri A, et al. Identification of major proteins secreted by cryptococcus neoformans. FEMS Yeast Res. 2006;6(4):645–651. [CrossRef]
- Cadieux B, Lian T, Hu G, et al. The mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus cryptococcus neoformans. J Infect Dis. 2013;207(8):1339–1347.
- Yu Chen-Hsin, Sephton-Clark Poppy, Tenor Jennifer L, et al. Gene expression of diverse cryptococcus isolates during infection of the human central nervous system. mBio. 2021;12(6):2313. [CrossRef]
- O'Meara TR, Norton D, Price MS, et al. Interaction of cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS pathogens. 2010;6(2):e1000776.
- O'Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. MBio. 2013;4(1):10.1128/mbio. 00522–12.
- Geddes JM, Croll D, Caza M, Stoynov N, Foster LJ, Kronstad JW. Secretome profiling of cryptococcus neoformans reveals regulation of a subset of virulence-associated proteins and potential biomarkers by protein kinase A. BMC microbiology. 2015;15:1–26.
- Geddes JMH, Caza M, Croll D, Stoynov N, Foster LJ, Kronstad JW. Analysis of the protein kinase A-regulated proteome of cryptococcus neoformans identifies a role for the ubiquitin-proteasome pathway in capsule formation. mBio. 2016;7(1):10.1128/mbio.01862–15. [CrossRef]
- Cao C, Wang K, Wang Y, Liu T, Rivera A, Xue C. Ubiquitin proteolysis of a CDK-related kinase regulates titan cell formation and virulence in the fungal pathogen cryptococcus neoformans. Nature communications. 2022;13(1):6397.
- Buzas EI. The roles of extracellular vesicles in the immune system. Nature Reviews Immunology. 2023;23(4):236–250.
- Kalluri R. The biology and function of extracellular vesicles in immune response and immunity. Immunity. 2024;57(8):1752–1768.
- Zhou Q, Ma K, Hu H, Xing X, Huang X, Gao H. Extracellular vesicles: Their functions in plant–pathogen interactions. Molecular plant pathology. 2022;23(6):760–771.
- de Oliveira HC, Castelli RF, Reis FC, Rizzo J, Rodrigues ML. Pathogenic delivery: The biological roles of cryptococcal extracellular vesicles. Pathogens. 2020;9(9):754.
- Rizzo J, Wong SSW, Gazi AD, et al. Cryptococcus extracellular vesicles properties and their use as vaccine platforms. J Extracell Vesicles. 2021;10(10):e12129. [CrossRef]
- Colombo AC, Rella A, Normile T, et al. Cryptococcus neoformans glucuronoxylomannan and sterylglucoside are required for host protection in an animal vaccination model. MBio. 2019;10(2):10.1128/mbio. 02909–18.
- Rella A, Mor V, Farnoud AM, et al. Role of sterylglucosidase 1 (Sgl1) on the pathogenicity of cryptococcus neoformans: Potential applications for vaccine development. Frontiers in microbiology. 2015;6:836.
- Del Poeta M, Wormley,Floyd L.,,Jr, Lin X. Host populations, challenges, and commercialization of cryptococcal vaccines. PLOS Pathogens. 2023;19(2):e1011115. [CrossRef]
- Wormley FL, J., Perfect JR, Steele C, Cox GM. Protection against cryptococcosis by using a murine gamma interferon-producing cryptococcus neoformans strain. Infect Immun. 2007;75(3):1453–1462. [CrossRef]
- Leopold Wager CM, Hole CR, Campuzano A, et al. IFN-γ immune priming of macrophages in vivo induces prolonged STAT1 binding and protection against cryptococcus neoformans. PLoS pathogens. 2018;14(10):e1007358.
- Van Dyke MCC, Chaturvedi AK, Hardison SE, et al. Induction of broad-spectrum protective immunity against disparate cryptococcus serotypes. Frontiers in Immunology. 2017;8:1359.
- Zhai B, Wozniak KL, Masso-Silva J, et al. Development of protective inflammation and cell-mediated immunity against cryptococcus neoformans after exposure to hyphal mutants. mBio. 2015;6(5):1433. [CrossRef]
- Lin J, Zhao Y, Ferraro AR, Yang E, Lewis ZA, Lin X. Transcription factor Znf2 coordinates with the chromatin remodeling SWI/SNF complex to regulate cryptococcal cellular differentiation. Communications Biology. 2019;2(1):412. [CrossRef]
- Tuyetnhu P, Yeqi L, Wendy W, Xiaorong L. Vaccination with a ZNF2oe strain of cryptococcus provides long-lasting protection against cryptococcosis and is effective in immunocompromised hosts. Infect Immun. 2023;91(7):198. [CrossRef]
- Yina W, Keyi W, Masso-Silva Jorge A, Amariliz R, Chaoyang X. A heat-killed cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio. 2019;10(6):10.1128/mbio.02145–19. [CrossRef]
- Yina W, Keyi W, Amariliz R, Chaoyang X. Development of a heat-killed fbp1 mutant strain as a therapeutic agent to treat invasive cryptococcus infection. Microbiology Spectrum. 2023;11(2):4955. [CrossRef]
- Keyi W, Vanessa E, Yina W, et al. Innate cells and STAT1-dependent signals orchestrate vaccine-induced protection against invasive cryptococcus infection. mBio. 2024;0(0):1944. [CrossRef]
- Wang Y, Wang K, Masso-Silva J, Rivera A, Xue C. A heat-killed cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model. mBio. 2019;10(6). [CrossRef]
- Masso-Silva J, Espinosa V, Liu TB, Wang Y, Xue C, Rivera A. The F-box protein Fbp1 shapes the immunogenic potential of cryptococcus neoformans. mBio. 2018;9(1). [CrossRef]
- Hester MM, Carlson D, Lodge JK, Levitz SM, Specht CA. Immune evasion by cryptococcus gattii in vaccinated mice coinfected with C. neoformans. Frontiers in Immunology. 2024;15. [CrossRef]
- Chen J, Shao J, Dai M, Fang W, Yang Y. Adaptive immunology of cryptococcus neoformans infections—an update. Frontiers in immunology. 2023;14:1174967.
- Wozniak KL, Young ML, Wormley Jr FL. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clinical and Vaccine Immunology. 2011;18(5):717–723.
- Wozniak KL, Ravi S, Macias S, et al. Insights into the mechanisms of protective immunity against cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PloS one. 2009;4(9):e6854.
- Normile TG, Rella A, Del Poeta M. Cryptococcus neoformans Δ sgl1 vaccination requires either CD4 or CD8 T cells for complete host protection. Frontiers in Cellular and Infection Microbiology. 2021;11:739027.
- Masso-Silva J, Espinosa V, Liu T, Wang Y, Xue C, Rivera A. The F-box protein Fbp1 shapes the immunogenic potential of cryptococcus neoformans. MBio. 2018;9(1):10.1128/mbio. 01828–17.
- Espinosa V, Dutta O, Heung LJ, et al. Cutting edge: Neutrophils license the maturation of monocytes into effective antifungal effectors. The Journal of Immunology. 2022;209(10):1827–1831.
- Espinosa V, Dutta O, McElrath C, et al. Type III interferon is a critical regulator of innate antifungal immunity. Science immunology. 2017;2(16):eaan5357.
- Espinosa V, Jhingran A, Dutta O, et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS pathogens. 2014;10(2):e1003940.
- Mukaremera L, Nielsen K. Adaptive immunity to cryptococcus neoformans infections. Journal of fungi. 2017;3(4):64.
- Specht Charles A, Ruiying W, Oliveira Lorena VN, et al. Immunological correlates of protection mediated by a whole organism, cryptococcus neoformans, vaccine deficient in chitosan. mBio. 2024;15(8):1746. [CrossRef]
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13(9):817–822.
- Robinson MJ, Osorio F, Rosas M, et al. Dectin-2 is a syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206(9):2037–2051.
- Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against candida albicans. Immunity. 2010;32(5):681–691.
- Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates toll-like receptor signaling via raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity. 2007;26(5):605–616.
- Gringhuis SI, Den Dunnen J, Litjens M, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through raf-1 and syk. Nat Immunol. 2009;10(2):203–213.
- LeibundGut-Landmann S, Groß O, Robinson MJ, et al. Syk-and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–638.
- Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17(4):356–363.
- Cheng S, Quintin J, Cramer RA, et al. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684.
- Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098.
- Divangahi M, Aaby P, Khader SA, et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat Immunol. 2021;22(1):2–6.
- Tarang S, Kesherwani V, LaTendresse B, Lindgren L, Rocha-Sanchez SM, Weston MD. In silico design of a multivalent vaccine against candida albicans. Scientific Reports. 2020;10(1):1066.
- Tarcha Eric J, Venkatesha B, Hung Chiung-Yu, Gardner Malcolm J, Cole Garry T. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect Immun. 2006;74(10):5802–5813. [CrossRef]
- Choudhury QJ, Ambati S, Link CD, Lin X, Lewis ZA, Meagher RB. Dectin-3-targeted antifungal liposomes efficiently bind and kill diverse fungal pathogens. Mol Microbiol. 2023;120(5):723–739. [CrossRef]
- Pham T, Shi R, Ambati S, Meagher R, Lin X. All hands on dec: Treating cryptococcosis with dectin decorated liposomes loaded with antifungals. iScience. 2024;27(7). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).