Submitted:
25 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
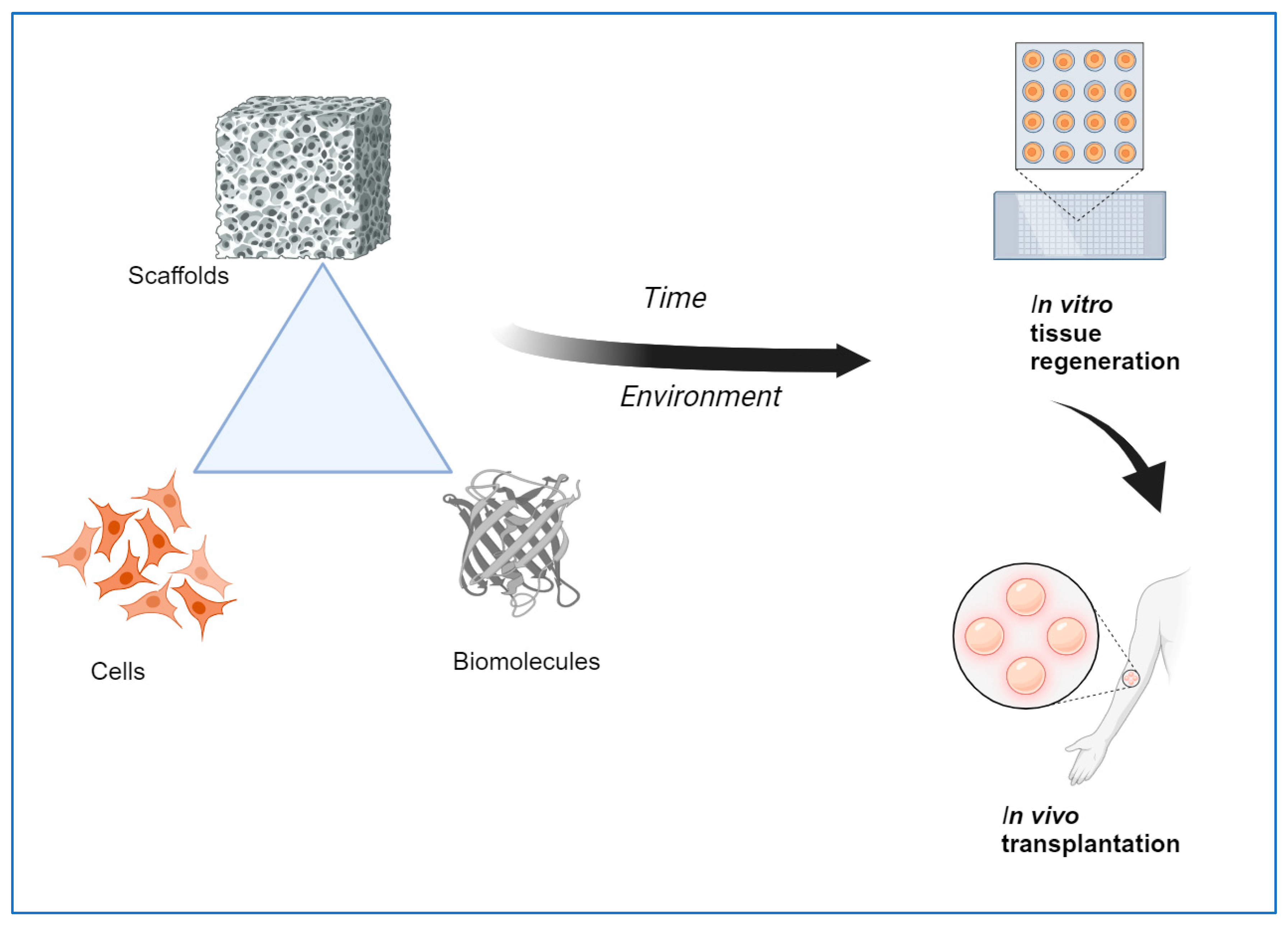
Tissue engineering is an interdisciplinary field that combines methods, materials, and biological molecules to fabricate newly formed tissues to replace or restore functional organs. Biomaterials-based scaffolds play a vital role in developing new tissue by interacting with human cells. Tissue Engineering scaffolds with ideal characteristics, namely, nontoxicity, biodegradability, and appropriate mechanical and surface properties, are vital for tissue regeneration applications. Although commonly utilized biomaterials can provide physical and chemical properties needed for tissue regeneration, inadequate biomimetic properties, as well as insufficient interactions of cells-scaffolds interaction, still need to be improved for the application of tissue engineering in vivo. Consequently, developing innovative biomaterials-based stimulus-responsive 3D scaffolds that can enhance the mediators of cell adhesion and cellular functioning and that can form functional tissues by providing structural integrity within an appropriate time is much needed. It is possible to achieve some essential features using a single material, so combining two or more materials may fulfill the requirements. In order to achieve a proper scaffold design, a suitable fabrication technique and combination of biomaterials with controlled micro or nanostructures are needed to achieve the proper biological responses. This review highlights the natural polymers, smart materials, and recent advanced techniques currently used to create emerging scaffolds for tissue regeneration applications.
Keywords:
1. Introduction
2. Natural, Synthetic, and Composite Materials Are Used to Fabricate TE Scaffolds
3. Scaffold Fabrication Techniques
| Methods | Summary | Reference |
|---|---|---|
| Increasing spinning time | In this way, an electrospun fiber membrane with a certain thickness will be obtained, which can reach hundreds of microns and become a 3D fibrous structure, although these methods may take a long time (for example, from 20 min to 20 h) till it grows to a sufficient 3D structure); mat thickness increases by increased spinning times leading to 3D fibrous thickness. Besides, multilayered with different materials can be fabricated by sequential electrospinning and co-electrospinning. This method's advantages are that each layer's fiber diameter, composition, and porosity can be controlled. | [35] |
| Assembly by post-processing of 2D electrospun fibrous structures | Examples are folding, layer-by-layer electrospinning, sintering, mechanical expansion such as peeling off the thin film from the collector, and then bending/folding or stacking the fiber layers into a 3D fibrous structure like pipe or thick mat | [36] |
| Direct assembly by an auxiliary factor | Examples are a 3D template, liquid, and collector. In addition, 3D fiber structures can also be successfully obtained through modification of the collector, for example, substituting the conventional 2D flat collector with a 3D collecting template and using liquid collection and removing microparticles filled between nanofibers have been reported, although a subsequent treatment to dry the as-prepared 3D structures or handle with the porogen is usually needed. | [37] |
| Self-assembly | A rapid growth of 3D fibrous macro without any additional assistance. Examples are fibrous yarns or spongiform fiber stacks. | [37] |
| SMPs | Technique/Mechanisms | Applications | Reference |
| Poly (N-isopropyl acrylamide) hydrogel matrix with 0.8 wt% nano fibrillated cellulose (NFC) | Biomimetic 4D printing. Reversible shape changes in water of varying temperature |
Composite hydrogel architectures were 4D bioprinted with localized, anisotropic swelling behavior that solves the inverse problem of designing the alignment patterns for prearranged target shapes generating complex three-dimensional morphologies for generating architectures for biomedical devices, tissue engineering, and soft robotics. | [41] |
| Bistrips/patches based on a poly(N-isopropyl acrylamide)-based hydrogel | Temperature-responsive swelling | A potential route for the development of self-folding stimuli-responsive micro-devices for biomedical applications. | [42] |
| Polylactic acid (PLA) and continuous carbon fiber-based continuous fiber fiber-reinforced thermoplastic Composites (CFRTPCs) |
Fused deposition modeling (FDM) | Light structures in the field of aviation and aerospace and biomedical applications. | [43] |
| Polybutylene succinate and polylactic acid (PBS/PLA) filament | 4 D printed and the graphene oxide (GO) functionalized shape memory PBS/PLA scaffolds | 4D printed PBS/PLA filament showed outstanding shape memory performance and demonstrated a promising prospect in the biomedical field. | [44] |
| Semicrystalline thermoplastic PLA pellets and Fe3O4 nanoparticles | Direct ink writing (DIW) | Minimally invasive medicine, biomedical devices | [45] |
| Polyethyleneimine (PEI); Hyaluronic acid; Gelatin; Human umbilical vein endothelial cells (HUVECs) | 4D inkjet printing | Tissue engineering | [46] |
| Polyethylene glycol diacrylate (PEGDA) hydrogel | Digital light processing (DLP) | Cardiac tissue regeneration | [47] |
| Thermoplastic polyurethane | Selective laser sintering (SLS). The shape-recovered scaffold facilitated directional cell adhesion and stimulated cell proliferation. | Bone tissue engineering | [48] |
4. Tissue-Engineered Product Applications
5. Importance of Continued Innovation in Scaffold Development Using Smart Materials
6. Tissue Engineering and the Use of Nanomaterials
7. Future Research Directions
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Garot C, Bettega G, Picart C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv Funct Mater. 2020 Oct 15;31(5):2006967. [CrossRef] [PubMed] [PubMed Central]
- Aldana AA, Abraham GA. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int J Pharm. 2017 May 25;523(2):441-453. [CrossRef] [PubMed]
- Gregor A, Filová E, Novák M, Kronek J, Chlup H, Buzgo M, Blahnová V, Lukášová V, Bartoš M, Nečas A, Hošek J. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng. 2017 Oct 16;11:31. [CrossRef] [PubMed] [PubMed Central]
- Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):14452-9. [CrossRef] [PubMed] [PubMed Central]
- Dzobo K, Thomford NE, Senthebane DA, Shipanga H, Rowe A, Dandara C, Pillay M, Motaung KSCM. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018 Jul 30;2018:2495848. [CrossRef] [PubMed] [PubMed Central]
- Kaul H, Ventikos Y. On the genealogy of tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2015 Apr;21(2):203-17. [CrossRef] [PubMed] [PubMed Central]
- Krishani M, Shin WY, Suhaimi H, Sambudi NS. Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review. Gels. 2023 Jan 23;9(2):100. [CrossRef] [PubMed] [PubMed Central]
- Marques CF, Diogo GS, Pina S, Oliveira JM, Silva TH, Reis RL. Collagen-based bioinks for complex tissue engineering applications: a comprehensive review. J Mater Sci Mater Med. 2019 Mar 6;30(3):32. [CrossRef] [PubMed]
- Silver, F. H., Jaffe, M., & Shah, R. G. Structure and behavior of collagen fibers. In Handbook of Properties of Textile and technical fibers (pp. 345–365). 2018. Woodhead Publishing.
- Fan, J.; Abedi-Dorcheh, K.; Sadat Vaziri, A.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Rastegar Adib, F.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef]
- Afewerki S, Sheikhi A, Kannan S, Ahadian S, Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: Towards natural therapeutics. Bioeng Transl Med. 2018 Dec 28;4(1):96-115. [CrossRef] [PubMed] [PubMed Central]
- Kuttappan S, Mathew D, Nair MB. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering - A mini-review. Int J Biol Macromol. 2016 Dec;93(Pt B):1390-1401. [CrossRef] [PubMed]
- Lavanya K, Chandran SV, Balagangadharan K, Selvamurugan N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020 Jun;111:110862. [CrossRef] [PubMed]
- Islam, S., Bhuiyan, M.A.R. & Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J Polym Environ 25, 854–866 (2017).
- Ahmed S, Annu, Ali A, Sheikh J. A review on chitosan-centred scaffolds and their applications in tissue engineering. Int J Biol Macromol. 2018 Sep;116:849-862. [CrossRef] [PubMed]
- Cen, L., Liu, W., Cui, L. et al. Collagen Tissue Engineering: Development of Novel Biomaterials and Applications. Pediatr Res 63, 492–496 (2008). [CrossRef]
- Gholap AD, Rojekar S, Kapare HS, Vishwakarma N, Raikwar S, Garkal A, Mehta TA, Jadhav H, Prajapati MK, Annapure U. Chitosan scaffolds: Expanding horizons in biomedical applications. Carbohydr Polym. 2024 Jan 1;323:121394.
- Echave, M. C., Pimenta-Lopes, C., Pedraz, J. L., Mehrali, M., Dolatshahi-Pirouz, A., Ventura, F., & Orive, G. Enzymatic crosslinked gelatin 3D scaffolds for bone tissue engineering. Int J Pharmaceutics, 2019. 562, 151-161.
- Dong C, Lv Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers (Basel). 2016 Feb 4;8(2):42.
- Eivazzadeh-Keihan, R., Noruzi, E. B., Mehrban, S. F., Aliabadi, H. A. M., Karimi, M., Mohammadi, A., & Maleki, A. Review: the latest advances in biomedical applications of chitosan hydrogel as a robust natural structure with eye-catching biological properties. J Mater Sci, 2022 57(6), 3855.
- Wong, S. K., Yee, M. M. F., Chin, K.-Y., & Ima-Nirwana, S. A review of the application of natural and synthetic scaffolds in bone regeneration. J Func Biomater, 2023, 14(5), 286. [CrossRef]
- Sowmya, S., Bumgardener, J. D., Chennazhi, K. P., Nair, S. V., & Jayakumar, R. (2013). Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Progress in Polymer Science, 38(10-11), 1748–1772.
- Prabhakaran, M. P., Venugopal, J., Chan, C. K., & Ramakrishna, S. (2008). Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology, 19(45), 455102.
- Turnbull, G., Clarke, J., Picard, F., Riches, P., Jia, L., Han, F., Li, B., & Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioactive Materials, 2018, 3(3), 278–314. [CrossRef]
- Gang, F., Ye, W., Ma, C., Wang, W., Xiao, Y., Liu, C., & Sun, X. 3D printing of PLLA/biomineral composite bone tissue engineering scaffolds. Materials, 2023, 15(12), 4280. [CrossRef]
- Kaviani, M., Geramizadeh, B. Basic Aspects of Skin Tissue Engineering: Cells, Biomaterials, Scaffold Fabrication Techniques, and Signaling Factors. J. Med. Biol. Eng. 43, 508–521 (2023).
- Kundu, J., Pati, F., Shim, J., & Cho, D. Rapid prototyping technology for bone regeneration. Rapid Prototyping of Biomaterials (Second Edition), 2014, 289-314.
- Joseph, B., Jose, C., Kavil, S. V., Kalarikkal, N. & Thomas, S. in Functional Biomaterials: Design and Development for Biotechnology, Pharmacology, and Biomedicine, 2 Volumes 371–394 (wiley, 2023).
- Capuana E, Lopresti F, Carfì Pavia F, Brucato V, La Carrubba V. Solution-Based Processing for Scaffold Fabrication in Tissue Engineering Applications: A Brief Review. Polymers (Basel). 2021 Jun 22;13(13):2041. [CrossRef] [PubMed] [PubMed Central]
- Sultana, N., Wang, M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterization of the scaffolds. J Mater Sci: Mater Med. 2008, 19, 2555–2561. [CrossRef]
- Ejiohuo, O. A Perspective on the Synergistic Use of 3D Printing and Electrospinning to Improve Nanomaterials for Biomedical Applications. Nano Trends, 2023,100025.
- El-Fiqi, A., Lee, J. H., Lee, E. J., & Kim, H. W. Collagen hydrogels incorporated with surface-aminated mesoporous nano bioactive glass: improved physicochemical stability and mechanical properties are adequate for complex tissue engineering. Acta Biomater, 2023, 9(12), 9508-9521.
- Lim, M. M., Sun, T, Sultana, N. In Vitro Biological Evaluation of Electrospun Polycaprolactone/Gelatine Nanofibrous Scaffold for Tissue Engineering, J Nanomater, 2015, vol. 2015, Article ID 303426, 10 pages, 2015. [CrossRef]
- Alshammari, A., Alabdah, F., Wang, W., & Cooper, G. Virtual Design of 3D-Printed Bone Tissue Engineered Scaffold Shape Using Mechanobiological Modeling: Relationship of Scaffold Pore Architecture to Bone Tissue Formation. Polymers, 2023, 15(19), 3918.
- Soliman, S., Pagliari, S., Rinaldi, A., Forte, G., Fiaccavento, R., Pagliari, F., … Traversa, E. (2010). Multiscale three-dimensional scaffolds for soft tissue engineering via multimodal electrospinning. Acta Biomaterialia, 6(4), 1227–1237.
- Subramanian, A., Krishnan, U. M., & Sethuraman, S. (2011). Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomedical Materials, 6(2), 025004.
- Sun, B., Long, Y. Z., Zhang, H. D., Li, M. M., Duvail, J. L., Jiang, X. Y., & Yin, H. L. (2014). Advances in three-dimensional nanofibrous macrostructures via electrospinning. Progress in Polymer Science, 39(5), 862–890.
- Gao, B., Yang, Q., Zhao, X., Jin, G., Ma, Y., & Xu, F. (2016). 4D Bioprinting for Biomedical Applications. Trends in Biotechnology, 34(9), 746-756. [CrossRef]
- Lai, J., Liu, Y., Lu, G., Yung, P., Wang, X., Tuan, R. S., & Li, Z. A. (2024). 4D bioprinting of programmed dynamic tissues. Bioactive Materials, 37, 348-377. [CrossRef]
- Yan, S., Zhang, F., Luo,L., Wang, L., Liu, Y., Leng.J., Shape Memory Polymer Composites: 4D Printing, Smart Structures, and Applications. Research. 2023;6:0234. [CrossRef]
- Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mater. 2016 Apr;15(4):413-8. [CrossRef] [PubMed]
- Ionov, L. (2014). Hydrogel-based actuators: Possibilities and limitations. Materials Today, 17(10), 494-503. [CrossRef]
- Tian, X., Liu, T., Yang, C., Wang, Q., & Li, D. (2016). Interface and performance of 3D printed continuous carbon fiber reinforced PLA composites. Composites Part A: Applied Science and Manufacturing, 88, 198-205. [CrossRef]
- Lin, C., Liu, L., Liu, Y., & Leng, J. (2022). 4D printing of shape memory polybutylene succinate/polylactic acid (PBS/PLA) and its potential applications. Composite Structures, 279, 114729. [CrossRef]
- Wei H, Zhang Q, Yao Y, Liu L, Liu Y, Leng J. Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite. ACS Appl Mater Interfaces. 2017;9(1):876–883.
- Cui C, Kim DO, Pack MY, Han B, Han L, Sun Y, Han LH. 4D printing of self-folding and cell-encapsulating 3D microstructures as scaffolds for tissue-engineering applications. Biofabrication. 2020;12(4): Article 045018.
- Wang Y, Cui H, Wang Y, Xu C, Esworthy TJ, Hann SY, Boehm M, Shen YL, Mei D, Zhang LG. 4D printed cardiac construct with aligned myofibers and adjustable curvature for myocardial regeneration. ACS Appl Mater Interfaces. 2021;13(11):12746–12758.
- Shuai C, Wang Z, Peng S, Shuai Y, Chen Y, Zeng D, Feng P. Water-responsive shape memory thermoplastic polyurethane scaffolds triggered at body temperature for bone defect repair. Mater Chem Front. 2022;6(36):1456–1469.
- Zheng X, Zhang P, Fu Z, Meng S, Dai L, Yang H. Applications of nanomaterials in tissue engineering. RSC Adv. 2021 May 26;11(31):19041-19058.
- Divakar, P., Moodie, K. L., Demidenko, E., Hoopes, P. J., & Wegst, U. G. Quantitative evaluation of the in vivo biocompatibility and performance of freeze-cast tissue scaffolds. Biomed. Mater., 2020, 15(5), 055003.
- Maleki, A., et al., Biomedical Applications of MXene-Integrated Composites: Regenerative Medicine, Infection Therapy, Cancer Treatment, and Biosensing. Advanced Functional Materials, 2022: p. 2203430.
- Iravani, S. and R.S. Varma, MXenes and MXene-based materials for tissue engineering and regenerative medicine: recent advances. Materials Advances, 2021. 2(9): p. 2906-2917.
- Hasan, A., Morshed, M., Memic, A., Hassan, S., Webster, T. J., & Marei, H. E. S. (2018). Nanoparticles in tissue engineering: Applications, challenges, and prospects. International Journal of Nanomedicine, 13, 5637–5655. [CrossRef]
- Khan, I., Saeed, K., & Khan, I. (2019). Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry, 12(7), 908–931. [CrossRef]
- Joudeh, N., & Linke, D. (2022). Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. Journal of Nanobiotechnology, 20(262). [CrossRef]
- Khan, Y., Sadia, H., Shah, S. Z. A., Khan, M. N., Shah, A. A., Ullah, N., Ullah, M. F., Bibi, H., Bafakeeh, O. T., Ben Khedher, N., Eldin, S. M., Fadhl, B. M., & Khan, M. I. (2022). Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: A review. Catalysts, 12(11), 1386. [CrossRef]
- Vasita, R., & Katti, D. S. (2006). Nanofibers and their applications in tissue engineering. International Journal of Nanomedicine, 1(1), 15–30. [CrossRef]
- Bramhill, J., Ross, S., & Ross, G. (2017). Bioactive nanocomposites for tissue repair and regeneration: A review. International Journal of Environmental Research and Public Health, 14(1), 66. [CrossRef]
- Bao, L., Cui, X., Mortimer, M., Wang, X., Wu, J., & Chen, C. (2023). The renaissance of one-dimensional carbon nanotubes in tissue engineering. Nano Today, 49, 101784. [CrossRef]
- Breuer T, Jimenez M, Humphrey JD, Shinoka T, Breuer CK. Tissue Engineering of Vascular Grafts: A Case Report From Bench to Bedside and Back. Arterioscler Thromb Vasc Biol. 2023 Mar;43(3):399-409.
- Antmen, E., Vrana, N. E., & Hasirci, V. The role of biomaterials and scaffolds in immune responses in regenerative medicine: macrophage phenotype modulation by biomaterial properties and scaffold architectures. Biomater. Sci. 2021. 9(24), 8090-8110.
- Eltom, A., Zhong, G., & Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv. Mater. Sci. Eng., 2019.
- Al-Abduljabbar, A., & Farooq, I. Electrospun polymer nanofibers: Processing, properties, and applications. Polymers, 2022, 15(1), 65. [CrossRef]
- Shukla, A., Dasgupta, N., Ranjan, S., Singh, S., & Chidambaram, R. Nanotechnology towards prevention of anemia and osteoporosis: from concept to market. Biotech Biotechnol Equip. 2017, 31(5), 863-879.
- Sun, J. L., Jiao, K., Niu, L. N., Jiao, Y., Song, Q., Shen, L. J., & Chen, J. H. Intrafibrillar silicified collagen scaffold modulates monocytes to promote cell homing, angiogenesis, and bone regeneration. Biomaterials, 2017, 113, 203-216.
- Sultana, N., Chang, H., Jefferson, S. et al. Application of conductive poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) polymers in potential biomedical engineering. J. Pharm. Investig. 50, 437–444 (2020). [CrossRef]
- Chang, H. C., Sun, T., Sultana, N., Lim, M. M., Khan, T. H., & Ismail, A. F. (2016). Conductive PEDOT:PSS coated polylactide (PLA) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) electrospun membranes: Fabrication and characterization. Materials Science and Engineering: C, 61, 396-410. [CrossRef]
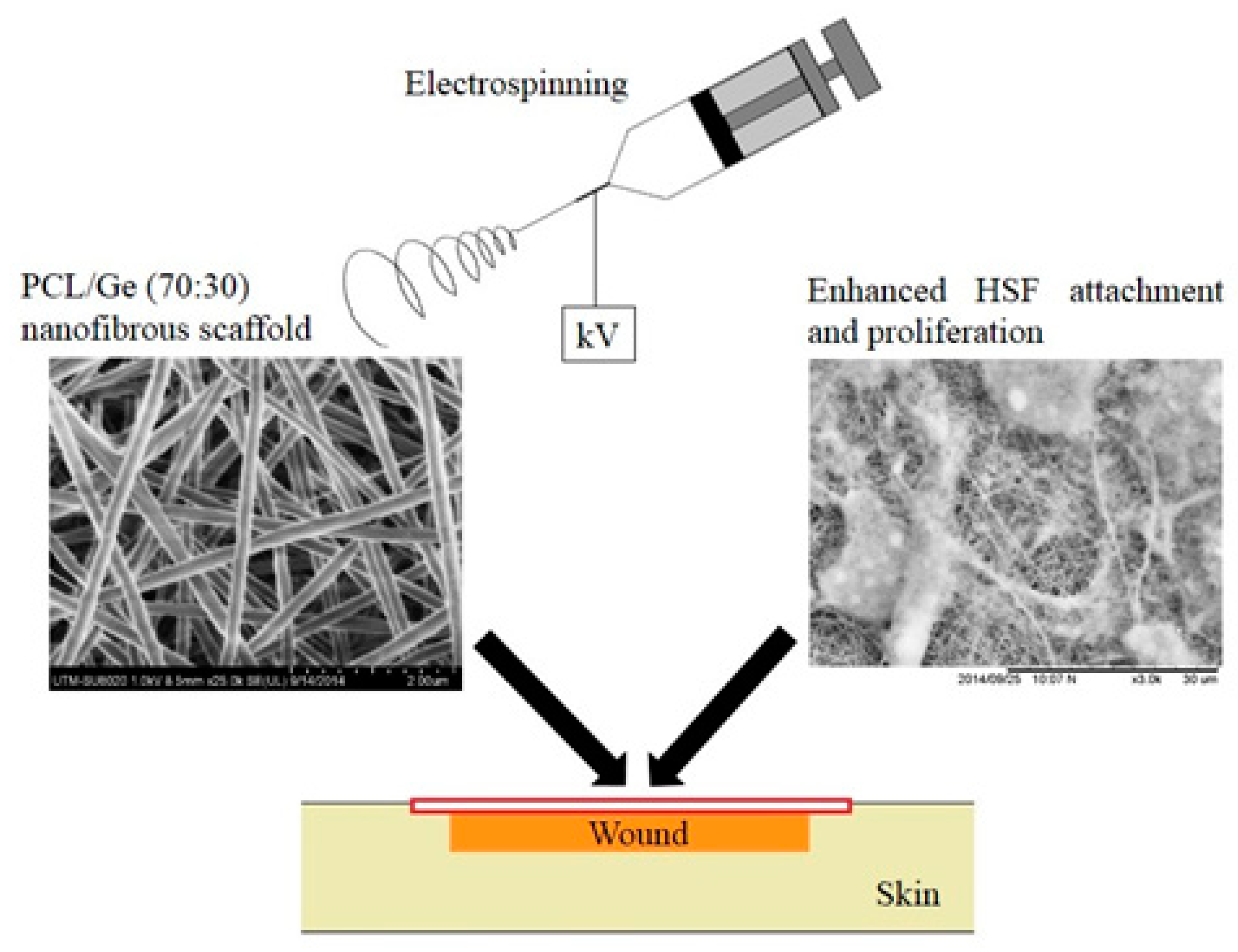
| Natural polymer | Structure/method of production | Biological properties | References |
|---|---|---|---|
| Collagen | Fibrillar structure, which contributes to the extracellular scaffolding | Promotes regeneration and angiogenesis of the bone through monocyte immunomodulation |
[16] |
| Chitosan | It contains an amine group, vital in pH sensitivity and functionality | Induces biological activity by showing excellent antimicrobial activity against bacteria | [17] |
| Gelatin | Composed of a freeze-dried fiber scaffold | Produces a scaffold that is enzymatically crosslinked to enhance bone regeneration. | [18] |
| Collagen | Consists of a triple helix chain formed by α chains | Offers low immunogenicity, a porous structure, permeability, good biocompatibility, and biodegradability | [16] |
| Collagen | Classical fibril-forming collagens, including types I, II, and III collagens | Crosslink formation can shield or modify major antigenic sites and, thus, reduce their capacity to interact with antibodies. | [19] |
| Chitosan | Semicrystalline biopolymer contains several hydrogen bonds forming functional groups, including amino and hydroxyl groups. | The hydrogels are pH-sensitive in aqueous media, so these stimuli-responsive hydrogels are the best choice for drug delivery. | [20] |
| Chitosan | Scaffolds, cells, and bio-signals, all together to minimize artificial and cellular environment | Cardiovascular tissue engineering | [15] |
| Gelatin | In its structure, amino acid sequences such as the arginine-glycine-aspartic acid (RGD) motif improve cell adhesion, differentiation, and proliferation. | Obtain different isometric points. | [18] |
| Pectin Systems | Method | Application |
|---|---|---|
| Low-methoxyl citrus pectin | UV photocrosslinking with peptide crosslinkers (cell-degradable) and adhesive ligands (integrin-specific); lyophilization | Skin tissue engineering |
| Sugar beet pectin (SBP) crosslinked by visible light | Applying 405 nm visible light in the presence of tris(bipyridine)ruthenium (II) chloride hexahydrate and sodium persulfate, rapid hydrogenation of SBP was obtained; 3D hydrogel constructs were obtained using 3D bioprinting | Promising for liver and other soft tissue engineering |
| Citrus peel’s pectin crosslinked with (3glycidyloxypropyl) trimethoxysilane (GPTMS) |
Freeze-drying or 3D bioprinting | Various tissue regeneration |
| Pectin/chitin/nano CaCO3 | Lyophilization | Bone regeneration |
| Pectin/chitosan | Freeze-drying | Bone tissue engineering |
| Pectin/strontium/hydroxyapatite | Solution-based chemical technique | Bone regeneration |
| Collagen/polyurethane/pectin | Semi-interpenetration process | Bone regeneration |
| Pectin/PVA | Freezing–thawing | Bone regeneration |
| Poly(L-lactide-co-ɛ-caprolactone) (PLCA)/pectin | Scaffolds functionalized with pectin | In vitro and in vivo bone regeneration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





