Submitted:
27 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
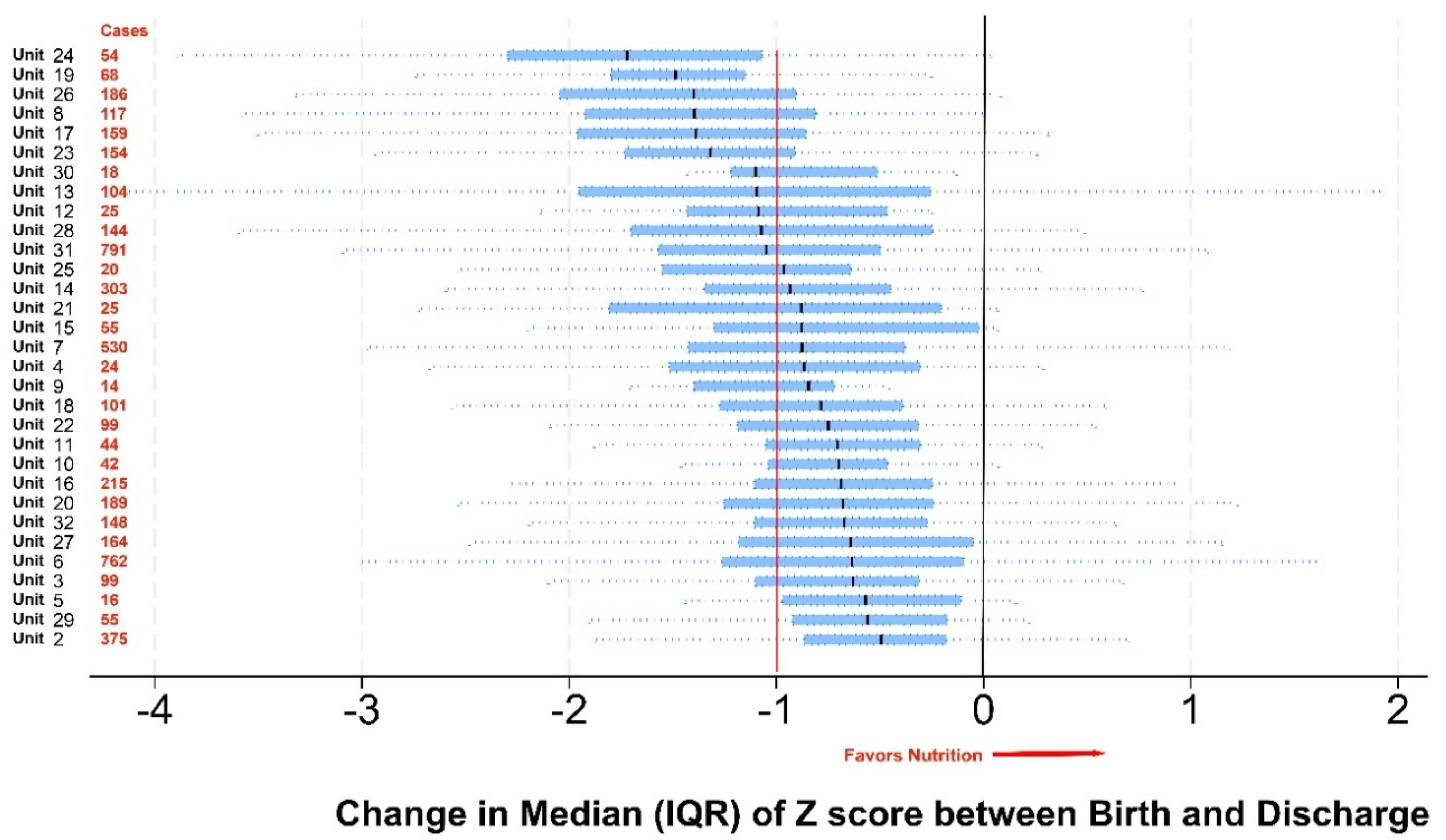
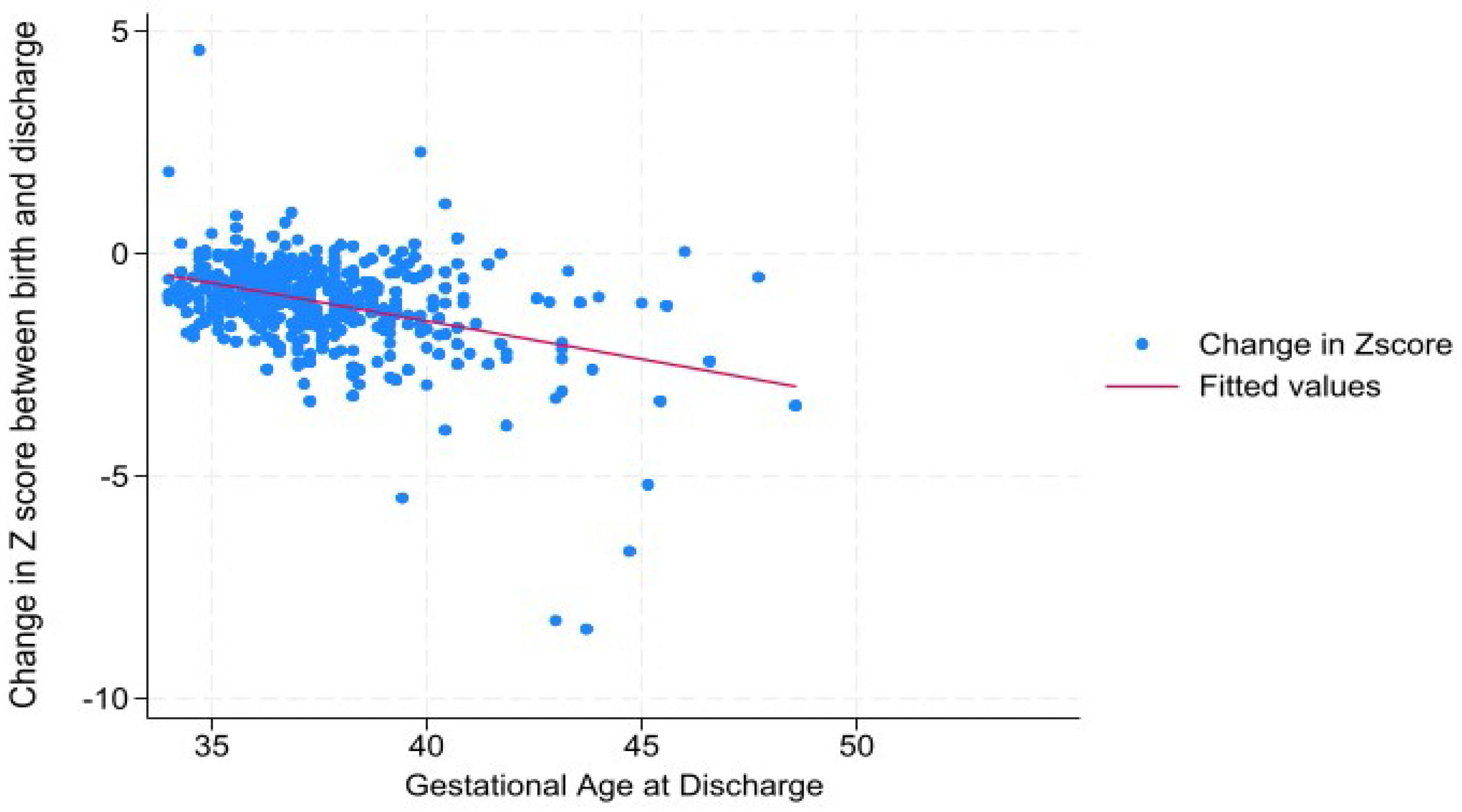
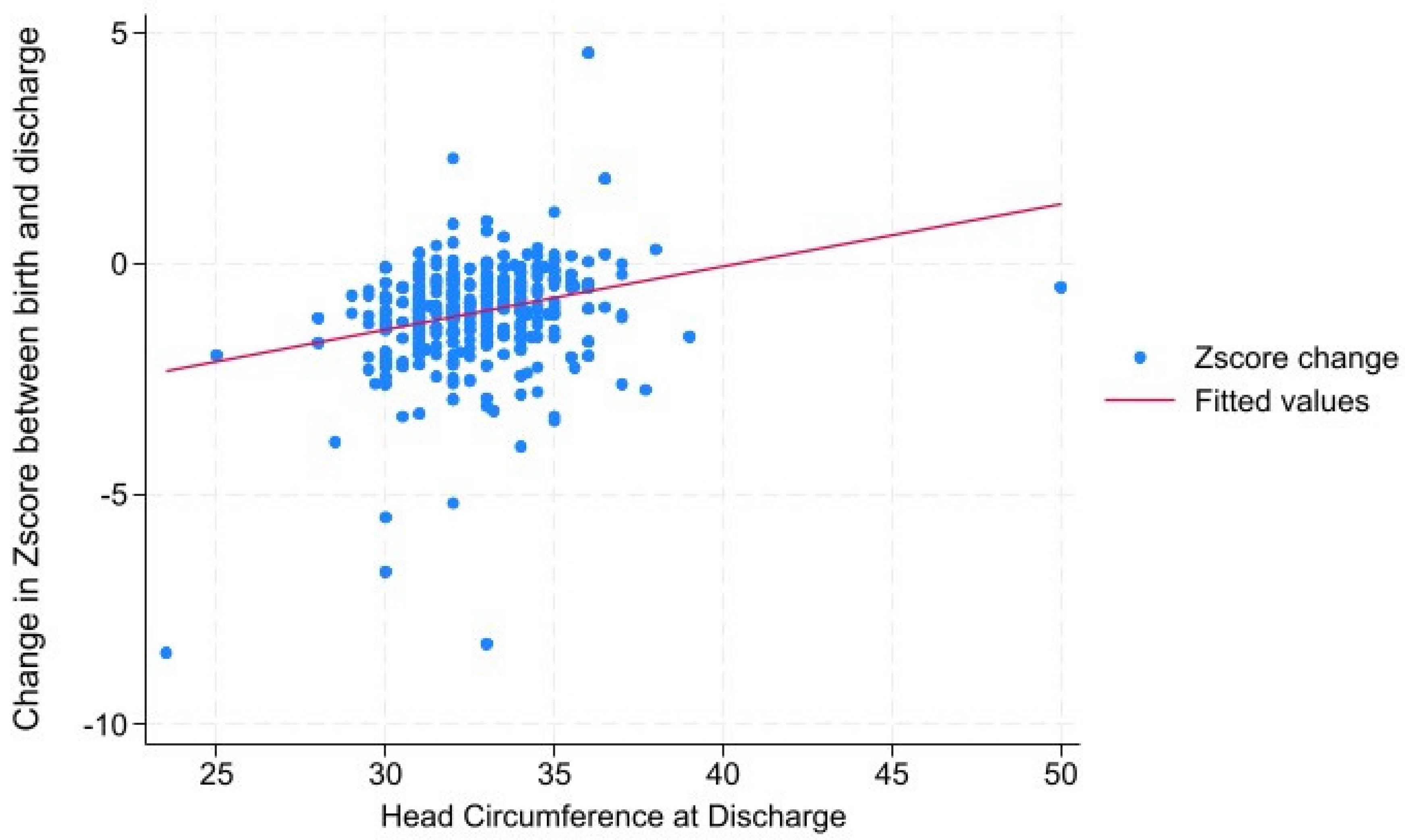
Results
Discussion
References
- Fenton, T. Z Score calculation using 2013 growth chart. https://www.ucalgary.ca/fenton/2013chart: Calgary University, Calgary, Canada; 2013.
- EpicLatino. EpicLatino Network Database. 2024.
- Rutledge A, Murphy HJ, Harer MW, Jetton JG. Fluid Balance in the Critically Ill Child Section: "How Bad Is Fluid in Neonates?". Front Pediatr 2021, 9, 651458. [Google Scholar] [CrossRef] [PubMed]
- Segar, JL. A physiological approach to fluid and electrolyte management of the preterm infant: Review. J Neonatal Perinatal Med 2020, 13, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Selewski DT, Gist KM, Nathan AT, et al. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr Res 2020, 87, 550–557. [Google Scholar] [CrossRef] [PubMed]
- González-López C, Solís-Sánchez G, Lareu-Vidal S, et al. Variability in Definitions and Criteria of Extrauterine Growth Restriction and Its Association with Neurodevelopmental Outcomes in Preterm Infants: A Narrative Review. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Salas AA, Gunawan E, Nguyen K, et al. Early Human Milk Fortification in Infants Born Extremely Preterm: A Randomized Trial. Pediatrics 2023, 152. [Google Scholar] [CrossRef]
- Starc M, Giangreco M, Centomo G, Travan L, Bua J. Extrauterine growth restriction in very low birth weight infants according to different growth charts: A retrospective 10 years observational study. PLoS One 2023, 18, e0283367. [Google Scholar] [CrossRef]
- Bracken JM, Pappas L, Wilkins J, Tracy K, Al-Rajabi TR, Abdelhadi RA. Measuring growth in critically ill neonates and children. Nutr Clin Pract. Oct 2023, 38 (Suppl 2), S28–S38. [Google Scholar] [CrossRef]
- Goldberg DL, Becker PJ, Brigham K, et al. Identifying Malnutrition in Preterm and Neonatal Populations: Recommended Indicators. J Acad Nutr Diet 2018, 118, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Lin Z, Green RS, Chen S, et al. Quantification of EUGR as a Measure of the Quality of Nutritional Care of Premature Infants. PLoS One 2015, 10, e0132584. [Google Scholar] [CrossRef]
- Selvanathan T, Guo T, Kwan E, et al. Head circumference, total cerebral volume and neurodevelopment in preterm neonates. Arch Dis Child Fetal Neonatal Ed 2022, 107, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Tan MJ, Cooke RW. Improving head growth in very preterm infants--a randomised controlled trial I: neonatal outcomes. Arch Dis Child Fetal Neonatal Ed 2008, 93, F337–F341. [Google Scholar] [CrossRef] [PubMed]
- Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 2011, 128, e899–e906. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–61. [Google Scholar] [CrossRef] [PubMed]
- Neubauer V, Griesmaier E, Pehböck-Walser N, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatr 2013, 102, 883–8. [Google Scholar] [CrossRef] [PubMed]
- Power VA, Spittle AJ, Lee KJ, et al. Nutrition, Growth, Brain Volume, and Neurodevelopment in Very Preterm Children. J Pediatr 2019, 215, 50–55.e3. [Google Scholar] [CrossRef] [PubMed]
| UNITS | CITY/COUNTRY |
| Centenario H. de Esp. Miguel Hidalgo | Aguascalientes, Mexico |
| Clínica Dávila | Santiago, Chile |
| Clínica de Santa María de Santiago | Santiago, Chile |
| Clínica del Country | Bogotá, Colombia |
| Clínica la Colina | Bogotá, Colombia |
| Clínica Materno Infantil San Luis | Bucaramanga, Colombia |
| Clínica San Felipe | Lima, Perú |
| Clínica Santa Bárbara | Quito, Ecuador |
| Clínica Somer | Rio Negro, Colombia |
| Clínica Universitaria Colombia | Bogotá, Colombia |
| Clínica Vespucio | Santiago, Chile |
| Colsanitas – Clínica Pediátrica UCI Neonatal | Bogotá, Colombia |
| Curaçao Medical Center | Willemstad, Curaçao |
| H Regional DR Rafael Pascacio Gamboa | Tuxtla Gutiérrez, México |
| Hospital Central Dr. Ignacio Morones Prieto | San Luis Potosí, México |
| Hospital Civil de Ipiales E.S.E | Ipiales, Colombia |
| Hospital de los Valles | Quito, Ecuador |
| Hospital Departamental San Vicente de Paul | Garzón, Huila, Colombia |
| Hospital Dr. Florencio Escardó | Tigre, Argentina |
| Hospital Español de Mendoza | Mendoza, Argentina |
| Hospital General EISS de Manta | Manta, Ecuador |
| Hospital Italiano de La Plata | La Plata, Argentina |
| Hospital Luis Lagomaggiore | Mendoza, Argentina |
| Hospital Metropolitano | Quito, Ecuador |
| Hospital Militar Central | Bogotá, Colombia |
| Hospital Regional Universitario de Colima | Colima, México |
| Hospital San Francisco de Quito | Quito, Ecuador |
| Hospital San José | Bogotá, Colombia |
| Hospital Santísima Trinidad | Asunción, Paraguay |
| Los Cobos Medical Center | Bogotá, Colombia |
| Maternidad Nuestra Sra. de las Mercedes | Tucumán, Argentina |
| S.E.S. Hospital de Caldas | Manizales, Colombia |
| VARIABLE | % Normal or Mean | p |
| Gestational Age at Birth | 29.6 ± 2.3 weeks | <0.0000 |
| No NEC | 93% | <0.0000 |
| Unit of origin | 32 units | <0.0000 |
| No ROM > 24 hours | 87% | 0.0190 |
| Temperature at Admission | 36.0oC ± 1 | 0.0350 |
| No IVH pathology | 71% | 0.0350 |
| Sex M | 55% | 0.056 |
| Presentation (cephalic) | 71% | 0.072 |
| Inborn | 89% | 0.091 |
| No Oxygen at 36w | 79% | 0.095 |
| Vaginal delivery | 22% | 0.146 |
| Receive Antenatal Corticosteroids | 72% | 0.218 |
| AGA | 89% | 0.234 |
| No Suspected Chorioamnionitis | 91% | 0.346 |
| Period (before/after 2020) | 45% | 0.636 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





