Submitted:
28 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Method
2.1. Ethics Approval and Consent to Participate
2.2. Participant Characteristics
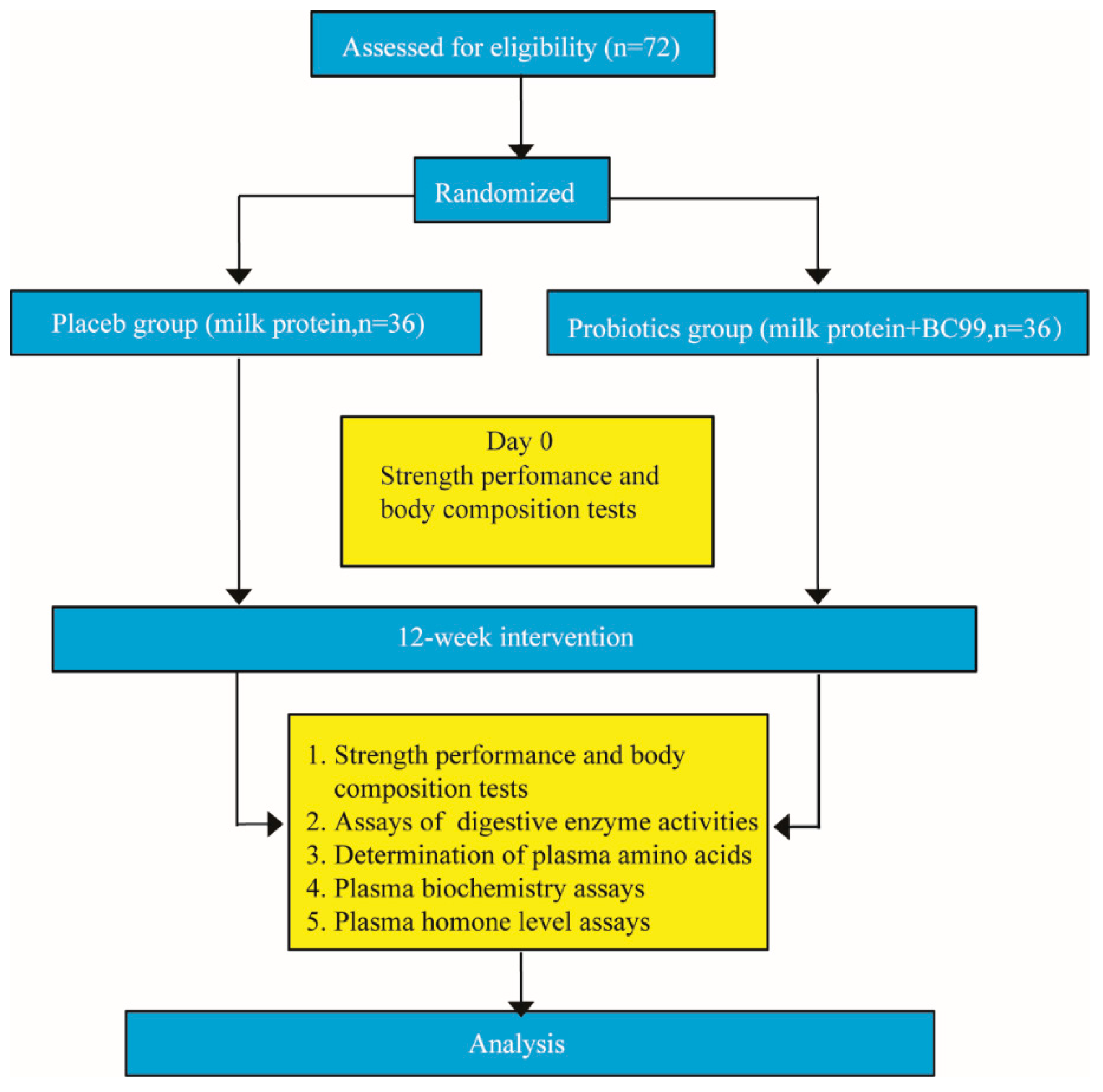
2.3. Experimental Design
2.4. Strength Performance and Body Composition
2.5. Enzyme Assays
2.6. Venous Blood Collection and Processing
2.7. Amino Acid Determination
2.8. Biochemistry Analysis
2.9. Hormone Assays
2.10. Statistical Analysis
3. Results
3.1. Effect of W. coagulans BC99 on strength performance
3.2. Effect of W. coagulans BC99 on Body Composition
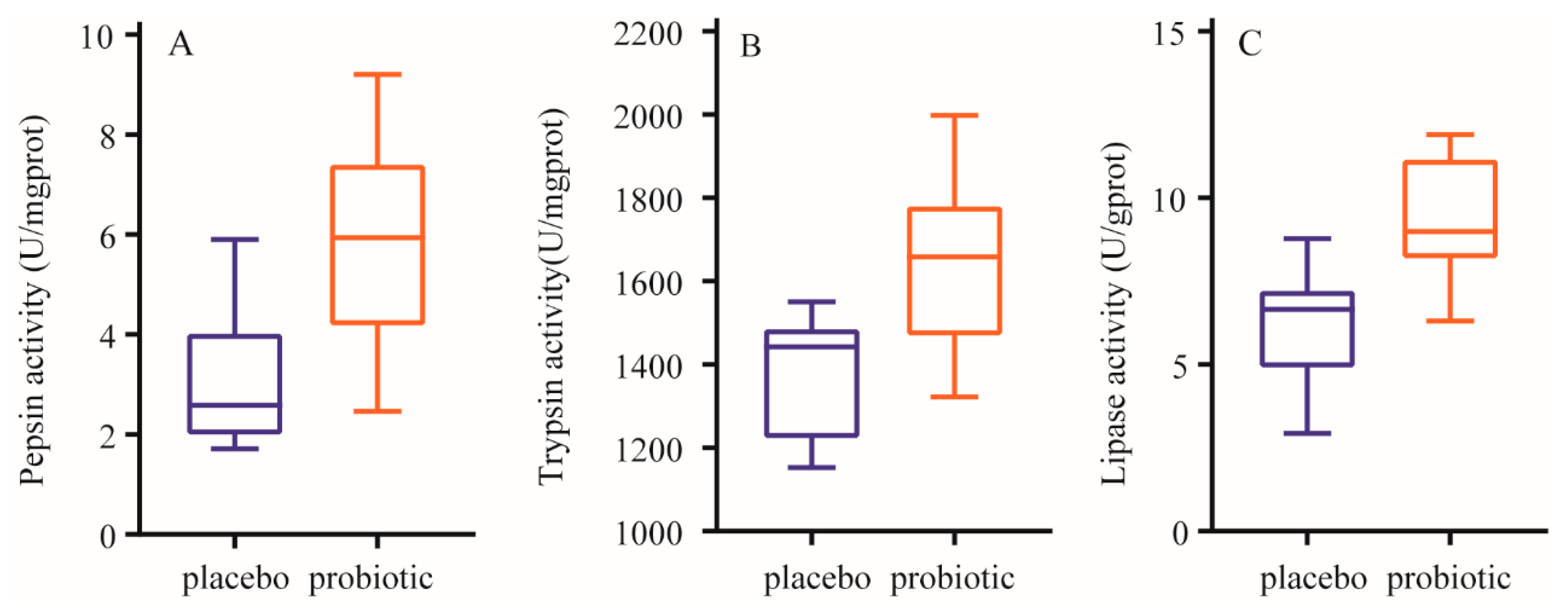
3.3. Effect of W. coagulans BC99 on Enzymatic Activity of Stool Samples
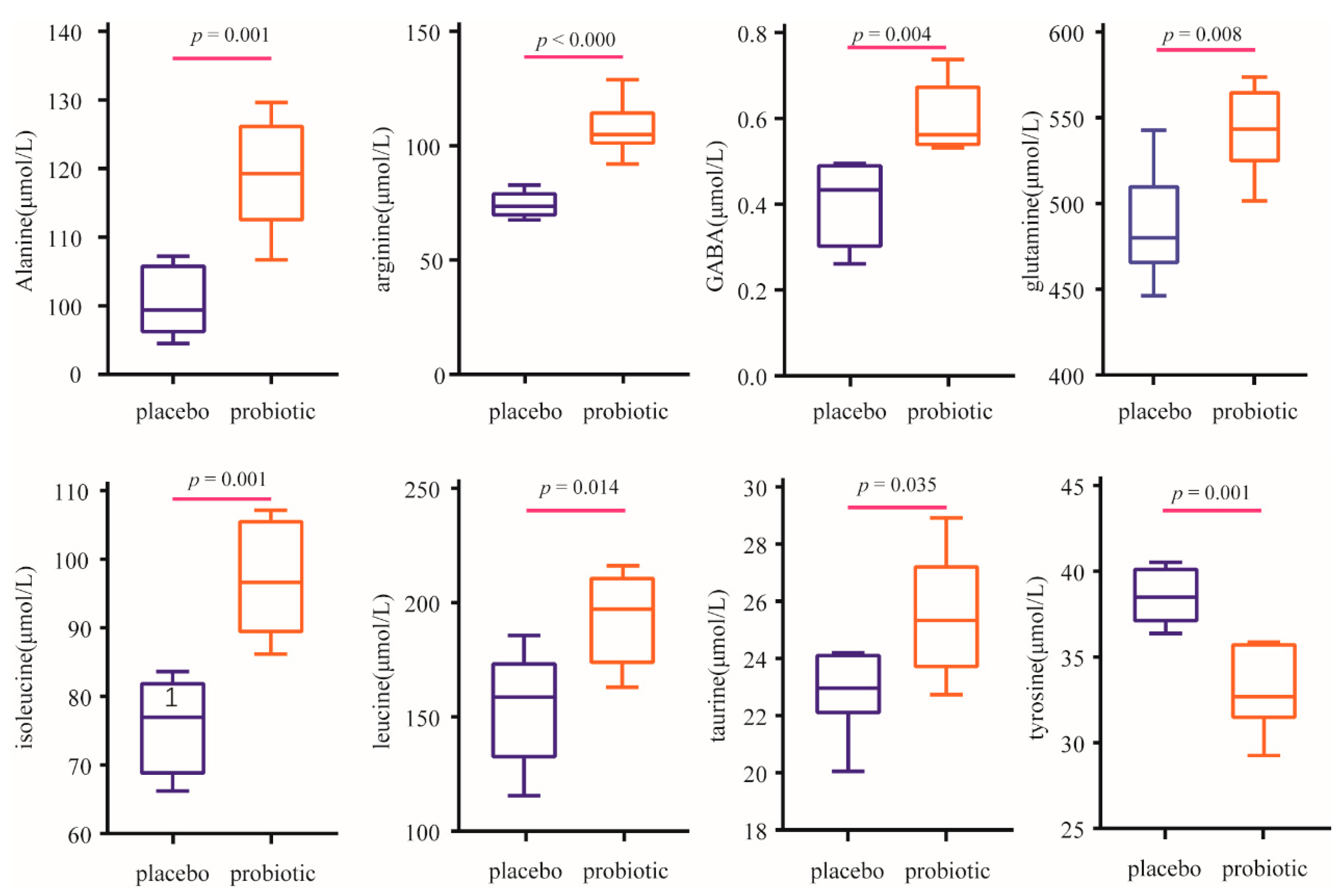
3.4. Effect of W. coagulans BC99 on Plasma Concentration of Amino Acids
3.5. Effect of W. coagulans BC99 on Plasma BIOCHEMICAL parameters
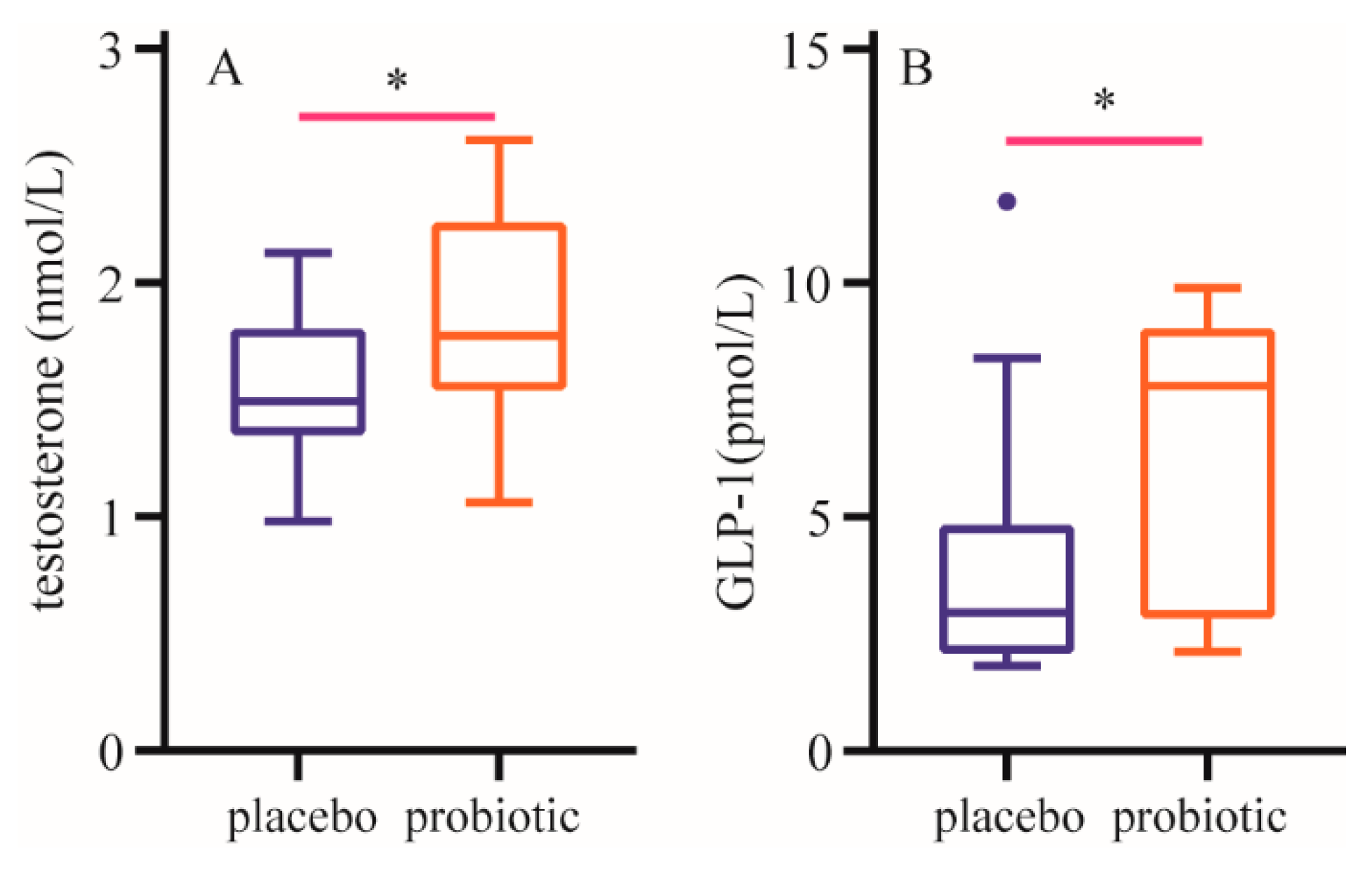
3.6. Effect of W. coagulans BC99 on plasma hormone level
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuchs, C.J.; Hermans, W.J.H.; Holwerda, A.M.; Smeets, J.S.J.; Senden, J.M.; Kranenburg, J.; Gijsen, A.P.; Wodzig, W.K.H.W.; Schierbeek, H.; Verdijk, L.B.; et al. Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial. Am. J. Clin. Nutr. 2019, 110, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Bröer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef]
- Lee, S.; Jo, K.; Jeong, H.G.; Yong, HI.; Choi, Y.S.; Kim, D.J.; Jung, S. ; Freezing-then-aging treatment improved the protein digestibility of beef in an in vitro infant digestion model. Food. Chem. 2021, 350, 129224. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; Lamers, W.H.; Broer, S.; Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.G.; Choi, G.; Kim, S.W.; Kim, B.Y.; Lee, S.; Park, H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. J. Int. Soc. Sport. Nutr. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.M.; Dyle, M.C.; Bullard, S.A.; Dierdorff, J.M.; Murry, D.J.; Fox, D.K.; Bongers, K.S.; Lira, V.A.; Meyerholz, D.K.; Talley, J.J.; et al. Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-dependent Pathway to Age-related Skeletal Muscle Weakness and Atrophy. J. Biol. Chem. 2015, 290, 25497–25511. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sport. Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Maughan, R.J. Nutritional ergogenic aids and exercise performance. Nutr. Res. Rev. 1999, 12, 255–80. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, TM.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, SM.; et al. International society of sports nutrition position stand: protein and exercise. J. Int. Soc. Sport. Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Holwerda, A.M.; Paulussen, K.J.M.; Overkamp, M.; Goessens, J.P.B.; Kramer, I.F.; Wodzig, W.K.W.H.; Verdijk, L.B.; Loon, L.J.C. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J. Nutr. 2019, 149, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F. Amino acid-derived bacterial metabolites in the colorectal luminal fluid: effects on microbial communication, metabolism, physiology, and growth. Microorganisms 2023, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y. Dietary protein intake and human health. Food. Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Sanders, ME.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastro. Hepat. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, M.; Tu, Y.; Zhang, N.F.; Deng, K.D.; Ma, T.; Diao, Q.Y. ; Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress-related indicators in Holstein calves. J. Anim. Physiol. Anim. Nutr. (Berl). 2016, 100, 33–38. [Google Scholar] [CrossRef]
- Keller, D.; Van Dinter, R.; Cash, H.; Farmer, S.; Venema, K. Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1). Benef. Microbes. 2017, 8, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis. Calcified. Tissue. Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Lustgarten, M.S. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 update. Front. Physiol. 2019, 10, 1435. [Google Scholar] [CrossRef]
- Bindels, L.B.; Delzenne, N.M. Muscle wasting: The gut microbiota as a new therapeutic target? Int. J. Biochem. Cell. 2013, 45, 2186–2190. [Google Scholar]
- Rezaee, N.; Rahmani-Nia, F.; Delfan, M.; Ghahremani, R. Exercise training and probiotic supplementation effects on skeletal muscle apoptosis prevention in type-I diabetic rats. Life. Sci. 2021, 285, 119973. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, OC.; Triantafyllidis, KK.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: a systematic review and meta-analysis of randomized controlled trials. J. Cachexia. Sarcopeni. 2023, 14, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Soares, MB.; Martinez, R.C.R.; Pereira, E.P.R.; Balthazar, C.F.; Cruz, A.G.; Ranadheera, C.S.; Sant'Ana, A.S. The resistance of and strains with claimed probiotic properties in different food matrices exposed to simulated gastrointestinal tract conditions. Food. Res. Int. 2019, 125, 108542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, J.; Fang, S.; Zhao, B. Complete genome sequence of Heyndrickxia (Bacillus) coagulans BC99 isolated from a fecal sample of a healthy infant. Microbiol. Resour. Ann. 2024, 13, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Mohan, M.; Saudagar, P.; Sable, C.; Shinde, S.; Bedade, D. In vitro and in vivo evaluation of probiotic potential and safety assessment of Bacillus coagulans SKB LAB-19 (MCC 0554) in humans and animal healthcare. Regul. Toxicol. Pharmacol. 2022, 133, 105218. [Google Scholar] [CrossRef] [PubMed]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of protein supplementation on performance andrecovery in resistance and endurance training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef]
- Huang, W.C.; Chang, Y.C.; Chen, Y.M.; Hsu, Y.J.; Huang, C.C.; Kan, N.W.; Chen, S.S. Whey protein improves marathon-induced injury and exercise performance in elite track runners. Int. J. Med. Sci. 2017, 14, 648–654. [Google Scholar] [CrossRef]
- Forbes, S.C.; Bell, G.J. Whey protein isolate supplementation while endurance training does not alter cycling performance or immune responses at rest or after exercise. Front. Nutr. 2019, 6, 19. [Google Scholar] [CrossRef]
- Williamson, E.; Kato, H.; Volterman, K.A.; Suzuki, K.; Moore, D.R. The effect of dietary protein on protein metabolism and performance in endurance-trained males. Med. Sci. Sport. Exer. 2019, 51, 352–360. [Google Scholar] [CrossRef]
- D'Lugos, A.C.; Luden, N.D.; Faller, J.M.; Akers, J.D.; McKenzie, A.I.; Saunders, M.J. Supplemental protein during heavy cycling training and recovery impacts skeletal muscle and heart rate responses but not performance. Nutrients 2016, 8, 550. [Google Scholar] [CrossRef]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J. Sarcopenia and its impact on quality of life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [PubMed]
- Stewart, C.; Rittweger, J. Adaptive processes in skeletal muscle: molecular regulators and genetic influences. J. Musculoskelet. Neuronal. Interact. 2006, 6, 73–86. [Google Scholar] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Katsanos, C.S.; Zhang, X.J.; Wolfe, R.R. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp. Gerontol. 2005, 4, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: advantage whey. J. Food. Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Dangin, M.; Boirie, Y.; Garcia-Rodenas, C.; Gachon, P.; Fauquant, J.; Callier, P.; Ballevre, O.; Beaufrere, B. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E340–E348. [Google Scholar] [CrossRef]
- Sharples, A.P.; Hughes, D.C.; Deane, C.S.; Saini, A.; Selman, C.; Stewart, C.E. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging. Cell. 2015, 14, 511e. [Google Scholar] [CrossRef]
- Church, D.D.; Hirsch, K.R.; Park, S.; Kim, I-Y.; Gwin, J.S.; Pasiakos, S.M.; Wolfe, R.R.; Ferrando, A.A. Essential amino acids and protein synthesis: insights to maximizing the muscle and whole-body response to feeding. Nutrients 2020, 12, 3717. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–82. [Google Scholar] [CrossRef]
- Cho, H.D.; Lee, J.H.; Jeong, J.H.; Kim, J.Y.; Yee, S.T.; Park, S.K.; Lee, M.K.; Seo, KI. Production of novel vinegar having antioxidant and anti-fatigue activities from Salicornia herbacea L. J. Sci. Food. Agr. 2016, 96, 1085–1092. [Google Scholar] [CrossRef]
- Xu, C.; Lv, J.L.; Lo, Y.M.; Cui, S.W.; Hu, X.; Fan, M. Effects of oat β-glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohyd. Polym. 2013, 92, 1159–1165. [Google Scholar] [CrossRef]
- Oh, H.A.; Kim, D.E.; Choi, H.J.; Kim, N.J.; Kim, D.H. Anti-fatigue effects of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol in mice. Biol. Pharm. Bull. 2015, 38, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.; Petersson, S.J.; Christensen, L.L.; Kristensen, J.M.; Sabaratnam, R.; Ørtenblad, N.; Andersen, M.; Højlund, K. Effect of long-term testosterone therapy on molecular regulators of skeletal muscle mass and fibre-type distribution in aging men with subnormal testosterone. Metabolism 2020, 112, 154347. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- Sinha-Hikim, I.; Cornford, M.; Gaytan, H.; Lee, M.L.; Bhasin, S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J. Clin. Endocrinol. Metab. 2006, 91, 3024–3033. [Google Scholar] [CrossRef]
- Caminiti, G.; Volterrani, M.; Iellamo, F.; Marazzi, G.; Massaro, R.; Miceli, M.; Mammi, C.; Piepoli, M.; Fini, M.; Rosano, G.M. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J. Am. Coll. Cardiol. 2009, 54, 919–927. [Google Scholar] [CrossRef]
- Katayama, T.; Takada, S.; Masaki, Y.; Kinugawa, S.; Matsumoto, J.; Furihata, T.; Fukushima, A.; Yokota, T.; Okita, K.; Tsutsui, H. The activation of glucagon-like peptide-1 improves the mitochondrial abnormalities in skeletal muscle and exercise intolerance in heart failure mice. J. Card. Fail. 2016, 22, S162–S162. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia. Sarcopenia. Muscle. 2019, 10, 903–918. [Google Scholar] [CrossRef]
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, H.; Yu, Z.H.; Zhang, F.; Liang, S.; Liu, H.; Chen, H.; Lü, M.H. The Gut microbiome and sex hormone-related diseases. Front. Microbiol. 2021, 12, 711137. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Influence of probiotics administration on gut microbiota core. J. Clin. Gastroenterol. 2018, 52, S50–S56. [Google Scholar] [CrossRef]
- Okuka, N.; Milinkovic, N.; Velickovic, K.; Polovina, S.; Sumarac-Dumanovic, M.; Minic, R.; Korčok, D.; Djordjevic, B.; Ivanovic, N.D. Beneficial effects of a new probiotic formulation on adipocytokines, appetite-regulating hormones, and metabolic parameters in obese women. Food. Funct. 2024, 15, 7658–7668. [Google Scholar] [CrossRef] [PubMed]
| Placebo (n = 36) | probiotic (n = 36) | |
|---|---|---|
| Age (years) | 20.25±1.03 | 20.19±0.79 |
| Height (cm) | 1.79±5.94 | 179.25±5.16 |
| Weigh (kg) | 73.55±8.73 | 73.61±8.24 |
| placebo | probiotics | ||||
|---|---|---|---|---|---|
| baseline | end | baseline | end | ||
| Bench press | 1-RM (kg) | 68.06±12.32 | 76.14±9.04* | 70.14±9.74 | 78.27±10.29* |
| 80%RM (times) | 11.42±3.54 | 10.07±2.36 | 11.31±3.09 | 11.41±1.84▲ | |
| Squat | 1-RM (kg) | 114.86±21.51 | 131.90±23.20* | 121.57±17.65 | 133.65±18.84* |
| 80%RM (times) | 10.33±5.39 | 10.91±2.96 | 10.54±4.14 | 13.04±4.01▲ | |
| placebo | probiotics | |||
|---|---|---|---|---|
| baseline | end | baseline | end | |
| Muscle mass | 34.44±3.78 | 34.15±2.93 | 34.47±3.34 | 36.16±3.12*▲ |
| Fat mass | 11.01±3.41 | 9.90±3.55 | 10.91±3.07 | 8.31±2.18**▲ |
| Fat free mass | 61.28±4.78 | 59.89±4.05 | 61.25±5.69 | 64.77±4.59*▲ |
| Body weight | 73.55±8.73 | 69.94±6.95 | 73.61±8.24 | 70.08±7.18 |
| BMI | 22.26±2.14 | 21.67±2.12 | 22.55±2.13 | 21.95±1.94 |
| Amino acids | Placebo (n = 36) | probiotic (n = 36) | p value |
|---|---|---|---|
| Alanine | 100.45±5.02 | 119.32±8.39 | 0.001 |
| Arginine | 74.30±5.75 | 107.43±12.13 | 0.000 |
| Asparagine | 36.16±3.34 | 40.53±2.84 | 0.210 |
| Aspartic acid | 7.18±1.15 | 7.76±0.39 | 0.273 |
| Cysteine | 7.19±1.16 | 7.78±0.40 | 0.261 |
| GABA | 0.41±0.09 | 0.59±0.08 | 0.004 |
| Glutamate | 1589.43±251.86 | 1704.56±431.67 | 0.008 |
| Glutamine | 486.67±32.71 | 542.75±25.63 | 0.585 |
| Glycine | 107.29±10.67 | 105.34±11.19 | 0.764 |
| Histidine | 87.89±8.82 | 87.60±7.49 | 0.951 |
| Isoleucine | 75.89±7.26 | 90.68±14.11 | 0.001 |
| Leucine | 156.03±22.01 | 192.79±19.41 | 0.014 |
| Lysine | 229.17±48.41 | 240.14±25.52 | 0.108 |
| Methionine | 29.45±2.74 | 32.64±3.89 | 0.132 |
| Phenylalanine | 55.28±2.05 | 53.47±5.23 | 0.449 |
| Proline | 118.92±9.57 | 132.49±12.34 | 0.059 |
| Serine | 83.33±11.38 | 83.15±15.97 | 0.983 |
| Taurine | 22.85±1.51 | 25.51±2.19 | 0.035 |
| Threonine | 190.11±13.40 | 205.05±28.42 | 0.271 |
| Tryptophan | 38.75±5.11 | 37.54±4.69 | 0.678 |
| Tyrosine | 38.54±1.67 | 33.07±2.51 | 0.001 |
| Valine | 120.32±9.28 | 131.25±8.96 | 0.065 |
| BCAA | 350.49±39.83 | 564.18±29.06 | 0.000 |
| EAA | 963.65±65.47 | 1222.31±65.83 | 0.000 |
| Total AA | 3643.83±254.01 | 4092.35±495.51 | 0.077 |
| Placebo (n = 36) | probiotic (n = 36) | |
|---|---|---|
| LDH(U/L) | 2036.90±273 | 1327.18±368* |
| CK(U/mL) | 0.19±0.04 | 0.09±0.03** |
| BUN(mmol/L) | 5.44±1.05 | 4.96±0.89* |
| Mb(ng/mL) | 1.74±0.33 | 1.60±0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





