1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a progressive respiratory condition that represents one of the leading causes of morbidity and mortality worldwide, affecting more than 300 million people and accounting for approximately three million deaths each year [
1,
4,
6,
8,
13]. This disorder is characterized by persistent airflow obstruction and a chronic inflammatory response in the lungs [
2,
4]. The management of COPD involves a complex therapeutic regimen that includes pharmacotherapy, pulmonary rehabilitation, and lifestyle modifications, with strict adherence to these treatments being essential to prevent exacerbations and improve patients' quality of life [
5].
However, achieving optimal adherence to prescribed interventions in COPD patients remains a significant challenge. Studies suggest that up to 50% of patients fail to adhere to their treatment [
6], which results in a higher risk of exacerbations, recurrent hospitalizations, and an accelerated decline in lung function [
7]. Among the multiple barriers to adherence are the complexity of the treatment, patients' negative perceptions of their disease, and logistical difficulties in accessing healthcare services [
8]. In this context, mobile health (mHealth) interventions have emerged as a promising tool to address these challenges, providing medication reminders, remote monitoring, and personalized educational support through mobile devices [
9,
10,
11]. mHealth interventions offer a unique opportunity to improve disease self-management and promote treatment adherence by providing continuous access to information and direct communication with healthcare providers[
11,
12]. However, despite their growing popularity, the effectiveness of these interventions in improving treatment adherence in COPD patients has yet to be fully established. The available studies present diverse, and in some cases inconsistent, results due to differences in study design, the types of interventions applied, and the methodological quality of the clinical trials [
13,
14,
15].
Given the lack of consensus regarding the efficacy of mHealth interventions in improving adherence in COPD patients, this systematic review aims to assess the effect of mobile phone-mediated interventions to improve adherence to prescribed treatment in COPD subjects, compared to conventional therapy.. Specifically, it will analyze studies that investigate the use of medication reminders, remote monitoring, and other mobile applications designed to support the management of COPD.
2. Materials and Methods
This systematic review was conducted according to the PRISMA guideline[
16] and the protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42023455996).
2.1. Criteria for Considering Studies for This Review
2.1.1. Types of Studies
We included randomized controlled trials (RCTs), with no restriction on language date of publication. Studies had to be available in full text, whether published or not.
2.1.2. Types of Participants
We included all RCTs in which participants had a medical diagnosis of COPD (as defined by the authors). We excluded studies that included a population with inability to use media based on mobile digital devices.
2.1.3. Types of Interventions
We included trials comparing mHealth-based educational programs—like internet and web-based learning platforms available on smartphones, tablets (including apps), other mobile devices, SMS, and social media-based education—versus other interventions or no intervention.
2.1.4. Types of Outcomes Measures
Adherence to treatment
Secondary outcomes
Physical activity
Pulmonary function
Quality of life
Days of hospitalizations
2.5. Search Methods for Identification of Studies
We searched the following databases for relevant trials up to July 2023:
MEDLINE (pubmed)
WEB OF SCIENCE
SCOPUS
The search strategy was designed based on relevant medical subject heading terms (Mesh) with the following combination:
#1 “Chronic Obstructive Pulmonary Diseases” OR “Chronic Obstructive Lung Disease” OR COAD OR COPD OR “Airflow Obstruction” OR “Chronic Airflow Obstructions” OR Smokers
#2 Mobile OR Cellphone OR phone OR Smartphone OR mhealth OR App OR Applications OR eHealth
#3 "Treatment Compliance" OR "Treatment Adherence" OR Adherence OR "Patient Compliance" OR "Self efficacy" OR Exacerbations OR "Physical activity" OR "Adverse events" OR "Emergency care" OR "Tobacco abstinence" OR Dyspnoea OR "Self care"
#1 AND #2 AND #3
2.6. Data Management
For data management, the Covidence software, in its free version, was used to eliminate duplicate records. Subsequently, the remaining files were exported to the open access Rayyan tool for screening the records. All references were stored in the Zotero bibliographic manager.
2.7. Study Selection Process and Data Extraction.
Two reviewers (C.C and E.S) worked in parallel and blindly on the synthesis of evidence, and in the case of discrepancies, these were resolved by a third reviewer (F.P) in conjunction with the previous two mentioned. After removing duplicate articles resulting from the database search strategy, both reviewers independently screened all remaining titles and abstracts. Finally, both reviewers performed the full-text review of the records to determine their inclusion in the review.
2.8. Data Extraction
Two reviewers (C.C and E.S.) extracted data independently, using a standardized form. Discrepancies were resolved by consensus; and when an agreement was not reached, a third author (F.P) was consulted in conjunction with the two aforementioned reviewers.
2.9. Assessment of Risk of Bias in Included Studies
Two reviewers independently assessed the risk of bias for each study using the Cochrane risk-of-bias tool for randomized trials (RoB 2)[
17]. This was then reviewed with the participation of the other two authors of the review (F.P and C.C). Risk of bias was assessed according to the following domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in outcome measurement, bias due selection of the reported result.
3. Results
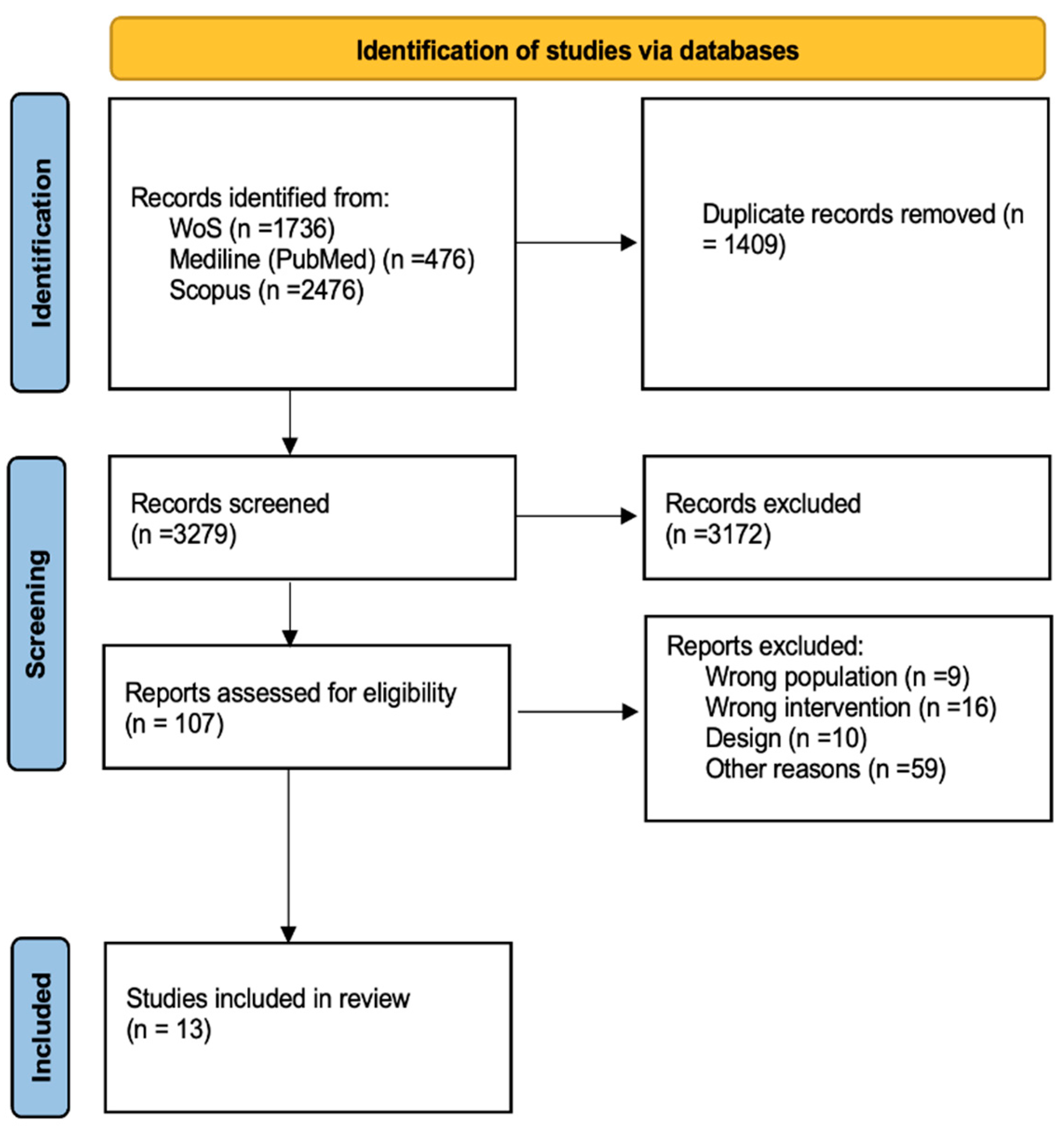
The literature search resulted in 4688 records. After duplication, 3279 records. During title and abstract screening, we excluded 3172 clearly irrelevant records. We proceeded to retrieve the full-text reports for 107 records. Of these, we excluded 94 studies for reasons summarized in
figure 1.
3.1. Characteristics of the Included Studies
The characteristics of the included studies are summarized in Table 1. A total of 1,167 participants were involved, of which 715 were men (61.26%). The average age was 67.12 years. All participants were diagnosed with COPD using spirometry, with the GOLD criteria being the most commonly used, followed by ATS . The average stage of the disease was between II and III.
Regarding the different interventions conducted in the included studies, three major categories were identified: follow-up calls, messaging services (either as reminders or to promote treatment adherence), and activity logging. As for the comparators, the most frequently used was usual care, followed by a comparison with groups that received no intervention (wait-and-see approach) .
Finally, in terms of the outcomes recorded across the different studies, the most prevalent was treatment adherence, followed by the ability to perform exercise or physical activity, and behavioral changes associated with the disease [
18,
19,
20,
21,
22,
23,
24,
27,
28,
29].
| Study |
Participant characteristics Population Age |
Objetives |
Intervention |
Outcomes |
Galdiz et al., (2020)
Wai-San et al. (2020)
Wang et al. (2021)
Çevirme et al.
(2020)
Sink et al. (2018)
Boer et al. (2018)
Vorrink et al. (2016)
Walters et al. (2013)
Tabak et al. (2013)
Chau et al. (2013)
Halpin et al. (2013)
Huong et al. (2009)
W.T.Liu et al. (2008)
|
Moderate to severe COPD patients (BODE index 3-7
Intervention group: 46, Control group: 48is
COPD patients ≥40 years, admitted for AECOPD
Intervention group: 68, Control group: 68
COPD patients aged 40-80 with smartphone access
Intervention group: 39, Control group: 39
COPD stage II patients for at least 6 months
Intervention group: 20, Control group: 20
COPD diagnosis in past 24 months, able to receive text/voice messages
Intervention group: 83, Control group: 85
COPD patients with ≥2 exacerbations in previous 12 months
intervention group: 43, Control group: 44
COPD patients (GOLD stage 2-3) who completed pulmonary rehab in last 6 months
Intervention group: 102, Control group: 81
COPD patients >45 years with confirmed spirometric diagnosis
Intervention group: 90, Control group: 92
COPD patients with confirmed diagnosis, no exacerbation in last 4 weeks
Intervention group: 18, Control group: 16
COPD patients ≥60 years with moderate to severe disease
Intervention group: 22, Control group: 18
COPD patients ≥40 years with confirmed spirometric diagnosis
Intervention group: 40, Control group: 39
Stable COPD patients with GOLD-defined moderate to severe disease
Intervention group: 9, Control group: 8
COPD patients with GOLD criteria, aged ≥40 years
Intervention group: 24, Control group: 24 |
Intervention: 62.3 years, Control: 63 years
Intervention: 76 years, Control: 74 years
Intervention: 63.2 years, Control: 64.4 years
Intervention: 66 years, Control: 61 years
Intervention: 59.89 years, Control: 61.9 years
Intervention: 69 years, Control: 65 years
Intervention: 62 years, Control: 63 years
intervention: 68 years, Control: 63 years
Intervention: 65 years, Control: 69 years
Intervention: 73 years, Control: 72 years
Intervention: 68 years, Control: 70 years
Intervention: 72 years, Control: 64 years
Intervention: 71 years, Control: 72 years |
Evaluate pulmonary rehabilitation with home kit
Reduce COPD hospital readmissions
Assess the impact of mHealth on self-management
Evaluate mobile-based education
Examine impact of daily mobile messages on COPD symptom management
Assess effect of mHealth tools alongside pulmonary rehabilitation
Evaluate impact of mHealth on physical activity levels
Evaluate effect of health mentoring via phone calls on COPD
Evaluate effect of mHealth app on activity and exacerbation self-management
Evaluate telemonitoring with a mobile kit
Evaluate smartphone-based intervention
Assess usability of mobile intervention and its impact on physical performance
Assess impact of music-tempo-guided walking via mobile on exercise |
SPulmonary rehabilitation with mobile phone, pulse oximeter, dumbbell, and bicycle at home
Standard care + bi-weekly mobile phone calls
mHealth program with educational modules and expert support
Mobile education for self-care and disease management
Daily automated calls or text messages for symptom tracking
Daily use of mHealth tools during pulmonary rehabilitation
mHealth with smartphone and physiotherapist support vs. usual care
Regular health mentoring calls vs. usual care
mHealth app with activity tracking and symptom monitoring
Mobile monitoring of oxygen saturation, pulse rate, respiratory rate 3x/day
BlackBerry smartphones for symptom monitoring, data collection
Daily symptom reporting and exercise monitoring via cell phone
Walking with music tempo guided by mobile phone for exercise training |
Primary outcome: Exercise tolerance
Changes in SF-36 quality of life questionnaire
Primary outcome: Hospital readmissions for AECOPD
Primary outcome: Health-related quality of life
Primary outcome: Changes in dyspnea and self-care
Primary outcome: Time until hospitalization
Primary outcome: Number of exacerbations
Primary outcome: Physical activity levels
Primary outcome: Changes in SF-36 quality of life
Primary outcome: Activity levels measured by pedometer
Primary outcome: Satisfaction and health-related quality of life
Primary outcome: Number of exacerbations
Primary outcome: Usability of intervention, changes in physical performance
Primary outcome: Hospital readmissions, quality of life, and physical activity |
| |
|
|
|
|
|
|
3.2. Risk of Bias of Included Studies
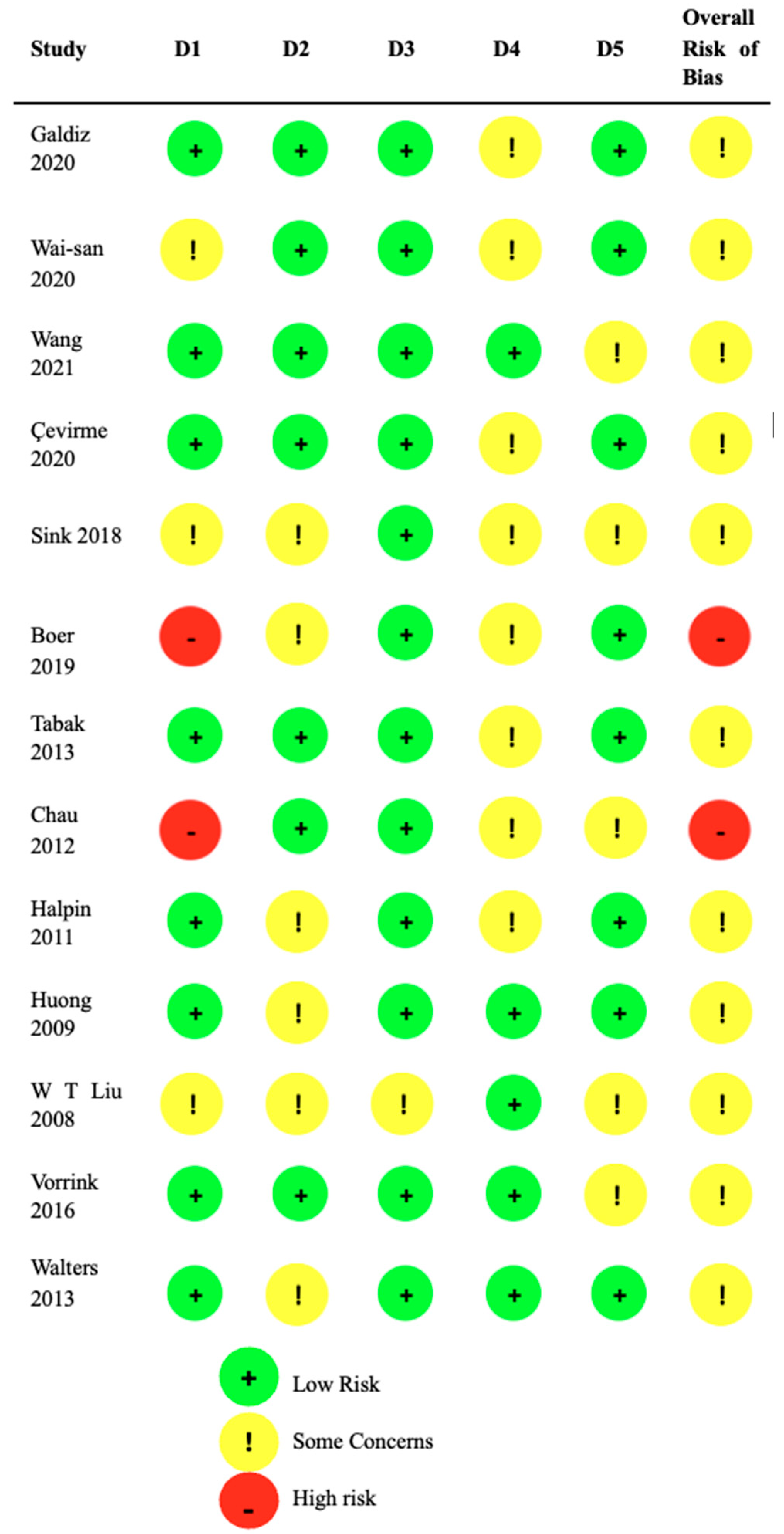
Figure 2 presents the individual risk of bias assessments for the included studies. Two studies were assessed as having a high risk of bias, while the remaining studies were considered to have "some concerns" according to the Cochrane tool.
3.3. Outcomes
3.3.1. Treatment Adherence
Two studies reported on treatment adherence. The first study [
18] used the number of attended follow-up appointments as an indicator, considering 12 patients non-adherent, resulting in a compliance rate of 60%. The second study [
25] utilized a platform to record symptoms and exercise data, finding that the intervention group showed higher adherence (87%) compared to the control group (66%) over six months.
3.3.2. Ability to Perform Physical Activity
Four studies assessed physical capacity related to treatment. Two studies [
21,
23] used pedometers to measure daily steps. In one study, both groups showed a decrease in steps over time, while in the other, the intervention had no significant effect, though the intervention group showed a non-significant increase in the first weeks [
25]. A third study [
28] used the 6-minute walk test, observing that the control group slightly increased their distance, while the intervention group decreased it. The last study [
25] used the Incremental Shuttle Walk Test (ISWT), finding a statistically significant increase in the distance covered at eight and 12 weeks in the intervention group.
3.3.3. Lung Function
Four studies evaluated lung function. Two of them measured Forced expiratory volume (FEV1) and Forced vital capacity (FVC); one found no significant differences [
26], while the other reported significant improvements in the intervention group [
20]. The other two studies assessed FEV1% and FEV1/FVC, finding no significant differences before and after the intervention in both groups [
27,
29].
3.3.4. Quality of Life
Eight studies evaluated quality of life using different questionnaires (CRQ, CCQ, SGRQ, and SF-12). Five studies observed improvements in the intervention group (IG) compared to the control group (CG). For instance, the study with the CRQ showed improvements in all dimensions in the IG [
18], while another using the SGRQ found a decrease in the CG over the short and long term but not in the IG [
22]. Two studies found no significant changes between groups [
23], and one study showed a decrease in the CG's quality of life, which was greater than in the IG according to the SGRQ [
29].
3.3.5. Hospitalizations
Five studies analyzed the number of hospitalizations. Three studies [
26,
27,
29] showed fewer hospitalizations in the IG, with readmission rates such as 13.7% in the IG versus 29.1% in the CG. Conversely, two studies [
24,
28] reported a higher number of hospitalizations in the IG compared to the CG.
4. Discussion
This systematic review identified two studies [
18,
25] that directly evaluated treatment adherence in COPD patients using mHealth interventions. Both studies showed that patients who used mHealth showed greater adherence to treatment compared to those who received conventional care. For example, in the first study [
18], the IG achieved 60% compliance on planned exercise days, with an appointment adherence of 92.4%, compared to 84.4% in the control group (CG). In the second study [
25], IG participants showed 87% adherence to recording their exercise and symptom data, compared to 66% in the CG. Additionally, a direct correlation was found between time spent on moderate to high-intensity physical activity and the frequency of physical activity and symptom recording, suggesting that greater engagement in physical activity promotes treatment adherence. In another 11 studies [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29], treatment adherence was indirectly assessed through the amount of exercise performed, symptom reporting, or mHealth use. For instance, one study [
26] showed greater adherence through a significant increase in time spent on intense physical activity in the IG.
4.1. Integrity and Applicability of Evidence
Most participants in the studies were men (61.26%), although in two studies, the majority were women (63.69% and 64.70%) [
25,
27]. Participant inclusion was based on diagnostic criteria such as spirometry and other medical examinations, although some studies considered additional criteria such as recent hospitalizations [
26] or previous COPD diagnosis [
27]. The results are relevant for various cultural and geographical contexts, as the studies included a wide range of ages and different approaches to treating COPD exacerbations. Mobile phone-based interventions can offer significant benefits, such as cost and resource reduction, and better care for patients who cannot regularly attend a medical center. However, as with other conventional therapies, implementing these interventions faces logistical and economic challenges that make it difficult to achieve optimal treatment adherence in the community.
4.2. Quality of Evidence
Of the 13 studies reviewed, 11 presented a moderate risk of bias due to problems in at least one domain, while two studies showed a high risk of bias, mainly in the allocation domain [
29,
30]. In the "some concerns" category, several studies presented problems such as lack of clarity in the assignment sequence (domain 1) [
20,
26,
28], insufficient data on blinding (domain 2) [
22,
23,
24,
25,
26,
27,
30], and lack of information on data loss and measurement methods (domains 3 and 4) [
26,
28]. Four studies had problems related to the absence of complete protocols (domain 5) [
19,
21,
28,
29]. Despite these limitations, all mHealth-based interventions were included to provide a comprehensive perspective on the topic.
4.3. Possible Biases in the Review Process
Although an exhaustive search was conducted without restrictions on language, region of origin, or publication date, there is a possibility of bias in the risk of bias assessment of the studies. The assessment of the 13 studies was conducted by a single reviewer using the RoB 2 Excel tool, and these assessments were reviewed by two other reviewers. Due to time and resource constraints, it was not possible to conduct an independent evaluation by two reviewers for each study, which could have introduced biases. However, steps were taken to minimize this risk through a multi-reviewer review process and the use of standardized tools. mHealth interventions show significant potential to improve treatment adherence in COPD patients. The reviewed studies suggest benefits in terms of treatment adherence and other health outcomes, but variability in study designs and the presence of biases limit the ability to generalize these results. Future studies should focus on improving methodological quality and reducing biases to confirm these findings and provide a stronger foundation on the effectiveness of mHealth interventions in managing COPD.
5. Conclusions
5.1. Implications for Practice
The available evidence does not currently allow for definitive conclusions about the effectiveness of mHealth-based interventions to improve treatment adherence in COPD patients. This uncertainty is due to several limitations, such as small sample sizes, short intervention durations, and heterogeneity in the methodological approaches of the reviewed studies. Although current results do not show statistically significant differences compared to conventional care, mHealth interventions present significant potential to provide important benefits, such as reducing costs and resources and improving access to healthcare for patients who face logistical barriers to regularly attending health centers. Therefore, these tools could play a crucial role in healthcare, especially in contexts where resources are limited, or access to healthcare services is restricted. However, it is essential to recognize that these conclusions are preliminary and could evolve as more high-quality evidence is generated.
5.2. Implications for Research
To conclusively determine the effectiveness of mHealth interventions in improving treatment adherence in COPD patients, it is imperative to conduct RCTs with a rigorous and well-structured design. These studies should strive to minimize the risk of bias by implementing rigorous randomization techniques, adequate methods for allocation concealment, and blinded outcome assessments to ensure impartiality in data interpretation. Additionally, it is crucial that these studies include sufficiently large sample sizes to ensure robust statistical power, allowing for the detection of real and significant differences between intervention and control groups. The studies should also incorporate an extensive follow-up period, preferably longer than six months, to evaluate both immediate and long-term effects of mHealth interventions. Future studies should not only evaluate the effectiveness of various modalities of mHealth interventions but also identify which are most effective in different subgroups of COPD patients, considering factors such as disease severity, comorbidities, and patient preferences. Furthermore, it is essential to include economic analyses that assess the cost-effectiveness and long-term sustainability of these interventions to determine their viability in general clinical practice. As more evidence accumulates, it will be possible to develop more precise and evidence-based recommendations for integrating mHealth technologies in COPD management, thereby optimizing health outcomes and efficiency in healthcare.
Author Contributions
Conceptualization, A.P and J.G.; methodology, J.G.; software, J.G.; validation, J.G., A.P. and A.P.D.; formal analysis, C.C, F.P. and E.S..; investigation, C.C, F.P. and E.S.; resources, A.P.; data curation, C.C, F.P. and E.S..; writing—original draft preparation, A.P, J.G and A.P.D..; writing—review and editing, A.P,J.G and A.P.D.; visualization, A.P,J.G and A.P.D.; supervision, J.G.; project administration, A.P.D.; funding acquisition, A.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 2020 Gold Reports. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/ (accessed 2023-04-26).
- Cosío, B. G. EPOC. Arch. Bronconeumol. 2007, 43, 15–23. [CrossRef]
- Tabaco. https://www.who.int/es/news-room/fact-sheets/detail/tobacco (accessed 2023-04-26).
- Arancibia H., F. Enfermedad Pulmonar Obstructiva Crónica y Tabaquismo. Rev. Chil. Enfermedades Respir. 2017, 33 (3), 225–229. [CrossRef]
- Hinojosa, F.; C, E. Enfermedad Pulmonar Obstructiva Crónica (EPOC). Acta Médica Peru. 2009, 26 (4), 188–191.
- 2023 GOLD Report. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/2023-gold-report-2/ (accessed 2023-04-13).
- Descripción y Epidemiología. DIPRECE. https://diprece.minsal.cl/garantias-explicitas-en-salud-auge-o-ges/guias-de-practica-clinica/enfermedad-pulmonar-obstructiva-cronica-de-tratamiento-ambulatorio/descripcion-y-epidemiologia/ (accessed 2023-04-26).
- Licencias Médicas. Biblioteca digital. Superintendencia de Salud. Gobierno de Chile. http://www.supersalud.gob.cl/documentacion/666/w3-propertyvalue-3748.html (accessed 2023-04-26).
- World Health Organization. Global Diffusion of eHealth: Making Universal Health Coverage Achievable: Report of the Third Global Survey on eHealth; World Health Organization: Geneva, 2016.
- Park, Y.-T. Emerging New Era of Mobile Health Technologies. Healthc. Inform. Res. 2016, 22 (4), 253. [CrossRef]
- Dolado Martín, C.; Berlanga-Fernández, S.; Fabrellas i Padrès, N.; Galimany Masclans, J. Uso de aplicaciones móviles de salud en usuarios de Atención Primaria. 2017.
- Green Paper on mobile health (“mHealth”) | Configurar el futuro digital de Europa. https://digital-strategy.ec.europa.eu/en/library/green-paper-mobile-health-mhealth (accessed 2023-04-26).
- Global health estimates: Leading causes of death. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed 2023-04-26).
- Telefonía. Subsecretaría de Telecomunicaciones de Chile. https://www.subtel.gob.cl/estudios-y-estadisticas/telefonia/ (accessed 2023-04-22).
- Stamenova, V.; Liang, K.; Yang, R.; Engel, K.; van Lieshout, F.; Lalingo, E.; Cheung, A.; Erwood, A.; Radina, M.; Greenwald, A.; Agarwal, P.; Sidhu, A.; Sacha Bhatia, R.; Shaw, J.; Shafai, R.; Bhattacharyya, O. Technology-Enabled Self-Management of Chronic Obstructive Pulmonary Disease with or without Asynchronous Remote Monitoring: Randomized Controlled Trial. J. Med. Internet Res. 2020, 22 (7). [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [CrossRef]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. [CrossRef]
- Galdiz, J. B.; Gómez, A.; Rodriguez, D.; Guell, R.; Cebollero, P.; Hueto, J.; Cejudo, P.; Ortega, F.; Sayago, I.; Chic, S.; Iscar, M.; Amado, C.; Rodríguez Trigo, G.; Cosio, B. G.; Bustamante, V.; Pijoan, J. I. Telerehabilitation Programme as a Maintenance Strategy for COPD Patients: A 12-Month Randomized Clinical Trial. Arch. Bronconeumol. 2021, 57 (3), 195–204. [CrossRef]
- Wang, L.; Guo, Y.; Wang, M.; Zhao, Y. A Mobile Health Application to Support Self-Management in Patients with Chronic Obstructive Pulmonary Disease: A Randomised Controlled Trial. Clin. Rehabil. 2021, 35 (1), 90–101. [CrossRef]
- Çevirme, A.; Gökçay, G. The Impact of an Education-Based Intervention Program (EBIP) on Dyspnea and Chronic Self-Care Management among Chronic Obstructive Pulmonary Disease Patients: A Randomized Controlled Study. Saudi Med. J. 2020, 41 (12), 1350–1358. [CrossRef]
- Vorrink, S. N. W.; Kort, H. S. M.; Troosters, T.; Zanen, P.; Lammers, J.-W. J. Efficacy of an mHealth Intervention to Stimulate Physical Activity in COPD Patients after Pulmonary Rehabilitation. Eur. Respir. J. 2016, 48 (4), 1019–1029. [CrossRef]
- Walters, J.; Cameron-Tucker, H.; Wills, K.; Schüz, N.; Scott, J.; Robinson, A.; Nelson, M.; Turner, P.; Wood-Baker, R.; Walters, E. H. Effects of Telephone Health Mentoring in Community-Recruited Chronic Obstructive Pulmonary Disease on Self-Management Capacity, Quality of Life and Psychological Morbidity: A Randomised Controlled Trial. BMJ Open 2013, 3 (9). [CrossRef]
- Tabak, M.; Vollenbroek-Hutten, M. M. R.; Van Der Valk, P. D. L. P. M.; Van Der Palen, J.; Hermens, H. J. A Telerehabilitation Intervention for Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Pilot Trial. Clin. Rehabil. 2014, 28 (6), 582–591. [CrossRef]
- Halpin, D. M. G.; Laing-Morton, T.; Spedding, S.; Levy, M. L.; Coyle, P.; Lewis, J.; Newbold, P.; Marno, P. A Randomised Controlled Trial of the Effect of Automated Interactive Calling Combined with a Health Risk Forecast on Frequency and Severity of Exacerbations of COPD Assessed Clinically and Using EXACT PRO. Prim. Care Respir. J. 2011, 20 (3), 324–331. [CrossRef]
- Nguyen, H. Q.; Gill, D. P.; Wolpin, S.; Steele, B. G.; Benditt, J. O. Pilot Study of a Cell Phone-Based Exercise Persistence Intervention Post-Rehabilitation for COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2009, 4, 301–313. [CrossRef]
- Ko, F. W.-S.; Tam, W.; Siu, E. H. S.; Chan, K.-P.; Ngai, J. C.-L.; Ng, S.-S.; Chan, T. O.; Hui, D. S.-C. Effect of Short-Course Exercise Training on the Frequency of Exacerbations and Physical Activity in Patients with COPD: A Randomized Controlled Trial. Respirology 2021, 26 (1), 72–79. [CrossRef]
- Sink, E.; Patel, K.; Groenendyk, J.; Peters, R.; Som, A.; Kim, E.; Xing, M.; Blanchard, M.; Ross, W. Effectiveness of a Novel, Automated Telephone Intervention on Time to Hospitalisation in Patients with COPD: A Randomised Controlled Trial. J. Telemed. Telecare 2020, 26 (3), 132–139. [CrossRef]
- Liu, W.-T.; Wang, C.-H.; Lin, H.-C.; Lin, S.-M.; Lee, K.-Y.; Lo, Y.-L.; Hung, S.-H.; Chang, Y.-M.; Chung, K. F.; Kuo, H.-P. Efficacy of a Cell Phone-Based Exercise Programme for COPD. Eur. Respir. J. 2008, 32 (3), 651–659. [CrossRef]
- Chau, J. P. C.; Lee, D. T. F.; Yu, D. S. F.; Chow, A. Y. M.; Yu, W.-C.; Chair, S.-Y.; Lai, A. S. F.; Chick, Y.-L. A Feasibility Study to Investigate the Acceptability and Potential Effectiveness of a Telecare Service for Older People with Chronic Obstructive Pulmonary Disease. Int. J. Med. Inf. 2012, 81 (10), 674–682. [CrossRef]
- Boer, L.; Bischoff, E.; van der Heijden, M.; Lucas, P.; Akkermans, R.; Vercoulen, J.; Heijdra, Y.; Assendelft, W.; Schermer, T. A Smart Mobile Health Tool versus a Paper Action Plan to Support Self-Management of Chronic Obstructive Pulmonary Disease Exacerbations: Randomized Controlled Trial. JMIR MHealth UHealth 2019, 7 (10). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).