Submitted:
25 October 2024
Posted:
28 October 2024
You are already at the latest version
Abstract
Keywords:
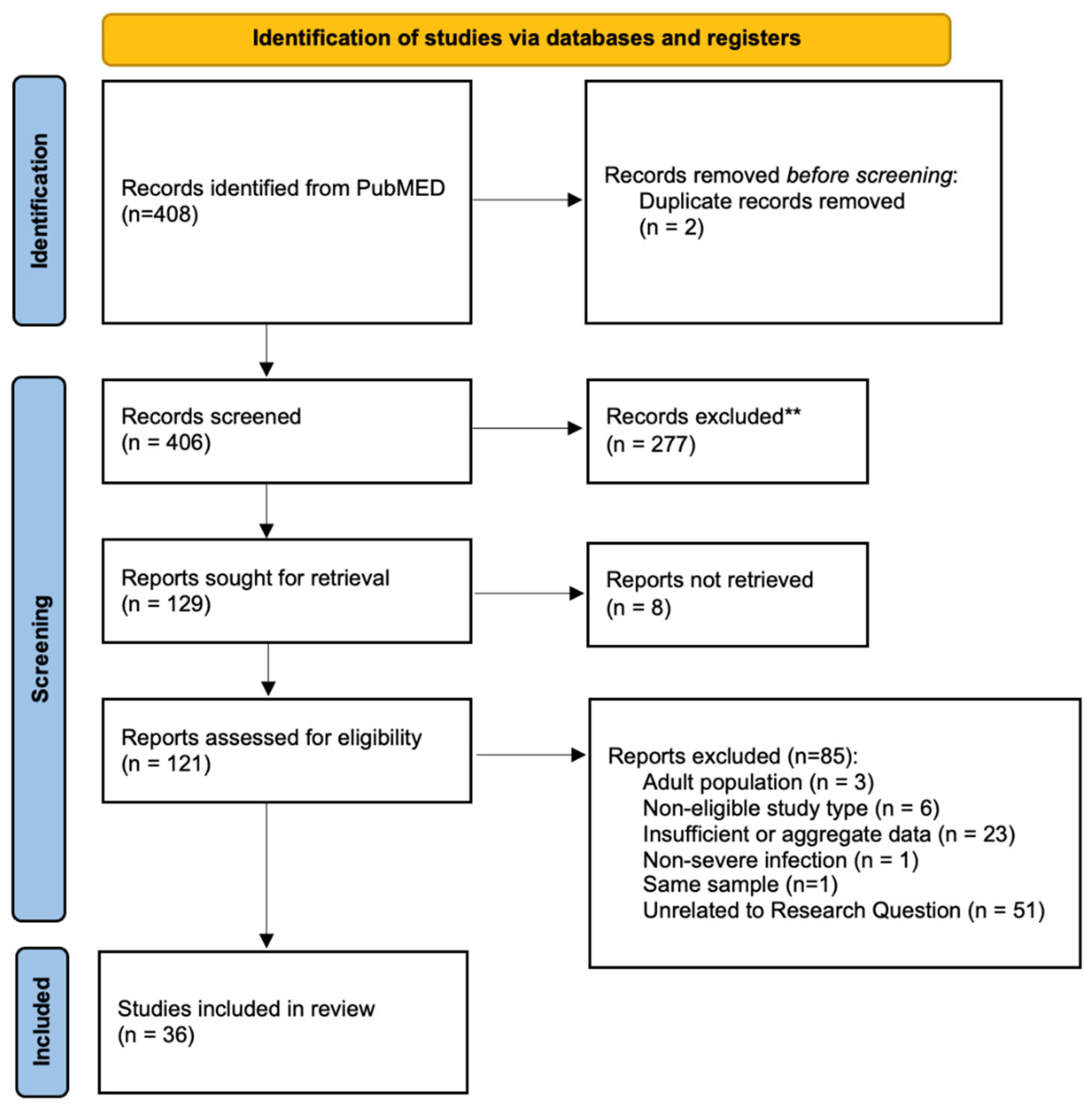
1. Background
2. Results
2.1. Case Report
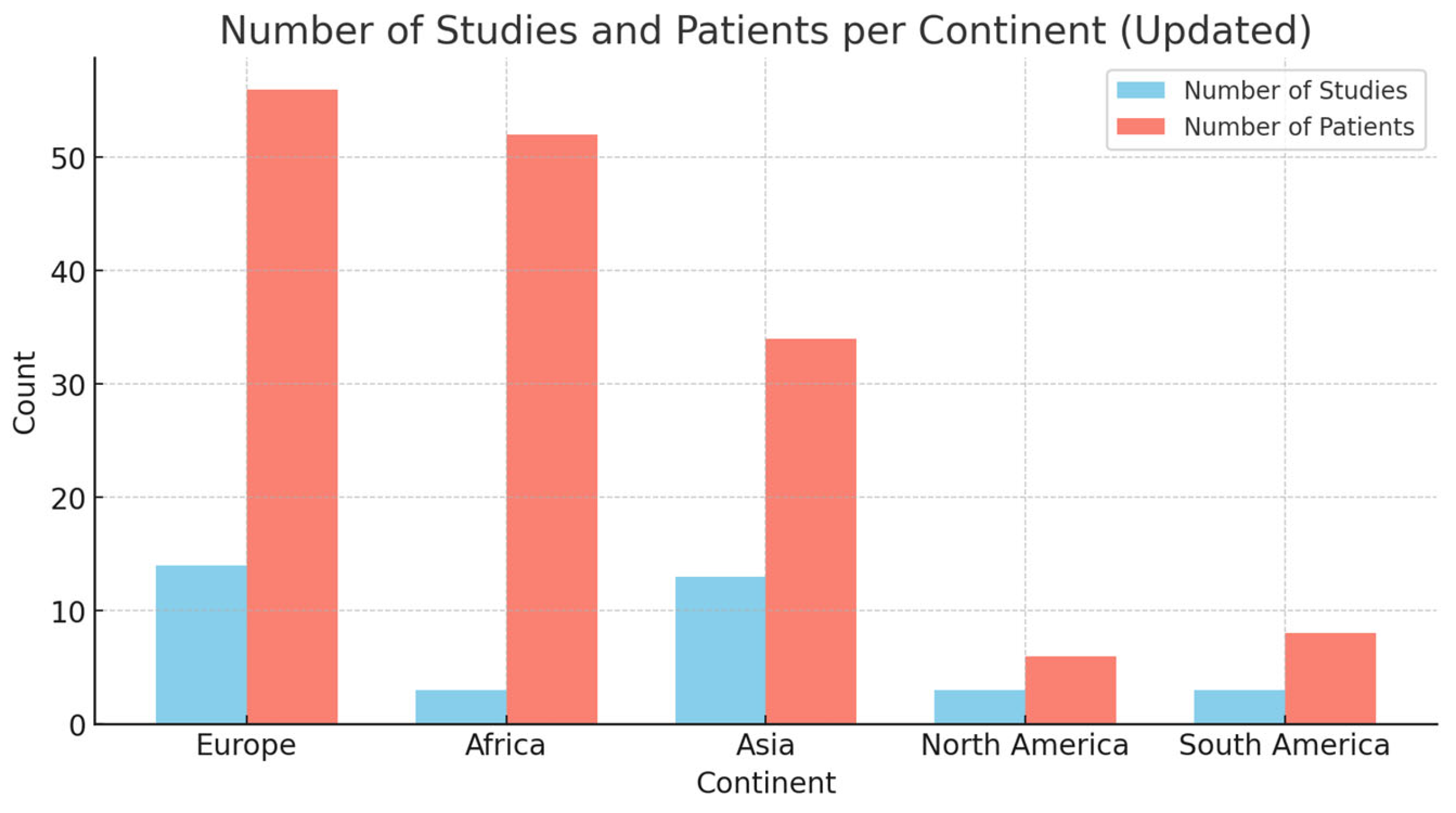
2.2. Literature Review
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonten M, Johnson JR, van den Biggelaar AHJ, Georgalis L, Geurtsen J, de Palacios PI, et al. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin Infect Dis. 2021 Apr 1;72(7):1211–9.
- Otto, M. Community-associated MRSA: what makes them special? Int J Med Microbiol IJMM. 2013 Aug;303(6–7):324–30.
- Castellazzi, M.L.; Bosis, S.; Borzani, I.; Tagliabue, C.; Pinzani, R.; Marchisio, P.; di Pietro, G.M. Panton-valentine leukocidin Staphylococcus aureus severe infection in an infant: a case report and a review of the literature. Ital. J. Pediatr. 2021, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Albiski MK, Lutz N, Ceroni D, NDele D, Zambelli PY, Bregou A. Paediatric musculoskeletal infections with Panton-Valentine leucocidin. Swiss Med Wkly [Internet]. 2018 Sep 23 [cited 2024 Aug 31]. Available online: https://smw.ch/index.php/smw/article/view/2519.
- Oumokhtar, B.; Moutaouakkil, K.; Abdellaoui, H.; Arhoune, B.; Atarraf, K.; El Fakir, S.; Yahyaoui, G.; Mahmoud, M.; Afifi, M.A. Paediatric osteoarticular infections caused by staphylococcus aureus producing panton–valentine leucocidin in morocco: Risk factors and clinical features. Afr. J. Paediatr. Surg. 2022, 19, 78–82. [Google Scholar] [CrossRef]
- Hoppe, P.-A.; Holzhauer, S.; Lala, B.; Bührer, C.; Gratopp, A.; Hanitsch, L.G.; Humme, D.; Kieslich, M.; Kallinich, T.; Lau, S.; et al. Severe infections of Panton-Valentine leukocidin positive Staphylococcus aureus in children. Medicine 2019, 98, e17185. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Matsudera, S.; Watanabe, S.; Yamaguchi, T.; Suzuki, K.; Ohkusu, M.; Ishiwada, N.; Yoshihara, S. Extensive Subcutaneous Abscess due to Panton-Valentine Leucocidin-Positive Community-Associated Methicillin-Resistant Staphylococcus aureus in an Infant. Tohoku J. Exp. Med. 2022, 258, 303–307. [Google Scholar] [CrossRef]
- Disseminated Panton-Valentine Leukocidin-Positive Staphylococcus aureus infection in a child. Arch Argent Pediatr [Internet]. 2016 Apr 1 [cited 2024 Aug 31]. Available online: http://www.sap.org.ar/docs/publicaciones/archivosarg/2016/v114n2a14e.pdf.
- Ahoyo TA, Martin-Odoom A, Bankolé HS, Baba-Moussa L, Zonon N, Prevost G, et al. EPIDEMIOLOGY AND PREVENTION OF NOSOCOMIAL PNEUMONIA ASSOCIATED WITH PANTON-VALENTINE LEUKOCIDIN (PVL) PRO- DUCING STAPHYLOCOCCUS AUREUS IN DEPARTMENTAL HOSPI- TAL CENTRE OF ZOU COLLINES IN BENIN. GHANA Med J. 2012;46(4).
- Hardy, C.; Osei, L.; Basset, T.; Elenga, N. Bone and joint infections with Staphylococcus aureus strains producing Panton–Valentine Leukocidin in French Guiana. Medicine 2019, 98, e16015. [Google Scholar] [CrossRef]
- Ogawa, E.; Shoji, K.; Uehara, Y.; Miyairi, I. Retropharyngeal Abscess Caused by Community-Acquired MRSA USA300 Clone in a 1-Year-Old Japanese Girl. Jpn. J. Infect. Dis. 2022, 75, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Hamada, H.; Hirose, S.; Shimoyama, K.; Fujimori, M.; Honda, T.; Yasukawa, K.; Ishiwada, N.; Ohkusu, M.; Takanashi, J.-I. PantoneValentine leukocidin-positive novel sequence type 5959 community-acquired methicillin-resistant Staphylococcus aureus meningitis complicated by cerebral infarction in a 1-month-old infant. J. Infect. Chemother. 2021, 27, 103–106. [Google Scholar] [CrossRef]
- Vanbiervliet, V.; Demeyer, I.; Claus, F.; Van Vaerenbergh, K. A case report: septic shock due to (tropical) pyomyositis and multiple metastatic embolisms caused by Panton Valentine Leukocidin-positive methicillin-sensitive staphylococcus aureus in a 12-year-old boy. Acta Clin. Belg. 2021, 77, 421–424. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Zhang, S.; Liang, Z.; Wang, Y.; Zhang, Y.; Zhou, G.; Jia, Y.; Chen, L.; She, D. Community-acquired necrotizing pneumonia caused by methicillin-resistant Staphylococcus aureus producing Panton–Valentine leukocidin in a Chinese teenager: case report and literature review. Int. J. Infect. Dis. 2014, 26, 17–21. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Nourse, C. Panton–Valentine leukocidin-associated Staphylococcus aureus necrotizing pneumonia in infants: a report of four cases and review of the literature. Eur. J. Pediatr. 2011, 171, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Irenji N, Pillai SKG, West-Jones JS. Serious life-threatening multifocal infection in a child, caused by Panton-Valentine leucocidin-producing Staphylococcus aureus (PVL-MSSA). BMJ Case Rep. 2018 Jun 5;bcr-2017-222138.
- Elledge, R.O.; Dasari, K.K.; Roy, S. Panton-Valentine leukocidin-positive Staphylococcus aureus osteomyelitis of the tibia in a 10-year-old child. J. Pediatr. Orthop. B 2014, 23, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, P.; Taccetti, G.; Montagnani, C.; Campana, S.; Galli, L.; Braggion, C.; de Martino, M. Evidence of transmission of a Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus clone: a family affair. Clin. Microbiol. Infect. 2013, 19, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Lehman, D.; Tseng, C.W.; Eells, S.; Miller, L.G.; Fan, X.; Beenhouwer, D.O.; Liu, G.Y. Staphylococcus aureusPanton-Valentine Leukocidin Targets Muscle Tissues in a Child with Myositis and Necrotizing Fasciitis. Clin. Infect. Dis. 2010, 50, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Green, K.; Chranioti, I.; Singh, S.; Jäger, H.R.; Drebes, A.; Gabbie, S.; Cohen, J. Panton-Valentine Leukocidin Producing Staphylococcus Aureus Facial Pyomyositis Causing Partial Cavernous Sinus Thrombosis. Pediatr. Infect. Dis. J. 2017, 36, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.G.; Dady, I.; Mukherjee, D. Unusual presentation of late-onset disseminated staphylococcal sepsis in a preterm infant. BMJ Case Rep. 2019, 12, e226325. [Google Scholar] [CrossRef] [PubMed]
- Gadelsayed, M.N.; Greally, P.; Elnazir, B. Methicillin-resistant Staphylococcus aureus (Panton-Valentine leucocidin) cavitating pneumonia in a healthy child. Arch. Dis. Child. 2012, 97, 980–981. [Google Scholar] [CrossRef]
- Fitzgerald, F.; Howard, J.; Bailey, F.; Soleimanian, S. Back pain in a previously healthy teenager. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef]
- Karlı, A.; Belet, N.; Yanık, K. ; Köken, ; Kilic, M.; Bilgici, M.C.; Şensoy, G. Panton-Valentine leukocidin positive Staphylococcus aureus infection in childhood: a case report.. 2015, 57, 615–617. [Google Scholar]
- Daskalaki, M.; Rojo, P.; Marin-Ferrer, M.; Barrios, M.; Otero, J.R.; Chaves, F. Panton–Valentine leukocidin-positive Staphylococcus aureus skin and soft tissue infections among children in an emergency department in Madrid, Spain. Clin. Microbiol. Infect. 2010, 16, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Geng W, Yang Y, Wu D, Zhang W, Wang C, Shang Y, et al. Community-acquired, methicillin-resistant Staphylococcus aureus isolated from children with community-onset pneumonia in China. Pediatr Pulmonol. 2010 Apr;45(4):387–94.
- Kechrid, A.; Pérez-Vázquez, M.; Smaoui, H.; Hariga, D.; Rodríguez-Baños, M.; Vindel, A.; Baquero, F.; Cantón, R.; del Campo, R. Molecular analysis of community-acquired methicillin-susceptible and resistant Staphylococcus aureus isolates recovered from bacteraemic and osteomyelitis infections in children from Tunisia. Clin. Microbiol. Infect. 2011, 17, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Nishisho, S.; Okada, H.; Shimono, R.; Kusaka, T. An infant with necrotizing pneumonia caused by methicillin-resistant Staphylococcus aureus strain USA300. Pediatr. Int. 2021, 64, e14658. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Larsen, A.R.; Petersen, A.; Schønheyder, H.C.D.; Westh, H.D.; Benfield, T.D.; Nygaard, U. Clinical Manifestations in Children with Staphylococcal Bacteremia Positive for Panton-Valentine Leucocidin A Nationwide Survey in a Low Methicillin-Resistance Setting. Pediatr. Infect. Dis. J. 2020, 39, E274–E276. [Google Scholar] [CrossRef] [PubMed]
- Mutale, W.; Sahay, K.M.; Hartley, J.; Thompson, D.; Ratnasinghe, D.; Hudson, L.; Hulse, E.; Fellows, G. Community acquired Panton-Valentine Leukocidin (PVL) positive Methicilin Resistant Staphylococcal aureus cerebral abscess in an 11-month old boy: a case study. BMC Res. Notes 2014, 7, 862. [Google Scholar] [CrossRef] [PubMed]
- E Bukhari, E.; E Al-Otaibi, F.; El-Hazmi, M.M.; Somily, A.M. Panton-Valentine leukocidin Staphylococcus aureus osteomyelitis of the femur in a Saudi child. . 2012, 33, 201–4. [Google Scholar]
- Ambrozova, H.; Maresova, V.; Fajt, M.; Pavlicek, P.; Rohacova, H.; Machova, I.; Petras, P. The first case of fatal pneumonia caused by Panton–Valentine leukocidin-producing Staphylococcus aureus in an infant in the Czech Republic. Folia Microbiol. 2012, 58, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Isobe, H.; Miyasaka, D.; Ito, T.; Takano, T.; Nishiyama, A.; Iwao, Y.; Khokhlova, O.E.; Okubo, T.; Endo, N.; Yamamoto, T. Recurrence of pelvic abscess from Panton-Valentine leukocidin-positive community-acquired ST30 methicillin-resistantStaphylococcus aureus. Pediatr. Int. 2013, 55, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Obando, I.; Valderrabanos, E.S.; A Millan, J.; Neth, O.W. Necrotising pneumonia due to influenza A (H1N1) and community-acquired methicillin-resistant Staphylococcus aureus clone USA300: successful management of the first documented paediatric case. Arch. Dis. Child. 2010, 95, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.L.; Eadie, P. Idiopathic neonatal necrotising fasciitis caused by community-acquired MSSA encoding Panton Valentine Leukocidin genes. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 1522–1524. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, C.d.O.; Chamon, R.C.; de Oliveira, T.L.R.; Nouér, S.A.; dos Santos, K.R.N. Systemic infection caused by the methicillin-resistant Staphylococcus aureus USA300-LV lineage in a Brazilian child previously colonized. Braz. J. Infect. Dis. 2023, 27, 102737. [Google Scholar] [CrossRef]
- Higuchi, W.; Takano, T.; Iwao, Y.; Ozaki, K.; Isobe, H.; Yamamoto, T.; Hung, W.-C.; Teng, L.-J.; Shimazaki, T.; Honda, A.; et al. Molecular characteristics of the Taiwanese multiple drug-resistant ST59 clone of Panton-Valentine leucocidin-positive community-acquired methicillin-resistant Staphylococcus aureus from pediatric cellulitis. J. Infect. Chemother. 2010, 16, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kefala-Agoropoulou, K.; Protonotariou, E.; Vitti, D.; Sarafidou, S.; Anastasiou, A.; Kollios, K.; Roilides, E. Life-threatening infection due to community-acquired methicillin-resistant Staphylococcus aureus: case report and review. Eur. J. Pediatr. 2009, 169, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Libert N, Batjom E, Cirodde A, de Rudnicki S, Grasser L, Borne M, et al. [Antitoxin treatments for necrotizing pneumonia due to Panton-Valentine leukocidin-secreting Staphylococcus aureus]. Med Mal Infect. 2009 Jan;39(1):14–20.
- Shallcross, L.J.; Fragaszy, E.; Johnson, A.M.; Hayward, A.C. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin--Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton–Valentine leukocidin. Mod. Pathol. 2007, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Couvé-Deacon E, Tristan A, Pestourie N, Faure C, Doffoel-Hantz V, Garnier F, et al. Outbreak of Panton-Valentine Leukocidin–Associated Methicillin-Susceptible Staphylococcus aureus Infection in a Rugby Team, France, 2010–2011 - Volume 22, Number 1—January 2016 - Emerging Infectious Diseases journal - CDC. [cited 2024 Sep 7]. Available online: https://wwwnc.cdc.gov/eid/article/22/1/15-0597_article.
- Gillet, Y.; Dumitrescu, O.; Tristan, A.; Dauwalder, O.; Javouhey, E.; Floret, D.; Vandenesch, F.; Etienne, J.; Lina, G. Pragmatic management of Panton–Valentine leukocidin-associated staphylococcal diseases. Int. J. Antimicrob. Agents 2011, 38, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Remington, F.P.; Bayer, A.S.; Diep, B.; Tan, N.; Bharadwa, K.; Tsui, J.; Perlroth, J.; Shay, A.; Tagudar, G.; et al. Clinical and Epidemiologic Characteristics Cannot Distinguish Community-Associated Methicillin-Resistant Staphylococcus aureus Infection from Methicillin-Susceptible S. aureus Infection: A Prospective Investigation. Clin. Infect. Dis. 2007, 44, 471–482. [Google Scholar] [CrossRef]
- Chiu, Y.-K.; Lo, W.-T.; Wang, C.-C. Risk factors and molecular analysis of Panton-Valentine leukocidin-positive methicillin-susceptible Staphylococcus aureus colonization and infection in children. J. Microbiol. Immunol. Infect. 2012, 45, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Shallcross LJ, Williams K, Hopkins S, Aldridge RW, Johnson AM, Hayward AC. Panton-Valentine leukocidin associated staphylococcal disease: a cross-sectional study at a London hospital, England. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2010 Nov;16(11):1644–8.
- Tinelli M, Monaco M, Vimercati M, Ceraminiello A, Pantosti A. Methicillin-Susceptible Staphylococcus aureus in Skin and Soft Tissue Infections, Northern Italy - Volume 15, Number 2—February 2009 - Emerging Infectious Diseases journal - CDC. [cited 2024 Oct 1]. Available online: https://wwwnc.cdc.gov/eid/article/15/2/08-0010_article.
- Rao, G.G.; Batura, R.; Nicholl, R.; Coogan, F.; Patel, B.; Bassett, P.; Kearns, A.M. Outbreak report of investigation and control of an outbreak of Panton-Valentine Leukocidin-positive methicillin-sensitive Staphylococcus aureus (PVL-MSSA) infection in neonates and mothers. BMC Infect. Dis. 2019, 19, 178. [Google Scholar] [CrossRef]
- Goemanne, S.; Tilmanne, A.; Biarent, D.; Smeesters, P.; Simoni, P.; Mahadeb, B.A.; Vicinanza, A. Severe Staphylococcus aureus infections in children: Case reports and management of positive Panton-Valentine leucocidin cases. Front. Pediatr. 2022, 10, 1003708. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, O.; Boisset, S.; Badiou, C.; Bes, M.; Benito, Y.; Reverdy, M.-E.; Vandenesch, F.; Etienne, J.; Lina, G. Effect of Antibiotics on Staphylococcus aureus Producing Panton-Valentine Leukocidin. Antimicrob. Agents Chemother. 2007, 51, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu O, Badiou C, Bes M, Reverdy ME, Vandenesch F, Etienne J, et al. Effect of antibiotics, alone and in combination, on Panton-Valentine leukocidin production by a Staphylococcus aureus reference strain. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2008 Apr;14(4):384–8.
- Health Protection Agency. Guidance on the Diagnosis and Management of PVL-Associated Staphylococcus aureus Infections (PVL-SA) in England. 2008.
- Lee, Y.-C.; Chen, P.-Y.; Wang, J.-T.; Chang, S.-C. A study on combination of daptomycin with selected antimicrobial agents: in vitro synergistic effect of MIC value of 1 mg/L against MRSA strains. BMC Pharmacol. Toxicol. 2019, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Reference | Patients | Age* | Immune compromission and comorbidities | Samples used for diagnosis | Methicillin Sensitivity | Clinical Picture | Complications and ICU | Treatment | HL (d) | Outcome |
| Castellazzi et al. (2021) [3] | F (1) | 0,5 | Left bronchus malformation, history of apnea, URTI, bronchiolitis. | Blood, drainage material, BAL. S. pneumoniae and Haemophilus influenzae detected in the BAL culture. | MRSA | Muscle abscesses, femoral osteomyelitis, lung nodules, pulmonary septic emboli, sepsis | PE, ICU | Cefotaxime ➔ Ceftaroline + Daptomycin + Clindamycin ➔ Ceftaroline ➔ Linezolid (os) | 49 | Full recovery |
| Albiński et al. (2018) [4] | M (1) | 12 | N/A | Blood | MSSA | Osteomyelitis (tibia) Sepsis | Absent | Cefuroxime + Flucloxacillin + Gentamicin | 39 | growth arrest |
| Albiński et al. (2018) | M (1) | 10 | N/A | Blood and drainage material | MSSA | Osteomyelitis (humerus) | Absent | Amoxicillin-clavulanic + Clindamycin + Flucloxacillin ➔ Clindamycin (os) | 16 | pathological fracture |
| Albiński et al. (2018) | M (1) | 12 | N/A | Blood and drainage material | MSSA | Panosteomyelitis (femur, tibia) | ICU | Amoxicillin-clavulanic + Amikacin + Flucloxacillin + Vancomycin ➔ Levofloxacin + Rifampicin (os) | 83 | suicidality |
| Albiński et al. (2018) | M (1) | 13 | Acute Lymphoid Leukemia | Blood and drainage material | MSSA | Necrotizing pandiaphysitis (tibia), sepsis | Absent | Cefuroxime + Flucloxacillin | 35 | growth arrest |
| Albiński et al. (2018) | M (1) | 10 | N/A | Blood and drainage material | MSSA | Arthritis (hip) | Absent | Flucloxacillin + Gentamicin ➔ Clindamycin (os) | 27 | Full recovery |
| Albiński et al. (2018) | M (1) | 15 | N/A | Blood and drainage material | MSSA | Arthritis (knee) | ICU | Amoxicillin-clavulanic + Clindamycin + Flucloxacillin + Imipenem + Vancomycin | 74 | Relapse |
| Albiński et al. (2018) | M (1) | 2 | N/A | Drainage material | MRSA | Abscess (foot) | Absent | Amoxicillin-clavulanic + Clindamycin + Flucloxacillin | 17 | Full recovery |
| Albiński et al. (2018) | F (1) | 2 | N/A | Drainage material | MSSA | Abscess (gluteal) | Absent | Amoxicillin-clavulanic + Clindamycin | 1 | Full recovery |
| Albiński et al. (2018) | F (1) | 12 | N/A | Drainage material | MSSA | Abscess (finger) | Absent | Flucloxacillin | 8 | Full recovery |
| Moutaouakkil et al. (2022) [5] | M (12) F (5) | 8,12 | NR | Blood cultures, articular fluids, synovial tissues and/orbone fragments. | MRSA | Osteomyelitis (17), STTI (8) | Absent | NR | 17,41 | Full recovery |
| Hoppe et al. (2019) [6] | M (6) F (4) | 5,73 | URTI (RSV-B3, Influenza A) (4); Neonatal drug withdrawal; congenital CMV; heart transplantation. | Blood, pleural exudate, sputum, and bronchial lavage samples. | MSSA (6) MRSA (4) | Necrotizing pneumonia (5), necrotizing fasciitis of the thorax (2), pyomyositis (2), axillary abscess (1), mastoiditis and cerebellitis (1), multiple and recurrent abscesses (1), preorbital cellulitis (1), purulent conjunctivitis (1). | DVT (2), ICU (4) | NR | 22,6 | FR (9), S (1); persisting post intensive care syndrome,including reduced lung capacity and critical illnesspolyneuropathy |
| Fujita et al. (2022)[7] | F (1) | 0,3 | N/A | Lumbar abscess drainage, nasal swab. Negative blood culture. | MRSA | Extensive subcutaneous abscess of the lumbar region. | Absent | Cefotaxime ➔ Vancomycin ➔ Oral Sulfamethoxazole-trimethoprim | 21 | Full recovery |
| Karli et al. (2016)[8] | M (1) | 12 | N/A | Blood | MSSA | Multiple peripherally localized cavitary round lesions in both lungs (CT). Left psoas muscle abscess and left femoral trochanter osteomyelitis. Sepsis | PE, ICU | Vancomycin ➔ Linezolid + Clindamycin | 30 | Full recovery |
| Ahoyo et al. (2012)[9] | NR (19) | NR | Malnourishment after severe malaria, anemia, hospitalization | Pleural fluid (6), nasal swab (2), BAL (11) | MSSA | Pneumonia (19) | NR | NR | NR | Full recovery (2); Deceased (17) |
| Hardy et al. (2019)[10] | F (1) | 4,6 | N/A | Blood, drainage material | MSSA | Acute osteomyelitis, dermohypodermitis, septic arthritis, multifocal lesions, cardiac tamponade. | DVT, ICU | Amoxicillin-clavulanic + Gentamicin ➔ Cefotaxime + Fosfomycin + Clindamycin | 11 | Deceased; cardiac tamponade |
| Hardy et al. (2019) | M (1) | 14,7 | N/A | Blood, drainage material.Staphylococcus haemolticus in pericardial effusion. | MSSA | Acute osteomyelitis, open skin wound. | Absent | Cefotaxime + Gentamicin + Fosfomycin ➔ Clindamycin + Ciprofloxacin ➔ Ciprofloxacin (os) + Rifampicin (os) | 14 | Full recovery |
| Hardy et al. (2019) | F (1) | 11,2 | N/A | Blood, drainage material | MSSA | Acute osteomyelitis, open skin wound. | Absent | Amoxicillin-clavulanate + Gentamicin ➔ Clindamycin + Ciprofloxacin + Fosfomycin ➔ Ciprofloxacin (os) + Rifampicin (os) | 18 | Full recovery |
| Hardy et al. (2019) | F (1) | 13 | Active chronic Hepatitis B | Blood, drainage material | MSSA | Acute osteomyelitis, dermohypodermitis, septic arthritis, multifocal lesions, necrotizing fasciitis, pyomyositis, necrotizing pneumonia. | ICU | Cefotaxime + Gentamicin + Vancomycin ➔ Cloxacillin + Clindamycin + Rifampicin ➔ Clindamycin (os) + Rifampicin (os) | 88 | Functional impairment (ankle) |
| Hardy et al. (2019) | M (1) | 8,4 | N/A | Blood, drainage material | MSSA | Acute osteomyelitis, septic arthritis, multifocal lesions. | Absent | Cefotaxime + Gentamicin ➔ Cefotaxime + Vancomycin ➔ Clindamycin (os) + Rifampicin (os) | 20 | Full recovery |
| Hardy et al. (2019) | M (1) | 6,5 | N/A | Blood, drainage material, sputum culture. | MSSA | Acute osteomyelitis, septic arthritis, multifocal lesions, necrotizing pneumonia. | Absent | Cefotaxime + Gentamicin + Vancomycin ➔ Cloxacillin + Clindamycin ➔ Clindamycin (os) + Rifampicin (os) | 42 | Functional impairment, chronic osteomyelitis |
| Ogawa et al. (2022)[11] | F (1) | 1 | N/A | Surgical drainage. | MRSA | Retropharyngeal abscess | ICU | Ampicillin/sulbactam + Vancomycin ➔ Clindamycin | 22 | Full recovery |
| Oshima et al. (2021)[12] | M (1) | 0,08 | N/A | Blood. | MRSA | SSTI, necrotizing pneumonia and cerebral infarction. Sepsis | DVT, ICU | Meropenem and Cefotaxime ➔ Vancomycin + Meropenem ➔ Linezolid | 68 | right-sided hemiparesis |
| Vanbiervliet et al. (2022)[13] | M (1) | 12 | N/A | Blood. | MSSA | Subperiosteal abscess, osteomyelitis (ankle), pyomyositis, septic cardiomyopathy. Sepsis | DVT, PE, ICU | Piperacillin-tazobactam + Vancomycin ➔ Flucloxacillin, Linezolid, Clindamycin, and Levofloxacin | 42 | amputation; hospitalization was probably longer |
| Chen et al. (2014)[14] | F (1) | 15 | N/A | Pleural fluid and sputum. | MRSA | Necrotizing pneumonia with cavitary lung lesions and bilateral pleural effusion. Sepsis | ICU | Linezolid + Fosfomycin + Teicoplanin | 62 | Full recovery |
| Schwartz et al. (2012)[15] | F (1) | 0,67 | N/A | Pleural fluid. | MSSA | Necrotizing pneumonia, Pulmonary hemorrhage. | ICU | Cefotaxime ➔ Flucloxacillin + Vancomycin ➔ Linezolid + Rifampicin + Gentamicin ➔ Flucloxacillin + Rifampicin (after MSSA confirmed) | 17 | Deceased |
| Schwartz et al. (2012) | M (1) | 1,25 | N/A | Blood, pleural fluid. | MRSA | Necrotizing pneumonia, pulmonary hemorrhage, cerebral septic emboli and abscesses, erythroderma. Sepsis | ICU | Flucloxacillin, Cefotaxime, Ampicillin, and Azithromycin ➔ Hydrocortisone and IVIG ➔ Flucloxacillin + Vancomycin | 28 | Deceased |
| Schwartz et al. (2012) | F (1) | 0,67 | N/A | Pleural fluid and tracheal aspirate. | MRSA | Necrotizing pneumonia with multiple pneumatoceles. | ICU | Clindamycin and Cefotaxime ➔ Vancomycin ➔ Clindamycin + Linezolid + Rifampicin | 7 | Full recovery |
| Schwartz et al. (2012) | M (1) | 0,42 | N/A | Scalp lesion, endotracheal tube, and blood. | MRSA | Necrotizing pneumonia, infarction of the right cerebral and cerebellar hemispheres, purulent subcutaneous scalp lesion with surrounding erythema. Sepsis | ICU | Cefotaxime, Flucloxacillin, and Vancomycin ➔ Linezolid + Lincomycin + Rifampicin ➔ Clindamycin | 7 | resolving hemiplegia |
| Irenji et al. (2018)[16] | M (1) | 13 | N/A | Blood, abscesses material. | MSSA | Extensive pelvic abscesses, bilateral pneumonia, pericardial effusion and osteomyelitis. Sepsis | ICU | Flucloxacillin + Cefotaxime ➔ Linezolid + Clindamycin | 41 | Full recovery |
| Elledge et al. (2014)[17] | M (1) | 10 | N/A | Blood. | MRSA | Osteomyelitis of the proximal tibia, history of boils on the buttocks and thighs. | Absent | Flucloxacillin + Rifampicin + Linezolid ➔ Flucloxacillin (os) + Rifampicin (os) ➔ Linezolid (os) | 14 | Full recovery |
| Cocchi et al. (2013)[18] | M (1) | 0,25 | N/A | Pleural drainage sample. | MRSA | Necrotizing pneumonia, pyopneumothorax. | ICU | Ampicillin-sulbactam + Gentamicin (initial treatment; adjustments not mentioned) | NR | NR |
| Lehman et al. (2010) [19] | M (1) | 6 | N/A | Blood, abscess drainage material. | MSSA | Necrotizing pneumonia and necrotizing fasciitis, septic osteomyelitis and arthritis, pulmonary consolidation. Sepsis | PE, ICU | Vancomycin + Cefotaxime ➔ Oxacillin + Gentamicin | 42 | Full recovery |
| Green et al. (2017) [20] | M (1) | 13 | Heterozygous factor V Leiden mutation | Blood. | MSSA | Periorbital cellulitis. | DVT | Ceftriaxone + Metronidazole ➔ Ceftriaxone + Clindamycin (os) | NR | Full recovery |
| Khattak et al. (2019)[21] | M (1) | 0 | Preterm infant (30th week) | Blood. | MSSA | Cavitating pneumonia, shoulder abscess, cerebral abscess, osteomyelitis. Sepsis | ICU | Cefotaxime + Vancomycin ➔ Flucloxacillin + Linezolid | 42 | Full recovery |
| Gadelsayed et al. (2012)[22] | F (1) | 11 | N/A | Nasal swab, BAL. | MRSA | Cavitating pneumonia. | Absent | Trimethoprim/sulfamethoxazole | NR | Full recovery |
| Fitzgerald et al. (2013)[23] | M (1) | 14 | N/A | Blood. | MSSA | Discitis with an epidural abscess at L3–L4, necrotizing pneumonia. Sepsis | ICU | Flucloxacillin, Clindamycin, and Gentamicin ➔ Ceftriaxone, Clindamycin and Clarithromycin ➔ Linezolid added + IVIG ➔ Ceftriaxone and Clindamycin (oral) | NR | Full recovery |
| Karli et al. (2015)[24] | M (1) | 12 | N/A | Blood. | MSSA | Necrotizing pneumonia, psoas abscess, cellulitis, and osteomyelitis. Sepsis | PE, ICU | Ceftriaxone + Vancomycin ➔ Vancomycin discontinued ➔ Linezolid ➔ Clindamycin (os) | 30 | Full recovery |
| Daskalaki et al. (2009)[25] | M (10) F (2) | 2,3 | N/A | Drainage material or skin samples (12). | MRSA (5) MSSA (7) | SSTI (12). Cellulitis, abscesses (9). | Absent | NR | NR | NR |
| Geng et al. (2010)[26] | NR (22) | 0,72 | Pneumonia with RSV (2), measles (2), CMV (1) | Sputum culture, pleural fluid and blood. | MRSA (22) | Necrotizing pneumonia (22), complicated in 3 cases with empyema, pneumopyothorax, septicemia. Sepsis (1) | Absent | NR | NR | Ful recovery (21) |
| Kechrid et al. (2010)[27] | M (13) F (3) | 6,18 | N/A | Blood or bone tissues. | MRSA (8) MSSA (8) | Osteomyelitis (10), bacteremia (6). Sepsis (1) | Absent | Oxacillin + Gentamicin (9); Teicoplanin + Gentamicin (3); Vancomycin + Gentamicin (1); Fosfomycin + Cefotaxime (1); Teicoplanin + Pristinamycin (2) | NR | Full recovery (14), Sequelae (2); Chronic osteomyelitis, necrosis of femur |
| Noguchi et al. (2021)[28] | M (1) | 0,92 | N/A | Sputum culture. Negative blood culture. | MRSA | Necrotizing pneumonia, left-sided abscess and pyothorax, DIC | ICU | Cefotaxime ➔ Meropenem and Vancomycin ➔ Linezolid and Clindamycin ➔ Vancomycin and Clindamycin | 31 | Full recovery |
| Bybeck et al. (2020)[29] | NR (15) | 9,06 | N/A | Blood (15), and surgical drainage in cases with abscesses. | MRSA (2) MSSA (13) | Pulmonary localization (6); osteoarticular localization (5); SSTI (2); pericardial effusion (1); DIC (1), pneumothorax (2), respiratory insufficiency (4), one rhabdomyolysis and one local abscess. Sepsis (1). | DVT (1), ICU (4) | NR | NR | Full recovery (11), Deceased (1); Sequelae (3); venous insufficiency and stenosis of the iliac vein (1); above-knee amputation (1); chronic heart failure (1 case, unrelated to infection) |
| Mutale et al. (2014)[30] | M (1) | 0,92 | N/A | Blood, brain abscess sample. Negative nasal swab. | MRSA | Parietal lobe abscess in the brain. | ICU | Ceftriaxone + RZHE ➔ Vancomycin + Amikacin + Metronidazole + Cefotaxime + Rifampicin ➔ Rifampicin + Vancomycin + Linezolid | 42 | Full recovery |
| Bukhari et al. (2012)[31] | F (1) | 0,75 | N/A | Blood, drainage material. | MSSA | Abscess formation and osteolytic lesions of the femur, left iliofemoral thrombosis | DVT | Ceftriaxone + Cloxacillin ➔ Clindamycin + Cloxacillin | 56 | Full recovery |
| Ambrozova et al. (2012)[32] | M (1) | 0,83 | Citrobacter youngae identified in stool culture | Blood, pleural fluid. | MSSA | Pleuropneumonia and empyema, mediastinitis, SSTI. Sepsis | ICU | Cefotaxime ➔ Cefotaxime, Clindamycin, Gentamicin, Fluconazole, Corticosteroids ➔ Oxacillin + Clindamycin + Gentamicin. | 16 | Deceased; progressive respiratory failure, pneumothorax, pneumoperitoneum, and circulatory failure, leading to death. |
| Isobe et al. (2013)[33] | F (1) | 17 | N/A | Drainage material. | MRSA | Osteomyelitis and multifocal pelvic (iliopsoas and piriformis) abscesses, adjacent to the sacroiliac joint. | Absent | Vancomycin + Pazufloxacin ➔ Post-discharge: Vancomycin + Minocycline (os)Second episode: Vancomycin + Fosfomycin ➔ Vancomycin (post-discharge) + Minocycline (os) | 27 | Relapse (3 episodes requiring hospitalization) |
| Obando et al. (2010)[34] | M (1) | 12 | Influenza A (H1N1) co-infection. | Pleural fluid culture. | MRSA | Necrotizing pneumonia and bilateral pleural empyemaand pneumothorax. | ICU | Ceftriaxone + Vancomycin + Clarithromycin ➔ Vancomycin + Clindamycin ➔ Linezolid + Clindamycin | 28 | Full recovery |
| Dunlop et al. (2011)[35] | M (1) | 0,03 | N/A | Cultures taken at the time of the first debridement. | MSSA | Necrotizing fasciitis. | ICU | Cefotaxime + Gentamicin + Flucloxacillin ➔ Vancomycin + Clindamycin ➔ High-dose Flucloxacillin + Clindamycin + Gentamicin | 35 | Full recovery |
| Whitaker et al. (2023)[36] | M (1) | 15 | N/A | Blood, drainage materials, nasal swab. | MRSA | Abscess in left psoas, subduralempyema in the sacral region extending to the lumbarspine. | PE | Vancomycin ➔ Daptomycin ➔ Sulfamethoxazole/trimethoprim (os) | 45 | Full recovery |
| Higuchi et al. (2010)[37] | M (1) | 7 | N/A | Drainage material. | MRSA | Abdominal cellulitis | Absent | Cefdinir (os) | 7 | Relapse |
| Kefala-Agoropoulou et al. (2010)[38] | F (1) | 10 | N/A | Blood, drainage material. | MRSA | Pneumonia, signs of encephalopathy, severe osteomyelitis on the whole right femur and pyomyositis and thrombosis of the right femoral andright external iliac vein. Sepsis | DVT, ICU | Cloxacillin ➔ Vancomycin + Clindamycin + Gentamicin ➔ Teicoplanin + Clindamycin (os) | 56 | Full recovery |
| Bloodstream (bacteremia) | 76 (49%) |
| Respiratory system | 73 (47%) |
| Skin and soft tissues | 59 (38%) |
| Osteo-articular system | 58 (37%) |
| Central Nervous System | 9 (6%) |
| Cardiovascular system | 6 (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





