1. Introduction
Dental caries remains one of the most prevalent chronic diseases worldwide, significantly impacting oral health and quality of life and potentially leading to dental morbidity if ineffectively managed [
1,
2]. Effective treatment planning depends on precise diagnosis and assessment of caries severity, as the caries process starts with the demineralization of dental tissues [
3]. This demineralization results in the dissolution of hydroxyapatite crystals, leading to the loss of calcium and phosphate from the dental structure, ultimately causing the exposure of dentin [
4,
5].
Dentin, which constitutes the majority of the tooth structure, is composed of an organic matrix, primarily type I collagen, non-collagenous proteins, and water, as well as an inorganic component composed by hydroxyapatite [
6]. Due to its tubular structure and higher organic content, dentin is more susceptible to acid penetration and has a higher dissolution rate compared to the enamel, which contains residual mineral crystallites that facilitate remineralization [
7]. Conditions such as caries, erosion, and developmental defects, including Molar Incisor Hypomineralization (MIH) [
8], further increase the susceptibility of dentin to mineral loss, leading to dentin hypersensitivity [
9] and significant discomfort for patients [
4,
6,
10,
11].
The challenge of dentinal remineralization has prompted advancements in dental technology aimed at preventing mineral loss and promoting tissue repair. Traditional fluoride-based products have proven effective in remineralization but are ineffective in promoting new mineral phase formation [
7,
12,
13,
14]. In particular, bioactive glasses have reported the ability to release ions such as calcium and phosphate, enhancing hydroxyapatite formation and improving dentin repair [
15,
16,
17]. Emerging bioactive products that deliver essential minerals directly to the site of repair have shown promising results in creating an environment conducive to natural dentin repair [
18,
19]. A biomimetic approach, which mimics the natural mineralization process using elements other than fluoride, increases remineralization and promotes tissue regeneration [
7,
14,
19,
20].
A recent review highlighted the role of silicon-substituted hydroxyapatite (Si-HA) in enhancing the bioactivity and mechanical properties of dentin and enamel [
21]. Si-HA has been reported not only to improve the stability of hydroxyapatite under acidic conditions but also to promote a more effective remineralization process, a determinant for the repair of demineralized dental tissues [
21]. This makes silicon an important ingredient in modern toothpaste formulations aimed at providing superior protection against caries and erosion [
11,
16,
17,
22]. Silicon-containing dental products have been shown to promote the formation of hydroxyapatite-like structures within enamel, offering long-lasting protection against erosion and caries [
20,
22].
This in vitro study aimed to evaluate the effectiveness of a Si-toothpaste with different concentrations of fluoride to reduce the depth of demineralized lesions in dentin after cariogenic pH cycling using different methods. The research hypothesis was that Si-toothpaste would positively contribute to remineralization and/or reduction in the depth of dentin caries lesions, irrespective of the concentration of fluoride.
2. Materials and Methods
This in vitro laboratory study was designed with random selection and followed an inductive approach, employing statistical and comparative procedures to evaluate the effectiveness of a Si-containing toothpaste with different concentrations of fluoride on dentin caries demineralization.
Specimen Preparation
Sixty dentin specimens, each measuring approximately 4 × 4 × 2 mm, were prepared from the middle third part of the roots of bovine incisor teeth previously sterilized in a 0.8% Thymol solution. The specimens were cut using a water-cooled rotating diamond wheel (Isomet, Buehler Ltd., Evanston, IL, USA). Each block was then embedded in self-curing acrylic resin and polished with progressively finer grit papers (600–1500 grades) under water irrigation. Final polishing was completed with 1µm diamond paste (Extec Corporation, Enfield, CT, USA) using a rotating polishing machine (PSK-2 V, Skill-tec Comércio e Manutenção Ltd.a., São Paulo, SP, Brazil).
Baseline surface hardness was assessed by performing three indentations spaced 100 µm apart at the center of each block using a Vickers indenter with a 25 g load for 10 seconds (HMV-G series Microhardness Tester, Shimadzu, Kyoto, Japan). The mean hardness value was calculated for each block, and only specimens with variations within 10% of the mean value (46 ± 10 Vickers hardness number) were included. The specimens were then randomly allocated into five groups (n = 12 per group), and the homogeneity of hardness across groups was confirmed by one-way ANOVA.
Toothpaste Selection
Five toothpaste formulations were selected based on the active ingredients and commercial availability. The test groups included three different formulations of a Si-containing toothpaste with varying fluoride concentrations: RGS
1 (1450 ppm F), RGS
2 (1100 ppm F), and RGS
3 (fluoride-free). A fluoride-free toothpaste served as the negative control (NC), while a standard sodium fluoride toothpaste was used as the positive control (PC). The composition and pH of the toothpaste slurries are displayed in
Table 1. In order to ensure blinding, the toothpastes were coded and stored by an independent researcher, with the codes undisclosed to the investigator conducting the study and analyzing the data.
Treatment and pH Cycling
Prior to the pH cycling treatment, half of each dentin specimen’s surface was covered with two layers of nail varnish (Risqué, Niasi, Taboão da Serra, São Paulo, Brazil) to serve as a reference for the sound (untreated) area. The specimens were first placed in a remineralizing solution for 24 h to simulate the oral environment [
13].
The pH cycling model, adapted from Hara et al. [
23], was performed over three days at 37°C to induce early-stage dentin demineralization. This model involved alternating between 1-hour demineralization phases and 23-hour remineralization phases each day. During the demineralization phase, the specimens were immersed in a solution containing 2.0 mM/L of calcium and phosphate in a 75 mM/L acetate buffer (pH = 4.7, 0.03 µg F/mL, 3 mL/mm²). For the remineralization phase, the specimens were placed in a solution containing 1.5 mM/L of calcium, 0.9 mM/L of phosphate, and 150 mM/L of potassium chloride in a 0.02 mM/L cacodylate buffer (pH = 7.0, 0.02 µg F/mL, 1 mL/mm²) [
13,
24].
Abrasion was performed twice daily at 10 AM and 2 PM using an automated brushing machine (MEV 3T-8XY, Odeme—Joaçaba, SC, Brazil). Each abrasion session involved 10 s of brushing with 30 mL of the toothpaste slurries, followed by 110 s of exposure to the toothpastes, completing a total of 2 min of treatment [
25]. The brushing machine was standardized to an average temperature of 37 °C and 11 cycles, corresponding to 10 s. Vertical zigzag movements were performed, with an amplitude (vertical and horizontal) of 20 mm and an axial load of 150 g. The toothpaste slurries were prepared before each use by mixing the toothpaste with deionized water in a 1:3 ratio (30 mL/specimen) under constant agitation to ensure complete homogenization. After each abrasion session, the specimens were rinsed with deionized water, and all solutions were refreshed daily.
Surface Microhardness test analysis (SMH)
The baseline surface microhardness (SMH0) was evaluated using a microhardness tester as previously described settings. The dentin surface microhardness after treatment (SMH1) with the toothpastes associated with pH cycling was also determined using the same parameters. For this determination, the acid-resistant nail varnish was removed, and the hardness of each half specimen was tested. Also, the percentage of surface hardness change (%SMHC) was then calculated, as follows (Eq. 1): %SMHC=(SH0−SH1)/SH0×100
Light-Induced Fluorescence (QLF) Analysis
The impact of the treatments on fluorescence loss in dentin carious lesions was evaluated using the Qraycam Pro device (Inspektor Research System BV, Amsterdam, The Netherlands). To prepare the specimens, the nail varnish covering the reference windows was carefully removed using a surgical blade and cotton swabs soaked in diluted acetone. The specimens were then rinsed with deionized water and air-dried. A standardized imaging setup was employed, ensuring consistent camera positioning and maintaining a fixed distance of 8 cm between the device and the specimens for all QLF measurements [
14]. The images were captured in a dark room, with exposure and contrast settings both set to 0 [
26,
27].
The Q-ray software (version 1.38, Inspektor Research System BV, Amsterdam, The Netherlands) was used to analyze fluorescence loss (ΔF) and the maximum lesion depth (ΔFmax). The ΔF value represents the average loss of fluorescence intensity when comparing the carious lesion to sound dentin, reflecting changes in the mineral content of the dentin [
27]. The ΔFmax value measures the maximum lesion depth by calculating the highest ΔF value within the lesion’s contour.
Scanning Electron Microscopy (SEM) Analysis
A subset of dentin specimens underwent SEM analysis to observe the morphological changes after one-time treatment with the different toothpastes. Before brushing, the specimens were acid-etched for 2 min with 0.05 M citric acid (pH 1.8) to remove the smear layer and expose the dentinal tubules [
14,
20,
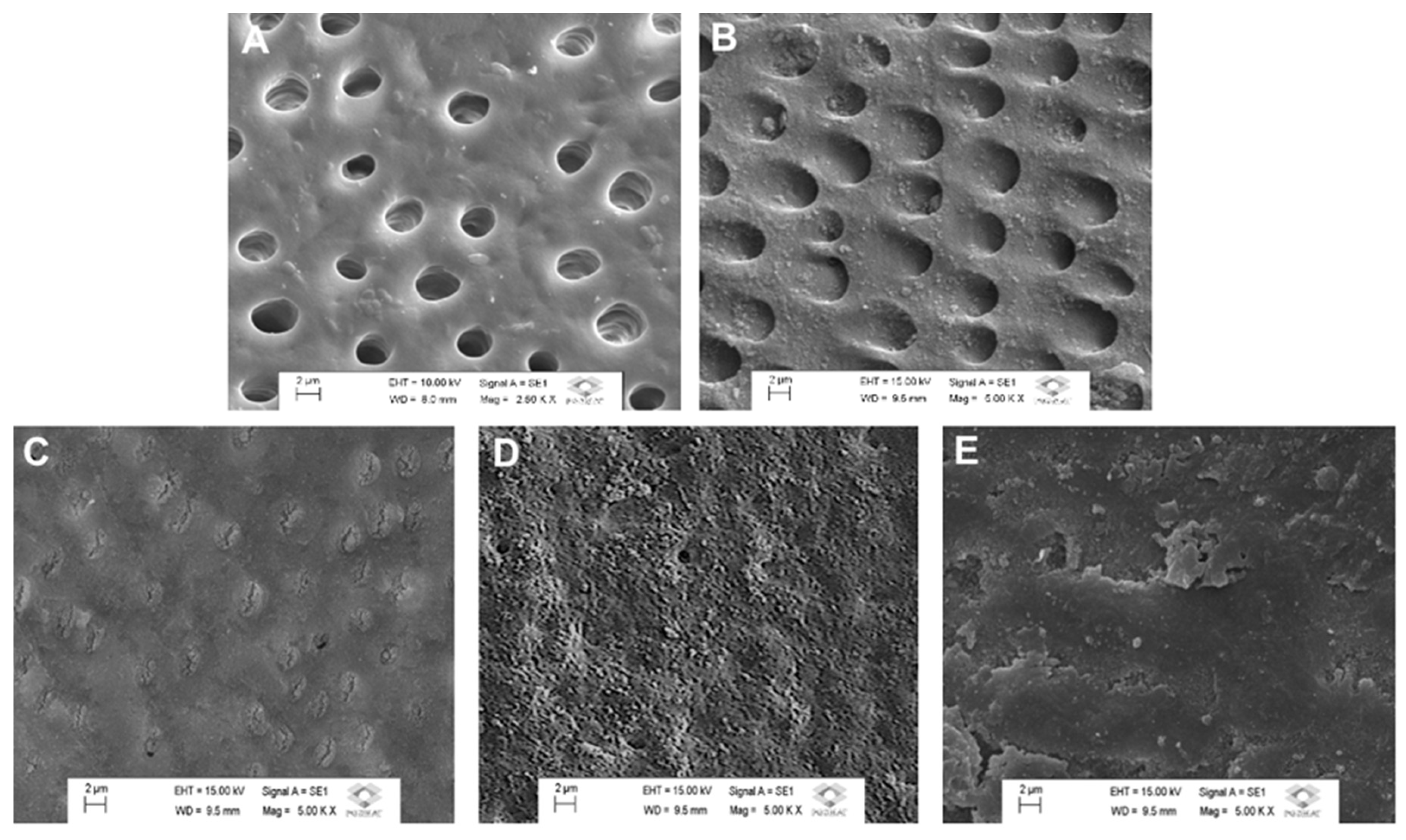
28]. The experimental groups for the dentin analysis were as follows: I, control, untreated group; II, morphological analysis immediately after brushing with the toothpaste. The SEM analysis was conducted using a scanning electron microscope (TESCAN VEGA3, LMU, Kohoutovice, Czech Republic) operating at 15 kV. The specimens were sputter-coated with gold using a vacuum evaporator (MED 010, Balzers, Liechtenstein) before microscopic examination. Photomicrographs at 5000x magnification were obtained to characterize the surface morphology of the treated dentin blocks, with representative images selected for analysis. Representative images of selected regions of the specimens were obtained for characterizing the morphological aspect of the surface.
Statistical Analysis
Statistical analyses were performed using the SPSS software package (version 21.0, SPSS Inc., Chicago, IL, USA). Normality and homogeneity of variances were assessed using the Shapiro–Wilk test and Levene’s test, respectively. Since the data showed a Gaussian distribution and equal variances, no data transformation was required. Differences between groups for the ΔF and ΔFmax variables were analyzed using one-way ANOVA followed by Tukey’s post hoc test, with a significance level set at 5%. Additionally, qualitative analysis was performed on the SEM images.
3. Results
The means (SD) of SMH before and after treatment, and also the surface microhardness change (%SMH
C), are presented in
Table 2. No significance was observed when the means of SMH at the baseline (SMH
0) were compared (p > 0.05). After treatment, all the mean groups were significantly different (p < 0.05). The mean of %SMH
C for the NC group was significantly lower compared to other experimental groups, which means that there was no mineral gain after treatment with the fluoride-free toothpaste. No significance was observed when the results of groups RGS
1 and RGS
2 were compared (p > 0.05). In addition, no significance was also observed comparing the results of groups RGS
3 and PC (p > 0.05). The results of %SMH
C can be ranked as follows: RGS
2 > RGS
1 > RGS
3 > PC.
The results of ΔF (fluorescence loss) and ΔF
max (lesion depth) using QLF analysis are displayed in
Table 3. The NC group exhibited significantly higher fluorescence loss and lesion depth compared to other experimental groups (p < 0.05). RGS
1 and RGS
2 groups presented significantly less fluorescence loss (ΔF) when compared to the PC group (p < 0.05), which was equivalent to that observed in RGS
3 group. RGS
1 and RGS
2 protected against fluorescence loss in 27.5% and 33.5% higher, respectively, compared to the PC group. Considering the analysis of ΔF
max, the Si-containing toothpastes irrespective of the fluoride concentrations (RGS
1, RGS
2, and RGS
3) presented a significantly higher reduction of demineralized lesion depth after treatment compared to both NC and PC groups (p < 0.05). Compared to the PC group, RGS
1 group showed a reduction of about 79.8% of demineralized lesion depth, whereas RGS
2 group demonstrated a reduction of 81.3%. Even the fluoride-free formulation (RGS
3) presented significantly better results compared to the PC group, offering 19.2% greater protection against fluorescence loss and a 51.6% reduction in lesion depth.
The morphological analysis provided detailed insights into the surface morphology of dentin after treatment with different toothpastes (
Figure 1). The untreated, demineralized dentin (control) exhibited widespread exposure of open dentinal tubules, indicating significant mineral loss. The PC group displayed partial mineral deposits but with insufficient tubule occlusion. In contrast, dentin treated with a Si- toothpaste associated with different fluoride concentrations (RGS
1, RGS
2, and RGS
3) exhibited occluded dentinal tubules resembling a protective mineral layer. Considering the occlusive effect obtained immediately after treatment, it can be speculated that the treatment with the Si-containing toothpaste was effective to mineralize the dentin surface, irrespective of the fluoride concentration. The dentin surface after treatment with RGS
1 and RGS
2 groups exhibited a uniform and more extensive tubule occlusion, suggesting a synergic effect between fluoride and silicon present in both toothpaste formulations.
Table 4 displays the elemental distribution in weight% observed in the remineralized dentin. The main elements observed in the mineralized layer formed onto the enamel were Si with a relative weight% 7.68%, 7.14%, 4.57%, respectively for RGS
1, RGS
2, and RGS
3, which apparently formed a chemical complexation with Ca (3.20 to 3.56%), P (1.85 to 2.30%), and O (38.03 to 32.68%) [
11].
4. Discussion
The findings of the present study demonstrated Si-toothpastes significantly enhanced the dentin remineralization and protection against demineralization compared to the traditional fluoride-based product, irrespective of the fluoride concentration. The superior performance of experimental groups RGS
1 and RGS
2 can be attributed to the synergic interaction between bioavailable silicon and fluoride ions, which facilitates the growth of hydroxyapatite crystals essential for repairing demineralized areas [
11,
14,
20,
28]. This synergism has been reported to induce the formation of a uniform and resilient mineral layer on the dentin surface, which is a key factor for preventing further demineralization and reinforcing the structural integrity of dental tissues [
20,
22,
29]. The SEM images and EDS data demonstrated the reasons why fluoride Si-toothpastes present superior results when reducing the depth of demineralized lesions in dentin after cariogenic pH cycling. After treatment with groups RGS
1 and RGS
2, the dentin surface exhibited a uniform and large-scale tubule occlusion. Furthermore, assessing the surface microhardness demonstrated that the treatment with the Si-toothpaste associated with fluoride in both concentrations (1100 and 1450 ppm) significantly increased the percentage of surface microhardness change. Specifically, there was a significant increase in the dentin surface hardness after treatment with the toothpastes associated with pH cycling.
Considering the lower hardness of the dentin tissue compared to that of the enamel, which contains a higher percentage of organic structure and is less mineralized, it can be speculated that the mineral layer that is formed on the dentin surface by the nucleation of Si (“enamel-like layer”) would be harder than that of the hardness of the subjacent dentin. Evaluating the mineral content in dentin according to the treated groups, fluoride Si-toothpastes were able to remineralize the dentin, leaving the structure with more minerals. In this manner, it seems that the presence of Si in the formulations enhances the ability to remineralize and regenerate the demineralized dentin, “building” new minerals. That is to say, the QLF and SMH analyses demonstrated the amount of new minerals present in the remineralized dentin and in the mineralized surface layer that were formed after the treatments. The group treated with the fluoride-free, Si-toothpaste (RGS3 group) presented significantly higher results of when compared to the group treated with conventional fluoride-containing toothpaste (PC, positive, control group). These results somehow highlight the significant impact of the bioactive action of the ions delivered when the Si-toothpaste is applied, indicating that this technology is effective in promoting substantial remineralization and dentin protection even in the absence of fluoride, which is particularly beneficial in situations where fluoride alone is ineffective.
These results align with previous studies demonstrating the efficacy of bioactive technologies in enhancing dentin protection and remineralization [
11,
16,
17,
19,
22]. Previous studies reported that oral care products containing bioactive glass such as Novamin—composed of sodium fluoride (NaF) and calcium sodium phosphosilicate (Na₂CaSiO₄)—and also Regenerate NR-5 technology, which combines calcium silicate (CaSiO₃) and sodium monofluorophosphate (Na₂PO₃F), have shown similar benefits in occluding dentin tubules and promoting hydroxyapatite formation in demineralized tissues [
11,
16,
17]. Comparatively, the commercial Si-toothpaste tested in the present study demonstrated superior results; it favored the formation of a more uniform mineral deposition on the dental tissues, creating a stronger protective barrier, particularly under the acidic conditions prevalent in the oral environment [
11,
19,
30]. In contrast to other technologies that work in alkaline environments, the fluoride Si-toothpaste contains a technology that is an acidified bioactive complex, primarily composed of silica and phosphate compounds [
11,
14,
23,
30]. When in contact with the oral environment, these compounds ionize, binding to the tooth structure and capturing available calcium particles, which allows the formation of the so-called “enamel-like layer” of silicon-enriched hydroxyapatite [
6,
11].
It has been reported that the silicon compound present in the formulation of this Si-toothpaste acts as a nucleating agent, inducing the formation of less soluble and more acid-resistant apatite crystals [
31]. This represents an evolving technology with advantages over traditional fluoride-only toothpastes, providing a biomimetic approach to dental remineralization, mimicking the natural process of hydroxyapatite formation [
6,
11,
15]. These findings suggested that Si-toothpaste provides a more effective protection against caries and acid challenges, particularly in cases where fluoride alone seems inadequate.
The SEM images from this study further supported these findings, demonstrating extensive coverage of dentin tubules and the formation of an enamel-like protective layer in the groups treated with the fluoride Si-toothpaste compared to traditional fluoride treatment (control NC group). The resulting mineral layer not only protects against acid challenges but also leads to a more robust dentin matrix, which enhances overall dentin protection and stability [
12,
13]. This morphological evidence is in consonance with other findings evaluating silicon-containing bioactive glasses, which also highlighted the ability of these materials to enhance the structural integrity of dental tissues by promoting more effective mineralization [
12,
13,
15].
The study emphasizes the importance of ion exchange within the hydroxyapatite structure. Hydroxyapatite, the principal mineral component of dentin, has a crystal structure that allows for the substitution of specific ions, which is crucial for its stability and resilience [
11,
13,
32]. F ions can replace hydroxyl ions (OH), forming fluorapatite, a more acid-resistant mineral that significantly enhances protection against caries [
12]. Similarly, phosphate ions (PO₄³⁻) can be replaced by carbonate (CO₃²⁻) or silicate (SiO₄⁴⁻) ions, further improving the mineral’s resistance to acid attacks and contributing to the durability and protective capabilities of the dentin [
13,
32]. Silicon substitution in particular leads to the formation of silicon-substituted hydroxyapatite (Si-HA), which enhances bioactivity, reduces crystallinity, and accelerates mineralization processes [
11,
13,
32]. This promoted greater surface reactivity, facilitating mineral deposition and dentinal tubule occlusion—key factors in the effectiveness of the fluoride Si-toothpaste [
11,
12,
13,
15]. Additionally, the interaction between silicon and the hydroxyapatite structure offers further benefits, improving biological integration and tissue regeneration [
15], which are clinically relevant for treating and preventing conditions such as dental caries, dental erosion, and dentin hypersensitivity [
6,
11,
18,
19,
24,
32]. The ability to nucleate calcium and phosphate ions and promote the natural reconstruction of mineralized tissue. In this manner, the use of this fluoride Si-toothpaste is an important conservative treatment in certain clinical cases such as patients with MIH or severe enamel defects [
8,
13,
22].
The present in vitro study demonstrated the effectiveness of a commercial Si-containing toothpaste associated with different fluoride concentrations to remineralize the dentin after cariogenic pH cycling. In this manner, the Si-toothpaste reduced the depth of demineralized tissue observed in the QLF analysis. It was also demonstrated that the Si was present in the dentin tissue in the experimental groups treated with the Si-containing toothpaste detected using EDS. The interaction of Si and Ca favored the mineral layer formed on the dentin surface, demonstrated in the morphological analysis with SEM. This layer allowed the significant increase in the surface microhardness observed in the groups treated with fluoride Si-toothpastes, regardless of the fluoride concentration. Considering the inherent limitations of in vitro studies, future research should explore alternative methodologies, while also incorporating gold standard products to enable more comparative analyses. In spite of these drawbacks, the findings of the present study corroborate previous in vitro results [
11,
14,
15,
23,
30,
33]. Considering these outcomes, further long-term clinical studies are necessary to assess its sustained effectiveness and applications. Investigating its performance across various clinical settings will be essential to fully understanding its potential in enhancing dental care.