1. Introduction
Increased pollution growth and high-water demand cause a decline in freshwater availability [
1]. This causes water resources to be scarce. In South Africa, a semi-arid country, freshwater quality deterioration continues to be a major problem. It is estimated that about 55,5% of rivers in the City of Cape Town metropole are polluted [
2]. Multiple sources of pollution, such as industrial effluents, agricultural runoff, catchment land-use changes, chemical contaminations, litter, freshwater flow modifications, commercial and domestic sewage, abstraction, and the introduction of alien vegetation species are responsible for this deterioration [
3]. Therefore, proper assessment of a water resource requires regular monitoring. Water quality monitoring in South Africa has mostly been done using physicochemical parameters and where biomonitoring has been used as a monitoring tool, macroinvertebrates have been used [
4]. Besides diatoms, other bio-monitoring indicators, such as fish and macroinvertebrates, have also been used [
5].
The study aimed at using diatoms as an indicator of water quality. This study also used historical physicochemical parameters obtained from the Department of Water and Sanitation from 2019 to 2021 as a reference of water quality conditions along the Kuils River. According to [
6], both diatoms and macroinvertebrates can equally predict water quality. However, diatoms are better associated with water quality while macroinvertebrates are regarded as better indicators of disturbances (land use, dissolved oxygen, acid and ammonia contamination) in river systems [
5,
7]. Diatoms are sensitive to a variety of ecological conditions. The tolerance and preference levels of diatoms to pH, conductivity, salinity, humidity, organic matter, trophic state, oxygen and current velocity in freshwater resources make them good indicators [
8]. Diatoms live in different types of aquatic habitats because of their ubiquitous nature. They are a well-defined group of algae belonging to the
Bacillariophyceae class. Their cell walls (skeletons) are made of silica which looks like a glass house, and they are both unicellular and autotrophic organisms [
9].
Diatoms require limited specialised equipment which makes water quality monitoring using diatoms, cost effective [
10]. For the quality of an environment in biology to be shown, a scale is used known as an index. The indices show the quality of an environment by indicating the types of organisms as well as the abundance of a particular organism in a representative environmental sample [
11]. Therefore, indices are commonly used to assess water quality in aquatic ecosystems (mainly marine and freshwater). An advantage of using diatom indices is that they summarise information (ecological and hydrological) that is given by diatom assemblages [
12].
In this study, Omnidia 6.1, a diatom database and software identification tool, was used to identify diatom indices. The software generated seventeen diatom indices, of which only three indices were used. All the developed indices have different functions. Trophic diatom index (TDI), Specific pollution index (SPI), and Generic diatom index (GDI) were used. These indices were chosen since they included more than 50% of the taxa that generated index values at all sites. The TDI measures inorganic nutrient concentrations whereas SPI and GDI are indices that measure organic pollution [
13]. This study used the following physicochemical parameters: pH, chemical oxygen demand, and phosphorus. These parameters were used as a reference for the conditions that commonly occur in the Kuils River.
2. Materials and Methods
2.1. Study Area
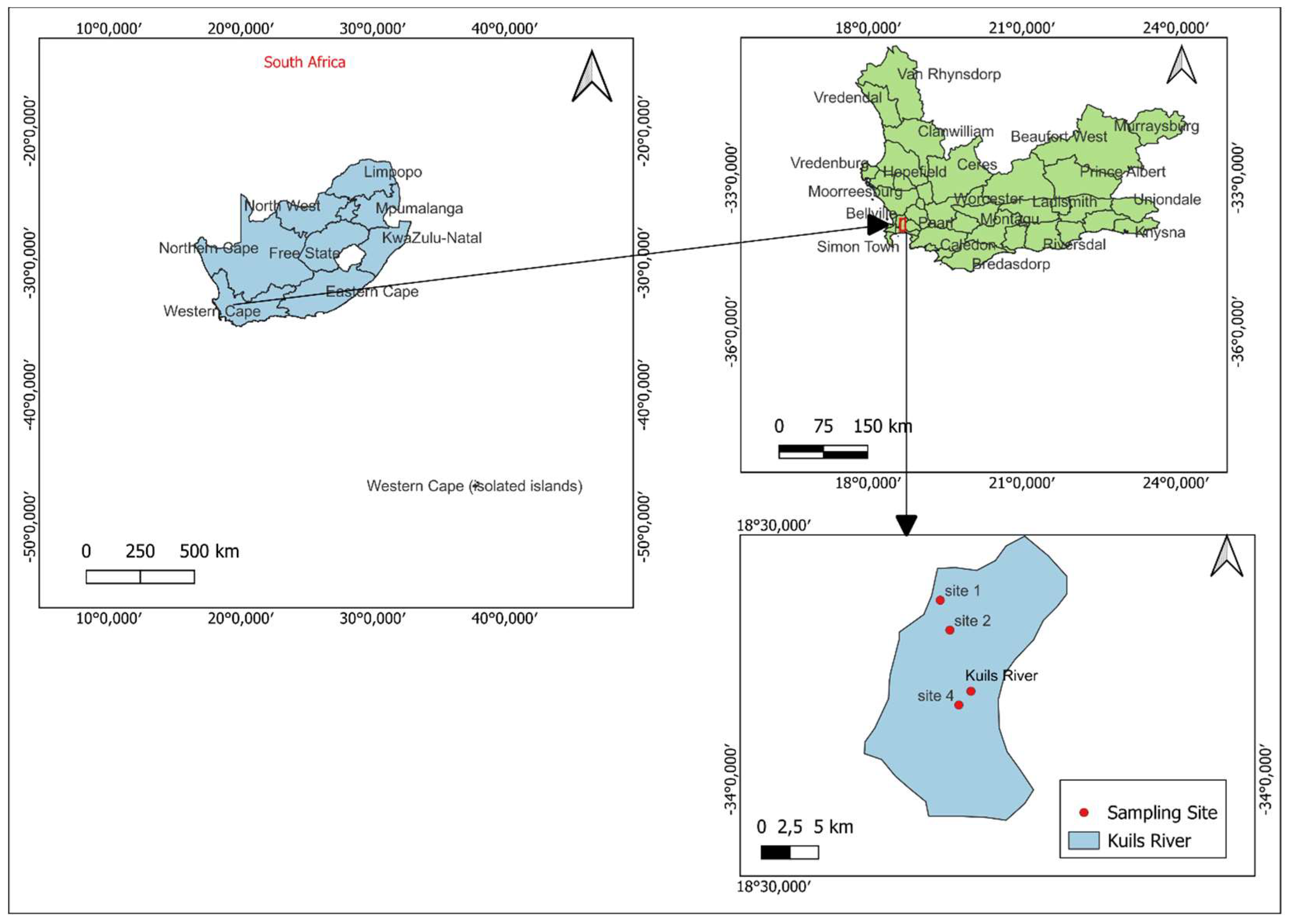
The Kuils River is located in the Western Cape Province of South Africa (
Figure 1). This area is in a Mediterranean climate with a mean annual precipitation of between 500 and 600 mm per year [
14]. This river is approximately 30 km in length, and river flows in a southward direction, and passes through the residential and industrial areas of Bellville and Kuils River downstream. The main tributary of the Kuils River is the Bottelary River. Land use is influenced by agricultural, residential, commercial, and industrial activities along the catchment. This river also receives treated and untreated wastewater from the Scottsdene, Bellville and Zandvliet Wastewater Treatment Works [
14]. Upstream, the river is made up of the sedimentary Malmesbury group of rocks, while downstream, it is made up of deposits of loose sand and dune formations underlain by extensive clay lenses [
15]. This study was conducted during both the dry (low water flow) and wet (high water flow) seasons.
2.2. Sample Collection
The study used the same four monitoring sampling points that are used by the Department of Water and Sanitation for physicochemical sampling to collect diatom samples. The diatom samples on all four sites were collected on the same day. Two to three submerged rocks or cobbles were randomly collected at each site. Diatoms were removed by scrubbing the upper surface of the substratum with a clean toothbrush to dislodge the diatom community. Only the upper side (the side most exposed to flowing water) of the rock was scrubbed to avoid contamination with sediment that might be present on the bottom part of the rock. The resulting diatom suspension, after scrubbing, was poured into a labelled 100 mL sample (Schott) bottle. Ethanol was used to preserve the diatom samples which were then taken to the laboratory for processing and analysis.
2.3. Physicochemical Analysis
The physical and chemical data used in the study were obtained from the National Water Monitoring Database which is owned by the Department of Water and Sanitation. The historical data obtained was from 2019 to 2021. The physical and chemical parameters measured at the four sampling sites include Chemical Oxygen demand, pH, and phosphorus. The data received from the Department of Water and Sanitation was used to calculate the arithmetic mean for each parameter in this study. Graphs were generated, making it easier to compare the change in water quality over time in the Kuils River.
2.4. Diatom Sample Preparation
The diatom samples were allowed to settle for 48 hours in the laboratory, after which the supernatant was discarded, and the remaining sample mixed well. 10 mL of the diatom suspension was placed in a clean beaker and 10 mL of potassium permanganate (KMnO4) was added to the sample. This solution was left to settle for 24 hours to allow for the oxidation of the organic material present in the sample. After 24 hours, 10 mL of concentrated hydrochloric acid was added to the beaker, containing the sample. The beakers were placed in a water bath at 90ºC for two hours to speed up the digestion process of potassium permanganate by the hydrochloric acid. The solutions were slowly heated until they cleared to a pale-yellow color. To check if the oxidation reaction was complete and that no organic matter remained in the sample, 1 mL of hydrogen peroxide was added. The observation of foam upon the addition of hydrogen peroxide was used to determine whether organic material was still present within the sample. If still present, the process had to be repeated again. When oxidation was complete, the samples were left to cool to room temperature before they were centrifuged.
The cooled samples were vigorously swirled and transferred into the centrifuge tubes. Thereafter, the samples were rinsed by centrifuging them with distilled water at 2500 rpm for seven minutes. After centrifugation, the supernatant was decanted, and the washing of the diatoms was repeated twice until the sample became circumneutral (clear). After the rinsing process was completed, the clear supernatant was removed from the testing tubes. The diatoms and small particles that settled at the bottom of the tube were resuspended by means of a jet of distilled water from the wash bottles. More distilled water was added until the test tubes were full. Furthermore, after the last wash, the diatoms were loosened again with distilled water, and the vortex mixer was used to mix and resuspend diatom samples. The final sample solution was poured into glass cylinders bearing the necessary sample information. After the centrifugation process was complete, slides and coverslips were thoroughly cleaned with detergent soap and stored in ethanol until needed. A portion of the cleaned sample was drawn from the numbered diatom suspension using a micropipette. The cleaned diatom suspension was then diluted until it appeared slightly cloudy to the naked eye. A single drop of ammonium peroxide (10% NH4) was added to neutralise electrostatic charges on the suspended particles and reduce aggregation. A 1.5 mL of this cleaned diatom suspension was placed on a clean dry coverslip and allowed to dry out overnight.
The dried coverslips were placed on a hot plate for approximately 2 minutes to remove the remaining ammonium peroxide. After the coverslips were cooled, they were briefly examined under the 40x objective lens to determine if the concentration of diatoms in the solution was adequate. According to [
16], at least 10 but not more than 40 valves should be visible per field. This is done so that when the sample is finally viewed under the 100x objective lens, the diatom valves ranging between 5 and 15 should be visible per field of view [
16]. Thus, if the diatom concentration was too high or too low, a new diatom slide was prepared and the whole process was repeated. Once the concentration of diatom samples present on coverslips was found to be correct or adequate, a previously dried and cleaned slide was lowered onto the coverslips, and sellotape was used to attach the coverslip to the slide. The completed slides were then ready for microscopic examination.
2.5. Diatom Counting and Data Processing
Diatom species were counted using a light microscope (Olympus CX21 LED). Diatoms were identified at the species level using the diatom guide that was developed by [
16]. Where possible, 300 diatoms were counted on each slide and recorded. The recorded information was then uploaded into the Omnidia 6.1 software. Seventeen different index values were calculated in the software. Only three indices were used in this study because they included more than 50% of the taxa which generated index values at all sample sites. The three chosen indices were the following: Generic diatom index (GDI), the Specific Pollution Index (SPI), and the Trophic Diatom Index (TDI).
3. Results
3.1. Physicochemical Parameters
Table 1 and
Table 2 represent the average historical physicochemical values that were obtained from the Department of Water and Sanitation over a period of three years. These values were obtained from the same sampling points that were used to obtain diatom samples. The pH levels during both high flow and low flow ranged between 6 and 8. These conditions correspond to the natural pH level found in riverine systems [
17]. The highest pH levels were recorded at site 1, which had a pH of 8.3 (during high flow), and site 4, which had a pH of 8.5 (during low flow). The lowest pH was 7.5 (Site 1) during high flow and 7.4 (Site 1) during low flow in 2019. These pH values fall within the range of natural conditions. Therefore, the Kuils River maintained a slightly alkaline water system over three years.
The observed level of pH in the water is attributed to the sedimentary rocks of the Malmesbury group that make up this river. These rocks are carbonate-rich and are composed of calcium and magnesium carbonates that are naturally alkaline [
18]. Therefore, carbonates from these rocks are dissolved when water comes into contact with these carbonate rocks. This results in increased alkalinity in the Kuils River. Another factor contributing to alkaline water in the Kuils River is groundwater and surface water interactions. During both high flow and low flow, areas along the Kuils River, where groundwater and surface water are interconnected, cause alkalinity to be transferred from the Malmesbury group of rocks to surface water through seepage.
The COD in uncontaminated waters is usually less than 20 mg/L, while water that receives effluents has a concentration of more than 200 mg/L [
19]. However, in industrial wastewater, the values could be as high as 60 000 mg/L. With regards to the current study,
Table 1 and
Table 2 show the highest COD concentration of 84 mg/L recorded at Site 1 in 2021. This high COD value was recorded during the low flow season. According to [
17], an increase in nutrients such as nitrates and phosphates is related to an increase in COD concentration. Thus, there is a link between COD and the input of organic matter and nutrients. The relationship between water flow fluctuations and nutrients proves that it is possible to use both these variables in determining the relationship with COD [
17]. The COD levels during high flow and low flow across all the sites over the three-year period were all above 20 mg/L. This means that all the sampled sites were experiencing a moderate increase in COD concentrations during high flow at site 1 from 2019 to 2021, whereas sites 2 and 3 remained constant.
The moderate increase in COD levels in the Kuils River is because of the location of the river. This river is surrounded by vineyards and fruit-growing farms. As a result of these surroundings, agricultural runoff causes an increase in COD levels present in the river. The chemicals used during agricultural activities to grow the fruits, including fertilizers, herbicides, and pesticides, contain organic compounds that are transferred into the Kuils River through runoff when precipitation occurs. Thus, the surrounding farms are contributing to the increased levels of COD in the Kuils River. Effluent discharge from Scottsdene, Bellville, and Zandvliet Wastewater Treatment Works also contributes to increased levels of COD in the Kuils River. According to [
14], these wastewater treatment plants discharge treated and untreated wastewater into the Kuils River. Untreated wastewaters contain organic compounds and once discharged into the Kuils River, it causes COD levels to rise.
The phosphate concentrations were slightly consistent and lower upstream during both high flow and low flow and slightly increased downstream (
Table 1 and
Table 2). The lower phosphate concentration upstream is a result of the Wastewater Treatment Works discharging effluent into the Kuils River, being located further downstream. This means that effluent, which is discharged into the river, contains pollutants that will cause a rise in phosphate levels downstream (Site 4). The presence of phosphorus in the Kuils River is a result of natural processes such as the Malmesbury group of rocks that make up this catchment. Rocks like shale from the Malmesbury group contain phosphate minerals and organic matter from agricultural activities that enter the Kuils River through weathering.
3.2. Diatom Species Composition
Table 3 indicates the dominant diatom species (>5%) that were observed during the study and their relative abundance at different sites. Since all four sites had alkaline waters, diatom species such as Fragilaria ulna var. acus were present and dominant at all the sites. This type of diatom is found all over the world and favours alkaline environments. Planothidium frequentissimum and Cocconeis engelbrechtii are examples of some of the diatom species that were in abundance at all four sites. The presence of these diatom species indicates that water conditions in the Kuils River are alkaline as these species favour such conditions. In addition, most diatoms are known to be sensitive to high levels of COD and organic pollution. In this study, the presence of dominant diatom species such as Nitzchia palea and Fragilaria ulna across all four sites indicate waters with moderate to high COD levels. Nitzchia palea and Fragilaria ulna are known as pollution-tolerant species and commonly dominate rivers with moderate to high COD levels. Therefore, the presence of these species in the Kuils River across all four sites indicates that water within the Kuils River contains increased levels of COD. These species also indicated organic pollution in the Kuils River as they reproduce and increase in abundance under moderate to high COD conditions. Cyclotella meneghiniana is a dominant diatom species that was found at Sites 2 and 3. The presence of this diatom gives an indication of low phosphorus concentrations at these sites as this species favour such conditions, whereas diatoms of the genus Nitzschia and Navicula were dominant at site 4. These species flourish under high phosphorus concentrations as they prefer nutrient-rich environments. Hence, their presence in abundance (Nitzschia and Navicula) at Site 4 gives an indication of increased phosphorus concentrations at Site 4.
3.2.1. Index Scores
The Omnidia 6.1 software calculates index scores for different indices. This software was developed because of the importance of indices to be designed in an easy manner, that enables data interpretation to be translated into information useful for management purposes [
20].
Table 4 and
Table 5 give an indication of how diatom index scores are interpreted as calculated by the Omnidia software. The range of the scores varies, with SPI & GDI (
Table 3) indicating bad water quality when the score is at zero, while TDI (
Table 4) indicates good water quality at a score of zero [
4,
21]).
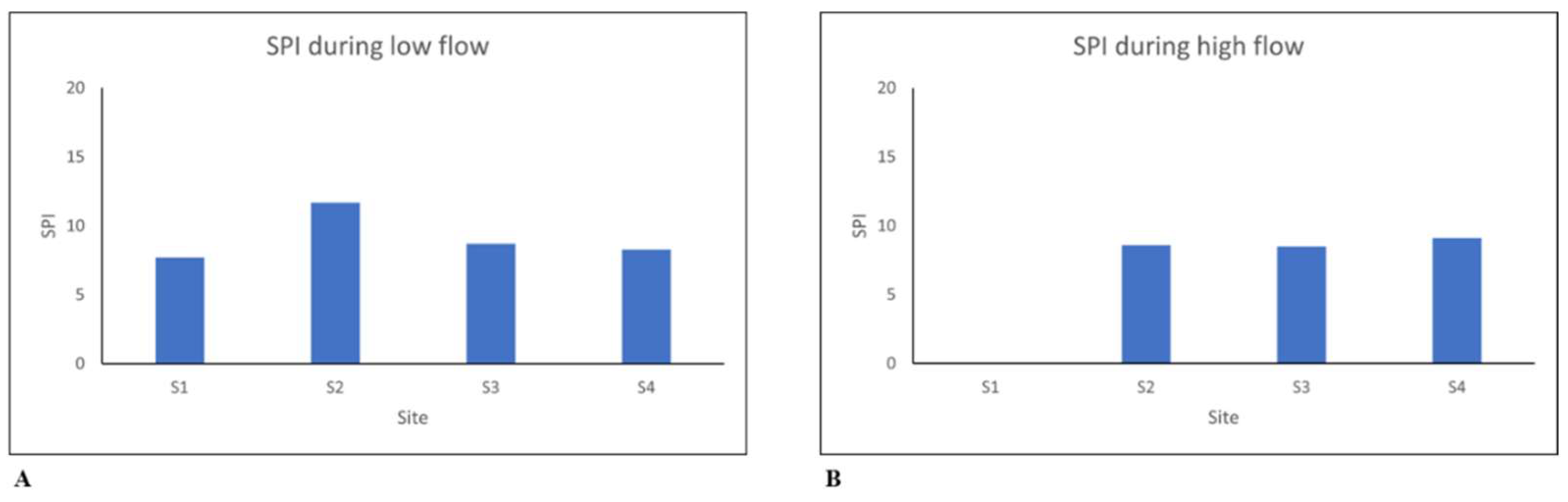
Figure 2,
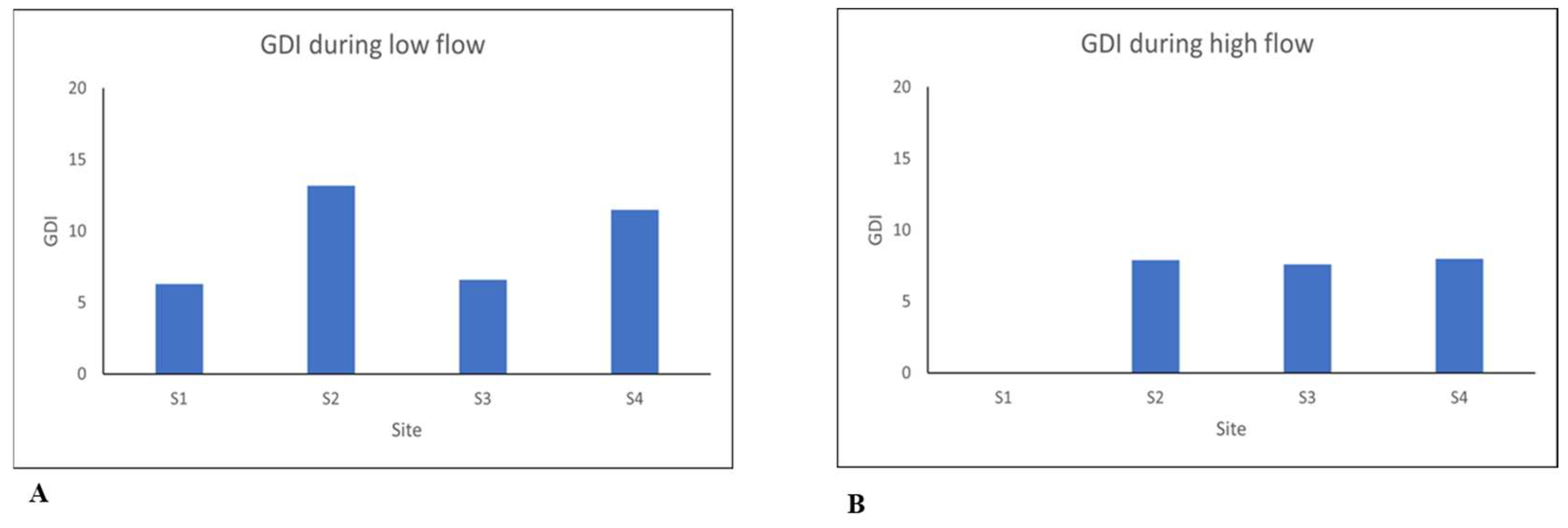
Figure 3, and
Figure 4 show index scores (SPI, GDI & TDI) that are interpreted according to
Table 4 and
Table 5. The SPI graphs (
Figure 2) indicate how both high flow and low flow values remained constant as they ranged between 7 and 9 in this study. A diminished water quality was observed across all the sites during all the seasons, as the values obtained were less than 9, as indicated in
Table 4. The water quality showed an improvement during the low flow season at Site 2 with a value of 11.7, even though the water quality remained poor at this site. Poor water quality in the high-flow season could be associated with the heavy rains that might have caused an increase in suspended solids and sediment yields [23, 17]. Factors such as runoff from stormwater drains and discharge from sewage pipes also contribute to the poor water quality in the river.
The GDI values in
Figure 3 indicate poor water quality at all sites across the river. The values are greater than 9, with the lowest value being 6.3 at Site 1. However, there was a slight change during the low flow season in which water quality improved to moderate quality, with values ranging between 12 to 15. This improvement occurred at Site 2 and could have been influenced by the presence of different diatom species that were encountered during the study in the low-flow season compared to those found in the high-flow season. The GDI accounts for the genera of taxa in which a genus may have different species with regard to their optimal conditions [
17]. The high flow season gives an indication of bad water quality in the river as the values in the graph (
Figure 3 (B)) are not greater than 9. According to [
22], GDI levels less than 9 are classified as bad water quality. The findings of GDI in this study corresponded to the findings from physicochemical parameters, as both results indicated the presence of pollutants in the river.
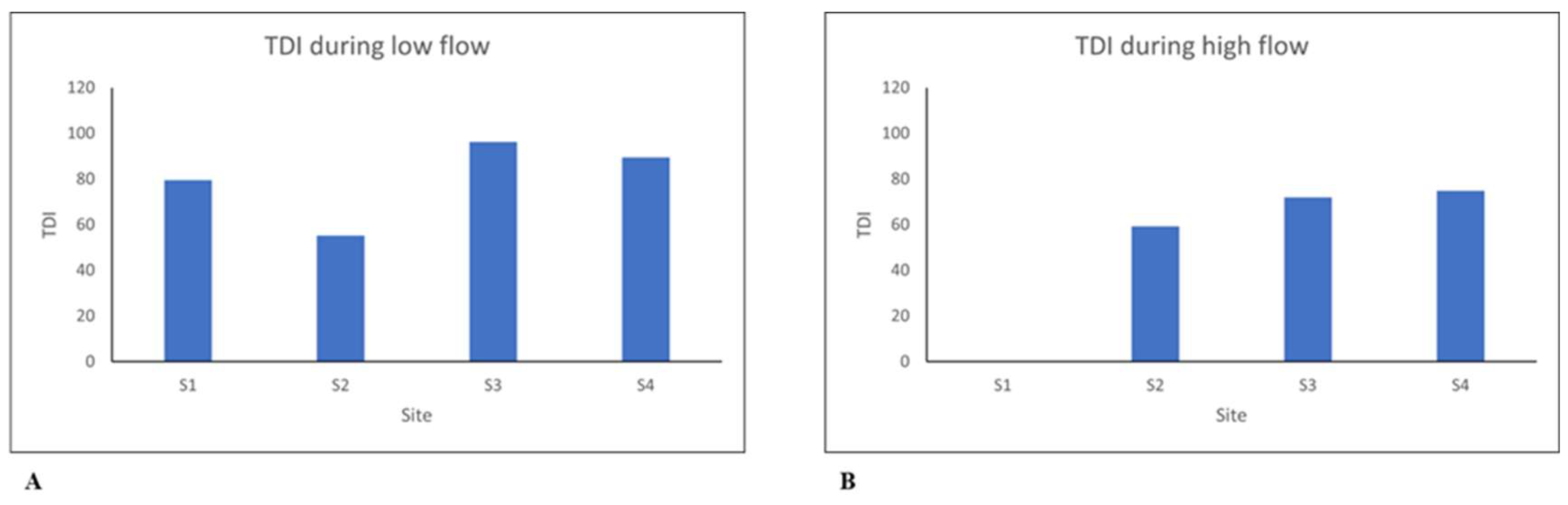
The TDI values were the lowest during low flow with a value of 55.2 at Site 2 (
Figure 5). Some of the highest recorded TDI values were 71.9, 74.9, 79.6 and 89.4. All these values indicate that the water quality during both high flow and low flow ranged between mesotrophic to eutrophic conditions. A rise in the TDI concentration was also noticeable as one moved downstream from one site to the next (
Figure 4). The high concentration in TDI indicated increased pollution along the river as one moved downstream. The findings from TDI, when compared to those obtained from physicochemical parameters, indicate similar conditions. Both results indicate poor water quality along the Kuils River and also showed that the pollutant concentrations increased downstream. The increased pollution inputs can be attributed to runoff from residential areas or the dilution of diatoms in the water taking place during high flow [
17]. Another reason for poor water quality could be nutrients entering the Kuils River through discharge from wastewater facilities, as well as farming activities along the catchment.
3.3. Observed Diatom Species
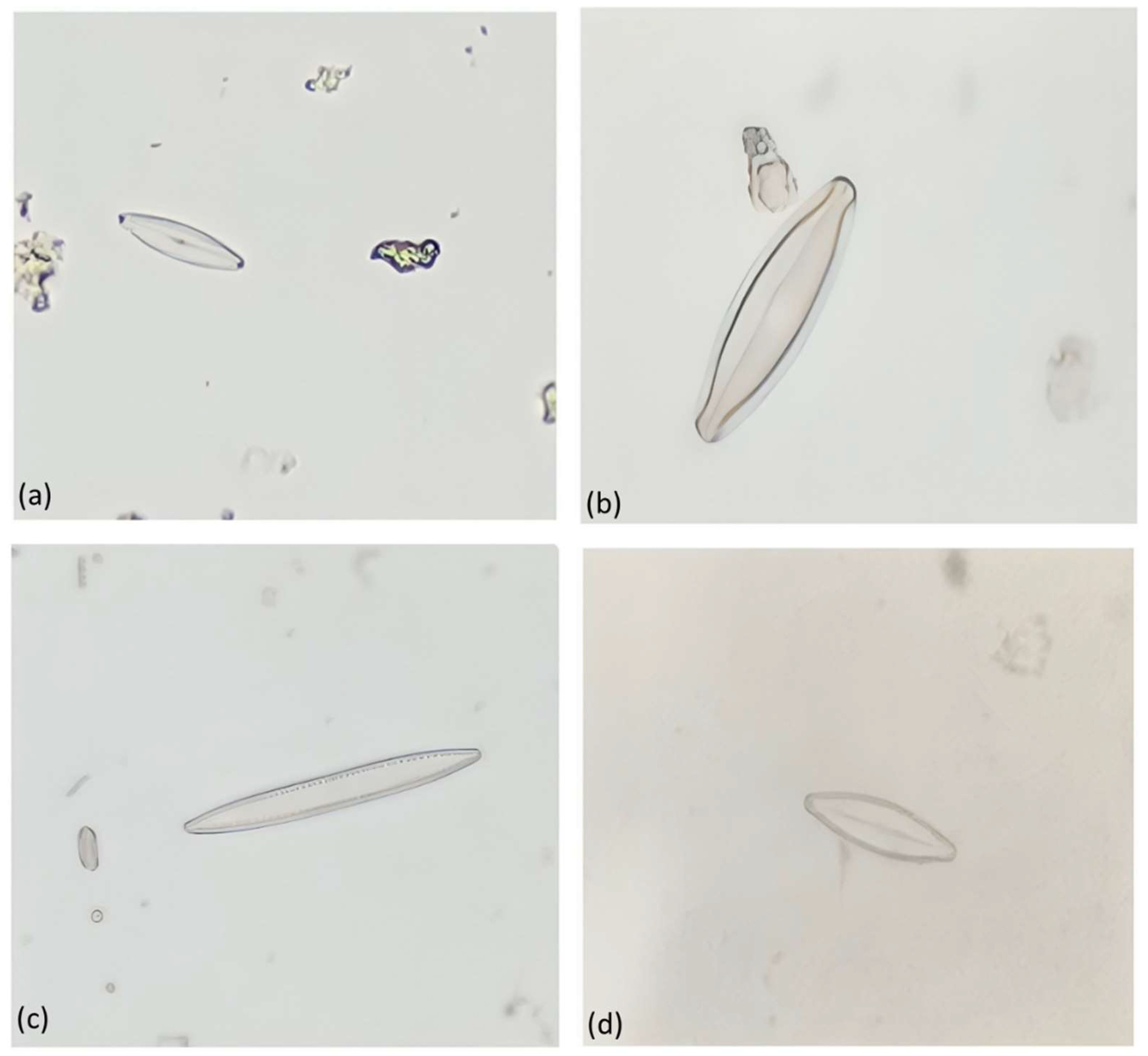
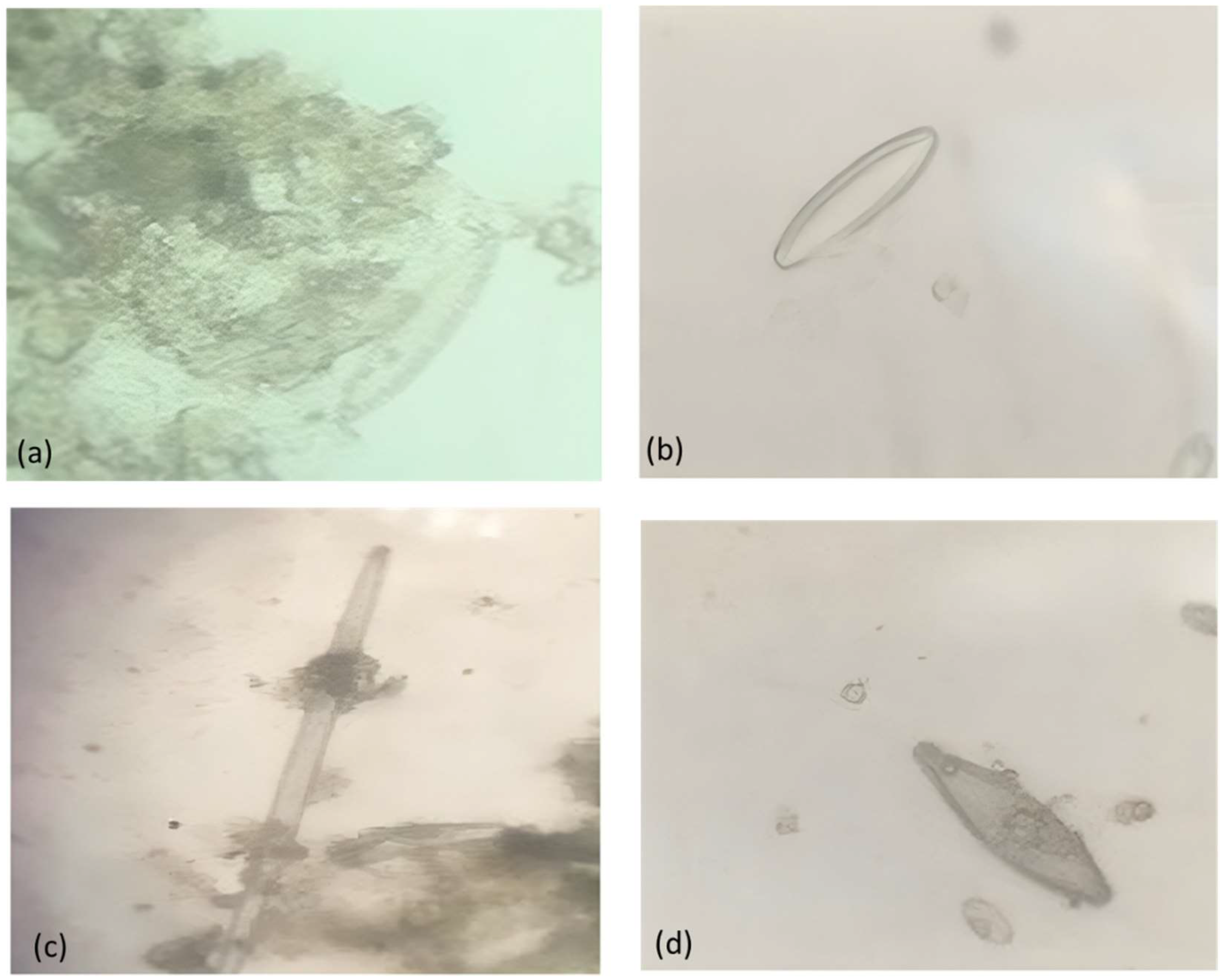
Photomicrographs of different types of diatoms observed in the samples are shown in
Figure 5 and
Figure 6. These photomicrographs show the diatom silica structure (cell wall or frustule). Centric and pennate diatoms are two groups of diatoms commonly found in freshwater systems [
16]. Centric diatoms are adapted to live in the water as part of phytoplankton, while pennate diatoms live in benthic habitats (on occasion temporarily re-suspended in the water column) [
16].
Figure 5 and
Figure 6 indicate diatoms belonging to the pennate group. Craticula cuspidata (
Figure 5a), which commonly occurs in eutrophic and brackish waters, is distinguished by parallel transverse striation. Additionally, Craticula cuspidata tolerates critical to heavy pollution levels [
16]. Nitzchia perspicua (
Figure 5b) contains weakly silicified frustule (cell wall), while its striae are not easily observable in light microscopy. Navicula viridula (
Figure 5c) favours eutrophic waters and tolerates critical levels of pollution. Nitzschia communis Rabenhorst (
Figure 5d), contains striae that is observable under light microscopy. It occurs in electrolyte-rich as well as brackish waters and is tolerant of extremely polluted conditions [
16].
Figure 6a illustrates Nitzschia palea, which is a type of diatom that is found all over the world. It thrives in eutrophic, heavily polluted to extremely polluted environments [
16]. Eunotia rhomboidea (
Figure 6b) contains small valves, and the frustule is visible in light microscopy. They are found in oligotrophic, electrolyte-poor waters. Fragilaria ulna var. acus (Kützing) Lange-Bertalot (
Figure 6c), is a cosmopolitan species that is found in the benthos of rivers and exists in alkaline freshwaters [
16]. Gomphonema pseudoaugur (
Figure 6d) has an oval shape and occurs in eutrophic waters. This diatom type is sensitive to more critical levels of pollution [
16].
Nitzschia communis Rabenhorst ( Figure 10), contains striae that is observable under light microscopy. It occurs in electrolyte rich as well as brackish waters and is tolerant of extremely polluted conditions (Taylor et al. 2007 [
16]. ). Figure 12 represents Planothidium engelbrechtii which has the ability to flourish in critical to heavily organic polluted environments. It is a cosmopolitan diatom that is found in the benthos of rivers or attached to substrates [
16].
4. Discussion
Dominant diatom species that were found in this study such as Planothidium engelbrechtii, Nitzschia frustulum, Nitzschia heufleriana and Craticula cuspidata are known to be tolerant to moderately critical pollution occurring in eutrophic waters as well as waters with high levels of pollution [
12,
16,
17]). The values obtained from diatom indices (SPI, GDI, and TDI) and physicochemical parameters indicate that these tools can be used to determine the water quality of a river. The findings from this study indicate that diatoms are able to determine water quality based on their dominance and abundance at a particular site along the river. This information reflects the conditions present in the river. For the purposes of water quality analysis, when used together physicochemical parameters and diatoms complement each other in giving an indication of water quality. This is because physicochemical parameters give an exact number of the conditions occurring at a particular site. For example, the physicochemical parameter method was able to record pH levels that ranged between 6 to 8 across the Kuils River, which indicated alkaline water, whereas the dominance of diatom species Fragilaria ulna var. indicated alkaline waters as this species prefers environments with moderate to high pH levels. Therefore, the information from both physicochemical parameters and diatoms work well together as they can determine the quality of water along the river.
The sources of pollution in the Kuils River can be attributed to anthropogenic activities and natural processes. Anthropogenic activities such as agricultural runoff, industrial effluents, and informal and formal settlements may have contributed to the poor quality of the river. Natural processes in the Kuils River may have contributed to poor water quality through the introduction of pollutants from the river’s bedrock, which causes groundwater and surface water interaction through seepage. The Malmesbury group of rocks, like shale, contain phosphate minerals and organic matter that enter the Kuils River through weathering. It is understood that this weathering occurring in the catchment increases the levels of phosphorus present in the river.
5. Conclusions
The study examined whether diatom-based water quality assessments align with traditional physicochemical methods, given the scarcity of diatom studies in South Africa, particularly in the Kuils River. Since no prior diatom data existed for this river, historical water quality parameters were used for comparison. The study found that diatoms, which respond to pollution levels, are reliable bioindicators for water quality monitoring. The results showed poor water quality in the Kuils River, especially downstream, due to nutrient runoff from rainfall, agriculture, sewage leaks, and wastewater treatment plants.
The study confirmed that both diatom analysis and physicochemical measurements complement each other, providing a comprehensive understanding of environmental conditions. Diatom communities reflect changes in water quality, and shifts in their composition indicate the presence of pollutants. The findings suggest that diatoms can be used effectively to monitor water quality alongside traditional methods, with regular long-term monitoring recommended.
The conclusions from this study indicate that diatoms are present in the Kuils River, and their distribution is influenced by physicochemical factors at each site. Diatoms play a crucial role in assessing water quality, as the dominant species at a given location reflect the environmental conditions that allow them to thrive, providing insights into current water quality. In the Kuils River, specific diatom communities are linked to eutrophic conditions, where nutrient-rich environments support species adapted to such settings. The ease of collecting diatom samples from river systems makes them valuable bioindicators. The study recommends monitoring diatom communities over an extended period, ideally longer than a year, to track changes in water quality over time. Additionally, future research should incorporate diatom analysis alongside traditional water quality assessments.
Author Contributions
Conceptualization, N.M. and L.K.; methodology, L.K.; software, L.K.; validation, L.K., N.M., A.P. and P.M.; formal analysis, N.M.; investigation, L.K., N,M., P.P., and A.P.; resources, N.M.; data curation, L.K., and N.M.; writing—original draft preparation, L.K.; writing—review and editing, N.M., and L.K.; visualization, N.M.; supervision, N.M., A.P., and P.P.; project administration, N.M,; funding acquisition, N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Cape Peninsula University of Technology Research Fund (URF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data generated, analyzed, and presented in this study are available upon request to the authors.
Acknowledgments
The authors thank Mr Ntyamtyambo Mavuso from the Department of Environmental and Occupational Studies, Cape Peninsula University of Technology, for his assistance in sample collection and lab work. The authors are also grateful to Mr Matheu Lecointe from the Omnidia Software Company in Monbazillac, France for his assistance with the software training.
References
- Gqomfa, B.; Maphanga, T.; Shale, K. The impact of informal settlement on water quality of Diep River in Dunoon. Sustain. Water Resour. Manag. 2022, 8, 27 [. [Google Scholar] [CrossRef]
- Daily Maverick. Cape Town’s rivers are open streams of sewage, yet the city is not spending its budget. Available online: https://www.dailymaverick.co.za/article/2019-09-26-cape-towns-rivers-are-open-streams-of-sewage-yet-the-city-is-not-spending-its-budget (accessed on 11 August 2022).
- Dallas H., F.; Day J., A. The effect of water quality variables on aquatic ecosystems: A review. Pretoria: Water Research Commission, South Africa, 2004; pp. 224. Available online: https://www.wrc.org.za/wp-content/uploads/mdocs/TT-244-04.pdf.
- Matlala, M. D. The use of diatoms to indicate water quality in wetlands: A South African perspective. Masters Thesis, North-West University, Potchefstroom, 2010. Available online: http://hdl.handle.net/10394/4410.
- Sonneman, J. A.; Walsh, C. J. ; Breen; P. F.; Sharpe, A. K. Effects of urbanization on streams of the Melbourne region, Victoria, Australia. II. Benthic diatom communities. Freshwater Biology, 2001, 46(4): 553-565. [CrossRef]
- Belore, M.L.; Winter, J.G.; Duthie, H.C. Use of diatoms and macroinvertebrates as bioindicators of water quality in southern Ontario rivers. Can. Water Resour. J. 2002, 27 (4): 457-484. [CrossRef]
- Geng, S.W.; Qu, X.D.; Zhang, Y.; Lin, K.D. Comparison and application of biological indices of macroinvertebrates in river health assessment. Huan Jing ke Xue=Huanjing Kexue 2012, 33, 2281–2287. Available online: https://pubmed.ncbi.nlm.nih. [PubMed]
- Lobo, E.A.; Heinrich, C.G.; Schuch, M.; Wetzel, C.E.; Ector, L. Diatoms as bioindicators rivers. River algae, 2016, 245-271. [CrossRef]
- Slingers, O. An analysis of diatoms as biological indicators of water quality in rivers of the Western Cape. Master's thesis, University of Cape Town, South Africa, 2015. Available online: http://hdl.handle.net/11427/19990.
- Musa, R. S. Relating epiphytic diatom community assemblage to water quality along the Nyl River floodplain, Limpopo, South Africa. Ph.D. Thesis, University of Johannesburg, South Africa, 2015. [Google Scholar]
- Ashbolt, N.J.; Grabow, W.O.K.; Snozzi, M. Indicators of Microbial Water Quality. In Water Quality: Guidelines, Standards and Health Risk Assessment and Management for Water-Related Infectious Disease; Chapter 13; Fewtrell, L., Bartram, J., Eds.; IWA Publishing: London, 2001; pp. 289–315, [924154533X.pdf (4.483Mb)]. [Google Scholar]
- De Almeida, S. F.P.; Gil, M.C.P. Ecology of freshwater diatoms from the central region of Portugal, Cryp-togamie algol, 2001, 22: 109-126. [CrossRef]
- Kelly, M.G.; Whitton, B.A. The Trophic Diatom Index: a new index for monitoring eutrophication in rivers. J Appl Phycol, 1995. [Google Scholar] [CrossRef]
- Hagen, B. Restoring the Kuils River: understanding the past to inform the future. Ph.D,Thesis, Stellenbosch University, Cape Town, South Africa, 2022. Available online: http://hdl.handle.net/10019.1/124601.
- Mwangi, F. N. Land use practices and their impact on the water quality of the upper Kuils River. Masters Thesis, University of Western Cape, Cape Town, South Africa, 2014. Available online: https://core.ac.uk/download/pdf/58914502.pdf.
- Taylor, J.C.; Harding, W. R.; Archibald, C.G. M. A methods manual for the collection, preparation and analysis of diatom samples, Version 1: 60, 2007; pp. 60. Available online: https://docs.niwa.co.nz/library/public/1770054839.pdf.
- Phungula, P. P. The use of diatoms in assessing water quality in the Nyl and Mogalakwena River system, in the Limpopo, South Africa. Masters Thesis, University of Johannesburg, South Africa, 2018. [Google Scholar]
- Kaushal, S.S.; Likens, G.E.; Utz, R.M.; Pace, M.L.; Grese, M.; Yepsen, M. Increased River alkalinization in the Eastern US. ES&T, [es401046s_si_001.pdf (1.27 MB)]. 2013, 47, 10302–10311. [Google Scholar]
- Chapman, D. V. Water quality assessments: a guide to the use of biota, sediments and water in environmental monitoring, 2nd Ed,; E&FN Spon; Great Britain, 1996; pp. 1- 609. Available online: https://iris.who.int/bitstream/10665/41850/1/0419216006_eng.pdf.
- Kelly, M.G. Use of the trophic diatom index to monitor eutrophication in rivers. Wat. Res, 1998, 32(1): 236-242. [CrossRef]
- De la Rey, P.A.; Taylor, J.C.; Laas, A.; Van Rensburg, L.; Vosloo, A. Determining the possible application value of diatoms as indicators of general water quality: A comparison with SASS 5. Water Sa 2004, 30, 325–332, [10.4314/wsa.v30i3.5080]. [Google Scholar] [CrossRef]
- Szczepocka, E.; Szulc, B. The use of benthic diatoms in estimating water quality of variously polluted rivers. Oceanol. Hydrobiol. Stud, 2009, 38 (1): 17-26. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).