1. Introduction
Hepatocellular carcinoma (HCC) is recognised as a major public health problem worldwide and is particularly prevalent among individuals with chronic liver disease [1,2]. The most common risk factors for HCC include chronic hepatitis B and C infections, alcohol use and non-alcoholic fatty liver disease (NAFLD). The incidence of HCC is higher in Asian and African regions where hepatitis B and C are endemic, but has also been increasing in Western countries in recent years, which is associated with an increase in NAFLD and alcohol consumption [3,4,5,6].
Curative treatment options such as surgical resection, ablation therapies and liver transplantation are available for HCC patients diagnosed at early stages [7,8]. However, since the majority of patients are diagnosed at an advanced stage, these curative options are not applicable and systemic therapies come to the fore for these patients. Liver transplantation is considered to be one of the most effective methods in the treatment of HCC, especially for suitable patients in the early stage. However, the risk of recurrence of HCC after transplantation is a critical factor that negatively affects the long-term survival of patients. In the literature, HCC recurrence rates after liver transplantation vary between 10% and 20%, which poses a significant challenge in clinical management [9,10,11].
Treatment options for HCC patients who relapse after liver transplantation are very limited and this patient group is usually excluded from clinical trials. The management of HCC recurrence after liver transplantation is particularly complex, as these patients are already on immunosuppressive therapy, which can impact the efficacy of various treatment modalities. Existing evidence suggests that the tyrosine kinase inhibitors sorafenib and regorafenib may have a role in the treatment of post-transplant HCC recurrence [12,13,14].
Sorafenib has been the standard of care for advanced, unresectable HCC for over a decade, demonstrating improved overall survival compared to placebo. However, the use of sorafenib in the post-transplant setting is complicated by potential drug-drug interactions with immunosuppressive agents and higher rates of treatment-related toxicity. [15,16]. After progression on sorafenib, the multikinase inhibitor regorafenib has shown efficacy as a second-line treatment option in HCC, including in the post-transplant setting [17,18,19]. Recent research has also explored the potential of immunotherapy agents, such as nivolumab, in HCC patients who have failed prior sorafenib treatment. However, the use of immunotherapy in the post-transplant setting remains limited due to concerns about graft rejection and insufficient clinical data [20,21,22].
Understanding the unique challenges and treatment strategies for HCC recurrence after liver transplantation is crucial, as this patient population is often excluded from large clinical trials. The sequential use of sorafenib and regorafenib in this setting may offer a viable treatment approach, but further research is needed to optimize outcomes and management of treatment-related toxicities in this complex patient group.
2. Materials and Methods
This study is a retrospective, multicenter analysis aimed at evaluating the effectiveness of sequential Sorafenib and Regorafenib treatments in patients with HCC recurrence following liver transplantation. The study was conducted on 73 patients diagnosed with recurrent HCC between 2012 and 2022. Patient data was collected from 11 oncology centers across 7 different cities in Turkey.
The study population included patients aged 18 years or older with a confirmed diagnosis of HCC recurrence following liver transplantation. Eligible patients began treatment with Sorafenib, followed by Regorafenib upon progression or intolerance to Sorafenib. Patients were excluded if they had incomplete medical records or had received systemic therapies other than Sorafenib or Regorafenib.
The study population consisted of patients aged 18 years or older with HCC who had undergone liver transplantation and experienced HCC recurrence after the transplant. Eligible patients had received Sorafenib as the first-line treatment following recurrence and were subsequently treated with Regorafenib either due to intolerance to Sorafenib or disease progression. Exclusion criteria included patients who had undergone multiple organ transplants, those with HCC recurrence within 3 months of liver transplantation, patients with severe organ failure (such as heart or renal failure) unrelated to HCC, and those who were participating in other clinical trials.
2.1. Data Collection
Clinical and demographic data, including patient age, sex, body mass index (BMI), date of liver transplantation, underlying liver disease, and cirrhosis etiology, were collected from medical records. Additionally, data on tumor characteristics, such as the size and number of recurrent lesions, as well as the time to recurrence after transplantation, were obtained. The performance status of patients was assessed using the Eastern Cooperative Oncology Group (ECOG) score, and laboratory data, including alpha-fetoprotein (AFP) levels and liver function tests (ALT, AST, bilirubin), were recorded. Liver function status was evaluated using the Child-Pugh score. Information on post-transplant immunosuppressive therapy regimens was also gathered. Treatment-related data included the dosage and duration of Sorafenib and Regorafenib, the reasons for treatment discontinuation (e.g., progression or intolerance), and any reported adverse effects, along with management strategies.
2.2. Treatment Protocol
All patients received Sorafenib as first-line therapy upon the detection of HCC recurrence following liver transplantation. Patients who experienced intolerance to Sorafenib, defined as the occurrence of grade 3 or higher toxicities unmanageable with dose adjustments, or disease progression, as determined by radiological evidence of tumor growth according to RECIST version 1.1 criteria, were transitioned to second-line treatment with Regorafenib. Radiological evaluations were performed every 8 to 12 weeks to monitor treatment response and disease progression. The dose and duration of each therapy were recorded, along with any necessary dose modifications.
2.3. Ethical Approval
The study was designed and conducted in accordance with internationally recognised principles of Good Clinical Practice and the Declaration of Helsinki. Ethical approval for the study was obtained from Akdeniz University Clinical Research Ethics Committee.
2.4. Outcome Measures
The primary endpoints were progression-free survival (PFS) for both Sorafenib (PFS1) and Regorafenib (PFS2), as well as overall survival (OS). PFS was defined as the time from the start of each treatment to disease progression or death from any cause. OS was measured from the date of recurrence diagnosis until death or last follow-up. The secondary endpoints included the incidence and severity of adverse effects, categorized according to the Common Terminology Criteria for Adverse Events (CTCAE).
2.5. Statistical Analysis
Survival analyses were performed using the Kaplan-Meier method to estimate PFS and OS. The Cox proportional hazards model was used to assess risk factors associated with progression and mortality, with hazard ratios (HR) and 95% confidence intervals (CI) calculated. Variables included in the analysis were ECOG performance status, Child-Pugh classification, AFP levels, and metastasis location. The analyses were performed with SPSS 26.0 programme and 95% confidence level was used. In the analyses, the relationship between categorical variables was analysed by Chi-square test. The analysis of the measurements in terms of categorical variables was analysed by t-test. The relationship between the measurements was analysed by Pearson's correlation test. Survival for PFS1, PFS2 and OS was analysed by Kaplan-Meier test.
3. Results
3.1. Patient Demographics and Clinical Characteristics
The 73 patients included in the study were diagnosed with recurrent hepatocellular carcinoma (HCC) after liver transplantation. Of these patients, 84,9% were male (n=62) and 15,1% were female (n=11). The mean age of the patients was 56,5 ± 11,4 years and the mean age at diagnosis was 52,3 ± 11 years. The AFP levels of the patients at the time of diagnosis were widely distributed and the mean AFP level was 1790,1 ± 8974,0 ng/mL. Furthermore, cirrhosis was detected in 75,3% of the patients and hepatitis B virus (HBV) was the most common etiology of cirrhosis among these patients (72.6%).
The patients' post-transplant period until relapse varied between (5-83.17 months), with a median of 13.72 months. The mean duration was found to be 20.19±19.06 months.
All patients received sorafenib as first-line treatment. Among patients who experienced progression with sorafenib or discontinued treatment due to toxicity, 45,2% (n=33) continued treatment with regorafenib. The mean follow-up period after recurrent disease was 39.65 months. (
Table 1)
Table 1.
Demographic and Clinical Characteristics of Patients with Recurrent HCC after Liver Transplantation.
Table 1.
Demographic and Clinical Characteristics of Patients with Recurrent HCC after Liver Transplantation.
| Parameter |
Value |
| Gender n (%) |
Female: 11 (15,1%), Male: 62 (84,9%) |
| Age (Mean±SD) Min-Max (Median) |
56.5±11,4 (22-74) [59] |
| Age at Diagnosis (Mean±SD) Min-Max (Median) |
52.3±11,0 (19-68) [54] |
| AFP at Diagnosis (Mean±SD) Min-Max (Median) |
17901±89740 (1-73593) [566] |
| ECOG at Diagnosis n (%) |
0: 29 (39,2%), 1: 44 (59,5%), 2: 1 (1,4%) |
| Cirrhosis at Diagnosis n (%) |
No: 18 (24,7%), Yes: 55 (75,3%) |
| Etiology of Cirrhosis n (%) |
HBV: 53 (72,6%), HCV: 7 (9,6%), Alcohol: 2 (2,7%), Other: 11 (15,1%) |
| Single Lesion n (%) |
Less than 5 cm: 42 (59,2%), Greater than 5 cm: 29 (40,8%) |
3.2. Sorafenib Treatment
The performance status of sorafenib-treated patients was categorised as ECOG 0 in 32,9%, ECOG 1 in 61,6% and ECOG 2 in 5,5%. The mean number of cures achieved during treatment was 8,6 ± 8,3 (median: 6). While 86,5% of patients had to discontinue treatment due to progression, 6,8% discontinued treatment due to toxicity. Clinical response rates with sorafenib were complete response (CR) 2,8%, partial response (PR) 19,7%, stable disease (SD) 33,8% and progressive disease (PD) 43.7%. (Table2)
Table 2.
Summary of Sorafenib Treatment Parameters and Outcomes in Patients with Recurrent HCC after Liver Transplantation.
Table 2.
Summary of Sorafenib Treatment Parameters and Outcomes in Patients with Recurrent HCC after Liver Transplantation.
| Parameter |
Value |
| AFP Before Sorafenib (Mean±SD) Min-Max (Median) |
21510±86322 (0.77-54000) [100] |
| Child Score Before Sorafenib n (%) |
A: 69 (94,5%), B: 4 (5,5%) |
| ECOG Before Sorafenib n (%) |
0: 24 (32,9%), 1: 45 (61,6%), 2: 4 (5,5%) |
| Number of Sorafenib Cycles (Mean±SD) Min-Max (Median) |
8.6±8,3 (1-38) [6] |
| Discontinuation of Sorafenib n (%) |
Progression: 64 (86,5%), Toxicity: 5 (6,8%), Continuing: 5 (6,8%) |
| Best Response to Sorafenib n (%) |
CR: 2 (2,8%), SD: 24 (33,8%), PR: 14 (19,7%), PD: 31 (43,7%) |
| Adverse Reactions n (%) |
No: 16 (21,6%), Yes: 58 (78,4%) |
| Hand and Foot Syndrome n (%) |
31 (41,9%) |
| Hand and Foot Syndrome Grade n (%) |
Grade 1: 15 (20,3%), Grade 2: 12 (16,2%), Grade 3: 2 (2,7%), Grade 4: 1 (1,4%) |
| Fatigue n (%) |
46 (62,2%) |
| Fatigue Grade n (%) |
Grade 1: 16 (21,6%), Grade 2: 20 (24,0%), Grade 3: 10 (13,5%) |
| Hypertension n (%) |
18 (24,3%) |
| Hypertension Grade n (%) |
Grade 1: 6 (8,1%), Grade 2: 12 (16,2%) |
| Diarrhea n (%) |
27 (36,5%) |
| Diarrhea Grade n (%) |
Grade 1: 10 (13,5%), Grade 2: 11 (14,9%), Grade 3: 5 (6,8%) |
| Rash n (%) |
8 (10,8%) |
| Rash Grade n (%) |
Grade 1: 3 (4,1%), Grade 2: 3 (4,1%), Grade 4: 2 (2,7%) |
3.3. Progression Free Survival 1 (PFS1) with Sorafenib
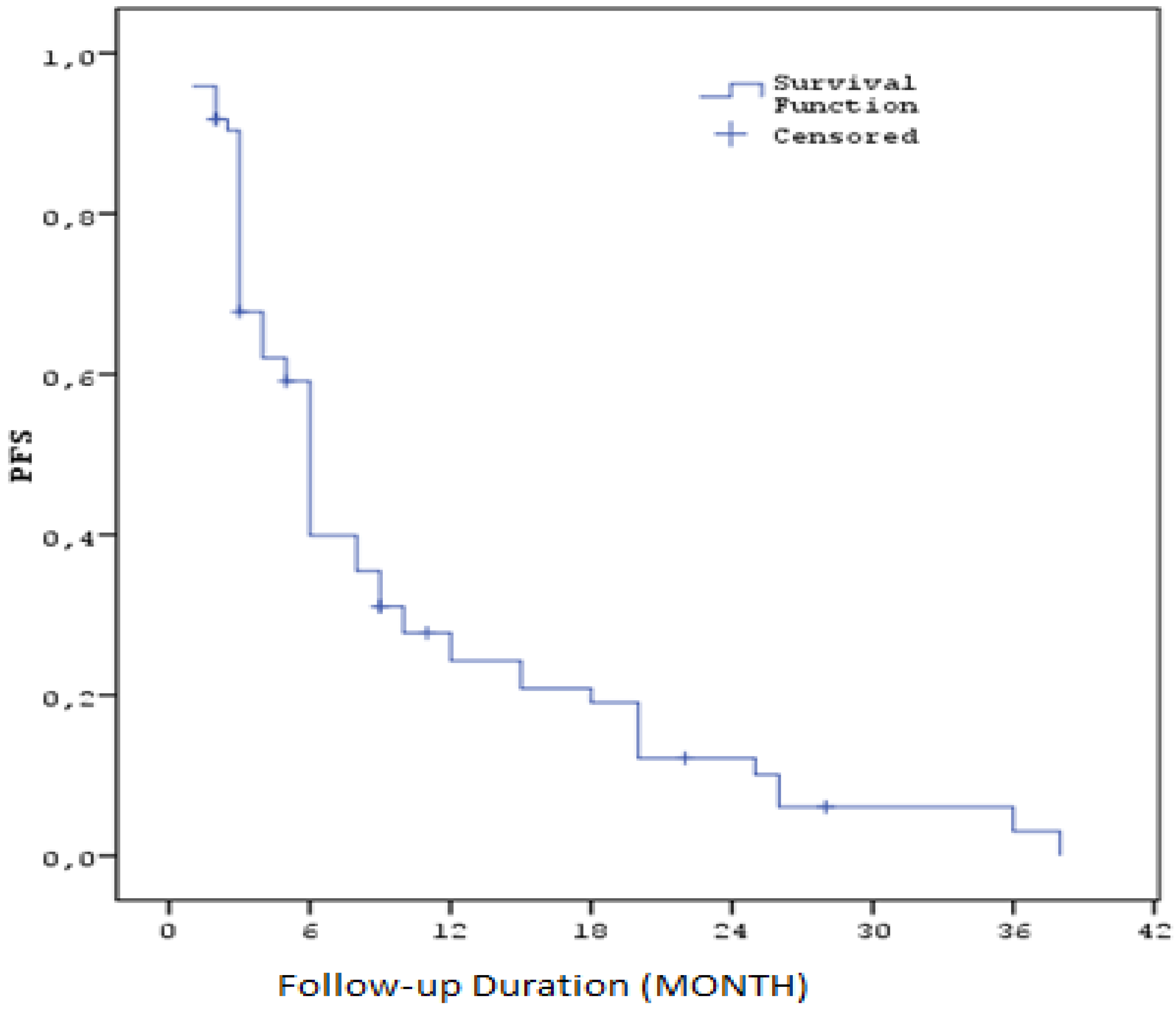
The median progression-free survival (PFS) calculated after sorafenib treatment was 5,6 months (SE: 0.3). Three-month PFS rate was 67.8% (SE: 5.5), six-month rate 39.9% (SE: 5.9), one-year rate 24.3% (SE: 5.3), two-year rate 12.2% (SE: 4.2) and three-year rate 3.0% (SE: 2.7). These results suggest that sorafenib treatment may be effective for a certain period of time in HCC patients, but long-term survival remains limited. (Table3) ( Figure1 )
Table 3.
PFS values for Sorafenib Treatment by Time.
Table 3.
PFS values for Sorafenib Treatment by Time.
| Parameter |
Value |
| PFS Duration (Months) Median (SE) / 95% CI |
5,6 (SE: 0,3) / 5,4 - 6,6 |
| PFS (%) at 3 months |
67.8% (SE: 5,5%) |
| PFS (%) at 6 months |
39.9% (SE: 5,9%) |
| PFS (%) at 1 year |
24.3% (SE: 5,3%) |
| PFS (%) at 2 years |
12.2% (SE: 4,2%) |
| PFS (%) at 3 years |
3.0% (SE: 2,7%) |
Figure 1.
Kaplan–Meier curves for progression-free survival with Sorafenib (PFS1).
Figure 1.
Kaplan–Meier curves for progression-free survival with Sorafenib (PFS1).
3.4. Regorafenib Treatment
The performance status of the patients who received regorafenib after sorafenib treatment was evaluated as ECOG 0 in 21,2%, ECOG 1 in 60,6% and ECOG 2 in 18.2%. The median duration of treatment was 7,6 cycles. The starting dose of regorafenib was generally 120 mg (45,5%) and 160 mg (24,2%), and 69,7% of patients received dose escalation during treatment. In terms of treatment responses, partial response (PR) rate was 51,5% and progressive disease (PD) rate was 48,5%. Treatment was discontinued in 87,9% of the patients due to progression and 6,1% due to toxicity.( Table4 )
Table 4.
Summary of Regorafenib Treatment Parameters and Outcomes.
Table 4.
Summary of Regorafenib Treatment Parameters and Outcomes.
| Parameter |
Value |
| ECOG Before Regorafenib n (%) |
0: 7 (21,2%), 1: 20 (60,6%), 2: 6 (18,2%) |
| Child Score Before Regorafenib n (%) |
A: 31 (93,9%), B: 2 (6,1%) |
| Number of Regorafenib Cycles (Mean±SD) Min-Max (Median) |
7.6±8,2 (2-36) [4] |
| Previous Treatment Lines Before Regorafenib n (%) |
1: 23 (71,9%), 2: 7 (21,9%), 3: 2 (6,3%) |
| Previous Treatments Before Regorafenib n (%) |
Sorafenib: 27 (81,8%), TAKE: 1 (3,0%), Sorafenib+TAKE: 3 (9,1%), Sorafenib+TARE: 1 (3,0%), Sorafenib+AKE+TARE: 1 (3,0%) |
| Initial Dose n (%) |
80 mg: 10 (30,3%), 120 mg: 15 (45,5%), 160 mg: 8 (24,2%) |
| Dose Increase During Follow-up n (%) |
No: 10 (30,3%), Yes: 23 (69,7%) |
| Maintenance Dose n (%) |
120 mg: 12 (36,4%), 160 mg: 21 (63,6%) |
| Dose Reduction n (%) |
No: 24 (72,7%), Yes: 9 (27,3%) |
| Treatment Discontinued n (%) |
No: 11 (33,3%), Yes: 22 (66,7%) |
| Reason for Discontinuation n (%) |
Progression: 29 (87,9%), Toxicity: 2 (6,1%), Continuing: 2 (6.1%) |
| Progression Location n (%) |
Liver: 10 (30,3%), Lung: 10 (30,3%), Bone: 9 (27,3%), Brain: 0 (0,0%), Abdomen: 12 (36,4%) |
| Best Response n (%) |
PR: 17 (51,5%), PD: 16 (48,5%) |
| Marker Response n (%) |
No: 18 (56,3%), Yes: 14 (43,8%) |
| Adverse Reactions n (%) |
No: 6 (18,2%), Yes: 27 (81,8%) |
| Fatigue n (%) |
26 (78,8%) |
| Fatigue Grade n (%) |
Grade 1: 8 (24,2%), Grade 2: 15 (45,5%), Grade 3: 3 (9,1%) |
| Hypertension n (%) |
11 (33,3%) |
| Hypertension Grade n (%) |
Grade 1: 5 (15,2%), Grade 2: 5 (15,2%), Grade 3: 1 (3,0%) |
| Diarrhea n (%) |
10 (34,5%) |
| Diarrhea Grade n (%) |
Grade 1: 6 (18,2%), Grade 2: 4 (12,1%) |
| Rash n (%) |
8 (24,2%) |
| Rash Grade n (%) |
Grade 1: 4 (12,1%), Grade 2: 3 (9,1%), Grade 3: 1 (3,0%) |
3.5. Progression Free Survival (PFS) with Regorafenib
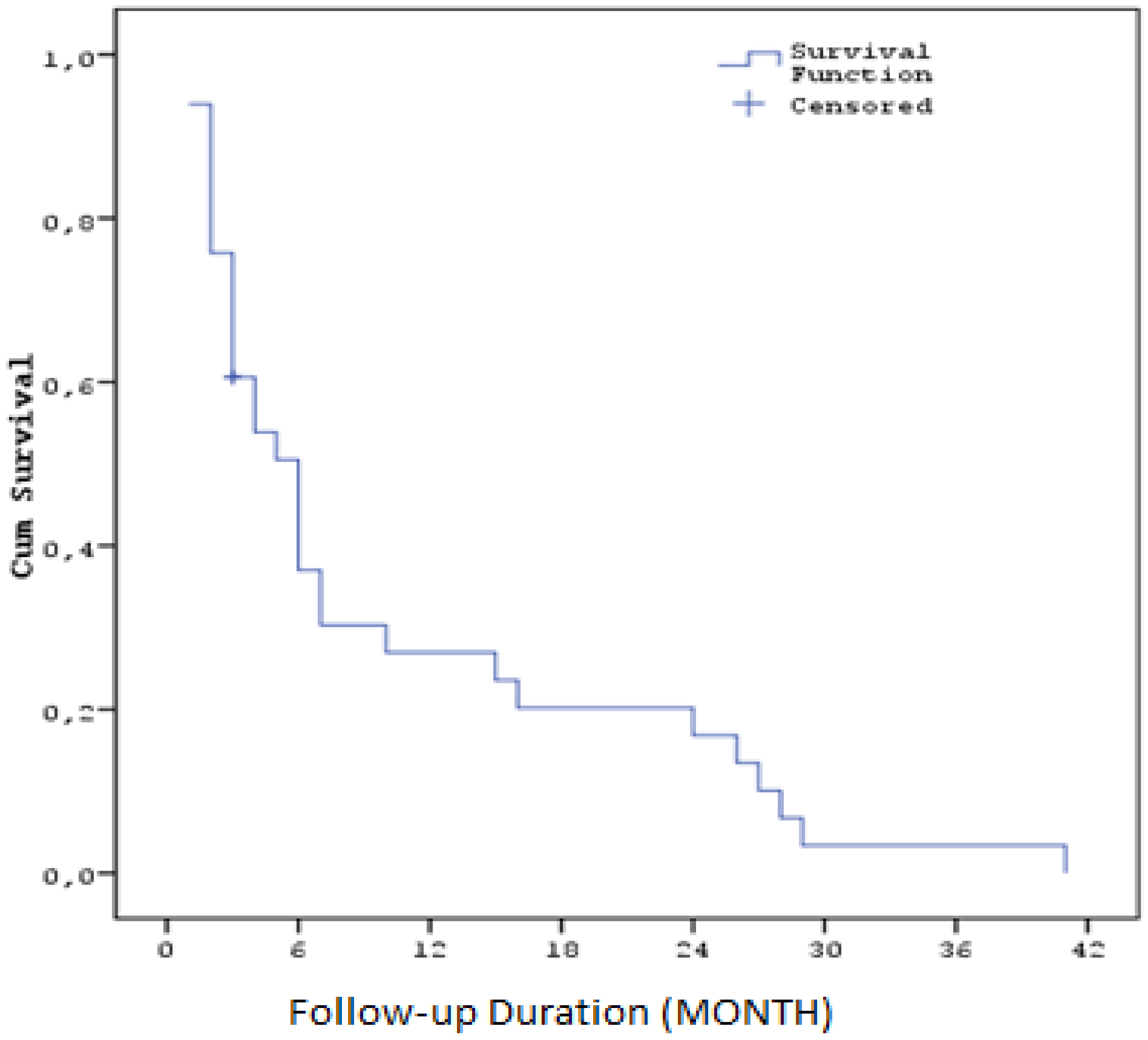
The median progression-free survival time after regorafenib treatment was calculated as 5,9 months (SE: 1,0). Three-month survival rate was 62,6% (SE: 8,6), six-month 38,2% (SE: 8,9), one-year 27,8% (SE: 8,3), two-year 17,4% (SE: 7,0) and three-year 3,5% (SE: 3,4). ( Table5 ) ( Figure2 )
Table 5.
PFS values for Regorafenib Treatment by Time.
Table 5.
PFS values for Regorafenib Treatment by Time.
| Parameter |
Value |
| PFS Duration (Months) Median (SE) / 95% CI |
5,9 (SE: 1,0) / 4 - 8 |
| PFS (%) at 3 months |
62.6% (SE: 8,6%) |
| PFS (%) at 6 months |
38.2% (SE: 8,9%) |
| PFS (%) at 1 year |
27.8% (SE: 8,3%) |
| PFS (%) at 2 years |
17.4% (SE: 7,0%) |
| PFS (%) at 3 years |
3.5% (SE: 3,4%) |
Figure 2.
Kaplan–Meier curves for progression-free survival with Regorafenib ( PFS2).
Figure 2.
Kaplan–Meier curves for progression-free survival with Regorafenib ( PFS2).
3.6. Overall Survival (OS)
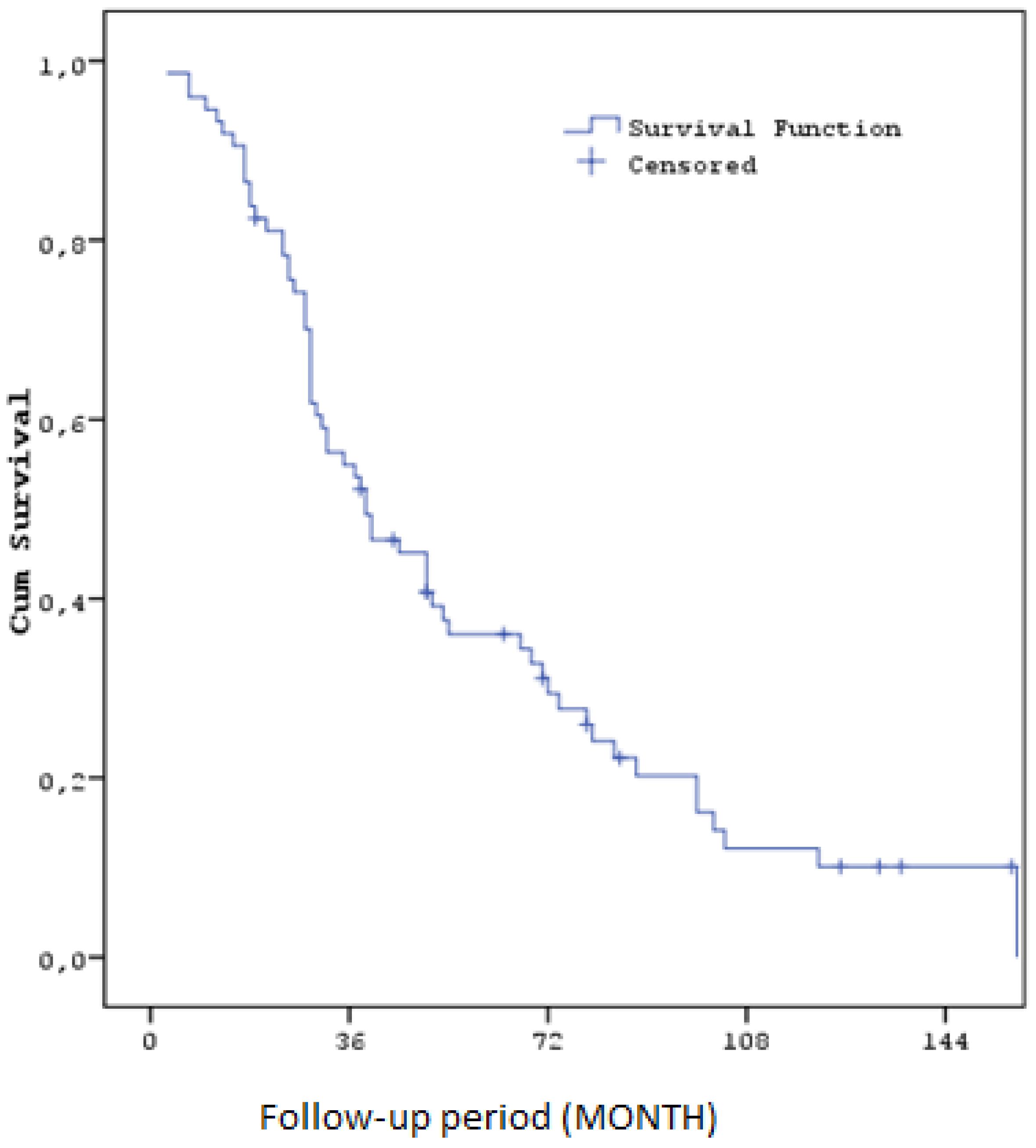
The median overall survival (OS) calculated throughout the study was 35,9 months (SE: 6,8). Overall survival rates were 93,2% (SE: 2,9) at one year, 55,0% (SE: 5,8) at three years, 36,0% (SE: 5,7) at five years and 12,1% (SE: 4,4) at ten years. These findings suggest that sorafenib and regorafenib can improve survival to a certain extent in patients with HCC recurrence after liver transplantation. ( Figure3) ( Table6)
Table 6.
Overall Survival (OS) Durations and Percentages at Various Time Points.
Table 6.
Overall Survival (OS) Durations and Percentages at Various Time Points.
| Parameter |
Value |
| OS Duration (Months) Median (SE) / 95% CI |
35,9 (SE: 6,8) / 25,7 - 52,3 |
| OS (%) at 1 year |
93.2% (SE: 2,9%) |
| OS (%) at 3 years |
55.0% (SE: 5,8%) |
| OS (%) at 5 years |
36.0% (SE: 5,7%) |
| OS (%) at 10 years |
12.1% (SE: 4,4%) |
Figure 3.
Kaplan–Meier curves for Overall Survival.
Figure 3.
Kaplan–Meier curves for Overall Survival.
3.7. Response to Treatment According to Child-Pugh Classification
Patients were divided into groups according to Child-Pugh classification to assess liver function. Patients in Child-Pugh A class had higher response rates to treatment and had significantly longer progression-free survival (PFS) and overall survival (OS) than patients in other classes. For example, while PFS was 14 months and OS was 22 months in Child-Pugh A patients before sorafenib treatment, PFS was 10 months and OS was 16 months in Child-Pugh B patients. When evaluated before regorafenib treatment, PFS was 12 months and OS was 18 months in Child-Pugh A patients.
During sorafenib treatment, 78,4% of patients reported various adverse reactions. The most common adverse reactions were hand-foot syndrome (41,9%), fatigue (62,2%)
4. Discussion
This study comprehensively evaluated the efficacy and survival effects of Sorafenib and Regorafenib in patients with HCC relapsed after liver transplantation. The findings indicate that PFS and OS times can be significantly improved in this patient group, but some remarkable differences and similarities also emerge when these findings are compared with the literature.
In our study, the median PFS duration (PFS1) after Sorafenib treatment was found to be 5,6 months. This finding is consistent with the PFS duration of Sorafenib reported in previous studies [23,24]. For example, in the SHARP study, the PFS duration of Sorafenib in patients with advanced HCC was reported as 5,5 months. This supports that Sorafenib may be effective in delaying disease progression in patients with advanced HCC. However, prognostic factors such as ECOG performance status and AFP levels were observed to have significant effects on PFS1. In the literature, it is frequently emphasised that these factors are critical factors affecting treatment response and survival times [25,26,27,28].
Regorafenib treatment was used as second-line therapy in patients who developed resistance to Sorafenib and the median PFS duration (PFS2) was 5,9 months. This finding indicates a longer duration compared to the PFS of 3,1 months reported in the RESORCE study. This difference may be explained by factors such as differences in the patient population and variations in the duration of follow-up. Regorafenib is known to be particularly beneficial in patients who show resistance to Sorafenib treatment, indicating that it may be an important option as a second-line treatment in this patient group [29,32].
In the study conducted by Iavarone et al., the median progression-free survival (PFS1) for sorafenib in post-liver transplant patients with recurrent hepatocellular carcinoma (HCC) was 3.0 months, reflecting the limited efficacy of sorafenib in this specific patient population. In contrast, our study reported a slightly longer median PFS1 of 5,6 months for sorafenib, which might be attributed to differences in patient characteristics or regional treatment protocols. Similarly, for regorafenib, Iavarone et al. observed a median PFS of 5,5 months, which closely aligns with the median PFS2 of 5,9 months reported in our study. When examining overall survival (OS), Iavarone et al. reported a total median OS of 28,8 months for patients treated with sorafenib followed by regorafenib. In comparison, our study observed a longer median OS of 35,9 months. The difference in OS may be influenced by variations in patient cohorts, follow-up durations, and treatment strategies, particularly considering the potential for improved liver function in post-transplant patients. Both studies highlight that the sequential use of sorafenib and regorafenib offers substantial survival benefits [33].
When the overall survival (OS) time was evaluated by an analysis including the use of both treatments, the median OS time was 35,9 months. This suggests, in line with data in the literature, that the use of Sorafenib followed by Regorafenib may provide long-term survival in patients with HCC relapsed after liver transplantation. This may be considered as a significant prolongation when compared with the SHARP and RESORCE studies; however, we think that the fact that the patients in our study also got rid of their cirrhotic livers with transplantation positively affected this survival time. This is a factor that is often overlooked in the literature and should be taken into consideration especially in the evaluation of survival time after transplantation [34,35,36].
In terms of side effects, side effects such as hand-foot syndrome, fatigue, hypertension and diarrhoea were frequently observed in both Sorafenib and Regorafenib treatments. These findings are in line with the side effect profiles reported in previous studies. The management of side effects is critical during the treatment process and careful monitoring of potential complications that may lead to disruption of the treatment process is required in these patients. In particular, a higher incidence of side effects in Sorafenib-treated patients may adversely affect treatment continuity and thus shorten survival times. In regorafenib treatment, the severity and frequency of side effects are more variable, but considering that this drug is used in patients who have previously developed resistance to treatment, the management of side effects gains greater importance [37,38].
5. Limitations
This study has several limitations that should be acknowledged. First, the retrospective design may introduce inherent biases related to data collection and patient selection. Despite efforts to include a representative cohort, variability in clinical practices across different centers and regions could affect treatment outcomes and follow-up intervals. Additionally, the relatively small sample size, especially of patients transitioning from sorafenib to regorafenib, limits the generalizability of our findings. The lack of a control group or comparison with alternative treatment strategies further constrains the ability to definitively conclude the superiority of the sequential use of sorafenib and regorafenib. Furthermore, while the study includes real-world data, the absence of detailed molecular profiling and biomarker analyses prevents a more nuanced understanding of individual treatment responses. This is especially relevant in the context of personalized medicine, where such data could guide more tailored therapeutic approaches.
6. Future Perspectives
Despite its limitations, this study offers valuable insights into the sequential use of sorafenib and regorafenib for managing HCC recurrence post-liver transplantation. Future research should prioritize larger, multicenter prospective trials to validate these findings and explore the role of molecular profiling in personalizing treatment approaches.
Given regorafenib's demonstrated efficacy in sorafenib-refractory patients, future therapeutic strategies should investigate combinations with novel agents such as immune checkpoint inhibitors and targeted therapies. Although concerns about graft rejection limit the use of immunotherapies in this setting, clinical trials assessing the safety of such combinations are essential.
Moreover, the development of new systemic therapies like lenvatinib, cabozantinib, and ramucirumab opens new avenues for comparison with regorafenib. Assessing these therapies' long-term outcomes, including survival and quality of life, will be crucial for optimizing treatment protocols for this unique patient population.
In conclusion, this study shows that Sorafenib and Regorafenib treatments provide a certain efficacy and may prolong survival times in patients with HCC relapsed after liver transplantation. However, the efficacy and side effect profiles of these treatments should be carefully evaluated in clinical practice. Our findings, supported by the literature, emphasise the critical importance of close follow-up and management of side effects in this patient group. Future studies are necessary to determine the optimal use of these therapies and to develop strategies to improve treatment outcomes in this patient group.
References
- Buonaguro, L. (2020, ). Human Hepatocellular Carcinoma (HCC). Multidisciplinary Digital Publishing Institute, 12(12), 3739-3739. 12 December. [CrossRef]
- Grandhi, M S., Kim, A K., Ronnekleiv-Kelly, S M., Kamel, I R., Ghasebeh, M A., & Pawlik, T M. (2016, ). Hepatocellular carcinoma: From diagnosis to treatment. Elsevier BV, 25(2), 74-85. 6 March. [CrossRef]
- Chang, B. , Tian, H., Huang, A., Zhai, X., Wang, L., Han, L., Jin, X., Gao, L., Liang, Q., Li, B., Lu, Y., Xie, H., Ji, D., & Zou, Z. (2024, ). Prevalence and prediction of hepatocellular carcinoma in alcohol-associated liver disease: a retrospective study of 136 571 patients with chronic liver diseases. BMJ, 2(1), e100036-e100036. 1 January. [CrossRef]
- Nassereldine, H. , Compton, K., Kendrick, P., Li, Z., Baumann, M M., Kelly, Y O., Schmidt, C., Sylte, D O., Motte-Kerr, W L., Daoud, F., Force, L M., McHugh, T A., Naghavi, M., Hay, S I., Shiels, M S., Rodriquez, E J., Mensah, G A., Nápoles, A M., Pérez-Stable, E J., Dwyer-Lindgren, L. (2024, ). Burden of liver cancer mortality by county, race, and ethnicity in the USA, 2000–19: a systematic analysis of health disparities. Elsevier BV, 9(3), e186-e198. 28 February. [CrossRef]
- Global hepatitis report 2024: action for access in low- and middle-income countries. (2024, ). https://www.who. 9 April 9789.
- Ansari, K K., & Jha, A. (2024, ). Causes of Cancer in the World: Comparative Risk Assessment of Nine Behavioral and Environmental Risk Factors. Cureus, Inc. 19 April. [CrossRef]
- Yang, J D., Hainaut, P., Gores, G J., Amadou, A., Plymoth, A., & Roberts, L R. (2019, ). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nature Portfolio, 16(10), 589-604. 22 August. [CrossRef]
- Ito, T. , & Nguyen, M. H. (2023). Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. H. ( 10, 413–428. [CrossRef]
- Hertl, M. , & Cosimi, A B. ( 2005, 10(4), 269–281. [Google Scholar] [CrossRef] [PubMed]
- Soong, R. , Yu, M., Chan, K., Chou, H., Wu, T., Lee, C., Wu, T., & Lee, C. (2011, ). Analysis of the recurrence risk factors for the patients with hepatocellular carcinoma meeting University of California San Francisco criteria after curative hepatectomy. BioMed Central, 9(1). 27 January. [CrossRef]
- Zimmerman, M A. ( 2008, 143(2), 182–182. [CrossRef]
- Straś, W. , Wasiak, D., Ła̧giewska, B., Tronina, O., Hreńczuk, M., Gotlib, J., Lisik, W., & Małkowski, P. (2022, ). Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. International Scientific Information Inc., 27. 26 January. [CrossRef]
- Sugawara, Y. , & Hibi, T. (2022, ). Liver transplantation for patients with hepatocellular carcinoma: Its current status and advances., 16(3), 207-211. 25 May. [CrossRef]
- Lee, H. , Yang, K., Choi, B H., Park, Y., Yoon, K T., Ryu, J H., & Chu, C W. (2016, ). Complete Regression of Recurrent Advanced Hepatocellular Carcinoma After Liver Transplantation in Response to Sorafenib Treatment: A Case Report. Elsevier BV, 48(1), 247-250. 1 January. [CrossRef]
- Almhanna, K. , & Philip, P A. ( 2009, November 1). Safety and efficacy of sorafenib in the treatment of hepatocellular carcinoma. Dove Medical Press, 261–261. [Google Scholar] [CrossRef]
- Sotiropoulos, G. , Nowak, K W. ( 2012, 44(9), 2754–2756. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S. , & Singal, A K. (2014, ). Regorafenib: an evidence-based review of its potential in patients with advanced liver cancer. Dove Medical Press, 81-81. 1 July. [CrossRef]
- Sherman, M. , & Sherman, B. (2018, ). Regorafenib for treatment of hepatocellular carcinoma. https://journals.lww. 24 January 0151. [Google Scholar]
- Waidmann, O. (2018, ). Recent developments with immunotherapy for hepatocellular carcinoma. Taylor & Francis, 18(8), 905-910. 20 July. [CrossRef]
- Okusaka, T. , & Ikeda, M. (2018, ). Immunotherapy for hepatocellular carcinoma: current status and future perspectives. Elsevier BV, 3, e000455-e000455. 1 January. [CrossRef]
- Jarroudi, O A., Ulusakarya, A., Almohamad, W., Afqir, S., & Morère, J. (2020, ). Anti-Programmed Cell Death Protein 1 (PD-1) Immunotherapy for Metastatic Hepatocellular Carcinoma After Liver Transplantation: A Report of Three Cases. Cureus, Inc. 25 October. [CrossRef]
- Kim, B H., & Park, J. (2018, ). Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy., 18(1), 17-22. 15 March. [CrossRef]
- Yoon, D H. ( 2010, 40(8), 768–773. [CrossRef]
- Mazzola, A. , Costantino, A., Petta, S., Bartolotta, T V., Raineri, S M., Sacco, R., Brancatelli, G., Cammà, C., & Cabibbo, G. (2015, ). Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Medicine, 11(21), 2923-2936. 28 September. [CrossRef]
- Llovet, J. M. , Ricci, S., Mazzaferro, V., Hilgard, P., Gane, E., Blanc, J.-F., de Oliveira, A. C., Santoro, A., Raoul, J.-L., Forner, A., Schwartz, M., Porta, C., Zeuzem, S., Bolondi, L., Greten, T. F., Galle, P. R., Seitz, J.-F., Borbath, I., Häussinger, D., Giannaris, T., Shan, M., Moscovici, M., Voliotis, D., & Bruix, J. (2008). Sorafenib in advanced hepatocellular carcinoma. The New England Journal of Medicine. [CrossRef]
- Sanoff, H K. ( 2016, 21(9), 1113–1120. [CrossRef]
- Terashima, T. , Yamashita, T., Takata, N., Nakagawa, H., Toyama, T., Arai, K., Kitamura, K., Yamashita, T., Sakai, Y., Mizukoshi, E., Honda, M., & Kaneko, S. (2015, ). Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Wiley, 46(7), 650-656. 6 October. [CrossRef]
- Otsuka, T. , Nakashita, S., Yanagita, K., Ario, K., Kawasoe, H., Kawazoe, S., Eguchi, Y., & Mizuta, T. (2015, ). Factors Associated with Post-Progression Survival in Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Multidisciplinary Digital Publishing Institute, 3(2), 68-77. 15 May. [CrossRef]
- Bruix, J. , Qin, S., Merle, P., Granito, A., Huang, Y.-H., Bodoky, G., Pracht, M., Yokosuka, O., Rosmorduc, O., Breder, V., Gerolami, R., Masi, G., Ross, P. J., Song, T., Bronowicki, J.-P., Ollivier-Hourmand, I., Kudo, M., Cheng, A.-L., Llovet, J. M.,... Han, G. (2017). Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 0064. [Google Scholar] [CrossRef]
- Ueshima, K. , Nishida, N., & Kudo, M. (2017, ). Sorafenib-Regorafenib Sequential Therapy in Advanced Hepatocellular Carcinoma: A Single-Institute Experience. Karger Publishers, 35(6), 611-617. 1 January. [CrossRef]
- Alsina, A. , Makris, A M., Nenos, V., Sucre, E., Arrobas, J., Franco, E., & Kemmer, N. (2014, ). Can Sorafenib Increase Survival for Recurrent Hepatocellular Carcinoma after Liver Transplantation? A Pilot Study. SAGE Publishing, 80(7), 680-684. 1 July. [CrossRef]
- Trojan, J. , & Waidmann, O. (2016, ). Role of regorafenib as second-line therapy and landscape of investigational treatment options in advanced hepatocellular carcinoma. Dove Medical Press, Volume 3, 31-36. 1 September. [CrossRef]
- Iavarone, M. , Invernizzi, F., Ivanics, T., Mazza, S., Zavaglia, C., Sanduzzi-Zamparelli, M., Fraile-López, M., Czauderna, C., Di Costanzo, G., Bhoori, S., Pinter, M., Manini, M. A., Amaddeo, G., Fernandez Yunquera, A., Piñero, F., Blanco Rodríguez, M. J., Anders, M., Aballay Soteras, G., Villadsen, G. E.,... Reig, M. (2021). Regorafenib efficacy after sorafenib in patients with recurrent hepatocellular carcinoma after liver transplantation: A retrospective study. Liver Transplantation, 27, 1778. [Google Scholar] [CrossRef]
- Chok, K. , Chan, S C., Cheung, T T., Chan, A C Y., Fan, S T., & Lo, C M. (2011, ). Late Recurrence of Hepatocellular Carcinoma after Liver Transplantation. Springer Science+Business Media, 35(9), 2058-2062. 19 May. [CrossRef]
- Roayaie, S. , Schwartz, J D., Sung, M W., Emre, S., Miller, C M., Gondolesi, G., Krieger, N., & Schwartz, M. (2004, ). Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Lippincott Williams & Wilkins, 10(4), 534-540. 1 January. [CrossRef]
- Rayya, F. , Harms, J. ( 2008, 40(4), 933–935. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. (2016, ). Regorafenib as Second-Line Systemic Therapy May Change the Treatment Strategy and Management Paradigm for Hepatocellular Carcinoma. Karger Publishers, 5(4), 235-244. 1 January. [CrossRef]
- Uchikawa, S. , Kawaoka, T. ( 2018, 6(11), 2217–2223. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).