1. Introduction
Developing countries in the world and more so in Africa, carry the greatest burden of Malaria disease [
1]. To ensure that this narrative changes, WHO has outlined key strategies which are to be owned by malaria endemic countries. These strategies are defined under the High Burden to High impact strategies with four key aims: i) Political will to reduce malaria deaths; (ii) Strategic information to drive impact, (iii) Better guidance, policies and strategies, and (iv) A coordinated national malaria response.[
2]
In achieving these goals, a key practical activity needed is accurate diagnosis of the parasites, to know the true burden of disease, foci of infections and how to prioritize and target these to reduce and prevent transmission, and to eliminate the disease [34]. Generally sub-Sharan African countries prefer the use of RDT as a routine diagnostic tool for malaria diagnosis because of ease of use at the outpatient departments (OPDs) by nurses and technicians, and by community health workers [56] . Confirmatory microscopy tests are done when treatments are to be initiated by an attending physician. However, for malaria elimination purposes, use of RDT is limiting with a malaria detection threshold of 100 – 200 parasites/µl and unable to detect non-falciparum malaria specifically [
7]. In addition, there is the problem of false negatives due to the prozone effect when excess infected blood overwhelm antigen binding sites, leading to poor binding with antibodies [
7]. Similarly, false negatives are high in malaria microscopy when performed by routine microscopists whose limit of detection is between 50-500 parasites/µl of blood [
8]. However, when done by experts, the limit of detection is significantly higher (10 parasites/µl of blood) and the data is much more reliable [
9,
10]. Microscopy enables differentiation of asexual from sexual parasites, parasite species and gives an insight into the transmission risks at different geographical locations [
11]. These can be achieved only by experts as routinely non-highly trained microscopist would fail to report on the parasite species that are not falciparum, thereby affecting programmatic decision making [
10]. Inaccurate diagnosis has the undesired outcome of unnecessary treatment when it is not required (false positives) and missed out treatment that could potentially end in fatality (in the case of false negatives) [
10,
12]. These scenarios are indicated in competency as sensitivity and specificity skills of malaria microscopist [
13].
The need for competent microscopy diagnostic skill set is therefore essential, as countries set their goals on elimination. Well trained and competent malaria microscopist need to be continually available for quality assurance and to serve as support base for training new groups of competent microscopists overtime for malaria disease surveillance [
14]. Several countries have reported on the positive impact of malaria microscopy training for staff of the Ministry of Health and specifically the National Health laboratories (NHL)/National Malaria Programs (NMPs). These included improved malaria epidemiological knowledge and competency in detection speciation and counting [15.16]
PAVON has one of its goals as training of competent microscopists using both theory and practice as the mode of teaching. The intent is to assist NMPs/NHLs to build the critical capacity for surveillance towards elimination. . Here we report on the impact of such training conducted in Botswana in collaboration with the National Health Laboratory and the National Malaria Program to provide personnel support for malaria elimination.
2. Materials and Methods
2.1. Training Design and Content
The training was prospective and included participants who worked in various health facilities across the country as laboratory technicians, technologist, or laboratory assistants. Some of the trainees had limited training on malaria microscopy and limited access to microscopes at their facilities. This prompted PAVON to initiate a program of donating microscopes as part of the training package. These donations were facilitated by Rotary Against Malaria-Global group (RAM-G) and Merck Global Health Institute, Geneva.
Training Site and Participants
Selection of the training site and participants was at the discretion of the Ministry of Health (MoH) and or National Health Laboratory. For the site, training was usually at a location away from distractions and close to a relatively well set up laboratory with space for slide preparation, buffer solution preparation, staining and drying. For the participants, the MoH/NHL took cognisance of district representation, the role of the participant in the laboratory at the district, gender balance and potential to learn new skills and/or improve on malaria microscopy skills. Each training session enrolled between 11 – 14 participants.
Training Approach
Each training session was residential and spanned two weeks. The scheme used the WHO guidelines and modified it to suit the local context and needs. This was with regards to the theoretical knowledge in malaria disease, skills and exposure to practical microscopy at the laboratories where they normally worked. All trainees held either biomedical science certificate or degree, or medical laboratory certificate or degree.
Training Facilitators
Facilitators were a combination of WHO certified Level 1 malaria microscopy experts (3) who also serve as national malaria microscopy and National Health Laboratory supervisors in their respective countries, and established malaria researchers (5).
Training Content
The theoretical training materials covered the following topics: basic epidemiology, malaria epidemiology, parasite life cycle, key metrics for malaria microscopy and definitions of key terminologies in epidemiology and microscopy. There was also a pre-test theory that focused on parasite detection, stage of development and speciation. For the practical, the content covered slide preparation, staining, and viewing under the microscope for parasite detection, stage of development, speciation and counting.
2.2. Basic Guidelines on Slide Preparation
The methods were consistent with the WHO recommendations already available in the guidelines, manuals and standard operating procedures for the preparation and staining of blood films that are of high quality [
14,
17]. Additional tips based on the experiences of the facilitators were shared, which included what to do if basic consumables and tools are unavailable in laboratories. Some of the recommendations done in Sri Lanka which had been tested under those circumstances and found practicable, were alluded to [
14,
17,
18]. These were (a). to wipe new slides, clean with a tissue before use for blood smears (b). thick and thin blood smears were to be prepared on the same slides but with the necessary precaution to ensure a gap of about 1 cm between the two smears to prevent fusion and methanol flow from the thin film into the thick film (c). labelling of the slides on the side facing away from the thin film to prevent wash offs and (d). collection of blood for the thick smear first before that of the thin smear, while keeping the standard preparation of the thin film first and then the thick film. For drying, the films were prepared just before the lunch break so that by the time lunch was over in approximately 135 minutes, the slides were dried enough for staining. In the absence of smooth-edged slides for spreading, participants were told of alternate methods including the use of sandpapers to smoothen the edges of the slides.
2.3. Criteria for Defining the Quality of Blood Smears
Following the guidelines of WHO for quality of slides, the blood films were designated as either good or bad. A good film was defined as having the following characteristics: correct thickness, good fixing, correct size, correct staining density, color, absence of staining granules, red, white blood cell nuclei and absence of stained particles. Specifically, a thick smear that has the correct thickness and is well fixed should allow for the examination of hundred microscope fields with a WBC density of 5- > 10 per field; b) it is of the correct size if it is sufficient to cover 100 fields; c) If WBC nuclei are red or purple then staining is of the correct degree; d) where staining granules are unable to obscure the presence of parasites they are said to be low. If any of the above was lacking, the smear was deemed as ‘bad’. Similarly, a thin smear was adjudged of good quality if: a) Two hundred microscope fields with a density of nearly 250 RBCs could be examined, b) staining makes WBC nuclei red or purple, c) stain granules and particles low to the extent that they do not obscure parasite identification. If any of the above was lacking, the thin smear was considered as of “bad quality” [
14,
17].
2.4. Set of Training Slides
The slide set used in the training were exactly as specified in the ECAMM training slide set which is presented in
Figure 1. These were 148 slides of carefully prepared and Giemsa-stained blood films that were either malaria positive with varying parasite densities, or malaria negative. The slides covered all the four common
Plasmodium parasites known to be infectious to humans (
P. falciparum, P. vivax, P. malariae and
P. ovale). There were additional negative slides of
Plasmodium parasites that included other parasites (
Babesia spp, Trypanosoma spp, Microfilariae) that could cause symptomatic infections as
Plasmodium species and may be mistaken for debris. The slides were obtained from the WHO Collaborating Centre for malaria diagnosis, Western Pacific Region at the Research Institute for Tropical Medicine (RITM), Muntinlupa City, Philippines. The slides are designed to assess a malaria microscopists’ competence in parasite detection, species identification and quantitation at the level of a patient.
Table 1.
Composition of the slides set used in the training.
Table 1.
Composition of the slides set used in the training.
| Malaria species |
Parasite density/µL |
Number of slides |
| Negative slide |
0 |
20 |
|
Pf low density |
80-200 |
5 |
|
Pf low density |
200-500 |
5 |
|
Pf medium density |
500-2000 |
5 |
|
Pv low density |
80-200 |
5 |
|
Pv low density |
200-500 |
10 |
|
Pv medium density |
500-2000 |
10 |
|
Pm low density |
80-200 |
10 |
|
Pm low density |
200-500 |
10 |
|
Pm medium density |
500-2000 |
10 |
|
Po low density |
80-200 |
10 |
|
Po low density |
200-500 |
10 |
|
Po medium density |
500-2000 |
5 |
| Mixed inf (Pf+Pm)
|
positive |
10 |
| Mixed inf (Pf+Po)
|
positive |
10 |
| Mixed inf (Pf+Pv)
|
positive |
10 |
| Mixed inf (Po+Pm)
|
positive |
10 |
| Mixed inf (Po+Pv)
|
positive |
10 |
| Mixed inf (Pv+Po)
|
positive |
10 |
| Other blood parasites (Babesia spp, Trypanosoma spp, Microfilariae ) |
Positive |
3 |
Combination of Slides Used for Detection and Developmental Stage
The number of slides used in the detection training were 20 negative slides and 20 positive slides all within the range of low to medium density p/µL of blood as specified in the slide types.
Combination of Slides Used for Speciation
The same number and set of slides used for detection were used for species identification.
Combination of Slides for Quantitation
The slides used for quantitation were only P. falciparum from ≥ 200 p/µL to ≤ 2000 p/µL.
Time Allocated for Each Slide Reading
Ten minutes was allocated to each slide reading irrespective of the category: detection and developmental stage, speciation and quantitation. In counting, if parasitaemia was estimated to within 20% of the approved range, it was deemed accurate.
2.5. Statistical Analysis
All the results were entered into a Microsoft excel file. Descriptive statistics (frequencies and percentages) were used to calculate categorical variables. For statistical analysis the Vassar statistics website tools were used (
www.vassarstats.net).The median and inter-quartile ranges were used as measures of central tendency to estimate overall scores. The Wilcoxon matched pairs signed rank test were used to compare median scores pre- and post-tests. The skills set of each participant was determined by calculating the sensitivity and specificity of the detection and speciation skills.
3. Results
The number of participants for the first, second and third trainings were, 11(3 males, 8 females), 12 (4 males, 9 females) and 14 (7 males, 7 females) respectively. All the participants from the first training had undergone previous online theoretical training. However, none of the participants in the subsequent two trainings had undergone a previous malaria microscopy training anywhere. In the first training 8 were from Government Hospital Laboratories, one from the National Health Laboratory, one from the malaria program and one from the Botswana Quality Assurance Laboratory. In the second training, 9 were from Government Hospital Laboratories and 3 from clinics. In the last training 10 were from Government Hospital Laboratories, one from the National Blood Transfusion Centre and 3 from clinics.
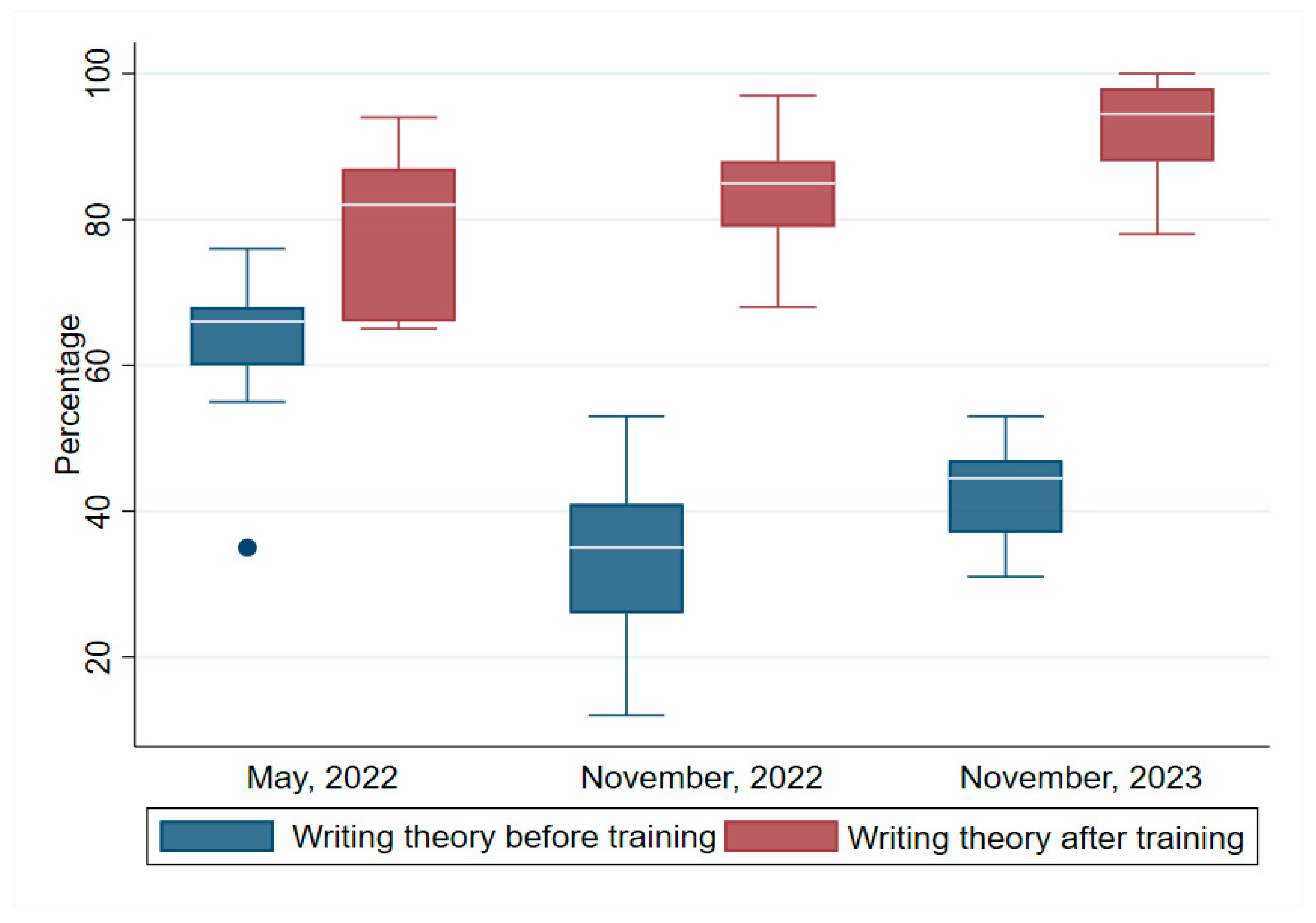
3.1. Writing Theory
Figure 1 shows the test scores pre- and post-test for writing. The median pre- and post-test scores for the first, second and third trainings were respectively (pre-test 66, IQR=60, 68), post-test (82, IQR=66, 87)}, (pre-test, 35, IQR=36, 41), post-test (85, IQR=79, 88)} and pre-test (45.5, IQR=37, 47), post-test (94.5, IQR=88, 98). To ascertain whether there was a statistically significant difference between the median writing theory test scores pre- and post-training, the Wilcoxon signed rank test was conducted. The test found a statistically significant difference between the pre- and post-training median writing theory for the first (z = 2.852, p <0.004), second (z = 3.184, p =0.002), and third (z = 3.301, p =0.001) trainings respectively. These results indicated that the training program significantly improved the knowledge of the participants in basic malaria epidemiology, life cycle and disease presentation.
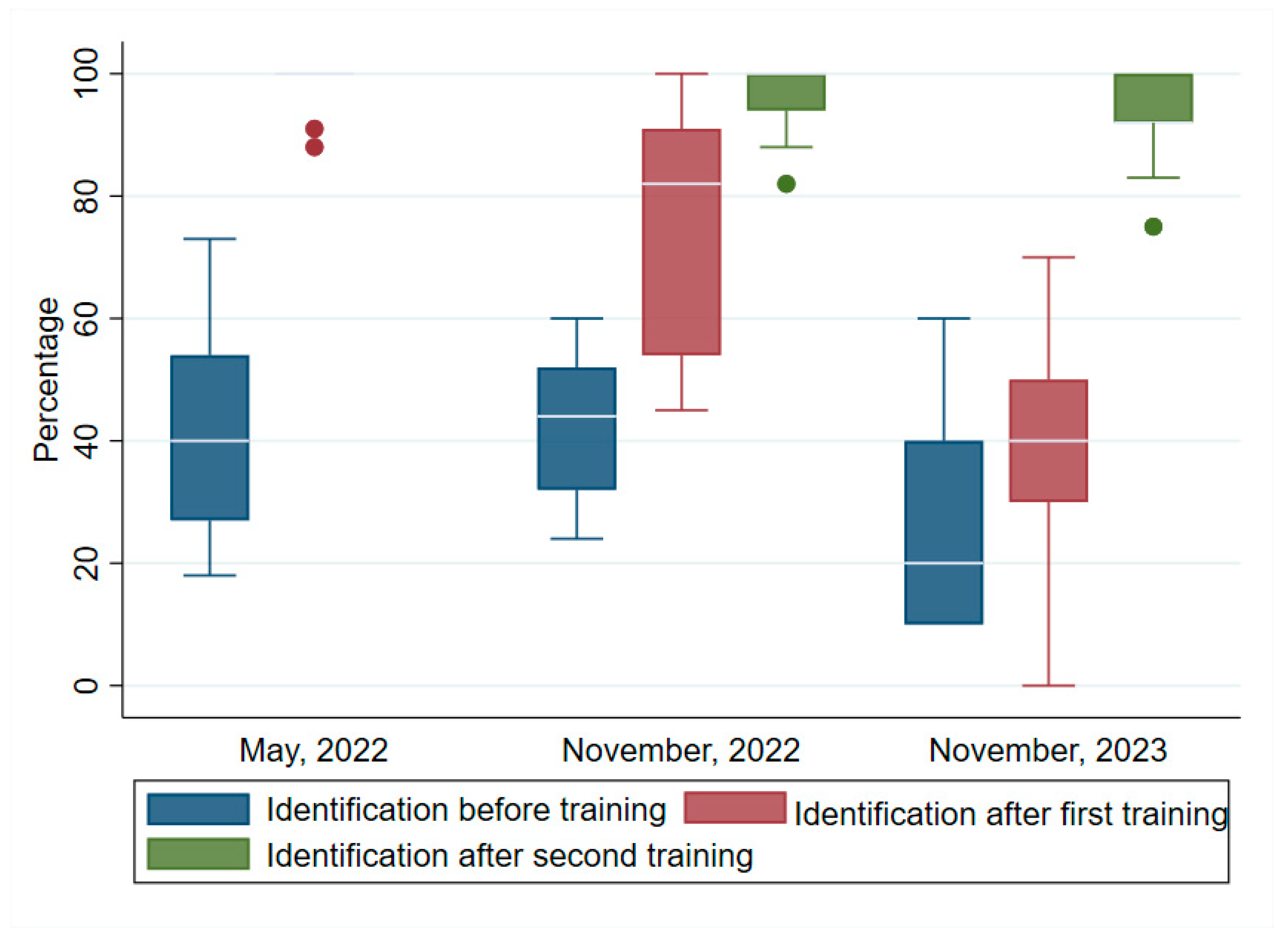
3.2. Identification (Detection)
Figure 2 shows the median scores in the identification of the parasite before and after the training programs. Notably, the median scores recorded after the training were higher than that before the training. For the first training, the median scores were pre-test: (40, IQR=27, 54), post-test: (100, IQR=94, 100). Two post-tests were conducted in the second and third trainings. The median scores were respectively, pretest: (44, IQR=32, 52), post-test1: (82, IQR=54, 91), post-test 2: (100, IQR=94, 100). In the third training, the pre-test median score was: (20, IQR=10, 40), while the post-tests 1 and 2 were: (40, IQR=30, 50) and (92, IQR=92, 100). Wilcoxon signed ranked tests showed that the increases in the post-tests in all three trainings were highly significant: (z = 2.937, p<0.003); (z = 3.110, p=0.002); and (z = 2.251, p=0.024) respectively.
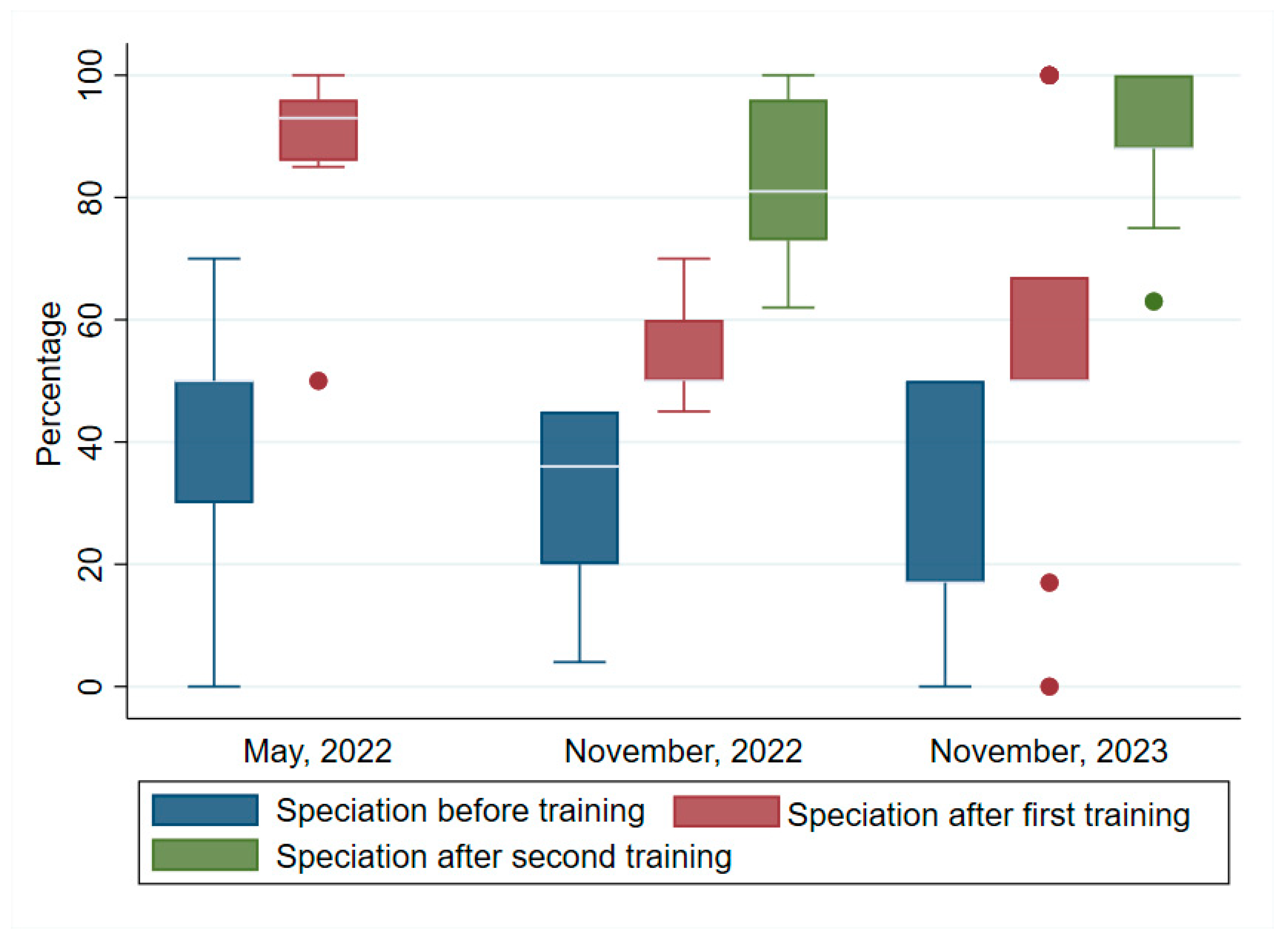
3.3. Speciation
Figure 3 shows the differences in the pre- and post-test median scores for speciation for the first, second and third trainings. Like the previous performances, post-test median scores were higher than the pre-test median scores. These were respectively, pre-test: (50, IQR=30, 50), post-test: (93, IQR=86, 96). The second training pre- and post-tests 1 and 2 median scores were, respectively: (36, IQR=20, 45). (50, IQR=50, 60) and (81, IQR=73, 96). The third training pre- and post-test median scores were respectively: (17, IQR=17, 50), (50, IQR=50, 67) and (88, IQR=88, 100). The Wilcoxon signed ranked test indicated that the increases were highly significant: first training, (z = 2.936, p p<0.003); second training, (z = 3.152, p=0.002); and third training (z = 3.237, p=0.001).
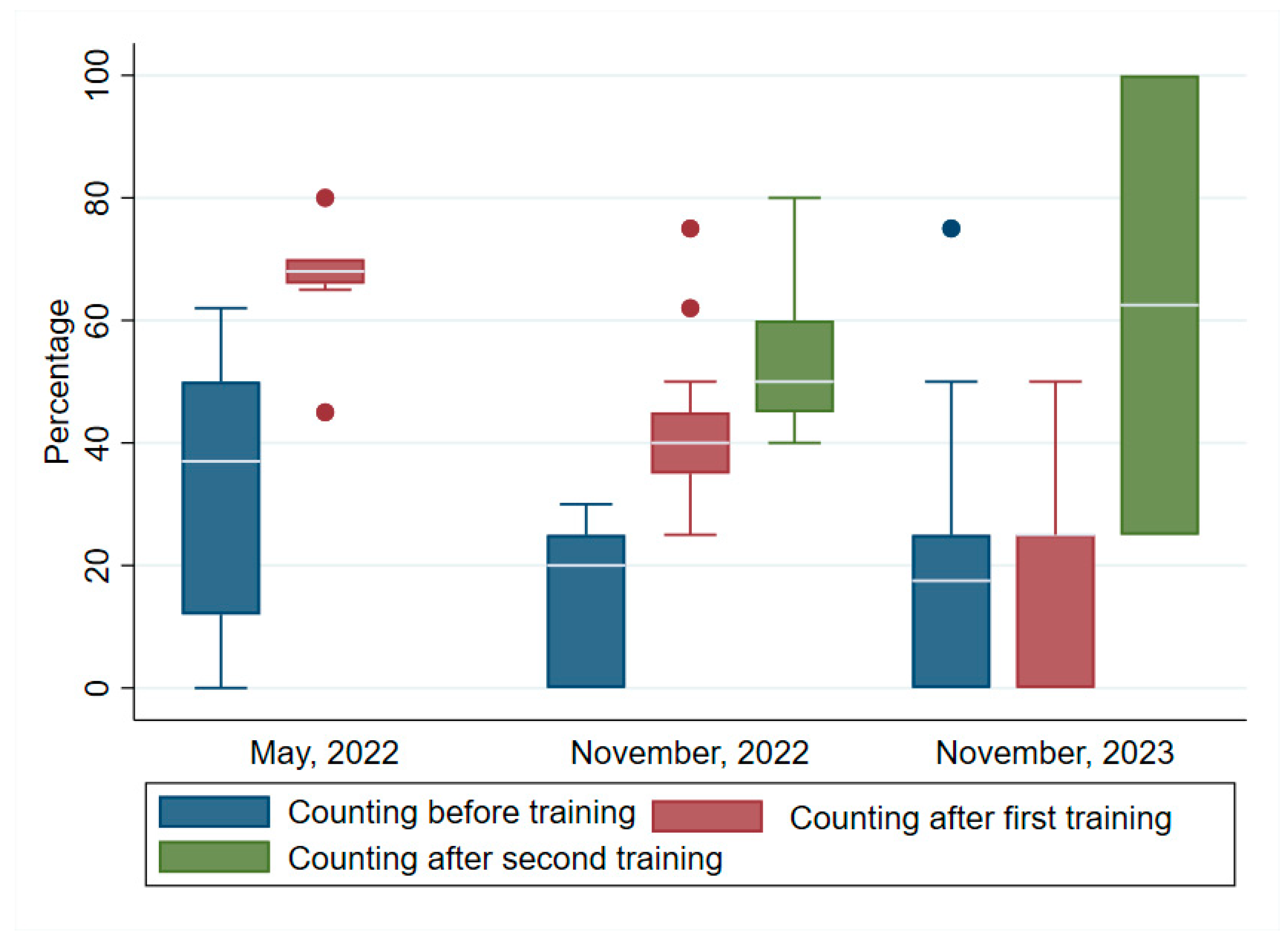
3.4. Counting
Figure 4 shows the differences in the median scores for counting pre/ and post/training. The median scores increased post training as in the previous skills set. The specific values were> first training pre- and post-test:(37, IQR=12, 50); (68, IQR=66, 70). The second training pre-and post-tests 1 and 2:(20, IQR=0, 25); test 1 (50, IQR=35, 45); and test 2 (50, IQR=45,60). In the third training the pre-and post-tests 1 and 2 scores were: (7.5, IQR=0, 25); (25, IQR=0, 25); and (62.5, IQR=25, 100). The increases in counting skills were highly significant for the first and second trainings but not the third. The Wilcoxon signed rank test respectively were: (z = 2.94, p= 0.003; (z = 3.114, p= 0.002; and (z = 0.662, p= 0.508).
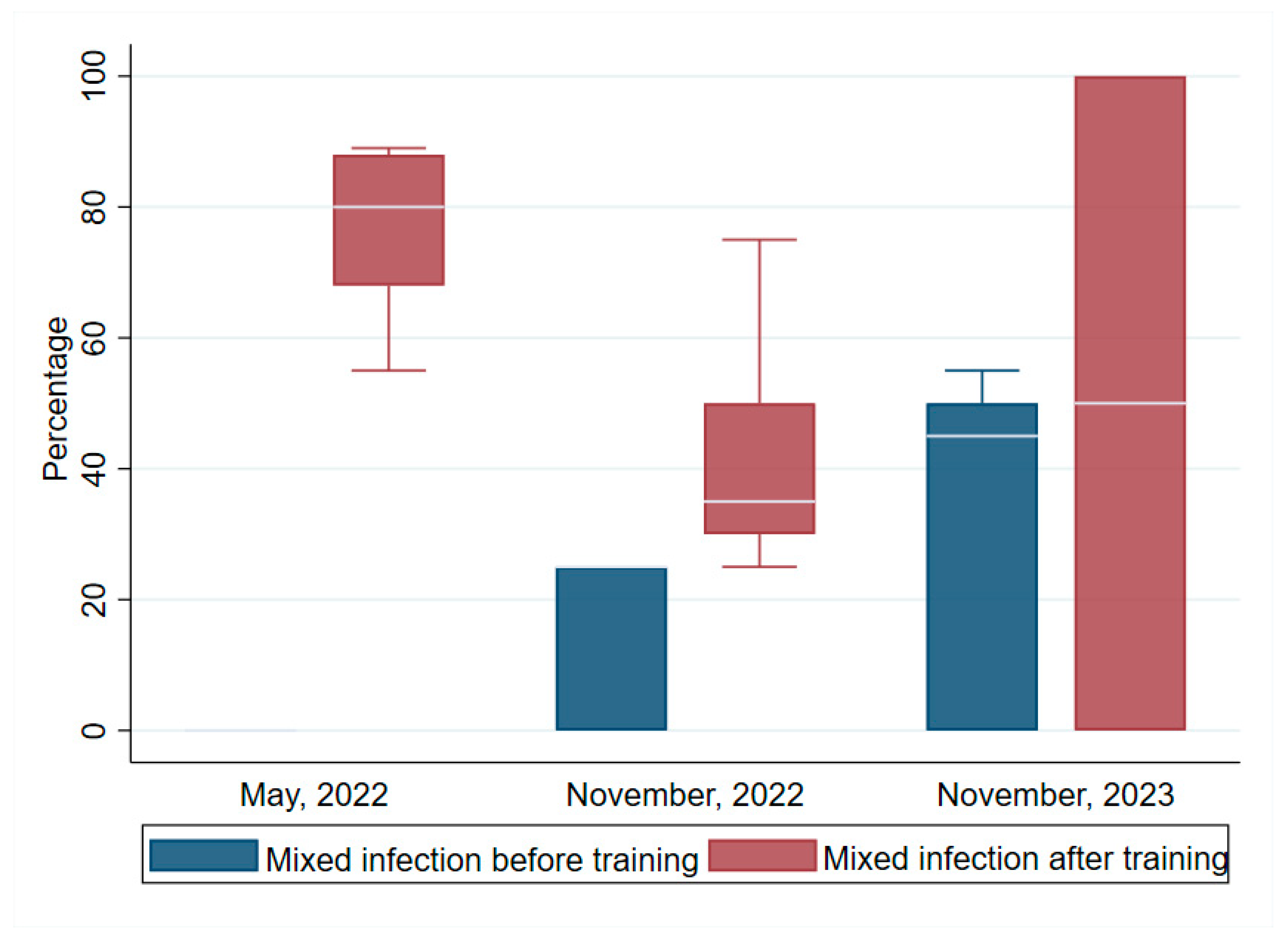
3.5. Mixed Infection
Figure 5 shows the differences in the median scores for mixed infection detection before and after the trainings. The median scores recorded after the post-tests were significantly higher than before the trainings as observed in the other skills set. The first training the median pre-and post-test scores were respectively:( 0), and (80, IQR=68, 88). For the second and third trainings the scores for pre- and post-tests were respectively: (25, IQR=0, 25) and (35, IQR=30; then (45, IQR=0, 50), and (50, IQR=0, 100). Wilcoxon signed ranked tests for the increases in scores regarding competency in the first, second and third training were highly significant: (z = 2.936, p =0.003), (z = 3.089, p =0.002). and (z = 2.618, p =0.008).
3.6. Sensitivity and Specificity of the Participants Skills in Detection and Speciation
The overall skills set of the participants in how they were able to avoid false positive (sensitivity (%)) and false negatives (specificity (%) were 98/97, 94/88, and 93/82 for the three trainings respectively. Participants from the first two groups who excelled and were selected to undergo the ECAMM training had median sensitivity and specificity of 98/98; and 97/98 for pre-and post-test respectively. The training competencies acquired through the training delivered by PAVON, was equivalent to that obtained through the ECAMM training.
4. Discussion
The present report provides evidence of the importance of malaria microscopy training to equip NMPs with the critical mass of competent staff who can be used in the malaria elimination agenda at all levels. The findings align with other trainings previously reported in sub-Saharan Africa [
19,
20,
21,
22]. Counting and detection of mixed infections were the most difficult for the trainees. This was not unique, which is why WHO training competency assessment cut off mark for counting and mixed infection is at > 50% compared to detection and speciation which are at > 90 % for Level1 and 80% for level 2 [
17]. In terms of specificity and sensitivity the suggested thresholds are 97 and 90% respectively for the highest competency level [
17,
23]. The main emphasis in clinical disease management of malaria is whether a slide is positive or negative for malaria, to trigger treatment. As a result, technicians in sub-Saharan Africa focus more on detection and in most cases detection of
P. falciparum and less on other species. It is clear from our training that significant increase in the competency level of technicians can be achieved if training is sustained. This will also impact on their ability to speciate correctly and efficiently. Outside of limited training opportunities, the sheer volume of work and poor supply of quality reagents could adversely reduce competency skills retention.
African Leaders Malaria Alliance (ALMA) have accented to the goal of malaria elimination, giving impetus to National Malaria Programs (NMPs) to see that malaria elimination is attainable [
23,
24,
25]. Such support is warranted as are collaborative activities between malaria focused research networks and NMPs to provide the skills set and competency for accurate data acquisition. Microscopy remains an important tool in malaria disease surveillance, case management, and anti-malarial drug efficacy and resistance [
26]. We found that participants who had gone through a previous online training (first training group) achieved higher competency scores than those who had not (second and third training groups). Nevertheless, an intensive hands-on training can help to improve those who are less exposed to additional trainings. This has also been seen in other training programs [
20,
26,
27]. Here, we emphasize that although video clips of malaria microscopy training are useful, repeated hands-on trainings are crucial for the acquisition and sustenance of competency skills. By providing good microscopes as a component of our training, we ensure that the programs have at least the minimum tools they can use to sustain further training.
It was notable that the participants who had attained Level A by our grading system achieved Level 1 status in the ECAMM competency assessment with similar sensitivity and specificity outcomes. This is remarkable and shows that malaria research networks that have established excellent training schemes can play vital roles in capacity building in malaria microscopy. Such networks could be recognized by WHO and RBM to augment capacity training in malaria competency. In this way NPMs will not be limited in their malaria microscopy capacity building agenda. Currently only AMREF and the University of Cheikh Anta Diop de Dakar (UCAD) are recognized by WHO for the ECAMM. We think that this is woefully limiting and needs to be expanded.
We conclude that to build a reference core of highly skilled microscopist to sustain competency skills training for NMPs, continued training would be necessary. This training must be validated by competency level awards so both the NMPs and trainees know the competency skills of staff and how they can be utilized in the malaria elimination agenda. We have shown here that institutions with such training capacity can significantly augment the availability of highly competent malaria microscopist for programs.
Author Contributions
Conceptualization IKQ; methodology, IKQ, AD, IS, DGM, JA, BG, MC; formal analysis, AZD, GKA, WKT; resources: ALL; data curation, IKQ, AD, DGM, JA, TKW, AZD; writing—original draft preparation, IKQ; writing—review and editing, ALL; funding acquisition, ALL. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Merck Global Health Institute, grant number SOW3.
Acknowledgments
The authors wish to acknowledge the Research Institute for Tropical Medicine, WHO Centre for the Western Pacific Region, Muntinlupa City, Philippines for providing us with the slides for the training and Rotary Club Against Malaria- Global (RAM-G).
Conflicts of interest: The authors declare no conflicts of interest.
References
- World Health Organization World malaria report 2023; World Health Organization: 2023;
- World Health Organization No title. High burden to high impact: a targeted malaria response 2018.
- Bannister, L.; Mitchell, G. The ins, outs and roundabouts of malaria. Trends Parasitol. 2003, 19, 209–213. [Google Scholar] [CrossRef] [PubMed]
- González-Sanz, M.; Berzosa, P.; Norman, F. F. Updates on malaria epidemiology and prevention strategies. Curr. Infect. Dis. Rep. 2023, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mbanefo, A.; Kumar, N. Evaluation of malaria diagnostic methods as a key for successful control and elimination programs. Tropical medicine and infectious disease 2020, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Oyegoke, O. O.; Maharaj, L.; Akoniyon, O. P.; Kwoji, I.; Roux, A. T.; Adewumi, T. S.; Maharaj, R.; Oyebola, B. T.; Adeleke, M. A.; Okpeku, M. Malaria diagnostic methods with the elimination goal in view. Parasitol. Res. 2022, 121, 1867–1885. [Google Scholar] [CrossRef] [PubMed]
- Gillet, P.; Mori, M.; Van Esbroeck, M.; Van den Ende, J.; Jacobs, J. Assessment of the prozone effect in malaria rapid diagnostic tests. Malar J. 2009, 8, 271–271. [Google Scholar] [CrossRef] [PubMed]
- Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Odhiambo, F.; Buff, A. M.; Moranga, C.; Moseti, C. M.; Wesongah, J. O.; Lowther, S. A.; Arvelo, W.; Galgalo, T.; Achia, T. O.; Roka, Z. G. Factors associated with malaria microscopy diagnostic performance following a pilot quality-assurance programme in health facilities in malaria low-transmission areas of Kenya, 2014. Malaria journal 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, P.; de Lucio, A.; Romay-Barja, M.; Herrador, Z.; Gonzalez, V.; Garcia, L.; Fernandez-Martinez, A.; Santana-Morales, M.; Ncogo, P.; Valladares, B.; Riloha, M.; Benito, A. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar J. 2018, 17, 333–4. [Google Scholar] [CrossRef] [PubMed]
- Kilian, A. H.; Metzger, W. G.; Mutschelknauss, E. J.; Kabagambe, G.; Langi, P.; Korte, R.; von Sonnenburg, F. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop. Med. Int. Health 2000, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dhorda, M.; Ba, E. H.; Kevin Baird, J.; Barnwell, J.; Bell, D.; Carter, J. Y.; Dondorp, A.; Ekawati, L.; Gatton, M.; González, I. Towards harmonization of microscopy methods for malaria clinical research studies. Malaria Journal 2020, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Malaria microscopy quality assurance manual-version 2; World Health Organization: 2016;
- Li, M.; Huang, F.; Yin, J.; Yan, H.; Zhou, S.; Xia, Z. Malaria microscopy competency in the subnational verification, China: implications for malaria elimination and the prevention of malaria reestablishment. Canadian Journal of Infectious Diseases and Medical Microbiology 2022, 2022, 8003845. [Google Scholar] [CrossRef] [PubMed]
- Kiggundu, M.; Nsobya, S. L.; Kamya, M. R.; Filler, S.; Nasr, S.; Dorsey, G.; Yeka, A. Evaluation of a comprehensive refresher training program in malaria microscopy covering four districts of Uganda. Am. J. Trop. Med. Hyg. 2011, 84, 820. [Google Scholar] [CrossRef] [PubMed]
- Obare, P.; Ogutu, B.; Adams, M.; Odera, J. S.; Lilley, K.; Dosoo, D.; Adhiambo, C.; Owusu-Agyei, S.; Binka, F.; Wanja, E. Misclassification of Plasmodium infections by conventional microscopy and the impact of remedial training on the proficiency of laboratory technicians in species identification. Malaria journal 2013, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Malaria microscopy quality assurance manual-version 2; World Health Organization: 2016;
- Fernando, S. D.; Ihalamulla, R. L.; Wickremasinghe, R.; de Silva, N. L.; Thilakarathne, J. H.; Wijeyaratne, P.; Premaratne, R. G. Effects of modifying the World Health Organization standard operating procedures for malaria microscopy to improve surveillance in resource poor settings. Malaria Journal 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, M.; Dwomoh, D.; Asamoah, A.; Kupeh, E. K.; Malm, K.; Nonvignon, J. Impact of malaria diagnostic refresher training programme on competencies and skills in malaria diagnosis among medical laboratory professionals: evidence from Ghana 2015-2019. Malar J. 2021, 20, 255–x. [Google Scholar] [CrossRef] [PubMed]
- Moura, S.; Fancony, C.; Mirante, C.; Neves, M.; Bernardino, L.; Fortes, F.; Sambo, M. d. R.; Brito, M. Impact of a training course on the quality of malaria diagnosis by microscopy in Angola. Malar J. 2014, 13, 437–437. [Google Scholar] [CrossRef] [PubMed]
- Aiyenigba, B.; Ojo, A.; Aisiri, A.; Uzim, J.; Adeusi, O.; Mwenesi, H. Immediate assessment of performance of medical laboratory scientists following a 10-day malaria microscopy training programme in Nigeria. Glob. Health. Res. Policy. 2017; 2, 32–x. eCollection 2017. [Google Scholar]
- Addo, K. K.; Yeboah-Manu, D.; Dan-Dzide, M.; Owusu-Darko, K.; Caulley, P.; Mensah, G. I.; Minamikawa, M.; Lienhardt, C.; Bonsu, F. A.; Ofori-Adjei, D. Diagnosis of tuberculosis in ghana: the role of laboratory training. Ghana Med. J. 2010, 44, 31–36. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; UNICEF Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin blood films in research settings (version 1.0): procedure: methods manual. 2015.
- Mswati, I. K. An Africa free of malaria. The Lancet 2019, 394, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Sambo, L. G.; Ki-Zerbo, G.; Kirigia, J. M. In In Malaria control in the African Region: perceptions and viewspoints on proceedings of the Africa Leaders Malaria Alliance (ALMA); BMC proceedings; Springer: 2011; Vol. 5, pp 1–8.
- Mbohou Nchetnkou, C.; Nyabeyeu Nyabeyeu, H.; Kojom Foko, L. P.; Lehman, L. G. Comparison of the fluorescence microscopy Cyscope® with light microscopy for malaria diagnosis in a small and active surveillance in Cameroon. Tropical Medicine and Health 2020, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Worges, M.; Whitehurst, N.; Saye, R.; Ndiaye, D.; Yamo, E.; Yukich, J. Performance outcomes from Africa-based malaria diagnostic competency assessment courses. Am. J. Trop. Med. Hyg. 2019, 100, 851. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).