Hippocampus View
The hippocampus is a structure situated in the medial temporal lobe and plays a crucial role in memory consolidation, learning processes, and mood regulation (Fan et al., 2018; van Strien et al., 2009). Anatomically, it is closely linked to the entorhinal cortex (EC) and, together with the cingulate gyrus, parahippocampal gyrus, amygdala, septal area, and hypothalamus, constitutes the limbic system, which governs emotions, behavior, and memory (AbuHasan et al., 2024; Hartley et al., 2014). Forming part of a larger structure known as the hippocampal formation, it encompasses four primary regions: (i) the dentate gyrus (DG); (ii) hippocampus proper or Cornu Ammonis (CA) subfields (CA3, CA2, and CA1); (iii) subicular complex (subiculum, presubiculum, and parasubiculum); (iv) and EC [
5]. Generally, the term “hippocampus” is used to collectively refer to the DG and the hippocampus proper (Spiers, 2012), further divided into layers distinguished by cell structure types: alveus, stratum oriens, stratum pyramidale, stratum radiatum, stratum lacunosum, and stratum moleculare (Maccaferri, 2005; Pang et al., 2019).
The DG comprises three layers, with the middle region housing densely packed small granular neurons, approximately 18 million in the human brain (Alkadhi, 2019). The dentate granular cell layer (DGCL) constitutes their cell bodies, while their extensive dendritic extensions form the molecular layer (ML). Additionally, the ML consists of Inner (IML), Middle (MML), and Outer lamina (OML). The inner polymorphic layer or hilus of DG, a third layer, lies between the blades of the granular layer (RajMohan & Mohandas, 2007). In the DG field, two principal excitatory glutamatergic cell types prevail: the abundant granular cells (GCs) and the hilar mossy cells (MCs), crucial in regulating the excitatory function of GCs (Amaral, 1978; Lothman et al., 1991; Scharfman, 2018; Scharfman & Myers, 2013).
Diverging from the small granule cells of the DG, pyramidal neurons in CA3, CA2, and CA1 are larger and represent the primary cells of this region, forming the stratum pyramidale (Hartley et al., 2014; Mercer et al., 2007). The CA subareas feature a layer of pyramidal cells that initiate in the hilus and curve upward away from the DG, terminating distally in the subiculum. The CA1 field, more distant from the DG and extending to the subiculum, stands out with a densely packed layer of small pyramidal cells, distinguishing it from the other two regions (Alkadhi, 2019; Amaral et al., 1991). Conversely, CA2 and CA3 areas consist of larger soma and less densely packed pyramidal cells (Lothman et al., 1991; Pang et al., 2019).
The EC serves as the site of a robust projection to DG and the hippocampus, forming the trisynaptic pathway. This pathway commences with EC neurons projecting apical dendrites into the ML of DG (RajMohan & Mohandas, 2007). Subsequently, granule cells dispatch their axons, mossy fibers, synapsing on the apical dendrites of CA3 pyramidal neurons. The axons of CA3 pyramidal neurons then form the Schaffer collaterals fibers, synapsing on the apical dendrites of CA1 pyramidal neurons (Alkadhi, 2019; Senzai, 2019).
In recent years, numerous studies have illuminated various pathologies that impact hippocampal plasticity, with a particular focus on temporal lobe epilepsy (TLE). TLE is characterized by spontaneous recurrent seizures (SRSs), which have the potential to induce permanent changes in the hippocampal circuitry, leading to hippocampal sclerosis (HS) (Cavazos et al., 2004; Leite et al., 1990; Sharma et al., 2007). These alterations encompass the neurodegeneration process (Castro et al., 2011; Melo et al., 2016), neurogenesis (Castro-Torres et al., 2019; Leibowitz et al., 2019; Parent et al., 1997a), mossy fiber sprouting (MFS) (Mello et al., 1993; Upadhya et al., 2019; Zhu et al., 2018), inflammation (Foresti et al., 2011; Vezzani et al., 2013), gliosis (Kodam et al., 2019; Morin-Brureau et al., 2018), angiogenesis (Benini et al., 2016; Feng et al., 2017; Romariz et al., 2014; Shu et al., 2016), and acquired channelopathies (McClelland et al., 2011; Simeone et al., 2013).
The hippocampus has garnered increasing interest from researchers due to its role in generating, maintaining, and spreading epileptic seizures (Castro et al., 2011; Cavalheiro et al., 1991; Melo et al., 2016; Turski et al., 1983, 1984). While it is acknowledged that the neuropathological condition compromises the hippocampal formation, many underlying mechanisms related to neuronal gain and loss remain elusive. Consequently, a deeper understanding of the hippocampal machinery affected by TLE and mechanistic insights into its pathophysiology may be crucial for exploring possible new therapeutic alternatives.
This review presents novel findings and repercussions of significant consequences caused by TLE in adult neurogenesis and cognition consequences. The exploration begins by examining how these processes impact different hippocampal areas, emphasizing changes in cellular morphology and molecular and electrophysiological properties. Furthermore, the article highlights both the positive and negative consequences of generating new brain cells in both physiological and pathological cases. Finally, the authors discuss some therapeutic approaches used to alleviate the effects of TLE.
Views on History of Epilepsy. A Necessary Contextualization
Observations regarding epilepsy have been documented since ancient times, with one of the earliest records dating back to the Mesopotamians. They attributed behaviors resembling epileptic seizures to supernatural forces (Panteliadis et al., 2017). The Greeks, in a dichotomous manner, viewed epilepsy sometimes as a sacred illness linked to the divine and genius, and at others times as a manifestation of evil spirits that invaded the body. Hippocrates, recognized as the father of medicine, challenged the notion of divine involvement, pioneering the idea that epilepsy stemmed from disorders in the brain (Magiorkinis et al., 2010).
For an extended period, various descriptions of the disease suggested a close association with insanity and wickedness. Contributing to this perception were references in Holy Scriptures, describing characteristic signs of convulsive seizures as possessions and mentions of lunatism (Budrys, 2007). Conversely, during the Middle Ages, epilepsy was considered to be possession and sorcery. The “symptoms of possession” outlined in the Malleus Maleficarum, a guide book for the Catholic Inquisition, could be related to epileptic manifestations. (Diamantis et al., 2010). Significant progress occurred during the Renaissance and modern age, as thoughts of the father of medicine were revisited, and various intellectuals proposed of new social, clinical and experimental views on epilepsy. Samuel Tissot wrote Traité de l’épilepsie which played a crucial role in expansion understanding and establishing the foundations of modern epileptology. (Kaculini et al., 2021) However, setbacks persisted, as exemplified by the case of Anneliese Michel, an epileptic patient who died in 1976 after enduring ten months of exorcism rituals by German priests (Kaculini et al., 2021).
In the 20th century United States, epileptic patients faced marginalization, ranging from marriage prohibition to suggestions of sterilization through legislative measures and eugenicist practices (Beyerstein, 1988). Advances in neuroscience during the same century, propelled by the efforts of scientists like Golgi, Cajal, Lennox, Dreifuss, and Kandel, coupled with developments in electroencephalogram electrophysiology studies, neuroimaging tests, and various treatment proposals, advances those collectively provided a robust scientific foundation for epileptology (Magiorkinis et al., 2014).
Towards the end of the 20th century, the World Health Organization, in collaboration with the International League Against Epilepsy (ILAE) and the International Bureau of Epilepsy (IBE), initiated the “Out of the Shadows Campaign” to rectify inaccurate information about epilepsy and its patients (Reis & Meinardi, 2002). Despite these efforts, lingering stigmas persist about the impact of epilepsy on the daily lives of patients. A thorough examination is required to comprehensively understand the relationship between epilepsy and cognition, along with exploring one of the most revolutionary concepts in neuroscience: adult neurogenesis.
The hippocampus, due to its high plasticity and the participation in generating, sustaining, and propagating epileptic seizures, has become a focal point for researchers. While it is acknowledged that the hippocampal formation is compromised by the neuropathological condition of TLE, many underlying mechanisms related to neuronal gain and loss remain elusive. Consequently, a deeper understanding of the hippocampal machinery affected by TLE, coupled with insights into its pathophysiology, is imperative for exploring potential therapeutic alternatives. In recent years, numerous studies have illuminated various pathologies that impact hippocampal plasticity, with a particular focus on temporal lobe epilepsy (TLE). TLE is characterized by spontaneous recurrent seizures (SRSs), which have the potential to induce lasting changes in the hippocampal circuitry, leading to hippocampal sclerosis (HS). These alterations encompass processes such as neurodegeneration, abnormal adult neurogenesis, mossy fiber sprouting (MFS), inflammation, gliosis, angiogenesis, and acquired channelopathies.
Neurogenesis, Reinterpreting the Brain
Biological tissues, in general, possess the capacity for repair facilitated by a group of diverse progenitor cell types known as stem cells. These cells play a crucial role in restoring senescent or damaged tissues. Although studies suggesting the formation of new cell types in the brains of adult mammals existed until the mid-20th century (Altman, 1962), it wasn’t until 1965 that Joseph Altman and Gopal demonstrated the origin of new neurons in the brains of adult rodents (ALTMAN & DAS, 1965). Utilizing autoradiographical evidence marked by a radiosensitizer thymidine-methyl-tritiated (an analogue of the widely used bromodeoxyuridine), they observed cell proliferation in the dentate gyrus of the hippocampus (Altman, 1969) and the olfactory bulb (Altman, 1969) of the animals.
In the late 90s, a study employing reliable neurogenesis marking techniques demonstrated that this phenomenon also occurs in the brains of adult human brains throughout their lives, in the same region observed in animal models thirty years earlier (Eriksson et al., 1998). Post-mortem tissues obtained from patients treated with 5-bromo-2’-deoxyuridine (BrdU) underwent thorough analysis, revealing a substantial increase in the number of newly generated neurons within the dentate gyrus.
The acknowledgment of adult neurogenesis marks a groundbreaking advancement in neuroscience, challenging long-held paradigms and overturning prevailing dogma of neuroscience (Andres-Barquin, 2002). For years, it was widely accepted that the central nervous system (CNS) lacked the capacity to produce new neurons beyond embryonic development, a notion initially challenged some six decades ago by Altman’s group. They presented autoradiographic evidence demonstrating cell proliferation in the hippocampus of adult rats. Significantly, nowadays adult neurogenesis isn’t limited to rodents (Kaplan & Hinds, 1977) but has been observed across various species, including birds (Nottebohm, 1989), primates (RAKIC, 1985), and even humans (Eriksson et al., 1998). This understanding has spurred intensive research endeavors aimed at comprehending adult hippocampal neurogenesis in humans (Moreno-Jiménez et al., 2019).
Neural Stem Cells
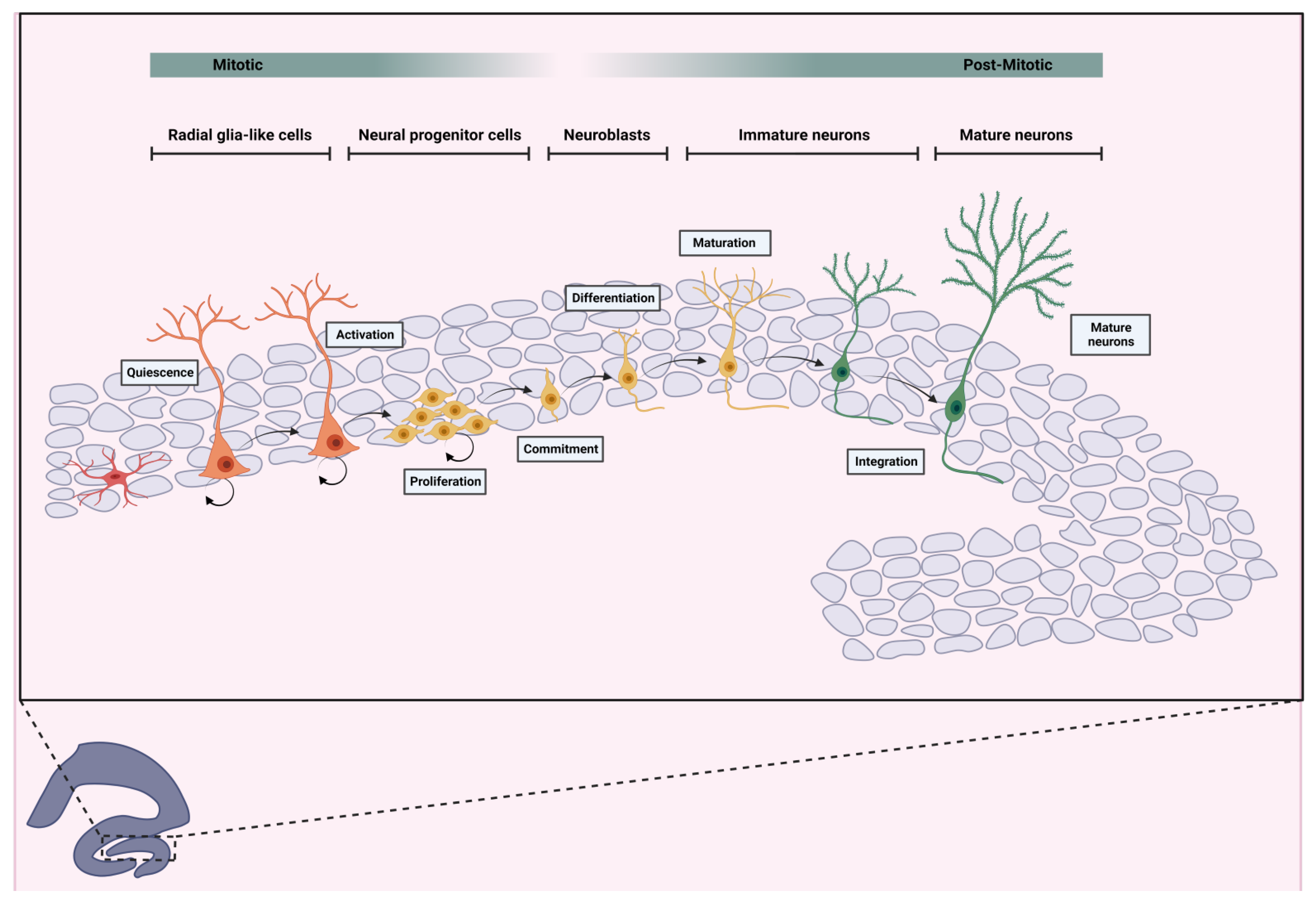
Typically, in the mammalian brain, adult neurogenesis is reported to occur primarily in two neurogenic niches: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ), a thin band between the granule cell layer and the hilus of the hippocampal DG. Both regions provide a unique microenvironment enabling adult neural stem cells (NSCs) to self-renew, proliferate, and differentiate into neurons or glia. A model for the development of these neural stem cells into mature granule neurons was proposed by Kempermann (2004) and consists of several distinct stages. The first stage involves the proliferation of stem cells, also known as Type 1 cells or Radial Glial Cells (RGC), through the process of mitosis. These cells have a characteristic morphology, with a triangular soma and a long apical dendrite that extends to the inner molecular layer. Additionally, radial cells exhibit unique electrophysiological properties and are positively marked for the markers GFAP, Nestin, Sox2, and Mash. It is believed that these cells can generate both neurons and glial cells (gliogenesis). These cells are quiescent and proliferate very little. However, during cell division, daughter cells can maintain the same characteristics (Type 1 cells, symmetric cell division) or acquire a different phenotype (asymmetric cell division), and are then designated as Type 2 cells. Type 2 cells are negative for GFAP but positive for Nestin, and have a dense and dysmorphic nucleus. These cells can be further subdivided into two subcategories: those that are negative for the immature neuron marker Doublecortin (DCX, Type 2a) and those that are positive for DCX (Type 2b), both highly proliferative.
Type 3 cells are equally positive for DCX and Ki-67 (a marker of cell proliferation expressed in the nucleus during the mitosis phases of the cell cycle, G1, S, and G2) but negative for Nestin, also exhibiting high proliferative capacity. These progenitor cells, which have a round nucleus and small cytoplasm, are suggested to exhibit advanced characteristics of developing neurons. The expression of DCX persists as cells become post-mitotic and enter the process of neuronal differentiation and maturation, known as neuroblasts. These neuroblasts exhibit transient expression of Calretinin, which later transforms into Calbindin expression, characteristic of some mature granule cells in the adult brain (Kempermann et al., 2004).
Newborn Neurons Development
From the second week of development, new neurons begin to acquire a morphology like that of granule neurons, with short dendrites that extend to the inner molecular layer and reach the end of the outer molecular layer by around the twenty-first day. During this development period, the axons of new neurons, also known as mossy fibers, begin to grow and branch through the hilus, projecting towards the CA3 region and reaching pyramidal cells in that area by around the sixteenth day after birth (Jessberger et al., 2007).
By around four weeks of age, young granule neurons show evidence of maturity, expressing characteristic markers of mature granule neurons such as Calbindin, Prox-1, and NeuN (van Praag et al., 1999a), and exhibiting specific electrophysiological properties of mature granule neurons (Deng et al., 2009; Ge et al., 2006; Overstreet-Wadiche et al., 2006). Although new granule neurons are physiologically indistinguishable from neighboring mature granule neurons after seven weeks, they continue to form new dendritic branches and spines, increasing the complexity of their connections until the fourth month (Jessberger et al., 2007).
Immature granule neurons, up to two weeks of age, initially receive GABAergic inputs, which play an excitatory role in this stage of neuronal development. After this period, new excitatory glutamatergic inputs emerge, and GABA switches to an inhibitory role. GABAergic inputs play a fundamental role in the maturation and integration of young neurons into the circuit (Ge et al., 2006).
While various studies have explored the modulation and maturation of neural progenitor cells, understanding whether pathologies alter radial neural stem cell (rNSC) migration and functionality remains unclear. Seizures appear to impact all populations of progenitor neurons in the SGZ, including radial (Type-1) cells and neuroblasts (Type-3 cells). Studies have indicated that seizures induce a massive activation of rNSCs, converting them into reactive astrocytes, ultimately depleting adult hippocampal neurogenesis (Hüttmann et al., 2003; Jessberger et al., 2005; Sierra et al., 2015; Steiner et al., 2008).
Notably, adult neurogenesis in the brain is highly sensitive to both internal and external changes. Genetic factors play a regulatory role in neurogenesis mechanisms, yet other factors such as aging, gender, stress, enriched environment, and voluntary exercise also exert critical influences (Brydges et al., 2018; Cameron & Mckay, 2001; Diederich et al., 2017; Huang et al., 2018; Hueston et al., 2017; Kempermann et al., 1997; Kuhn et al., 1996; Mirescu et al., 2004; Ohline et al., 2018; Pinar et al., 2018; Schoenfeld et al., 2017; Smith et al., 2018; Takei, 2019; Tanapat et al., 1999; Tarasova et al., 2018; van Praag et al., 1999b; Yagi & Galea, 2019; Yamada et al., 2018; Zang et al., 2017; Zarif et al., 2018). Moreover, evidence associates adult hippocampal neurogenesis with various pathologies, including Alzheimer’s disease, Parkinson’s disease, and temporal lobe epilepsy (TLE), among others (Choi et al., 2018; Höglinger et al., 2004; Lim et al., 2018; Mirochnic et al., 2009; Mishra et al., 2019; Radad et al., 2017; Rudnitskaya et al., 2019; Winner et al., 2012)
In the subsequent sections, we will delve into the repercussions of hippocampal neurogenesis and its implications in TLE, exploring its potential contributions to mossy fiber sprouting (MFS), the presence of basal dendrites, and the migration of newborn neurons to ectopic places in the DG hilus.
BrdU, a mitotic marker, is integrated into DNA during the S phase of cell replication, given its short half-life (Hancock et al., 2009). Researchers concluded that BrdU-positive neurons resulted from cell division during the marker’s administration period (Eriksson et al., 1998). These findings challenge a longstanding dogma raise questions about one of the main dogmas of neuroscience over the last few centuries: the idea that neurogenesis only occurs during the embryonic and early postnatal periods and not during adulthood. Faced with this new way of interpreting the brain, it has become important to study and understand all the new possibilities that these discoveries entail, given that the notion of the brain being able to replace populations of neurons has emerged with great enthusiasm as a new form of intervention for a series of neurodegenerative diseases.
Embryonic neurogenesis was the first to be unraveled, providing essential knowledge about the nervous system, cell diversity, and adult neurogenesis. (Gage & Temple, 2013) The prenatal process involves a complex interplay of biochemical, physical, environmental and genetic factors. (Accogli et al., 2020). Neural stem cells (NSCs) share a common function of self-renewing and proliferation with others stem cells, ultimately differentiating into neurons and glia cells in the CNS. (Gage, 2000). Neural embryogenesis occurs in the early gestation, involving the formation of the neural plate, neural tube and two crucial proliferative regions: the ventricular zone (VZ) and the subventricular zone (SVZ). (Zhang & Jiao, 2015). In this way, the process of neurogenesis progresses through several stages:
Step 1: Cell proliferation. Specific molecular signals induce neuroepithelial cell division to increase quantitatively.
Step 2: Formation of radial glial cells: Neuroepithelial cells differentiate into radial glial cells, positioning themselves in the VZ and extending radial fibers to the neural tube’s outer surface.
Step 3: Cell differentiation. Radial glial cells undergo asymmetric division to produce neuronal progenitors.
Step 4: Migration: Neurons migrate along radial glial fibers to form the cortical layers.
Step 5: Gliogenesis: Radial glial cells proliferate and produce oligodendrocytes and astrocytes. Most radial glia cells differentiate into ependymal cells after brain structure formation. Closer to birth, some cells transition into NSCs, facilitating adult neurogenesis and embryogenesis (Zhang & Jiao, 2015).
The process of adult neurogenesis is distinguishable by specific cell types and markers expressed during differentiation and maturation. Here’s a model outlining key points of the process based on cell type and biomarkers. Adult neurogenesis comprises two major stages: the mitotic stage, in which the cells are capable of self-renewal, and the post-mitotic stage, in which the formed cells do not carry out replicative processes. Like embryonic neurogenesis, adult neurogenesis comprises a number of steps divided into the mentioned two stages:
Mitotic Stage
Step 1: Quiescent multipotent NSCs in the adult hippocampus can be activated by various regulators (Urbán et al., 2019). Dentate gyrus NSCs, also known as Radial Glia-Like Cells (RGLs), are triangular cells with long radial processes (Denoth-Lippuner & Jessberger, 2021). Following activation, two pathways are possible: (1) symmetric replication for RGLs selfrenew (2) asymmetric replication generating a new self-renewing RGL and a non-radial glia-like cell (Pilz et al., 2018) Nestin and glial fibrillary acidic protein (GFAP) are prominently expressed at this stage (
Figure 1).
Step 2: Non-radial glia-like cells, or neural progenitor cells (NPCs), are transit-amplifying cells crucial for proliferation. NPCs are cells committed to differentiation, with a defined lineage and limited self-renewal capacity. They are nestin-positive but GFAP-negative (Kempermann et al., 2004). Two subtypes can be distinguished: nestin-positive/DCX-negative and nestin-positive/DCX-positive (Kronenberg et al., 2003). Asymmetric proliferation of NPCs into replicated NPCs and neuroblasts is notable at this stage (
Figure 1).
Step 3: Neuroblasts, immature cells with neuronal differentiation potential, are formed. It is important to note, like NPCs, they are transiently amplifying progenitor cells, DCX-positive and nestin-negative, although they are highly committed to differentiation into neurons (Kempermann et al., 2004) (
Figure 1).
Step 4: Neuroblasts migrate guided by intrinsic mechanisms and chemical signals, organized by astrocytes, form the subgranular layer to the granular layer of the hippocampal dentate gyrus, as evidenced by doublecortin expression (
Figure 1).
Post-Mitotic Stage
Step 5: Neuroblast differentiation leads to immature neurons capable of maturation into fully functional ones. During their maturation, these young immature neurons growth the mossy fibers (axon from granular neurons) to form synapses with interneurons at hilus and pyramidal neurons at CA3, and dendritic extensions towards the molecular layer to form connections with the entorhinal cortex (Toda et al., 2019), they are also glutamatergic neurons (Toni et al., 2008) (
Figure 1).
Step 6: Mature granule cells are incorporated into neural networks, contributing to memory and learning. NeuN and calbindin expression mark this maturity stage (Kempermann et al., 2004). Brain-derived neurotrophic factor (BDNF) plays a crucial role in neuronal survival and plasticity during this process (Huang & Reichardt, 2001). (
Figure 1).
While the discovery of neurogenesis was revolutionary for neuroscience, its therapeutic potential is limited due to the relatively small number of newly formed neurons compared to existing ones (Spalding et al., 2013). Additionally, neurogenesis decreases with age (Knoth et al., 2010), and integration of new neurons into neural circuits is costly process. Nevertheless, understanding neurogenesis function and modulation remains a topic of interest for potential supplementary therapies.
Epilepsy
Over the decades, the understanding of epilepsy has evolved significantly, leading to changes in its definitions and classifications (Tejada et al., 2013). In 2014, the International League Against Epilepsy (ILAE), established a committee to redefine epilepsy as a brain disorder characterized by a pathological and persistent tendency to generate recurrent, unprovoked, or reflex epileptic seizures (Falco-Walter et al., 2018). These seizures are defined by excessive and/or synchronous abnormal neural activity in neuronal populations in the brain and serve as one of the primary manifestations, behavioral or otherwise, for diagnosing epilepsy (Engel, 1995; Falco-Walter et al., 2018)
More specifically, the new classification of epilepsy is determined when an individual presents at least one of these possibilities:
“1) experiences at least two unprovoked or reflex seizures separated by more than 24 hours, 2) has one unprovoked or reflex seizure and a probability of experiencing another seizure similar to the general recurrence risk after two unprovoked seizures (greater than 60%) over the next 10 years, or 3) presents with an epilepsy syndrome (Fisher et al., 2014). It is crucial to identify and classify the type of seizure, type of epilepsy, and epilepsy syndrome for accurate diagnosis (Scheffer et al., 2017).
Globally, epilepsy affects approximately 50 million individuals and rank as the second neurological disorder with the greatest economic impact (Falco-Walter et al., 2018; Fiest et al., 2017). Epidemiological studies have observed a bimodal distribution in terms of age, with peaks in early childhood and around the age of 50 (Thijs et al., 2019). Although there are no significant differences based on biological sex, there is a slight male predominance (Beghi, 2020). The incidence and prevalence of epilepsy are higher in low-income countries, possibly due to socioeconomic factors and limited access to healthcare (Neligan et al., 2012; Thijs et al., 2019).
Epilepsy remains a complex condition with diverse signs and symptoms. Therefore, it’s essential to identify the comorbidities and etiological factors at each stage of diagnosis to determine the most appropriate treatment. The causes of epilepsy are multifaceted and can be genetic, structural, metabolic, infectious, immunologic and unknown. Examples include hippocampal sclerosis, pathogenic variant of SCN1A (associated with Dravet syndrome), febrile seizures in childhood, and encephalitis (Scheffer et al., 2017). Epilepsies often lead to comorbidities with physiological, cognitive, psychological and social consequences for affected individuals (Falco-Walter et al., 2018; Fiest et al., 2014; Tellez-Zenteno et al., 2007). These issues can interact synergistically, leading to significant impairment, as demonstrated by the relationship between neurogenesis and cognition, both vital for learning, memory, information integration, and other functions, potentially resulting in serious comorbidities such as cognitive decline and depression.
Temporal Lobe Epilepsy
The temporal lobe encompasses critical areas such as the hippocampus, amygdala, and adjacent regions, which are prone to seizures and referred to as “epileptogenic zones” (Bertram, 2009; Engel, 1996). This susceptibility gives rise to one of the most prevalent forms of epilepsies worldwide, known as temporal lobe epilepsy (TLE), which is categorized as a focal epilepsy (Englot et al., 2020). The initial insult, including trauma, tumors, strokes, and cortical dysplasia, typically initiate the seizure progression (Bertram, 2009).
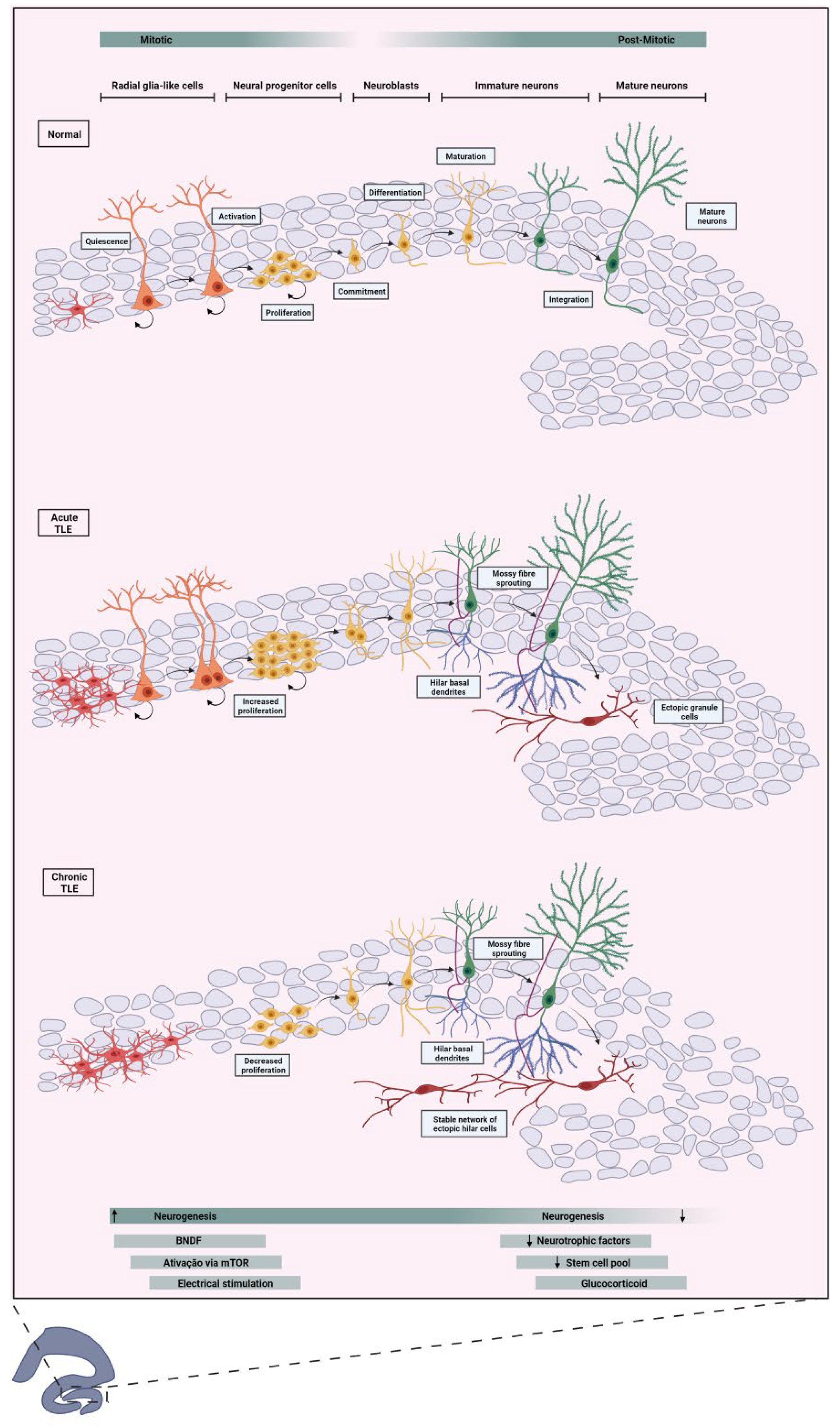
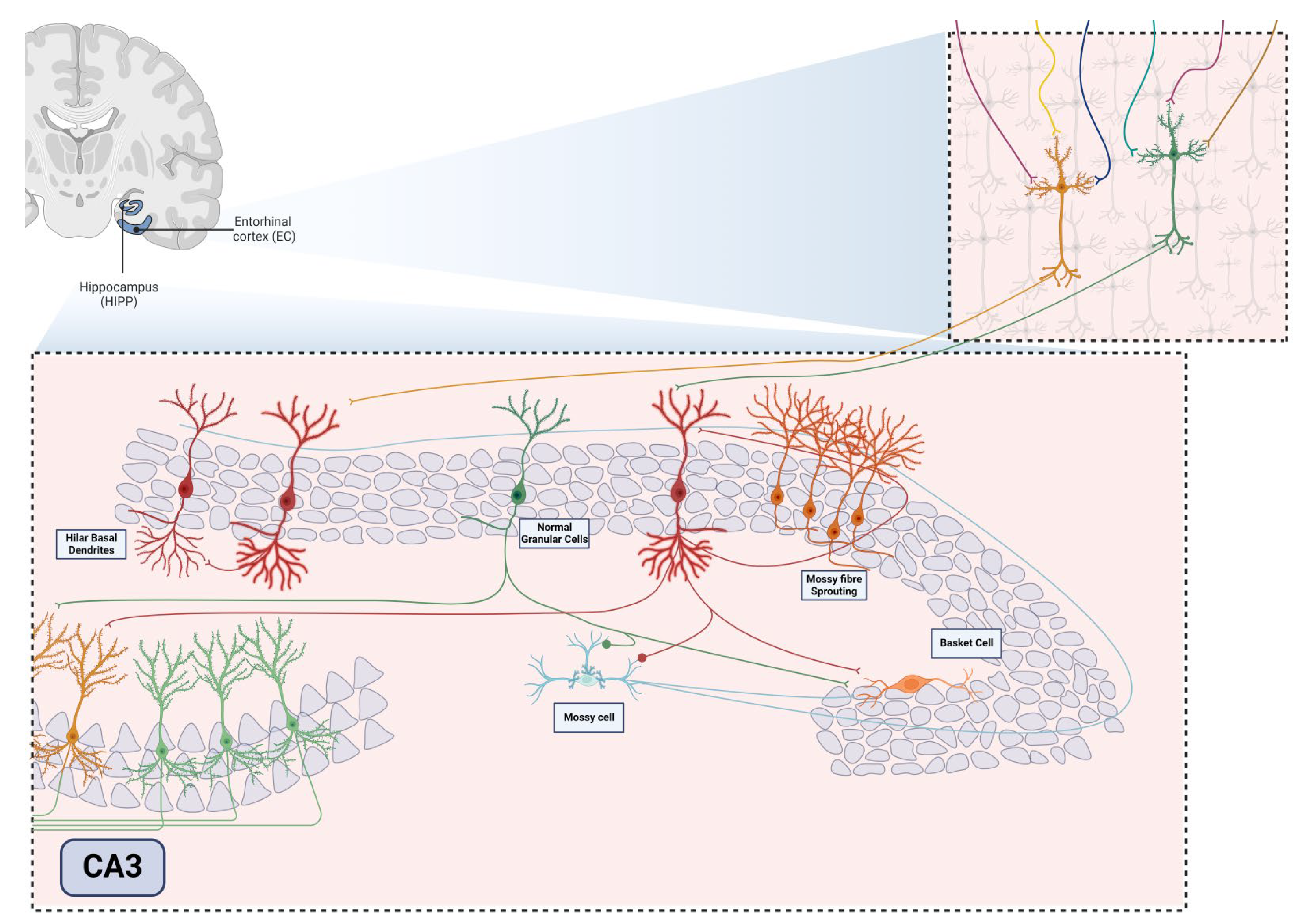
Consequently, structures within the temporal lobe undergo cytoarchitectural and molecular changes. For instance, the hippocampus exhibits atrophy degeneration, termed hippocampal sclerosis, along with aberrant mossy fiber sprouting, granular cell dispersion, gliosis, and notably, aberrant neurogenesis (see
Figure 2) (Ammothumkandy et al., 2022; Houser, 1990; Steinhäuser et al., 2012; Sutula et al., 1989). Notably, TLE has been associated with a poorer prognosis compared to other extratemporal lobe epilepsies, particularly when characterized by a singular lesion such as hippocampal sclerosis, which yields lower rates of seizure freedom (11% seizure-free) compared to other epilepsies (24% seizure-free) (Semah et al., 1998).
The temporal lobe plays integral roles in memory, learning, auditory processing, and emotion regulation (Santos et al., 2021). Consequently, individuals with TLE not only face a poorer prognosis but also experience comorbidities related to these functions, including anxiety, depression, and notably, deficits in memory, language, and learning. These cognitive impairments contribute to a decline in overall cognitive functions (Vrinda et al., 2019).
More Neurons Are Not Always a Good Sign, Analyzing Neurogenesis on Temporal Lobe Epilepsy
The occurrence of increased neurogenesis is not always indicative of a healthy brain. Natural rates of neurogenesis are subject to modulation by various external factors, such as physical exercise (van Praag et al., 1999b) and environmental enrichment (Nilsson et al., 1999), both positive regulators of neurogenesis inducing the formation of more newborn neurons. Otherwise, some processes sometimes are negative modulators, as with the use of drugs of abuse (Avchalumov & Mandyam, 2021) which induce the reduction in the number of newborn neurons. Conversely, epileptic seizures can leave a range of sequelae, one of which is sclerosis of the horn of Ammon, a common histopathologic condition observed in individuals with TLE (MD et al., 2002). However, it is not the sole structural lesion implicated in the seizure genesis.
In TLE, a notable augmentation of cell proliferation and neurogenesis typically seem into SGZ acutely after seizures or Status Epilepticus (SE). This often results in not only aberrant neurogenesis but also altered cell morphologies and issues with the migration and synaptic connections of newborn neurons within the hippocampal network (Parent et al., 1997a). Granule cells generated from aberrant neurogenesis can migrate to ectopic sites, such as and the hilus, the inner molecular of the dentate gyrus and the cell layer of CA3. (Scharfman et al., 2000). These cells are believed to contribute to pro-epileptic activity due to their increased number of excitatory inputs, which elevate hippocampal excitability (Myers et al., 2013; Pun et al., 2012; Scharfman et al., 2000).
Another common occurrence is the sprouting of mossy fibers, which creates aberrant connections between granular cells, further increasing cell excitability and leading to recurrence of seizures (Krook-Magnuson et al., 2015). Additionally, some granular cells generated in this context project an extra dendrite toward the hilus, known as the hilar basal dendrite (Ribak et al., 2000).
This aberrant neurogenesis induced by seizures appears to partially redefine the circuitry in the dentate gyrus, increasing susceptibility to seizures (Figure 2). However, the effects extend beyond seizure recurrence. Aberrant neurogenesis, with its integration errors and overexcitation of ectopic granule cells, has been shown in experimentally induced seizures to affect mechanisms of cardiovascular physiology, increasing the likelihood of sudden unexpected death in epilepsy (SUDEP) (Scorza et al., 2008).
Studies on human brains have demonstrated the aggressive impact of chronic epilepsy on reducing neurogenesis compared to non-epileptic patients. These findings align with animal model studies showing that neurogenesis increases after the seizure but decreases after some time (Parent et al., 1997a). Additionally, suppression of neurogenesis in pilocarpine-induced animal models has been shown to suppress spontaneous recurrent seizures for almost a year and potentially normalize others epilepsy-related sequelae (Cho et al., 2015).
Understanding the stages of neurogenesis most affected by epilepsy is crucial, though current limitations hinder in-depth conclusions. Research has shown that excitation, through increased extracellular potassium or depolarization with glutamate, can promote an increase in the proportion of stem/progenitor cells that become neurons (Deisseroth et al., 2004). This suggests that stem/progenitor cells might be stimulated by heightened electrical activity, such as during an epileptic seizure. Another factor potentially amplifying neurogenesis is the increase in brain-derived neurotrophic factor (BDNF) (Vezzani et al., 1999) following epileptic seizures, which has been shown to increase neurogenesis in the dentate gyrus and ectopic neurogenesis (Scharfman, 2005).
One pathway that regulates cell growth, survival, and development, the mechanistic target of rapamycin (mTOR), is intrinsically linked to epilepsy and its effects on aberrant neurogenesis. Epilepsy induces hyperactivation of the mTOR pathway (Devinsky et al., 2018), which reduces cell self-renewal and enhances lineage differentiation and expansion, leading to aberrant migration of daughter cells and severe alterations in dendrite formation and synaptic integration (LiCausi & Hartman, 2018).
Additionally, the persistence of epileptic activity can disrupt neurogenic processes due the alterations in the pool of stem/progenitor cells, excitatory neurotransmission and neuronal calcium influx.
One effect that occurs in epileptic patients is a dispersion of cells in the granular layer, related to a deficiency of reelin (Heinrich et al., 2006), a glycoprotein that regulates neuronal migration. However, another study has shown that loss of reelin expression may promote aberrant neurogenic migration in epilepsy (Gong et al., 2007). The formation of hilar basal dendrites may be related to changes in the glial scaffold or reduced expression of neurotrophic factors (Jessberger & Parent, 2015).
Morphological abnormalities in neurons born from epilepsy-induced neurogenesis led to ectopia and faulty connections, but this integration, although abnormal, is still stable (Jessberger et al., 2007). This supports the view that aberrant neurogenesis significantly alters neural circuits in the dentate gyrus, with cognitive manifestations becoming increasingly apparent as the disease progresses.
Cognition and Cognitive Impairment
Cognition encompasses the full range of conscious and unconscious processes used to acquire and retain knowledge, and to evaluate and process sensory experiences. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines six cognitive domains: (1) Complex attention, which involves to the practice and maintenance of sustained, divided, and selective attention; (2) Executive function, which includes planning, decision making, working memory, and mental flexibility; (3) Learning and memory, which covers the formation of immediate, recent, and long-term memory; (4) Language, which includes expressive and receptive language skills; (5) Perceptual-motor, which includes visual perception, and (6) Verbal-motor, which includes verbal and nonverbal language skills: (5) Perceptual Motor, which includes visual perception skills, visuoconstructive abilities, perceptual motor integration, and gnosia; (6) Social Cognition, which pertains to the ability to recognize emotions and consider the mental state of others (APA, 2014).
Cognitive impairment, while not a disease itself, describes a group of neurocognitive disorders that cause mild to severe disability. These disorders are categorized into three broad groups: delirium, mild cognitive impairment, and severe neurocognitive impairment (Sachdev et al., 2014). Over time, or with the development of comorbid conditions, these impairments can become increasingly disruptive to daily activities, making it progressively harder for individuals to perform routine tasks independently.
Symptoms and Signs of TLE
Epilepsy can cause a few comorbidities that are often more complex than the disease itself due to the morphologic and functional changes induced by epileptic seizures. While scientific advances have increased the life expectancy of patients with epilepsy, other challenges have come to the forefront because of the significant impact on quality of life. Studies have shown that patients with temporal lobe epilepsy can experience progressive cognitive impairment as they age [
80]. Approximately 70% of epileptic patients experience cognitive impairment, and nearly 60% suffer from neuropsychological dysfunction related to mood (Helmstaedter & Witt, 2017).
Memory is one of the essential cognitive functions for work and social living, and it is particularly affected in TLE (Helmstaedter, 2002). Data indicate that the persistence of epilepsy can permanently worsen memory, with a cumulative degradation of this capacity (Landi et al., 2019). The functions performed by the temporal lobe, which are related to memory, learning, and emotional regulation, are primarily impaired in TLE, often affecting the hippocampus (Vrinda et al., 2019). For example, verbal memory impairment, indicative of damage to the temporal lobe, is highly disabling (Blume, 2006), as it directly impacts basic communication abilities.
The DSM-5 highlights several signs and symptoms of cognitive impairment, including disturbances in attention and consciousness, memory deficits, disorientation, language and perception problems, substantial limitations in learning cognitive performance, preferably documented by a standardized neuropsychological test (APA, 2014). The presence of one or more of these signs and symptoms, combined with diagnoses of other neuropsychiatric conditions such as depressive and anxiety disorders, reflects the sequelae caused by chronic epilepsy (Sachdev et al., 2014). Cognitive impairments in TLE patients can be categorized into three profiles: minimally impaired, memory impairment, and those with impairments in memory, executive functions and processing speed (Sen et al., 2018) This complexity underscores the challenges in diagnosing and classifying cognitive comorbidities in TLE patients.
Diagnosing cognitive decline TLE is complex because deficits depend on the type and location of the underlying neuropathology and are therefore heterogeneous (Vrinda et al., 2019). Diagnosis typically involves quantitative imaging tools such as magnetic resonance imaging (MRI) with voxel-based morphometry and diffusion tensor techniques, which allow more sensitive identification of the brain regions affected in TLE. Characteristics analyzed include the volume of specific brain areas, gyrification indices, cortical thickness, damaged surface area, and white matter integrity (Bell et al., 2011). Clinical assessment, including tests like the Montreal Cognitive Assessment complement the diagnosis by correlating behaviors with visible brain lesions.
Atrophy in the prefrontal cortex is usually associated with poor executive function (Keller et al., 2009). Damage to both hemispheres can lead to problems with language, visual and verbal memory, and the presence of hallucinations, delusions, or gross behavioral abnormalities post-seizures (Vrinda et al., 2019). A study by Marques et al., (2007) found a correlation between cognitive decline, seizure duration, and the presence of hippocampal sclerosis, demonstrating that changes in this area can lead to cognitive consequences and, therefore, a poor quality of life for individual with TLE (Marques et al., 2007).
Cognitive decline in animal models of ELT is analyzed using behavioral tests such as the Morris water maze, radial arm maze, and place recognition test (Cho et al., 2015; Vrinda et al., 2019). Histological analyses showing hippocampal sclerosis, cortical atrophy or aberrant neurogenesis confirms cognitive decline in these models, and new technologies are being explore to reduce this impairment (Cho et al., 2015; Lévesque & Avoli, 2013).
It is worth noting that broad-spectrum antiepileptic drugs, such as valproate and phenobarbital, can significantly reduce hippocampal cells and other limbic structures, potentially exacerbate the cognitive decline in TLE (Voorhies, 1988). Therefore, there is a need to find new effective treatments for seizures that reduce cognitive decline.
Epilepsy, Cognitive Decline and Adult Neurogenesis Relation
Adult hippocampal neurogenesis is subject to a variety of modifications influenced by intrinsic and extrinsic factors such as genetic factors, aging, enriched environment, stress and pathological conditions, in particular ELT (Santos et al., 2021). Because of these influences, this highly plastic region is capable of generating neurons with atypical characteristics, a phenomenon known as aberrant neurogenesis. These changes include increased proliferation of neural precursors, production of ectopic granule cells (hEGCs), mossy fiber sprouting (MFS), and persistence of hilar basal dendrites in adult granule neurons (
Figure 2) (Cameron & Mckay, 2001; Cho et al., 2015; Pun et al., 2012; Scharfman, 2002).
These changes, which occur at the biochemical, physical, and behavioral levels, can lead to memory deficits, learning difficulties, and, consequently, cognitive impairment (Cho et al., 2015; Parent et al., 1997a). This complexity of neurobiological changes highlights the importance of understanding the interactions between aberrant neurogenesis and conditions such as ELT in order to develop more effective strategies for treating associated cognitive impairments (
Figure 2).
In ectopic, granule cells generated by aberrant neurogenesis have electrophysiological properties similar to those of cells generated under normal conditions. However, these cells have highly synchronous behavior with CA3 pyramidal cells due to their aberrant integration (Jessberger & Parent, 2015). This abnormal integration may be the cause of the subsequent information processing errors that together produce the cognitive deviations mentioned above (
Figure 2).
Furthermore, inhibition of hippocampal neurogenesis by genetic suppression or pharmacological approaches normalizes the cognitive decline associated with epilepsy, suggesting that targeting abnormal neurons generated by aberrant neurogenesis could modulate hippocampal memory dysfunction in pathological conditions (Cho et al., 2015).
Suppressing neurogenesis, however, could reduce the long-term neuroplasticity of the brain and affect the individual in a similar way. In this sense, Jessberger and Parent suggested that aberrant neurogenesis could be an attempt by the damaged brain to repair itself (Jessberger & Parent, 2015) as epileptic seizures have remarkable consequences on neural tissue (Scharfman, 2007). It has also been observed that modulation of proliferation and migration pathways in the postnatal brain can control the recruitment of neuroblasts (Bressan & Saghatelyan, 2021). Some studies have already presented interesting data on how the variation of factors in these mechanisms modulates the behavior of cells. Changes in the levels of the polysialylated form of the neural cell adhesion molecule (PSA-NCAM) have the potential to modulate the presence of ectopic granule cells (Pekcec et al., 2007). In addition, PSA-NCAM is known to be strongly associated with neuronal plasticity (Wainwright & Galea, 2013). Another study showed that reelin, a protein that plays an important role in cell movement as discussed above, is present at lower levels after seizures, and this effect is accompanied by a greater appearance of ectopic granular cells from aberrant neurogenesis (Gong et al., 2007).
Overall, gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the developed brain, but in newborn neurons it plays a depolarizing and then hyperpolarizing role. The function of GABA depends on the intracellular chloride concentration, which determines the electrochemical gradient of the membrane. It has already been shown that this neurotransmitter is essential as a regulator of the transit of new neurons and that the premature hyperpolarization of newborn neurons is responsible for altering the normal development of the cell (Faigle & Song, 2013). In light of these studies and many others that can be performed, we propose that the search for a better understanding of the pathways involved in this process seems necessary to provide new therapeutic targets to modulate neurogenesis in order to reduce the deviations caused by the natural course of the disease.
In addition, the sprouting of mossy fibers is another feature of aberrant neurogenesis that may exacerbate cellular changes and congenital consequences (
Figure 2) (Scharfman et al., 2003). Under normal conditions, mossy fibers are axons of the granule cells of the DG, which, with their collateral branches, can innervate cells of the hilus, such as mossy cells and/or interneurons (Amaral et al., 2007). These cells then send their fibers to the basket cells, which in turn inhibit the granule cells (Amaral et al., 2007). In this way, the granule cells regulate their own excitability.
However, pathologies such as TLE have been associated with MFS. It is still unclear whether MFS arises from the excitability of the DG or whether MFS initiates the generation of seizures (Santos et al., 2021). However, it is already known that MFS involves sending fibers to the inner molecular layer of the DG (Parent et al., 1997a). Therefore, studies have shown that MFS generates strong recurrent excitatory feedback loops and drives seizure generation (Pun et al., 2012; Scharfman et al., 2003). Therefore, studies have shown that MFS generates strong recurrent excitatory feedback loops and drives seizure generation (Althaus et al., 2016; Kron et al., 2010).
Another structure associated with aberrant neurogenesis is the hilar basal dendrites (Santos et al., 2021), which are transient under normal conditions as they decrease in maturing neurons (Seress & Pokorny, 1981), but increase dramatically in ELT and remain during neuronal maturation (Franck et al., 1995). In addition, these dendrites receive axons from the mossy fibers of other granule cells, allowing the formation of stable exitory networks that can lead to seizures (Ribak et al., 2000). Thus, abnormal connection formation may be related to cognitive decline, but more studies are needed to prove that reducing MFS and HBDs benefits memory and learning (
Figure 2). It is worth noting, however, that when adult neurogenesis was ablated before inducing seizures in animals, there was no reduction in MFS, so it may not be directly related to cognition since the animals recovered their memory (Cho et al., 2015).
Environmental Enrichment
The idea that an enriched environment (EE) might imply some correlation with cognitive function was observed by Hebb in 1947 (Khalife et al., 2022). In EE, there is exposure to complex and stimulating environments that are also satisfactory for simpler physiological conditions such as food, hydration, and sleep.
Hebb’s studies were the first to establish a link between the environment and neuroplasticity. Another study showed that changes in the amount of pre- and post-synaptic proteins, such as synaptophysin and PSD-95, in animals exposed to an enriched environment are indicative of synaptic plasticity resulting from this event (Nithianantharajah, 2004), suggesting that EE is capable of stimulating the formation of functional synapses.
In addition, environmental enrichment is an important factor in the positive regulation of hippocampal neurogenesis, which promotes significant improvements in memory and learning (Denoth-Lippuner & Jessberger, 2021).
Several beneficial effects of EE have been observed in animal models of epilepsy, ranging from a reduction in seizure frequency to relief of depressive and hyperactivity symptoms (Khalife et al., 2022). Another interesting finding showed that EE was able to attenuate memory and learning problems after seizures in animal models, also demonstrating increased neurogenesis in the hippocampus (Gorantla et al., 2019).
In addition, several other studies have shown how EE has proven to be an ally in the treatment of epilepsy-associated neurodisorders (Vrinda et al., 2019).
Furthermore, EE therapy has been observed to be regulated by brain-derived neurotrophic factor (BDNF) and extracellular signal-regulated kinase (ERK) (Akyuz & Eroglu, 2021).
In this sense, environmental enrichment takes a prominent role as a possible modulator of neurogenesis and neural plasticity in the search for alternatives to treat cognitive decline in TLE patients. These studies will have to investigate not only the cognitive profile established, but also the configuration of the integration of these new neurons into the brain circuitry. However, it seems promising to consider the association of environmental enrichment with antiepileptic drugs in the treatment of epilepsy and its associated comorbidities.
Phytocannabinoids
Cannabidiol has been used in studies with seizure models since the 1970s, showing effective results against seizures (Izquierdo et al., 1973; Turkanis et al., 1979), and has been confirmed in more recent studies with other animal models and doses (Jones et al., 2012; Leo et al., 2016). n vitro studies have shown a neuroprotective effect of CBD, suggesting that it is a powerful antioxidant and a good treatment (Hampson et al., 1998). Human studies have generally shown positive results for seizure control (Cunha et al., 1980; Devinsky et al., 2016; Thiele et al., 2018).
In addition, when associated with neurogenesis, chronic use has been shown to increase the number of cells positive for Ki-67, BrdU, and DCX, and to improve anxious and depressive behaviors (Campos et al., 2013; Schiavon et al., 2016). The mechanism by which this happens involves the endocannabinoid system, because when CBD and CB1 and CB2 antagonists were added to hippocampal progenitor cell cultures, cell proliferation did not occur, unlike what happens when CBD alone is added (Campos et al., 2013). In addition, this phytocannabinoid increases BNDF, which may promote neurogenesis (Gaston et al., 2021). Animal models of ELT that received CBD showed improvement in reference and working memory tests (Patra et al., 2019). This highlights the potential of CBD to improve cognitive decline associated with TLE by promoting hippocampal neurogenesis and influencing the mechanisms underlying this phenomenon. In addition, its efficacy in reducing seizures stands out, consolidating it as a viable therapy for this specific condition.
Abnormal Trinity of Newborn Neurons of Dentate Gyrus Could Drive DG Pattern Separation into Patter Completion? Abnormal Newborn Neurons Drive DG Pattern Separation to Completion?
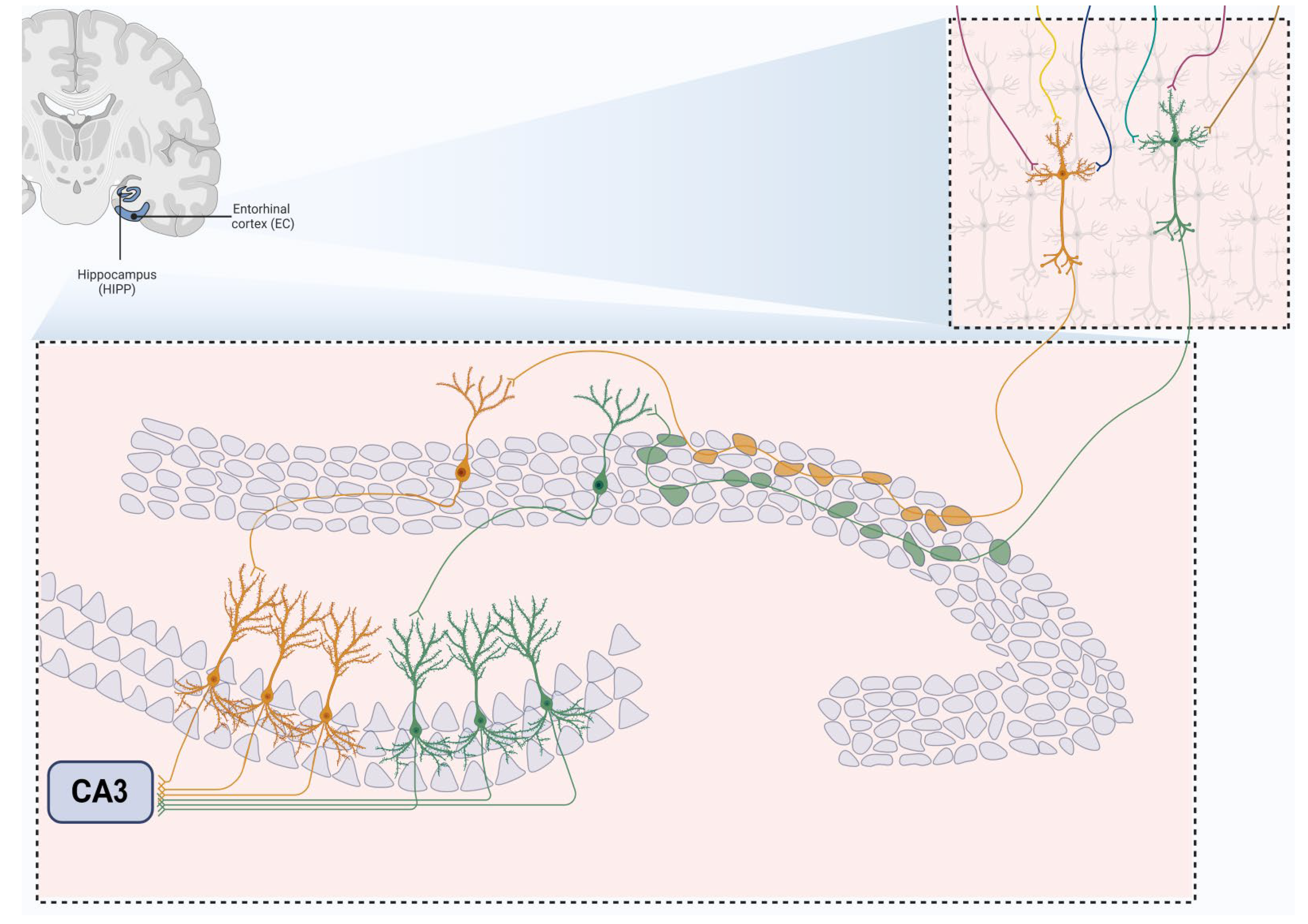
The hippocampus plays a central role in memory processing, particularly through its CA3 and DG regions. The CA3 region is crucial for both the storage and recall of memories. This is due to its extensive network of recurrent connections among pyramidal cells, which allows for the encoding of complex memory patterns. According to computational models, these recurrent connections help form “cell assemblies,” a concept originally proposed by Hebb. This mechanism enables the CA3 to store input patterns through modifiable synapses, which become stronger as patterns are repeated. One of the key players in this process is the mossy fibers, which originate in the DG and make strong, excitatory connections with CA3 pyramidal cells. These connections, known as “teaching inputs,” are thought to be instrumental in initiating the long-term changes needed for memory storage. Empirical studies support this idea, showing that a single mossy fiber can have enough influence to activate CA3 cells and strengthen synaptic connections. Once a memory pattern is stored, the CA3 region has the ability to complete or reconstruct this pattern even if only a partial or distorted version is presented. This ability, known as “pattern completion,” is essential for recognizing familiar stimuli and recalling memories accurately (
Figure 3).
The DG, on the other hand, is involved in a different but complementary process called “pattern separation.” This process alters incoming information to ensure that it is represented in a way that reduces overlap with other patterns (
Figure 3). By making memory representations sparser and more distinct, the DG helps prevent confusion between similar stimuli and supports more precise memory encoding and retrieval in CA3. Research continues to validate these theoretical concepts, showing that tasks requiring differentiation between similar stimuli engage both the DG and CA3. This reinforces the idea that the DG’s role in pattern separation and the CA3’s role in pattern completion is critical for effective memory processing.
While a few hEGCs are present in healthy rodents, their numbers increase significantly in diseases such as temporal lobe epilepsy. For example, in TLE, the number of hEGCs can rise up to tenfold compared to normal levels. The increase in hEGCs is linked to the response of the hippocampus to injury or seizures. After an initial insult or status epilepticus—a prolonged seizure event—there is a surge in the proliferation of progenitor cells. These progenitors can differentiate into various cell types, including GCs. This abnormal proliferation is driven by factors such as upregulated genes and excitotoxic damage to neurons.
Overall, the presence of hEGCs in the hilus represents a significant alteration in the hippocampal architecture, associated with various pathological conditions. These ectopic cells contribute to the abnormal neural activity observed in diseases such as TLE, impacting the hippocampus’s function and potentially influencing disease progression. In computational models investigating the role of hEGCs in the hippocampus, researchers observed that these ectopic cells have a dominant influence over the entire granule cell (GC) population. In the Standard model, which includes a typical distribution of mature GCs, only a small percentage (around 2%) of these cells fire in response to input from the perforant pathway. This model provides a baseline for normal GC activity.
The dominance of hEGCs results in reduced variability in the GC output to CA3. Most entorhinal cortex input patterns activate only the small subset of dominant hEGCs, impairing the hippocampus’s ability to perform pattern completion effectively. This is because the excessive dominance of hEGCs narrows the range of GC output, affecting the diversity of input patterns processed by CA3 (
Figure 4). In contrast, when immature GCs are placed in the GC layer (as in the Intermediate model), they exhibit firing rates comparable to mature GCs and do not significantly affect the activity of mature GCs. This is due to the inhibitory control present in the GC layer, which prevents the immature GCs from becoming as dominant as the hEGCs in the hilus (
Figure 5). The findings suggest that normal DG–CA3 function relies on a balanced activity across the GC population. When a subset of GCs, like hEGCs, becomes overly dominant, it disrupts hippocampal function. This dominance can lead to repetitive activation of the same CA3 pyramidal cells, potentially strengthening these connections over time and impairing the network’s ability to perform pattern separation and completion. The ‘dominant’ GC hypothesis posits that such dominance results in a skewed activation of both GCs and CA3 pyramidal cells, ultimately diminishing the hippocampus’s ability to process and recall complex patterns, impairing memory process like pattern separation and driving the dentate gyrus produce more completion (
Figure 5) The ‘dominant’ GC hypothesis indicates that such dominance skews hippocampal activation and impairs memory processing.
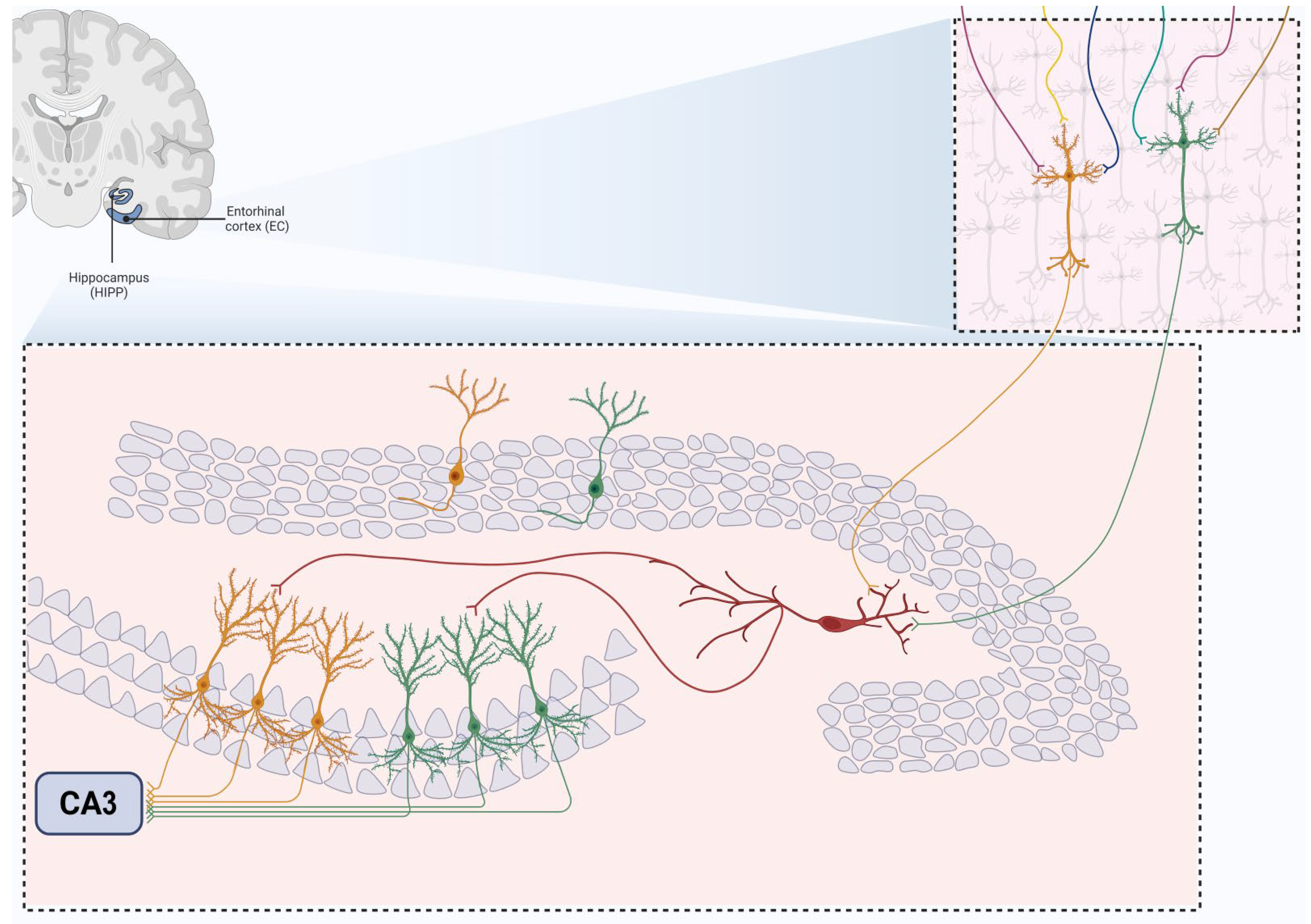
In the hilus, mossy fibers produce numerous collaterals that innervate excitatory mossy cells (MCs) and inhibitory interneurons. MC axons then extend to inhibitory basket cells, which, in turn, inhibit granule cells. Over the years, studies have highlighted the complex relationship between TLE and abnormal MFS in the hippocampus. In TLE, mossy fibers can sprout into the dentate inner molecular layer (ML) of DG, forming recurrent excitatory synapses with granule cells. This abnormal sprouting might result from TLE-induced damage (
Figure 6). While some studies emphasize the excitatory role of aberrant MFS in TLE, others suggest inhibitory effects, leading to ongoing debate about whether MFS has “epileptogenic” or “restorative” consequences. In the early 1990s, the “mossy fiber sprouting hypothesis” emerged, closely related to the “dormant basket cell hypothesis.” The death of MCs in the hilus leads to pathological rearrangement of granule cells, which become more excitable and activate recurrent excitatory circuits. Similarly, kainate-induced MFS has been shown to increase DG excitability through abnormal excitatory inputs to granule cells. In contrast, studies involving direct stimulation of the commissural and trisynaptic pathways indicate that commissural projections can innervate inhibitory MCs, providing negative feedback to granule cells through an alternative route. The relationship between MFS and neurogenesis is also complex. While some studies find a positive correlation between MFS and neurogenesis, others do not. Ongoing postnatal neurogenesis continually reshapes hippocampal circuitry, contributing to seizure-related plasticity. After seizures, increased numbers of newborn granule cells may alter local circuitry and promote MFS. Notably, MFS does not appear until newborn neurons mature, typically around four weeks post-seizure. In the rat seizure model induced by pilocarpine (PILO TLE), both neonatal and adult-born granule cells contribute to aberrant axonal reorganization, though they may not have the same impact on abnormal network formation and hyperexcitability. For example, research has shown that while sprouting correlates with seizure frequency, it does not always establish functionally active connections capable of driving recurrent excitation. Ablating hippocampal neurogenesis reduces seizure frequency but does not eliminate MFS.
Overall, MFS plays a critical role in seizure-related plasticity. However, the relationship between MFS and adult neurogenesis remains unclear, highlighting the need for further research to better understand the effects of MFS on epilepsy.
Human and nonhuman primates typically have basal dendrites in about 15% of GCs in the adult DG, which is considered normal. However, TLE significantly increases the number of basal dendrites. In rodent GCs, basal dendrites are transient during development, appearing around postnatal days 5 and 6 and disappearing in mature GCs. Interestingly, newly generated GCs, identified using DCX, a marker for neuroblasts, often display recurrent basal dendrites. This supports the notion that basal dendrites are a temporary feature of newly born GCs in adult rats, similar to their developmental stage. Epileptiform activity can alter the morphology of GCs, impacting the entire DG circuitry. After TLE induced by perforant pathway stimulation, basal dendrites extending into the hilus were observed in Golgi-impregnated GCs. These hilar basal dendrites are postsynaptic targets of mossy fiber collaterals and contribute to recurrent excitatory circuits and neuroplastic changes associated with TLE (
Figure 6). Similarly, adult-generated GCs with HBDs are integrated into the pathological recurrent circuitry of the epileptic brain due to increased mossy fiber input in their apical dendrites. Whole-cell patch recordings have shown increased recurrent excitation in GCs with basal dendrites in hippocampal slices from monkeys. Various classical epilepsy models, such as pilocarpine or kainic acid-induced status epilepticus (SE), have demonstrated the role of HBDs in consolidating recurrent excitatory circuits.
HBDs exhibit a time-dependent pattern in the pathological circuitry of the epileptic brain. For example, one study found that HBDs appear one week after PILO-induced SE. Another detailed study on time-dependent changes in adult-generated GCs after PILO-induced epileptogenesis highlighted the presence of abnormal apical dendritic morphology and basal dendrites in both immature and newborn GCs during SE. Notably, aberrant HBDs were more prevalent in newborn GCs than in immature ones, with only 9% of mature GCs displaying HBDs. However, other studies have shown that neurons generated five days after PILO injection were morphologically indistinguishable from control neurons. However, this did not prevent cognitive deficits.
Taken together, these abnormalities — basal dendrites, mossy fiber sprouting, and aberrant neurogenesis — appear to drastically alter the circuitry and functionality of the DG. We propose the hypothesis that these changes may shift the DG’s role from pattern separation to pattern completion. This shift would likely lead to an increase in excitatory collaterals among granule neurons, raising the basal activity of the DG and consequently elevating the firing rate from its typical 5% to a higher level (
Figure 6). This heightened DG activity could, in turn, amplify activity in the CA3 region, further disrupting the balance between excitation and inhibition in the hippocampus.
Conclusions and Future Perspectives
The discovery of adult neurogenesis in the 20th century allowed a reinterpretation of the brain and the distortion of one of the oldest and most widely accepted premises of neuroscience. If, on the one hand, the new knowledge offered the possibility of using mechanisms contained in the own organ to try to correct disorders, the same resource is subject to failures and imbalances that can lead to various other disorders.
It has been widely documented that impaired cognitive function is one of the most common conditions in patients with epilepsy (Blume, 2006; Elger et al., 2004; Holmes, 2015; Motamedi & Meador, 2003) and has a progressive potential, even when seizures are under control (Blume, 2006). It has been observed that neurogenesis in this scenario does not occur as expected, manifesting aberrant proliferation and integration that contribute to recurrent excitatory circuits (Parent et al., 1997b), and its suppression can bring animal models of ELT back to behavioral normality. However, most of the mechanisms underlying this condition remain unknown.
However, the overwhelming majority of the mechanisms that underlie this condition are still unknown. Although the multifactorial aspect of aberrant neurogenesis in ELT is a considerable challenge, a better understanding of the cellular mechanisms underlying the integration of aberrant neurons, as well as chemoattractant and chemorepulsive indicators, may help to propose new ways to modulate neural plasticity, the migration of these cells, and the composition of associated therapies. This could lead to a more effective approach to reduce and control the damage resulting from epilepsy, instilling a sense of reassurance and confidence in the future of epilepsy treatment.
References
- AbuHasan, Q., Reddy, V., & Siddiqui, W. (2024). Neuroanatomy, Amygdala.
- Accogli, A., Addour-Boudrahem, N., & Srour, M. (2020). Neurogenesis, neuronal migration, and axon guidance (pp. 25–42). [CrossRef]
- Akyuz, E., & Eroglu, E. (2021). Envisioning the crosstalk between environmental enrichment and epilepsy: A novel perspective. Epilepsy & Behavior, 115, 107660. [CrossRef]
- Alkadhi, K. A. (2019). Cellular and Molecular Differences Between Area CA1 and the Dentate Gyrus of the Hippocampus. Molecular Neurobiology, 56(9), 6566–6580. [CrossRef]
- Althaus, A. L., Zhang, H., & Parent, J. M. (2016). Axonal plasticity of age-defined dentate granule cells in a rat model of mesial temporal lobe epilepsy. Neurobiology of Disease, 86, 187–196. [CrossRef]
- Altman, J. (1962). Are New Neurons Formed in the Brains of Adult Mammals? Science, 135(3509), 1127–1128. [CrossRef]
- Altman, J. (1969). Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. Journal of Comparative Neurology, 137(4), 433–457. [CrossRef]
- ALTMAN, J., & DAS, G. D. (1965). Post-Natal Origin of Microneurones in the Rat Brain. Nature, 207(5000), 953–956. [CrossRef]
- Amaral, D. G. (1978). A golgi study of cell types in the hilar region of the hippocampus in the rat. Journal of Comparative Neurology, 182(5), 851–914. [CrossRef]
- Amaral, D. G., Dolorfo, C., & Alvarez-Royo, P. (1991). Organization of CA1 projections to the subiculum: A PHA-L analysis in the rat. Hippocampus, 1(4), 415–435. [CrossRef]
- Amaral, D. G., Scharfman, H. E., & Lavenex, P. (2007). The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) (pp. 3–790). [CrossRef]
- Ammothumkandy, A., Ravina, K., Wolseley, V., Tartt, A. N., Yu, P.-N., Corona, L., Zhang, N., Nune, G., Kalayjian, L., Mann, J. J., Rosoklija, G. B., Arango, V., Dwork, A. J., Lee, B., Smith, J. A. D., Song, D., Berger, T. W., Heck, C., Chow, R. H., … Bonaguidi, M. A. (2022). Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nature Neuroscience, 25(4), 493–503. [CrossRef]
- Andres-Barquin, P. J. (2002). Santiago Ramón y Cajal and the Spanish school of neurology. The Lancet Neurology, 1(7), 445–452. [CrossRef]
- APA, A. P. A.-. (2014). Manual diagnóstico e estatístico de transtornos mentais: DSM-5 (Artmed, Ed.; 5th ed., Vol. 5).
- Avchalumov, Y., & Mandyam, C. D. (2021). Plasticity in the Hippocampus, Neurogenesis and Drugs of Abuse. Brain Sciences, 11(3), 404. [CrossRef]
- Beghi, E. (2020). The Epidemiology of Epilepsy. Neuroepidemiology, 54(2), 185–191. [CrossRef]
- Bell, B., Lin, J. J., Seidenberg, M., & Hermann, B. (2011). The neurobiology of cognitive disorders in temporal lobe epilepsy. Nature Reviews Neurology, 7(3), 154–164. [CrossRef]
- Benini, R., Roth, R., Khoja, Z., Avoli, M., & Wintermark, P. (2016). Does angiogenesis play a role in the establishment of mesial temporal lobe epilepsy? International Journal of Developmental Neuroscience, 49(1), 31–36. [CrossRef]
- Bertram, E. H. (2009). Temporal lobe epilepsy: Where do the seizures really begin? Epilepsy & Behavior, 14(1), 32–37. [CrossRef]
- Beyerstein, B. L. (1988). Neuropathology and the Legacy of Spiritual Possession. 12, 248–262.
- Blume, W. T. (2006). The Progression of Epilepsy. Epilepsia, 47(s1), 71–78. [CrossRef]
- Bressan, C., & Saghatelyan, A. (2021). Intrinsic Mechanisms Regulating Neuronal Migration in the Postnatal Brain. Frontiers in Cellular Neuroscience, 14. [CrossRef]
- Brydges, N. M., Moon, A., Rule, L., Watkin, H., Thomas, K. L., & Hall, J. (2018). Sex specific effects of pre-pubertal stress on hippocampal neurogenesis and behaviour. Translational Psychiatry, 8(1), 271. [CrossRef]
- Budrys, V. (2007). Neurology in Holy Scripture. European Journal of Neurology, 14(7). [CrossRef]
- Cameron, H. A., & Mckay, R. D. G. (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology, 435(4), 406–417. [CrossRef]
- Campos, A. C., Ortega, Z., Palazuelos, J., Fogaça, M. V, Aguiar, D. C., Díaz-Alonso, J., Ortega-Gutiérrez, S., Vázquez-Villa, H., Moreira, F. A., Guzmán, M., Galve-Roperh, I., & Guimarães, F. S. (2013). The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. International Journal of Neuropsychopharmacology, 16(6), 1407–1419. [CrossRef]
- Castro, O. W., Furtado, M. A., Tilelli, C. Q., Fernandes, A., Pajolla, G. P., & Garcia-Cairasco, N. (2011). Comparative neuroanatomical and temporal characterization of FluoroJade-positive neurodegeneration after status epilepticus induced by systemic and intrahippocampal pilocarpine in Wistar rats. Brain Research, 1374, 43–55. [CrossRef]
- Castro-Torres, R. D., Landa, J., Rabaza, M., Busquets, O., Olloquequi, J., Ettcheto, M., Beas-Zarate, C., Folch, J., Camins, A., Auladell, C., & Verdaguer, E. (2019). JNK Isoforms Are Involved in the Control of Adult Hippocampal Neurogenesis in Mice, Both in Physiological Conditions and in an Experimental Model of Temporal Lobe Epilepsy. Molecular Neurobiology, 56(8), 5856–5865. [CrossRef]
- Cavalheiro, E. A., Leite, J. P., Bortolotto, Z. A., Turski, W. A., Ikonomidou, C., & Turski, L. (1991). Long-Term Effects of Pilocarpine in Rats: Structural Damage of the Brain Triggers Kindling and Spontaneous I Recurrent Seizures. Epilepsia, 32(6), 778–782. [CrossRef]
- Cavazos, J. E., Jones, S. M., & Cross, D. J. (2004). Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy. Neuroscience, 126(3), 677–688. [CrossRef]
- Cho, K.-O., Lybrand, Z. R., Ito, N., Brulet, R., Tafacory, F., Zhang, L., Good, L., Ure, K., Kernie, S. G., Birnbaum, S. G., Scharfman, H. E., Eisch, A. J., & Hsieh, J. (2015). Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nature Communications, 6(1), 6606. [CrossRef]
- Choi, S. H., Bylykbashi, E., Chatila, Z. K., Lee, S. W., Pulli, B., Clemenson, G. D., Kim, E., Rompala, A., Oram, M. K., Asselin, C., Aronson, J., Zhang, C., Miller, S. J., Lesinski, A., Chen, J. W., Kim, D. Y., van Praag, H., Spiegelman, B. M., Gage, F. H., & Tanzi, R. E. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science, 361(6406). [CrossRef]
- Cunha, J. M., Carlini, E. A., Pereira, A. E., Ramos, O. L., Pimentel, C., Gagliardi, R., Sanvito, W. L., Lander, N., & Mechoulam, R. (1980). Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology, 21(3), 175–185. [CrossRef]
- Deisseroth, K., Singla, S., Toda, H., Monje, M., Palmer, T. D., & Malenka, R. C. (2004). Excitation-Neurogenesis Coupling in Adult Neural Stem/Progenitor Cells. Neuron, 42(4), 535–552. [CrossRef]
- Deng, W., Saxe, M. D., Gallina, I. S., & Gage, F. H. (2009). Adult-Born Hippocampal Dentate Granule Cells Undergoing Maturation Modulate Learning and Memory in the Brain. The Journal of Neuroscience, 29(43), 13532–13542. [CrossRef]
- Denoth-Lippuner, A., & Jessberger, S. (2021). Formation and integration of new neurons in the adult hippocampus. Nature Reviews Neuroscience, 22(4), 223–236. [CrossRef]
- Devinsky, O., Marsh, E., Friedman, D., Thiele, E., Laux, L., Sullivan, J., Miller, I., Flamini, R., Wilfong, A., Filloux, F., Wong, M., Tilton, N., Bruno, P., Bluvstein, J., Hedlund, J., Kamens, R., Maclean, J., Nangia, S., Singhal, N. S., … Cilio, M. R. (2016). Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. The Lancet Neurology, 15(3), 270–278. [CrossRef]
- Devinsky, O., Vezzani, A., O’Brien, T. J., Jette, N., Scheffer, I. E., de Curtis, M., & Perucca, P. (2018). Epilepsy. Nature Reviews Disease Primers, 4(1), 18024. [CrossRef]
- Diamantis, A., Sidiropoulou, K., & Magiorkinis, E. (2010). Epilepsy during the Middle Ages, the Renaissance and the Enlightenment. Journal of Neurology, 257(5), 691–698. [CrossRef]
- Diederich, K., Bastl, A., Wersching, H., Teuber, A., Strecker, J.-K., Schmidt, A., Minnerup, J., & Schäbitz, W.-R. (2017). Effects of Different Exercise Strategies and Intensities on Memory Performance and Neurogenesis. Frontiers in Behavioral Neuroscience, 11. [CrossRef]
- Elger, C. E., Helmstaedter, C., & Kurthen, M. (2004). Chronic epilepsy and cognition. The Lancet Neurology, 3(11), 663–672. [CrossRef]
- Engel, J. (1995). Concepts of Epilepsy. Epilepsia, 36(s1), 23–29. [CrossRef]
- Engel, J. (1996). Introduction to temporal lobe epilepsy. Epilepsy Research, 26(1), 141–150. [CrossRef]
- Englot, D. J., Morgan, V. L., & Chang, C. (2020). Impaired vigilance networks in temporal lobe epilepsy: Mechanisms and clinical implications. Epilepsia, 61(2), 189–202. [CrossRef]
- Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A.-M., Nordborg, C., Peterson, D. A., & Gage, F. H. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4(11), 1313–1317. [CrossRef]
- Faigle, R., & Song, H. (2013). Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochimica et Biophysica Acta (BBA) - General Subjects, 1830(2), 2435–2448. [CrossRef]
- Falco-Walter, J. J., Scheffer, I. E., & Fisher, R. S. (2018). The new definition and classification of seizures and epilepsy. Epilepsy Research, 139, 73–79. [CrossRef]
- Fan, S., Sun, A., & Liu, L. (2018). Epigenetic modulation during hippocampal development (Review). Biomedical Reports. [CrossRef]
- Feng, L., Shu, Y., Wu, Q., Liu, T., Long, H., Yang, H., Li, Y., & Xiao, B. (2017). EphA4 may contribute to microvessel remodeling in the hippocampal CA1 and CA3 areas in a mouse model of temporal lobe epilepsy. Molecular Medicine Reports, 15(1), 37–46. [CrossRef]
- Fiest, K. M., Birbeck, G. L., Jacoby, A., & Jette, N. (2014). Stigma in Epilepsy. Current Neurology and Neuroscience Reports, 14(5), 444. [CrossRef]
- Fiest, K. M., Sauro, K. M., Wiebe, S., Patten, S. B., Kwon, C.-S., Dykeman, J., Pringsheim, T., Lorenzetti, D. L., & Jetté, N. (2017). Prevalence and incidence of epilepsy. Neurology, 88(3), 296–303. [CrossRef]
- Fisher, R. S., Acevedo, C., Arzimanoglou, A., Bogacz, A., Cross, J. H., Elger, C. E., Engel, J., Forsgren, L., French, J. A., Glynn, M., Hesdorffer, D. C., Lee, B. I., Mathern, G. W., Moshé, S. L., Perucca, E., Scheffer, I. E., Tomson, T., Watanabe, M., & Wiebe, S. (2014). ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia, 55(4), 475–482. [CrossRef]
- Foresti, M. L., Arisi, G. M., & Shapiro, L. A. (2011). Role of glia in epilepsy-associated neuropathology, neuroinflammation and neurogenesis. Brain Research Reviews, 66(1–2), 115–122. [CrossRef]
- Franck, J. E., Pokorny, J., Kunkel, D. D., & Schwartzkroin, P. A. (1995). Physiologic and Morphologic Characteristics of Granule Cell Circuitry in Human Epileptic Hippocampus. Epilepsia, 36(6), 543–558. [CrossRef]
- Gage, F. H. (2000). Mammalian Neural Stem Cells. Science, 287(5457), 1433–1438. [CrossRef]
- Gage, F. H., & Temple, S. (2013). Neural Stem Cells: Generating and Regenerating the Brain. Neuron, 80(3), 588–601. [CrossRef]
- Gaston, T. E., Martin, R. C., & Szaflarski, J. P. (2021). Cannabidiol (CBD) and cognition in epilepsy. Epilepsy & Behavior, 124, 108316. [CrossRef]
- Ge, S., Goh, E. L. K., Sailor, K. A., Kitabatake, Y., Ming, G., & Song, H. (2006). GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature, 439(7076), 589–593. [CrossRef]
- Gong, C., Wang, T.-W., Huang, H. S., & Parent, J. M. (2007). Reelin Regulates Neuronal Progenitor Migration in Intact and Epileptic Hippocampus. The Journal of Neuroscience, 27(8), 1803–1811. [CrossRef]
- Gorantla, V. R., Thomas, S. E., & Millis, R. M. (2019). Environmental Enrichment and Brain Neuroplasticity in the Kainate Rat Model of Temporal Lobe Epilepsy. Journal of Epilepsy Research, 9(1), 51–64. [CrossRef]
- Hampson, A. J., Grimaldi, M., Axelrod, J., & Wink, D. (1998). Cannabidiol and (−)Δ 9 -tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences, 95(14), 8268–8273. [CrossRef]
- Hancock, A., Priester, C., Kidder, E., & Keith, J. R. (2009). Does 5-bromo-2′-deoxyuridine (BrdU) disrupt cell proliferation and neuronal maturation in the adult rat hippocampus in vivo? Behavioural Brain Research, 199(2), 218–221. [CrossRef]
- Hartley, T., Lever, C., Burgess, N., & O’Keefe, J. (2014). Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1635), 20120510. [CrossRef]
- Heinrich, C., Nitta, N., Flubacher, A., Müller, M., Fahrner, A., Kirsch, M., Freiman, T., Suzuki, F., Depaulis, A., Frotscher, M., & Haas, C. A. (2006). Reelin Deficiency and Displacement of Mature Neurons, But Not Neurogenesis, Underlie the Formation of Granule Cell Dispersion in the Epileptic Hippocampus. The Journal of Neuroscience, 26(17), 4701–4713. [CrossRef]
- Helmstaedter, C. (2002). Effects of chronic epilepsy on declarative memory systems (pp. 439–453). [CrossRef]
- Helmstaedter, C., & Witt, J.-A. (2017). Epilepsy and cognition – A bidirectional relationship? Seizure, 49, 83–89. [CrossRef]
- Höglinger, G. U., Rizk, P., Muriel, M. P., Duyckaerts, C., Oertel, W. H., Caille, I., & Hirsch, E. C. (2004). Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nature Neuroscience, 7(7), 726–735. [CrossRef]
- Holmes, G. L. (2015). Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disorders, 17(2), 101–116. [CrossRef]
- Houser, C. R. (1990). Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Research, 535(2), 195–204. [CrossRef]
- Huang, Y.-Q., Wu, C., He, X.-F., Wu, D., He, X., Liang, F.-Y., Dai, G.-Y., Pei, Z., Xu, G.-Q., & Lan, Y. (2018). Effects of Voluntary Wheel-Running Types on Hippocampal Neurogenesis and Spatial Cognition in Middle-Aged Mice. Frontiers in Cellular Neuroscience, 12. [CrossRef]
- Hueston, C. M., Cryan, J. F., & Nolan, Y. M. (2017). Stress and adolescent hippocampal neurogenesis: diet and exercise as cognitive modulators. Translational Psychiatry, 7(4), e1081–e1081. [CrossRef]
- Hüttmann, K., Sadgrove, M., Wallraff, A., Hinterkeuser, S., Kirchhoff, F., Steinhäuser, C., & Gray, W. P. (2003). Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. European Journal of Neuroscience, 18(10), 2769–2778. [CrossRef]
- Izquierdo, I., Orsingher, O. A., & Berardi, A. C. (1973). Effect of cannabidiol and of other Cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia, 28(1), 95–102. [CrossRef]
- Jessberger, S., & Parent, J. M. (2015). Epilepsy and Adult Neurogenesis. Cold Spring Harbor Perspectives in Biology, a020677. [CrossRef]
- Jessberger, S., Römer, B., Babu, H., & Kempermann, G. (2005). Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Experimental Neurology, 196(2), 342–351. [CrossRef]
- Jessberger, S., Zhao, C., Toni, N., Clemenson, G. D., Li, Y., & Gage, F. H. (2007). Seizure-Associated, Aberrant Neurogenesis in Adult Rats Characterized with Retrovirus-Mediated Cell Labeling. The Journal of Neuroscience, 27(35), 9400–9407. [CrossRef]
- Jones, N. A., Glyn, S. E., Akiyama, S., Hill, T. D. M., Hill, A. J., Weston, S. E., Burnett, M. D. A., Yamasaki, Y., Stephens, G. J., Whalley, B. J., & Williams, C. M. (2012). Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure, 21(5), 344–352. [CrossRef]
- Kaculini, C. M., Tate-Looney, A. J., & Seifi, A. (2021). The History of Epilepsy: From Ancient Mystery to Modern Misconception. Cureus. [CrossRef]
- Kaplan, M. S., & Hinds, J. W. (1977). Neurogenesis in the Adult Rat: Electron Microscopic Analysis of Light Radioautographs. Science, 197(4308), 1092–1094. [CrossRef]
- Keller, S. S., Baker, G., Downes, J. J., & Roberts, N. (2009). Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy & Behavior, 15(2), 186–195. [CrossRef]
- Kempermann, G., Jessberger, S., Steiner, B., & Kronenberg, G. (2004). Milestones of neuronal development in the adult hippocampus. Trends in Neurosciences, 27(8), 447–452. [CrossRef]
- Kempermann, G., Kuhn, H. G., & Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature, 386(6624), 493–495. [CrossRef]
- Khalife, M. R., Scott, R. C., & Hernan, A. E. (2022). Mechanisms for Cognitive Impairment in Epilepsy: Moving Beyond Seizures. Frontiers in Neurology, 13. [CrossRef]
- Knoth, R., Singec, I., Ditter, M., Pantazis, G., Capetian, P., Meyer, R. P., Horvat, V., Volk, B., & Kempermann, G. (2010). Murine Features of Neurogenesis in the Human Hippocampus across the Lifespan from 0 to 100 Years. PLoS ONE, 5(1), e8809. [CrossRef]
- Kodam, A., Ourdev, D., Maulik, M., Hariharakrishnan, J., Banerjee, M., Wang, Y., & Kar, S. (2019). A role for astrocyte-derived amyloid β peptides in the degeneration of neurons in an animal model of temporal lobe epilepsy. Brain Pathology, 29(1), 28–44. [CrossRef]
- Kron, M. M., Zhang, H., & Parent, J. M. (2010). The Developmental Stage of Dentate Granule Cells Dictates Their Contribution to Seizure-Induced Plasticity. The Journal of Neuroscience, 30(6), 2051–2059. [CrossRef]
- Kronenberg, G., Reuter, K., Steiner, B., Brandt, M. D., Jessberger, S., Yamaguchi, M., & Kempermann, G. (2003). Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. Journal of Comparative Neurology, 467(4), 455–463. [CrossRef]
- Krook-Magnuson, E., Armstrong, C., Bui, A., Lew, S., Oijala, M., & Soltesz, I. (2015). In vivo evaluation of the dentate gate theory in epilepsy. The Journal of Physiology, 593(10), 2379–2388. [CrossRef]
- Kuhn, H., Dickinson-Anson, H., & Gage, F. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. The Journal of Neuroscience, 16(6), 2027–2033. [CrossRef]
- Landi, S., Petrucco, L., Sicca, F., & Ratto, G. M. (2019). Transient Cognitive Impairment in Epilepsy. Frontiers in Molecular Neuroscience, 11. [CrossRef]
- Leibowitz, J. A., Natarajan, G., Zhou, J., Carney, P. R., & Ormerod, B. K. (2019). Sustained somatostatin gene expression reverses kindling-induced increases in the number of dividing Type-1 neural stem cells in the hippocampi of behaviorally responsive rats. Epilepsy Research, 150, 78–94. [CrossRef]
- Leite, J. P., Bortolotto, Z. A., & Cavalheiro, E. A. (1990). Spontaneous recurrent seizures in rats: An experimental model of partial epilepsy. Neuroscience & Biobehavioral Reviews, 14(4), 511–517. [CrossRef]
- Leo, A., Russo, E., & Elia, M. (2016). Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacological Research, 107, 85–92. [CrossRef]
- Lévesque, M., & Avoli, M. (2013). The kainic acid model of temporal lobe epilepsy. Neuroscience & Biobehavioral Reviews, 37(10), 2887–2899. [CrossRef]
- LiCausi, F., & Hartman, N. (2018). Role of mTOR Complexes in Neurogenesis. International Journal of Molecular Sciences, 19(5), 1544. [CrossRef]
- Lim, J., Bang, Y., & Choi, H. J. (2018). Abnormal hippocampal neurogenesis in Parkinson’s disease: relevance to a new therapeutic target for depression with Parkinson’s disease. Archives of Pharmacal Research, 41(10), 943–954. [CrossRef]
- Lothman, E. W., Bertram, E. H., & Stringer, J. L. (1991). Functional anatomy of hippocampal seizures. Progress in Neurobiology, 37(1), 1–82. [CrossRef]
- Maccaferri, G. (2005). Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. The Journal of Physiology, 562(1), 73–80. [CrossRef]
- Magiorkinis, E., Diamantis, A., Sidiropoulou, K., & Panteliadis, C. (2014). Highights in the History of Epilepsy: The Last 200 Years. Epilepsy Research and Treatment, 2014, 1–13. [CrossRef]
- Magiorkinis, E., Sidiropoulou, K., & Diamantis, A. (2010). Hallmarks in the history of epilepsy: Epilepsy in antiquity. Epilepsy & Behavior, 17(1), 103–108. [CrossRef]
- Marques, C. M., Caboclo, L. O. S. F., da Silva, T. I., da Silva Noffs, M. H., Carrete, H., Lin, K., Lin, J., Sakamoto, A. C., & Yacubian, E. M. T. (2007). Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy & Behavior, 10(3), 477–485. [CrossRef]
- McClelland, S., Flynn, C., Dubé, C., Richichi, C., Zha, Q., Ghestem, A., Esclapez, M., Bernard, C., & Baram, T. Z. (2011). Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Annals of Neurology, 70(3), 454–465. [CrossRef]
- MD, I. B., MRCPath, M. T., & Wiestler, O. D. (2002). Ammon’s Horn Sclerosis: A Maldevelopmental Disorder Associated with Temporal Lobe Epilepsy. Brain Pathology, 12(2), 199–211. [CrossRef]
- Mello, L. E. A. M., Cavalheiro, E. A., Tan, A. M., Kupfer, W. R., Pretorius, J. K., Babb, T. L., & Finch, D. M. (1993). Circuit Mechanisms of Seizures in the Pilocarpine Model of Chronic Epilepsy: Cell Loss and Mossy Fiber Sprouting. Epilepsia, 34(6), 985–995. [CrossRef]
- Melo, I. S., Santos, Y. M. O., Costa, M. A., Pacheco, A. L. D., Silva, N. K. G. T., Cardoso-Sousa, L., Pereira, U. P., Goulart, L. R., Garcia-Cairasco, N., Duzzioni, M., Gitaí, D. L. G., Tilelli, C. Q., Sabino-Silva, R., & Castro, O. W. (2016). Inhibition of sodium glucose cotransporters following status epilepticus induced by intrahippocampal pilocarpine affects neurodegeneration process in hippocampus. Epilepsy & Behavior, 61, 258–268. [CrossRef]
- Mercer, A., Trigg, H. L., & Thomson, A. M. (2007). Characterization of Neurons in the CA2 Subfield of the Adult Rat Hippocampus. The Journal of Neuroscience, 27(27), 7329–7338. [CrossRef]
- Mirescu, C., Peters, J. D., & Gould, E. (2004). Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience, 7(8), 841–846. [CrossRef]
- Mirochnic, S., Wolf, S., Staufenbiel, M., & Kempermann, G. (2009). Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus, 19(10), 1008–1018. [CrossRef]
- Mishra, A., Singh, S., Tiwari, V., Parul, & Shukla, S. (2019). Dopamine D1 receptor activation improves adult hippocampal neurogenesis and exerts anxiolytic and antidepressant-like effect via activation of Wnt/β-catenin pathways in rat model of Parkinson’s disease. Neurochemistry International, 122, 170–186. [CrossRef]
- Moreno-Jiménez, E. P., Flor-García, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., Ávila, J., & Llorens-Martín, M. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nature Medicine, 25(4), 554–560. [CrossRef]
- Morin-Brureau, M., Milior, G., Royer, J., Chali, F., Le Duigou, C., Savary, E., Blugeon, C., Jourdren, L., Akbar, D., Dupont, S., Navarro, V., Baulac, M., Bielle, F., Mathon, B., Clemenceau, S., & Miles, R. (2018). Microglial phenotypes in the human epileptic temporal lobe. Brain, 141(12), 3343–3360. [CrossRef]
- Motamedi, G., & Meador, K. (2003). Epilepsy and cognition. Epilepsy & Behavior, 4, 25–38. [CrossRef]
- Myers, C. E., Bermudez-Hernandez, K., & Scharfman, H. E. (2013). The Influence of Ectopic Migration of Granule Cells into the Hilus on Dentate Gyrus-CA3 Function. PLoS ONE, 8(6), e68208. [CrossRef]
- Neligan, A., Hauser, W. A., & Sander, J. W. (2012). The epidemiology of the epilepsies (pp. 113–133). [CrossRef]
- Nilsson, M., Perfilieva, E., Johansson, U., Orwar, O., & Eriksson, P. S. (1999). Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. Journal of Neurobiology, 39(4), 569–578. [CrossRef]
- Nithianantharajah, J. (2004). Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiology of Learning and Memory, 81(3), 200–210. [CrossRef]
- Nottebohm, F. (1989). From Bird Song to Neurogenesis. Scientific American, 260(2), 74–79. [CrossRef]
- Ohline, S. M., Wake, K. L., Hawkridge, M.-V., Dinnunhan, M. F., Hegemann, R. U., Wilson, A., Schoderboeck, L., Logan, B. J., Jungenitz, T., Schwarzacher, S. W., Hughes, S. M., & Abraham, W. C. (2018). Adult-born dentate granule cell excitability depends on the interaction of neuron age, ontogenetic age and experience. Brain Structure and Function, 223(7), 3213–3228. [CrossRef]
- Overstreet-Wadiche, L. S., Bensen, A. L., & Westbrook, G. L. (2006). Delayed Development of Adult-Generated Granule Cells in Dentate Gyrus. The Journal of Neuroscience, 26(8), 2326–2334. [CrossRef]
- Pang, C. C.-C., Kiecker, C., O’Brien, J. T., Noble, W., & Chang, R. C.-C. (2019). Ammon’s Horn 2 (CA2) of the Hippocampus: A Long-Known Region with a New Potential Role in Neurodegeneration. The Neuroscientist, 25(2), 167–180. [CrossRef]
- Panteliadis, C. P., Vassilyadi, P., Fehlert, J., & Hagel, C. (2017). Historical documents on epilepsy: From antiquity through the 20th century. Brain and Development, 39(6), 457–463. [CrossRef]
- Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S., & Lowenstein, D. H. (1997a). Dentate Granule Cell Neurogenesis Is Increased by Seizures and Contributes to Aberrant Network Reorganization in the Adult Rat Hippocampus. The Journal of Neuroscience, 17(10), 3727–3738. [CrossRef]
- Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S., & Lowenstein, D. H. (1997b). Dentate Granule Cell Neurogenesis Is Increased by Seizures and Contributes to Aberrant Network Reorganization in the Adult Rat Hippocampus. The Journal of Neuroscience, 17(10), 3727–3738. [CrossRef]
- Patra, P. H., Barker-Haliski, M., White, H. S., Whalley, B. J., Glyn, S., Sandhu, H., Jones, N., Bazelot, M., Williams, C. M., & McNeish, A. J. (2019). Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia, 60(2), 303–314. [CrossRef]
- Pekcec, A., Mühlenhoff, M., Gerardy-Schahn, R., & Potschka, H. (2007). Impact of the PSA-NCAM system on pathophysiology in a chronic rodent model of temporal lobe epilepsy. Neurobiology of Disease, 27(1), 54–66. [CrossRef]
- Pilz, G.-A., Bottes, S., Betizeau, M., Jörg, D. J., Carta, S., Simons, B. D., Helmchen, F., & Jessberger, S. (2018). Live imaging of neurogenesis in the adult mouse hippocampus. Science, 359(6376), 658–662. [CrossRef]
- Pinar, C., Yau, S., Sharp, Z., Shamei, A., Fontaine, C. J., Meconi, A. L., Lottenberg, C. P., & Christie, B. R. (2018). Effects of Voluntary Exercise on Cell Proliferation and Neurogenesis in the Dentate Gyrus of Adult FMR1 Knockout Mice. Brain Plasticity, 4(2), 185–195. [CrossRef]
- Pun, R. Y. K., Rolle, I. J., LaSarge, C. L., Hosford, B. E., Rosen, J. M., Uhl, J. D., Schmeltzer, S. N., Faulkner, C., Bronson, S. L., Murphy, B. L., Richards, D. A., Holland, K. D., & Danzer, S. C. (2012). Excessive Activation of mTOR in Postnatally Generated Granule Cells Is Sufficient to Cause Epilepsy. Neuron, 75(6), 1022–1034. [CrossRef]
- Radad, K., Moldzio, R., Al-Shraim, M., Kranner, B., Krewenka, C., & Rausch, W.-D. (2017). Recent Advances on the Role of Neurogenesis in the Adult Brain: Therapeutic Potential in Parkinson’s and Alzheimer’s Diseases. CNS & Neurological Disorders - Drug Targets, 16(7). [CrossRef]
- RajMohan, V., & Mohandas, E. (2007). The limbic system. Indian Journal of Psychiatry, 49(2), 132. [CrossRef]
- RAKIC, P. (1985). DNA Synthesis and Cell Division in the Adult Primate Braina. Annals of the New York Academy of Sciences, 457(1), 193–211. [CrossRef]
- Reis, R., & Meinardi, H. (2002). ILAE/WHO “Out of the Shadows Campaign” Stigma: does the flag identify the cargo? Epilepsy & Behavior, 3(6), 33–37. [CrossRef]
- Ribak, C. E., Tran, P. H., Spigelman, I., Okazaki, M. M., & Nadler, J. V. (2000). Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. The Journal of Comparative Neurology, 428(2), 240–253. [CrossRef]
- Romariz, S. A., Garcia, K. de O., Paiva, D. de S., Bittencourt, S., Covolan, L., Mello, L. E., & Longo, B. M. (2014). Participation of bone marrow-derived cells in hippocampal vascularization after status epilepticus. Seizure, 23(5), 386–389. [CrossRef]
- Rudnitskaya, E. A., Kozlova, T. A., Burnyasheva, A. O., Kolosova, N. G., & Stefanova, N. A. (2019). Alterations of hippocampal neurogenesis during development of Alzheimer’s disease-like pathology in OXYS rats. Experimental Gerontology, 115, 32–45. [CrossRef]
- Sachdev, P. S., Blacker, D., Blazer, D. G., Ganguli, M., Jeste, D. V, Paulsen, J. S., & Petersen, R. C. (2014). Classifying neurocognitive disorders: the DSM-5 approach. Nature Reviews Neurology, 10(11), 634–642. [CrossRef]
- Santos, V. R., santana Melo, I., Pacheco, A. L. D., & de Castro, O. W. (2021). Life and death in the hippocampus: What’s bad? Epilepsy & Behavior, 121, 106595. [CrossRef]
- Scharfman, H. E. (2002). Review: Epilepsy as an Example of Neural Plasticity. The Neuroscientist, 8(2), 154–173. [CrossRef]
- Scharfman, H. E. (2005). Brain-derived Neurotrophic Factor and Epilepsy—A Missing Link? Epilepsy Currents, 5(3), 83–88. [CrossRef]
- Scharfman, H. E. (2007). The neurobiology of epilepsy. Current Neurology and Neuroscience Reports, 7(4), 348–354. [CrossRef]
- Scharfman, H. E. (2018). Advances in understanding hilar mossy cells of the dentate gyrus. Cell and Tissue Research, 373(3), 643–652. [CrossRef]
- Scharfman, H. E., Goodman, J. H., & Sollas, A. L. (2000). Granule-Like Neurons at the Hilar/CA3 Border after Status Epilepticus and Their Synchrony with Area CA3 Pyramidal Cells: Functional Implications of Seizure-Induced Neurogenesis. The Journal of Neuroscience, 20(16), 6144–6158. [CrossRef]
- Scharfman, H. E., & Myers, C. E. (2013). Hilar mossy cells of the dentate gyrus: a historical perspective. Frontiers in Neural Circuits, 6. [CrossRef]
- Scharfman, H. E., Sollas, A. L., Berger, R. E., & Goodman, J. H. (2003). Electrophysiological Evidence of Monosynaptic Excitatory Transmission Between Granule Cells After Seizure-Induced Mossy Fiber Sprouting. Journal of Neurophysiology, 90(4), 2536–2547. [CrossRef]
- Scheffer, I. E., Berkovic, S., Capovilla, G., Connolly, M. B., French, J., Guilhoto, L., Hirsch, E., Jain, S., Mathern, G. W., Moshé, S. L., Nordli, D. R., Perucca, E., Tomson, T., Wiebe, S., Zhang, Y., & Zuberi, S. M. (2017). <scp>ILAE</scp> classification of the epilepsies: Position paper of the <scp>ILAE</scp> Commission for Classification and Terminology. Epilepsia, 58(4), 512–521. [CrossRef]
- Schiavon, A. P., Bonato, J. M., Milani, H., Guimarães, F. S., & de Oliveira, R. M. W. (2016). Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 27–34. [CrossRef]
- Schoenfeld, T. J., McCausland, H. C., Morris, H. D., Padmanaban, V., & Cameron, H. A. (2017). Stress and Loss of Adult Neurogenesis Differentially Reduce Hippocampal Volume. Biological Psychiatry, 82(12), 914–923. [CrossRef]
- Scorza, F. A., Cysneiros, R. M., Arida, R. M., Scorza, C. A., de Almeida, A.-C. G., Schmidt, B., & Cavalheiro, E. A. (2008). Adult hippocampal neurogenesis and sudden unexpected death in epilepsy: Reality or just an attractive history? Medical Hypotheses, 71(6), 914–922. [CrossRef]
- Semah, F., Picot, M.-C., Adam, C., Broglin, D., Arzimanoglou, A., Bazin, B., Cavalcanti, D., & Baulac, M. (1998). Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology, 51(5), 1256–1262. [CrossRef]
- Sen, A., Capelli, V., & Husain, M. (2018). Cognition and dementia in older patients with epilepsy. Brain, 141(6), 1592–1608. [CrossRef]
- Senzai, Y. (2019). Function of local circuits in the hippocampal dentate gyrus-CA3 system. Neuroscience Research, 140, 43–52. [CrossRef]
- Seress, L., & Pokorny, J. (1981). Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. Journal of Anatomy, 133(Pt 2), 181–195.
- Sharma, A. K., Reams, R. Y., Jordan, W. H., Miller, M. A., Thacker, H. L., & Snyder, P. W. (2007). Mesial Temporal Lobe Epilepsy: Pathogenesis, Induced Rodent Models and Lesions. Toxicologic Pathology, 35(7), 984–999. [CrossRef]
- Shu, Y., Xiao, B., Wu, Q., Liu, T., Du, Y., Tang, H., Chen, S., Feng, L., Long, L., & Li, Y. (2016). The Ephrin-A5/EphA4 Interaction Modulates Neurogenesis and Angiogenesis by the p-Akt and p-ERK Pathways in a Mouse Model of TLE. Molecular Neurobiology, 53(1), 561–576. [CrossRef]
- Sierra, A., Martín-Suárez, S., Valcárcel-Martín, R., Pascual-Brazo, J., Aelvoet, S.-A., Abiega, O., Deudero, J. J., Brewster, A. L., Bernales, I., Anderson, A. E., Baekelandt, V., Maletić-Savatić, M., & Encinas, J. M. (2015). Neuronal Hyperactivity Accelerates Depletion of Neural Stem Cells and Impairs Hippocampal Neurogenesis. Cell Stem Cell, 16(5), 488–503. [CrossRef]
- Simeone, T. A., Simeone, K. A., Samson, K. K., Kim, D. Y., & Rho, J. M. (2013). Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiology of Disease, 54, 68–81. [CrossRef]
- Smith, L. K., White, C. W., & Villeda, S. A. (2018). The systemic environment: at the interface of aging and adult neurogenesis. Cell and Tissue Research, 371(1), 105–113. [CrossRef]
- Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., Boström, E., Westerlund, I., Vial, C., Buchholz, B. A., Possnert, G., Mash, D. C., Druid, H., & Frisén, J. (2013). Dynamics of Hippocampal Neurogenesis in Adult Humans. Cell, 153(6), 1219–1227. [CrossRef]
- Spiers, H. J. (2012). Hippocampal Formation. In Encyclopedia of Human Behavior (pp. 297–304). Elsevier. [CrossRef]
- Steiner, B., Zurborg, S., Hörster, H., Fabel, K., & Kempermann, G. (2008). Differential 24 h responsiveness of Prox1–expressing precursor cells in adult hippocampal neurogenesis to physical activity, environmental enrichment, and kainic acid–induced seizures. Neuroscience, 154(2), 521–529. [CrossRef]
- Steinhäuser, C., Seifert, G., & Bedner, P. (2012). Astrocyte dysfunction in temporal lobe epilepsy: K + channels and gap junction coupling. Glia, 60(8), 1192–1202. [CrossRef]
- Sutula, T., Cascino, G., Cavazos, J., Parada, I., & Ramirez, L. (1989). Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Annals of Neurology, 26(3), 321–330. [CrossRef]
- Takei, Y. (2019). Age-dependent decline in neurogenesis of the hippocampus and extracellular nucleotides. Human Cell, 32(2), 88–94. [CrossRef]
- Tanapat, P., Hastings, N. B., Reeves, A. J., & Gould, E. (1999). Estrogen Stimulates a Transient Increase in the Number of New Neurons in the Dentate Gyrus of the Adult Female Rat. The Journal of Neuroscience, 19(14), 5792–5801. [CrossRef]
- Tarasova, A. Yu., Perepelkina, O. V., Lil’p, I. G., Revishchin, A. V., Pavlova, G. V., & Poletaeva, I. I. (2018). Influence of “Enriched Environment” on Behavior and Neurogenesis in Mice Selected by Cognitive Trait. Bulletin of Experimental Biology and Medicine, 164(5), 583–586. [CrossRef]
- Tejada, J., Costa, K. M., Bertti, P., & Garcia-Cairasco, N. (2013). The epilepsies: Complex challenges needing complex solutions. Epilepsy & Behavior, 26(3), 212–228. [CrossRef]
- Tellez-Zenteno, J. F., Patten, S. B., Jetté, N., Williams, J., & Wiebe, S. (2007). Psychiatric Comorbidity in Epilepsy: A Population-Based Analysis. Epilepsia, 48(12), 2336–2344. [CrossRef]
- Thiele, E. A., Marsh, E. D., French, J. A., Mazurkiewicz-Beldzinska, M., Benbadis, S. R., Joshi, C., Lyons, P. D., Taylor, A., Roberts, C., Sommerville, K., Gunning, B., Gawlowicz, J., Lisewski, P., Beldzinska, M. M., Szewczyk, K. M., Steinborn, B., Zolnowska, M., Hughes, E., McLellan, A., … Wilfong, A. (2018). Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet, 391(10125), 1085–1096. [CrossRef]
- Thijs, R. D., Surges, R., O’Brien, T. J., & Sander, J. W. (2019). Epilepsy in adults. The Lancet, 393(10172), 689–701. [CrossRef]
- Toda, T., Parylak, S. L., Linker, S. B., & Gage, F. H. (2019). The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry, 24(1), 67–87. [CrossRef]
- Toni, N., Laplagne, D. A., Zhao, C., Lombardi, G., Ribak, C. E., Gage, F. H., & Schinder, A. F. (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nature Neuroscience, 11(8), 901–907. [CrossRef]
- Turkanis, S. A., Smiley, K. A., Borys, H. K., Olsen, D. M., & Karler, R. (1979). An Electrophysiological Analysis of the Anticonvulsant Action of Cannabidiol on Limbic Seizures in Conscious Rats. Epilepsia, 20(4), 351–363. [CrossRef]
- Turski, W. A., Cavalheiro, E. A., Bortolotto, Z. A., Mello, L. M., Schwarz, M., & Turski, L. (1984). Seizures produced by pilocarpine in mice: A behavioral, electroencephalographic and morphological analysis. Brain Research, 321(2), 237–253. [CrossRef]
- Turski, W. A., Cavalheiro, E. A., Schwarz, M., Czuczwar, S. J., Kleinrok, Z., & Turski, L. (1983). Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behavioural Brain Research, 9(3), 315–335. [CrossRef]
- Upadhya, D., Hattiangady, B., Castro, O. W., Shuai, B., Kodali, M., Attaluri, S., Bates, A., Dong, Y., Zhang, S.-C., Prockop, D. J., & Shetty, A. K. (2019). Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proceedings of the National Academy of Sciences, 116(1), 287–296. [CrossRef]
- Urbán, N., Blomfield, I. M., & Guillemot, F. (2019). Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron, 104(5), 834–848. [CrossRef]
- van Praag, H., Kempermann, G., & Gage, F. H. (1999a). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience, 2(3), 266–270. [CrossRef]
- van Praag, H., Kempermann, G., & Gage, F. H. (1999b). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience, 2(3), 266–270. [CrossRef]
- van Strien, N. M., Cappaert, N. L. M., & Witter, M. P. (2009). The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nature Reviews Neuroscience, 10(4), 272–282. [CrossRef]
- Vezzani, A., Aronica, E., Mazarati, A., & Pittman, Q. J. (2013). Epilepsy and brain inflammation. Experimental Neurology, 244, 11–21. [CrossRef]
- Vezzani, A., Ravizza, T., Moneta, D., Conti, M., Borroni, A., Rizzi, M., Samanin, R., & Maj, R. (1999). Brain-derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. Neuroscience, 90(4), 1445–1461. [CrossRef]
- Voorhies, T. (1988). Cognitive and Behavioral Effects of Antiepileptic Drugs. Seminars in Neurology, 8(01), 35–41. [CrossRef]
- Vrinda, M., Arun, S., Srikumar, B. N., Kutty, B. M., & Rao, B. S. S. (2019). Temporal lobe epilepsy-induced neurodegeneration and cognitive deficits: Implications for aging. Journal of Chemical Neuroanatomy, 95, 146–153. [CrossRef]
- Wainwright, S. R., & Galea, L. A. M. (2013). The Neural Plasticity Theory of Depression: Assessing the Roles of Adult Neurogenesis and PSA-NCAM within the Hippocampus. Neural Plasticity, 2013, 1–14. [CrossRef]
- Winner, B., Regensburger, M., Schreglmann, S., Boyer, L., Prots, I., Rockenstein, E., Mante, M., Zhao, C., Winkler, J., Masliah, E., & Gage, F. H. (2012). Role of α-Synuclein in Adult Neurogenesis and Neuronal Maturation in the Dentate Gyrus. The Journal of Neuroscience, 32(47), 16906–16916. [CrossRef]
- Yagi, S., & Galea, L. A. M. (2019). Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology, 44(1), 200–213. [CrossRef]
- Yamada, J., Nadanaka, S., Kitagawa, H., Takeuchi, K., & Jinno, S. (2018). Increased Synthesis of Chondroitin Sulfate Proteoglycan Promotes Adult Hippocampal Neurogenesis in Response to Enriched Environment. The Journal of Neuroscience, 38(39), 8496–8513. [CrossRef]
- Zang, J., Liu, Y., Li, W., Xiao, D., Zhang, Y., Luo, Y., Liang, W., Liu, F., & Wei, W. (2017). Voluntary exercise increases adult hippocampal neurogenesis by increasing GSK-3β activity in mice. Neuroscience, 354, 122–135. [CrossRef]
- Zarif, H., Nicolas, S., Guyot, M., Hosseiny, S., Lazzari, A., Canali, M. M., Cazareth, J., Brau, F., Golzné, V., Dourneau, E., Maillaut, M., Luci, C., Paquet, A., Lebrigand, K., Arguel, M.-J., Daoudlarian, D., Heurteaux, C., Glaichenhaus, N., Chabry, J., … Petit-Paitel, A. (2018). CD8+ T cells are essential for the effects of enriched environment on hippocampus-dependent behavior, hippocampal neurogenesis and synaptic plasticity. Brain, Behavior, and Immunity, 69, 235–254. [CrossRef]
- Zhang, J., & Jiao, J. (2015). Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. BioMed Research International, 2015, 1–14. [CrossRef]
- Zhu, G., Meng, D., Chen, Y., Du, T., Liu, Y., Liu, D., Shi, L., Jiang, Y., Zhang, X., & Zhang, J. (2018). Anterior nucleus of thalamus stimulation inhibited abnormal mossy fiber sprouting in kainic acid-induced epileptic rats. Brain Research, 1701, 28–35. [CrossRef]
Figure 1.
Adult hippocampal neurogenesis. Schematic representation illustrating the process of adult hippocampal neurogenesis, demonstrating the formation of newborn neurons and their integration into the circuitry within the granular layer.
Figure 1.
Adult hippocampal neurogenesis. Schematic representation illustrating the process of adult hippocampal neurogenesis, demonstrating the formation of newborn neurons and their integration into the circuitry within the granular layer.
Figure 2.
Progression of Aberrant Neurogenesis. Simplified diagram of the hippocampal neurogenesis system in which: Normal: Represents the standard neurogenesis of the adult hippocampus. Acute LTE: Shows how acute temporal lobe epilepsy disrupts hippocampal neurogenesis, with increased cell proliferation (highlighted by the greater number of cells), hilar basal dendrites (in blue), mossy fiber sprouting (in purple), and cell ectopic for the hilus (represented by the red cell). These effects are called aberrant. Chronic LTE: Demonstrates the chronic effects of temporal lobe epilepsy on hippocampal neurogenesis, with depletion of stem/progenitor cells (evidenced by the lower number of cells), hilar basal dendrites (in blue), sprouting of mossy fibers (in purple) and stable network of the ectopic hilar cells (represented by the two red cells). Below are factors that influence increases and decreases in neurogenesis in the course of temporal lobe epilepsy.
Figure 2.
Progression of Aberrant Neurogenesis. Simplified diagram of the hippocampal neurogenesis system in which: Normal: Represents the standard neurogenesis of the adult hippocampus. Acute LTE: Shows how acute temporal lobe epilepsy disrupts hippocampal neurogenesis, with increased cell proliferation (highlighted by the greater number of cells), hilar basal dendrites (in blue), mossy fiber sprouting (in purple), and cell ectopic for the hilus (represented by the red cell). These effects are called aberrant. Chronic LTE: Demonstrates the chronic effects of temporal lobe epilepsy on hippocampal neurogenesis, with depletion of stem/progenitor cells (evidenced by the lower number of cells), hilar basal dendrites (in blue), sprouting of mossy fibers (in purple) and stable network of the ectopic hilar cells (represented by the two red cells). Below are factors that influence increases and decreases in neurogenesis in the course of temporal lobe epilepsy.
Figure 3.
Pattern separation and pattern completion under normal conditions. The DG transforms incoming information to reduce overlap with other patterns, creating sparser and more distinct representations. The CA3 region completes stored memory patterns, facilitating the recognition of familiar stimuli and the accurate retrieval of memories.
Figure 3.
Pattern separation and pattern completion under normal conditions. The DG transforms incoming information to reduce overlap with other patterns, creating sparser and more distinct representations. The CA3 region completes stored memory patterns, facilitating the recognition of familiar stimuli and the accurate retrieval of memories.
Figure 4.
Ectopic granule cells (hEGCs) in epileptogenic conditions exert a dominant influence, impairing pattern separation. This dominance makes it difficult for CA3 to perform pattern completion, which contributes to the abnormal neural activity observed in conditions such as temporal lobe epilepsy (TLE).
Figure 4.
Ectopic granule cells (hEGCs) in epileptogenic conditions exert a dominant influence, impairing pattern separation. This dominance makes it difficult for CA3 to perform pattern completion, which contributes to the abnormal neural activity observed in conditions such as temporal lobe epilepsy (TLE).
Figure 5.
Under normal conditions, immature granule neurons (GCs) exhibit similar firing rates to mature granule neurons, as represented in the shower where ‘water’ (information) flows preferentially between immature neurons. However, inhibitory control prevents these cells from becoming dominant. In contrast, under epileptogenesis conditions (temporal lobe epilepsy - TLE), ectopic granule cells (hEGCs) dominate the flow of information, resulting in a higher and less controlled ‘flow rate,’ which compromises the pattern separation.
Figure 5.
Under normal conditions, immature granule neurons (GCs) exhibit similar firing rates to mature granule neurons, as represented in the shower where ‘water’ (information) flows preferentially between immature neurons. However, inhibitory control prevents these cells from becoming dominant. In contrast, under epileptogenesis conditions (temporal lobe epilepsy - TLE), ectopic granule cells (hEGCs) dominate the flow of information, resulting in a higher and less controlled ‘flow rate,’ which compromises the pattern separation.
Figure 6.
In epileptiform conditions, aberrant mossy fiber sprouting forms recurrent excitatory synapses with the dendrites of granule cells in the dentate inner molecular layer, basket cells, and mossy cells. Additionally, hilar basal dendrites, which are postsynaptic targets of mossy fiber collaterals, contribute to this synaptic rearrangement, leading to the creation of recurrent excitatory circuits that promote neuroplastic changes and increase abnormal neural activity associated with temporal lobe epilepsy (TLE).
Figure 6.
In epileptiform conditions, aberrant mossy fiber sprouting forms recurrent excitatory synapses with the dendrites of granule cells in the dentate inner molecular layer, basket cells, and mossy cells. Additionally, hilar basal dendrites, which are postsynaptic targets of mossy fiber collaterals, contribute to this synaptic rearrangement, leading to the creation of recurrent excitatory circuits that promote neuroplastic changes and increase abnormal neural activity associated with temporal lobe epilepsy (TLE).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).