1. Introduction
African swine fever (ASF) is a highly contagious and fatal hemorrhagic disease in pigs caused by the African swine fever virus (ASFV). Since its first report in China in 2018, ASF has spread rapidly and has posed a devastating impact on the pig industry in the following years [
1,
2]. Despite the domestic and international efforts to develop a vaccine, there is currently no safe and effective commercial vaccine available [
3,
4,
5,
6]. Therefore, the prevention and control of ASF mainly rely on rapid detection and early diagnosis.
ASFV is a double-stranded DNA virus, with a large genome of approximately 170-190 kb, encoding over 150 viral proteins [
7]. The structure of ASFV is complex, which can be divided from the outside to the inside into the outer membrane, capsid, inner membrane, core shell, and nucleoid [
8]. The p17 protein encoded by ORF D117L is an important component of the ASFV capsid [
9]. ASFV p17 plays a critical role in viral morphogenesis, the absence of p17 hinders the proteolysis of the polyproteins pp220 and pp62, thereby affecting the assembly of the viral particles [
10].
ASFV p17 not only affects the morphogenesis and assembly of viral particles but also participates in regulation of host cell function and immune evasion. In eukaryotic cells, p17 is located on the endoplasmic reticulum (ER) membrane, and its expression can cause ER stress, leading to the release of reactive oxygen species, which inhibits cell proliferation by arresting the cell cycle [
11]. The binding of p17 to TOMM70 promotes the binding of the autophagy receptor SQSTM1 to TOMM70, resulting in mitophagy and decreased expression of MAVS, thereby suppressing the innate immune response [
12]. The p17 is involved in regulation of the innate immune response mediated by cGAS-STING signaling pathway. On the one hand, p17 binds to STING, inhibiting the interactions between STING and TBK1/IKKɛ, and blocking the production of type I IFN [
13]. On the other hand, p17 downregulates the phosphorylation of TBK1 and IRF3 by recruiting the host scaffold protein PR65A and inducing partial degradation of STING via apoptosis [
14].
As one of the main capsid proteins, p17 has a high abundance in ASFV particles and possesses a good immunogenicity [
7], making it a potential target for detecting ASFV infection. There has been a report of p17 expression in CHO cells and establishment of a specific indirect ELISA based on the purified p17 from CHO cells [
15]. The p17 monoclonal antibodies (mAbs) were generated from eukaryotic p17 protein immunized mice and the mAb recognized linear epitopes are located at the N-terminus of p17 [
15,
16]. In this study, we expressed and purified the prokaryotic p17 to produce p17 mAbs from immunized mice. Two p17 mAbs generated could recognize a conserved linear epitope at 72-78 amino acids in the middle of p17. In addition, the epitope-based indirect ELISA was established, and it was able to specifically detect ASFV antibodies in the serum samples of pigs with ASFV infection. Our results not only provide useful tools for ASFV research, but also establish a new method for the detection and prevention of ASF.
2. Materials and Methods
2.1. Mice, Cells, Viruses, Sera and Reagents
The BALB/c mice of 6- to 8-weeks old were from the Laboratory Animal Center of Yangzhou University. The animal experiment was in strict accordance with the Guidance for the Care and Use of Laboratory Animals of Yangzhou University (SYXK(JS)-2021-0026). HEK-293T cells (ATCC Cat # CRL-3216), Marc-145 cells (Cellosaurus Cat # CVCL_4540), Vero cells (ATCC Cat # CCL-81), PK-15 cells (ATCC Cat # CCL-33), MDCK cells (ATCC Cat # CCL-34) and myeloma cell line SP2/0 (ATCC Cat # CRL-1581) were cultured in Dulbecco modified Eagle medium (DMEM, Hyclone Laboratories, USA) containing 10% fetal bovine serum (FBS) and 100 IU/ml of penicillin plus 100 μg/ml streptomycin. Primary porcine alveolar macrophages (PAMs) and 3D4/21 cells (ATCC cat# CRL-2843) were cultured in RPMI 1640 medium (Hyclone Laboratories) which contains 100 IU/mL of penicillin plus 100 μg/mL streptomycin and 10% FBS. All cells were grown at 37°C in a 5% CO2 humidified incubator. The ASFV strain (genotype II, GenBank accession ON456300) was stored in the animal biosafety level 3 (ABSL-3) of Yangzhou University approved by the Ministry of Agriculture and Rural Affairs (07140020201109-1), whereas the porcine reproductive and respiratory syndrome virus (PRRSV), recombinant PRRSV expressing p17 (PRRSV-p17), porcine epidemic diarrhea virus (PEDV), porcine delta coronavirus (PDCoV), swine influenza virus (SIV) and porcine serum samples were kepted in our lab. The African swine fever virus ELISA antibody detection kit was bought from Putai Biology Technology Co., Ltd. The RFP mAb (ab185921) was acquired from Abcam (Cambridge, UK). The anti-β-actin mAb (5057) were acquired from Cell Signaling Technology (Boston, MA, USA). The anti-PRRSV N mAb, anti-PEDV N mAb and anti-SIV NP mAb were produced and stored in our lab. The anti-PDCoV NS7 mAb was a gift from Prof. Zhenhai Chen of Yangzhou University.
2.2. Expression and Purification of p17 Protein
The ASFV p17 C-terminal intramembrane region (61-171 aa) gene coding sequence D117L was amplified by PCR from pCAGGS-p17-HA stored in our laboratory and cloned into Xho I and EcoR I sites of pCold-TF vector or Sal I and Xba I sites of pCold-MBP vector by Seemless/In-Fusion Cloning (2×MultiF Seamless Assembly Mix, Abclonal, Wuhan, China). The plasmids were transformed into BL21/DE3 E. coli competent cells and treated with 1 mM isopropyl-β-d-1-thiogalactoside (IPTG) at 16 °C or 37 °C for 24 h to induce p17 expressions. The expressed p17 fusion proteins were purified by gel recovery method. Specifically, the bacteria pellet was collected after centrifugation, resuspended in PBS and sonicated on ice. Following centrifugation, the supernatant was retained and run in SDS-PAGE as much as possible. After staining with Coomassie brilliant blue and subsequent complete de-staining, the target gel piece was cut out and the ground gel was placed in a dialysis bag for electrophoresis at 120 V for 2 h. Finally, the liquid in the dialysis bag was transferred and dialyzed in PBS overnight at 4°C, and concentrated with PEG 20000.
2.3. Production of Anti-p17 Monoclonal Antibodies (mAbs)
BALB/c mice were first immunized subcutaneously at multiple points on the back with 100 μg purified TF-p17 protein mixed with Montanide gel (SEPPIC SA Cedex, France) at a volume ratio of 10:1. The second and third immunizations were performed at 14 d and 21 d after the first immunization, with each 50 µg protein mixed with same adjuvant. Five days later, tail vein blood was drawn and serum p17 antibody was measured by p17 protein indirect ELISA. Mouse exhibiting the highest antibody titer was chosen for the final boost (50 µg p17 protein injection intraperitoneally without adjuvant). Subsequently, the mouse was sacrificed three days later for cell fusion with SP2/0 cells. The hybridomas secreting p17 specific antibodies were screened out by MBP-p17 based indirect ELISA, followed by Western blotting confirmation. Positive hybridomas were subjected for limited dilution subcloning three times and tested further for antibody secretion.
2.4. Mapping of the Precise Linear B Cell Epitope of p17 Protein
The pCAGGS-p17-HA and pDsRed-C1-p17 plasmids were previously constructed and used in this study [
11,
13].First, the C-terminal intramembrane region of p17 (aa 61-117) was separated into three fragments which were tested for the reactivity with p17 mAbs. Second, based on the reaction information of the fragment, progressive truncations at both N and C terminal ends were performed from full length p17. The reactivity of different fragments with p17 mAbs was tested, the critical amino acids of both N and C ends for reactivity with p17 mAbs were determined, and thus, the minimal epitope was deduced. Totally, 14 p17 fragments (P1-P14) were designed, with all cloning PCR primers listed in
Table S1. All p17 fragments were amplified by PCR using the template pCAGGS-p17-HA, and then cloned into the
Bgl II and
EcoR I sites of pDsRed-C1-express vector by Seemless/In-Fusion Cloning. All the constructed plasmids were transfected into 293T cells, and the reactivity of different truncated p17 proteins expressed in transfected 293 T cells with p17 mAbs was tested using Western blotting.
2.5. Western Blotting
The reactivity of anti-p17 mAbs with p17 expressed in transfected 293T cells, PRRSV-p17 infected Marc-145 cells and ASFV infected PAMs, as well as the p17 fragments in transfected 293T cells was evaluated using Western blotting. The cell protein samples were separated by 6-10 % SDS-PAGE, and transferred to PVDF membranes. The membranes were then blocked using 5% non-fat dry milk TBS solution for 1 h, with 0.1% Tween-20 (TBST). Next, the membrane was incubated with the primary mAbs (1 ∶ 1000 anti-RFP and p17 hybridoma ascites) at 4 ℃ overnight. Next, the membrane was incubated with HRP-conjugated Goat Anti-Mouse IgG (1: 10000, BBI, Shanghai, China) for 1 h. Protein signals was visualized and captured by Western blot imaging system.
2.6. Immunofluorescence Assay
3D4/21 cells were transfected with pCAGGS-p17-HA or pdsRed-C1-p17/truncated mutants for 24 h, while Marc-145 cells were infected with PRRSV-p17 with a multiplicity of infection (MOI) of 0.1 for 72 h. The cells were fixed in 4 % paraformaldehyde for 30 min, permeabilized with 0.5% Triton X-100, and blocked with 5% BSA. The treated cells were incubated with p17 mAbs (1: 200 ascites) overnight, and Donkey anti-Mouse IgG (H+L) Alexa Fluor 488 (1: 500, Invitrogen, Shanghai, China) for 1 h, followed by DAPI staining (Beyotime, Shanghai, China) for 15 min. Cells were visualized by fluorescence microscope at the excitation wavelengths 405 nm, 488 nm and 535 nm, respectively.
2.7. Enzyme-Linked Immunosorbent Assay
For p17 protein indirect ELISA, the ELISA wells were coated with p17 purified protein (MBP-p17) diluted in PBS at a concentration of 0.3125 μg/mL at 4°C overnight, followed by washing and blocking with 5% skim milk at 37°C for 2 h. Hybridoma supernatant (100 μL/well) was added to each well and incubated for 2 h at 37 °C. After washing with PBST, secondary antibody Goat anti-Mouse IgG-HRP (1:10000 dilution, 100 μL/well, TransGen Biotech, Beijing, China) was added and incubated at 37°C for 1 h. Next, TMB (50 μL/well) was added and incubated for 15 min at 37 °C in the dark. The reaction was stopped with 0.5 M H2SO4 and the optical density at 450 nm (OD450) was measured. The ratios of hybridoma supernatants to SP2/0 supernatant (P/N) were calculated, with P/N ≥ 2.0 considered as positive.
For p17 epitope indirect ELISA, ELISA wells were coated with antigenic epitope peptide diluted with PBS at the concentration of 0.3125-10 μg/mL at 4°C overnight, followed by washing and blocking with 5% BSA at 37 °C for 2 h. Diluted porcine serum (1:5-1:800, 100 μL/well) was added and incubated at 37 °C for 2 h. After washing with PBST, secondary antibody Goat anti-Swine IgG-HRP (1:10000, 100 μL/well, Proteintech, Wuhan, China) was added and incubated at 37 °C for 1 h, followed by addition of substrate TMB, stopping with 0.5 M H2SO4 and OD450 measurement. The ratios of positive sera to negative normal serum (P/N) were calculated.
2.8. Bioinformatics Analysis
2.9. Quantification and Statistical Analysis
Statistical analyses was performed by GraphPad Prism 8 software (GraphPad Software, Inc.), with the data presented as means ± SEM. The p values were calculated by using unpaired t test. Statistical significance was represented as: not significant (NS): p > 0.05, *: p ≤ 0.05, and **: p ≤ 0.01. The p ≤ 0.05 was considered as statistically significant.
4. Discussion
African Swine Fever (ASF) has a history of over 100 years since its first discovery in Kenya in 1921 [
1]. Over the following decades, ASF made the leap from Africa to Europe, spreading widely until that it entered China in 2018 [
2]. It is well known that China is a major country for pig farming and product consumption, with pig farming and inventory accounting for more than half of the global total [
17]. The prevalence of ASF in China has caused the mutation and recombination of the ASFV [
18,
19]. At present, the recombinant ASFV of genotype I and II have been found in China, some of which, although classified as genotype I, have inserted multiple virulence genes of genotype II and exhibit high virulence and transmissibility in pigs [
18]. These pose a huge challenge to the prevention and control of ASF.
The inactivated vaccine for ASF has shown poor protective efficacy [
20], and the gene-deleted vaccine has been observed to undergo gene recombination in the field, carrying the risk of virulence reversion [
19,
21]. For the prevention and control of ASF, detection has become a crucial procedure [
22]. Currently, the detection of ASF primarily focuses on the detection of the viral genome or particles, as well as the detection of antibodies related to ASFV [
23]. In subacute and chronic infections, serological detection of ASFV antibodies is a reliable means [
23]. The production of mAbs against ASFV structural proteins, the identification of antigenic epitopes, and the subsequent establishment of detection methods mainly concentrated on structural proteins such as p72 [
24,
25,
26,
27], p54 [
28,
29,
30,
31,
32], p30 [
33,
34,
35], and CD2v [
36,
37,
38], with only few reports on p17. ASFV p17 closely surrounds p72 in a trimeric form and is crucial for the assembly of the ASFV capsid and the formation of the icosahedral morphology [
9]. Besides mediating viral morphogenesis and immune evasion, the expression of p17 also affects various host cell events, including host cell endocytosis, ubiquitin-mediated proteolysis, N-glycan biosynthesis, and apoptosis [
39].
Due to the poor prokaryotic expression of full length p17 (not shown), we discarded the hydrophobic transmembrane region and selected the hydrophilic intramembrane region for fusion with two molecular chaperone proteins, TF and MBP, respectively (
Figure 1). By immunizing with TF-p17 protein and screening of the hybridomas by MBP-p17 based indirect ELISA to reduce the non-specificity, we obtained two p17 specific mAbs, which work successfully in multiple immune assays including ELISA, Western blotting and Immunofluorescence (
Figure 2-4 and
Figure S1), demonstrating the value of broad application. Notably, the mAb recognized p17 in transfected cells and PRRSV-17 infected cells are about 23 kD, whereas the mAb recognized p17 in ASFV infected cells is about 17 kD (
Figure 3A). The size difference of p17 in different expression systems indicated the disparity of post translational modification of p17 protein under different conditions, which is interesting and deserves for further investigation.
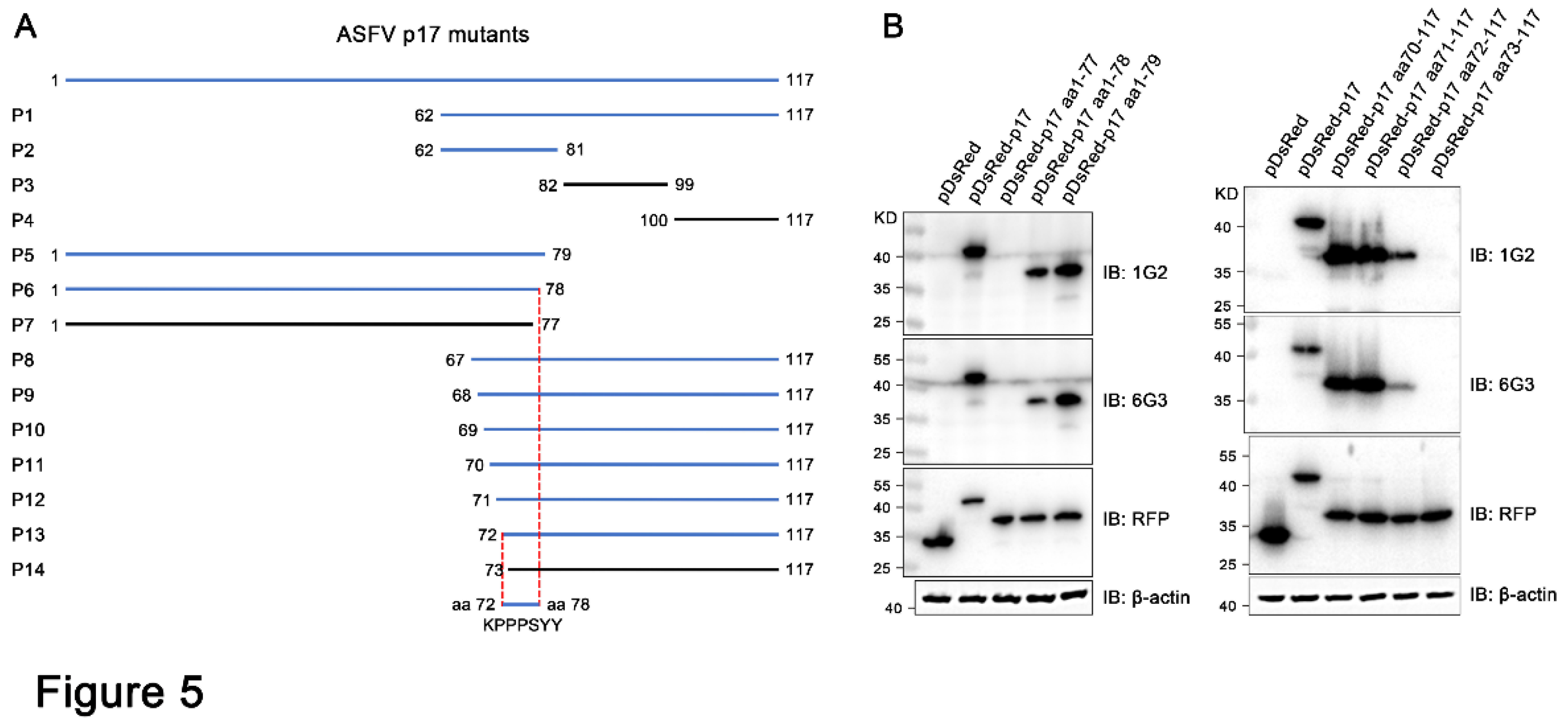
We identified the minimal antigenic epitope recognized by these two p17 mAbs that is
72KPPPSYY
78 (
Figure 5 and
Figure S2). Previous studies identified two antigenic epitopes in p17 that are
3TETSPLLSH
11 and
8LLSHNLSTREGIK
20, by using p17 mAbs [
15,
16]. Consistent with our study, a recent study predicted and confirmed that
63TIDCKSSIPKPPPSYYVQQPEPHH
86 is one of most immunogenic and immunoreactive epitopes [
40], further confirmed the validity of this antigenic epitope. The identification of precise antigenic epitope justified the utilization of the epitope in the serological detection of ASFV infection. Based on the highly conserved antigenic epitope recognized by p17 mAbs (
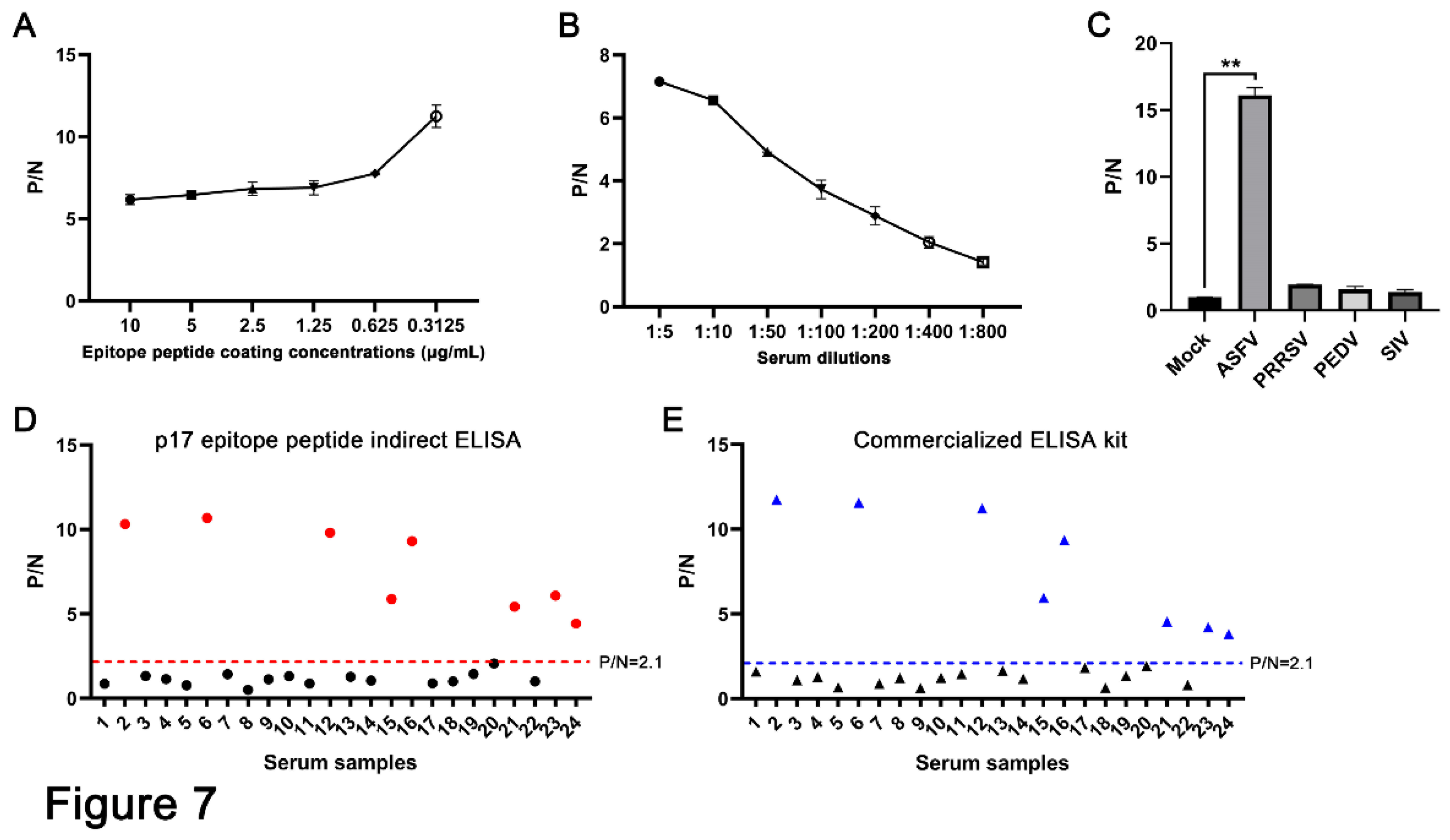
Figure 6), we established an indirect ELISA to detect the antibodies against ASFV (
Figure 7). In detection of 24 clinical serum samples from pigs, 9 of 24 serum samples were detected as positive (
Figure 7D). The results were totally consistent with that of commercial ELISA kit (
Figure 7E), suggesting the effectiveness and validity of our peptide ELISA assay and the potential for practical application in clinic. Recently, our team has developed both RPA-LbCas12a and RPA-LwCas13a detection methods based on the ASFV structural protein p17 gene (D117L), reaching a sensitivity as high as two gene copies, showing no cross-reactivity with other common pig viruses [
41,
42]. Thus, the combined detections of nucleic acid and antibody based on the ASFV structural D117L gene / p17 protein can greatly improve the detection of ASFV infection, achieving convenience and precision.
Collectively, we generated two specific mAbs of ASFV p17, identified a highly conserved B-cell epitope of p17, and established an epitope based ELISA detecting ASFV antibodies. These tools not only provide effective means for in-depth research on the structural p17 protein of ASFV, but also contribute to the prevention and control of ASF.
Figure 1.
Production and identification of the p17 truncated fusion proteins. (A-C) Analysis of the transmembrane region (A), hydrophilicity (B), and B cell epitopes (C) of p17 protein using online tools, as described in Methods. (D and E) The TF-p17 jd (D) and MBP-p17 jd (E) were induced by IPTG at a final concentration of 1mM at 37 ℃ and 16 ℃. Whole cell lysate, supernatant, and precipitate were examined for p17 protein expressions by SDS-PAGE and Coomassie blue staining, with the major band about 68 kD (D) and 53 kD (E) which are red boxed. (F) The purified p17 fusion proteins were verified by SDS-PAGE and Coomassie blue staining against standard bovine serum albumin (BSA). The concentration of TF-p17 jd protein is greater than 1 μg/μL, while the concentration of MBP-p17 jd protein is approximately 500 ng/μL.
Figure 1.
Production and identification of the p17 truncated fusion proteins. (A-C) Analysis of the transmembrane region (A), hydrophilicity (B), and B cell epitopes (C) of p17 protein using online tools, as described in Methods. (D and E) The TF-p17 jd (D) and MBP-p17 jd (E) were induced by IPTG at a final concentration of 1mM at 37 ℃ and 16 ℃. Whole cell lysate, supernatant, and precipitate were examined for p17 protein expressions by SDS-PAGE and Coomassie blue staining, with the major band about 68 kD (D) and 53 kD (E) which are red boxed. (F) The purified p17 fusion proteins were verified by SDS-PAGE and Coomassie blue staining against standard bovine serum albumin (BSA). The concentration of TF-p17 jd protein is greater than 1 μg/μL, while the concentration of MBP-p17 jd protein is approximately 500 ng/μL.
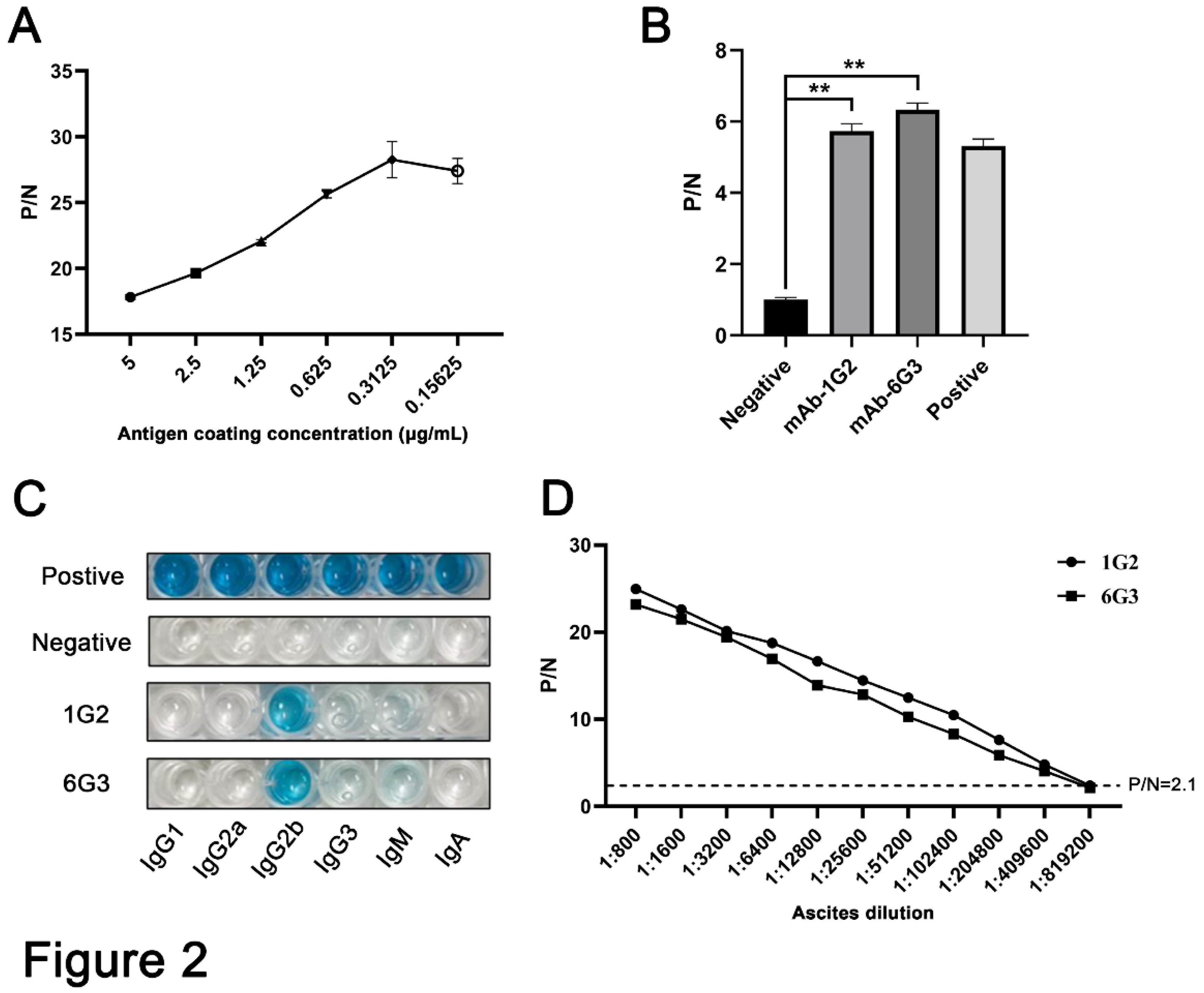
Figure 2.
Characterization of anti-p17 monoclonal antibodies. (A) Optimization of the amount of coating MBP-p17 jd protein in indirect ELISA for detection of ASFV positive serum. (B) The mAbs were tested in MBP-p17 protein based indirect ELISA. The cell supernatants of hybridoma clones 1G2 and 6G3 were used as the primary antibodies, the SP2/0 cell supernatant was used as the negative control, and the serum of immunized mice was used as positive control. **p < 0.01. (C) Subclass of p17 mAbs 1G2 and 6G3 was determined by the monoclonal antibody subclass identification kit (C060101) from CELLWAY-LAB (Luoyang, China). (D) Measurement of the titers of ascite mAbs 1G2 and 6G3 by MBP-p17 protein indirect ELISA. The dotted line denotes the P/N value of 2.1.
Figure 2.
Characterization of anti-p17 monoclonal antibodies. (A) Optimization of the amount of coating MBP-p17 jd protein in indirect ELISA for detection of ASFV positive serum. (B) The mAbs were tested in MBP-p17 protein based indirect ELISA. The cell supernatants of hybridoma clones 1G2 and 6G3 were used as the primary antibodies, the SP2/0 cell supernatant was used as the negative control, and the serum of immunized mice was used as positive control. **p < 0.01. (C) Subclass of p17 mAbs 1G2 and 6G3 was determined by the monoclonal antibody subclass identification kit (C060101) from CELLWAY-LAB (Luoyang, China). (D) Measurement of the titers of ascite mAbs 1G2 and 6G3 by MBP-p17 protein indirect ELISA. The dotted line denotes the P/N value of 2.1.
Figure 3.
Analysis of specific reactivity of p17 mAbs by Western blotting. (A-C) 293T cells were transfected with pCAGGS-p17-HA (1μg/mL) (A) and pDsRed-p17 (1μg/mL) (B) with pCAGGS vector and pDsRed vector as the controls. Marc-145 cells were infected with PRRSV-p17 at a multiplicity of infection (MOI) of 0.1 for 72 h (C). Cell were harvested and cell lysates were detected for exogenous p17 by Western blotting with 1G2 and 6G3 mAbs as primary antibodies. (D) Primary PAMs were infected with ASFV (0.1 MOI) or mock infected for 96 h, and cell lysates were detected for endogenous p17 by Western blotting with the mAbs 1G2 and 6G3 as primary antibodies. (E) Primary PAMs, Marc-145 cells, Vero cells, PK15 cells and MDCK cells were infected with ASFV (MOI 0.1), PRRSV (MOI 0.1), PEDV (MOI 0.1), PDCoV (MOI 0.1) and SIV (MOI 0.1), respectively. Cell were harvested and cell lysates were detected the specificity of p17 mAbs by Western blotting with 1G2 and 6G3 mAbs as primary antibodies. The replications of PRRSV, PEDV, PDCoV and SIV were detected by Western blotting with the indicated antibodies.
Figure 3.
Analysis of specific reactivity of p17 mAbs by Western blotting. (A-C) 293T cells were transfected with pCAGGS-p17-HA (1μg/mL) (A) and pDsRed-p17 (1μg/mL) (B) with pCAGGS vector and pDsRed vector as the controls. Marc-145 cells were infected with PRRSV-p17 at a multiplicity of infection (MOI) of 0.1 for 72 h (C). Cell were harvested and cell lysates were detected for exogenous p17 by Western blotting with 1G2 and 6G3 mAbs as primary antibodies. (D) Primary PAMs were infected with ASFV (0.1 MOI) or mock infected for 96 h, and cell lysates were detected for endogenous p17 by Western blotting with the mAbs 1G2 and 6G3 as primary antibodies. (E) Primary PAMs, Marc-145 cells, Vero cells, PK15 cells and MDCK cells were infected with ASFV (MOI 0.1), PRRSV (MOI 0.1), PEDV (MOI 0.1), PDCoV (MOI 0.1) and SIV (MOI 0.1), respectively. Cell were harvested and cell lysates were detected the specificity of p17 mAbs by Western blotting with 1G2 and 6G3 mAbs as primary antibodies. The replications of PRRSV, PEDV, PDCoV and SIV were detected by Western blotting with the indicated antibodies.
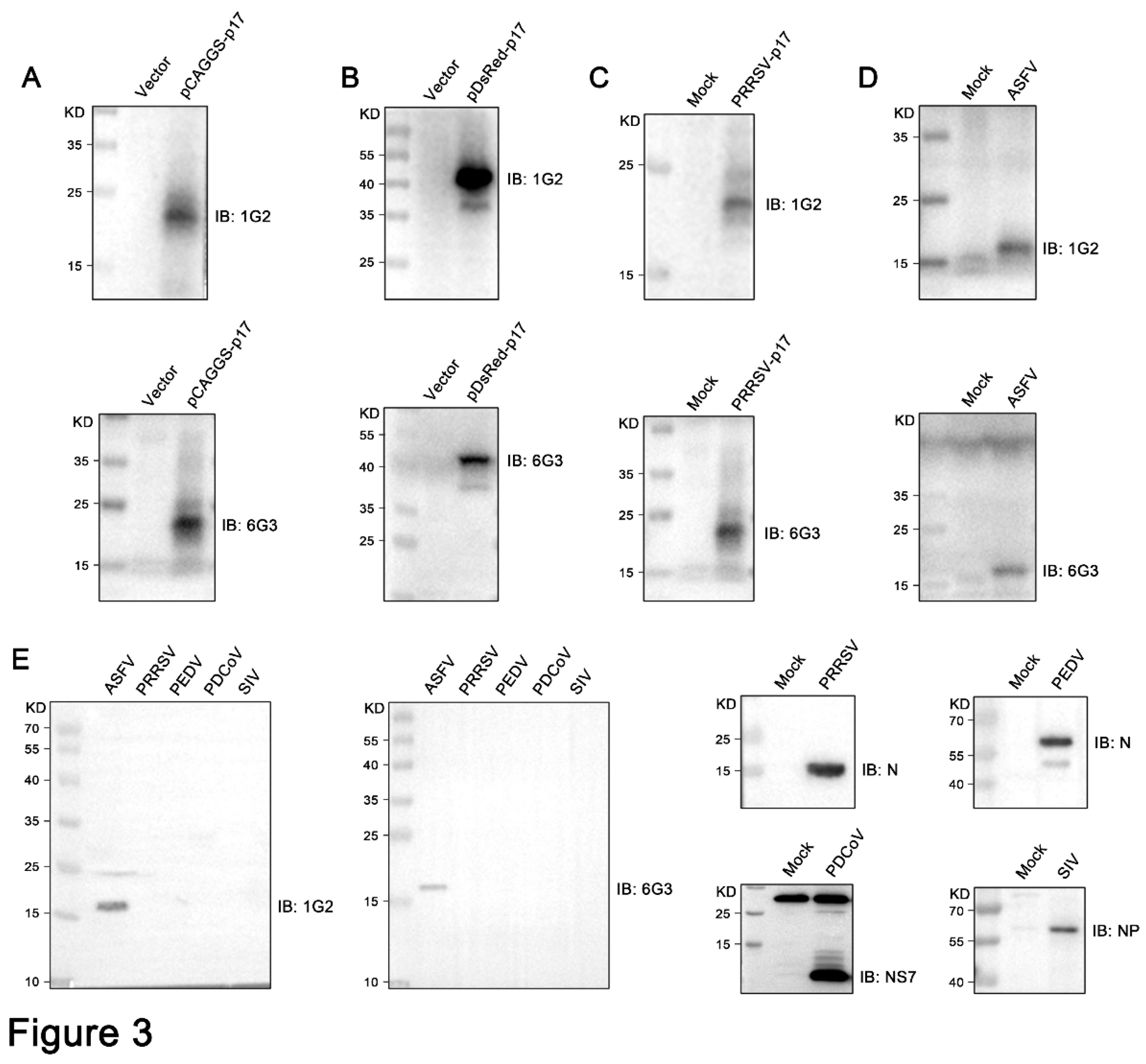
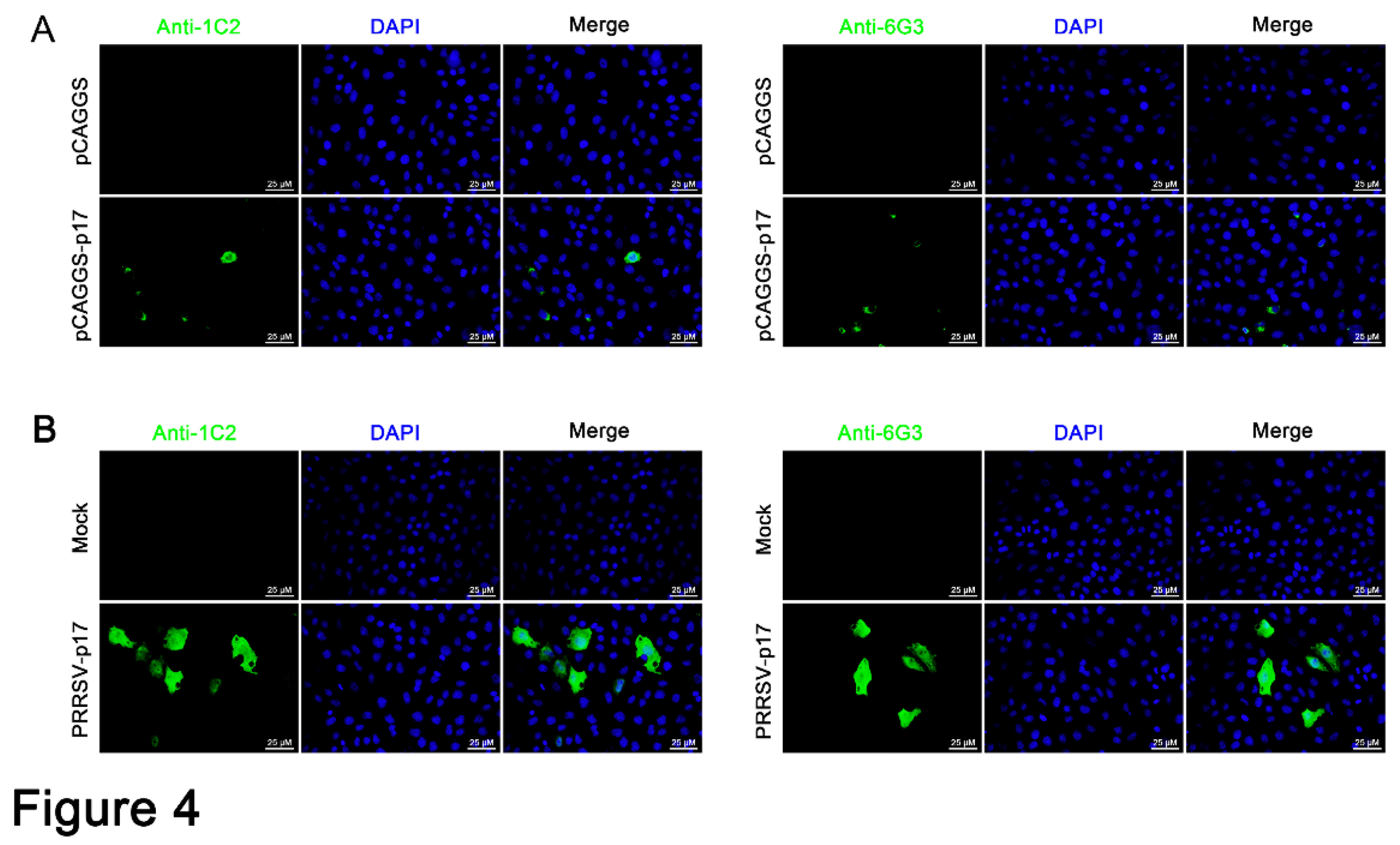
Figure 4.
Analysis of specific reactivity of p17 mAbs by Immunofluorescence. (A) 3D4/21 cells were transfected pCAGGS-p17-HA and pCAGGS vector, respectively. Cells were fixed at 24 h post-transfection and stained with 1G2 or 6G3 mAbs, together with Goat anti-mouse IgG H&L Alexa Fluor 488. Cellular nuclei were counterstained with 4’,6’-diamidino-2-phenylindole (DAPI). (B) Marc-145 cells were infected with PRRSV-p17 (MOI 0.1). Cells were fixed at 72 h post-infection and stained with 1G2 or 6G3, together with secondary antibody and DAPI.
Figure 4.
Analysis of specific reactivity of p17 mAbs by Immunofluorescence. (A) 3D4/21 cells were transfected pCAGGS-p17-HA and pCAGGS vector, respectively. Cells were fixed at 24 h post-transfection and stained with 1G2 or 6G3 mAbs, together with Goat anti-mouse IgG H&L Alexa Fluor 488. Cellular nuclei were counterstained with 4’,6’-diamidino-2-phenylindole (DAPI). (B) Marc-145 cells were infected with PRRSV-p17 (MOI 0.1). Cells were fixed at 72 h post-infection and stained with 1G2 or 6G3, together with secondary antibody and DAPI.
Figure 5.
Precise mapping of the antigenic epitopes recognized by p17 monoclonal antibodies. (A) Schematic strategy for precise mapping the epitope. The fragments with reactivity with p17 mAbs are blue marked, whereas those without reactivity with mAbs are black marked. (B) Western blotting analysis of the critical C terminal amino acid (left) and N terminal amino acid (right) for reactivity of p17 proteins with the two mAbs 1G2 and 6G3. The aa is an abbreviation for amino acid.
Figure 5.
Precise mapping of the antigenic epitopes recognized by p17 monoclonal antibodies. (A) Schematic strategy for precise mapping the epitope. The fragments with reactivity with p17 mAbs are blue marked, whereas those without reactivity with mAbs are black marked. (B) Western blotting analysis of the critical C terminal amino acid (left) and N terminal amino acid (right) for reactivity of p17 proteins with the two mAbs 1G2 and 6G3. The aa is an abbreviation for amino acid.
Figure 6.
Conservation analysis of the identified novel linear epitope of the p17 protein. (A) Alignment of the epitope (72KPPPSYY78) in 30 representative genotypes I and II ASFV strains. The red box indicated the identified conserved epitope. (B) Prediction of the p17 structure by using Alphafold2 online web tools and visualization using PyMOL. The epitope recognized by two mAbs is displayed in pink color, and shown in the front view (a) and in flipping 180° along the X-axis (b), respectively.
Figure 6.
Conservation analysis of the identified novel linear epitope of the p17 protein. (A) Alignment of the epitope (72KPPPSYY78) in 30 representative genotypes I and II ASFV strains. The red box indicated the identified conserved epitope. (B) Prediction of the p17 structure by using Alphafold2 online web tools and visualization using PyMOL. The epitope recognized by two mAbs is displayed in pink color, and shown in the front view (a) and in flipping 180° along the X-axis (b), respectively.
Figure 7.
Establishment of epitope indirect ELISA for detecting of ASFV antibodies. (A) The p17 epitope peptide (synthesized by GeneCreate Wuhan China) was used for coating at the concentrations from 0.3125-10 μg/mL. The epitope ELISA was tested for detection of ASFV positive serum (1:5 dilution) and negative normal pig serum to determine the optimized concentration of coating epitope peptide. (B) The optimization of serum dilution in indirect ELISA with peptide coating concentration at 0.3125 μg/mL. (C) The specificity of the established epitope based indirect ELISA. PRRSV, PEDV, SIV and ASFV positive pig sera and negative pig serum were used. **p < 0.01. (D and E) 24 clinical pig serum samples were tested using our established indirect epitope peptide ELISA (D) and the commercial ASFV ELISA detection kit (E), with pig negative serum as the control. The samples marked in red (D) and blue (E) are those with positive detection results.
Figure 7.
Establishment of epitope indirect ELISA for detecting of ASFV antibodies. (A) The p17 epitope peptide (synthesized by GeneCreate Wuhan China) was used for coating at the concentrations from 0.3125-10 μg/mL. The epitope ELISA was tested for detection of ASFV positive serum (1:5 dilution) and negative normal pig serum to determine the optimized concentration of coating epitope peptide. (B) The optimization of serum dilution in indirect ELISA with peptide coating concentration at 0.3125 μg/mL. (C) The specificity of the established epitope based indirect ELISA. PRRSV, PEDV, SIV and ASFV positive pig sera and negative pig serum were used. **p < 0.01. (D and E) 24 clinical pig serum samples were tested using our established indirect epitope peptide ELISA (D) and the commercial ASFV ELISA detection kit (E), with pig negative serum as the control. The samples marked in red (D) and blue (E) are those with positive detection results.