Submitted:
28 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
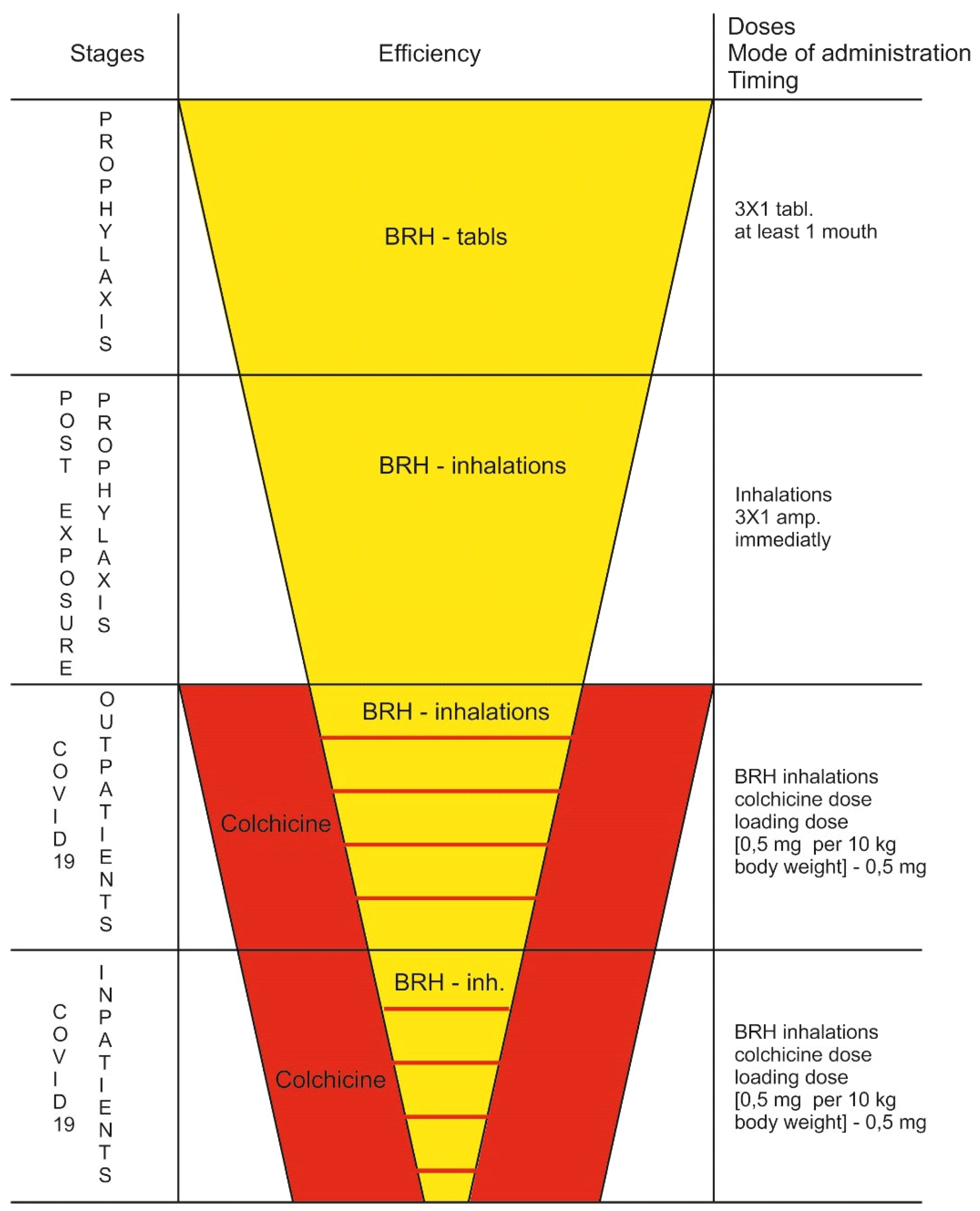
Prophylactic Role of BRH
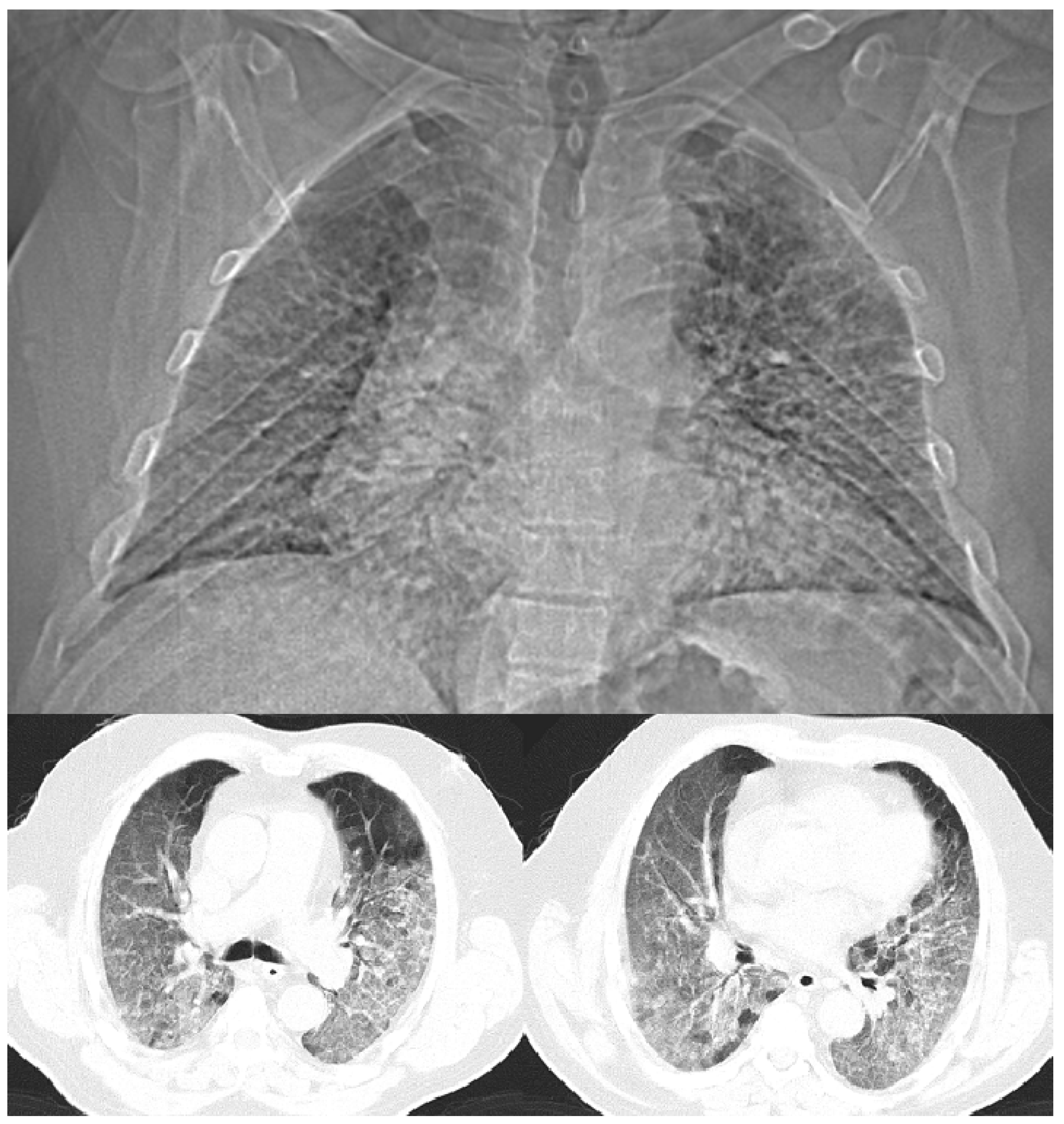
Life-Saving Effect of High-Dose Colchicine
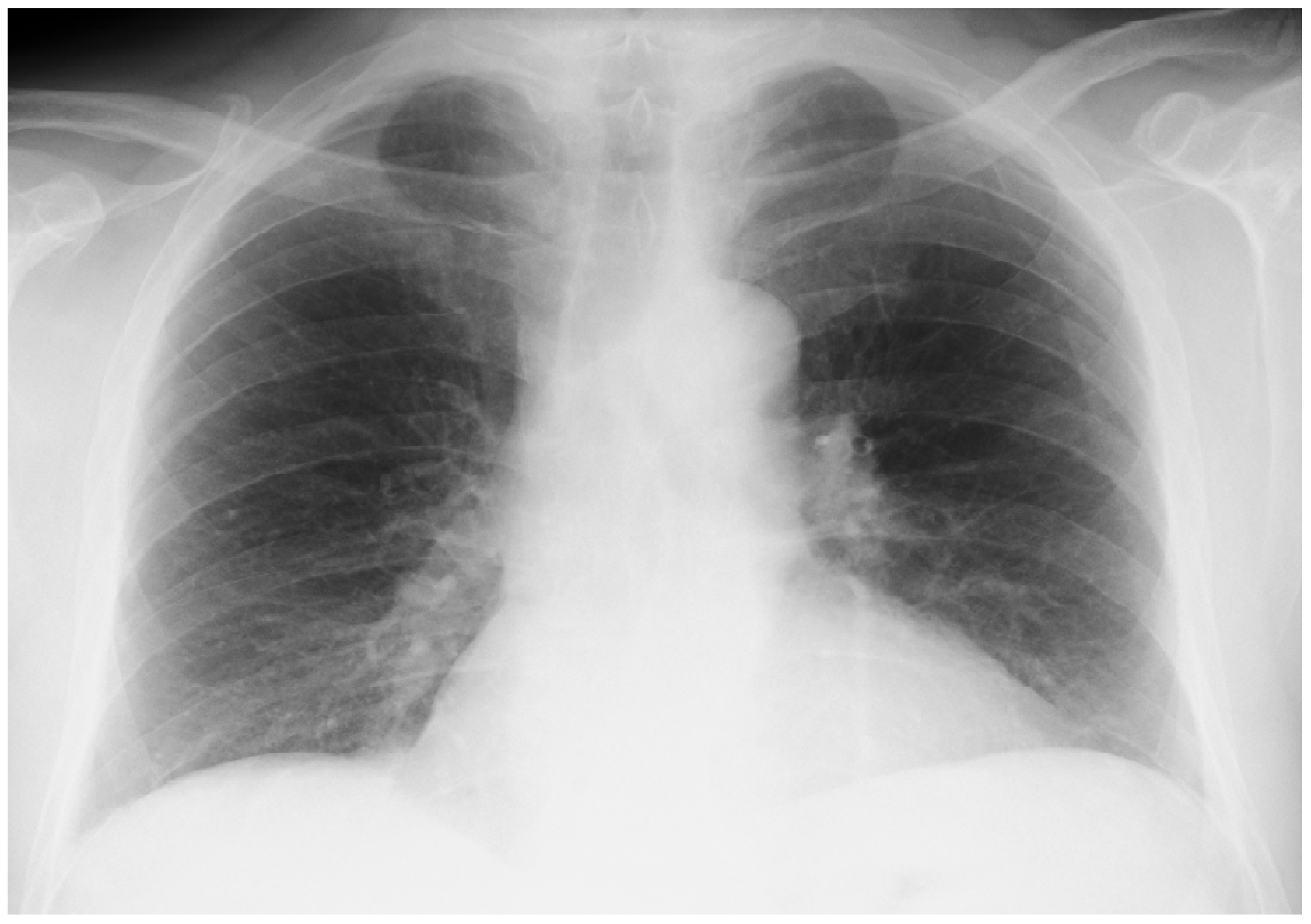
Onset of the Disease
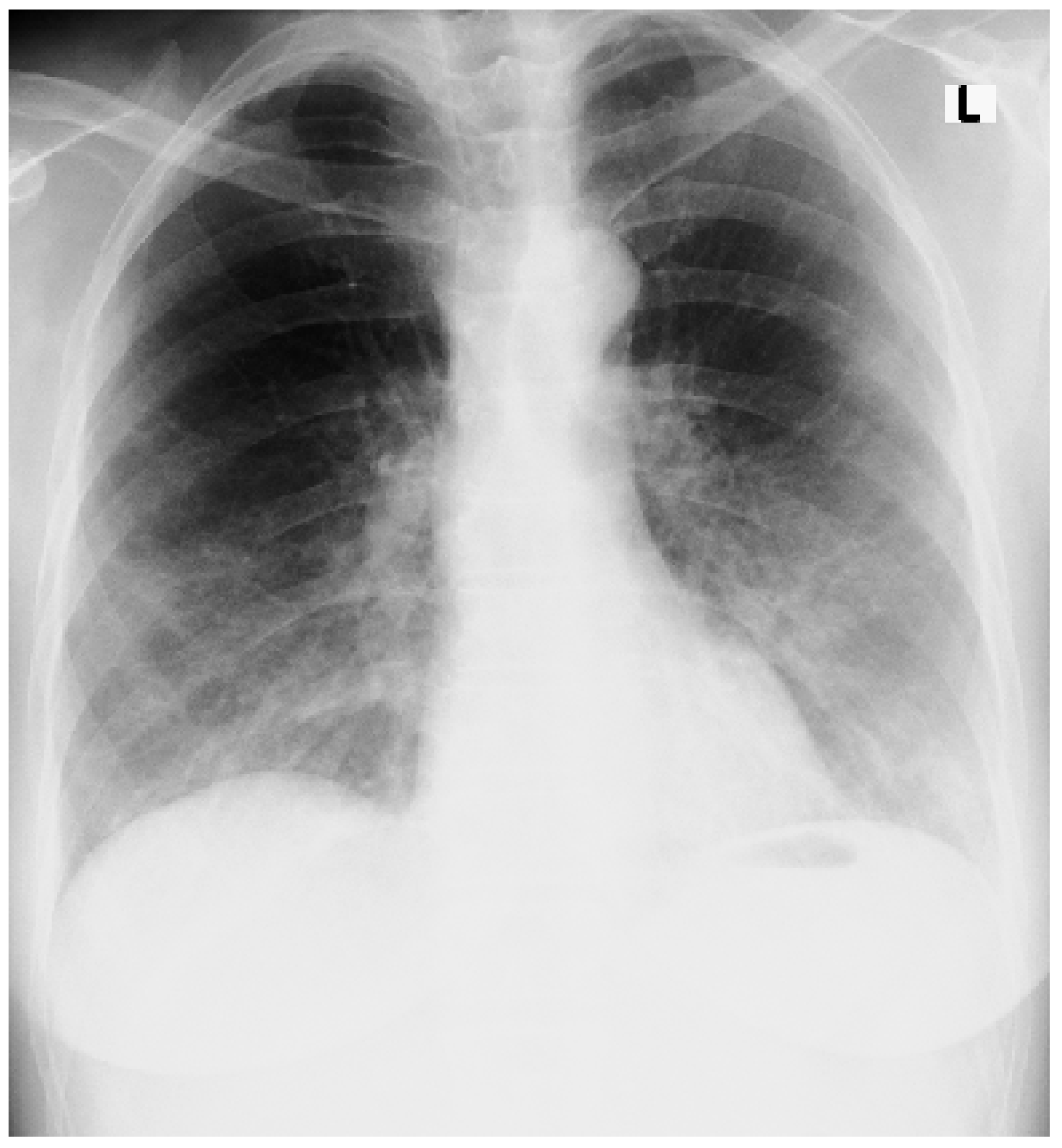
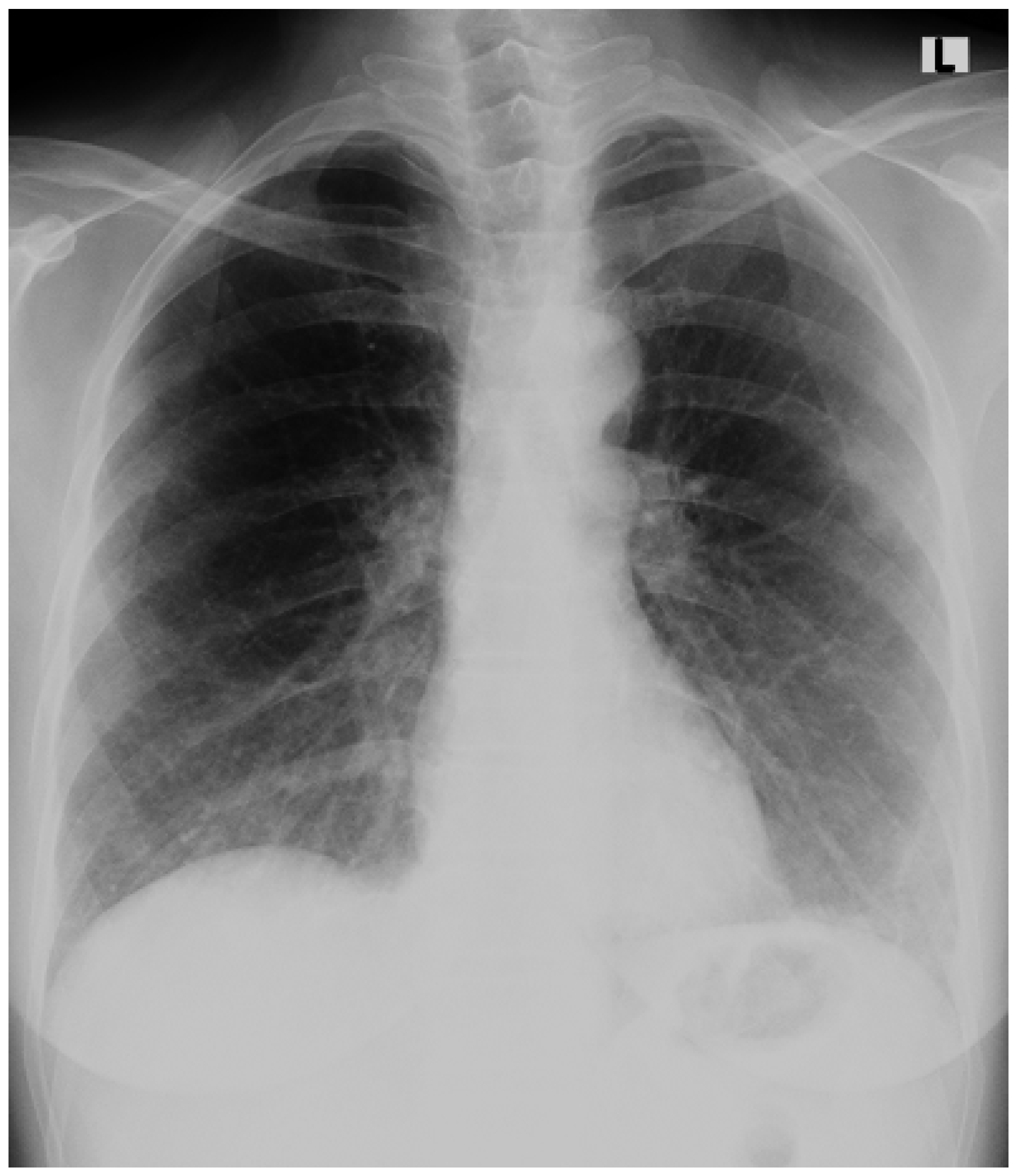
An Accidental Overdose of Colchicine Results in Immediate Recovery of the Patient
Discussion
Acknowledgements
References
- Morgenstern, J. Paxlovid evidence: Still very little reason to prescribe. First10EM. 2024. [CrossRef]
- Sax, P. The Rise and Fall of Paxlovid—HIV and ID Observations. NEJM Journal Watch. 2024. Available online: https://blogs.jwatch.org (accessed on 3 June 2024).
- Mitev, V. Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib. Pharmacia. 2023, 70:1177-1193. [CrossRef]
- Mondeshki, T.; Bilyukov, R.; Tomov, T.; et al. Complete, rapid resolution of severe bilateral pneumonia and acute respiratory distress syndrome in a COVID-19 patient: role for a unique therapeutic combination of inhalations with bromhexine, higher doses of colchicine, and hymecromone. Cureus. 2022, 14:e30269. [CrossRef]
- Mitev, V.; Mondeshki, T.; Marinov, K.; et al. Colchicine, bromhexine, and hymecromone as part of COVID19 treatment - cold, warm, hot. Current Overview on Disease and Health Research. Khan BA (ed): BP International, London, UK; 2023, 10:106-13.
- Tiholov, R.; Lilov, AI.; Georgieva, G.; et al. Effect of increasing doses of colchicine on thetreatment of 333 COVID-19 inpatients. Immun Inflamm Dis. 2024, 12: e1273.
- Mondeshki, T.; Bilyukov, R.; Mitev, V. Effect of an Accidental Colchicine Overdose in a COVID-19 Inpatient With Bilateral Pneumonia and Pericardial Effusion. Cureus. 2023, 15:e35909. [CrossRef]
- Mitev, V. What is the lowest lethal dose of colchicine? Biotechnol & Biotechnol Equip. 2023, 37, 1:2288240. [CrossRef]
- Lilov, A.; Palaveev, K.; Mitev, V. High Doses of Colchicine Act As “Silver Bullets” Against Severe COVID-19. Cureus. 2024, 16:e54441. [CrossRef]
- Mondeshki, T.; Mitev, V. High-Dose Colchicine: Key Factor in the Treatment of Morbidly Obese COVID-19 Patients. Cureus. 2024, 16:e58164. [CrossRef]
- Bulanov, D.; Yonkov, A.; Arabadzhieva, E.; et al. Successful Treatment With High-Dose Colchicine of a 101-Year-Old Patient Diagnosed With COVID-19 After an Emergency Cholecystectomy. Cureus. 2024, 16:e63201. [CrossRef]
- Mitev, V. Colchicine—The Divine Medicine against COVID-19. J Pers Med. 2024, 14:756. [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020, 181:271–280. [CrossRef]
- Lucas, JM.; Heinlein, C.; Kim, T.; et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microen-vironment and promotes prostate cancer metastasis. Cancer Discovery. 2014, 4:1310–1325. [CrossRef]
- Depfenhart, M.; De Villiers, D.; Lemperle, G.; et al. Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy? Intern Emerg Med. 2020, 15:801–812. [CrossRef]
- Tolouian, R.; Moradi, O.; Mulla, ZD.; et al. Bromhexine for post-exposure COVID-19 prophylaxis: A randomized, double-blind, placebo-controlled trial. Jundishapur J Microbiol. 2022, 15:e130198.
- Mikhaylov, EN.; Lyubimtseva, TA.; Vakhrushev, AD.; et al. Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: A randomized open-label study. Interdiscip Perspect Infect Dis. 2022, 4693121. [CrossRef]
- Mitev, V.; Mondeshki, T.; Miteva, A.; et al. COVID-19 Prophylactic effect of Bromhexine Hydrochloride Preprints. 2024. [CrossRef]
- Freeman, TL.; Swartz, TH. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11:1518. [CrossRef]
- Leung, YY.; Hui, LLY.; Kraus, VB. Colchicine—update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015, 45:341-350. [CrossRef]
- Rabbani, AB.; Oshaughnessy, M.; Tabrizi, R.; et al. Colchicine and COVID-19: A Look Backward and a Look Ahead. Medical Research Archives. 2024, 12 (9).
| Marker | Unit | Min. | Max. | Day 1 | Day 2 | Day 5 |
| 4751-PCR test for COVID-19 (SARS-CoV-2 RNA) | (+) positive | |||||
| Leukocytes (Leu) | 109/L | 3.5 | 10.5 | 5.0 | 5.7 | 8.8 |
| Erythrocytes (Er) | 1012/L | 4.4 | 5.9 | 4.8 | 4.7 | 5.1 |
| Hemoglobin (Hb) | g/L | 135 | 180 | 160 | 153 | 150 |
| Hematocrit (Ht) | g/L | 0.4 | 0.53 | 0.44 | 0.45 | 0.44 |
| MCV | fL | 82 | 96 | 91 | 87 | 88 |
| MCH | pg | 27 | 33 | 34 | 31 | 30 |
| MCHC | g/L | 300 | 360 | 366 | 356 | 345 |
| Platelets (Tr) | 109/L | 130 | 440 | 180 | 160 | 141 |
| NE % - Neutrophil granulocytes % | % | 40 | 70 | 82.9 | 84.6 | 88.2 |
| EO % - Eosinophilic granulocytes % | % | 0 | 6.5 | 0 | 0.0 | 0.0 |
| BA% - Basophilic granulocytes % | % | 0 | 2 | 1 | 0 | 0 |
| MO % - Monocytes % | % | 1 | 11 | 5 | 6 | 6 |
| LY % - Lymphocytes % | % | 20 | 48 | 11 | 9 | 5.2 |
| NE# -Neutrophil granulocytes-count | 109/L | 2 | 7 | 4.16 | 4.82 | 7.76 |
| ЕО# -Eosinophilic granulocytes-count | 109/L | 0 | 0.5 | 0.1 | 0.0 | 0.0 |
| BA# -Basophilic granulocytes-count | 109/L | 0 | 0.14 | 0.05 | 0 | 0.00 |
| MO# -Monocytes-count | 109/L | 0 | 0.8 | 0.25 | 0.34 | 0.53 |
| LY# -Lymphocytes-count | 109/L | 1 | 4 | 1.4 | 0.51 | 0.46 |
| IG (%) - Immature granulocytes - % | % | 0 | 5 | 0.1 | 0.4 | 0.6 |
| IG # - Immature granulocytes - count | 109/L | 0 | 0.7 | 0.0 | 0.02 | 0.0 |
| D-dimer | μg/L | 0 | 0.55 | 1.2 | 2.1 | 3.1 |
| Creatinine - serum | μmol/L | 62 | 106 | 196 | 201 | 210 |
| pH | 7.35 | 7.45 | 7.4 | 7.45 | 7.3 | |
| pCO2 | kPa | 4.67 | 6 | 3.5 | 4.11 | 3.9 |
| pO2 | kPa | 10 | 13 | 6.75 | 5.6 | 5.2 |
| SB | mmol/L | 21 | 25 | 20.5 | 23.7 | 28 |
| BE (w) | mmol/L | -2.5 | 2.5 | -3.3 | 4.1 | 5.1 |
| O2 Sat | % | 94 | 98 | 91 | 60 | 55 |
| tCO2 | mmol/L | 20 | 27 | 19.5 | 21.5 | 25 |
| CRP | mg/L | 0 | 5 | 56 | 148.1 | 231 |
| LDH | E/L | 140 | 370 | 577 | 1893 | 2012 |
| ESR | mm/h | 0 | 20 | 92 | ||
| ALAT | U/L | 0 | 40 | 66 | 84 | 102 |
| ASAT | U/L | 0 | 40 | 56 | 68 | 83 |
| Marker | Unit | Min. | Max. | Day 1 | Day 4 | Day 8 |
| 4751-PCR тест за COVID-19 (SARS-CoV-2 RNA) | (+) positive | (-) negative | ||||
| Leukocytes (Leu) | 109/L | 3.5 | 10.5 | 7.8 | 8.1 | 9.2 |
| Erythrocytes (Er) | 10^12/L | 4.4 | 5.9 | 4.8 | 4.9 | 4.7 |
| Hemoglobin (Hb) | g/L | 135 | 180 | 123 | 121 | 120 |
| Hematocrit (Ht) | g/L | 0.4 | 0.53 | 0.39 | 0.41 | 0.4 |
| MCV | fL | 82 | 96 | 81 | 82 | 85 |
| MCH | pg | 27 | 33 | 26 | 28 | 27 |
| MCHC | g/L | 300 | 360 | 315 | 324 | 315 |
| Platelets (Tr) | 109/L | 130 | 440 | 316 | 340 | 354 |
| NE % - Neutrophil granulocytes % | % | 40 | 70 | 78.4 | 74.9 | 62 |
| EO % - Eosinophilic granulocytes % | % | 0 | 6.5 | 2.1 | 0.0 | 0.0 |
| BA% - Basophilic granulocytes % | % | 0 | 2 | 1 | 0 | 0 |
| MO % - Monocytes % | % | 1 | 11 | 7.1 | 8 | 10.5 |
| LY % - Lymphocytes % | % | 20 | 48 | 11 | 15.9 | 26 |
| NE# -Neutrophil granulocytes-count | 109/L | 2 | 7 | 6.12 | 6 | 5.7 |
| ЕО# -Eosinophilic granulocytes-count | 109/L | 0 | 0.5 | 0.16 | 0.0 | 0.0 |
| BA# -Basophilic granulocytes-count | 109/L | 0 | 0.14 | 0.08 | 0.01 | 0.00 |
| MO# -Monocytes-count | 109/L | 0 | 0.8 | 0.55 | 0.65 | 0.96 |
| LY# -Lymphocytes-count | 109/L | 1 | 4 | 0.86 | 1.28 | 2.39 |
| IG (%) - Immature granulocytes - % | % | 0 | 5 | 0.4 | 1.2 | 1.5 |
| IG # - Immature granulocytes - count | 109/L | 0 | 0.7 | 0.0 | 0.1 | 0.14 |
| D-dimer | μg/L | 0 | 0.55 | 1.5 | 1.1 | 0.85 |
| Creatinine - serum | μmol/L | 62 | 106 | 89 | 95 | 82 |
| pH | 7.35 | 7.45 | 7.4 | 7.41 | 7.39 | |
| pCO2 | kPa | 4.67 | 6 | 4.1 | 4.5 | 4.5 |
| pO2 | kPa | 10 | 13 | 7.8 | 8.1 | 8.4 |
| SB | mmol/L | 21 | 25 | 20.5 | 24 | 25 |
| BE (w) | mmol/L | -2.5 | 2.5 | 2.8 | 2.5 | 2.3 |
| O2 Sat | % | 94 | 98 | 91 | 92 | 94 |
| tCO2 | mmol/L | 20 | 27 | 23 | 25 | 24 |
| CRP | mg/L | 0 | 5 | 22 | 15 | 9 |
| LDH | E/L | 140 | 370 | 572 | 511 | 480 |
| ESR | mm/h | 0 | 20 | 40 | 22 | |
| ALAT | U/L | 0 | 40 | 45 | 41 | 42 |
| ASAT | U/L | 0 | 40 | 35 | 32 | 38 |
| blood glucose | mmol/L | 3.9 | 5.6 | 5.5 | 6.5 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





