1. Introduction
The genus
Oncidium comprises approximately 330 species within the subtribe Oncidiinae of the Orchidaceae family [
1]. The
Oncidesa Gower Ramsey varieties 'Honey Angel' (HA), 'Golden Star' (GS), and 'Apollo' (AP) are extensively cultivated in Taiwan, covering approximately 250 hectares. Annually, this results in the production of 57 million cut flowers, with an output value of around US
$15 million. Taiwan primarily exports
Oncidium cut flowers to countries such as Japan and the Netherlands, with a market share of up to 95% in Japan. Taiwan's original cultivation environment was well-suited for
Oncidium growth. However, due to climate change, rising temperatures and extended summers, now lasting up to seven months, have introduced challenges in
Oncidium production. This has compelled farmers to adopt more precise and scientific production models to mitigate these impacts. These cultivars typically undergo a juvenile phase lasting 1.5 to 2 years, after which flowering occurs at the end of each growth cycle when the pseudobulb reaches full maturity.
Oncidium species exhibit heteroblastic sympodial growth. During vegetative shoot development, an inflorescence bud is initiated prior to the expansion of the pseudobulb. Inflorescence elongation takes place as the pseudobulb approaches full expansion, with the inflorescence emerging either from the base of the pseudobulb or from the leaf axil. Flower sizes can range from 1 cm to 12.5 cm [
2]. Typically, a single inflorescence emerges from one site, although, in some cases, two inflorescences may develop. In
Honey Angel plants, pseudobulb development occurs between the third and fourth leaves from the top, and inflorescences are produced twice a year [
3]. Li and Chang (2023) [
3] observed that in
Honey Angel, the pseudobulb begins to elongate once the current shoot reaches 20 cm, coinciding with a marked increase in inflorescence bud length and node number. Moreover, when the shoot length reaches 30 cm, the pseudobulb emerges from the leaf sheath (unsheathing), which accelerates inflorescence bud development. Thus, assessing the current shoot length provides a reliable indicator of the developmental stage of the inflorescence bud.
Light is a critical abiotic factor influencing plant growth, development, morphogenesis, and physiological characteristics.
Oncidium species are semi-shade plants that generally thrive under indirect or filtered light conditions, with optimal daytime and nighttime temperatures of 26.6 °C and 12.8 °C, respectively [
4]. In Taiwan, most
Oncidium are cultivated under 50-80% shade during the summer months in polythene sheet or shade net screenhouses, where relative humidity is maintained at 75%. Previous observations showed that
Honey Angel (HA) plants experienced stunted growth during hot summer afternoons, with seasonal fluctuations in solar radiation negatively impacting production schedules by reducing flower yield and quality. To address this, a double-layer shade net was used to lower temperatures; however, this intervention led to a decline in stemming rates and suboptimal cut flower quality [
4]. Subsequent studies were conducted to assess the growth and flowering quality of HA and
Golden Star (GS) cultivars under different fertilization treatments, and at light intensities (LI) of 40% and 30% photosynthetic photon flux density (PPFD) over a 5-month period in a greenhouse. The results indicated significant differences in growth traits and cultivar responses to varying LIs. Optimal growth, development, and flowering performance of
Oncidium were achieved by adjusting fertilization treatments according to LI levels. Notably, HA produced a significantly higher number of branches and florets per plant under 40% LI compared to the control [
4].
In addition, we evaluated the growth and flowering characteristics of
Honey Angel (HA) pseudobulbs at three growth stages under two supplementary lighting treatments: red/white-medium blue and red/white-low blue, applied for 1-hour and 2-hour intervals [
5]. The results demonstrated that these lighting treatments had a significant effect on pseudobulb length and flower number at different stages of growth. These findings suggest that HA plants may adjust their circadian rhythms and oscillator systems in response to artificial supplementary lighting, allowing them to develop adaptive mechanisms suited to tropical environments and maintain consistent growth across various stages. Our previous study also demonstrated that both varieties were well-suited for prolonged irradiation (1 hr) at 500 µmol photons m⁻² s⁻¹, resulting in faster dark recovery of the photosystem and reduced photoinhibition effects. Consequently, the intensity of artificial lighting in light cultivation technologies should be maintained within the range of 300 to 500 µmol photons·m⁻²·s⁻¹ to prevent damage to the photosynthetic system from severe light-induced inhibition [
6].
In this study, we employed cultural practices to regulate the growth and flowering characteristics of Oncidium by manipulating the development of vegetative shoots and inflorescences in a greenhouse environment. Pseudobulbs and flowers at various developmental stages may exhibit differential responses to diverse canopy treatments, thereby influencing axillary bud development and morphological traits, ultimately enhancing inflorescence productivity in Oncidium. Optimizing plant growth and flowering can be achieved through the application of artificial supplemental lighting, which facilitates year-round flower production, allows for precise control of flowering timing, and enables growers to regulate flowering in alignment with market demand for Oncidium.
2. Materials and Methods
2.1. Plant Materials and Cultural Practices
The experiment was conducted over a 5-month period, from March to September 2023. The
Oncidesa cultivars used in the study had been cultivated long-term at our nurseries at Da-Lin Farm, located at the National Chia-Yi Station in Chia-Yi City, Taiwan (23°35'51.7"N, 120°30'13.7"E). When the plants reached two years of age and grew to a height of 10–20 cm with four to five leaves, they were transplanted into 16 cm free-draining plastic pots (1.6 L capacity, one plant per pot) containing a commercial potting mix of crushed stone and charcoal in a 3:1 ratio. The plants were then placed in an environmentally controlled greenhouse at Da-Lin Farm, maintained under an 8-hour photoperiod, with day/night temperatures set to 30/25°C, relative humidity at 80%, and a PPFD of 320 μmol·m⁻²·s⁻¹. The pots were evenly spaced to ensure uniform growth and size among the plants. Pseudobulbs emerged from the leaf sheaths as the shoots reached their maximum growth (
Figure 1). Cultural practices followed those outlined in our previous study [
4]. In brief, plants were watered once per week, and a water-soluble compound fertilizer (N: P₂O₅: K₂O, 20:20:20; Scott, Marysville, OH, USA) was applied weekly at a concentration of 1 g L⁻¹. For the experiment, plants of uniform size from each cultivar were selected and randomly divided into four groups for the canopy treatment study. Microclimate stations were centrally located within each plot, and PPFD values were recorded using a HOBO Temperature/Light/External Data Logger U12-012 (Onset Computer Corporation, Massachusetts, USA) throughout the experiment. The greenhouse conditions were consistent for all groups, with an average temperature of 26.8°C and a 12-hour photoperiod during the 5-month experimental period.
2.2. Greenhouse Cover Material Treatments
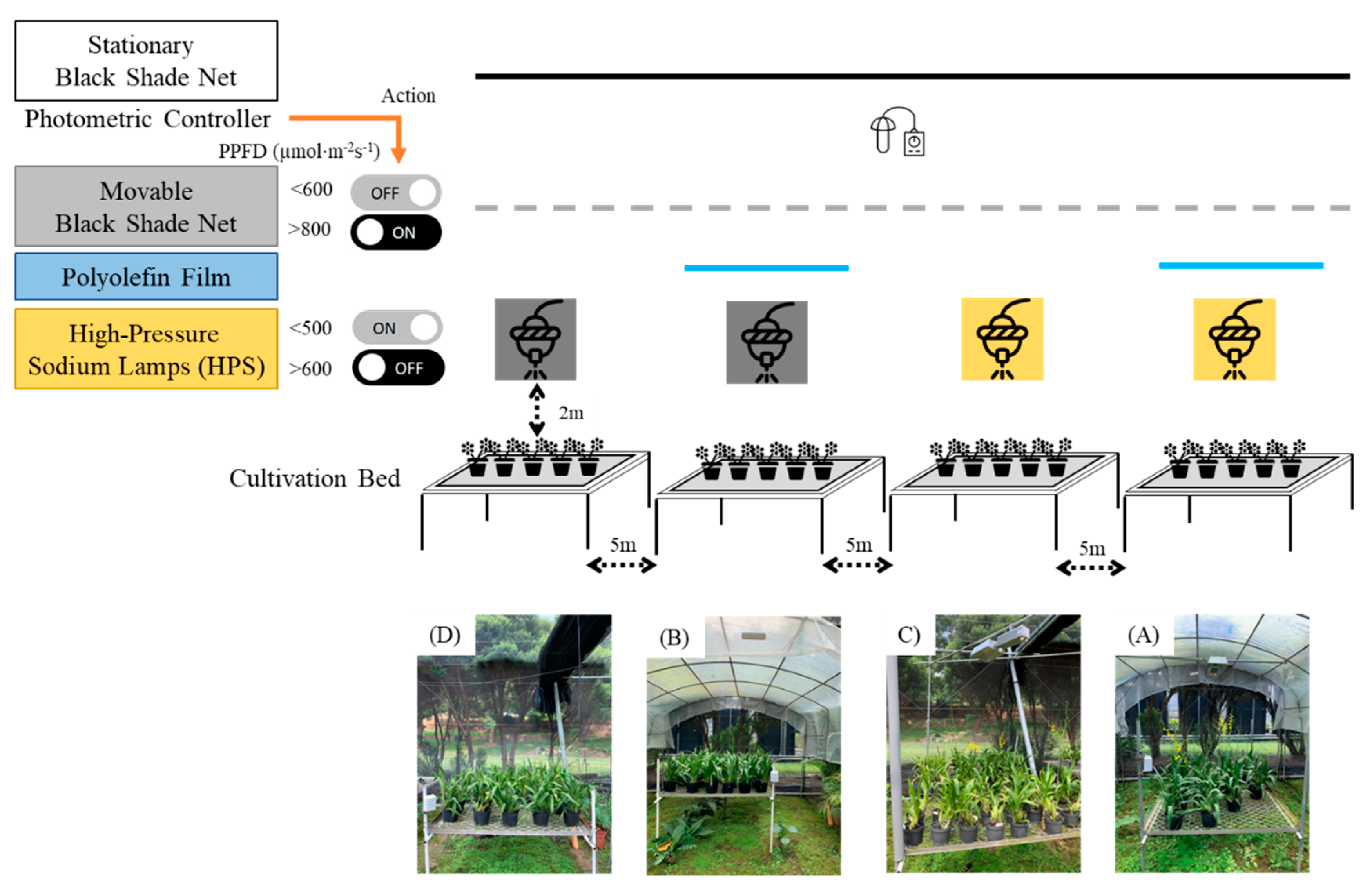
Four different types of canopies (A ~ D) were applied to plants until the end of the experiment as follows (
Figure 2).
A canopy — The greenhouse was covered with stationary black shade nets and a polyolefin (PO) transparent plastic film screen with a light transmittance of approximately 70–80%. Supplemental lighting was provided by high-pressure sodium (HPS) lamps positioned about 2 m above the cultivation beds, delivering a LI of approximately 140 µmol·m⁻²·s⁻¹ at leaf level;
B canopy — The greenhouse was sheltered with stationary black shade nets with PO plastic film;
C canopy — The greenhouse was sheltered with stationary black shade nets and an HPS lamp as a supplemental light source;
D canopy — The greenhouse was sheltered with stationary black shade nets.
In the D canopy treatment, a rigid frame covered with 50% light-reducing black shade nets was used as a control to limit natural light penetration from the top of the greenhouse. For both A and D canopy treatments, a transparent plastic screen made of PO plastic film (0.15 mm thickness, 5.45 m × 4 m coverage area) was installed on the sides of the greenhouse to further reduce natural light penetration. Additionally, daily supplemental lighting with HPS lamps (600W, 220V) was provided to the plants in both A and C canopies. These supplemental intermittent light sources were mounted on a rotating system installed on the plant culture racks and independently controlled by a central processor. Rack dimensions were customized with six layers (layer number/layer height/layer length/layer width: 6/40 cm/1200 cm/600 cm). The mean photosynthetic daily light integral measured at each location in the greenhouse under A, B, C, and D canopy treatments was 150, 50, 200, and 100 μmol·m⁻²·s⁻¹ PPFD, respectively.
The operation of the movable shade net and HPS lamps was controlled by a light sensor fixed within the stationary shade net. When the LI dropped below 500 µmol·m⁻²s⁻¹, the HPS lamps provided supplementary lighting, which stopped when the intensity exceeded 600 µmol·m⁻²s⁻¹. If LI fell below 600 µmol·m⁻²·s⁻¹, the movable shade net was retracted to provide more sunlight; if it exceeded 800 µmol·m⁻²·s⁻¹, the shade net was extended to reduce sunlight exposure. Daytime lighting treatment was conducted by intermittent lighting.
2.3. Plant Growth and Flowering Quality Assessments
The following ten traits per plant were recorded and calculated when 80% of florets became mature and fully blooming, approximately 1.5~2 months from pseudobulb maturity until the end of the five-month experimental period.
1. Pseudobulb length (PL), measured as the long axis (mm) between the base and the top of a pseudobulb with a Vernier caliper;
2. Pseudobulb width (PW), measured as the short/major axis (girth, mm) of a pseudobulb with a Vernier caliper;
3. Pseudobulb thickness (PT), measured as the maximum thickness (mm) of a pseudobulb with a Vernier caliper;
4. Leaf width (LW) measured as the maximum width (mm) of the second leaf with a Vernier caliper;
5. Leaf thickness (LT) measured as the thickness (mm) of the second leaf (in the middle of the leaf and avoiding veins) with a thickness gauges (PC-480S2, Teclock, Japan);
6. Leaf length (LL) measured as the maximum length (cm) of the second leaf with a Vernier caliper;
7. Chlorophyll content measured as a SPAD value with a chlorophyll meter (SPAD-502 plus, Minolta Camara Co., Japan);
8. Inflorescence length (FL), measured as the length (cm) between the base to the top of a flower stalk with a Vernier caliper;
9. Number of branches (FB), recorded as all the branches of a flower stalk;
10. Number of florets (FN), recorded as all the florets (unopened buds and opened florets) of a flower stalk.
Generally, bud and plantlet vegetative stages lasted 3~4 months, followed by the unsheathing phase for one more month, and the further stage of pseudo-bulb with florescence for 1.5~2 months [
5]. Fifteen plants were randomly assigned to each individual canopy treatment, and the experiment assumed interdependence between each canopy treatment during the growing stage.
2.4. Statistical Analysis
The experiment included three varieties (Honey Angel, Golden Star, and Apollo) and four treatments, following a factorial design. A total of 28 varieties and 21 canopy treatments (repeated) were used, with a total of 132 pots in the study. Ten traits were measured, and their significance was evaluated using analysis of variance (ANOVA). Treatment means were compared using Tukey's HSD test at p ≤ 0.05, performed with CoStat 6.4 (CoHort Software, Monterey, CA, USA).
3. Results
This study evaluated the impacts of four canopy treatments (C) on three cultivars (V) by measuring changes in ten horticultural traits. All measured traits exhibited significant differences at the 1%, 5%, or 0.01% levels for main effects and interaction effects, except that both PL and FB traits exhibited non-significant differences among V effects, and the LW trait displayed non-significant differences among the C effect and V x C interaction (
Table 1).
Results of the horticultural evaluation of the three cultivars under the four canopy treatments are presented in
Table 2. Phenotypic variations among cultivars were apparent, except that PL and FB did not show any significance among cultivars. The mean values of LW, LL, SPAD, and FN of AP plants were significantly higher (respectively, 33.96 mm, 37.09 cm, 66.94, and 85.94) than those of GS and HA plants. Nevertheless, the maximum value of PW (41.50 mm) and PT (33.53 mm) occurred in the HA cultivar, and the longest FL (112.9 cm) and shortest LT (0.93 mm) were displayed in GS and HA cultivars, respectively, compared to other cultivars in all treatments.
Table 3 illustrates the differences in measured traits among the four canopy treatments, suggesting that canopy variations in the cultivars and all treatments seemed to strongly affect all traits except leaf width (LW). Significantly higher PW (42.99 mm) and PT (34.41 mm) but lower SPAD (57.96) values were detected under the C canopy compared to other treatments. Furthermore, C canopy treated plants had the longest PL (98.17 mm) and LL (35. 88 cm) but least FN (66.36) compared to other treatments. However, the largest LT (1.01 mm) and FB (8.23) were found in the D- and A-canopies, respectively, compared to other treatments.
Results of canopy treatment horticultural traits evaluation of genotypes cultivated at pseudobulb, leaf, and flower stages are presented in
Figure 3,
Figure 4 and
Figure 5, respectively.
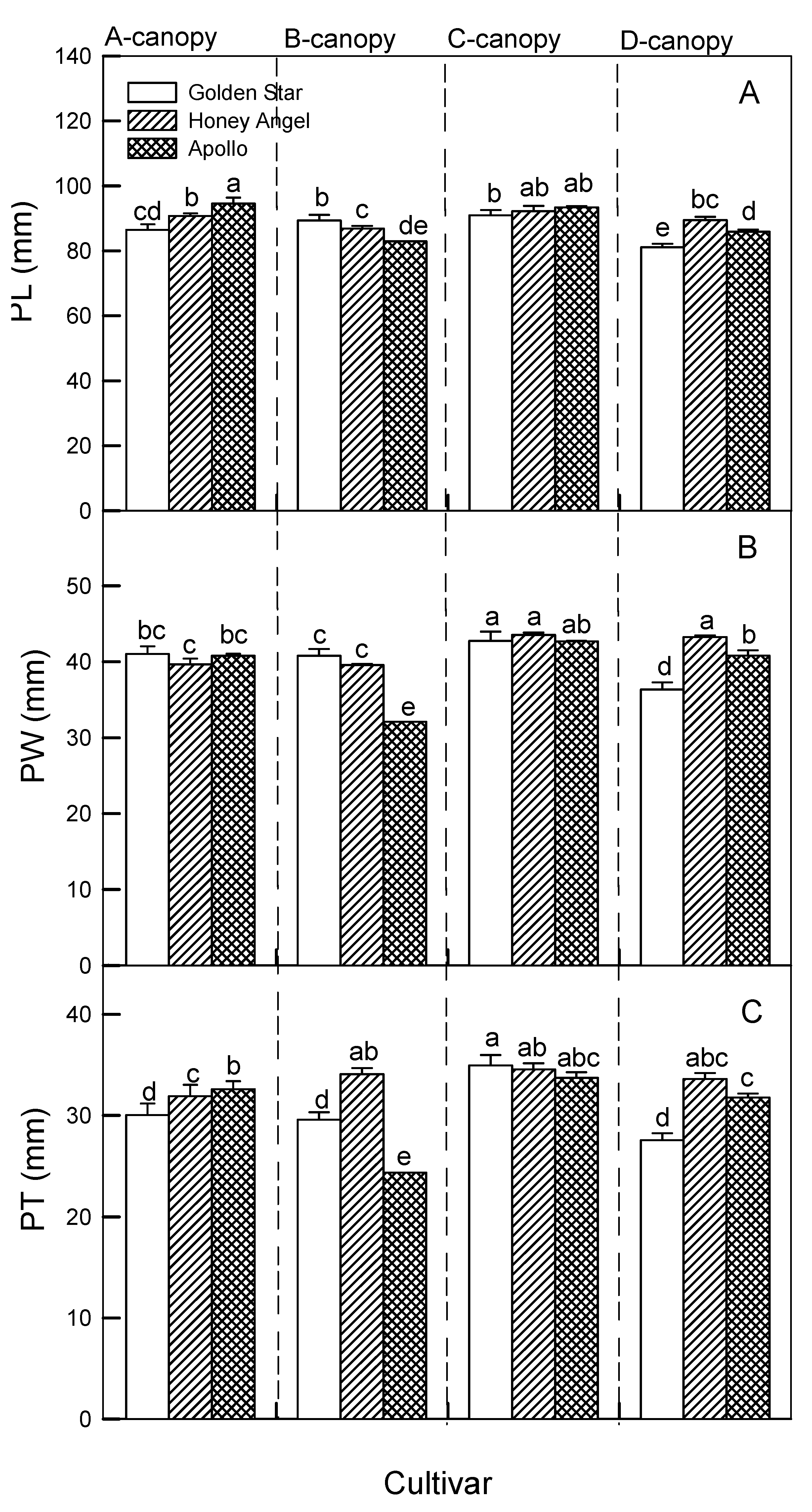
Figure 3A demonstrates that the mean values of PL in B- and C-canopy treatments of GS (90.05 mm and 92.00 mm, respectively) were significantly higher than for other treatments, whereas relatively higher PL occurred in C-canopy treatments of HA (96.25 mm) compared to other treatments, and significantly higher PL were found in A- and C-canopy treatments of AP (96.05 mm and 94.00 mm, respectively) than in other treatments. All plants treated with C-canopy exhibited remarkably higher PW (42.89 ~ 45.01 mm) and PT (33.89 ~ 35.01 mm) than other treatments (
Figure 3B,C), indicating that different canopy treatments affected pseudobulb traits differently.
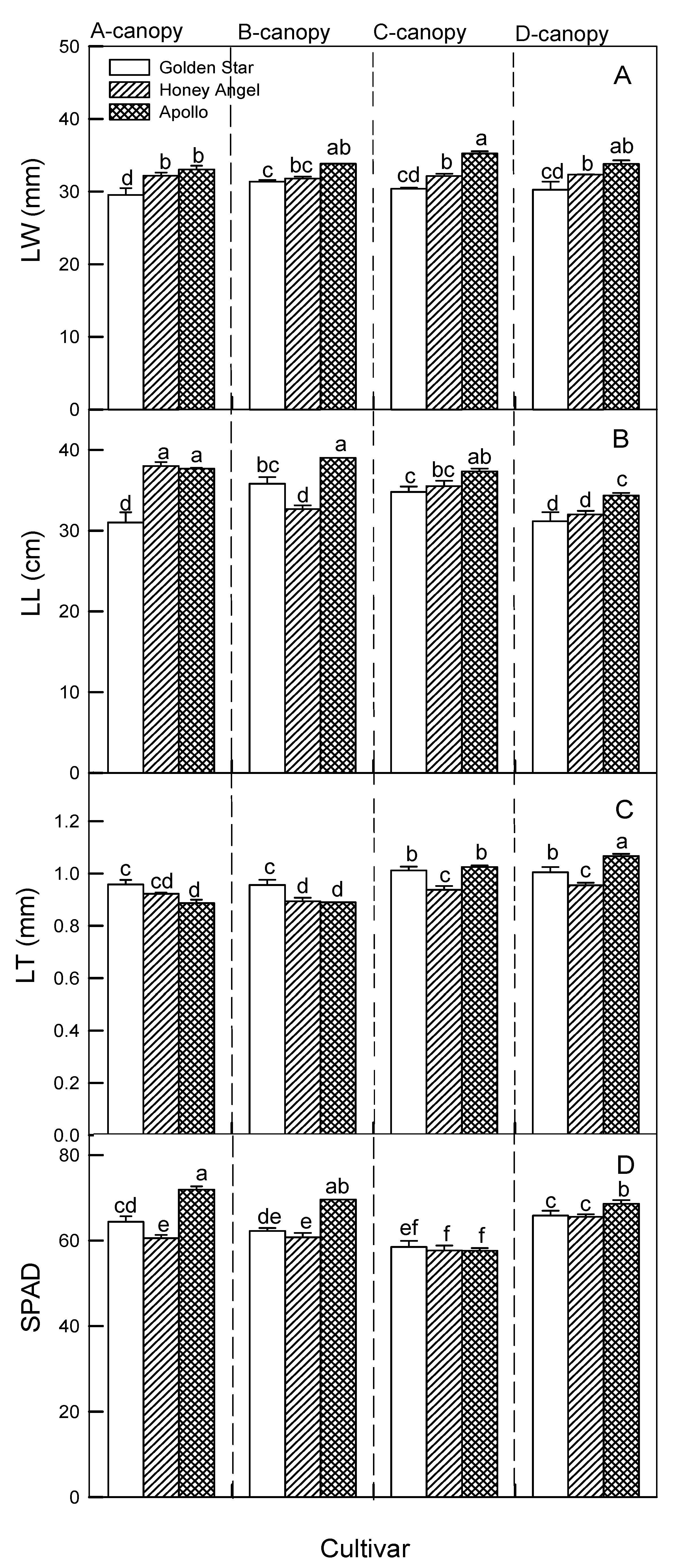
When cultivars were compared across canopy treatments, the AP cultivar had notably increased LW, LL, and SPAD values compared to other genotypes, and AP treated with C, B, and A canopies exhibited the highest LW, LL, and SPAD values at 36.65 mm, 38.90 cm, and 73.01, respectively (
Figure 4A,B,D), indicating that different treatments affected leaf development differently. Interestingly, the longest LT was observed in AP plants under the control condition (1.10 mm), but LT of all plants from the C canopy treatment (0.89 ~1.00 mm) were relatively longer than both A and B canopy treatments (
Figure 4C).
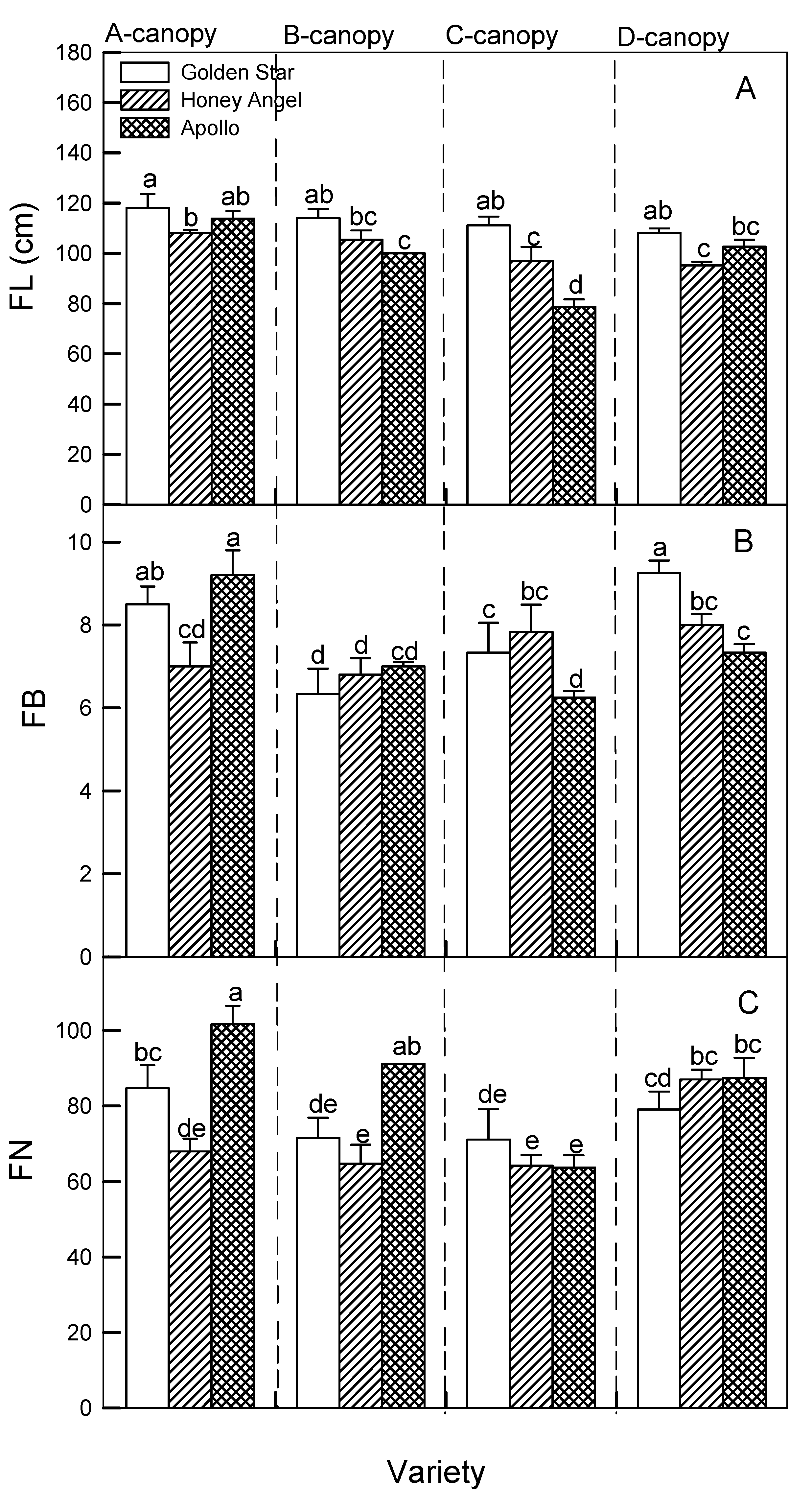
Figure 5A shows that A-canopy treatment had a prominent role by increasing FL in HA and AP plants (115.02 cm and 118.36 mm, respectively) compared to other treatments, whereas FL in GS plants was non-significantly observed in all treatments (119.02~ 120.03 cm).
Figure 5B illustrates that the FB values of GS plants under A and D canopy treatments (8.3 and 9.5, respectively) were significantly higher than those under B and C canopy treatments (8.3 and 9.5, respectively), whereas HA plants treated with C and D canopies had relatively more FB (7.6 and 7.9, respectively) than A and B canopies (6.9 and 7.0, respectively). Furthermore, significantly more FB was observed in AP plants treated with the A canopy (9.6) compared to other treatments (6.2 ~6.5). FN values of GS and AP plants significantly increased under A canopy treatment compared to other treatments, and the highest FN values of GS and AP plants under the A canopy were recorded as 85 and 100, respectively, whereas a significant increase in FN in HA occurred in the control at 86 compared to other treatments at 65~ 68, suggesting that this genotype has a different adaptability to canopy treatment (
Figure 5C).
Means in the same row within varieties followed by different letters are significantly different at p ≤ 0.05 by ANOVA. Each cultivar was assumed to be dependent on the other. n = 28.
Means in the same row within canopies followed by different letters are significantly different at p ≤ 0.05 by ANOVA. Each canopy treatment was assumed to be dependent on the other. n = 7.
4. Discussion
Oncidium cultivars are adapted to intermediate and warmer climates and exhibit photoperiod responses, where the length of the skotoperiod (dark period) regulates flowering and development [
7]. Economically significant species within the Oncidiinae subtribe are particularly sensitive to day/night lengths for transitioning into the reproductive stage of their life cycle. In short-day plants, nighttime interruption by additional lighting inhibits floral differentiation, and these plants will only flower when exposed to light periods shorter than a specific threshold. Conversely, in long-day plants, supplemental lighting during nighttime interruption promotes flower bud production [
8]. In our study, different cultivars exhibited distinct morphological characteristics under varying light conditions. AP plants treated with A canopy showed relatively higher pseudobulb length (PL), pseudobulb thickness (PT), leaf width (LW), chlorophyll content (SPAD), number of branches (FB), and number of florets (FN) compared to other cultivars, indicating that black shading nets combined with transparent plastic screens and daily supplemental lighting are crucial for the growth of AP plants. Generally, plants treated under C canopy conditions displayed superior pseudobulb growth traits across various cultivars compared to other treatments, while A and D canopy treatments resulted in better flowering quality. These findings suggest that different
Oncidesa cultivars require specific canopy treatments to optimize flowering, and that the use of supplemental lighting is essential for enhancing pseudobulb growth and flowering quality.
These cultivars may utilize supplemental lighting to enhance photosynthesis and create an inductive photoperiod, thereby improving plant growth and flowering quality. Interestingly, SPAD values and FN for all plants were relatively lower under the C canopy treatment compared to other treatments, suggesting that total chlorophyll content and floret production are sensitive to supplementary lighting in greenhouses equipped with black shading nets. Photoperiod and temperature likely also influenced SPAD values and FN, thereby affecting the plants' responses to canopy treatments under shade net cultivation in the greenhouse. Additionally, we observed that GS, HA, and AP plants exposed to C canopy conditions developed lighter green leaves and produced fewer flowers when subjected to higher light intensities and day lengths exceeding a certain threshold. This response suggests that these plants tend to reduce chlorophyll content to avoid excessive light capture and potential damage to thylakoid membranes [
9]. Under the C canopy treatment, relatively higher LW, LL, and LT were observed, which increased under high light conditions, enhancing light penetration into deeper leaf layers [
10,
11,
12]. Photoperiods and temperatures associated with the C canopy treatment, characterized by higher heat emission from HPS lamps and higher temperatures with shorter day lengths, likely contributed to the decline in chlorophyll content and FN in the plants. Although vegetative buds emerged in the leaf axils, fewer developing inflorescences were noted under the C canopy treatment, with only a few visible inflorescence buds emerging from the leaf axils.
Li et al. (2014) [
13] found that canopy lighting enhances the photosynthetic capacity of the middle and lower leaves by promoting a more uniform light distribution, which is advantageous given the curvilinear response of leaf photosynthesis to light intensity (LI). Diffused light penetration into the deeper parts of the canopy increases the LI available to the lower leaves. Oncidium plants are particularly sensitive to abrupt or prolonged changes in LI, which can significantly influence their growth and flowering characteristics. Although Oncidium orchids can tolerate high light levels, it is recommended that growers use shade cloths providing 30–50% shade in greenhouses [
14]. Under appropriate light conditions, orchids develop short, robust stems and bright green, leathery leaves. However, excessive light results in scorched, yellowed, and stunted plants, while too much shade leads to dark green, soft, succulent leaves with thin, spindly stems [
15].
Cattleya orchids flourish under medium to bright light with irradiance levels between 400 and 600 μmol·m⁻²·s⁻¹ and benefit from using 40% shade cloth. Most Oncidium orchids perform optimally when exposed to sunlight for several hours daily, with an ideal light intensity of around 500 μmol·m⁻²·s⁻¹. Cymbidium plants, on the other hand, prefer bright, dappled shade in the afternoon during summer and full sunlight in winter. Mature Cymbidium plants, however, require 50–55% shade during hot weather. During the growing season, they need light intensities between 1,000 and 1,200 μmol·m⁻²·s⁻¹, while during the flowering season, they thrive at 400–600 μmol·m⁻²·s⁻¹ [
15].
Based on these findings, Oncidium cultivars in this study may modify their periodic and oscillator systems under artificial greenhouse canopy conditions, developing adaptive mechanisms suited to tropical and subtropical environments, and maintaining resilience during minimal fluctuations. The four types of canopy treatments resulted in significant differences in various horticultural traits, and the interactive effects between cultivars and treatments were notably distinct, suggesting that each cultivar has specific requirements for canopy conditions. Therefore, subsequent cultivation environments, periods, and growth stages should be tailored to optimize the growth and flowering quality of each Oncidium cultivar.
5. Conclusions
The application of four different canopy treatments to three Oncidium cultivars led to significant variations in pseudobulb growth and flower quality traits, except for leaf width. Each cultivar exhibited specific canopy treatment requirements to achieve optimal growth and flowering performance. Apollo plants under canopy A demonstrated relatively greater pseudobulb length and thickness, leaf width, chlorophyll content, and a higher number of branches and florets compared to Honey Angel and Golden Star. Canopy C-treated plants showed superior growth traits across all cultivars, while those under canopy A and D treatments exhibited enhanced flowering quality. The effects of canopy treatments on growth and flowering traits varied among cultivars, reflecting their differing adaptability to specific canopy conditions. The use of customized greenhouse canopies in orchid cultivation offers significant potential, as each cultivar's morpho-physiological characteristics are influenced by their light environment and responsiveness to unpredictable conditions, such as light fluctuations, irregular light regimes, and climate change. Further research is needed to determine optimal combinations of light quality, intensity, and duration across the entire life cycle of Oncidium cultivars.
Author Contributions
C.M. Chang, K.H. Lin analyzed the data and wrote the manuscript. M.Y. Huang conducted the experiments, analyzed the data, and wrote the manuscript. C.I. Chen conducted the experiments and analyzed the data. W.H. Huang, and C.W. Wang developed the research concept, conducted the experiments, analyzed the data, wrote the manuscript, and supervised the entire study. All authors have read and approved the final manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, D.; Zhao, X.W.; Li, Y.Y.; Ke, S. J.; Yin, W.L.; Lan, S.; Liu, Z J. Advances and prospects of orchid research and industrialization. Hortic. Res, 2022, 9, uhac220.
- De, L.C. Good Agricultural Practices of Oncidium orchids. Biot. Res. Today, 2022, 4, 233–237.
- Li, Y. T.; Chang, Y.C.A. Relationship between the growth of current shoot and the development of inflorescence and vegetative buds in Oncidesa Gower Ramsey ‘Honey Angel’leaf axils. HortScience, 2023, 58(3), 268-273.
- Chang, C.-M.; Lin, K.-H.; Huang, M.-Y.; Chen, C.-I.; Hsueh, M.-L.; Wang, C.-W.; Yeh, K.-W. Growth and Flowering Characteristics of Oncidium Gower Ramsey Varieties under Various Fertilizer Management Treatments in Response to Light Intensities. Agronomy 2021, 11, 2549.
- Chang, C.-M.; Wang, C.-W.; Huang, M.-Y.; Chen, C.-I.; Lin, K.-H.; Shen, C.-P. The Effects of Light Treatments on Growth and Flowering Characteristics of Oncidesa Gower Ramsey ‘Honey Angel’ at Different Growth Stages. Agriculture 2023, 13, 1937.
- Chang, C.-M.; Chen, C.-I.; Shen, J.-Y.; Lin, C.-Y.; Lai, Y.-H.; Wang, C.-W. Photoprotection and Photoinhibition During Light Induction in Two Oncidium Gower Ramsey Varieties. TW J. of Biodivers. 2022, 24(2):17-37.
- Blanchard, M.G.; Erik, S.R. Intermittent light from a rotating high-pressure sodium lamp promotes flowering of long-day plants. HortScience 2010, 45, 236–241.
- Ganesh, S.; Jawaharlal, M.; Rajamani, K.; Visalakshi, M.; Karthikeyan, S.; Ganga, M.; Eevera, T. Investigating the physiological effects of leds with combined spectral emittances in floriculture. App. Ecol. Environ. Res. 2024, 22: 17-40.
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science, 2016, 354 (6314), 857-861.
- Abhijita S.; Dalal, V.K.; Misra, A.N. In: A Closer Look at Photosynthesis. Editors: Vijay Kumar Dalal and Amarendra Narayan Misra. Inc. Chapter 2: The Effects of Light Quality and Quantity on Photosynthesis. Nova Science Publishers, 2023, 46-81.
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants, 2018, 10(5), ply052.
- Ichiro, T.; Hiroki, O.; Takashi, F.; Riichi, O. Light environment within a leaf. II. Progress in the past one-third century. Journal of Plant Research, 2016, 129, 353-363.
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of crop photosynthesis by diffuse light: quantifying the contributing factors. Annals of botany, 2014, 114(1), 145-156.
- De, L.C.; Sailo, N.; Singh, D.R. Growth and developmental aspects in commercial orchids. Floric. Sci. 2018, 2, 6–15.
- De, L.C. Good agricultural practices of commercial orchids. Vigyan Varta, 2020, 1, 53–64.
Figure 1.
(A) Plant morphology among Oncidesa Gower Ramsey, ‘Golden Star’ (GS, left), ‘Honey Angel’ (HA, middle), and ‘Apollo’ (AP, right). (B) The pseudobulb, branches of a flower stalk, and florets of a flower stalk among GS, HA, and AP cultivars displayed from the top to bottom, respectively.
Figure 1.
(A) Plant morphology among Oncidesa Gower Ramsey, ‘Golden Star’ (GS, left), ‘Honey Angel’ (HA, middle), and ‘Apollo’ (AP, right). (B) The pseudobulb, branches of a flower stalk, and florets of a flower stalk among GS, HA, and AP cultivars displayed from the top to bottom, respectively.
Figure 2.
Schematic illustration of canopy treatments in greenhouse culture. Four different types of canopies (A ~ D) were applied to plants in the greenhouse. The A canopy was designed as the greenhouse sheltered with black shade nets and transparent plastic screen film, provided with supplemental light sources. The B canopy was designed as the greenhouse sheltered with black shading nets with transparent plastic screen added. The C canopy was designed as the greenhouse sheltered with black shading nets plus supplemental light sources. The D canopy was designed as the greenhouse sheltered with black shading nets (control).
Figure 2.
Schematic illustration of canopy treatments in greenhouse culture. Four different types of canopies (A ~ D) were applied to plants in the greenhouse. The A canopy was designed as the greenhouse sheltered with black shade nets and transparent plastic screen film, provided with supplemental light sources. The B canopy was designed as the greenhouse sheltered with black shading nets with transparent plastic screen added. The C canopy was designed as the greenhouse sheltered with black shading nets plus supplemental light sources. The D canopy was designed as the greenhouse sheltered with black shading nets (control).
Figure 3.
The responses of four canopy treatments (A ~ D canopy) on pseudobulb length (PL, panel A), pseudobulb width (PW, panel B), and pseudobulb thickness (PT, panel C) in ‘Honey Angel’ (HA), ‘Golden Star’ (GS), and ‘Apollo’ (AP). Means within the same trait of all canopy treatments in those three varieties followed by different lowercase letters significantly differ at p ≤ 0.05 by Tukey’s HSD test. Each treatment was assumed to be dependent on the other. Vertical bars represent the mean ± standard errors (n = 7).
Figure 3.
The responses of four canopy treatments (A ~ D canopy) on pseudobulb length (PL, panel A), pseudobulb width (PW, panel B), and pseudobulb thickness (PT, panel C) in ‘Honey Angel’ (HA), ‘Golden Star’ (GS), and ‘Apollo’ (AP). Means within the same trait of all canopy treatments in those three varieties followed by different lowercase letters significantly differ at p ≤ 0.05 by Tukey’s HSD test. Each treatment was assumed to be dependent on the other. Vertical bars represent the mean ± standard errors (n = 7).
Figure 4.
The responses of four canopy treatments (A ~ D canopy) on leaf width (LW, panel A), leaf length (LL, panel B), leaf thickness (LT, panel C), and chlorophyll content (SPAD value, panel D) in ‘Honey Angel’ (HA), ‘Golden Star’ (GS), and ‘Apollo’ (AP). Means within the same trait of all canopy treatments in those three cultivars followed by different lowercase letters significantly differ at p ≤ 0.05 by Tukey’s HSD test. Each treatment was assumed to be dependent on the other. Vertical bars represent the mean ± standard errors (n = 7).
Figure 4.
The responses of four canopy treatments (A ~ D canopy) on leaf width (LW, panel A), leaf length (LL, panel B), leaf thickness (LT, panel C), and chlorophyll content (SPAD value, panel D) in ‘Honey Angel’ (HA), ‘Golden Star’ (GS), and ‘Apollo’ (AP). Means within the same trait of all canopy treatments in those three cultivars followed by different lowercase letters significantly differ at p ≤ 0.05 by Tukey’s HSD test. Each treatment was assumed to be dependent on the other. Vertical bars represent the mean ± standard errors (n = 7).
Figure 5.
The responses of four canopy treatments (A ~ D canopy) on inflorescence length (FL, panel A), number of pedicels (FB, panel B), and floret numbers (FN, panel C) in ‘Honey Angel’ (HA), ‘Golden Star’ (GS), and ‘Apollo’ (AP). Means within the same trait of all canopy treatments in those three varieties followed by different lowercase letters significantly differ at p ≤ 0.05 by Tukey’s HSD test. Each treatment was assumed to be dependent on the other. Vertical bars represent the mean ± standard errors (n = 7).
Figure 5.
The responses of four canopy treatments (A ~ D canopy) on inflorescence length (FL, panel A), number of pedicels (FB, panel B), and floret numbers (FN, panel C) in ‘Honey Angel’ (HA), ‘Golden Star’ (GS), and ‘Apollo’ (AP). Means within the same trait of all canopy treatments in those three varieties followed by different lowercase letters significantly differ at p ≤ 0.05 by Tukey’s HSD test. Each treatment was assumed to be dependent on the other. Vertical bars represent the mean ± standard errors (n = 7).
Table 1.
Pseudobulb length (PL), pseudobulb width (PW), pseudobulb thickness (PT), leaf width (LW), leaf thickness (LT), leaf length (LL), chlorophyll content (SPAD value), inflorescence length (FL), number of branches (FB), and floret numbers (FN) per plant were examined by ANOVA for the effects of cultivar (V), canopy (C), and their interactions (V×C).
Table 1.
Pseudobulb length (PL), pseudobulb width (PW), pseudobulb thickness (PT), leaf width (LW), leaf thickness (LT), leaf length (LL), chlorophyll content (SPAD value), inflorescence length (FL), number of branches (FB), and floret numbers (FN) per plant were examined by ANOVA for the effects of cultivar (V), canopy (C), and their interactions (V×C).
| Trait |
Main and interaction effect |
| cultivarvariety (V) |
canopy (C) |
V x C |
| F and p value with significance |
|---|
| F |
p |
F |
p |
F |
p |
| PL (mm) |
2.62 |
0.08NS
|
12.17 |
0.0000**** |
7.99 |
0.0000**** |
| PW (mm) |
3.41 |
0.04* |
11.84 |
0.0000**** |
21.49 |
0.0000**** |
| PT (mm) |
7.35 |
0.001** |
9.57 |
0.0000**** |
13.73 |
0.0000**** |
| LW (mm) |
44.51 |
0.0000**** |
0.92 |
0.43 |
1.76 |
0.11 NS
|
| LL (cm) |
14.94 |
0.0000**** |
6.95 |
0.0003*** |
10.80 |
0.0000**** |
| LT (mm) |
6.43 |
0.002** |
19.87 |
0.0000**** |
8.42 |
0.0000**** |
| SPAD |
11.75 |
0.0000**** |
20.55 |
0.0000**** |
8.94 |
0.0000**** |
| FL(cm) |
13.08 |
0.0000**** |
10.00 |
0.0000**** |
6.58 |
0.0000**** |
| FB |
0.90 |
0.41 NS
|
8.25 |
0.0000**** |
5.95 |
0.0000**** |
| FN |
7.75 |
0.001** |
8.43 |
0.0000**** |
5.88 |
0.0000**** |
Table 2.
Means of ten traits (PL, PW, PT, LW, LL, LT, SPAD, FL, FB, and FN) per plant in each cultivar (GS, HA, and AP) over a five-month period after two months of pseudobulb maturity cultivation was established.
Table 2.
Means of ten traits (PL, PW, PT, LW, LL, LT, SPAD, FL, FB, and FN) per plant in each cultivar (GS, HA, and AP) over a five-month period after two months of pseudobulb maturity cultivation was established.
| Trait |
Golden Star |
Honey Angel |
Apollo |
| PL (mm) |
86.96 a
|
89.82 a
|
89.20 a
|
| PW (mm) |
40.22 ab
|
41.50 a
|
39.10 b
|
| PT (mm) |
30.54 b
|
33.53 a
|
30.61 b
|
| LW (mm) |
30.38c
|
32.10 b |
33.96 a |
| LL (cm) |
33.19 b |
34.54 b
|
37.09 a
|
| LT (mm) |
0.98 a
|
0.93b
|
0.97 a
|
| SPAD |
62.76 b
|
61.17 b
|
66.94 a
|
| FL(cm) |
112.90 a
|
101.45b
|
98.80 b
|
| FB |
7.85 a
|
7.41 a
|
7.45 a
|
| FN |
76.58 b
|
70.98 b
|
85.94 a
|
Table 3.
Means of ten traits (PL, PW, PT, LW, LL, LT, SPAD, FL, FB, and FN) per plant exposed to different canopy treatments over a five-month period after two months of pseudobulb maturity cultivation was established.
Table 3.
Means of ten traits (PL, PW, PT, LW, LL, LT, SPAD, FL, FB, and FN) per plant exposed to different canopy treatments over a five-month period after two months of pseudobulb maturity cultivation was established.
| Trait |
A-canopy |
B-canopy |
C-canopy |
D-canopy |
| PL (mm) |
90.57 a
|
86.38 b
|
92.17 a
|
85.51 b
|
| PW (mm) |
40.49 b
|
37.48 c
|
42.99 a
|
40.13 b
|
| PT (mm) |
31.51 b
|
29.35 b
|
34.41 a
|
30.98 b
|
| LW (mm) |
31.57 a
|
32.31 a
|
32.58 a
|
32.13 a
|
| LL (cm) |
35.56 a
|
35.82 a
|
35.88 a
|
32.50 b
|
| LT (mm) |
0.92 b
|
0.91 b
|
0.99 a
|
1.01 a
|
| SPAD |
65.63 a
|
64.21 a
|
57.96 b
|
66.69 a
|
| FL(cm) |
113.38 a
|
106.47 a
|
95.64 b
|
102.06 ab
|
| FB |
8.23 a
|
6.71 b
|
7.14 b
|
8.19 a
|
| FN |
84.78 a
|
75.75 ab
|
66.36 b
|
84.44 a
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).