Submitted:
29 October 2024
Posted:
30 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Coffee Composition
3. Bioactive Compounds in Coffee and Its Potential to Prevent Atherosclerosis
3.1. The Impact of Coffee Consumption on Obesity
3.1.1. Coffee Consumption and Gut Microbiota in Weigh Loss
3.2. Anti-Atherogenic Effects of Bioactive Compounds of Coffee
3.3. Coffee Consumption on Lipid Metabolism and Inflammation
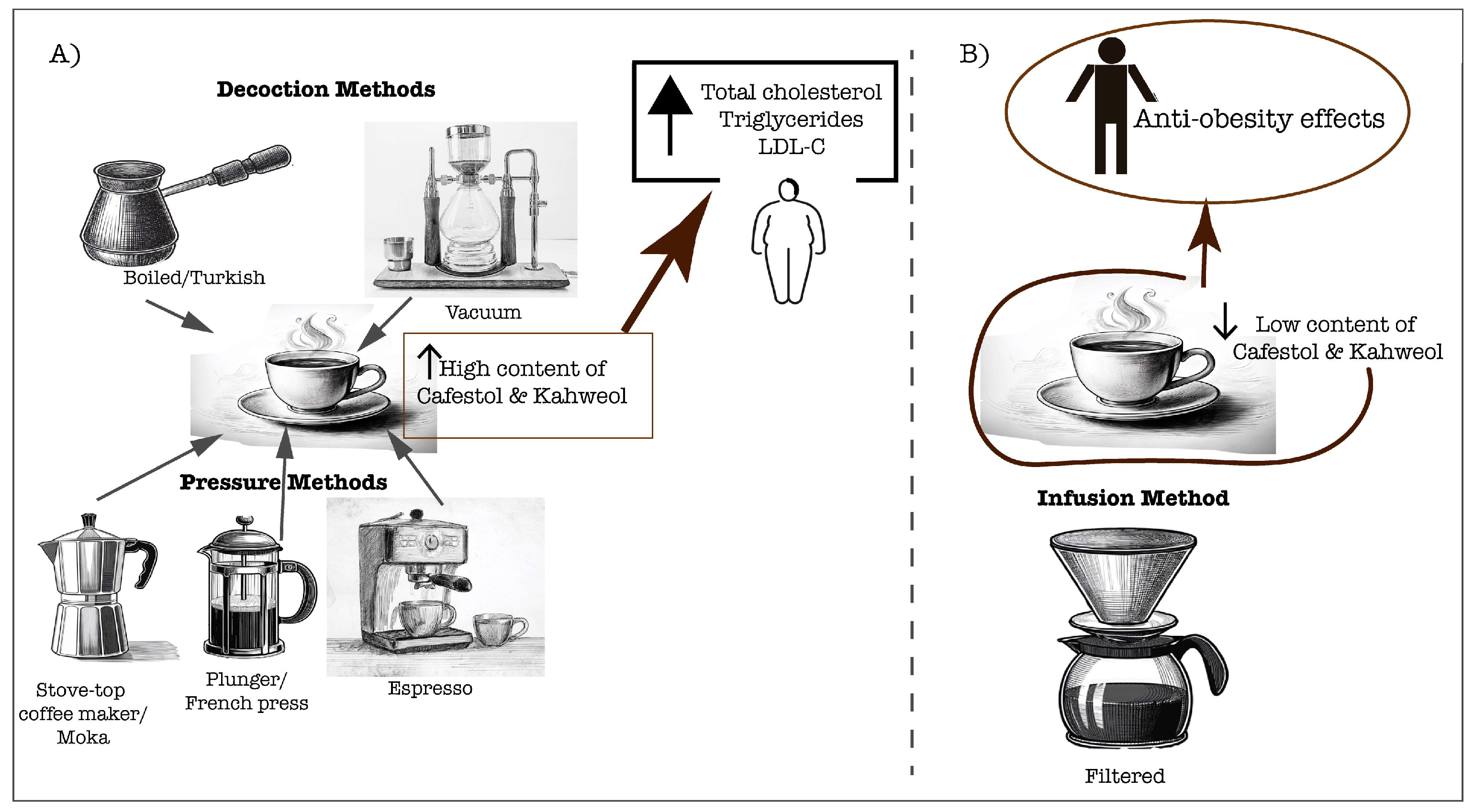
3.4. Coffee Extraction Method Influence the Lipid Profile
| Coffee/Coffee Bioactive | Study Model | Main findings | Study details | References |
|---|---|---|---|---|
|
Caffeine and CGA⇧ |
3T3-F422A preadipocyte cell line |
⇩ PPAR-γ expression ⇩c/EBP-α |
Caffeine 1 mM + CGA 0.5 mM loaded into solid lipid nanoparticles |
Uner and Celebi, 2023 [12] |
|
Caffeine and CGA |
Female ICR mice |
⇩ Adipose tissue ⇩ Body weight ⇩Total cholesterol (serum and hepatic) ⇩Triglycerides ⇩ Leptin levels ⇧ AMPK activation ⇩ PPAR-γ2 liver expression |
Diet containing: 0.2% CGA 0.03% caffeine For 24 weeks |
Zheng et al., 2014 [13] |
|
Green coffee bean extract |
Individuals, over the age of 18, (n=103) |
⇩ Body weight Lipid profile improvement |
500 mg/day green coffee extract Supplementation for at least 1 week to 8 weeks |
Kanchanasurakit et al., 2023 [17] |
|
Green coffee extract |
Overweight/ obese patients with type 2 diabetes (n=44) |
⇩ Body weight ⇩ Body mass index ⇩ Systolic blood pressure ⇩ C-reactive protein ⇩ Triglycerides ⇧ HDL levels |
800 mg/day green coffee extracct supplementation for 10 weeks |
Khalili-Moghadam et al., 2023 [18] |
|
Coffee |
Kidney transplant recipients aged 49.5 years (n=170) |
⇧ Body adiposity (central adiposity) Lower muscle quality |
Median coffee consumption 200 mL/day 2 years-follow up |
Costa et al., 2023 [21] |
|
Coffee |
Individuals with metabolic syndrome (n= 1,483) |
⇩ Total fat tissue ⇩ Trunk fat ⇩ Visceral adipose tissue |
Moderate coffee consumption (1-7 cups/week) 3 years-follow up |
Henn et al., 2023 [22] |
|
Instant coffee |
High-fat fed rats (Male Sprague Dawley) |
⇩ Body weight ⇩ Adiposity ⇧ Insulin resistance ⇩Firmicutes (F)-to-Bacteroidetes ratio and Clostridium Cluster XI ⇧ Enterobacteria |
Instant caffeinated coffee (20 g/L) for 10 weeks |
Cowan et al., 2014 |
|
CGA |
C57BL/6 male mice fed a high fat diet |
⇩ Body weight ⇩ Subcutaneous and visceral weight ⇧ short chain fatty acid producers (Dubosiella, Romboutsia, Mucispirillum, and Faecalibaculum) ⇧ Akkermansia |
150 mg/Kg CGA solution for 20 weeks |
Ye et al., 2021 [25] |
|
Green coffee extract |
Apo-/- mice fed antiatherogenic diet |
⇩ Adiposity ⇩ Weigh gain ⇩ Inflammatory infiltrate in adipose tissue Improved microbiota diversity ⇧ Desulfovibrio ⇧ Mogibacteriaceae |
Green coffee extract 220 mg/Kg for 14 weeks |
Caro-Gómez et al., 2019 [27] |
|
Freeze-dried coffee solution |
Wistar rats fed high-fat diet |
⇧ Bifidobacterium spp. ⇧ HDL-C reverse cholesterol transport ⇩ II1b mRNA Did not improved weight gain |
Freeze-dried coffee solution at a dose of 0.39 g/100 g for 8 weeks |
Cavalcanti et al., 2022 [28] |
| Caffeic acid, 1-methyluric acid and 1,3,7-trimethyluric acid |
In vitro and ex vivo study on plasma from healthy indivi-duals |
Prevention of LDL oxidation by cooper |
0.5 μM caffeic acid, 3 μM 1,3,7-trimethyluric acid, 30 μM 1-methyluric acid, caffeic acid |
Gómez-Ruiz et al., 2007 [33] |
|
Acute coffee consumption (400 mg CGA) |
In vitro and ex vivo experiments on plasma from healthy volunteers (n=20) after drinking coffee |
⇧ Antioxidant capacity of plasma Prevention of LDL oxidation |
Acute coffee consumption containing 420 mg of CGA (400 mL of coffee |
Lara-Guzmán et al., 2016 [23] |
|
Coffee |
Healthy male volunteers aged 20 to 31 (n=11) | ⇩ Total cholesterol ⇩ LDL-C ⇩ MDA ⇩ LDL oxidation |
Coffee intake, 24 g total per day for 1 week |
Yukawa et al., 2004 [35] |
|
Filtered Coffee /caffeic acid |
Ex vivo and in vitro experiments in plasma from healthy volunteers (n=10) |
⇩ LDL oxidation Incorporation of caffeic, p-coumaric, and ferulic acids into LDL |
Coffee consumption (200 mL) In vitro: 1, 10, 100 nmol/L caffeic acid incubated with isolated LDL from healthy subjects |
Natella et al., 2007 [36] |
|
Coffee (high content of polyphenols) |
Healthy subjects aged 20 years or older (n= 169) |
⇩ plasma LysoPC levels |
Low coffee consumption (≤100 mL/day), high coffee consumption >100 mL/day) |
Miranda et al., 2017 [37] |
|
Filtered coffee |
Habitual coffee drinkers (n=47) |
⇩ plasma LysoPC levels |
First month: no coffee consumption Second month: 4 cups of paper-filtered coffee/day Third month: 8 cups of paper-filtered coffee/day |
Kuang et al., 2018 [38] |
|
Filtered coffee |
Healthy volunteers (n=20) |
⇧ SOD ⇧ Catalase ⇧ GPx Did not reduce ox-LDL levels |
482 ± 61 mL/day medium light roast or medium roast paper-filtered for 4 weeks |
Corrêa et al., 2012 [40] |
|
Filtered coffee with high content of CGA and low content of kahweol and cafestol/DHFA in in vitro experiments |
Subjects (n=74) aged between 20 and 60 years. In vitro experiments in THP-1 monocyte-derived macrophages |
⇩ Oxylipins levels in plasma ⇩ Lipid peroxidation markers ⇩ Inflammatory markers No significant differences on ox-LDL levels in plasma In vitro data: ⇩ Ox-LDL uptake ⇩ CD36 expression ⇩ SR-A expression ⇩ LOX-1 expression ⇩ ROS production ⇩ oxylipins profile |
Consumption of coffee A containing 787 mg CGA (n=24), coffee B containing 407 mg CGA (n=25), 400 mL/day for 8 weeks. In vitro experiment: 25 μg/mL ox-LDL, 1μM DHFA, and 1μM phenolic acid |
Lara-Guzmán et al., 2020 [41] |
|
DHFA |
Culture human macrophages |
⇩ ROS production ⇩ 8-Isoprostane ⇩ Ox-LDL uptake ⇩ CD36 expression ⇩ inflammatory mediators (TNF-α, IL-6, and IL-17) ⇧ IL-10 ⇧ PGE1 |
THP-1 monocyte-derived macrophages were exposed to 50 μg/mL oxLDL, 10 ng/mL LPS or 20 μM 7KC treated with 1 μM DHFA |
Lara-Guzmán et al., 2024 |
|
Filtered coffee |
Habitual coffee drinkers (n=47) younger than 65 years with elevated risk of type 2 diabetes |
⇩ IL-18 ⇩ 8-Isoprostane ⇧ adiponectin ⇧ Caffeine in serum ⇧ CGA in serum ⇧ Caffeic acid metabolites in serum ⇧HDL ⇩LDL/HDL ratio |
First month: no coffee Second month: 4 cups/day Third month: 8 cups/day |
Kempf et al., 2010 [43] |
|
Green and roasted coffee |
Normocholesterolemic (n=25) and hypercholesterolemic (n=27) subjects aged 18 to 45 years |
⇩ Total cholesterol ⇩ LDL-C ⇩ VLDL-C ⇩ Triglycerides ⇧ Plasma antioxidant capacity ⇩ MDA levels ⇩ Carbonylation ⇩ CRP |
Moderate coffee consumption (3 cups per day) for 8 weeks |
Martínez-López et al., 2019 [4] |
|
Kahweol |
INS-1 cells |
⇩ NF-κB ⇧ Antioxidant enzymes (Hemeoxygenase-1) ⇧ p-AKT ⇧ BCL-2 |
Cells were exposed to 3mM streptozotocin and pre-incubated with 2.5 and 5 μM Kahweol |
El-Huneidi et al., 2021 [44] |
|
Kahweol |
AREc32 cells |
⇧Nrf2 |
0.02 and 30 μM Kahweol |
Wu et al., 2014 [45] |
|
Caffeine |
RAW264.7 cells |
⇩ NF-κB ⇩ pho-p38MAPK |
Cells were exposed to 1 μg/mL LPS and treated with caffeine (0, 100, 400, 800, 1000, and 1200 μM) |
Hwang et al., 2016 [46] |
|
Caffeine |
Peripheral Blood Mononuclear Cells isolated from 3 healthy individuals |
⇩ STAT1 expression ⇩ TNF expression ⇩ IFNG expression ⇩ PPARG expression ⇩ IL-8, IL-4, IL10, and TNF-α levels |
Caffeine (0.019 mM, 0.102 mM, and 1.16 mM) |
Iris et al., 2018 [47] |
|
Coffee pulp extract/CGA/caffeine |
Raw 264.7 cells |
⇩ TNF-α, IL-6, iNOS, COX-2, and PGE2 expression ⇩NFκB activation ⇩ MAPK signaling |
Cells were stimulated with 1μg/mL LPS and treated with 1000 μg/mL coffee pulp extract, 13.38 μg/mL CGA, and 3.82 μg/mL caffeine |
Ontawong et al., 2023 [48] |
|
Coffee/Green coffee |
C57BL6 male mice |
⇩ Body weight ⇩ Mesenteric fat weight ⇩ Atf3, Fos, and Socs3 ⇩ Hsp70 |
High fat diet 2% freeze-dried caffeinated coffee, decaffeinated coffee, or green coffee for 9 weeks |
Jia et al., 2014 [49] |
|
Instant organic coffee |
C57BL6 male mice |
Improved glucose metabolism ⇩ Adipose tissue inflammation ⇩ Hypertrophy ⇩ Macrophage infiltration ⇩IL-6, TNF-α ⇧ Adapative thermogenesis ⇧ Mitochondrial biogenesis |
High-fat diet +consumption of instant organic coffee (0.1% v/v) for 4 weeks |
Martins et al., 2023 [20] |
|
Caffeine |
Subjects with (n=40) and without coronary artery disease (n=40) |
⇩ CRP in plasma Improvement of brachial endothelial function. |
200 mg Acute C caffeine ingestion |
Shechter et al., 2011 [50] |
|
Caffeinated and decaffeinated coffee |
N= 15,551 women (Nurse’s Health Study) and n= 7,397 men (Health Professionals) | ⇩ CRP ⇩Leptin ⇩ IL-6 ⇩C-peptide ⇩ Estrone, total estradiol, free estradiol ⇧Adiponectin |
Regular coffee consumption; Follow-up between 9 to 14 years |
Hang et al., 2019 [51] |
|
Filtered coffee |
Healthy women (n=730) and women with type 2 diabetes (n=663) aged 43-70 years |
⇩ CRP Prevent endothelial dysfunction ⇩ E-selectin |
Regular caffeinated and decaffeinated coffee consumption. Follow-up of 14 to 15 years |
Lopez-Garcia et al., 2006 [52] |
|
High-CGA coffee |
Cyclists subjects Men (n=10), women (n=5) aged 19 to 51 years |
⇧ Antioxidant capacity in plasma It did not decrease post-exercise inflammation |
High-CGA coffee consumption (300 ml/day) for 2 weeks. Coffee was prepared using the Turkish method. Participation in a 50-Km cycling time trial |
Nieman et al., 2018 [53] |
|
Caffeine/Coffee |
Resistance-trained Iranian men (n=15) around 21 years old. Russian healthy physically active subjects (n=134) aged 28 to 31 years. | ⇩Myeloperoxi-dase ⇩Acetylcholines-terase Association of ADORA2A gene polymorphism with anti-inflammatory effects of caffeine |
6 mg/Kg Acute caffeine consumption before resistance exercise. Regular coffee intake in the physically active subjects. |
Rahimi et al., 2023 [54] |
|
Coffee/caffeine |
Peripheral blood mononuclear cells isolated from 8 healthy individuals |
⇩ Inflammatory markers in some individuals ⇧ inflammatory markers in some individual |
Cells were isolated before and after coffee consumption (3 capsules of coffee containing 165 mg caffeine). Exposed to 1 μg/mL LPS and 5 μg/mL phytohaemagglutinin. Cells were treated with 200 ng/mL caffein in vitro |
Muqaku et al., 2016 [55] |
|
Caffeine |
Healthy subjects: men (n=112) and women (n=132) aged 18 to 55 years | ⇩ CRP in plasma ⇩ Body fatt total and visceral ⇧ Adiponectin ⇧ Il-10 ⇩ IL-6, TNF-α |
Habitual caffeine intake |
Rodas et al., 2020 [59] |
|
Coffee |
Individuals (n=109) aged 22 to 70 years |
⇧ Total cholesterol ⇧ Triglycerides ⇧ LDL-C ⇧VLDL-C |
Regular coffee consumption (Turkish method and instant coffee) |
Saad Al-Fawaeir et al., 2023 [63] |
|
Coffee |
Women with vitamin D deficiency (n=270) aged 18 to 65 years |
⇧ Total cholesterol/HDL ratio |
Turkish coffee consumption during 3 previous months. Moderate consumption (1-2 cups/day). High consumption (⩾ 3 cups/day). 150 mg caffeine per cup |
Habash et al., 2022 [66] |
|
Coffee |
Healthy volunteers (n=3000) |
Filtered coffee: ⇩ Serum cholesterol ⇩ Triglyceride Unfiltered coffee: ⇧ Serum cholesterol ⇧ Triglycerides |
Filtered and unfiltered coffee consumption (1-5 cups/day) |
Naidoo et al., 2011 [67] |
|
Coffee |
Healthy volunteers (n=1272) over the age of 30. |
⇧ HDL-C levels |
Regular plain black coffee consumption (5 cups per week). Fo-llow-up of 13 years |
Chang et al., 2010 [68] |
|
Filtered coffee |
ELSA-Brasil cohort (n=4732) |
⇧ Total cholesterol ⇧ Triglycerides ⇧ VLDL-C ⇧ Triglyceride-rich lipoprotein particles |
Regular high-consumption of filtered coffee (more than 3 cups/day) |
Miranda et al., 2022 [69] |
|
Coffee |
Tromø Study in Norther Norway (n=21083) aged 40 years |
⇧ Total cholesterol levels |
Espresso coffee 3 to 5 cups per day. Boiled/plunger coffee more than 6 cups per day |
Svatun et al., 2020 [70] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Coffee Organization [ICO]. Coffee Report and Outlook, December 2023 [PDF]. 2023. Retrieved from https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf.
- Farag, M.A.; Zayed, A.; Sallam, I.E.; Abdelwareth, A; Wessjohann, L.A. Metabolomics-Based Approach for Coffee Beverage Improvement in the Context of Processing, Brewing Methods, and Quality Attributes. Foods 2022, 11(6):864. [CrossRef]
- Higgins, L.G.; Cavin, C.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol Appl Pharmacol 2008, 226:328–337. [CrossRef]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur J Nutr 2019, 58(2):865-878. [CrossRef]
- Shanmugam, G. Polyphenols: potent protectors against chronic diseases. Nat Prod Res 2024:1-3. [CrossRef]
- Reis, J.P.; Loria, C.M.; Steffen, L.M.; Zhou, X.; van Horn, L.; Siscovick, D.S.; Jacobs, D.R. Jr.; Carr, J.J. Coffee, decaffeinated coffee, caffeine, and tea consumption in young adulthood and atherosclerosis later in life: the CARDIA study. Arterioscler Thromb Vasc Biol 2010, 30(10):2059-66. [CrossRef]
- Khalili-Moghadam, S.; Hedayati, M.; Golzarand, M.; Mirmiran, P. Effects of green coffee aqueous extract supplementation on glycemic indices, lipid profile, CRP, and malondialdehyde in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Front Nutr 2023, 10:1241844. [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv Food Nutr Res 2020, 91:65-96. [CrossRef]
- Purushothaman, A.; Babu, S.S.; Naroth, S.; Janardanan, D. Antioxidant activity of caffeic acid: thermodynamic and kinetic aspects on the oxidative degradation pathway. Free Radic Res 2022, 56(9-10):617-630. [CrossRef]
- Engin, A. Endothelial Dysfunction in Obesity. Adv Exp Med Biol 2017, 960:345-379. [CrossRef]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9(11):2367. [CrossRef]
- Uner, B.; Macit Celebi, M.S. Anti-obesity effects of chlorogenic acid and caffeine- lipid nanoparticles through PPAR-γ/C/EBP-ɑ pathways. Int J Obes (Lond) 2023, 47(11):1108-1119. [CrossRef]
- Zheng, G.; Qiu, Y.; Zhang, Q.F.; Li, D. Chlorogenic acid and caffeine in combination inhibit fat accumulation by regulating hepatic lipid metabolism-related enzymes in mice. Br J Nutr 2014, 112(6):1034-40. [CrossRef]
- Wang, Q.; Hu, G.; Lu, Q.; Hong, D.; Al-Romaima, A.; Qiu, M.; Xiong, W. Identification and screening of novel diterpenoids from roasted arabica coffee in the regulation of lipid content in white adipocytes. Food Funct 2023, 14(11):5138-5150. [CrossRef]
- Quarta, S.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Damiano, F.; Siculella, L.; Wabitsch, M.; Verri, T.; Favari, C.; Del Rio, D.; Mena, P.; De Caterina, R.; Massaro, M. Coffee Bioactive N-Methylpyridinium Attenuates Tumor Necrosis Factor (TNF)-α-Mediated Insulin Resistance and Inflammation in Human Adipocytes. Biomolecules 2021, 11(10):1545. [CrossRef]
- Duangjai, A.; Nuengchamnong, N.; Suphrom, N.; Trisat, K.; Limpeanchob, N.; Saokaewm S. Potential of Coffee Fruit Extract and Quinic Acid on Adipogenesis and Lipolysis in 3T3-L1 Adipocytes. Kobe J Med Sci 2018, 64(3):E84-E92.
- Kanchanasurakit, S.; Saokaew, S.; Phisalprapa, P.; Duangjai, A. Chlorogenic acid in green bean coffee on body weight: a systematic review and meta-analysis of randomized controlled trials. Syst Rev 2023, 12(1):163. [CrossRef]
- Khalili-Moghadam, S.; Hedayati, M.; Golzarand, M.; Mirmiran, P. Effects of green coffee aqueous extract supplementation on glycemic indices, lipid profile, CRP, and malondialdehyde in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Front Nutr 2023, 10:1241844. [CrossRef]
- Van Schaik, L.; Kettle, C.; Green, R.; Irving, H.R.; Rathnerm J.A. Effects of Caffeine on Brown Adipose Tissue Thermogenesis and Metabolic Homeostasis: A Review. Front Neurosci 2021, 15:621356. [CrossRef]
- Martins, B.C.; Soares, A.C.; Martinsm F.F.; Resende, A.C.; Inada, K.O.P., Souza-Mello, V.; Nunes, N.M.; Daleprane, J.B. Coffee consumption prevents obesity-related comorbidities and attenuates brown adipose tissue whitening in high-fat diet-fed mice. J Nutr Biochem 2023, 117:109336. [CrossRef]
- Costa, M.S.D.; Pontes, K.S.D.S.; Guedes, M.R.; Barreto Silva, M.I.; Klein, M.R.S.T. Association of habitual coffee consumption with obesity, sarcopenia, bone mineral density and cardiovascular risk factors: A two-year follow-up study in kidney transplant recipients. Clin Nutr 2023, 42(10):1889-1900. [CrossRef]
- Henn, M.; Babio, N.; Romaguera, D.; Vázquez-Ruiz, Z.; Konieczna, J.; Vioque, J.; Torres-Collado, L.; Razquin, C.; Buil-Cosiales, P.; Fitó, M.; Schröder, H.; Hu, F.B.; Abete, I.; Zulet, M.Á.; Fernández-Villa, T.; Martín, V.; Estruch, R.; Vidal, J.; Paz-Graniel, I.; Martínez, J.A.; Salas-Salvadó, J.; Martínez-González, M.A.; Ruiz-Canela, M. Increase from low to moderate, but not high, caffeinated coffee consumption is associated with favorable changes in body fat. Clin Nutr 2023, 42(4):477-485. [CrossRef]
- Lara-Guzmán, O.J.; Álvarez-Quintero, R.; Osorio, E.; Naranjo-Cano, M, Muñoz-Durango K. GC/MS method to quantify bioavailable phenolic compounds and antioxidant capacity determination of plasma after acute coffee consumption in human volunteers. Food Res Int 2016, 89(Pt 1):219-226. [CrossRef]
- Cowan, T.E.; Palmnäs, M.S.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: impact on gut microbiota and serum metabolomics. J Nutr Biochem 2014, 25(4):489-95. [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front Endocrinol (Lausanne) 2021, 12:762691. [CrossRef]
- Jonsson, A.L.; Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 2017, 14(2):79-87. [CrossRef]
- Caro-Gómez, E.; Sierra, J.A.; Escobar, J.S.; Álvarez-Quintero, R.; Naranjo, M.; Medina, S.; Velásquez-Mejía, E.P.; Tabares-Guevara, J.H.; Jaramillo, J.C.; León-Varela, Y.M.; Muñoz-Durango, K.; Ramírez-Pineda, J.R. Green Coffee Extract Improves Cardiometabolic Parameters and Modulates Gut Microbiota in High-Fat-Diet-Fed ApoE-/- Mice. Nutrients 2019, 11(3):497. [CrossRef]
- Cavalcanti, M.H.; Roseira, J.P.S.; Leandro, E.D.S.; Arruda, S.F. Effect of a freeze-dried coffee solution in a high-fat diet-induced obesity model in rats: Impact on inflammatory response, lipid profile, and gut microbiota. PLoS One 2022, 17(1):e0262270. [CrossRef]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr Metab Cardiovasc Dis 2018, 28(1):44-52. [CrossRef]
- Stegemann, C.; Pechlaner, R.; Willeit. P.; Langley, S.R.; Mangino, M.; Mayr, U.; Menni, C.; Moayyeri, A.; Santer, P.; Rungger, G.; Spector, T.D.; Willeit, J.; Kiechl, S.; Mayr, M. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129(18):1821-31. [CrossRef]
- Bojic, L.A.; McLaren, D.G.; Harms, A.C.; Hankemeier, T.; Dane, A.; Wang, S.P.; Rosa, R.; Previs, S.F.; Johns, D.G.; Castro-Perez, J.M. Quantitative profiling of oxylipins in plasma and atherosclerotic plaques of hypercholesterolemic rabbits. Anal Bioanal Chem 2016, 408(1):97-105. [CrossRef]
- Zmysłowski, A.; Szterk, A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis 2017, 16(1):188. [CrossRef]
- Gómez-Ruiz, J.A.; Leake, D.S.; Ames, J.M. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem 2007, 55(17):6962-9. Erratum in: J Agric Food Chem. 2007;55(20):8284. [CrossRef]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of Total Phenols and Antioxidants in Coffee Samples of Different Origins and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2022, 27(5):1591. [CrossRef]
- Yukawa, G.S.; Mune, M.; Otani, H.; Tone, Y.; Liang, X.M.; Iwahashi, H.; Sakamoto, W. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry (Mosc) 2004, 69(1):70-4. [CrossRef]
- Natella, F.; Nardini, M.; Belelli, F.; Scaccini, C. Coffee drinking induces incorporation of phenolic acids into LDL and increases the resistance of LDL to ex vivo oxidation in humans. Am J Clin Nutr 2007, 86(3):604-9. [CrossRef]
- Miranda, A.M.; Carioca, A.A.F.; Steluti, J.; da Silva, I.D.C.G.; Fisberg, R.M.; Marchioni, D.M. The effect of coffee intake on lysophosphatidylcholines: A targeted metabolomic approach. Clin Nutr 2017, 36(6):1635-1641. [CrossRef]
- Kuang, A.; Erlund, I.; Herder, C.; Westerhuis, J.A.; Tuomilehto, J. Cornelis, M.C. Lipidomic Response to Coffee Consumption. Nutrients. 2018;10(12):1851. [CrossRef]
- Tung, W.C.; Rizzo, B.; Dabbagh, Y.; Saraswat, S.; Romanczyk, M.; Codorniu-Hernández, E.; Rebollido-Rios, R.; Needs, P.W.; Kroon, P.A.; Rakotomanomana, N.; Dangles, O.; Weikel, K.; Vinson, J. Polyphenols bind to low density lipoprotein at biologically relevant concentrations that are protective for heart disease. Arch Biochem Biophys 2020, 694:108589. [CrossRef]
- Corrêa, T.A.; Monteiro, M.P.; Mendes, T.M.; Oliveira, D.M.; Rogero, M.M.; Benites, C.I.; Vinagre, C.G.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; César, L.A.; Torres, E.A. Medium light and medium roast paper-filtered coffee increased antioxidant capacity in healthy volunteers: results of a randomized trial. Plant Foods Hum Nutr 2012, 67(3):277-82. [CrossRef]
- Lara-Guzmán, O.J.; Medina, S.; Álvarez, R.; Oger, C.; Durand, T.; Galano, J.M.; Zuluaga, N.; Gil-Izquierdo, Á.; Muñoz-Durango, K. Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells. Free Radic Biol Med 2020, 160:604-617. [CrossRef]
- Lara-Guzmán, O.J.; Arango-González, Á.; Rivera, D.A.; Muñoz-Durango, K.; Sierra, J.A. The colonic polyphenol catabolite dihydroferulic acid (DHFA) regulates macrophages activated by oxidized LDL, 7-ketocholesterol, and LPS switching from pro- to anti-inflammatory mediators. Food Funct 2024, 15(20):10399-10413. [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; Tuomilehto, J. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr 2010, 91(4):950-7. [CrossRef]
- El-Huneidi, W.; Anjum, S.; Bajbouj, K.; Abu-Gharbieh, E.; Taneera, J. The Coffee Diterpene, Kahweol, Ameliorates Pancreatic β-Cell Function in Streptozotocin (STZ)-Treated Rat INS-1 Cells through NF-kB and p-AKT/Bcl-2 Pathways. Molecules 2021, 26(17):5167. [CrossRef]
- Wu, K.C.; McDonald, P.R.; Liu, J.; Klaassen, C.D. Screening of natural compounds as activators of the keap1-nrf2 pathway. Planta Med 2014, 80(1):97-104. [CrossRef]
- Hwang, J.H.; Kim, K.J.; Ryu, S.J.; Lee, B.Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem Biol Interact 2016, 248:1-7. [CrossRef]
- Iris, M.; Tsou, P.S.; Sawalha, A.H. Caffeine inhibits STAT1 signaling and downregulates inflammatory pathways involved in autoimmunity. Clin Immunol 2018, 192:68-77. [CrossRef]
- Ontawong, A.; Duangjai, A.; Vaddhanaphuti, C.S.; Amornlerdpison, D.; Pengnet, S.; Kamkaew, N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon 2023, 9(3):e13917. [CrossRef]
- Jia, H.; Aw, W.; Egashira, K.; Takahashi, S.; Aoyama, S.; Saito, K.; Kishimoto, Y.; Kato, H. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr 2014, 9(3):389. [CrossRef]
- Shechter, M.; Shalmon, G.; Scheinowitz, M.; Koren-Morag, N.; Feinberg, M.S.; Harats, D.; Sela, B.A.; Sharabi, Y.; Chouraqui, P. Impact of acute caffeine ingestion on endothelial function in subjects with and without coronary artery disease. Am J Cardiol 2011, 107(9):1255-61. [CrossRef]
- Hang, D.; Kværner, A.S.; Ma, W.; Hu, Y.; Tabung, F.K.; Nan, H.; Hu, Z.; Shen, H.; Mucci, L.A.; Chan, A.T.; Giovannucci, E.L.; Song, M. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am J Clin Nutr 2019,109(3):635-647. [CrossRef]
- Lopez-Garcia, E.; van Dam, R.M.; Qi, L.; Hu, F.B. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr 2006, 84(4):888-93. [CrossRef]
- Nieman, D.C.; Goodman, C.L.; Capps, C.R.; Shue, Z.L.; Arnot, R. Influence of 2-Weeks Ingestion of High Chlorogenic Acid Coffee on Mood State, Performance, and Postexercise Inflammation and Oxidative Stress: A Randomized, Placebo-Controlled Trial. Int J Sport Nutr Exerc Metab 2018, 28(1):55-65. [CrossRef]
- Rahimi, M.R.; Semenova, E.A.; Larin, A.K.; Kulemin, N.A.; Generozov, E.V.; Łubkowska, B.; Ahmetov, I.I.; Golpasandi, H. The ADORA2A TT Genotype Is Associated with Anti-Inflammatory Effects of Caffeine in Response to Resistance Exercise and Habitual Coffee Intake. Nutrients 2023, 15:1634. [CrossRef]
- Muqaku, B.; Tahir, A.; Klepeisz, P.; Bileck, A.; Kreutz, D.; Mayer, R.L.; Meier, S.M.; Gerner, M.; Schmetterer, K.; Gerner, C. Coffee consumption modulates inflammatory processes in an individual fashion. Mol Nutr Food Res 2016, 60(12):2529-2541. [CrossRef]
- Yang, A.; Palmer, A.A.; de Wit, H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology (Berl) 2010, 211(3):245-57. [CrossRef]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 2013, 12(4):265-86. [CrossRef]
- Volz, N.; Boettler, U.; Winkler, S.; Teller, N.; Schwarz, C.; Bakuradze, T.; Eisenbrand, G.; Haupt, L.; Griffiths, L.R.; Stiebitz, H.; Bytof, G.; Lantz, I.; Lang, R.; Hofmann, T.; Somoza, V.; Marko, D. Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J Agric Food Chem 2012, 60(38):9631-41. [CrossRef]
- Rodas, L.; Riera-Sampol, A.; Aguilo, A.; Martínez, S.; Tauler, P. Effects of Habitual Caffeine Intake, Physical Activity Levels, and Sedentary Behavior on the Inflammatory Status in a Healthy Population. Nutrients 2020, 12(8):2325. [CrossRef]
- Guercia, E.; Berti, F.; De Zorzi, R.; Navarini, L.; Geremia, S.; Medagli, B.; De Conto, M.; Cassetta, A.; Forzato, C. On the Cholesterol Raising Effect of Coffee Diterpenes Cafestol and 16-O-Methylcafestol: Interaction with Farnesoid X Receptor. Int J Mol Sci 2024, 25(11):6096. [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, L.F.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends in Food Science and Technology 2020, 96:45-60. [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2020, 9(2):121. [CrossRef]
- Saad, Al-Fawaeir.; Jafar, M.; Alawneh, Ibrahim, Al-Odat. Influence of coffee consumption on serum lipid profile parameters: Can coffee consumption lead to health consequences in humans? J Agr Food Res 2023, 14:100904.
- Post, S.M.; de Wit, E.C.; Princen, H.M. Cafestol, the cholesterol-raising factor in boiled coffee, suppresses bile acid synthesis by downregulation of cholesterol 7 alpha-hydroxylase and sterol 27-hydroxylase in rat hepatocytes. Arterioscler Thromb Vasc Biol 1997, 17(11):3064-70. [CrossRef]
- Cai, L.; Ma, D.; Zhang, Y.; Liu, Z.; Wang, P. The effect of coffee consumption on serum lipids: a meta-analysis of randomized controlled trials. Eur J Clin Nutr 2012, 66(8):872-7. [CrossRef]
- Habash, M.; Al-Shakhshir, S.; Abusamak, M.; Mohammad, M.Y.; AbuSamak, M. The association of coffee consumption rate with serum 25-hydroxyvitamin D, non-HDL levels, and TC/HDL ratio in females with vitamin D deficiency. Womens Health (Lond) 2022, 18:17455057221112268. [CrossRef]
- Naidoo, N.; Chen, C.; Rebello, S.A.; Speer, K.; Tai, E.S.; Lee, J.; Buchmann, S.; Koelling-Speer, I.; van Dam, R.M. Cholesterol-raising diterpenes in types of coffee commonly consumed in Singapore, Indonesia and India and associations with blood lipids: a survey and cross sectional study. Nutr J 2011, 10:48. [CrossRef]
- Chang, H.C.; Nfor, O.N.; Ho, C.C.; Chen, P.H.; Kung, Y.Y.; Hsu, S.Y.; Tantoh, D.M.; Liaw, Y.C.; Hsieh, C.F.; Liaw, Y.P. Changes in High-Density Lipoprotein Cholesterol Levels in Relation to Coffee Consumption Among Taiwanese Adults. J Multidiscip Healthc 2020, 13:1427-1432. [CrossRef]
- Miranda, A.M.; Goulart, A.C.; Generoso, G.; Bittencourt, M.S.; Santos, R.D.; Toth, P.P.; Jones, S.R.; Benseñor, I.M.; Lotufo, P.A.; Marchioni, D.M. Association between coffee consumption with serum lipid profile in ELSA-Brasil study: a metabolomic approach. Eur J Nutr 2022, 61(8):4205-4214. [CrossRef]
- Svatun, Å.L.; Løchen, M.L.; Thelle, D.S.; Wilsgaard, T. Association between espresso coffee and serum total cholesterol: the Tromsø Study 2015-2016. Open Heart 2022, 9(1):e001946. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





