1. Introduction
More than 50% of stroke patients experience gait problems and have a reduced likelihood of regaining their previous motor skills [
1]. Limited ankle dorsiflexion (DF) in stroke patients is one of the main causes of functional limitations after stroke [
2,
3]. The causes of limited DF are spastic plantar flexors and weakened dorsiflexors, which make it difficult for stroke patients to perform active DF [
4]. Limited DF in stroke patients can restrict fascia, ligaments, and muscles, resulting in loss of normal joint range of motion (ROM) and other accessory movements of the ankle joint and adjacent joints [
5,
6]. Insufficient DF causes asymmetric weight bearing on the affected leg, which reduces the balance and gait of stroke patients and ultimately interferes with their ability to perform activities of daily living (ADL) or participate socially [
7].
Regaining efficient gait is a major goal for stroke survivors. Therefore, to overcome mobility impairment in stroke patients, interventions that improve ankle DF are commonly used during rehabilitation, including calf muscle stretching, ankle foot orthoses, ankle ROM exercises, robot training, and functional electrical stimulation of the dorsiflexors [
8,
9,
10,
11,
12]. Recently, there has been increased research on the effectiveness of the floss band at improving DF. A floss band is a simple intervention that involves wrapping a joint or muscle with a thick elastic band to cause vascular occlusion and then performing active exercises for 1~3 min [
13]. The mechanism of the floss band technique include fascial shearing and occluding blood flow to the muscle [
14]. Driller and Overmayer (2017) reported that using a floss band on either ankle increased DF, plantarflexion, and jump performance in 52 recreational athletes. Vogrin et al. (2020b) found that the floss band technique improved ROM and muscle function for 45 minutes. In addition, athletes who used the floss band technique exhibited improvement in DF in a weight-bearing posture that was sustained for 72 hours [
17].
Studies have consistently shown the potential of floss band application in improving ankle ROM in athletes. However, no studies have applied a floss band to the ankles of stroke patients. Therefore, this study analyzed the effects of a floss band applied to the ankle joint in chronic stroke patients on ROM, balance, and gait, and compared these outcomes with those of a sham group.
2. Materials and Methods
2.1. Participants
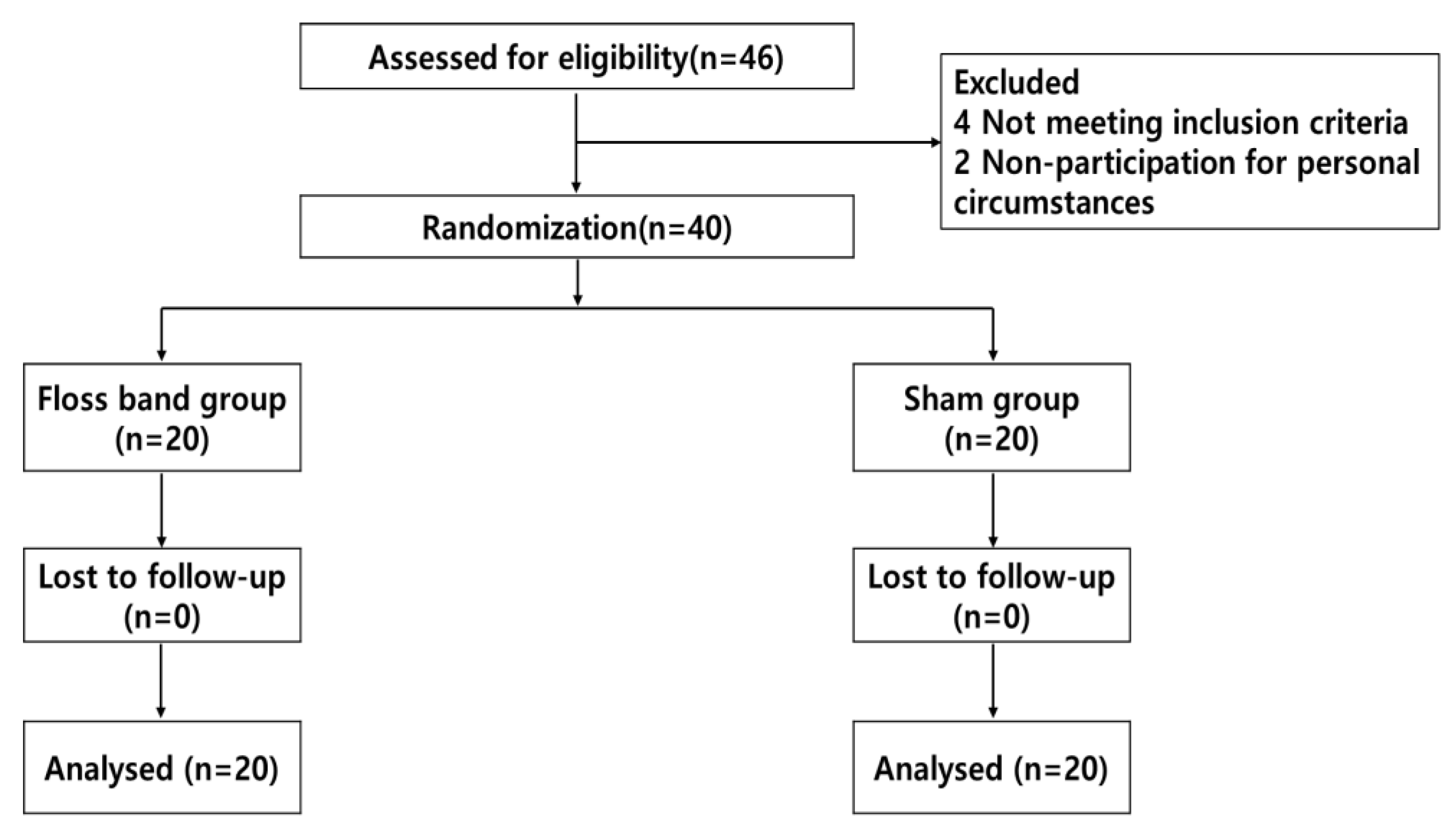
This randomized controlled trial used a two-by-two factorial design (Figure. 1). G*Power software (ver. 3.1 for Windows; Franz Faul, University of Kiel, Kiel, Germany) was used to calculate the sample size needed assuming a moderate effect size of 0.25 for repeated measures, with both within and between-group analyses, an alpha error probability of 0.05, and a power of 0.80. A total sample size of 34 subjects was estimated. We enrolled 40 people assuming a dropout rate exceeding 10%. Subjects were recruited from H Rehabilitation Hospital, Republic of Korea. All subjects were diagnosed with ischemic or hemorrhagic stroke, as confirmed by computed tomography or magnetic resonance imaging. The study enrolled chronic patients 6 months after stroke onset who were able to walk 10 meters independently, were unable to dorsiflex the ankle on the affected side, had a modified Ashworth scale (MAS) score < G2 for the ankle joint, and had adequate cognitive status (Mini-Mental State Examination (MMSE) score of ≥ 24. The exclusion criteria were impaired lower extremity function due to other causes, dizziness, hemianopia, or other symptoms indicating vestibular dysfunction, serious heart disease or use of a pacemaker, and unable to tolerate the floss band intervention. The study was approved by the Ethics Committee of Nambu University (1041478-2021-HR-022). Patients provided informed consent before participating in the study.
Figure 1.
Flow chart of the study.
Figure 1.
Flow chart of the study.
2.2. Procedures
This was a cross-sectional study. The clinical information collected included age, height, weight, gender, affected side, stroke type, Korean Mini-Mental State examination (K-MMSE) score, MAS, Brunnstrom recovery stage (BRS), Berg Balance Scale (BBS) score, modified Barthel index (MBI), and timed up and go (TUG) performance. The patients were selected using a randomization program (
www.randomizer.org) and randomly assigned to the floss band (n = 20) or sham (n = 20) group. The results before and immediately after using the floss band were compared.
2.3. Interventions
All patients received conventional treatment, which included functional electric stimulation (FES), joint mobilization, passive stretching, balance training, and gait training. Floss bands were applied by qualified therapists with at least 5 years of experience.
2.3.1. Floss Band intervention
The floss band intervention used the standard floss band technique, wrapping a floss band (lime green Sanctband Comprefloss™, 2” × 3.5 m; PENTEL, Shah Alam, Malaysia) made of natural rubber tightly around the ankle on the affected side. It was started at the fifth metatarsal and wrapped horizontally around the metatarsals twice, in a figure eight to the medial malleolus, over the Achilles tendon and the lateral malleolus three times, and around the medial malleolus again, before being passed twice from the medial malleolus over the Achilles tendon to the lateral malleolus, forming an end knot (
Figure 2). The participants were instructed to perform low-intensity active exercise involving ankle DF and plantar flexion for 2 minutes after applying the floss band. Then, the floss band was removed and the patient was asked to walk lightly on level ground for about 1 minute to allow reperfusion to normalize blood flow.
2.3.2. Sham Floss Band Intervention
The wrap used in the sham group lacked the elasticity of a floss band; the ankle was wrapped in the same manner, but loosely to facilitate blood circulation. Similarly, the participants were instructed to perform low-intensity active exercise involving ankle DF and plantar flexion for 2 minutes after applying the sham floss band. Finally, the sham floss band was removed, and the patient was asked to walk lightly on level ground for about 1 minute under the same conditions as in the floss band group.
2.4. Outcome Measurements
The participants were assessed before and immediately after the band intervention (floss or sham), including in terms of ankle passive ROM, weight-bearing lunge test (WBLT) performance, static balance, and gait ability.
2.4.1. Ankle Passive Range of Motion
Ankle passive ROM was measured using a universal goniometer in a non-weight bearing position. Subjects were prone with the knee joint at 90° [
18]. The ankle joint was set at 0° of eversion and inversion. The goniometer axis was placed beneath the lateral malleolus, and the stationary arm was positioned parallel to the fibula. The movable arm was positioned parallel to the fifth metatarsal, with the ankle in a neutral position. The measurement was repeated three times, and the average value was calculated. The intrarater reliability of the passive ankle DF had an intraclass correlation coefficient (ICC) of 0.92–0.96 [
19,
20].
2.4.2. Weight-Bearing Lunge Test
The WBLT was performed to assess DF in a functional ankle joint. A measuring tape was placed horizontally on the floor perpendicular to a wall. The participants placed their affected-side foot on the tape with their big toe contacting the wall, and were instructed to touch the wall with the knee on the affected side. While maintaining this position, they were instructed to perform lunges by bending their knee, aiming for contact between their knee and the wall while keeping their heel firmly fixed on the floor. Once they were able to maintain knee and heel contact, the affected side foot was moved away from the wall, and they repeated the lunge test. The test was performed with 1-cm increases until knee and heel contact was no longer maintained. The maximum lunge distance was the farthest distance from the wall to the big toe with the foot staying on the floor (without heel lifting) when the knee touched the wall. Three practice trials of the WBLT were conducted, followed by three test trials. For data analysis, the average of the three trials was calculated. The intrarater reliability of the WBLT was high (ICC = 0.98–0.99) [
21].
2.4.3. Static Balance Ability
Static balance was assessed using the APDM Mobility Lab™ Opal inertial sensor system (APDM, Portland, OR, USA). The test was conducted in a quiet treatment room. During the test, participants were barefoot and wore three Opal inertial sensors: one over their clothing at the level of the fifth lumbar vertebra and one on each ankle. Each subject was instructed to maintain their balance as stably as possible in a barefoot standing position (10 cm between heels, toe-out of 5°) for 30 seconds. The test was repeated three times at 30-second intervals. The static balance outcome measure was the postural sway area (cm/s2). The signal was sampled, processed automatically, and streamed to a laptop using Mobility Lab™ software (Mobility Lab, Arlington, VA, USA). Balance assessed with the APDM had a high ICC or 0.60–0.89 [
22].
2.4.4. Gait Ability
The APDM Mobility Lab™ Opal inertial sensor system (APDM) was used to assess gait based on the foot strike (FS) and toe-off (TO) angles. Data were collected from the sensor wirelessly at a sampling rate of 128 Hz and processed to quantify postural sway parameters. The test was conducted in a quiet treatment room. During the test, participants were barefoot and wore three Opal inertial sensors: one over their clothing at the level of the fifth lumbar vertebra and at each ankle. Verbal instructions were given to ensure accuracy. The subject was told to stand still at the start line until the first long tone was heard, at which time they started walking at a comfortable natural pace. When a second tone was heard after 2 minutes, the participants were asked to stop walking. After practicing for 30 seconds to become familiar with the test, participants were asked to walk back and forth along a straight 10-meter corridor at their usual pace for 2 minutes without a walking assist. All procedures were performed by an experienced physical therapist who stood nearby and ensured the participants’ safety. Gait parameters extracted for analysis included the FS and TO angles. Signals were streamed to a laptop for automatic processing using Mobility Lab™ software. The APDM gait had excellent reliability, with an ICC of 0.905–0.991 [
23].
2.5. Statistical Analysis
The data were analyzed using IBM SPSS Statistics 22.0 (Chicago, IL, USA). Descriptive statistics (mean ± standard deviation) were calculated for age, height, weight, K-MMSE, MAS, BRS, BBS, MBI, and TUG. Frequency analysis was used to assess gender, affected side, and stroke type. Normality was tested using the Kolmogorov–Smirnov test. Two-way repeated-measures ANOVA was used to make comparisons between groups (floss vs. sham) and times (before vs. after), with the paired t-test applied if significant main effects were present. Statistical significance was set at p < 0.05.
3. Results
We enrolled 40 stroke patients (22 men, 18 women). There were no serious adverse events or patient withdrawals.
3.1. Baseline
Table 1 summarizes the patients’ demographics and stroke characteristics. There were no significant baseline differences between groups in age, height, weight, gender, affected side, stroke type, K-MMSE, MAS, BRS, BBS, MBI, or TUG.
3.2. Passive ROM
The passive ROM of DF improved in both the floss band and sham groups after the intervention (F = 146.745,
p = 0.000), but the improvement was significantly greater in the floss band group (F = 13.728,
p = 0.002). In addition, a significant time × group interaction effect was found (F = 114.000,
p = 0.000). Patients treated with the floss band had a greater improvement in passive DF ROM than those treated with the sham floss band. However, the passive ROM of plantar flexion showed no significant differences according to time or group, and there was no significant time × group interaction effect (
p > 0.05) (
Table 2).
3.3. WBLT
The WBLT improved in both groups after the intervention (F = 27.143, p = 0.000), but the improvement was significantly greater in the floss band group (F = 6.234, p = 0.022). However, there was no significant time × group interaction effect (F = 3.276, p = 0.086) (
Table 2).
3.4. Balance ability
The sway area of static balance improved in both groups after the intervention (F = 27.143, p = 0.000), but improvement was significantly greater in the floss band group (F = 6.234, p = 0.022). In addition, there was a significant time × group interaction effect (F = 7.878, p = 0.011). Patients treated with the floss band had greater improvement in static balance than those treated with the sham floss band (
Table 2).
3.5. Gait ability
FS improved in both groups after the intervention (F = 15.422, p = 0.001), and there was no significant difference in the degree of improvement between the two groups (F = 2.076, p = 0.166). There was a significant time × group interaction effect (F = 12.694, p = 0.002). Patients treated with the floss band had a greater improvement in FS. There was no significant main effect of time or group, and no significant time × group interaction effect (p > 0.05), on TO (
Table 2).
4. Discussion
This study is the first to investigate the effects of applying floss bands to the ankle joint on DF and plantarflexion, functional joint ROM under weight-bearing conditions, static balance, and gait ability in stroke patients. We found improvements in DF, static balance, and gait ability when floss bands were applied to the ankle joint. These significant effects of the floss band may have practical implications for ADL in stroke patients, given the improvements in balance and gait abilities.
Applying floss bands to the ankle joint in stroke patients significantly improved DF compared to the placebo band. There was a significant intervention × time interaction effect on the DF, with superior outcomes seen in the floss band group compared to the sham group. This is consistent with Driller and Overmayer (2017), who reported that floss bands improved ankle DF in athletes and recommended the application of floss bands before sports events to increase the ankle DF and help prevent injury. Stroke patients often experience limited ankle DF compared to healthy individuals, in turn leading to functional limitations. The physiological mechanism of floss bands involves compressing the muscles, stimulating the nervous system to separate the fascia, and allowing the fascia to move freely [
24]. Stroke often involves paralysis around the ankle, limiting ankle ROM, causing muscle weakness, and restricting sliding of the fascia. Therefore, the application of floss bands to stroke patients is believed to improve the limited DF through fascial separation via muscle compression.
Our results also showed that applying floss bands in stroke patients improved WBLT significantly. The WBLT is used to assess ankle DF, flexibility, stability, and balance in a closed kinetic chain. One plausible explanation is that the floss band strengthens the ankle muscles, which in turn improves functional ankle DF. Stevenson et al. (2019) achieved improvements in WBLT performance using a floss band at the ankle, suggesting that the increased functional ROM facilitates treatment. Although the mechanism of floss bands remains known, Vogrin et al. (2020a) showed that blood flow restriction leads to the accumulation of metabolites, synthesis of muscle proteins, and increase in growth hormones. The subsequent reperfusion normalizes the supply of nutrients and hyaluronic acid to local areas, increasing ROM [
25,
27]. We postulate that the floss band increased WBLT via this mechanism.
In our subjects, the sway area of static balance was reduced significantly more with the floss band compared to the placebo, and there was a significant intervention × time interaction. The static balance of stroke patients is related to ankle muscle strength [
29,
30], flexibility [
30], and proprioception [
31]. Kim & Kim (2018) reported that static balance when standing was correlated with ankle ROM and lower extremity muscle strength. We postulated that the application of a floss band affected balance by increasing the ankle ROM. Chang et al. (2021) reported that applying a floss band to the knee in men improved lower extremity flexibility, quadriceps strength, and balance. Application of a floss band relaxes soft tissues and increases muscle elasticity, thereby reducing joint stiffness and improving balance. Therefore, the application of a floss band in stroke patients can improve static balance while standing by improving ankle ROM, muscle strength around the ankle, and coordination on the affected side.
We observed a significantly greater improvement in FS during gait with the floss band compared to the placebo, and there was a significant intervention × time interaction. FS refers to the initial contact during the gait cycle and is closely related to the range of DF [
34]. Ravichandran & Janakiraman (2021) claimed that improving the functional range of DF is essential for post-stroke rehabilitation because limited ankle DF is common in most chronic stroke patients and is related to balance and gait. Stroke patients often have limited ankle DF, and abnormal initial contact occurs at the forefoot or outer edge of the foot, negatively affecting the stability of the loading response phase [
35]. Kaneda et al. (2020b) reported that applying a floss band to the gastrocnemius improved flexibility, the rate of force development, and DF. It is thought that the application of a floss band increased DF by reducing the tension in the calf muscle and surrounding soft tissues, thereby increasing the FS angle. Stroke results in complex sensorimotor deficits, including muscle weakness, impaired muscle control, spasticity, and proprioceptive deficits that affect balance and gait. Cho et al. (2021) reported that ankle muscle contraction and active-resistive strength training in chronic stroke improved ankle muscle strength, proprioception, balance, and gait. Similar to previous authors, we think that the application of a floss band increased DF by relaxing the calf muscle and surrounding soft tissues, thereby increasing the FS angle. Since the application of a floss band enhances DF and balance, it should help to improve the gait of stroke patients.
We observed a significant effect of the floss band on plantar flexion and TO angle in stroke patients. We postulate that there was no difference in plantar flexion between the groups in this study because the plantar flexion angle of the stroke patients was within the normal ROM. TO refers to the initial swing during the gait cycle and is closely related to plantar flexion [
37].
This study has several limitations. First, it analyzed the immediate effect of the floss band, and further research needs to examine changes over time. Second, we applied a floss band only to the ankle of stroke patients; further studies need to examine the effects on other joints in these patients. Lastly, our results may not be generalizable to all patients with stroke as we studied stroke patients in BRS 4 or 5.
5. Conclusions
This study investigated the effects of a floss band on the ankle ROM , WBLT performance, balance, and gait of stroke patients. The floss band group had significantly improved DF, FS, and balance compared to the control group. Therefore, a floss band is a feasible therapeutic method for improving ankle DF, balance, and FS, to in turn improving the gait of chronic stroke patients undergoing rehabilitation. The study findings serve as a valuable reference that could aid the clinical application of floss bands.
Author Contributions
Conceptualization, J.K. and B.M.; formal analysis, B.M.; investigation, J.K.; data curation, B.M.; writing—original draft preparation, B.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported( in part) by research funds from Nambu University, 2023
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Nambu University (1041478-2021-HR-022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choi, S.H.; Lim, C.G. Immediate effects of ankle non-elastic taping on balance and gait ability in patients with chronic stroke: a randomized, controlled trial. Journal of Manipulative and Physiological Therapeutics 2020, 43, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.H.; Keenan, M.A. Gait analysis to assist walking after stroke. The Lancet 2007, 369, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Park, J.; Song, G.; Lee, S.; Jo, Y.; Jin, M.; Lee, G. Usability of the Thera-Band® to improve foot drop in stroke survivors. NeuroRehabilitation 2018, 42, 505–510. [Google Scholar] [CrossRef]
- Park, D.; Lee, J. H.; Kang, T.W.; Cynn, H.S. Immediate effects of talus-stabilizing taping on balance and gait parameters in patients with chronic stroke: A cross-sectional study. Topics in Stroke Rehabilitation 2018, 25, 417–423. [Google Scholar] [CrossRef]
- Boffeli, T.J.; Collier, R.C. Minimally invasive soft tissue release of foot and ankle contracture secondary to stroke. The Journal of Foot and Ankle Surgery 2014, 53, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, H.; Janakiraman, B. The Effects of Ankle Joint Mobilization on Dorsiflexion Range and Gait Parameters in Chronic Stroke Survivors: A Systematic Review and Meta-analysis. Journal of Stroke Medicine 2021, 4, 15–24. [Google Scholar] [CrossRef]
- Mirshams Shahshahani, P.; Ashton-Miller, J.A. On the importance of the hip abductors during a clinical one legged balance test: A theoretical study. Plos one 2020, 15, e0242454. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ren, Y.; Roth, E.J.; Harvey, R.; Zhang, L.Q. Effects of repeated ankle stretching on calf muscle–tendon and ankle biomechanical properties in stroke survivors. Clinical biomechanics 2011, 26, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Park, S.J. Effects of joint mobilization and stretching on the range of motion for ankle joint and spatiotemporal gait variables in stroke patients. Journal of Stroke and Cerebrovascular Diseases 2020, 29, 104933. [Google Scholar] [CrossRef]
- Choo, Y.J.; Kim, J.K.; Kim, J.H.; Chang, M.C.; Park, D. Machine learning analysis to predict the need for ankle foot orthosis in patients with stroke. Scientific Reports 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Johnston, T.E.; Keller, S.; Denzer-Weiler, C.; Brown, L. A Clinical Practice Guideline for the Use of Ankle-Foot Orthoses and Functional Electrical Stimulation Post-Stroke. Journal of Neurologic Physical Therapy 2021, 45, 112–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Zhou, M.; Li, D.; Yan, J.; Liu, Q.; Long, J. Ankle rehabilitation robot training for stroke patients with foot drop: Optimizing intensity and frequency. NeuroRehabilitation 2023, 53, 567–57. [Google Scholar] [CrossRef] [PubMed]
- Kalc, M.; Mikl, S.; Žökš, F.; Vogrin, M.; Stöggl, T. Effects of different tissue flossing applications on range of motion, maximum voluntary contraction, and h-reflex in young martial arts fighters. Frontiers in Physiology 2021, 12, 752641. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Forte, P.; Dewaele, E.; Branquinho, L.; Teixeira, J.E.; Ferraz, R.; Monteiro, A.M. Effect of blood flow restriction technique on delayed onset muscle soreness: a systematic review. Medicina 2022, 58, 1154. [Google Scholar] [CrossRef] [PubMed]
- Driller, M.W.; Overmayer, R.G. The effects of tissue flossing on ankle range of motion and jump performance. Physical Therapy in Sport 2017, 25, 20–24. [Google Scholar] [CrossRef]
- Vogrin, M.; Novak, F.; Licen, T.; Greiner, N.; Mikl, S.; Kalc, M. Acute effects of tissue flossing on ankle range of motion and tensiomyography parameters. Journal of Sport Rehabilitation 2020, 30, 129–135. [Google Scholar] [CrossRef]
- Rosier, E. The Long-Term Effects of Tissue Flossing on Ankle Dorsiflexion Range of Motion in Athletes with Chronic Ankle Instability, Illinois State University, Illinois, USA, March 31 2020.
- Koshino, Y.; Takabayashi, T.; Akuzawa, H.; Mizota, T.; Numasawa, S.; Kobayashi, T.; Edama, M. Differences and relationships between weightbearing and non-weightbearing dorsiflexion range of motion in foot and ankle injuries. Journal of Orthopaedic Surgery and Research 2024, 19, 115. [Google Scholar] [CrossRef]
- Clapper, M.P.; Wolf, S.L. Comparison of the reliability of the Orthoranger and the standard goniometer for assessing active lower extremity range of motion. Physical Therapy 1988, 68, 214–218. [Google Scholar] [CrossRef]
- Abu El Kasem, S.T.; Aly, S.M.; Kamel, E.M.; Hussein, H.M. Normal active range of motion of lower extremity joints of the healthy young adults in Cairo, Egypt. Bulletin of Faculty of Physical Therapy 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Cady, K.; Croix, M.D.S.; Deighan, M. Back foot influence on dorsiflexion using three different positions of the weight bearing lunge test. Physical Therapy in Sport 2021, 47, 1–6. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Review of Medical Devices 2016, 13, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, S.; Li, J.; Komal, S.; Li, K. Reliability and Validity of a Wearable Inertial Sensor System for Gait Assessment in Healthy Young Adults. In 2021 14th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI) 2021, 1-6.
- Kaneda H; Takahira N; Tsuda K; Tozaki K; Kudo S; Takahashi Y. Effects of tissue flossing and dynamic stretching on hamstring muscles function. Journal of Sports Science & Medicine 2020, 19, 681–689. [Google Scholar]
- Stevenson, P.J.; Stevenson, R.K.; Duarte, K.W. Acute effects of the voodoo flossing band on ankle range of motion. J Med Biomed Appl Sci 2019, 7, 244–253. [Google Scholar]
- Vogrin, M.; Kalc, M.; Ličen, T. Acute effects of tissue flossing around the upper thigh on neuromuscular performance: a study using different degrees of wrapping pressure. Journal of Sport Rehabilitation 2020, 30, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.; Kraemer, R.; Hollander, D.; Clavier, J.; Thomas, C.; Francois, M. Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. Journal of Applied Physiology 2006, 101, 1616–1622. [Google Scholar] [CrossRef]
- In, T.; Jin, Y.; Jung, K.; Cho, H.Y. Treadmill training with Thera-Band improves motor function, gait and balance in stroke patients. NeuroRehabilitation 2017, 40, 109–114. [Google Scholar] [CrossRef]
- Cho, J.E.; Lee, W.H.; Shin, J.H.; Kim, H. Effects of bi-axial ankle strengthening on muscle co-contraction during gait in chronic stroke patients: a randomized controlled pilot study. Gait & Posture 2021, 87, 177–183. [Google Scholar]
- Yoo, D.; Kim, D.H.; Seo, K.H.; Lee, B.C. The effects of technology-assisted ankle rehabilitation on balance control in stroke survivors. IEEE transactions on neural systems and rehabilitation engineering 2019, 27, 1817–1823. [Google Scholar] [CrossRef]
- Cho, J.E.; Kim, H. Ankle proprioception deficit is the strongest factor predicting balance impairment in patients with chronic stroke. Archives of rehabilitation research and clinical translation 2021, 3, 100165. [Google Scholar] [CrossRef]
- Kim, S. G.; Kim, W. S. Effect of ankle range of motion (ROM) and lower-extremity muscle strength on static balance control ability in young adults: a regression analysis. Medical science monitor: international medical journal of experimental and clinical research 2018, 24, 3168. [Google Scholar] [CrossRef]
- Chang, N.J.; Hung, W.C.; Lee, C.L.; Chang, W.D.; Wu, B.H. Effects of a single session of floss band intervention on flexibility of thigh, knee joint proprioception, muscle force output, and dynamic balance in young adults. Applied Sciences 2021, 11, 12052. [Google Scholar] [CrossRef]
- Aali, S.; Rezazadeh, F.; Badicu, G.; Grosz, W.R. Effect of heel-first strike gait on knee and ankle mechanics. Medicina 2021, 57, 657. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Liu, Y.; Liang, W.; Zhang, L.; Zhou, F.; Yuan, X. The effect of electromyographic feedback functional electrical stimulation on the plantar pressure in stroke patients with foot drop. Frontiers in Neuroscience 2024, 18, 1377702. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, H.; Takahira, N.; Tsuda, K.; Tozaki, K.; Sakai, K.; Kudo, S.; Kenmoku, T. The effects of tissue flossing and static stretching on gastrocnemius exertion and flexibility. Isokinetics and Exercise Science 2020, 28, 205–213. [Google Scholar] [CrossRef]
- Inai, T.; Kobayashi, Y.; Huang, C.; Fujita, K.; Fujimoto, M.; Nihey, F.; Kudo, S. Identification of characteristics of foot position and angle during swing phase in fallers using principal component analysis. Frontiers in Bioengineering and Biotechnology 2023, 11, 1117884. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).