1. Introduction
1.1. Observational Studies can Inform the Real-World Impact of Tobacco and Nicotine Product Use
Smoking kills 480,000 people per year in the US alone (American Lung Association, 2024). Pharmaceutical cessation products have been proven to help stop smoking, and yet in the US, 28 million adults still smoke, and global smoking prevalence exceeds 1.3 billion (Cornelius, 2023; WHO, 2023). For adults who can’t or won’t quit smoking conventional combusted cigarettes (CC), non-combusted tobacco and nicotine products were developed as potentially harm-reducing alternatives. Electronic cigarettes (EC) are the most prevalent of these products, with use by 11-17 million adults in the US (Erhabor et al., 2023), and this review is focused on best practices for evaluating the risk-inducing and risk-reducing health effects of EC use. However, the principles discussed in this review are broadly applicable to tobacco and nicotine products.
EC have been widely available for over a decade, and data from national cross-sectional and longitudinal studies, including the Population Assessment of Tobacco and Health Study (PATH), Behavioral Risk Factor Surveillance System (BRFSS), National Health Interview Survey (NHIS), and National Health and Nutrition Examination Survey (NHANES), can increasingly provide information about the health effects associated with extended and chronic use of tobacco products (Boakye et al., 2023). These non-randomized observational studies of exposure can complement evidence from randomized controlled trials with health outcomes associated with real-world actual-use patterns (Black, 1996; Lindson et al., 2023).
1.2. Precision and Accuracy Challenges and Opportunities for Observational Studies of Tobacco and Nicotine Products
Non-randomized observational studies assessing the risk-inducing and risk-reducing effects of tobacco and nicotine product use encompass several research questions, including the evaluation of incremental risk (vs. non-use), relative risk of EC vs. CC, risk reversal (associated with quitting or product switching), and the risk associated with dual use of EC and CC. Answering these questions with a high degree of accuracy and precision is challenging because these studies, by definition, compare outcomes across unbalanced heterogeneous samples. The characteristics of these samples often differ in terms of demographic, environmental and background risk factors, which can introduce bias and conflate risk. To reduce this bias, unadjusted odds ratios (OR) are typically adjusted (aOR) with the goal of accounting for potentially confounding imbalances between cohorts.
Beyond these typical adjustments, evaluation of the impact of tobacco product use involves disambiguating between multiple sources of exposure (EC, CC, and non-tobacco sources of risk) across populations which span different histories of use. A study subject can be a never-, former- or current-user of a given product, and their use state can transition over time (Brouwer, Jeon, et al., 2022; Brouwer, Levy, et al., 2022). The harm associated with each product (EC,CC) is dependent on cumulative use history (dose per use, frequency of use, duration and timing of use, and quit duration). Capturing the cumulative use history of these tobacco products is essential for assessing harm and harm reduction, and the primary focus of this review is on outlining ways to increase precision and accuracy of these assessments (Munafò, 2024).
2. Methods
This review was informed by the ROBINS (Risk Of Bias In Non-randomized Studies) framework, developed through expert consensus to evaluate and mitigate statistical bias in non-randomized studies (D’Andrea et al., 2021; Sterne et al., 2016). The principles from the ROBINS framework were applied to a recent meta-analysis, chosen because it represented possibly the most extensive recent meta-analysis in the field, spanning 107 observational studies that assessed the relative risk of exclusive and dual EC and CC use, across multiple categories of harm including cardiovascular disease, stroke, metabolic dysfunction, asthma, COPD (chronic obstructive pulmonary disease), and oral disease (Glantz et al., 2024).
During the preparation of this review, feedback was solicited from multiple experts in the field (see Acknowledgements). In addition, an initial draft has been posted on the public preprint server Preprints.org. Further supporting data are available in the
Supplementary Materials. The authors invite and welcome timely feedback on the pre-publication draft from anyone in the research community.
3. Definitions
3.1. Harm Reduction Encompasses the Concepts of Relative Risk and Risk Reduction
Observational studies of the health impact of EC use commonly address one or more of the research questions enumerated in
Table 1:
To address these research questions, populations representative of several categories of tobacco use histories are commonly utilized: exclusive use of EC use, product switching and former CC use, and dual EC and CC use (see also
Figure 2). It should be noted that since it can be challenging to identify large samples of exclusive adult EC users who never used CC products, “EC cohorts” often include a mix of former and sometimes also current CC use histories, therefore Questions 2 and 3 are sometimes inherent in answering Question 1.
Question 3, characterizing the risk of dual-use (DU), is an extension of Question 1, where both EC and CC are concurrently used, but it can also incorporate question 2, to the extent that CC use is displaced by EC use in the sample studied. Thus, assessment of DU relative risk requires particular attention to avoiding potential sources of confounding and statistical bias.
3.2. Measures of Incremental Risk and Risk Reduction
In an observational study, non-tobacco users often represent the normative control group for odds adjustments. The non tobacco using control sample should therefore have an adjusted odds ratio (aOR) of 1.0, reflecting the normalized background risk rate due to non-tobacco use sources. These risk sources may include genetic factors, lifestyle and environmental factors such as poor diet and exercise, alcohol, cannabis and other drug use, and exposure to pollution and secondhand smoke (Martin et al., 2024).
The aOR observed in people who use tobacco products includes the baseline risk (of 1.0) from non-tobacco use sources, plus incremental excess risk due to use of the tobacco product (risk in excess of 1.0, see Supplement I, Equations SI-1 and SI-2). For example, CC use has been causally linked with increased risk of cardiovascular disease (CVD), stroke, and other respiratory diseases (Centers for Disease Control and Prevention (US) et al., 2010; Department of Health and Human Services, 2014). Relative risk is the ratio of these excess risks due to product use (Equation SI-3).
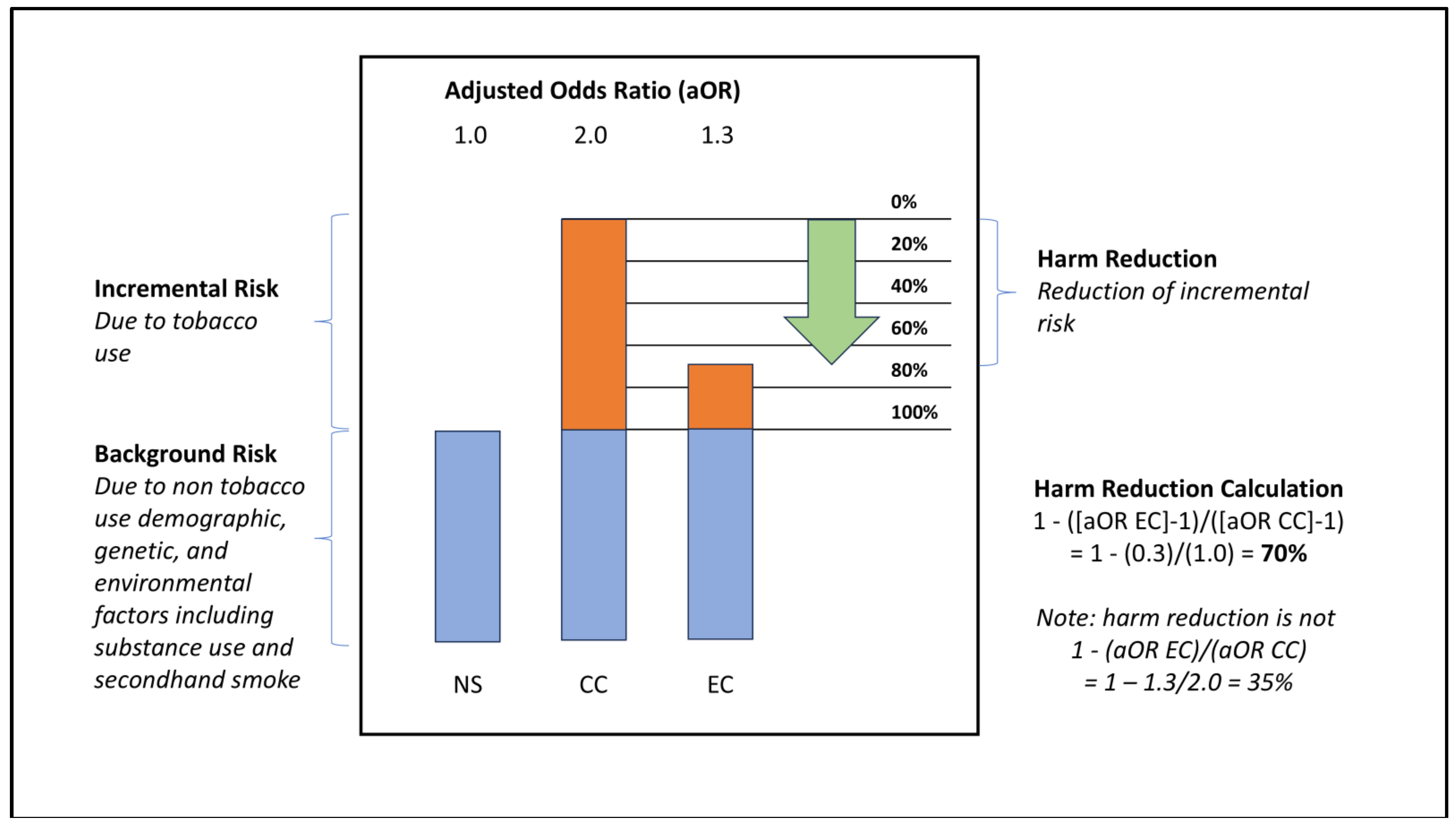
Harm reduction refers to the reduction in the excess risk due to tobacco use, when a less harmful tobacco product is used instead of a more harmful tobacco product. The equation describing harm reduction for use of EC compared to CC is derived in Equation SI-4, and an illustrative example is provided in
Figure 1, below. Further considerations regarding the definitions of risk and harm reduction are discussed in Supplement I.
In this illustrative example, a non-tobacco user (NS) has an adjusted odds ratio (aOR) of 1.0 for a given harm, reflecting the background risk rate. If CC smoking doubles the risk of harm, a subject engaging in CC smoking would have aOR of 2.0. In other words, they would incur a risk of 1.0 from non-tobacco sources, and an additional incremental risk of 1.0 from CC smoking. Likewise, if using EC caused 30% of the incremental risk of CC smoking, a typical study subject using EC would have aOR of 1.3. This aOR would be comprised of risk of 1.0 from non-tobacco sources and 0.3 from EC use. In this example, if the subject had used EC instead of smoking CC, their aOR would be 1.3 instead of 2.0. The harm reduction associated with EC would be 70%, due to the incremental risk of 0.3 (i.e. 1.3 - 1.0) with EC vs. 1.0 for CC (i.e. 2.0 - 1.0). Note that reduction in all sources of risk is 35% for EC vs. CC users (1.3 vs. 2.0), but harm reduction is 70% (0.3 vs. 1.0 incremental risk due specifically to tobacco product use).
4. An Overview of Factors Impacting Precision and Accuracy of Observational Studies
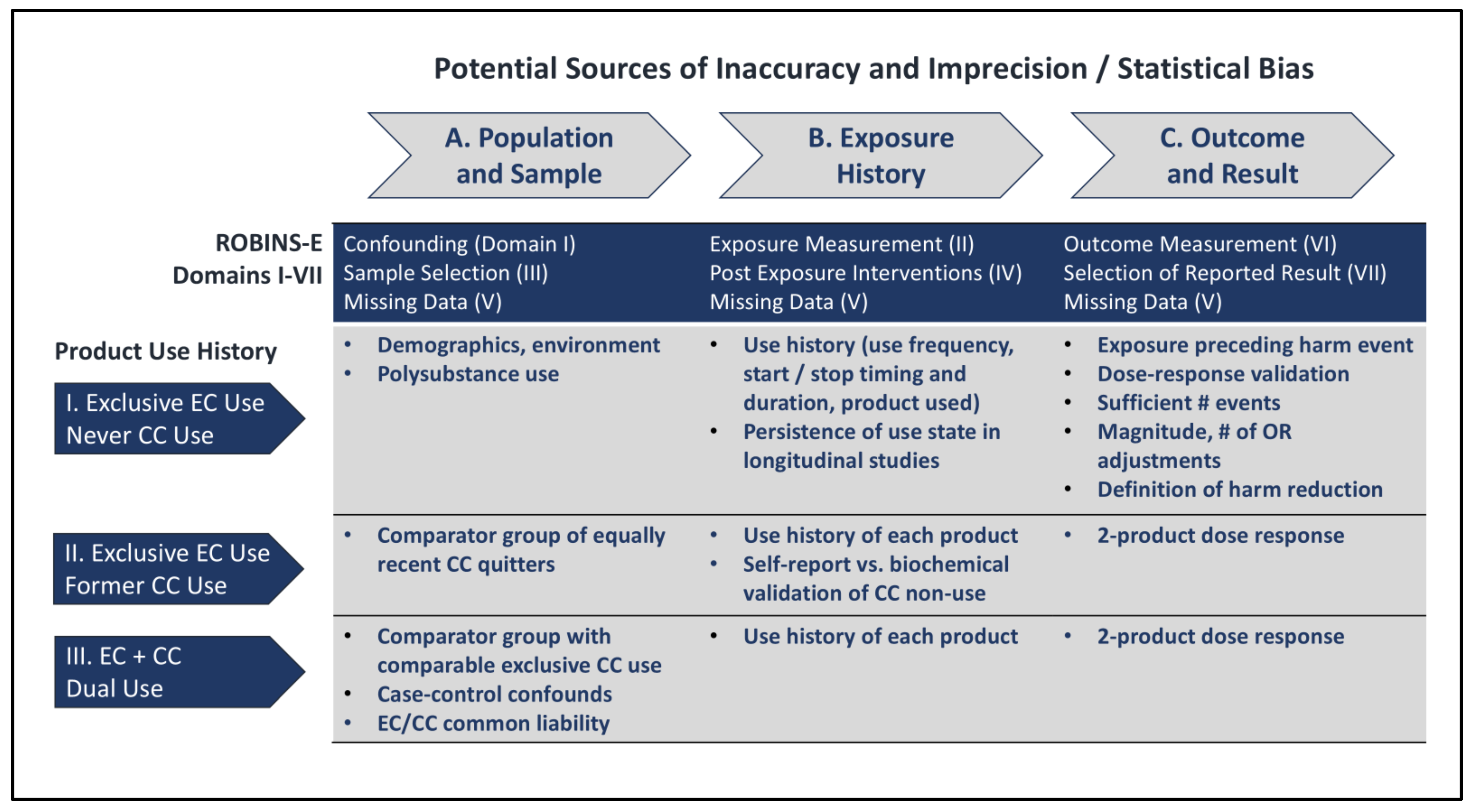
The ROBINS framework identifies seven domains which can impact precision and accuracy of observational studies. Broadly, these sources of potential bias pertain to three groupings: definitions of population and sample, characterization of exposure history, and selection of outcomes and results, as enumerated in
Figure 2, and discussed below.
5. What is the Risk Associated with Exclusive Product Use?
One way to minimize statistical bias in observational studies is by restricting analyses to samples that have only ever used one product to a degree sufficient to impact risk. However, significant biases may still arise if there is not a comprehensive characterization of exposure history, validation that exposure precedes harm in retrospective studies, confirmation of persistence of use state in prospective studies, and sufficiency of outcome sample size. Statistical precision and accuracy can be further validated through convergence of multiple lines of evidence, including dose-responses and consideration of counterfactuals (Munafò & Smith, 2018).
5.1. Population and Sample-Related Considerations
5.1.1. Sample Definition
Samples termed “EC users” often include a history of former CC use, and sometimes even current CC dual-use. A recommendation in these cases is to use more precise terminology, to prevent an inaccurate perception or interpretation that there was no previous or concurrent exposure to other products. Furthermore, EC cohorts which also have former or current exposure to CC tobacco product use require additional considerations to minimize the impact of confounders, as discussed further in this paper, in
Section 6 and
Section 7.
The PATH Wave 6 data set provides an illustrative example. Of older (35+ years old) current exclusive EC users (past 30-day use of only EC), 91% were former CC smokers. Older current EC users who were never CC users (<100 lifetime cigarettes smoked) only represented ~80 participants in this survey of over 30,000 participants (see Figure SI-1). This highlights the challenges in accurately evaluating diseases which may manifest after decades of former CC use.
5.2. Exposure History-Related Considerations
5.2.1. What Product Was Used?
Electronic cigarettes represent a heterogeneous and evolving category. Exposure to nicotine and toxicants, as well as efficacy in displacing CC, may vary dramatically across products, therefore specification of which products were used can be an important consideration in the precision and generalizability of any results. Firstly, it is important to identify which active molecule was vaped. EC use in some survey questions may encompass cannabis use, and in other cases is nicotine-free (Selya et al., 2024). Nicotine can be formulated as a free-base or as nicotine salts with potentially different abuse liability profiles. In general, earlier generations of EC utilized lower concentrations of free-base nicotine, while current generations utilize higher concentrations of nicotine salt formulations, with higher nicotine flux. There may be an inverse relationship between nicotine concentration and toxicant exposure due to nicotine titration (El Hourani et al., 2022). Newer products may be associated with increased efficacy in switching (Kasza et al., 2021). Toxicant exposure may be higher in products which are not temperature regulated, or which do not utilize quality system practices and manufacturing processes which pass FDA review. An extreme example was the phenomenon of EVALI-related harm arising from use of Vitamin E as a cost-cutting solubilizing agent by some manufacturers of FDA-unregulated THC-containing vapes (Marrocco et al., 2022). Flavorants may impact emission chemistry and toxicant profile. The impact of exposure to nicotine analogue molecules such as methylated nicotines is also not well understood (Erythropel et al., 2024). Depending on the specificity of the analysis needed, brand name descriptors, flavor profile, source of purchase, or visual confirmation can all help confirm which product was used. Lastly, “usual product” may or may not be representative of all products used, unless it is confirmed that the usual product was used exclusively.
5.2.1. Comprehensive History of Use
Risk typically increases with repeated exposure to tobacco products. This was demonstrated in the Atherosclerosis Risk in Communities (ARIC) prospective multi-decade longitudinal study that evaluated the predictive value of CC use history metrics on subsequent cardiovascular disease (CVD) risk (Lubin, Couper, et al., 2016). This study found that:
”Pack-years remained the primary determinant of smoking-related CVD risk…smoking fewer cigarettes/day for longer duration was more deleterious than smoking more cigarettes/day for shorter duration…no single metric, cigarettes/day, smoking duration, or pack-years, fully characterizes smoking-related risks.”
Similarly, for chronic obstructive pulmonary disease (COPD), duration of smoking is more predictive of risk than cigarettes per day (Bhatt et al., 2018). In summary, for some observational studies, it may be sufficient to characterize CC use history by either use duration or pack-years. However, depending on the analytic precision required, more granular exposure metrics may be necessary (Lubin, Albanes, et al., 2016).
For quantifying EC exposure, there is no standardized equivalent to the CC pack-years metric. Past 30-day frequency of use data may not be representative of cumulative exposure. Concerningly, most major national surveys, including BRFSS, NHIS, and NHANES, do not report past duration of EC use. Without accounting for the frequency and duration of EC use, studies are limited in their precision and accuracy of measuring the impact of EC exposure.
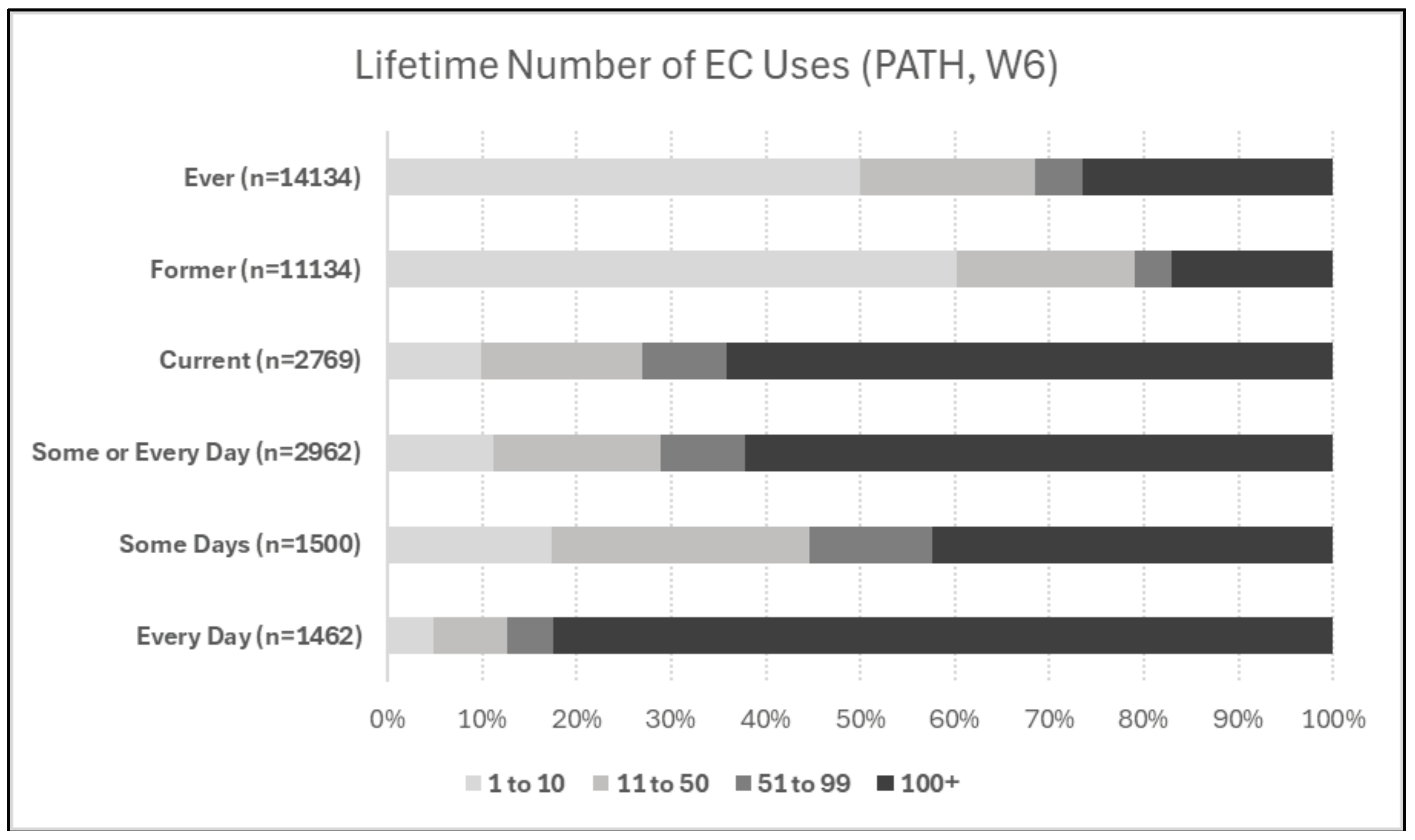
When available, it is instructive to consider frequency, duration, and timing of use information when characterizing EC exposures. For instance, PATH does include time of first ever- or regular-use of EC, as well as lifetime number of uses (Boakye et al., 2023).
Figure 3, below, shows lifetime number of uses of EC for the following categories: ever-use, former use, current use, use some-days or every-day, use some days, and use every day. Of the ever-use sample, 50% reported 10 or fewer lifetime uses, 23% reported 11 to 99 uses, and 27% reported ever-use of 100+ times. Likewise, 58% of current some-day users reported fewer than 100 lifetime uses, while 83% of current every-day users reported 100+ lifetime uses.
5.2.3. Use State Persistence or Transitions in Prospective Studies
Prospective studies typically segment cohorts based on their product use state at the start of the study. Over the course of the prospective period, participants may continue their product use, stop use, and/or transition to other products. A precise assessment of exposure should capture these ongoing product use patterns, in addition to current and past use, as they may exacerbate or ameliorate risk.
5.3. Outcome and Result-Related Considerations
5.3.1. Timing of Harm vs. Exposure
Exposure can only be causal for harm events that occur after the exposure, and the exposure should be of sufficient dose and duration to plausibly drive the underlying physiological disease process. Consequently, retrospective studies should carefully evaluate the timing and duration of exposure, and relative timing at which harm events occurred. For example, PATH Wave 1 (W1, fielded in 2013-14) contains a sample of n=1,684 individuals who reported COPD ('ever in life'). Of these, 1,252 (74%) reported that the harm event occurred 4 or more years earlier, meaning that they had the outcome before EC were widely available in the marketplace.
For assessment of relative risk of exclusive use of EC vs. CC use or vs. non-use, for some databases or endpoints, EC usage data may not be of sufficient duration for precise analysis (Cummings et al., 2024).
For instance, COPD is most typically seen after 40 or more pack-years of smoking. Criteria for diagnosis of early COPD are in development, but an exemplar assessment framework still included a minimum of 10 pack-years of CC use (Curtis et al., 2024). Generalized indicators of health may be predictive of earlier chronic changes in pulmonary function, but may lack predictive accuracy (Rennard & Drummond, 2015). Consequently, potentially more sensitive and specific assessment instruments of early pulmonary health changes are in development (Shiffman et al., 2023).
5.3.2. Self-Reported Metrics and Categorization of Exposure And harm Events
Self-reported metrics of exposure and harm are most accurate when verified through additional means. For instance, in a recent large RCT of EC and nicotine replacement therapy (NRT) for smoking cessation, 26% of self-reports of 7-day cigarette abstinence conflicted with exhaled CO measurements in the EC group, and 33% of abstinence self-reports conflicted in the NRT group (Auer et al., 2024). Likewise, self-report of diagnosis of some health conditions can be confirmed with concordance of corresponding drug prescriptions, although it is also true that some prescriptions go unfilled in real-world settings.
5.3.3. Quantification of risk and risk reduction
Reporting of risk involves differentiating between background risks, not associated with tobacco use, and incremental risks associated with tobacco product use. Harm reduction involves the displacement of incremental risk associated with one product with use by another product with a lesser incremental risk impact. This is described in more detail in
Figure 1.
5.3.4. Verification of dose-response
Verification of a dose-response relationship between exposure and outcome is one of the most important approaches for verifying the accuracy of results. Lack of dose-response relationship should lead to examination of potential counterfactual explanations. A common dose-response comparison is between every-day, some-day, former, and never users, but as discussed previously, precision may be limited without incorporation of duration of use or cumulative exposures to all products (i.e., pack-years of CC and duration of regular use of EC, or lifetime number of EC uses).2
Another validation opportunity, at least in studies using databases such as PATH which capture timing of initiation of regular use, could be to confirm that risk was not elevated until after exposure began. For example, in a population which uses EC, aOR of events in the time before starting EC should be 1.0 if covariate adjustments have canceled out all sources of bias. In other words, zero exposure dose should correspond to zero incremental risk.
5.3.5. Was There Sufficient Sample Size and Quantity of Harm Events for Model Validity?
Sample size sufficiency can be a challenge in non-randomized observational studies of tobacco and nicotine exposure. A generally accepted rule of thumb is that the EPV ratio (events per variable, e.g. the number of hazard events per odds adjustment regression variable) should be at least 10 for linear regression models to avoid “major problems” (Peduzzi et al., 1996).3 Issues which can arise from insufficient EPVs include bias of regression coefficients, confidence limits which don’t properly cover the data, and paradoxical associations (significance in the wrong direction). A definitive exploration is beyond the scope of this review, but there is a need to more deeply explore this issue.
Generally, the greater the demographic imbalance between samples, and the greater the magnitude of adjustments vs. the precision of the aOR effect size and confidence interval, the more closely confounding and counterfactuals should be considered, particularly if directionality of association inverts after adjustment. Transparent reporting of the number of hazard events observed in each sample in the raw data, along with the number and magnitude of adjustment variables, can help to verify EPV ratio sufficiency and accuracy of results.
5.3.6. Accuracy of Comparisons Across Populations with Minimal Demographic Overlap
Adjustments of odds ratios in the field of tobacco research are often derived from application of a linear fit in a regression model. However, linear fits may introduce confounding if variables are not independent or don’t follow linear relationships. One of the most common examples is the effect of age, where a linear fit (i.e., adjustment = m*age + b) may not be representative of effects that take years to accumulate and then manifest increasingly rapidly with age. If an EC sample is much younger than a control sample, then linear adjustments may result in an under- or overestimate of risk. Likewise, correlation between CC and EC use is higher in older adults (see Supplement I) and thus correlation of CC harm may be higher if these interdependencies are not accounted for. One mitigation strategy is to stratify age into deciles and adjust for each decile independently. While stratification may seemingly reduce analytic power by increasing the confidence interval associated with the age adjustment, it may in actuality increase accuracy of odds adjustments because of “apples to apples” comparisons.
5.3.7. Transparent Peer Review and Consideration of Counterfactuals
ROBINS framework authors suggest that counterfactuals always be analyzed and recommend that:
“it is very important that experts in both subject matter and epidemiological methods are included in any team evaluating a (non-randomized study). The risk of bias assessment should begin with consideration of what problems might arise, in the context of the research question, in making a causal assessment of the effect of the intervention(s) of interest on the basis of non-randomized studies.” (Sterne et al., 2016).
Likewise, peer review of completed analyses from experts spanning perspectives can help to verify and validate study precision and accuracy.
6. What is the Relative Risk Associated with Displacing One Product with Another?
6.1. Population and Sample-Related Considerations
When analyzing individuals who have formerly used CC and switched to EC, careful consideration of the previous issues associated with exclusive EC use hold. In addition, the impact of exposure to two sources of harm needs to be considered.
6.1.1. Verification of Stopping CC Use
Self-reported rates of stopping smoking are typically higher than biochemically verified rates. Unfortunately, while the PATH study does capture certain biomarkers, COHb is not available (indicating exposure to combustion products), and this would be a valuable future addition to the survey. Likewise, biochemical verification of cannabis and other drug use and non-use would be valuable in the PATH study.
6.2. Exposure-Related Considerations
6.2.1. Duration of CC Use and Recency of Stopping CC Use
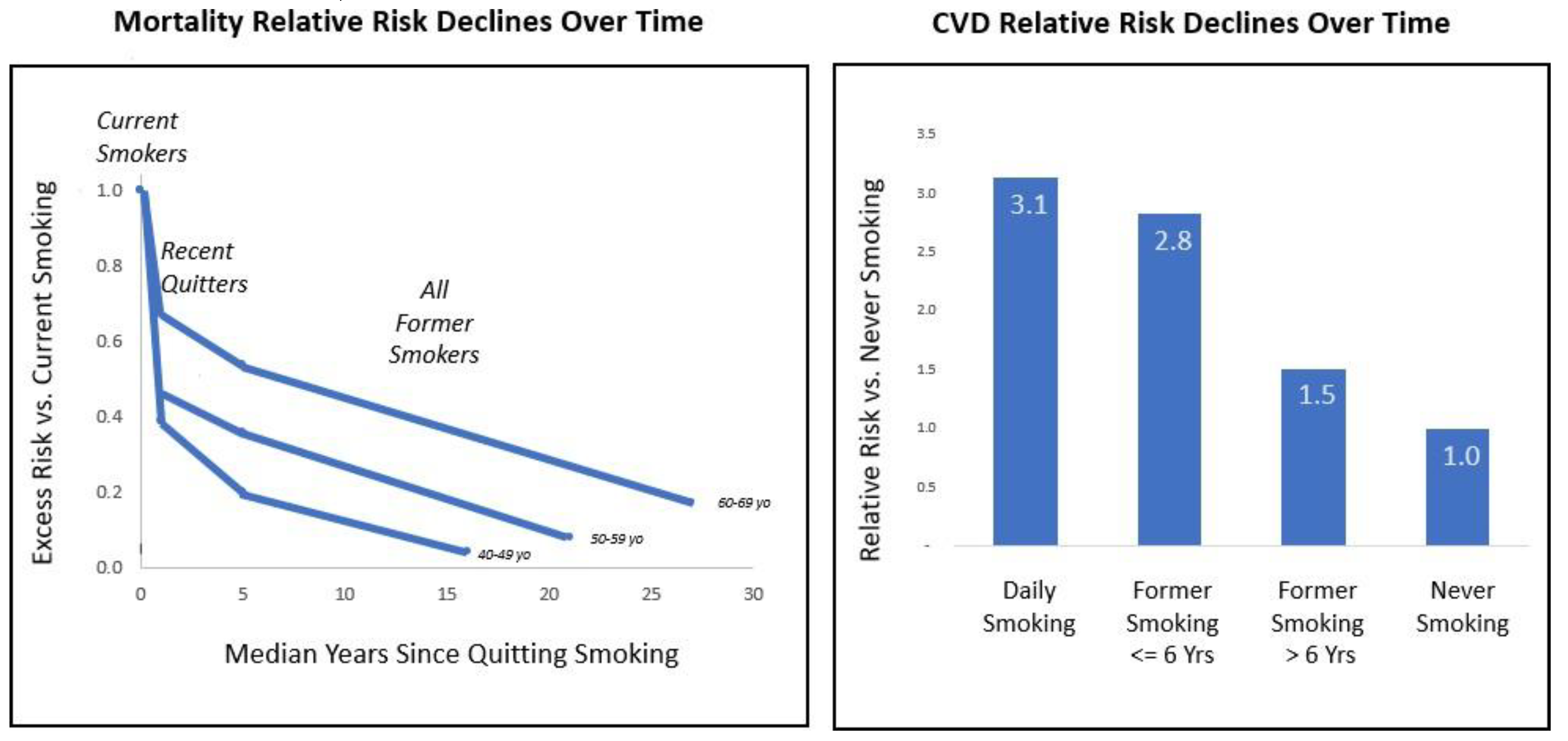
An important source of potential statistical bias is accounting for the time course with which risk rises over extended use and then falls off after the exposure ends. As shown in
Figure S3, mortality risk is elevated in smokers (left panel). After quitting smoking, risk trends downwards over a course of years and decades, with risk decrementing more slowly with older smokers, possibly due to more pack-years of harm accumulation (Cho et al., 2024; Klonizakis et al., 2022). The right panel of the figure shows that CVD risk is markedly lower in former smokers who stopped CC more than six years previously compared to those who are more recent quitters (Farsalinos et al., 2019). Several other studies have also reported that CVD risk declines over a time course of decades after stopping smoking (Duncan et al., 2019; Lubin, Couper, et al., 2016). Likewise, for COPD, model accuracy increased when time since quitting CC was incorporated as a predictive factor (Chang et al., 2021).
As time since switching increases, this upper limit for harm reversal approaches closer to the level of never smoking. In studies of samples which had on average stopped smoking a decade or more earlier, time since stopping smoking was less predictive for CVD risk than other metrics such as cigarettes per day (Duncan et al., 2019; Nance et al., 2017).
EC switching impact should be measured against the maximum harm reversal which would be possible in that time period, for instance if quitting with traditional pharmaceutical approaches or with abstinence (“cold turkey”). Therefore, when calculating aOR among ‘exclusive’ EC users who formerly smoked CC, the correct OR adjustment comparator is not necessarily the typical ex-smoker, who may have quit a decade or more ago, but a cohort of ex-smokers which has quit on average as recently as the EC sample after a similar number of pack-years of CC exposure.
7. What is the Risk Associated with Dual-Use of Two Products?
When comparing dual-use vs. exclusive use of CC or EC, it is especially important to comprehensively and transparently characterize exposure to both products as there are multiple opposing confounds.
7.1. Population and Sample-Related Considerations
In populations which include both EC and CC use history, isolation of DU, CC and EC use into separate samples can increase accuracy and precision of evaluation. due to multiple confounding factors, which include both correlations and anti-correlations between EC and CC use patterns.
7.1.1. Correlation of Likelihood of Use of EC and CC
Common liability, switching patterns, and reverse causation (switching to EC use after a harm diagnosis arising from CC use) cause a positive association between ever-use of CC and EC (Khouja et al., 2021; Kim & Selya, 2020). CDC data (2021 NHIS survey) showed that current EC users were 2.8x more likely than current EC non-users to be current CC users and 1.8x more likely to be former CC smokers, while 54% less likely to be never CC users (CDC, 2023). Furthermore, EC use frequency is higher among people who have a history of smoking more CC (Levy et al., 2017). See Supplement I for more information on this topic.
7.1.2. Anti-Correlation of Frequency of Use of EC and CC
At the same time, multiple studies have reported that frequency of CC use is negatively associated with EC use, when EC are used for quitting cigarettes (Cohen et al., 2024; Harlow et al., 2022; Kasza et al., 2024; Wang et al., 2021).
7.1.3. Risk of Confounding with Case-Control Cohorts
Case-control designs (harm vs. no-harm cohort designs) are highly subject to confounding. Because of correlation of use patterns of EC and CC, along with common liability influences, it is important to stratify populations into EC, CC, and dual use samples rather than stratifying into samples that have experienced harm vs. no harm. The case study discussed below includes two cautionary examples of studies which utilized a case-control approach to segment into harm vs. no-harm cohorts. These studies consequently reported that CC use caused no cardiovascular harm (with former CC use being protective in one study) and attributed the entirety of tobacco product use harm to EC use. (El-Shahawy et al., 2022; Gathright et al., 2020).
7.2. Exposure-Related Considerations
7.2.1. Measurement of Exposure to Two Products
Biomarker studies suggest that EC use which displaces CC use causes a reduction in exposure to CC toxins (Holt et al., 2023). The counterargument has been made that each type of product presents unique risks and so DU risk is EC risk x CC risk (Alzahrani et al., 2018). For precision and accuracy in assessing the impact of DU vs. CC or EC use, it is critical to comprehensively characterize the exposure history of both products in all samples. Evaluating the dose response for each type of exposure (CC pack-years vs. EC use frequency x duration of regular use) may help to differentiate between these two hypotheses.
7.3. Outcome and Result-Related Considerations
7.3.1. Accuracy Considerations for Mixed Linear Models
In some non-randomized observational studies, a mixed linear model approach is used to estimate risk associated with EC use and CC use as independent variables, without measuring DU risk directly (Alzahrani et al., 2018). Because of the positively and negatively-correlated interactions between EC and CC use, the standard error of fit for each of these regression adjustment coefficients may be higher in the edge cases where dual-use occurs. Consequently, the impact of DU should be directly measured as an independent sample rather than extrapolated from multiplication of aOR EC * aOR CC. At a minimum, the goodness of fit and actual confidence interval for the subsample of dual users must be verified to validate the precision and accuracy of imputation in a mixed linear model.
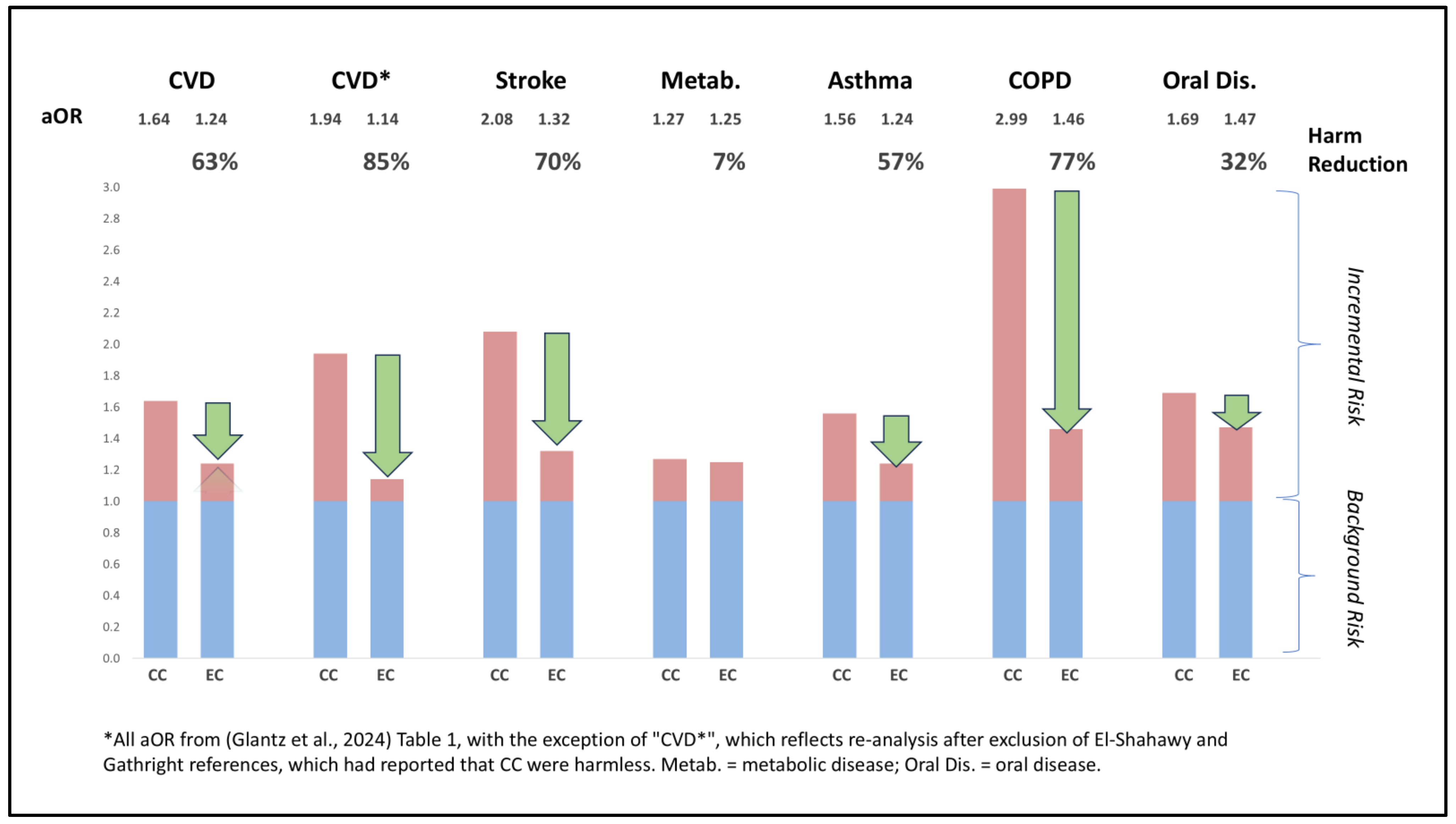
8. Case Study (Glantz et al., 2024)
The preceding considerations are next discussed within the context of a case study of a recent meta-analysis of non-randomized observational studies of exposure to EC and CC. 124 primary citations spanning 107 unique studies were cited. The meta-analysis was designed to determine: 1) whether EC are harm-reduced relative to CC as single agents, and 2) whether DU of both products is more harmful than exclusive smoking of CC. The authors had reported odds for all-source (tobacco and non-tobacco) risks in people who use EC vs. CC. The actual magnitude of tobacco-related harm reduction indicated by these references is now reported here in
Figure 5.
The meta-analysis included 18 citations (spanning 15 references) pertaining to CVD or stroke risk. The authors concluded that EC are not safer than CC with respect to CVD and stroke, but also reported that each of these conclusions was brittle with respect to a single study. Upon inspection, both of these studies contained major errors or characteristics which should have disqualified them from inclusion (El-Shahawy et al., 2022; Patel et al., 2022). The magnitude of EC and DU risks reported in the primary references incorporated multiple confounds impacting accuracy and precision, in particular in terms of measuring duration and timing of EC and CC use (Supplement II explores these confounds in detail, with focus on the areas of CVD and stroke risk). Additionally, the authors imputed 55 of 113 data points describing the risk of DU vs. CC use by asserting they were mathematically substitutable with the risk of EC vs. non-use. These 55 imputed data points were found to provide up to four-fold higher estimates of incremental DU risk in contradistinction to 58 data points where the data was reported in the original study. Furthermore, in 37 complete data point sets (aOR available for EC, CC and DU) which were reported in primary references, the incremental OR impact of DU when imputed had no correlation with the actual values (see Supplement II)
9. Discussion
A careful consideration of the ROBINS framework can help to inform best practices when examining the harm-inducing and harm-reducing effects of EC and other nicotine products through non-randomized studies of exposure. These considerations span sample selection and confounding, exposure measurement and outcome definition, and selection of results. Precise, comprehensive and transparent characterization of the dose-response relationship between exposure and outcome is perhaps the central theme, and requires capturing dose, duration and timing of use and stopping of use of two separate sources of exposure.
Opportunities for maximizing precision and accuracy of observational studies include the following, enumerated in
Table 2.
Beyond the scope of this review, but important to mention, biomarkers of potential health impact (BOPH), measured in blood and urine, can also be corroborative of risk, as they reflect the impact of exposure to toxicants on downstream physiological systems and thus can have relevant diagnostic sensitivity and specificity. For some diseases which may take decades to develop, biomarkers may also be predictive of future events. Notably, the PATH study captures biomarkers of exposure (BOE), BOPH, and harm outcomes in an integrated longitudinal record, and offers an important data source for ongoing and future research (Anic et al., 2022; Holt et al., 2023).
An accurate assessment of the relative risk of ECs is important for the study and regulation of tobacco use, and for the protection of public health. This includes understanding the health impact of EC when used by adults, both relative to non-use and when used to displace CCs. The findings have important implications for tobacco regulatory science, as promoting smoking cessation at every stage of life can help to ameliorate the short and longer-term consequences of cigarette smoking.
As of 2020, 28% of US adults believed that CC are definitely harm-reduced products relative to EC, and 52% believed that CC are definitely or possibly harm-reduced, while only 11% believed that EC are definitely harm-reduced (National Cancer Institute, 2023). An analysis of the PATH study showed that of adults who smoked CC, a belief that EC were harm-reduced was associated with an odds ratio (OR) of 2.24 for switching to EC, and for successful switchers was associated with protective OR of 0.55 vs. relapsing back to smoking (Kim et al., 2022). Similarly, dual-users of EC and CC who believed EC were less harmful had aOR of 2.9 for stopping smoking and aOR 1.1 for stopping nicotine use altogether vs. dual-users who did not believe EC were less harmful (Persoskie et al., 2019).
In other words, if EC are not harm-reduced, then their use should be discouraged. However, if EC are harm reduced in actuality but analyses are not performed accurately, misinformation may keep smokers from switching and increase DU prevalence vs. exclusive EC use. Conversely, if EC are harm reduced and adults who smoke are informed, it may help to reduce CC use and DU prevalence, and save lives (Hannel et al., 2024; Yong et al., 2017). Therefore, it is critical to consider which methodological details which can impact the statistical validity of non-randomized studies of exposure to tobacco and nicotine products.
10. Conclusion
Non-randomized studies of exposure to tobacco and nicotine products provide an important source of real-world data that helps to characterize the relative risk and potential risk reversal associated with different patterns of use, including exclusive use, switching, and stopping tobacco and nicotine product use. By carefully incorporating principles from the ROBINS framework into the construction of EC and other tobacco product exposure variables, researchers will be able to maximize the precision of exposure measurement, thereby improving the accuracy of the results. In so doing, this will help to inform regulatory science and improve public health, representing the best PATH forward.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org,
Funding
G.C. is a salaried employee of Rose Research Center (RRC). S.C.’s research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health (NIH) and FDA Center for Tobacco Products (CTP) under Award Number 2U54CA229974. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. This review was not funded by nor commissioned by any other entities.
Acknowledgements
The authors thank Marcus Munafò, Mike Cummings and Jed Rose for comments on the manuscript. The authors also thank Floe Foxon, Saul Shiffman and David Levy for discussion on early findings preceding the writing of the manuscript. Floe Foxon and G.C. previously jointly evaluated the findings from a peer-reviewed manuscript (Patel et al., 2022), which informed the current research (Joelving, 2024).
Conflicts of Interest
S.C. has no disclosures to declare. G.C. is an employee of RRC, an independent contract research organization that performs studies pertaining to smoking cessation and tobacco harm reduction. Other research support: National Institute on Drug Abuse; Global Action to End Smoking, Inc. (formerly Foundation for a Smoke-Free World, Inc.), a US nonprofit 501(c)(3) private foundation; Nicotine BRST LLC; JUUL Labs; Altria; Embera Neurotherapeutics, Inc.; Otsuka Pharmaceutical; Swedish Match, Philip Morris International. G.C. was previously a Principal Scientist at JUUL Labs. He also was employed at Nektar Therapeutics, whose pipeline included an inhaled NRT. Stock holdings in Qnovia, a developer of an inhaled NRT, and JUUL Labs.
Notes
- 1
in Figure 2, current use (past 30 day use) sample size does not exactly correspond to the sum of current some-day and current every-day use, and ever-use does not exactly correspond to the sum of former and current use, because of inconsistencies in participant answers across different questions.
- 2
A technical consideration is that lifetime smoking of fewer than 100 CC is typically considered “non-use”, while “even one or two puffs” is typically included in EC ever-use. For evaluations of the impact of chronic use of tobacco products, this difference may be immaterial, but studies seeking to precisely identify a health impact of ever-use or former-use of EC may be confounded by unaccounted-for CC smoking of under 100 cigarettes which could equal or exceed the limited exposure to EC in this sample.
- 3
In some cases, 5-9 events per adjustment variable may be sufficient, for instance with use of penalized regression approaches, or when the event fraction (incidence of harm events) is greater than ~10% (Lu et al., 2016). In other cases, an EPV of 20-50 may be necessary (Austin & Steyerberg, 2017; Pavlou et al., 2015; Van Der Ploeg et al., 2014; Vittinghoff & McCulloch, 2007).
Appendix A
| Terms |
|
| CC |
Combusted / conventional cigarette |
| EC |
Electronic cigarette; electronic nicotine delivery system |
| NRSE |
Non-randomized study of exposure; longitudinal parallel cohort study |
| OR |
Odds ratio;
aOR is adjusted to normalize for covariate imbalances across cohorts |
| |
|
| National Surveys Mentioned |
| BRFSS |
Behavioral Risk Factor Surveillance System (CDC) |
| NHANES |
National Health and Nutrition Examination Survey (CDC) |
| NHIS |
National Health Interview Survey (CDC) |
| PATH |
Population Assessment of Tobacco and Health (FDA, NIH, NIDA) |
References
- Alzahrani, T. , Pena, I., Temesgen, N., & Glantz, S. A. (2018). Association Between Electronic Cigarette Use and Myocardial Infarction. A. ( 55(4), 455–461. [CrossRef]
- American Lung Association. (2024). Tobacco Facts | State of Tobacco Control.
- Anic, G. M. , Rostron, B. L., Hammad, H. T., van Bemmel, D. M., Del Valle-Pinero, A. Y., Christensen, C. H., Erives, G., Faulcon, L. M., Blount, B. C., Wang, Y., Wang, L., Bhandari, D., Calafat, A. M., Kimmel, H. L., Everard, C. D., Compton, W. M., Edwards, K. C., Goniewicz, M. L., Wei, B., … Chang, C. M. (2022). Changes in Biomarkers of Tobacco Exposure among Cigarette Smokers Transitioning to ENDS Use: The Population Assessment of Tobacco and Health Study, 2013–2015. International Journal of Environmental Research and Public Health, 1462. [Google Scholar] [CrossRef]
- Auer, R. , Schoeni, A., Humair, J.-P., Jacot-Sadowski, I., Berlin, I., Stuber, M. J., Haller, M. L., Tango, R. C., Frei, A., Strassmann, A., Bruggmann, P., Baty, F., Brutsche, M., Tal, K., Baggio, S., Jakob, J., Sambiagio, N., Hopf, N. B., Feller, M., … Berthet, A. (2024). Electronic Nicotine-Delivery Systems for Smoking Cessation. New England Journal of Medicine. [CrossRef]
- Austin, P. C. , & Steyerberg, E. W. (2017). Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Statistical Methods in Medical Research. [CrossRef]
- Bhatt, S. P. , Kim, Y., Harrington, K. F., Hokanson, J. E., Lutz, S. M., Cho, M. H., DeMeo, D. L., Wells, J. M., Make, B. J., Rennard, S. I., Washko, G. R., Foreman, M. G., Tashkin, D. P., Wise, R. A., Dransfield, M. T., & Bailey, W. C. (2018). Smoking duration alone provides stronger risk estimates of chronic obstructive pulmonary disease than pack-years. Thorax. [CrossRef]
- Black, N. (1996). Why we need observational studies to evaluate the effectiveness of health care. BMJ, 1215. [Google Scholar] [CrossRef]
- Boakye, E. , Erhabor, J., Obisesan, O., Tasdighi, E., Mirbolouk, M., Osuji, N., Osei, A. D., Lee, J., DeFilippis, A. P., Stokes, A. C., Hirsch, G. A., Benjamin, E. J., Robertson, R. M., Bhatnagar, A., El Shahawy, O., & Blaha, M. J. (2023). Comprehensive review of the national surveys that assess E-cigarette use domains among youth and adults in the United States. The Lancet Regional Health - Americas. [CrossRef]
- Brouwer, A. F. , Jeon, J., Hirschtick, J. L., Jimenez-Mendoza, E., Mistry, R., Bondarenko, I. V., Land, S. R., Holford, T. R., Levy, D. T., Taylor, J. M. G., Fleischer, N. L., & Meza, R. (2022). Transitions between cigarette, ENDS and dual use in adults in the PATH study (waves 1–4): Multistate transition modelling accounting for complex survey design. Tobacco Control. [CrossRef]
- Brouwer, A. F. , Levy, D. T., Jeon, J., Jimenez-Mendoza, E., Sanchez-Romero, L. M., Mistry, R., & Meza, R. (2022). The Impact of Current Tobacco Product Use Definitions on Estimates of Transitions Between Cigarette and ENDS Use. Nicotine & Tobacco Research, 1762. [Google Scholar] [CrossRef]
- CDC. (2023). QuickStats: Percentage Distribution of Cigarette Smoking Status† Among Current Adult E-Cigarette Users by Age Group—National Health Interview Survey, United States, 2021. MMWR. Morbidity and Mortality Weekly Report. [CrossRef]
- Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US), & Office on Smoking and Health (US). (2010). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General, /: Disease Control and Prevention (US). http, 5301.
- Chang, J. T. , Meza, R., Levy, D. T., Arenberg, D., & Jeon, J. (2021). Prediction of COPD risk accounting for time-varying smoking exposures. PLOS ONE, 2485. [Google Scholar] [CrossRef]
- Cho, E. R. , Brill, I. K., Gram, I. T., Brown, P. E., & Jha, P. (2024). Smoking Cessation and Short- and Longer-Term Mortality. NEJM Evidence. [CrossRef]
- Cohen, G. , Rose, J. E., & Polosa, R. (2024). Personalized and Adaptive Interventions for Smoking Cessation: Emerging Trends and Determinants of Efficacy. iScience.
- Cornelius, M. E. (2023). Tobacco Product Use Among Adults – United States, 2021. MMWR. Morbidity and Mortality Weekly Report. [CrossRef]
- Cummings, M. , Rigotti, N. A., Benowitz, N., & Hatsukami, D. (2024). An Exchange about “Population-Based Disease Odds for E-Cigarettes and Dual Use versus Cigarettes.” NEJM Evidence, 3(8). 3. [CrossRef]
- Curtis, J. L. , Bateman, L. A., Murray, S., Couper, D. J., Labaki, W. W., Freeman, C. M., Arnold, K. B., Christenson, S. A., Alexis, N. E., Kesimer, M., Boucher, R. C., Kaner, R. J., Barjaktarevic, I., Cooper, C. B., Hoffman, E. A., Barr, R. G., Bleecker, E. R., Bowler, R. P., Comellas, A., … Martinez, F. J. (2024). Design of the SPIROMICS Study of Early COPD Progression: SOURCE Study. Chronic Obstructive Pulmonary Diseases: Journal of the COPD Foundation. [CrossRef]
- D’Andrea, E. , Vinals, L., Patorno, E., Franklin, J. M., Bennett, D., Largent, J. A., Moga, D. C., Yuan, H., Wen, X., Zullo, A. R., Debray, T. P. A., & Sarri, G. (2021). How well can we assess the validity of non-randomised studies of medications? A systematic review of assessment tools. BMJ Open, 4396. [Google Scholar] [CrossRef]
- Department of Health and Human Services, U. (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, /: of Health and Human Services, CDC. http.
- Duncan, M. S. , Freiberg, M. S., Greevy, R. A., Kundu, S., Vasan, R. S., & Tindle, H. A. (2019). Association of Smoking Cessation With Subsequent Risk of Cardiovascular Disease. A. ( 322(7), 642. [CrossRef]
- El Hourani, M. , Shihadeh, A., Talih, S., & Eissenberg, T. (2022). Comparison of Nicotine Emissions Rate, “Nicotine Flux”, from Heated, Electronic, and Combustible Tobacco Products. Tobacco Control, 2021. [Google Scholar] [CrossRef]
- El-Shahawy, O. , Shah, T., Obisesan, O. H., Durr, M., Stokes, A. C., Uddin, I., Pinjani, R., Benjamin, E. J., Mirbolouk, M., Osei, A. D., Loney, T., Sherman, S. E., & Blaha, M. J. (2022). Association of E-Cigarettes With Erectile Dysfunction: The Population Assessment of Tobacco and Health Study. American Journal of Preventive Medicine. [CrossRef]
- Erhabor, J. , Boakye, E., Obisesan, O., Osei, A. D., Tasdighi, E., Mirbolouk, H., DeFilippis, A. P., Stokes, A. C., Hirsch, G. A., Benjamin, E. J., Rodriguez, C. J., El Shahawy, O., Robertson, R. M., Bhatnagar, A., & Blaha, M. J. (2023). E-Cigarette Use Among US Adults in the 2021 Behavioral Risk Factor Surveillance System Survey. JAMA Network Open, 3408. [Google Scholar] [CrossRef]
- Erythropel, H. C. , Jabba, S. V., Silinski, P., Anastas, P. T., Krishnan-Sarin, S., Zimmerman, J. B., & Jordt, S. E. (2024). Variability in Constituents of E-Cigarette Products Containing Nicotine Analogues. JAMA. [CrossRef]
- Farsalinos, K. E. , Polosa, R., Cibella, F., & Niaura, R. (2019). Is e-cigarette use associated with coronary heart disease and myocardial infarction? Insights from the 2016 and 2017 National Health Interview Surveys. Therapeutic Advances in Chronic Disease, 2231. [Google Scholar] [CrossRef]
- Gathright, E. C. , Wu, W.-C., & Scott-Sheldon, L. A. J. (2020). Electronic cigarette use among heart failure patients: Findings from the Population Assessment of Tobacco and Health study (Wave 1: 2013–2014). Heart & Lung. [CrossRef]
- Glantz, S. A. , Nguyen, N., & Oliveira Da Silva, A. L. (2024). Population-Based Disease Odds for E-Cigarettes and Dual Use versus Cigarettes. NEJM Evidence. [CrossRef]
- Hannel, T. , Wei, L., Muhammad-Kah, R. S., Largo, E. G., & Sarkar, M. (2024). Modeling the population health impact of accurate and inaccurate perceptions of harm from nicotine. Harm Reduction Journal. [CrossRef]
- Harlow, A. F. , Stokes, A. C., Brooks, D. R., Benjamin, E. J., Leventhal, A. M., McConnell, R. S., Barrington-Trimis, J. L., & Ross, C. S. (2022). Prospective association between e-cigarette use frequency patterns and cigarette smoking abstinence among adult cigarette smokers in the United States. Addiction, 3129. [Google Scholar] [CrossRef]
- Holt, N. M. , Shiffman, S., Black, R. A., Goldenson, N. I., Sembower, M. A., & Oldham, M. J. (2023). Comparison of biomarkers of exposure among US adult smokers, users of electronic nicotine delivery systems, dual users and nonusers, 2018–2019. Scientific Reports. [CrossRef]
- Joelving, F. (2024). Prescription for controversy. Science. [CrossRef]
- Kasza, K. A. , Edwards, K. C., Kimmel, H. L., Anesetti-Rothermel, A., Cummings, K. M., Niaura, R. S., Sharma, A., Ellis, E. M., Jackson, R., Blanco, C., Silveira, M. L., Hatsukami, D. K., & Hyland, A. (2021). Association of e-Cigarette Use With Discontinuation of Cigarette Smoking Among Adult Smokers Who Were Initially Never Planning to Quit. JAMA Network Open, 1408. [Google Scholar] [CrossRef]
- Kasza, K. A. , Rivard, C., Goniewicz, M. L., Fong, G. T., Hammond, D., Cummings, K. M., & Hyland, A. (2024). E-Cigarette Characteristics and Cigarette Cessation Among Adults Who Use E-Cigarettes. JAMA Network Open, 2396. [Google Scholar] [CrossRef]
- Khouja, J. N. , Wootton, R. E., Taylor, A. E., Davey Smith, G., & Munafò, M. R. (2021). Association of genetic liability to smoking initiation with e-cigarette use in young adults: A cohort study. PLOS Medicine, 0035. [Google Scholar] [CrossRef]
- Kim, S. , & Selya, A. S. (2020). The Relationship Between Electronic Cigarette Use and Conventional Cigarette Smoking Is Largely Attributable to Shared Risk Factors. Nicotine & Tobacco Research. [CrossRef]
- Kim, S. , Shiffman, S., & Sembower, M. A. (2022). US adult smokers’ perceived relative risk on ENDS and its effects on their transitions between cigarettes and ENDS. BMC Public Health. [CrossRef]
- Klonizakis, M. , Gumber, A., McIntosh, E., & Brose, L. S. (2022). Medium- and longer-term cardiovascular effects of e-cigarettes in adults making a stop-smoking attempt: A randomized controlled trial. BMC Medicine. [CrossRef]
- Levy, D. , Yuan, Z., & Li, Y. (2017). The Prevalence and Characteristics of E-Cigarette Users in the U.S. International Journal of Environmental Research and Public Health. [CrossRef]
- Lindson, N. , Theodoulou, A., Ordóñez-Mena, J. M., Fanshawe, T. R., Sutton, A. J., Livingstone-Banks, J., Hajizadeh, A., Zhu, S., Aveyard, P., Freeman, S. C., Agrawal, S., & Hartmann-Boyce, J. (2023). Pharmacological and electronic cigarette interventions for smoking cessation in adults: Component network meta-analyses. The Cochrane Database of Systematic Reviews, 0152. [Google Scholar] [CrossRef]
- Lu, M. , Zhong, W., Liu, Y., Miao, H., Li, Y., & Ji, M. (2016). Sample Size for Assessing Agreement between Two Methods of Measurement by Bland-Altman Method. The International Journal of Biostatistics. [CrossRef]
- Lubin, J. H. , Albanes, D., Hoppin, J. A., Chen, H., Lerro, C. C., Weinstein, S. J., Sandler, D. P., & Beane Freeman, L. E. (2016). Greater Coronary Heart Disease Risk With Lower Intensity and Longer Duration Smoking Compared With Higher Intensity and Shorter Duration Smoking: Congruent Results Across Diverse Cohorts. Nicotine & Tobacco Research. [CrossRef]
- Lubin, J. H. , Couper, D., Lutsey, P. L., Woodward, M., Yatsuya, H., & Huxley, R. R. (2016). Risk of Cardiovascular Disease from Cumulative Cigarette Use and the Impact of Smoking Intensity: Epidemiology, 27(3), 395–404. [CrossRef]
- Marrocco, A. , Singh, D., Christiani, D. C., & Demokritou, P. (2022). E-cigarette vaping associated acute lung injury (EVALI): State of science and future research needs. Critical Reviews in Toxicology. [CrossRef]
- Martin, S. S. , Aday, A. W., Almarzooq, Z. I., Anderson, C. A. M., Arora, P., Avery, C. L., Baker-Smith, C. M., Barone Gibbs, B., Beaton, A. Z., Boehme, A. K., Commodore-Mensah, Y., Currie, M. E., Elkind, M. S. V., Evenson, K. R., Generoso, G., Heard, D. G., Hiremath, S., Johansen, M. C., Kalani, R., … on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. (2024). 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. [CrossRef]
- Munafò, M. R. (2024, March 22). SRNT 2024 Plenary, /: Lecture: How to Avoid Being Precisely Wrong: Triangulation and Causal Inference in Observational Epidemiology. https, 22 March 2024. [Google Scholar]
- Munafò, M. R. , & Smith, G. (2018, January 25). Comment: Repeating experiments is not enough. Nature, 25 January.
- Nance, R. , Delaney, J., McEvoy, J. W., Blaha, M. J., Burke, G. L., Navas-Acien, A., Kaufman, J. D., Oelsner, E. C., & McClelland, R. L. (2017). Smoking intensity (pack/day) is a better measure than pack-years or smoking status for modeling cardiovascular disease outcomes. Journal of Clinical Epidemiology. [CrossRef]
- National Cancer Institute. (2023). HINTS 5 Cycle 4 (2020), 1282.
- Patel, U. , Patel, N., Khurana, M., Parulekar, A., Patel, A., Ortiz, J. F., Patel, R., Urhoghide, E., Mistry, A., Bhriguvanshi, A., Abdulqader, M., Mehta, N., Arumaithurai, K., & Shah, S. (2022). Effect Comparison of E-Cigarette and Traditional Smoking and Association with Stroke—A Cross-Sectional Study of NHANES. Neurology International. [CrossRef]
- Pavlou, M. , Ambler, G., Seaman, S. R., Guttmann, O., Elliott, P., King, M., & Omar, R. Z. (2015). How to develop a more accurate risk prediction model when there are few events. Z. ( 2015). How to develop a more accurate risk prediction model when there are few events. BMJ, h3868. [CrossRef]
- Peduzzi, P. , Concato, J., Kemper, E., Holford, T. R., & Feinstein, A. R. (1996). A simulation study of the number of events per variable in logistic regression analysis. R. ( 49(12), 1373–1379. [CrossRef]
- Persoskie, A. , O’Brien, E. K., & Poonai, K. (2019). Perceived relative harm of using e-cigarettes predicts future product switching among US adult cigarette and e-cigarette dual users. Addiction, 2197. [Google Scholar] [CrossRef]
- Rennard, S. I. , & Drummond, M. B. (2015). Early chronic obstructive pulmonary disease: Definition, assessment, and prevention. The Lancet, 1778. [Google Scholar] [CrossRef]
- Selya, A. , Kim, S., Shiffman, S., Gitchell, J., & Foxon, F. (2024). What Substances Are Adolescents Vaping? Estimating Nicotine-Specific and Cannabis-Specific Vaping from US National Youth Surveys. Substance Use & Misuse. [CrossRef]
- Shiffman, S. , McCaffrey, S. A., Hannon, M. J., Goldenson, N. I., & Black, R. A. (2023). A New Questionnaire to Assess Respiratory Symptoms (The Respiratory Symptom Experience Scale): Quantitative Psychometric Assessment and Validation Study. JMIR Formative Research. [CrossRef]
- Sterne, J. A. , Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., Henry, D., Altman, D. G., Ansari, M. T., Boutron, I., Carpenter, J. R., Chan, A.-W., Churchill, R., Deeks, J. J., Hróbjartsson, A., Kirkham, J., Jüni, P., Loke, Y. K., Pigott, T. D., … Higgins, J. P. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. [CrossRef]
- Van Der Ploeg, T. , Austin, P. C., & Steyerberg, E. W. (2014). Modern modelling techniques are data hungry: A simulation study for predicting dichotomous endpoints. BMC Medical Research Methodology. [CrossRef]
- Vittinghoff, E. , & McCulloch, C. E. (2007). Relaxing the Rule of Ten Events per Variable in Logistic and Cox Regression. E. ( 165(6), 710–718. [CrossRef]
- Wang, R. J. , Bhadriraju, S., & Glantz, S. A. (2021). E-Cigarette Use and Adult Cigarette Smoking Cessation: A Meta-Analysis. American Journal of Public Health. [CrossRef]
- WHO. (2023). WHO report on the global tobacco epidemic, 2023: Protect people from tobacco smoke.
- Yong, H.-H. , Hitchman, S. C., Cummings, K. M., Borland, R., Gravely, S. M. L., McNeill, A., & Fong, G. T. (2017). Does the Regulatory Environment for E-Cigarettes Influence the Effectiveness of E-Cigarettes for Smoking Cessation?: Longitudinal Findings From the ITC Four Country Survey. Nicotine & Tobacco Research, 1276. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).