1. Introduction
Soil microbes play a crucial role in ecosystem processes by driving the earth's biogeochemical cycles [
1,
2]. This includes soil organic matter (SOM) decomposition, maintenance of plant diversity, and atmospheric concentrations of greenhouse gases [
2,
3,
4,
5]. Shifts in the soil microbial community composition and structure and the richness of functional genes specific to recalcitrant carbon (C) decomposition in response to nitrogen (N) availability affect the nutrient cycles [
6,
7]. Biological N fixation (BNF) and atmospheric N deposition (ND) are estimated to be twice as large as N input throughout natural ecosystems [
8,
9]. Therefore, the responses of these microbial groups to external N sources such as BNF and atmospheric ND may have a considerable impact on global C and N cycling.
Approximately 80% of BNF worldwide is fixed by legumes annually [
10]. When conditions are conducive to the growth of legumes, such as when the grasslands release enough resource space after being disturbed, the symbiotic N fixation rate of leguminous plants is as high as 50 kg N ha
−1 yr
−1 [
11]. Legume plants are the most important N sources for terrestrial ecosystems and play an important role in the study of BNF [
12,
13,
14]. Legumes positively affect soil N content and availability [
15,
16,
17] and soil C accumulation, compared with non-N-fixing species [
18]. Soil microbes can be affected by soil C and N availability and plant species richness [
19,
20,
21], and the introduction of legumes is expected to influence soil microbial communities. In a field experiment in subtropical China, Huang et al. [
22] found that legumes affected soil microbial communities in the rhizosphere after the establishment of planting systems. Legume plants enhance the structure and complexity of the soil food web, which are the food sources, including leaf litter, dead roots, and root exudates for soil microbes, and may exert a bottom-up control on soil microbial communities [
23,
24,
25]. Soil microbes use plant litter and SOM as their sources of C and N, respectively, and the roots of legumes provide extracellular enzymes that help soil microbes break down SOM during plant litter decomposition [
26,
27]. The introduction of legume plants can influence the soil microbial community structure because fungi and bacteria use organic matter with different C: N ratios, and fungi are the main decomposers of plant residues [
28].
The atmospheric ND is increasing annually worldwide and is expected to rise to 600 Tg N yr
−1 by 2100 [
29,
30]. ND can increase plant productivity by enhancing N availability [
31,
32]. However, excessive ND can induce soil acidification [
33,
34], decrease plant diversity [
35], and alter plant biomass C, N, and phosphorus cycles [
36,
37]. Various effects of ND on soil microbial communities have been reported, including negative [
38,
39,
40,
41], positive [
42], and neutral effects [
43]. Increased N reduces fungal biomass via changes in plant-specific exudates and alterations in nutrient competition between plants and rhizosphere microbes [
44]. However, a low addition of nitrogen had a short-term positive effect on microbial biomass but no long-term effect [
42]. These conflicting results may be due to differences in soil N [
41,
45,
46], SOM [
38,
47,
48], and pH [
45,
49,
50,
51]. Microbial biomass C decreases following N fertilization because of decreased soil pH, resulting in greater osmotic potential and increased solubility of Al, which is toxic to soil microbes [
50]. However, the soil of the karst natural grassland ecosystem has high calcium and magnesium contents and a strong acid-base neutralization ability. Within a certain range, pH changed slightly with adding soil nitrogen. Therefore, the effects of legume plant introduction and ND on the soil microbial communities in karst grassland ecosystems remain unclear. However, relatively few studies have compared their effects on soil physicochemical properties and soil microbial community structure.
In this study, we aimed to examine the effects of BNF. We simulated ND on soil nematode community structures, as indicated by phospholipid fatty acids (PLFA), in karst grassland ecosystems in Southwest China. The BNF was established by introducing a legume species (
I. atropurpurea) into the site. The total atmospheric ND is high (approximately 3 g N m
−2 yr
−1 in this area) [
52]. Southwest China's extremely fragile karst ecosystem is one of the largest exposed carbonate rock areas worldwide at more than 0.54 million km
2 [
53]. Karst grassland ecosystems are N-limited [
54] and rich in legume plants [
55]. We hypothesized that 1) legume plant introduction would increase the bacterial and fungal biomass and change the soil microbial community composition in karst grassland ecosystems, and 2) ND would suppress the soil microbial community biomass in karst grassland ecosystems.
2. Materials and Methods
2.1. Study Site and Experimental Design
The study was conducted at the Huanjiang Observation and Research Station for Karst Ecosystems of the Chinese Academy of Sciences (107°51’-108°43’E, 24°44’-25°33’N), which is in Huanjiang County, Guangxi Province, Southwest China. These sites experience a typical subtropical monsoon climate with mean annual temperature and precipitation of 19 ℃ and 1389 mm, mainly from May to September. The region in the depression area is a gentle valley surrounded by hills. The brown calcareous soil developed from a dolostone base [
56,
57]. According to the Chinese Soil Taxonomy system, this is a primitive soil [
58]. The soil pH was approximately 7.38, the total atmospheric ND was approximately 37 kg N ha
−1 yr
−1 [
52], and the NHx and NOy deposition ratio was close to 1 [
59].
The experimental site was a grassland developed from an abandoned maize–soybean field in 1982. The dominant species were
Apluda mutica,
Microstegium vagans, and
Imperata cylindrica. The experimental area consisted of nine plots (5 × 4 m) in June 2014. All the plots were separated by 15 cm wide concrete walls. The walls were placed 50 cm below the ground to block underground plant roots and soil moisture migration and 20 cm above the land surface to prevent surface runoff. The plots were laid out in a randomized block design with three replicate blocks. Each replicate block included three treatments, that is, no nitrogen deposition simulation, no leguminous plant introduction as a control (CK), atmospheric N deposition simulation (N
D: 10 g N m
−2 yr
−1), and a leguminous plant introduction system (N
L). Since June 2015, 16.6 g of N as NH
4NO
3 was dissolved in 1.5 L of tap water and applied monthly to each ND treatment plot near the soil surface using a backpack sprayer. Due to the area's high rainfall, no water was sprayed onto the CK and N
L treatment plots. The leguminous plant used in the present study was
Indigofera atropurpurea, a native N-fixing shrub in the area [
55]. We collected seeds of
I. atropurpurea from the wild and cultivated seedlings. In November 2014, seedlings with the same rhizome and height were transplanted (1 × 1 m) into the N
L treatment plots. N fixation
I. atropurpurea was approximately 16.3 g N m
−2 yr
−1 in the N
L treatment plots (Liu et al. unpublished data).
2.2. Sampling and Physicochemical Analyses
The soil was sampled in July 2014 before treatment application and in July 2015, 2016, 2017, and 2018, respectively. Soil cores (5 cm in diameter) were collected at 0–10 cm depths from five random locations within each plot. Five cores from each plot were combined to form a composite. The litter above each sampling area was removed before the cores were collected. Samples were transported to the laboratory in insulated boxes and passed through a 2-mm sieve to remove stones and roots. Subsamples of fresh soil samples were kept in a refrigerator at -20 ℃ and used for PLFA analysis.
The plants were sampled in October 2014, 2015, 2016, 2017, and 2018. Two 1 × 1 m subplots were randomly selected and installed to monitor the plant biomass in each 20 m2 plot. In October, the ground plant tissues in each subplot were cut, and their fresh weight was measured. Then, a 20 g fresh sample was baked in an oven at 80 ℃ for 24 h to constant weight. The plot's dry weight was measured and converted into plant biomass (kg m−2).
Soil pH was measured in deionized water using a glass electrode. Soil organic carbon (SOC, g kg
−1 dried soil) was measured using the Walkley–Black method, and total N (TN, g kg
−1 dried soil) was measured after micro-Kjeldahl digestion using a flow injection autoanalyzer (FIA, Lachat Instruments, USA) [
60].
PLFAs were extracted from fresh soil equivalent to 8 g dry weight and analyzed using the method described by Bossio and Scow [
61] to determine each fraction's soil microbial community composition. The concentration of each PLFA was calculated based on the concentration of the 19:0 internal standard. The PLFAs used as bacterial biomass were i14:0, 15:0, i15:0, a15:0, i16:0, 16:1ω7c, 17:0, a17:0, i17:0, cy17:0, 18:1ω7c, and cy19:0 [
62]. 18:2ω6, 9c, and 18:1ω9c represented fungal PLFAs [
63,
64]. 10 Me 16:0, 10 Me 17:0, and 10 Me 18:0 represented actinomycete PLFAs [
57,
65]. 16:1ω5c indicated arbuscular mycorrhizal fungi (AMF) [
66]. Other PLFAs, such as 14:0, 16:0, 17:1w8c, 16:1 2OH, and 16:1ω9c, were also used to analyze the composition of microbial communities.
2.3. Statistical Analyses
Data were tested for normality and homogeneity before analysis and were naturally log-transformed when necessary. Repeated analysis of variance (ANOVA) was used to determine the treatment effects (CK, N
D, and N
L) on plant biomass, soil chemical properties, and soil microbial communities for each sampling event. Pearson’s correlation analysis was used to identify the relationships between soil microbial communities, plant biomass, and the soil C: N ratio. Repeated measures ANOVA and Pearson’s correlation analyses were performed using SPSS (version 16.0; SPSS Inc., Chicago, IL, USA). Statistical significance was determined at
p < 0.05 with the LSD test. The principal response curve (PRC) method was used to determine the temporal trends of soil microbial community composition for each treatment using CANOCO 4.5 (Ithaca). The results are presented as a diagram showing the first Principal Component of the variance explained by treatment on the y-axis and the sampling periods on the x-axis. The control treatment was treated as the zero baseline (horizontal line). The treatment effect is represented by the deviation of each fluctuating line (N
D, N
L) from the zero baseline over time. The treatments and sampling times were converted to nominal (0, 1) environmental variables [
67,
68]. Two non-parametric multivariate statistical tests of dissimilarity (Adonis and multi-response permutation procedures, MRPP) were performed to test the dissimilarity of soil microbial communities between ND and biological nitrogen fixation using the “vegan” package in R statistical software [
69].
3. Results
3.1. Plant Biomass and Soil Chemical Properties
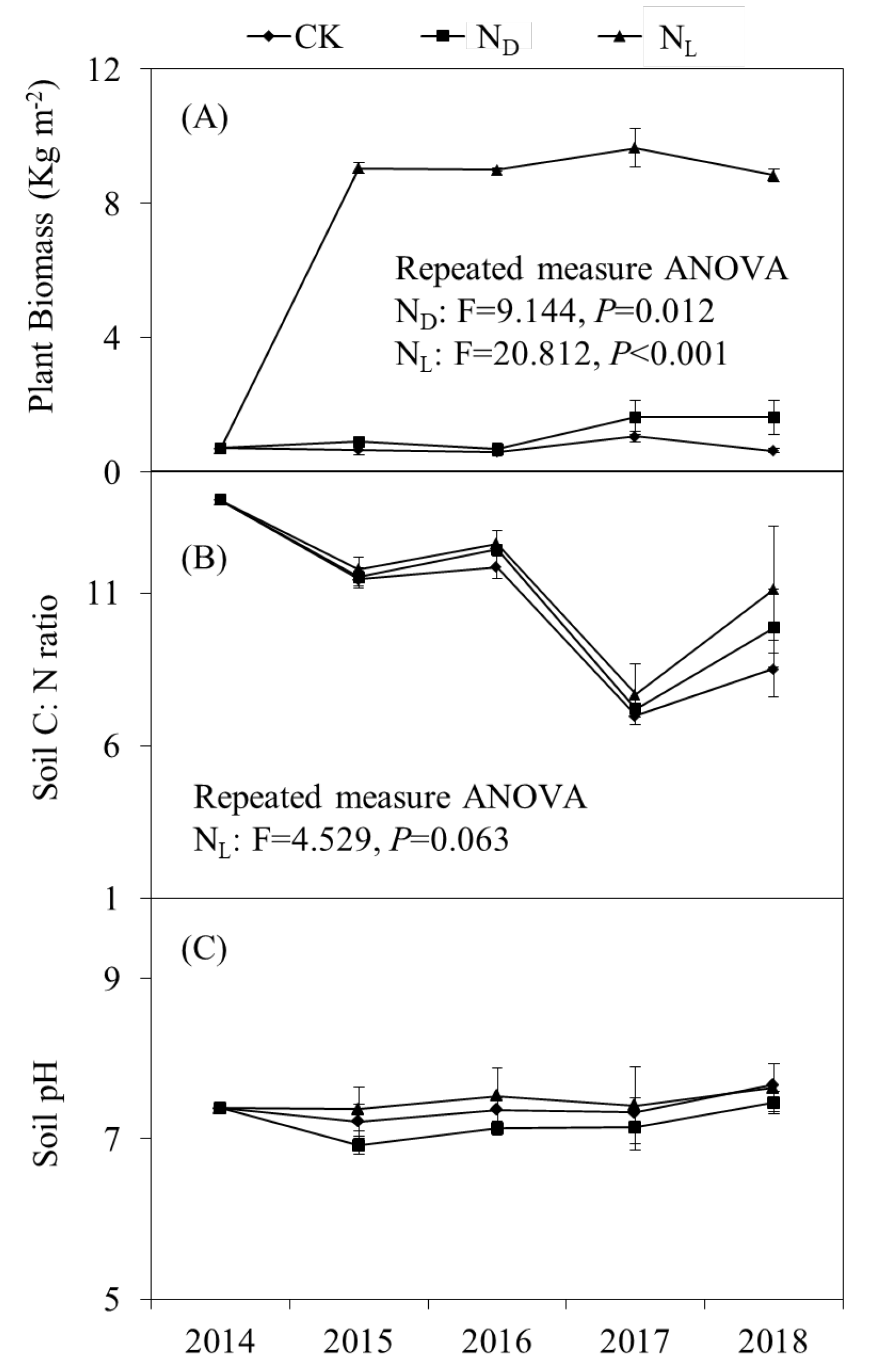
Repeated measures ANOVA showed that plant biomass was significantly increased by legume plant introduction and ND and was significantly higher under the treatment of legume plants than under the ND treatment throughout the experimental period (Fig. 1A). The introduction of legume plants significantly increased the soil C: N ratio throughout the study period (Fig. 1B). Soil pH decreased insignificantly with ND (Fig. 1C).
Figure 1.
Changes in (A) plant biomass, (B) soil C: N ratio, i.e., the ratio of soil organic carbon and total nitrogen, and (C) soil pH under control (CK), nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). Bars indicate standard errors of means.
Figure 1.
Changes in (A) plant biomass, (B) soil C: N ratio, i.e., the ratio of soil organic carbon and total nitrogen, and (C) soil pH under control (CK), nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). Bars indicate standard errors of means.
3.2. Soil Microbial Community Abundance and Structure
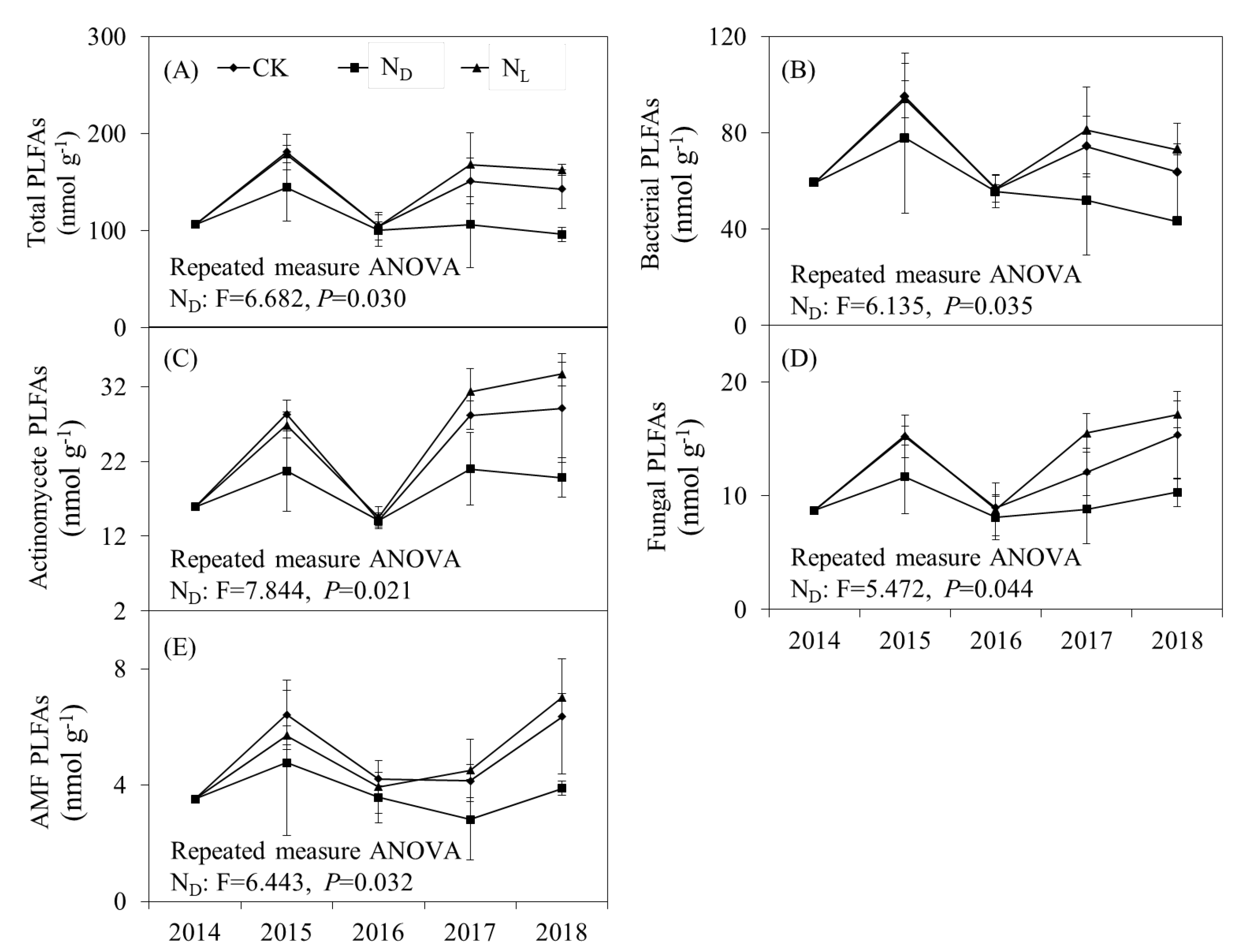
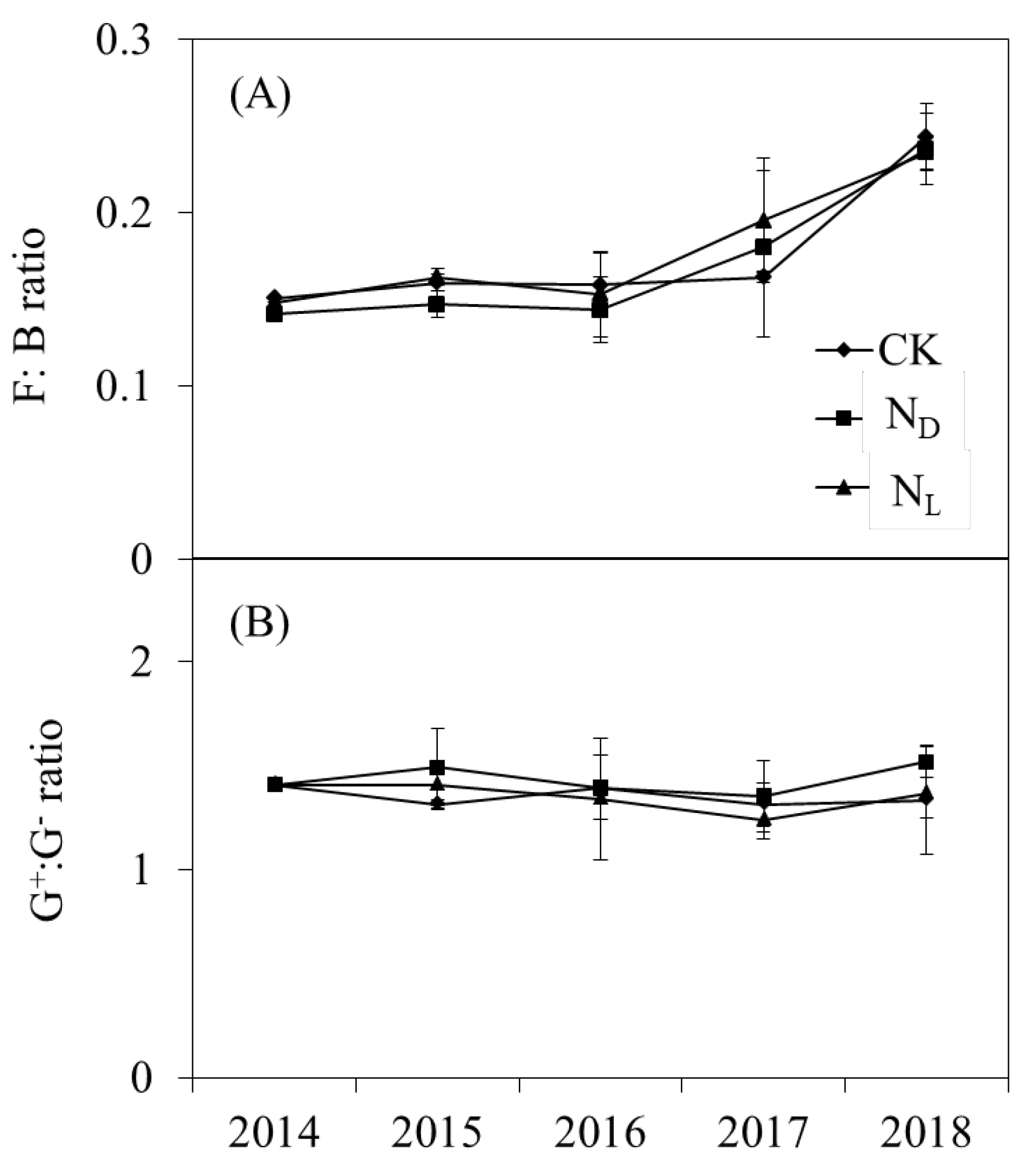
The abundance of total PLFAs (P = 0.030), bacterial PLFAs (P = 0.035), actinomycete PLFAs (P = 0.021), fungal PLFAs (P = 0.044), and AMF PLFAs (P = 0.032) were significantly decreased by ND throughout the experimental period (Fig. 2). Legume plant introduction had no significant effect on the soil microbial community abundance. However, the soil microbial community abundance was higher in the legume plant introduction plots than in the ND plots. Legume plant introduction and ND did not significantly affect the soil microbial community structure. The ratio of fungal to bacterial PLFAs (F: B ratio) and Gram-positive to Gram-negative bacterial PLFAs (G+:G− ratio) was not significantly influenced by ND or legume plant introduction treatments (Fig. 3).
Figure 2.
Abundance of (A) total phospholipid fatty acids (PLFAs), (B) bacterial PLFAs, (C) actinomycete PLFAs, (C) fungal PLFAs, and (D) arbuscular mycorrhizal fungi (AMF) PLFAs (E) under control (CK), nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). Bars indicate standard errors of means.
Figure 2.
Abundance of (A) total phospholipid fatty acids (PLFAs), (B) bacterial PLFAs, (C) actinomycete PLFAs, (C) fungal PLFAs, and (D) arbuscular mycorrhizal fungi (AMF) PLFAs (E) under control (CK), nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). Bars indicate standard errors of means.
Figure 3.
(A) Ratio of fungal to bacterial phospholipid fatty acids (PLFAs) (F:B ratio) and (B) the ratio of gram-positive to gram-negative bacteria PLFAs (G+:G- ratio) under control (CK) nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). Bars indicate standard errors of means.
Figure 3.
(A) Ratio of fungal to bacterial phospholipid fatty acids (PLFAs) (F:B ratio) and (B) the ratio of gram-positive to gram-negative bacteria PLFAs (G+:G- ratio) under control (CK) nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). Bars indicate standard errors of means.
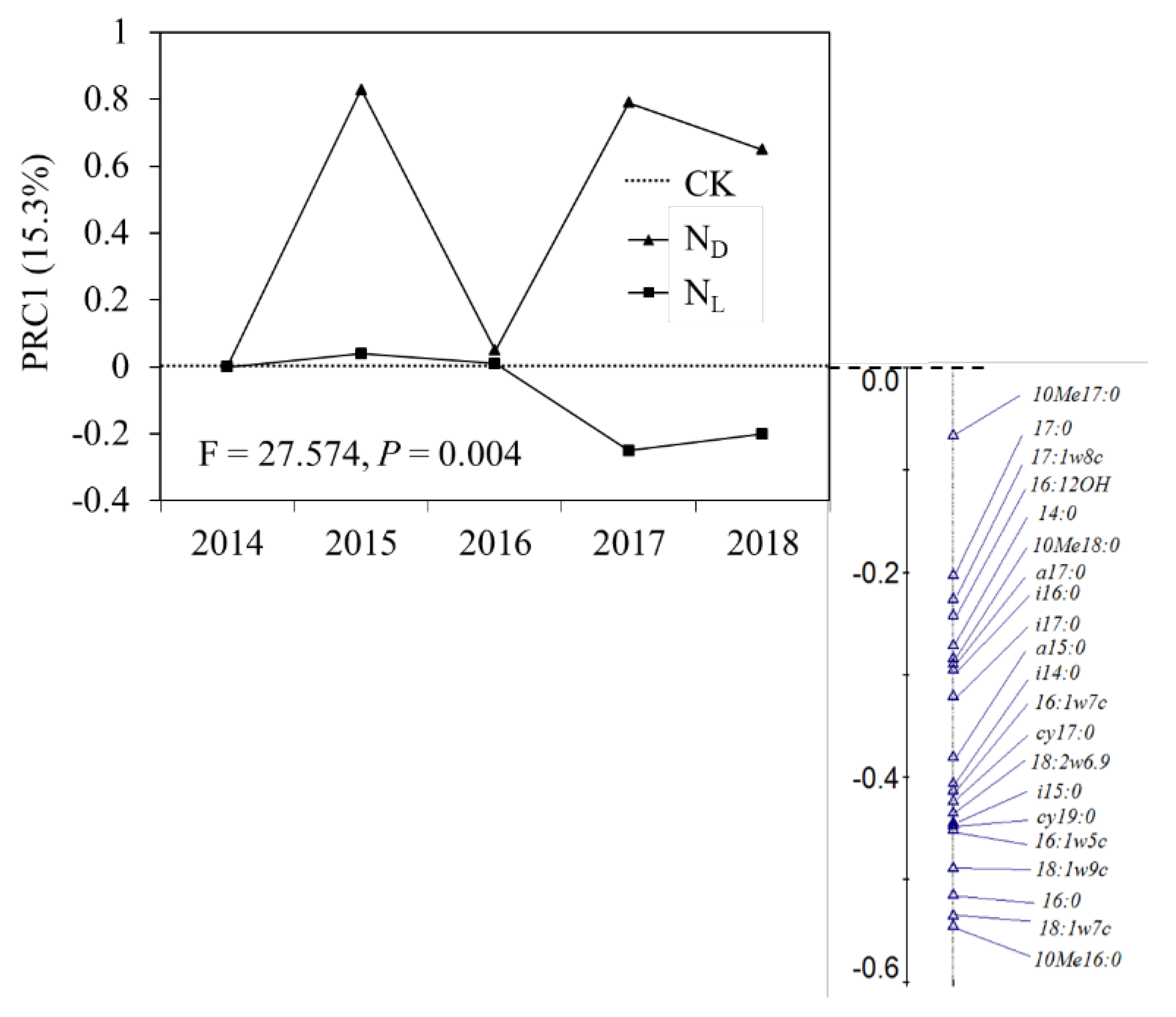
Adonis and MRPP analyses showed that the effects of ND and legume plant introduction on the soil microbial communities were significantly different (
Table 1). PRC analysis showed that the effect of legume plant introduction on the soil microbial community composition was more powerful than the effect of ND (Fig. 4). The soil microbial community composition did not respond to the introduction of legume plants in the early years (2014, 2015, and 2016), but showed a positive relationship with legume plant introduction in the fourth (2017) and fifth years (2018). In addition, there was no clear relationship between ND and the soil microbial community composition (Fig. 4).
Figure 4.
Principal response curves (PRC) with weights of the density of each soil microbial under Control (CK), nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). The horizontal axis represents the control treatment.
Figure 4.
Principal response curves (PRC) with weights of the density of each soil microbial under Control (CK), nitrogen deposition (ND), and legume plant introduction (NL) treatments in each sampling event (2014, 2015, 2016, 2017, and 2018). The horizontal axis represents the control treatment.
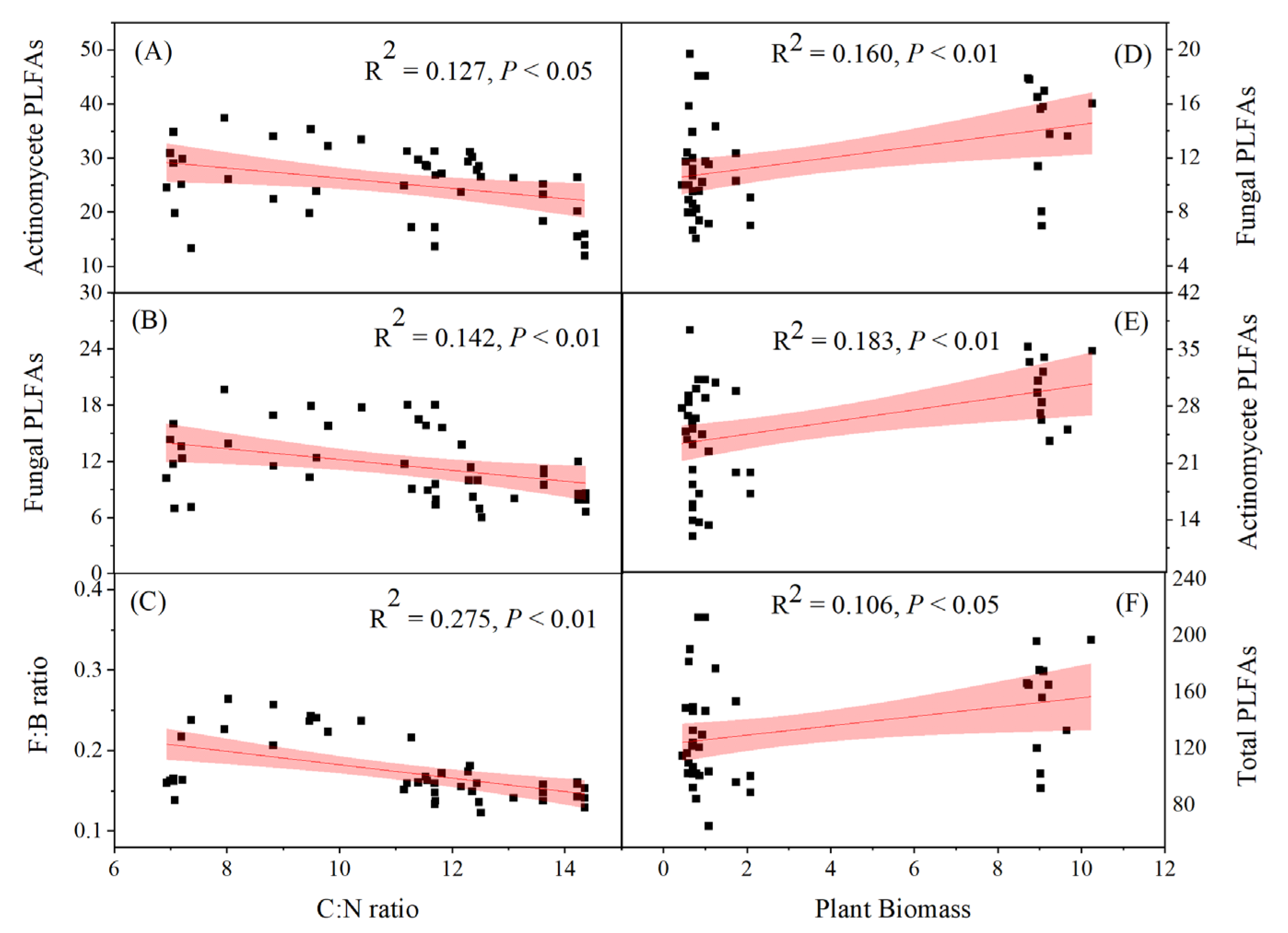
3.3. Relationships Between Soil Microbial Variables and Plant Biomass, Soil C: N Ratio
The soil C: N ratio exhibited a highly significant negative correlation with fungal PLFAs and the F:B ratio (P < 0.01), and a significant negative correlation with actinomycete PLFAs (P < 0.05) (Fig. 5 A, B, and C). Plant biomass had a significant positive correlation with actinomycete PLFAs and fungal PLFAs (P < 0.01) and a significant positive correlation with total PLFAs (P < 0.05) (Fig. 5 D, E, and F).
Figure 5.
Soil microbial variables as a function of soil C: N ratio and plant biomass. Fitted regressions and their 95% confidence interval (shaded) and corresponding significance levels (P) are presented. F:B ratio: fungal to bacterial phospholipid fatty acids (PLFAs); C: N: soil organic carbon and total nitrogen ratio.
Figure 5.
Soil microbial variables as a function of soil C: N ratio and plant biomass. Fitted regressions and their 95% confidence interval (shaded) and corresponding significance levels (P) are presented. F:B ratio: fungal to bacterial phospholipid fatty acids (PLFAs); C: N: soil organic carbon and total nitrogen ratio.
4. Discussion
4.1. Effects of Legumes on the Soil Microbial Community
Legume plants increased the biomass of soil microbial communities, which is consistent with our hypothesis. Similarly, N-fixers have been reported to exhibit significant positive effects on soil microbial biomass [
57,
65,
70]. For example, legumes increased soil microbial biomass and activity on heavy clay soil near Wageningen [
70]. This is mainly because legume plants enhance the structure and complexity of the soil food web by providing main food sources for soil microbes, such as leaf litter, dead roots, and root exudates, which may exert bottom-up control on soil organisms [
25,
26,
27]. Bacteria, fungi, and AMF are attracted to and feed on rhizomes, such as the nutrients, exudates, border cells, and mucilage released by plant roots [
71]. The soil microbial community is primarily influenced by belowground C and N inputs [
65]. Due to their ability to fix atmospheric N, legume plants have been commonly used to improve net primary production (NPP) and are widely used to improve soil C and N storage [
72,
73,
74,
75]. Therefore, legume plants can enhance the activities of soil microorganisms. They can alter the soil microenvironment under the canopy, such as the microtopography, soil temperature, humidity, water content, and structure. Dutta and Agrawal [
76] found that N-fixing shrubs significantly increased tailings soil's clay and silt content, reduced the sand content, increased the field water-holding capacity, and reduced soil acidity. The increase in soil structure caused by legume plants can enhance the activity of soil microbes [
23,
24]. For example, 5-year-old
Tectona grandis increased soil microbial C by 92% [
24]. The soil respiration rate under the Inga crown cover increased by 15% compared with the control [
23]. These results suggest that legume plants play an important role in affecting the SOM, with SOC and TN being the two major SOM components, and soil structure, and thus shape the microbial community composition.
In this study, legume plants did not significantly affect soil microbial diversity. This may be because of the short introduction time of the legume plants. I. atropurpurea require time to accept other microorganisms, such as mycorrhizal fungi, to form multiple symbiotic relationships that affect soil microbial diversity.
4.2. Effects of ND on the Soil Microbial Community
Our study found that bacterial-, actinobacterial-, and AMF PLFAs were significantly reduced by ND, which is consistent with our hypothesis. Consistent with our study and its findings, most studies have reported that continuous N input negatively affects microbial activity [
38,
39,
40,
41]. Tian et al. [
39] found that ND significantly negatively affects microbial biomass and community composition in subtropical forests in China. First, soil acidification caused by ND leads to soil microbial biomass loss [
49,
77], and soil pH is a significant driver of microbial biomass variation [
20,
45,
51]. Li et al. [
78] found that ND caused significant changes in bacterial diversity and community composition and enriched the copiotrophic bacteria. However, it reduced the oligotrophic groups by increasing the soil inorganic N content and C availability and decreasing the soil pH. The ND resulted in a decline of soil pH by 0.3 units compared with the controls. However, karst regions have a high buffering capacity for soil acidification because the soil is rich in Ca
2+ and Mg
2+ [
53]. Soil pH was not significantly reduced but was maintained at neutral levels under ND after three years. We found that soil pH could not explain the variations in bacterial-, actinobacterial-, and AMF PLFAs. This indicates that the decrease in these soil microbes in N
D may be caused by factors other than soil pH in our study area.
The effect of inorganic N addition on soil microbes is related to the C source [
38,
47,
48]. Under ND, the amount of photosynthetic products transferred from the aboveground to the belowground level decreased. High levels of inorganic N addition decreased fine root production [
79] and increased the fraction of recalcitrant compounds, such as melanins and lignin [
6]. This resulted in less C availability to soil microbes. Williams et al. [
48] found that AMF needed to obtain more C from plants under N fertilization. Due to the high energy cost required to obtain a C source, soil microbes may decline with continuous ND [
80]. According to the enzyme inhibition hypothesis, N addition could inhibit the enzymes involved in soil recalcitrant C decomposition because soil microbes are mainly C-limited. The decrease in labile C inputs is expected to reduce soil microbial activity and biomass [
72,
81]. Ramirez et al. [
38] found that some soil bacterial groups consistently decreased after one year of N addition in 28 soils in North America. N addition could decrease soil bacterial activities by altering the metabolic capabilities of soil bacterial communities. Our results showed that soil DOC and the C: N ratio were the major factors affecting soil bacterial and AMF PLFAs. Therefore, the significant decreases in bacterial-, actinobacterial-, and AMF PLFAs under ND may have been caused by soil C availability rather than soil pH in karst grassland ecosystems.
As microbial PLFA diversity may incorrectly represent the true microbial diversity [
57], we used the term “microbial PLFA diversity” rather than “microbial diversity” in this study. While recognizing this potential limitation, it is useful to make cautious inferences regarding microbial diversity based on the PLFA analysis. In the present study, ND significantly increased the species richness index, indicating that ND could enhance species richness. The diversity index showed a growing trend, and the dominant index showed a downward trend under ND (Fig. 2). The diversity index gives weight to rarer species. In contrast, the Simpson index gives weight to more abundant species [
57]. This suggests that ND could help to increase the abundance of rare species in karst areas.
4.3. Differences Between ND and Legumes on Soil Microbial Community Structure
ND and legume plant introduction were equivalent to the different exogenous N inputs for soil microbes. The soil microbial community structure changes with different exogenous N inputs [
38,
82,
83]. Using high-throughput pyrosequencing, Ramirez et al. [
38] found that inorganic N addition consistently altered the microbial community structure, increasing the relative abundance of Actinobacteria and Firmicutes and decreasing the relative abundance of Acidobacteria and Verrucomicrobia.
In our study, SEM showed that N
D indirectly negatively affected soil microbial community structure by directly affecting soil pH. Similarly, Tian et al. [
39] found that ND decreased the F: B ratio in subtropical forests, likely due to decreased soil pH. Compared with N
L, soil pH was decreased from 7.37 to 6.92 by N
D. A systematic study across a large pH gradient ranging from 8.3 to 4.5 has observed that different microbial species respond differentially to soil pH [
84]. The relatively large effect of soil pH on fungal PLFA compared with the marginal response of bacterial PLFA to pH results in a similar F: B ratio pattern with fungal PLFAs [
84]. Our individual contribution analysis showed that soil pH was the main factor explaining the variation in fungal PLFAs and the F: B ratio, except for bacterial PLFAs.
Meanwhile, N addition affects fungal growth rates more negatively than bacterial growth rates. Fungal PLFA decreases more than bacterial PLFA because of N addition [
85]. A strong correlation exists between fungal growth rate and biomass, but bacterial biomass remains stable when the bacterial growth rate changes significantly [
86]. PRC showed that N
D resulted in the biomass of actinomycetes, 10 Me 17:0, and bacteria, that is, 15:0, dominating the soil microbial community. Therefore, by decreasing soil pH, N
D significantly negatively affected the microbial community structure, owing to the decreasing fungal PLFAs in karst grasslands.
Differences in N
D and N
L also caused the dominance of fungal PLFAs, that is, 18:1ω7c, in the soil microbial community. The SEM showed that N
L positively affects the soil microbial community structure. Legume plants can influence the soil structure [
23,
24], increase soil porosity, reduce soil water content, and make the soil environment conducive to fungal growth. In contrast, legume plant tissues have high N content, and leaf litter, thin roots, and root nodules can reduce the entire litter layer's C: N ratio and accelerate the litter's decomposition and turnover. This can provide more C and N sources to soil microbes [
36,
87]. He et al. [
88] found that the continuous addition of exogenous substrates changed the soil microbial community structure, leading to the alternate growth of bacteria and fungi. Over time, the microbial community became increasingly balanced in meeting C demand. As fungi can effectively decompose inflexible C components, fungal activity is often associated with root systems and large nutrient patches [
28,
89]. The quantity and structure of fungi are susceptible to plant residues, and the stimulatory effect of plant residues on fungal growth is significantly higher than that on bacteria [
28]. In complex soil environments, the response of the microbial community structure to external N sources strongly depends on the availability of C sources and C–N coupling [
47]. Compared with ND, legumes improve soil N sources and provide sufficient C sources for soil microbes. This indicates that the introduction of legume plants is beneficial for stabilizing the soil microbial community structure in karst grassland ecosystems.
5. Conclusions
ND significantly decreased the abundance of bacteria, Actinobacteria, and AMF PLFAs. The total amounts of PLFAs and bacterial, fungal, actinobacterial, and AMF biomasses were significantly higher in NL than in ND. The F: B ratio was not significantly different between ND and NL but showed an increasing trend in NL. PRC analysis showed significant differences in the temporal dynamics of the soil microbial community composition within the ND and NL plots. ND negatively affects soil microbial community structure by decreasing soil pH. Legume plants positively affect soil microbial community structure through their effects on the soil environment and SOM, such as increased SOC and TN. This suggests that the introduction of legume plants into karst grassland ecosystems can increase the biomass of soil microbes and contribute to maintaining the stability of the soil microbial community structure. Thus, more attention should be paid to the process of subsurface N transfer by soil microbial networks (such as AMF) in the rhizosphere of legumes to reveal the mechanism whereby legumes promote the stability of soil microbial community in karst grassland ecosystems.
Author Contributions
Conceptualization, W.Z. and K.W.; formal analysis, R.Y.; investigation, D.X.; data curation, X.L.; writing—original draft preparation, X.L., R.Y. and J.Z.; writing—review and editing, X.L., W.Z., J.Z. and X.H.; supervision, X.H. and H.C.; project administration, W.Z. and K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 42361144886, U20A2011, and U23A20155), and Natural Science Foundation of Shandong Province (ZR2023QC298).
Data Availability Statement
All relevant data are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pellegrinetti, T.A.; Cotta, S.R.; Feitosa, Y.B.; et al. The role of microbial communities in biogeochemical cycles and greenhouse gas emissions within tropical soda lakes. Sci. Total Environ. 2024, 947.
- Xiang, L.; Achen, W.; Wenjie, W.; et al. High salinity inhibits soil bacterial community mediating nitrogen cycling. Appl. Environ. Microbiol. 2021, 87(21), e0136621.
- Campbell, T.P.; Ulrich, D.; Toyoda, J.G.; et al. Microbial communities influence soil dissolved organic carbon concentration by altering metabolite composition. Front. Microbiol. 2022, 12.
- Karhu, K.; Auffret, M.D.; Dungait, J.A.; Hopkins, D.W.; Prosser, J.I.; Singh, B.K.; et al. Temperature sensitivity of soil respiration rates enhanced by microbial community response. Nature 2014, 513, 81–84.
- Teste, F.P.; Kardol, P.; Turner, B.L.; Wardle, D.A.; Zemunik, G.; Renton, M.; et al. Plant-soil feedback and the maintenance of diversity in Mediterranean-climate shrublands. Science 2017, 355, 173–176.
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828.
- Freedman, Z.; Eisenlord, S.D.; Zak, D.R.; Xue, K.; He, Z.; Zhou, J. Towards a molecular understanding of N cycling in northern hardwood forests under future rates of N deposition. Soil Biol. Biochem. 2013, 66, 130–138.
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645.
- Zheng, M.; Zhou, Z.; Luo, Y.; Zhao, P.; Mo, J. Global pattern and controls of biological nitrogen fixation under nutrient enrichment: A meta-analysis. Glob. Change Biol. 2019, 25(9), 3018–3030.
- Zhang, Z.Q.; Su, W.R.; Liao, W.B.; Lan, C.Y. Role of legume species in revegetation of mined wastelands. Chin. J. Ecol. 2002, 21(2), 47–52.
- Frankow-Lindberg, B.E.; Dahlin, A.S. N2 fixation, N transfer, and yield in grassland communities including a deep-rooted legume or non-legume species. Plant Soil 2013, 370, 567–581.
- Cheng, Q. Perspectives in biological nitrogen fixation research. J. Integr. Plant Biol. 2008, 50(7), 786–798.
- Sprent, J.I.; Parsons, R. Nitrogen fixation in legume and non-legume trees. Field Crops Res. 2000, 65, 183–196.
- Tian, J.; Bu, L.; Zhang, M.; Yuan, J.; Zhang, Y.; Wei, G.; Wang, H. Soil bacteria with distinct diversity and functions mediate soil nutrients after introducing leguminous shrub in desert ecosystems. Glob. Ecol. Conserv. 2021, 31, e01841.
- Forrester, D.I.; Bauhus, J.; Khanna, P.K. Growth dynamics in a mixed-species plantation of Eucalyptus globulus and Acacia mearnsii. For. Ecol. Manag. 2004, 193, 81–95.
- Rothe, A.; Binkley, D. Nutritional interactions in mixed species forest: a synthesis. Can. J. For. Res. 2001, 31, 1855–1870.
- Kelty, M.J. The role of species mixtures in plantation forestry. For. Ecol. Manag. 2006, 233, 195–204.
- Resh, S.C.; Binkley, D.; Parrotta, J.A. Greater soil carbon sequestration under nitrogen-fixing trees compared with Eucalyptus species. Ecosystems 2002, 5, 217–231.
- Xiao, D.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Wang, K. Effects of different legume species and densities on arbuscular mycorrhizal fungal communities in a karst grassland ecosystem. Sci. Total Environ. 2019, 678, 551–558.
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133.
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072.
- Huang, X.; Liu, S.; Wang, H.; et al. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48.
- Rhoades, C.C.; Eckert, G.E.; Coleman, D.C. Effect of pasture trees on soil nitrogen and organic matter: Implications for tropical Montane forest restoration. Restor. Ecol. 1998, 6(3), 262–270.
- Singh, A.N.; Singh, J.S. Experiments on ecological restoration of coalmine spoil using native trees in a dry tropical environment, India: a synthesis. New For. 2006, 31, 25–39.
- Rachel, P.; Ann, M.N.; Unkovich, M.M.P.N. Biological nitrogen fixation by legume cover plants in oil palm plantations: calibration of the ureide technique and effects of plantation age and soil nitrate. Plant Soil 2023, 491(1/2), 665–680.
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; et al. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries: A review. Agron. Sustain. Dev. 2012, 32(2).
- Zhou, Z.; Wang, G.; Yu, G.D.G. The leguminous Hedysarum shrubs effectively drive the diversity and structural composition of soil bacterial community through rhizocompartments in the process of desertification reversal. Land Degrad. Dev. 2023, 34(16), 4833–4846.
- Bai, Z.; Bodé, S.; Huygens, D.; Boeckx, P.; Zhang, X. Kinetics of amino sugar formation from organic residues of different quality. Soil Biol. Biochem. 2013, 57, 814–821.
- Fowler, D.; Steadman, C.E.; Stevenson, D.; et al. Effects of global change during the 21st century on the nitrogen cycle. Atmos. Chem. Phys. 2015, 15, 13849–13893.
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; Fangmeier, A.; Zhang, F. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462.
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379.
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17.
- Bowman, W.D.; Cleveland, C.C.; Halada, L.; Hresko, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770.
- Maljanen, M.; Yli-Pirila, P.; Hytonen, J.; Joutsensaari, J.; Martikainen, P.J. Acidic northern soils as sources of atmospheric nitrous acid (HONO). Soil Biol. Biochem. 2013, 67, 94–97.
- Yang, H.; Yang, L.I.; Mingyu, W.U.; Zhang, Z.; Linghao, L.I.; Wan, S. Plant community responses to nitrogen addition and increased precipitation: the importance of water availability and species traits. Glob. Change Biol. 2011, 17, 2936–2944.
- Hu, J.; Chen, H.; Yue, B.C.D. Elevational gradient regulates the effects of short-term nutrient deposition on soil microorganisms and SOM decomposition in subtropical forests. Plant Soil 2023, 489(1/2), 225–238.
- Fang, Z.; Yu, H.; Wang, B.; et al. Response of plant-bacteria-soil system to phosphorus addition under simulated nitrogen deposition: evidence from a dryland ecosystem. Plant Soil 2023, 489(1-2), 593–611.
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Change Biol. 2012, 18, 1918–1927.
- Tian, D.; Jiang, L.; Ma, S.; Fang, W.; Schmid, B.; Xu, L.; Zhu, J.; Li, P.; Losapio, G.; Jing, X.; Zheng, C.; Shen, H.; Xu, X.; Zhu, B.; Fang, J. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China. Sci. Total Environ. 2017, 607–608, 1367–1375.
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825.
- Treseder, K.K. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120.
- Zhang, Q.; Zak, J.C. Effects of water and nitrogen amendment on soil microbial biomass and fine root production in a semi-arid environment in West Texas. Soil Biol. Biochem. 1998, 30, 39–45.
- Mo, J.; Zhang, W.E.I.; Zhu, W.; Gundersen, P.E.R.; Fang, Y.; Li, D.; Wang, H.U.I. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Change Biol. 2008, 14, 403–412.
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; et al. Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol. 2012, 196, 807–815.
- Compton, J.E.; Watrud, L.S.; Porteous, L.A.; Degrood, S. Response of soil microbial biomass and community composition to chronic nitrogen additions at Harvard Forest. For. Ecol. Manag. 2004, 196, 143–158.
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322.
- Blagodatskaya, E.V.; Blagodatsky, S.A.; Anderson, T.-H.; Kuzyakov, Y. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl. Soil Ecol. 2007, 37, 95–105.
- Williams, A.; Manoharan, L.; Rosenstock, N.P.; Olsson, P.A.; Hedlund, K. Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol. 2017, 213, 874–885.
- Wang, H.; Liu, S.R.; Zhang, X.; Mao, Q.; Li, X.; You, Y.; Wang, J.; Zheng, M.; Zhang, W.; Lu, X.; Mo, J. Nitrogen addition reduces soil bacterial richness, while phosphorus addition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol. Biochem. 2018, 127, 22–30.
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 1997, 7, 737–750.
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019.
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785.
- Wang, S.; Liu, Q.; Zhang, D. Karst Rocky Desertification in Southwestern China: Geomorphology, Landuse, Impact and Rehabilitation. Land Degrad. Dev. 2004, 15, 115–121.
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91.
- Liu, L.; He, X.; Xie, Q.; Wang, K. Genetic diversity of rhizobia isolated from common legumes in the karst area, Northwest Guangxi. Chinese J. Appl. Ecol. 2015, 26(12), 3633–3669.
- Chen, H.; Liu, J.; Wang, K.; Zhang, W. Spatial distribution of rock fragments on steep hillslopes in the karst region of northwest Guangxi, China. Catena 2010, 84(1–2), 21–28.
- Zhao, J.; Zeng, Z.; He, X.; Chen, H.; Wang, K. Effects of monoculture and mixed culture of grass and legume forage species on soil microbial community structure under different levels of nitrogen fertilization. Eur. J. Soil Biol. 2015, 68, 61–68.
- Gong, Z. Chinese Soil Taxonomy; Science Press: Beijing, China, 1999.
- Yu, G.; Jia, Y.; He, N.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 387–394.
- Liu, G.; Jiang, N.; Zhang, L.; Liu, Z. Soil physical and chemical analysis and description of soil profiles. China Standard Methods Press: Beijing, 1996.
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278.
- Frostegård, Å.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65.
- Hu, Y.; Xiang, D.; Veresoglou, S.D.; Chen, F.; Chen, Y.; Hao, Z.; Zhang, X.; Chen, B. Soil organic carbon and soil structure are driving microbial abundance and community composition across the arid and semi-arid grasslands in northern China. Soil Biol. Biochem. 2014, 77, 51–57.
- Li, D.; Liu, J.; Chen, H.; Zheng, L.; Wang, K. Soil microbial community responses to forage grass cultivation in degraded karst soils, southwest China. Land Degrad. Dev. 2018, 27, 456–464.
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48.
- Olsson, P.A. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol. Ecol. 1999, 29, 303–310.
- Framptona, G.K.; Van den Brink, P.J.; Gould, P.J.L. Effects of spring precipitation on a temperate arable collembolan community analysed using Principal Response Curves. Appl. Soil Ecol. 2000, 14, 231–248.
- Van den Brink, P.J.; Ter Braak, C.J.F. Principal response curves: Analysis of time dependent multivariate responses of a biological community to stress. Environ. Toxicol. Chem. 1999, 18, 138–148.
- Oksanen, J. Multivariate analysis of ecological communities. R: Vegan Tutorial, 2011, 1(7), pp. 11–12 (R Package Version).
- Vries, F.T.D.; Goede, R.D.; Hassan, M.R.; et al. Soil biota in grass and grass-legume mixtures. Aspects Appl. Biol. 2012, 115.
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799.
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; et al. Nitrogen cycles: past, present, and future. Biogeochemistry 2004, 70, 153–226.
- Peoples, M.B.; Herridge, D.F.; Ladha, J.K. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agriculture production. Plant Soil 1995, 174, 3–28.
- Drinkwater, L.E.; Wagoner, P.; Sarrantonio, M. Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 1998, 396, 262–265.
- Liu, X.; Zhang, W.; Wu, M.; Ye, Y.; Wang, K.; Li, D. Changes in soil nitrogen stocks following vegetation restoration in a typical karst catchment. Land Degrad. Dev. 2019, 30, 60–72.
- Dutta, R.K.; Agrawal, M. Effects of tree plantations on the soil characteristics and microbial activity of coalmine spoil land. Tropical Ecology 2002, 43(2), 315–324.
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63.
- Li, H.; Xu, Z.; Yang, S.; Li, X.; Top, E.M.; Wang, R.; Zhang, Y.; Cai, J.; Yao, F.; Han, X.; Jiang, Y. Responses of soil bacterial communities to nitrogen deposition and precipitation increment are closely linked with aboveground community variation. Microbial Ecology 2016, 71, 974–989.
- Peng, Y.; Guo, D.; Yang, Y. Global patterns of root dynamics under nitrogen enrichment. Global Ecol. Biogeogr. 2017, 26, 102–114.
- Berthrong, S.T.; Yeager, C.M.; Gallegos-Graves, L.; Steven, B.; Eichorst, S.A.; Jackson, R.B.; Kuske, C.R. Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl. Environ. Microbiol. 2014, 80, 3103–3112.
- Fierer, N.; Strickland, M.S.; Liptzin, D.; Bradford, M.A.; Cleveland, C.C. Global patterns in belowground communities. Ecol. Lett. 2009, 12(11), 1238–1249.
- Blagodatskaya, E.V.; Kuzyakov, Y.; Blagodatsky, S.A.; Anderson, T.-H. Contrasting effects of glucose, living roots and maize straw on microbial growth kinetics and substrate availability in soil. Eur. J. Soil Sci. 2009, 60(2), 186–197.
- Milcu, A.; Heim, A.; Ellis, R.J.; Scheu, S.; Manning, P. Identification of general patterns of nutrient and labile carbon control on soil carbon dynamics across a successional gradient. Ecosystems 2011, 14(5), 710–719.
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596.
- Demoling, F.; Nilsson, L.O.; Bååth, E. Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol. Biochem. 2008, 40(2), 370–379.
- Meidute, S.; Demoling, F.; Bååth, E. Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol. Biochem. 2008, 40(9), 2334–2343.
- Killingbeck, K.T. Nutrients in senesced leaves: keys to the search for potential resorption and resorption efficiency. Ecology 1996, 77, 1716–1727.
- He, H.; Zhang, W.; Zhang, X.; Xie, H.; Zhuang, J. Temporal responses of soil microorganisms to substrate addition as indicated by amino sugar differentiation. Soil Biol. Biochem. 2011, 43(6), 1155–1161.
- Baldrian, P.; Kolařík, M.; Štursová, M.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6(2), 248–258.
Table 1.
Significance tests using nonparametric multivariate statistical approaches (Adonis and MRPP) to assess the effects of different treatments on soil microbial community.
Table 1.
Significance tests using nonparametric multivariate statistical approaches (Adonis and MRPP) to assess the effects of different treatments on soil microbial community.
| Comparisons |
Adonis |
MRPP |
| R2 |
F |
P |
Observed δ |
Expected δ |
P |
| CK VS ND
|
0.15 |
3.871 |
0.052 |
0.177 |
0.187 |
0.064 |
| CK VS NL
|
0.016 |
0.367 |
0.719 |
0.146 |
0.143 |
0.898 |
| ND VS NL
|
0.234 |
6.703 |
0.005** |
0.167 |
0.186 |
0.008** |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).