Submitted:
29 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population and Sampling
2.2. Data Assessment
2.3. Statistical Analysis
3. Results
3.1. HCFMUSP Population
3.1.1. General Characteristics
3.1.2. Histological Diagnosis
3.1.3. Optical Microscopy - HCFMUSP
3.1.4. Characterization of Immune Deposits - HCFMUSP
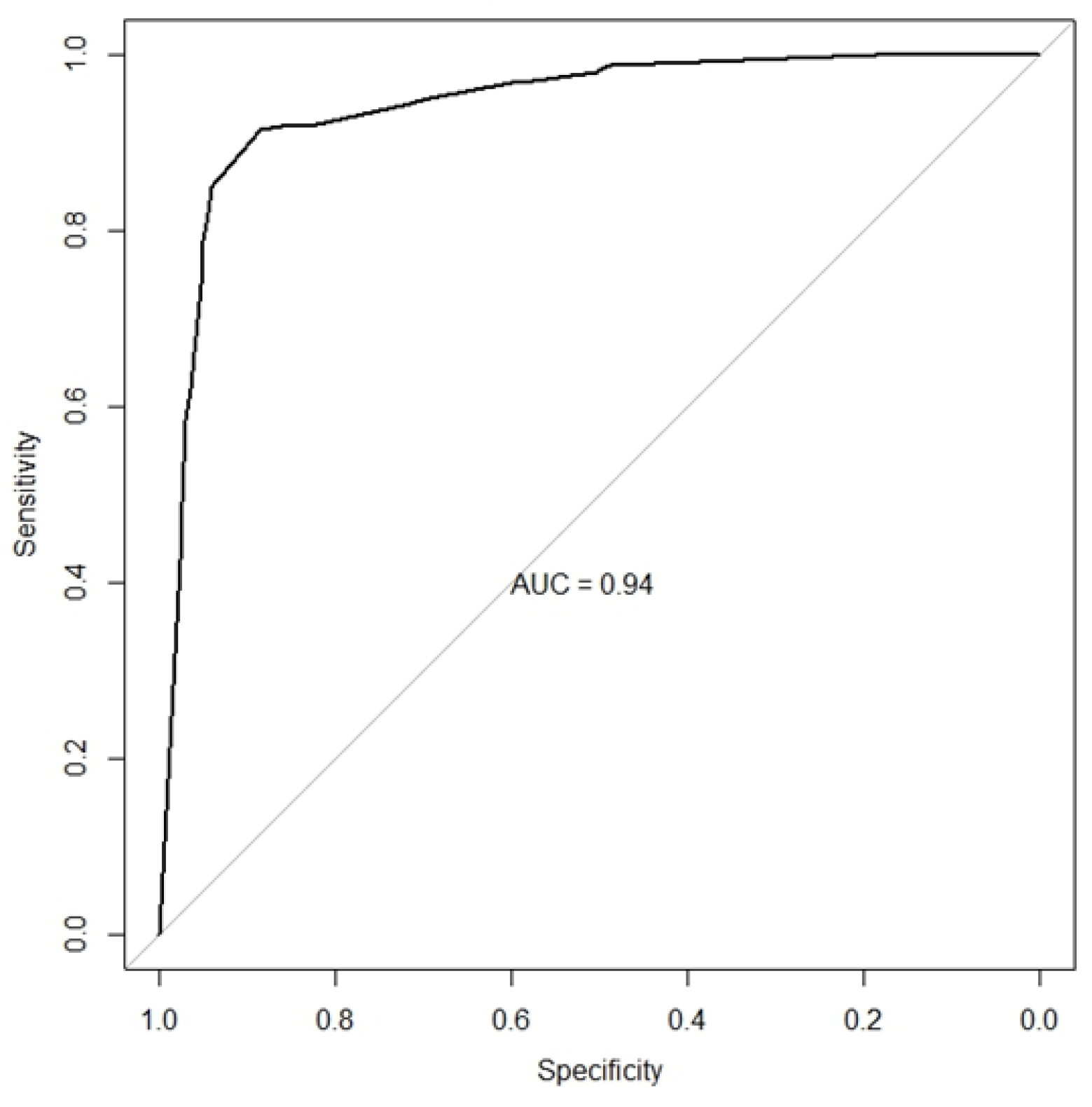
3.1.5. Histopathological Features Diagnostic Performance - HCFMUSP
3.1.6. LN Histopathological Criteria Combination - HCFMUSP
3.1.7. Criteria for Differentiating Class V LN and Non-Lupus Membranous Nephropathy - HCFMUSP
3.2. HAN Population
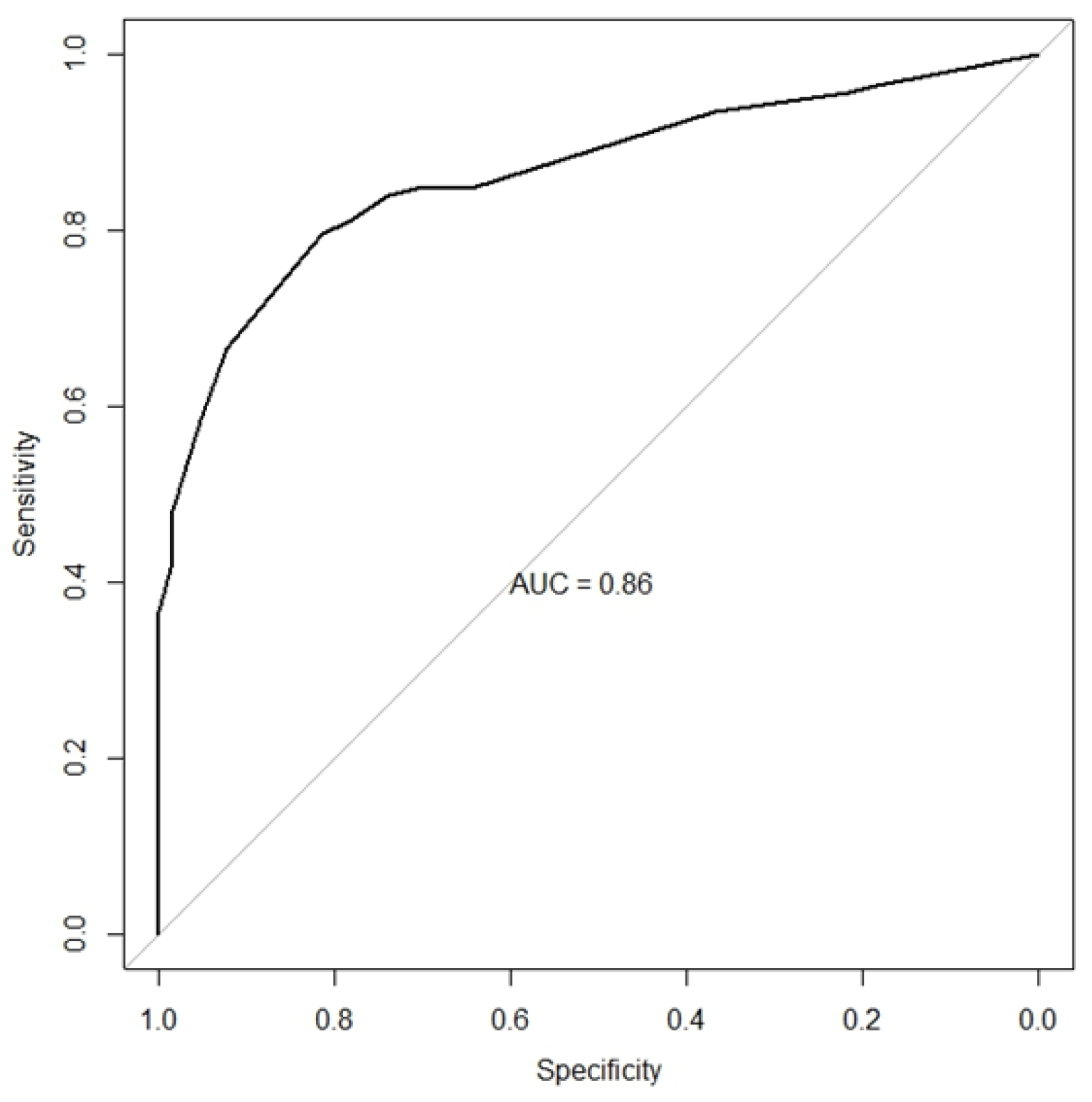
3.2.1. Histopathological Features Diagnostic Performance - HAN
3.2.2. LN Histopathological Criteria Combination - HAN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tektonidou, M.G., A. Dasgupta, and M.M. Ward, Risk of End-Stage Renal Disease in Patients With Lupus Nephritis, 1971-2015: A Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol, 2016. 68(6): p. 1432-41.
- Bernatsky, S., et al., Mortality in systemic lupus erythematosus. Arthritis Rheum, 2006. 54(8): p. 2550-7.
- Faurschou, M.; Dreyer, L.; Kamper, A.; Starklint, H.; Jacobsen, S. Long-term mortality and renal outcome in a cohort of 100 patients with Lupus Nephritis. Arthritis Care Res. 2010, 62, 873–880. [CrossRef]
- O’Shaughnessy, M.M.; Hogan, S.L.; Thompson, B.D.; Coppo, R.; Fogo, A.B.; Jennette, J.C. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol. Dial. Transplant. 2017, 33, 661–669. [CrossRef]
- Polito, M.G.; de Moura, L.A.R.; Kirsztajn, G.M. An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9617 native kidney biopsies. Nephrol. Dial. Transplant. 2009, 25, 490–496. [CrossRef]
- Dos-Santos, W.L.C., et al., Current distribution pattern of biopsy-proven glomerular disease in Salvador, Brazil, 40 years after an initial assessment. J Bras Nefrol, 2017. 39(4): p. 376-383. [CrossRef]
- Malafronte, P.; Mastroianni-Kirsztajn, G.; Betônico, G.N.; Romão, J.E.; Alves, M.A.R.; Carvalho, M.F.; Neto, O.M.V.; Cadaval, R.A.M.; Bérgamo, R.R.; Woronik, V.; et al. Paulista registry of glomerulonephritis: 5-year data report. Nephrol. Dial. Transplant. 2006, 21, 3098–3105. [CrossRef]
- Alarcón, G.S., Multiethnic lupus cohorts: what have they taught us? Reumatol Clin, 2011. 7(1): p. 3-6.
- Yap, D.Y.; Tang, C.S.; Ma, M.K.; Lam, M.F.; Chan, T.M. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol. Dial. Transplant. 2012, 27, 3248–3254. [CrossRef]
- Weening, J.J.; ON Behalf of the International Society of Nephrology and Renal Pathology Society Working Group on the Classification Oflupus Nephritis; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [CrossRef]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus ery-thematosus. Arthritis Rheum. 1997, 40, 1725. [CrossRef]
- Petri, M.; Orbai, A.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [CrossRef]
- Wang, H.; Gao, Y.; Ma, Y.; Cai, F.; Huang, X.; Lan, L.; Ren, P.; Wang, Y.; Chen, J.; Han, F. Performance of the 2019 EULAR/ACR systemic lupus erythematosus classification criteria in a cohort of patients with biopsy-confirmed lupus nephritis. Lupus Sci. Med. 2021, 8, e000458. [CrossRef]
- Jennette, J.C.; Iskandar, S.S.; Dalldorf, F.G. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int. 1983, 24, 377–385. [CrossRef]
- Kudose, S.; Santoriello, D.; Bomback, A.S.; Stokes, M.B.; D’agati, V.D.; Markowitz, G.S. Sensitivity and Specificity of Pathologic Findings to Diagnose Lupus Nephritis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1605–1615. [CrossRef]
- Ahmed, M.; Love, T.; Moore, C.; Le, T.H.; Jean-Gilles, J.; Goldman, B.; Choung, H.Y.G. The spectrum of renal diseases with lupus-like features: a single-center study. Ren. Fail. 2022, 44, 581–593. [CrossRef]
- IBGE), I.B.d.G.e.E., Censo demográfico 2022: identificação étnico-racial da população, por sexo e idade: resultados do universo. 2023, IBGE: Rio de Janeiro.
- Rijnink, E.C.; Teng, Y.O.; Kraaij, T.; Dekkers, O.M.; Bruijn, J.A.; Bajema, I.M. Validation of the Systemic Lupus International Collaborating Clinics classification criteria in a cohort of patients with full house glomerular deposits. Kidney Int. 2018, 93, 214–220. [CrossRef]
- Rijnink, E.C.; Teng, Y.O.; Kraaij, T.; Wolterbeek, R.; Bruijn, J.A.; Bajema, I.M. Idiopathic non-lupus full-house nephropathy is associated with poor renal outcome. Nephrol. Dial. Transplant. 2017, 32, 654–662. [CrossRef]
- Zahir, Z.; Wani, A.; Gupta, A.; Agrawal, V. Clinicopathological pattern of non-lupus full house nephropathy. Indian J. Nephrol. 2020, 30, 301–306. [CrossRef]
- Ruggiero, B.; Vivarelli, M.; Gianviti, A.; Pecoraro, C.; Peruzzi, L.; Benetti, E.; Ventura, G.; Pennesi, M.; Murer, L.; Coppo, R.; et al. Outcome of childhood-onset full-house nephropathy. Nephrol. Dial. Transplant. 2016, 32, gfw230–1204. [CrossRef]
- Caza, T.N.; Storey, A.J.; Hassen, S.I.; Herzog, C.; Edmondson, R.D.; Arthur, J.M.; Kenan, D.J.; Larsen, C.P. Discovery of seven novel putative antigens in membranous nephropathy and membranous lupus nephritis identified by mass spectrometry. Kidney Int. 2023, 103, 593–606. [CrossRef]
- Debiec, H. and P. Ronco, PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med, 2011. 364(7): p. 689-90.
- Seifert, L.; Hoxha, E.; Eichhoff, A.M.; Zahner, G.; Dehde, S.; Reinhard, L.; Koch-Nolte, F.; Stahl, R.A.; Tomas, N.M. The Most N-Terminal Region of THSD7A Is the Predominant Target for Autoimmunity in THSD7A-Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 1536–1548. [CrossRef]
- Sethi, S.; Madden, B.J.; Debiec, H.; Charlesworth, M.C.; Gross, L.; Ravindran, A.; Hummel, A.M.; Specks, U.; Fervenza, F.C.; Ronco, P. Exostosin 1/Exostosin 2–Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2019, 30, 1123–1136. [CrossRef]
| Lupus Nephritis (n 269) |
Control (n 219) |
|||
|---|---|---|---|---|
| Demographic data: | ||||
| Age | 31.5 (25-40) | 44 (32-58) | p<0.001 | |
| Sex | p<0.001 | |||
| Male | 41 (15.2) | 121 (55.2) | ||
| Female | 228 (84.8) | 98 (44.8) | ||
| Race/Ethnicity | p<0.001 | |||
| Black | 49 (18.2) | 14 (6.4) | ||
| Mixed | 18 (6.7) | 9 (4.1) | ||
| White | 183 (68.0) | 168 (76.7) | ||
| Yellow | 0 | 5 (2.3) | ||
| Unknown | 19 (7.1) | 23 (10.5) | ||
| Clinical data: | ||||
| Creatinine (mg/dl): | 1.00 (0.73-2.03) | 1.96 (1.12-3.41) | p<0.001 | |
| eGFR (CKD-EPI): | 73 (29-110) | 34 (17-71) | p<0.001 | |
| Hemoglobin (g/dl): | 10.9 (9.6-12.2) | 11.1 (9.6-13.2) | p=0.06 | |
| Albumin (g/dl): | 3.0 (2.4-3.5) | 3.2 (2.5-3.6) | p=0.15 | |
| Cholesterol (mg/dl): | 218 (173-274) | 200 (164-252) | p=0.06 | |
| Proteinuria (g/d or g/g): | 2.010 (0.900-4.093) | 2.300 (1.200-4.453) | p=0.29 | |
| Hematuria (%): | 186 (72.7) | 144 (75.4) | p=0.51 | |
| Positive ANA (%): | 214 (98.2) | 57 (32.7) | p<0.001 | |
| Renal syndrome (%): | p<0.001 | |||
| Acute glomerulonephritis | 35 (13.7) | 47 (25.0) | ||
| Nephrotic syndrome | 15 (5.9) | 25 (13.3) | ||
| Nephritic/nephrotic syndrome | 74 (29.0) | 32 (17.0) | ||
| Rapidly progressive GN | 13 (5.1) | 25 (13.3) | ||
| Unknown etiology renal injury | 15 (5.9) | 17 (9.0) | ||
| Asymptomatic urinary abnormalities | 103 (40.4) | 42 (22.3) | ||
|
All data are displayed as median (interquartile range) or N (%). HCFMUSP, Hospital das Clínicas of the University of São Paulo Medical School; eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; ANA, anti-nuclear antibody; GN, glomerulonephritis |
||||
| Immune deposit sites | Lupus nephritis (n 269) | Control (n 219) | IgA nephropathy (n 88) | MN (n 47) | MPGN (n 31) | Pauci-immune GN (n 32) | Proliferative GN (n 21) | |
|---|---|---|---|---|---|---|---|---|
| Mesangial | 220 (87.3) | 165 (77.8) | 88 (100) | 19 (42.2) | 25 (86.2) | 17 (53.1) | 16 (88.9) | |
| Subendothelial | 194 (73.8) | 52 (24.2) | 10 (11.4) | 2 (4.3) | 29 (93.5) | 1 (3.1) | 10 (55.6) | |
| Subepithelial | 130 (49.2) | 51 (23.5) | 0 | 47 (100) | 1 (3.3) | 0 | 3 (15.0) | |
| Subendothelial and subepithelial | 78 (29.8) | 4 (1.8) | 0 | 2 (4.3) | 1 (3.3) | 0 | 1 (5.0) | |
| Tubular | 36 (14.5) | 3 (1.4) | 0 | 0 | 1 (3.6) | 0 | 2 (11.1) | |
| Vascular | 7 (2.8) | 3 (1.4) | 1 (1.1) | 0 | 0 | 1 (3.1) | 1 (5.6) | |
| Extraglomerular | 39 (15.7) | 6 (2.8) | 1 (1.1) | 0 | 1 (3.5) | 1 (3.1) | 3 (16.7) | |
| Immunofluorescence positivity | ||||||||
| IgA | 141 (56.8) | 102 (49.0) | 88 (100) | 8 (17.8) | 4 (14.8) | 0 | 2 (12.5) | |
| IgG | 242 (97.6) | 83 (40.3) | 11 (12.8) | 45 (100) | 14 (51.8) | 4 (12.5) | 9 (56.2) | |
| IgM | 172 (69.6) | 89 (43.2) | 36 (41.9) | 15 (33.3) | 23 (85.2) | 10 (32.3) | 5 (29.4) | |
| Kappa | 148 (59.9) | 66 (32.0) | 17 (19.8) | 36 (80.0) | 12 (44.4) | 0 | 1 (5.9) | |
| Lambda | 222 (89.9) | 133 (64.9) | 73 (85.9) | 41 (93.2) | 15 (55.6) | 0 | 4 (23.5) | |
| C3 | 227 (91.5) | 157 (75.5) | 74 (85.1) | 27 (60.0) | 22 (84.6) | 19 (59.4) | 14 (82.3) | |
| C1q | 215 (86.7) | 38 (18.3) | 8 (9.3) | 10 (22.2) | 15 (55.6) | 2 (6.2) | 3 (17.6) | |
| Fibrinogen | 27 (10.8) | 11 (5.3) | 5 (5.7) | 0 | 0 | 4 (12.9) | 2 (11.8) | |
| Other | ||||||||
| Full-house staining | 91 (36.8) | 8 (3.8) | 2 (2.3) | 3 (6.7) | 2 (7.4) | 0 | 1 (5.9) | |
| Dominant IgG | 227 (91.5) | 66 (32.5) | 0 | 45 (100) | 11 (40.7) | 3 (9.7) | 7 (43.7) | |
| C1q ≥ 1+ | 197 (79.4) | 23 (11.1) | 2 (2.3) | 8 (17.8) | 11 (40.7) | 1 (3.1) | 1 (5.9) | |
| IgG/C3/C1q plus IgA or IgM | 176 (71.3) | 13 (6.2) | 2 (2.3) | 3 (6.7) | 7 (25.9) | 0 | 1 (5.9) | |
| ≥ 4 elements staining in IF | 187 (75.7) | 22 (10.7) | 10 (11.6) | 4 (8.9) | 7 (25.9) | 0 | 1 (6.2) | |
| Note: Data are presented as n (%). Missing data were excluded from the analysis. HCFMUSP, Hospital das Clínicas of the University of São Paulo Medical School; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; IF, immunofluorescence. | ||||||||
| Feature | Sensitivity (IC 95%) | Specificity (IC 95%) | Accuracy (IC 95%) | PPV (IC 95%) | NPV (IC 95%) | Positive LR (IC 95%) | Negative LR (IC 95%) | |
|---|---|---|---|---|---|---|---|---|
| Mesangial proliferation | 0.89 (0.84, 0.92) | 0.36 (0.30, 0.43) | 0.65 (0.61, 0.69) | 0.63 (0.58, 0.68) | 0.72 (0.63, 0.81) | 1.39 (1.25, 1.55) | 0.31 (0.21, 0.46) | |
| Endocapillary hypercellularity | 0.64 (0.58, 0.70) | 0.63 (0.57, 0.70) | 0.64 (0.59, 0.68) | 0.68 (0.62, 0.74) | 0.59 (0.53, 0.65) | 1.75 (1.44, 2.13) | 0.57 (0.47, 0.68) | |
| Mesangial deposits | 0.87 (0.83, 0.91) | 0.22 (0.17, 0.28) | 0.58 (0.53, 0.62) | 0.57 (0.52, 0.62) | 0.59 (0.48, 0.70) | 1.12 (1.03, 1.22) | 1.12 (1.03, 1.22) | |
| Subendothelial deposits | 0.74 (0.68, 0.79) | 0.76 (0.70, 0.81) | 0.75 (0.71, 0.79) | 0.79 (0.73, 0.84) | 0.70 (0.64, 0.76) | 3.05 (2.38, 3.91) | 0.35 (0.28, 0.43) | |
| Subepithelial deposits | 0.49 (0.43, 0.55) | 0.76 (0.70, 0.82) | 0.62 (0.57, 0.66) | 0.72 (0.65, 0.78) | 0.55 (0.50, 0.61) | 2.10 (1.60, 2.74) | 0.66 (0.58, 0.76) | |
| Combined subendothelial and subepithelial deposits | 0.30 (0.24, 0.36) | 0.98 (0.95, 0.99) | 0.61 (0.56, 0.65) | 0.95 (0.88, 0.99) | 0.54 (0.48, 0.59) | 16.08 (5.98, 43.20) | 0.72 (0.66, 0.78) | |
| Extraglomerular deposits | 0.16 (0.11, 0.21) | 0.97 (0.94, 0.99) | 0.53 (0.48, 0.58) | 0.87 (0.73, 0.95) | 0.49 (0.44, 0.54) | 5.51 (2.38, 12.75) | 0.87 (0.82, 0.92) | |
| Positive IgA | 0.57 (0.50, 0.63) | 0.51 (0.44, 0.58) | 0.54 (0.49, 0.59) | 0.58 (0.52, 0.64) | 0.50 (0.43, 0.57) | 1.16 (0.97, 1.38) | 0.85 (0.70, 1.03) | |
| Positive IgG | 0.98 (0.95, 0.99) | 0.60 (0.53, 0.66) | 0.80 (0.76, 0.84) | 0.74 (0.69, 0.79) | 0.95 (0.90, 0.98) | 2.42 (2.05, 2.86) | 0.04 (0.02, 0.09) | |
| Positive IgM | 0.70 (0.63, 0.75) | 0.57 (0.50, 0.64) | 0.64 (0.59, 0.68) | 0.66 (0.60, 0.72) | 0.61 (0.54, 0.68) | 1.61 (1.35, 1.92) | 0.53 (0.43, 0.67) | |
| Positive C3 | 0.92 (0.87, 0.95) | 0.25 (0.19, 0.31) | 0.61 (0.56, 0.65) | 0.59 (0.54, 0.64) | 0.71 (0.59, 0.81) | 1.21 (1.11, 1.32) | 0.35 (0.22, 0.55) | |
| Positive C1q | 0.87 (0.82, 0.91) | 0.82 (0.76, 0.87) | 0.84 (0.81, 0.88) | 0.85 (0.80, 0.89) | 0.84 (0.78, 0.88) | 4.72 (3.53, 6.32) | 0.16 (0.12, 0.23) | |
| C1q ≥ 1+ | 0.79 (0.74, 0.84) | 0.89 (0.84, 0.93) | 0.84 (0.80, 0.87) | 0.90 (0.85, 0.93) | 0.78 (0.72, 0.83) | 7.15 (4.84, 10.56) | 0.23 (0.18, 0.30) | |
| Dominant IgG | 0.92 (0.87, 0.95) | 0.67 (0.61, 0.74) | 0.81 (0.77, 0.84) | 0.77 (0.72, 0.82) | 0.87 (0.80, 0.92) | 2.82 (2.30, 3.44) | 0.13 (0.08, 0.19) | |
| Full-house staining | 0.37 (0.31, 0.44) | 0.96 (0.93, 0.98) | 0.64 (0.60, 0.69) | 0.92 (0.85, 0.96) | 0.56 (0.51, 0.62) | 9.73 (4.84, 19.57) | 0.65 (0.59, 0.72) | |
| IgG/C3/C1q plus IgA or IgM | 0.71 (0.65, 0.77) | 0.94 (0.90, 0.97) | 0.82 (0.78, 0.85) | 0.93 (0.89, 0.96) | 0.73 (0.68, 0.79) | 11.46 (6.73, 19.51) | 0.31 (0.25, 0.37) | |
| ≥ 4 elements staining in IF | 0.76 (0.70, 0.81) | 0.89 (0.84, 0.93) | 0.82 (0.78, 0.85) | 0.89 (0.84, 0.93) | 0.75 (0.70, 0.81) | 7.09 (4.75, 10.59) | 0.27 (0.22, 0.34) | |
| HCFMUSP, Hospital das Clinicas of the University of São Paulo Medical School; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; IF, immunofluorescence. | ||||||||
| No. of Features* | Sensitivity (IC 95%) | Specificity (IC 95%) | Accuracy (IC 95%) | PPV (IC 95%) | NPV (IC 95%) | Positive LR (IC 95%) | Negative LR (IC 95%) |
|---|---|---|---|---|---|---|---|
| One or more | 1.00 (0.99, 1.00) | 0.19 (0.14, 0.25) | 0.63 (0.59, 0.68) | 0.60 (0.55, 0.65) | 1.00 (0.91, 1.00) | 1.23 (1.15, 1.31) | 0.00 (0.00, NA) |
| Two or more | 0.96 (0.93, 0.98) | 0.61 (0.54, 0.67) | 0.80 (0.76, 0.84) | 0.75 (0.70, 0.79) | 0.93 (0.87, 0.97) | 2.44 (2.06, 2.90) | 0.06 (0.03, 0.12) |
| Three or more | 0.91 (0.87, 0.94) | 0.87 (0.81, 0.91) | 0.89 (0.86, 0.92) | 0.89 (0.85, 0.93) | 0.89 (0.84, 0.93) | 6.84 (4.81, 9.74) | 0.10 (0.07, 0.15) |
| Four or more | 0.74 (0.68, 0.80) | 0.96 (0.92, 0.98) | 0.84 (0.80, 0.87) | 0.95 (0.91, 0.98) | 0.75 (0.70, 0.81) | 16.76 (8.81, 31.87) | 0.27 (0.22, 0.33) |
| Five | 0.46 (0.40, 0.53) | 0.98 (0.94, 0.99) | 0.69 (0.65, 0.74) | 0.96 (0.90, 0.99) | 0.60 (0.54, 0.65) | 18.73 (7.80, 44.97) | 0.55 (0.49, 0.62) |
| *Mesangial proliferation, subendothelial deposits, C1q ≥ 1+, dominant IgG and 4 or more positive elements in immunofluorescence. HCFMUSP, Hospital das Clinicas of the University of São Paulo Medical School; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; | |||||||
| Feature | Sensitivity (IC 95%) | Specificity (IC 95%) | Accuracy (IC 95%) | PPV (IC 95%) | NPV (IC 95%) | Positive LR (IC 95%) | Negative LR (IC 95%) |
|
|---|---|---|---|---|---|---|---|---|
| Mesangial proliferation | 0.68 (0.52, 0.81) | 0.60 (0.44, 0.74) | 0.64 (0.53, 0.74) | 0.61 (0.46, 0.75) | 0.67 (0.50, 0.80) | 1.69 (1.13, 2.52) | 0.53 (0.33, 0.87) | |
| Mesangial deposits | 0.76 (0.61, 0.87) | 0.58 (0.42, 0.72) | 0.67 (0.56, 0.77) | 0.65 (0.51, 0.77) | 0.70 (0.53, 0.84) | 1.80 (1.23, 2.63) | 0.41 (0.23, 0.73) | |
| Positive IgA | 0.43 (0.29, 0.59) | 0.82 (0.68, 0.92) | 0.63 (0.52, 0.73) | 0.71 (0.51, 0.87) | 0.59 (0.46, 0.71) | 2.45 (1.20, 4.97) | 0.69 (0.52, 0.92) | |
| Positive IgM | 0.70 (0.54, 0.82) | 0.67 (0.51, 0.80) | 0.68 (0.58, 0.78) | 0.68 (0.53, 0.81) | 0.68 (0.52, 0.81) | 2.09 (1.32, 3.29) | 0.46 (0.28, 0.74) | |
| Positive C1q | 0.76 (0.61, 0.87) | 0.78 (0.63, 0.89) | 0.77 (0.67, 0.85) | 0.78 (0.63, 0.89) | 0.76 (0.61, 0.87) | 3.42 (1.94, 6.06) | 0.31 (0.18, 0.53) | |
| C1q ≥ 1+ | 0.65 (0.50, 0.79) | 0.82 (0.68, 0.92) | 0.74 (0.63, 0.82) | 0.79 (0.63, 0.90) | 0.70 (0.56, 0.82) | 3.67 (1.89, 7.12) | 0.42 (0.28, 0.64) | |
| Full-house staining | 0.22 (0.11, 0.36) | 0.93 (0.82, 0.99) | 0.57 (0.46, 0.67) | 0.77 (0.46, 0.95) | 0.54 (0.42, 0.65) | 3.26 (0.96, 11.08) | 0.84 (0.71, 1.00) | |
| IgG/C3/C1q plus IgA or IgM | 0.57 (0.41, 0.71) | 0.93 (0.82, 0.99) | 0.75 (0.65, 0.83) | 0.90 (0.73, 0.98) | 0.68 (0.55, 0.79) | 8.48 (2.76, 26.04) | 0.47 (0.33, 0.65) | |
| ≥ 4 elements staining in IF | 0.61 (0.45, 0.75) | 0.91 (0.79, 0.98) | 0.76 (0.66, 0.84) | 0.88 (0.71, 0.96) | 0.69 (0.56, 0.81) | 6.85 (2.61, 17.95) | 0.43 (0.30, 0.62) | |
| HCFMUSP, Hospital das Clinicas of the University of São Paulo Medical School; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; IF, immunofluorescence. | ||||||||
| Feature | Sensitivity (IC 95%) | Specificity (IC 95%) | Accuracy (IC 95%) | PPV (IC 95%) | NPV (IC 95%) | Positive LR (IC 95%) | Negative LR (IC 95%) |
|---|---|---|---|---|---|---|---|
| Mesangial proliferation | 0.69 (0.61, 0.76) | 0.43 (0.32, 0.55) | 0.60 (0.54, 0.67) | 0.71 (0.64, 0.78) | 0.40 (0.30, 0.51) | 1.21 (0.97, 1.50) | 0.73 (0.52, 1.02) |
| Endocapillary hypercellularity | 0.74 (0.67, 0.81) | 0.61 (0.49, 0.72) | 0.70 (0.64, 0.76) | 0.80 (0.72, 0.86) | 0.53 (0.43, 0.64) | 1.89 (1.42, 2.53) | 0.42 (0.31, 0.58) |
| Mesangial deposits | 0.70 (0.62, 0.77) | 0.36 (0.25, 0.49) | 0.59 (0.52, 0.66) | 0.71 (0.63, 0.78) | 0.35 (0.24, 0.47) | 1.09 (0.89, 1.34) | 0.84 (0.56, 1.24) |
| Subendothelial deposits | 0.74 (0.66, 0.80) | 0.58 (0.45, 0.70) | 0.69 (0.62, 0.75) | 0.80 (0.72, 0.86) | 0.49 (0.38, 0.61) | 1.75 (1.31, 2.35) | 0.46 (0.33, 0.64) |
| Subepithelial deposits | 0.49 (0.41, 0.57) | 0.55 (0.43, 0.67) | 0.51 (0.44, 0.58) | 0.71 (0.61, 0.79) | 0.33 (0.25, 0.42) | 1.10 (0.82, 1.48) | 0.92 (0.71, 1.19) |
| Combined subendothelial and subepithelial deposits |
0.24 (0.18, 0.31) | 0.90 (0.80, 0.96) | 0.44 (0.37, 0.51) | 0.84 (0.71, 0.94) | 0.34 (0.27, 0.41) | 2.37 (1.11, 5.04) | 0.85 (0.75, 0.95) |
| Extraglomerular deposits | 0.33 (0.26, 0.41) | 1.00 (0.95, 1.00) | 0.54 (0.48, 0.61) | 1.00 (0.93, 1.00) | 0.41 (0.34, 0.49) | Inf (NaN, Inf) | 0.67 (0.60, 0.75) |
| Positive IgA | 0.63 (0.55, 0.71) | 0.55 (0.42, 0.67) | 0.60 (0.53, 0.67) | 0.75 (0.66, 0.82) | 0.41 (0.31, 0.52) | 1.39 (1.04, 1.86) | 0.68 (0.50, 0.92) |
| Positive IgG | 0.96 (0.91, 0.98) | 0.35 (0.24, 0.48) | 0.76 (0.70, 0.82) | 0.76 (0.69, 0.82) | 0.79 (0.60, 0.92) | 1.47 (1.23, 1.76) | 0.12 (0.05, 0.29) |
| Positive IgM | 0.65 (0.57, 0.73) | 0.44 (0.32, 0.57) | 0.59 (0.51, 0.65) | 0.71 (0.62, 0.79) | 0.38 (0.27, 0.49) | 1.17 (0.91, 1.49) | 0.79 (0.55, 1.12) |
| Positive C3 | 0.84 (0.77, 0.90) | 0.32 (0.21, 0.45) | 0.68 (0.61, 0.74) | 0.73 (0.66, 0.80) | 0.49 (0.33, 0.65) | 1.25 (1.04, 1.50) | 0.48 (0.29, 0.81) |
| Positive C1q | 0.69 (0.61, 0.77) | 0.77 (0.65, 0.87) | 0.72 (0.65, 0.78) | 0.87 (0.79, 0.93) | 0.54 (0.43, 0.64) | 3.06 (1.93, 4.83) | 0.40 (0.30, 0.52) |
| C1q ≥ 1+ | 0.64 (0.55, 0.72) | 0.83 (0.72, 0.91) | 0.70 (0.63, 0.76) | 0.89 (0.82, 0.95) | 0.51 (0.42, 0.61) | 3.83 (2.20, 6.67) | 0.43 (0.34, 0.55) |
| Dominant IgG | 0.85 (0.78, 0.90) | 0.48 (0.36, 0.61) | 0.73 (0.66, 0.79) | 0.77 (0.70, 0.84) | 0.60 (0.46, 0.74) | 1.65 (1.29, 2.10) | 0.31 (0.20, 0.50) |
| Full-house staining | 0.40 (0.32, 0.49) | 0.97 (0.89, 1.00) | 0.58 (0.51, 0.65) | 0.97 (0.88, 1.00) | 0.43 (0.35, 0.52) | 13.20 (3.32, 52.46) | 0.62 (0.54, 0.71) |
| IgG/C3/C1q plus IgA or IgM | 0.58 (0.49, 0.66) | 0.95 (0.87, 0.99) | 0.70 (0.63, 0.76) | 0.96 (0.90, 0.99) | 0.51 (0.42, 0.60) | 12.47 (4.09, 38.00) | 0.44 (0.36, 0.54) |
| ≥ 4 elements staining in IF | 0.64 (0.55, 0.72) | 0.80 (0.68, 0.89) | 0.69 (0.62, 0.75) | 0.87 (0.79, 0.93) | 0.51 (0.41, 0.61) | 3.20 (1.94, 5.29) | 0.45 (0.35, 0.58) |
| HAN, Hospital Ana Nery; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; IF, immunofluorescence. | |||||||
| No. of features* | Sensitivity (IC 95%) | Specificity (IC 95%) | Accuracy (IC 95%) | PPV (IC 95%) | NPV (IC 95%) | Positive LR (IC 95%) | Negative LR (IC 95%) |
|---|---|---|---|---|---|---|---|
| One or more | 0.96 (0.92, 0.99) | 0.18 (0.10, 0.30) | 0.71 (0.65, 0.77) | 0.71 (0.64, 0.78) | 0.71 (0.44, 0.90) | 1.18 (1.05, 1.33) | 0.20 (0.07, 0.54) |
| Two or more | 0.85 (0.78, 0.90) | 0.65 (0.52, 0.76) | 0.78 (0.72, 0.84) | 0.83 (0.76, 0.89) | 0.67 (0.54, 0.78) | 2.39 (1.71, 3.35) | 0.24 (0.15, 0.37) |
| Three or more | 0.66 (0.58, 0.74) | 0.92 (0.83, 0.97) | 0.75 (0.68, 0.81) | 0.95 (0.88, 0.98) | 0.57 (0.47, 0.67) | 8.64 (3.69, 20.21) | 0.36 (0.28, 0.47) |
| Four | 0.36 (0.28, 0.45) | 1.00 (0.94, 1.00) | 0.57 (0.50, 0.64) | 1.00 (0.93, 1.00) | 0.43 (0.35, 0.51) | Inf (NaN, Inf) | 0.64 (0.56, 0.72) |
| *Subendothelial deposits, C1q ≥ 1+, dominant IgG and 4 or more positive elements in immunofluorescence. HAN, Hospital Ana Nery; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio; IF, immunofluorescence. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





