Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Access to MWF and Residue Samples
2.2. Fungicides
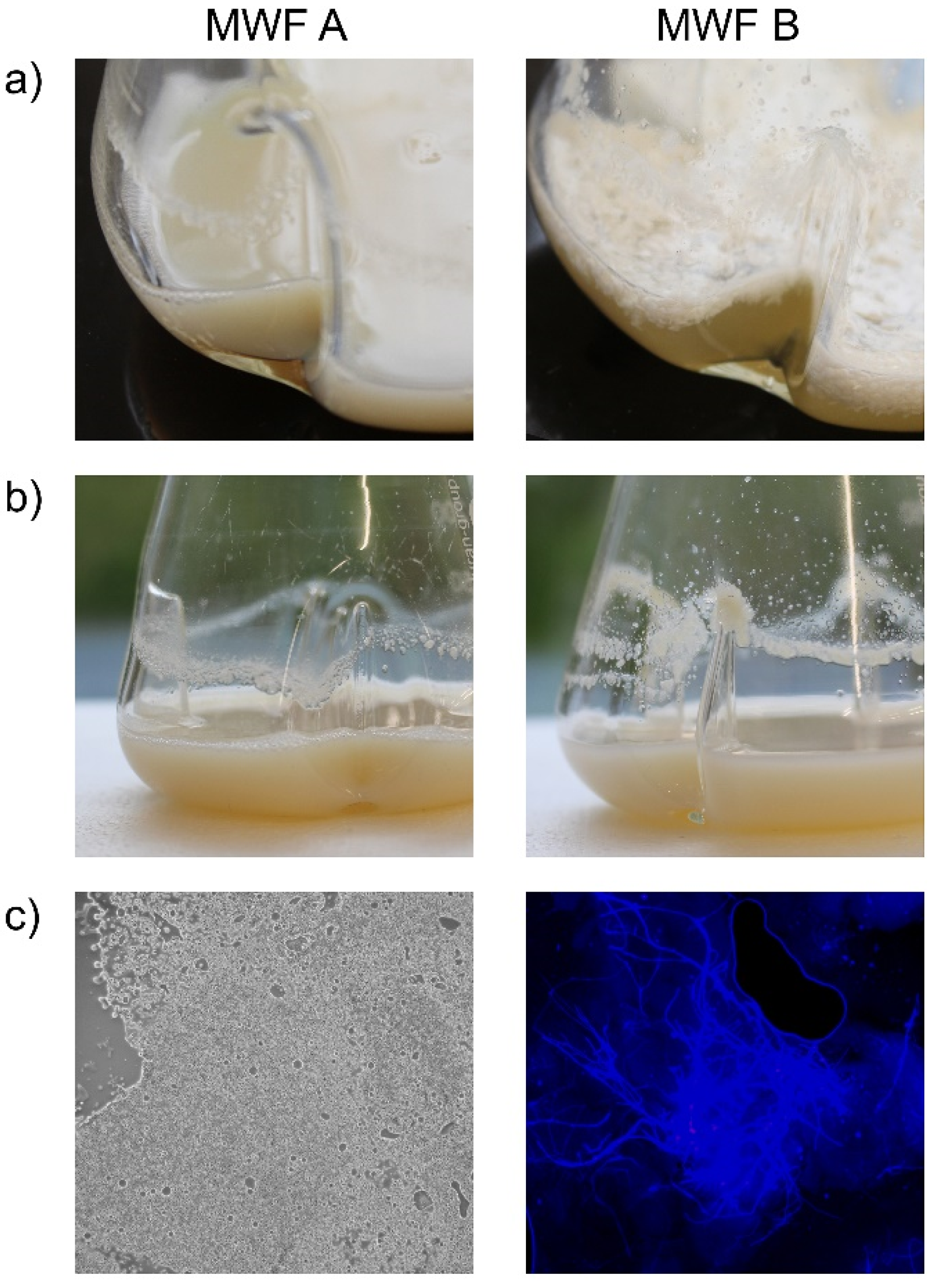
2.3. Metalworking Fluids
2.4. Examination and Isolation of Fungi from MWF Samples
2.5. Examination of Residue Samples
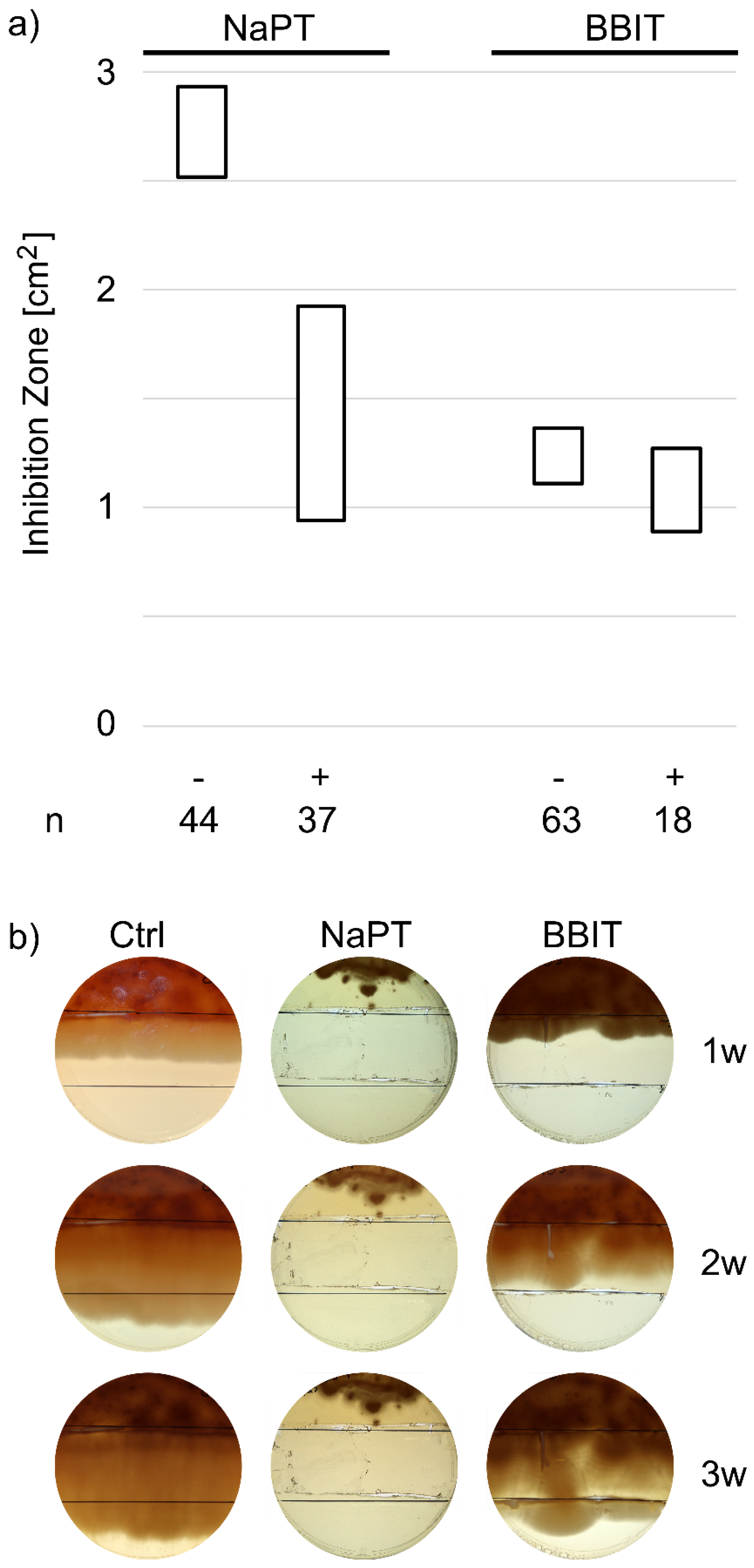
2.6. Zone of Inhibition Tests
2.7. Adaptation to Fungicides
2.8. Sporulation Assays
2.9. Stability Assays
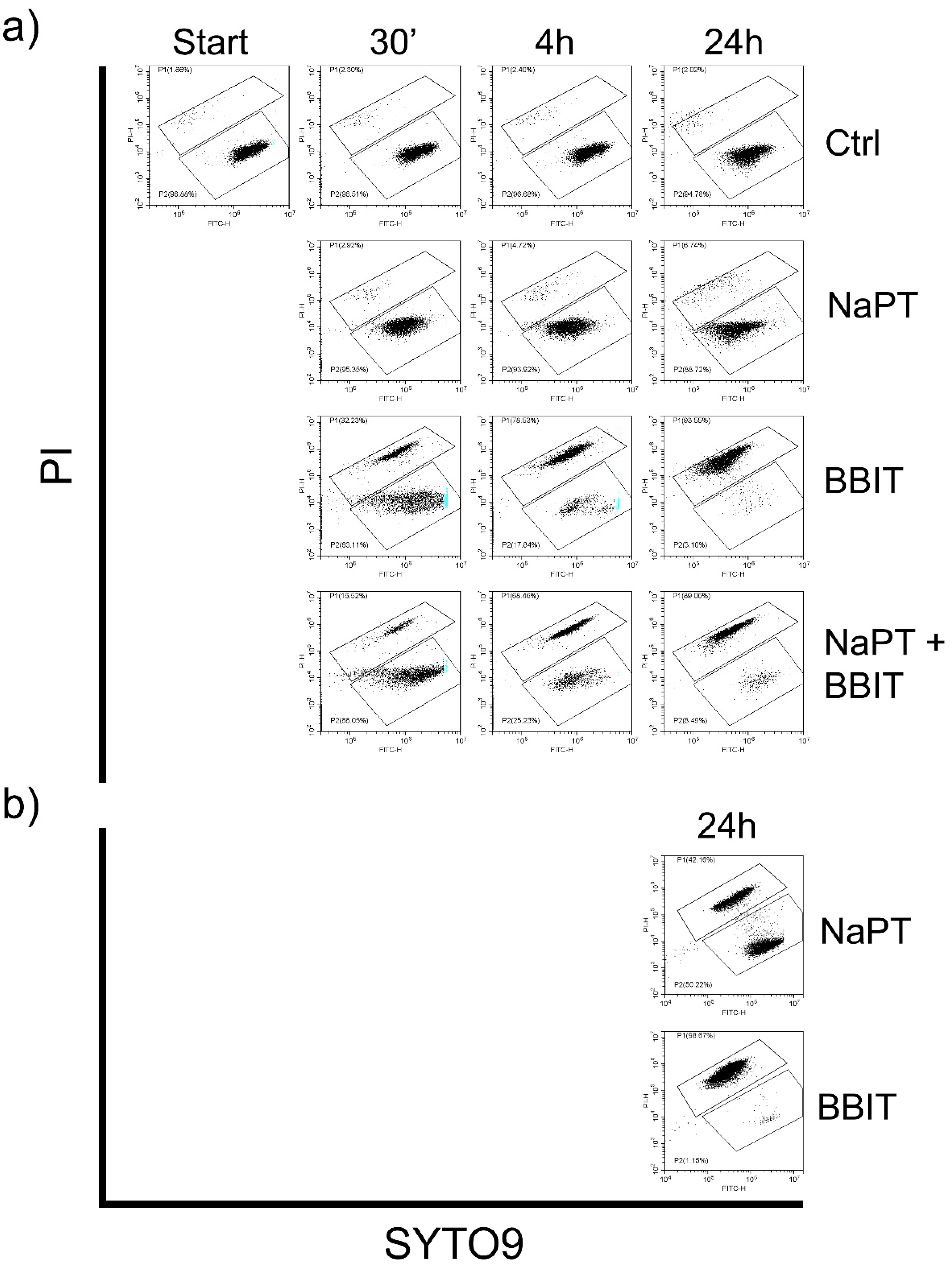
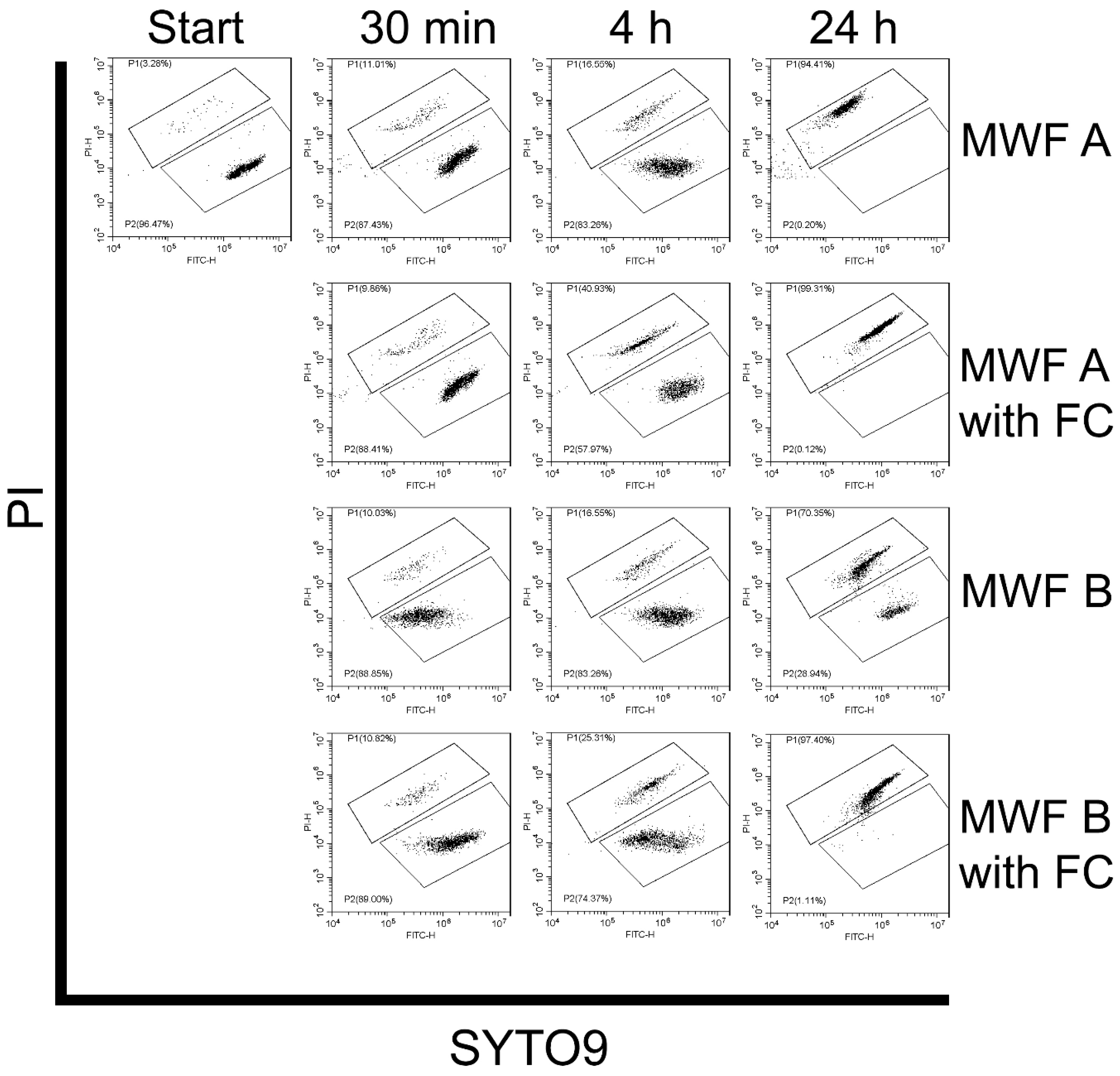
2.10. Quantification of Spores and Viability Assays
2.11. Volatilization of Fungicides-Assays
3. Results
3.1. Fungal Occurrence in MWFs and Machining Systems
3.1.1. In MWFs
3.1.2. In residue Samples
3.1.3. Detected Species
3.2. Escape from Fungicide-Toxicity in MWFs
3.2.1. Resistance Formation in in-Use Samples
3.2.2. Adaptation to NaPT and BBIT
3.3. Impact of Fungicides on Sporulation
3.3.1. Quantities of Released Spores
3.3.2. Effect of Fungicides on Spore Viability
3.3.3. Effect of MWFs on Spore Viability
3.3.4. Effect of Delayed Spore Inactivation
3.4. Impact of Fungicide-Volatiles
4. Discussion
Author Contributions
Conflict of Interest
References
- Canter, N. M. The chemistry of metalworking fluids. In: Metalworking Fluids, 3rd Ed.; Byers, J. P. Ed.; CRC Press, Boca Raton FL, USA, 2017, pp. 143–169. [CrossRef]
- Di Martino, P. Ways to improve biocides for metalworking fluid. AIMS Microbiol. 2021, 7, 13-27. [CrossRef]
- Canter, N. M. Antimicrobial pesticides (microbicides): Additives needed to extend the use of metalworking fluids. Tribol. Lubr. Technol. September 2023, 14-24. http://digitaleditions.walsworthprintgroup.com/publication/?m=5716&i=799455&p=16&ver=html5.
- Passman, F.J.; Küenzi, P. Microbiology in metalworking fluids. Tribol. Transact. 2020, 6, 1147–1171. [Google Scholar] [CrossRef]
- The Ultimate Guide to Central Coolant Filtration Systems. Available online: https://www.edjetech.com/blog/guide-to-central-coolant-filtration-systems (accessed on 25 October 2024).
- STLE MWF Education & Training Committee. Metalworking fluid basics. Tribol. Lubr. Technol. March 2023, 54-61. http://digitaleditions.walsworthprintgroup.com/publication/?m=5716&i=783486&p=56&ver=html5.
- Passman, F.J. Microbiology of metalworking fluids. In: Metalworking Fluids, 3rd ed.; Byers, J. P. Ed.; CRC Press, Bocca Ranton, USA, 2018, pp. 241 - 284. [CrossRef]
- Kidd, G.H.; Wolf, F.T. Dimorphism in a pathogenic Fusarium. Mycologia 1973, 6, 1371–1375. [Google Scholar] [CrossRef]
- Sánchez-Martínez, C.; Pérez-Martín, J. Dimorphism in Fungal Pathogens: Candida albicans and Ustilago maydis--similar inputs, different outputs. Curr. Opin. Microbiol. 2001, 4, 214–221. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal Dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef]
- Elansky, S.N.; Chudinova, E.M.; Elansky, A.S.; Kah, M.O.; Sanzhieva, D.A.; Mukabenova, B.A.; Dedov, A.G.). Microorganisms in spent water-miscible metalworking fluids as a resource of strains for their disposal. J. Clean. Prod. 2022, 350, 131438. [Google Scholar] [CrossRef]
- Harris, S.D. Branching of fungal hyphae: regulation, mechanisms and comparison with other branching systems. Mycologia 2008, 100, 823–832. [Google Scholar] [CrossRef]
- Raza, T.; Qadir, M.F.; Khan, K.S.; Eash, N.S.; Yousuf, M.; Chatterjee, S.; Manzoor, R.; Ur Rehman, S.; Oetting, J.N. Unraveling the potential of microbes in decomposition of organic matter and release of carbon in the ecosystem. J. Environ. Manag. 2023, 344, 118529. [Google Scholar] [CrossRef]
- Dantigny, P.; Nanguy, S.P. Significance of the physiological state of fungal spores. Int. J. Food Microbiol. 2009, 134, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Trafny, E.L. Microorganisms in metalworking fluids: current issues in research and management. Int. J. Occup. Med. Environ. Health. 2013, 26, 4–15. [Google Scholar] [CrossRef]
- Kapoor, R.; Selvaraju, S. B.; Yadav, J.S. Extended tracking of the microbial community structure and dynamics in an industrial synthetic metalworking fluid system. FEMS Microbiol. Ecol. 2014, 87, 664–677. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. Methods Mol. Biol. 2017, 1542, 51–106. [Google Scholar] [CrossRef] [PubMed]
- Cighir, A.; Mare, A.D.; Vultur, F.; Cighir, T.; Pop, S.D.; Horvath, K.; Man, A. Fusarium spp. in human disease: exploring the boundaries between commensalism and pathogenesis. Life (Basel). 2023, 13, 1440. [CrossRef]
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health risks associated with exposure to filamentous fungi. Int. J. Environ. Res. Public Health. 2017, 14, 719. [Google Scholar] [CrossRef] [PubMed]
- Special report: Antimicrobial pesticides (microbicides): Additives needed to extend the use of metalworking fluids. Available online: https://www.tribonet.org/news/general-topics/special-report-antimicrobial-pesticides-microbicides-additives-needed-to-extend-the-use-of-metalworking-fluids/ (accessed on 25 October 2024).
- Hoch, H.C.; Galvani, C.D.; Szarowski, D.H.; Turner, J.N. Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia. 2005, 97, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, C.; Xue, B.; Ji, P.; Li, Y.; Sun, L.; Wang, S. Development of a rapid sporulation method of Fusarium graminearum using liquid cultivation. Plant. Dis. 2022, 106, 34–38. [Google Scholar] [CrossRef]
- Vanhauteghem, D.; Demeyere, K; Callaert, N.; Boelaert, A.; Haesaert, G.; Audenaert, K.; Meyer, E. Flow cytometry is a powerful tool for assessment of the viability of fungal conidia in metalworking fluids. Appl. Environ. Microbiol. 2017, 83, e00938-17. [CrossRef]
- Lodhi, A.P.S.; Kumar, D. Natural ingredients based environmental friendly metalworking fluid with superior lubricity. Colloids Surf. A. 2021, 613, 126071. [Google Scholar] [CrossRef]
- Pitkäranta, M.; Meklin, T.; Hyvärinen, A.; Paulin, L.; Auvinen, P.; Nevalainen, A.; Rintala, H. Analysis of fungal flora in indoor dust by ribosomal DNA sequence analysis, quantitative PCR, and culture. App. Environ. Microbiol. 2008, 74, 233–244. [Google Scholar] [CrossRef]
- Soliman, S.S.M. Editorial: Candida spp.-transmission, pathogenesis, host-pathogen interaction, prevention, and treatment. Front Microbiol. 2023, 14, e1258837. [CrossRef]
- Mahalingam, S.R.R.; Madhavan, P.; Pei Pei, C. Diutina (Candida) rugosa complex: The biofilm ultrastructure, extracellular matrix, cell wall component and antifungal susceptibility to amphotericin B, caspofungin, fluconazole and voriconazole. BioRxiv, 2020. [CrossRef]
- Ko, J.K.; Lee, S.M. Advances in cellulosic conversion to fuels: engineering yeasts for cellulosic bioethanol and biodiesel production. Curr. Opin. Biotechnol. 2018, 50, 72–80. [Google Scholar] [CrossRef]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for controlling the sporulation in Fusarium spp. J. Fungi (Basel). 2022, 9, 10. [Google Scholar] [CrossRef]
- Quirce, S.; Vandenplas, O.; Campo, P.; Cruz, M.J.; de Blay, F.; Koschel, D.; Moscato, G.; Pala, G.; Raulf, M.; Sastre, J.; Siracusa, A.; Tarlo, S.M.; Walusiak-Skorupa, J.; Cormier, Y. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy 2016, 71, 765–779. [Google Scholar] [CrossRef]
- Al-Shaarani, A.A.Q.A.; Pecoraro, L. A review of pathogenic airborne fungi and bacteria: unveiling occurrence, sources, and profound human health implication. Front. Microbiol. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Cause and effect. Newly emerging infectious diseases. Nat. Rev. Microbiol. 2006, 4, 414. [CrossRef]
- Gordon, T. Metalworking fluid-the toxicity of a complex mixture. J. Toxicol. Environ. Health A. 2004, 67, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Metalworking Fluids: Safety and Health Best Practices Manual. Available online: https://www.osha.gov/metalworking-fluids/manual (accessed on 25 October 2024).
| Year | Fluid Samples | Residue Samples | ||
| n | Positives | n | Positives | |
| 2014 | 5,223 | 280 (5.4%) | 45 | 17 (37.8%) |
| 2015 | 5,188 | 387 (7.5%) | 27 | 10 (37.0%) |
| 2016 | 5,053 | 289 (5.7%) | 52 | 31 (59.6%) |
| 2017 | 6,399 | 325 (5.1%) | 61 | 44 (72.1%) |
| 2018 | 5,882 | 348 (5.9%) | 40 | 18 (45.0%) |
| 2019 | 5,131 | 392 (7.6%) | 48 | 32 (66.7%) |
| 2020 | 3,791 | 248 (6.5%) | 38 | 22 (57.9%) |
| 2021 | 3,897 | 156 (4.0%) | 33 | 15 (45.5%) |
| 2022 | 4,229 | 131 (3.1%) | 37 | 21 (56.8%) |
| 2023 | 3,902 | 190 (4.9%) | 36 | 19 (52.8%) |
| Total | 48,695 | 2,746 (5.6%) | 417 | 229 (54.9%) |
| Genus | n | Species | n |
|---|---|---|---|
| Fusarium | 45 | F. solani | 21 |
| F. keratoplasticum | 7 | ||
| F. nierenbergiae | 6 | ||
| F. languescens | 4 | ||
| F. petroliphilium | 4 | ||
| Fusarium spp | 3 | ||
| Paecilomyces | 1 | P. lilacinus | 1 |
| Diutina | 28 | D. neorugosa | 17 |
| D. rugosa | 11 | ||
| Candida | 9 | C. tropicalis | 3 |
| C. haemulonii | 3 | ||
| Candida spp. | 3 | ||
| Yarrowia | 18 | Y. lipolytica | 18 |
| Unknown | 2 |
| System | Spore quantity [Log10 mL-1] |
|---|---|
| Buffer (Ctrl) | 6.22 ± 0.32 |
| NaPT | 6.16 ± 0.42 |
| BBIT | 6.19 ± 0.31 |
| NaPT + BBIT | 6.21 ± 0.34 |
| MWF A | 6.29 ± 0.35 |
| MWF A with FC | 6.20 ± 0.32 |
| MWF B | 6.13 ± 0.25 |
| MWF B with FC | 6.01 ± 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




