1. Introduction
Human papillomavirus (HPV), the most common sexually transmitted infection (STI), is the primary cause of cervical cancer (CC), which has been the fastest growing cancer in Canada (+3.7% per year) since 2015 [
1]. Risk factors for HPV exposure include early sexual activity (before 16 years old), multiple sexual partners, co-infection with other STIs like HIV, smoking, and high parity (having three or more children). These factors, along with low socioeconomic status, significantly increase the risk of developing and dying from cervical cancer [
2,
3,
4,
5,
6,
7]. CC ranked among the top five cancers diagnosed in women aged 25-44 in Ontario [
2]. In Canada, cervical cancer continues invading and taking women’s lives with recent estimates of 400 cervical cancer-related deaths and 1600 new cervical cancer diagnoses in 2024 alone [
2]. Similarly, current estimates in Ontario, one of the most populated provinces in Canada, indicate that 660 women will be diagnosed and 150 will die from cervical cancer in 2024 [
8].
Cervical cancer is highly preventable through appropriate screening, such as the Papanicolaou (Pap) test, which allows for the early detection of malignant and precancerous lesions. Despite this, participation in screening remains disappointedly low among structurally marginalized women and individuals with cervix, including those who are racialized immigrants and refugees, Indigenous people, members of 2SLGBTQ+, sex workers and ex prisoners. These groups face numerous structural and individual barriers, including racism, gender expression discrimination, language barriers, lack of healthcare access, cultural stigma surrounding STIs, including HPV, transportation costs, limited knowledge of cervical cancer screening, and demanding life priorities [
9,
10,
11,
12,
13,
14,
15].
Our study focused on sex workers, and ex-prisoners, who are not only at elevated risk for HPV but also encounter multiple institutional and systemic barriers. These include stigma, cultural taboos surrounding sex work and incarceration, discriminatory and judgmental attitudes by healthcare providers, as well as logistical issues like service availability (e.g., no family physician, inconvenient clinic hours, long wait times). The persistence of these disparities suggest that innovative methods are needed to address these barriers and improve screening rates among equity-deserving groups like sex workers, and ex-prisoners [
15,
16,
17,
18,
19].
HPV self-sampling (HPV-SS) as a primary screening method has the potential to overcome these barriers [
20,
21,
22,
23,
24,
25,
26,
27]. HPV-SS is an easy, user-friendly tool that has shown high acceptability among diverse groups of women [
28,
29,
30,
31,
32,
33,
34]. Women are taught to use swabs to self-collect vaginal sample that are analyzed for high-risk HPV strains, responsible for causing CC. This alternative method of screening can be done at a woman’s chosen time and location with no third parties involved. Furthermore, it provides a sense of empowerment for those who are often excluded due to social stigma surrounding their social location and identity. There is solid evidence of the validity and acceptability of HPV-SS compared to clinician-collected cervical samples [
35,
36,
37]. The HPV-SS kits have been tested successfully among non-responders in Europe and in Canada among under-housed women in British Columbia, and under or never screened (UNS) women in Manitoba and Ontario [
30,
31,
38,
39,
40].
Guided by these findings, our community-based mixed-methods pilot study aimed to assess the accessibility and feasibility of HPV self-sampling for UNS sex workers, and ex-prisoners in the Greater Toronto Area (GTA) in Ontario. This paper reports only the findings from the quantitative component of the study.
2. Methods &Materials
The quantitative component of our pilot community-based mixed methods study involved the recruitment of 84 sex workers, and ex-prisoners through community centers serving this population in Greater Toronto Area (GTA) (i.e., Maggie’s Toronto -A sex workers support organization and PASAN- a community-based prisoner health and harm reduction organization).
The study protocol was reviewed and approved by Toronto Metropolitan University (REB# 2023-165) and University of Toronto (REB# 45488).
Eligible participants were recruited by two peer associates/community champions identified by our collaborating community centers. They received a half-day Zoom training from our research team, covering the study protocol, eligibility criteria, ethical considerations, information about cervical cancer risk factors and screening, and HPV-SS.
The study involved two groups: participants who agreed to use the HPV-SS kit and those who did not. The inclusion criteria were as follows: aged 25-69; self-identified as a current or former sex worker and/or having a history of incarceration; self-reported time since the last Pap test was >4 years (including no history of a Pap test); no prior history of cervical cancer or hysterectomy; not pregnant or having given birth in the last 3 months; residing in the GTA; able to read, write, and communicate in English; able to provide informed consent; and willing to share contact information with the study team. Study participants were recruited from March 2024 to June 2024.
Study participants were approached by trained peer associates/community champions at the selected community centers (Maggie’s Toronto and PASAN). As trusted community members, they discussed the study with potential participants and assessed eligibility. Eligible individuals who agreed to be contacted were referred to our Research Associate (RA), a trained nurse. The RA followed up via phone or zoom to provide further information and address any questions. Those interested in participating and with computer access were emailed a link to review the online consent form. Participants without computer access were invited to use designated computers at the community centers.
After providing consent, participants were granted access to an online self-administered questionnaire. The survey collected information on sociodemographic and clinical characteristics, sexual health practices, knowledge of cervical cancer screening (Pap tests and HPV), and experiences of stigma related to sex work or incarceration in medical and social contexts. It also assessed their intention to use HPV-SS and perceived facilitators and barriers to accessing HPV-SS. The questionnaire took approximately 30 to 45 minutes to complete.
Upon receiving notification of completed questionnaires, the RA reviewed participants’ responses about their intention in undertaking HPV-SS. The RA then contacted those interested in HPV-SS to arrange a meeting at one of the two collaborating centers for them to conduct HPV-SS.
All participants were provided links to cervical cancer screening educational materials and short videos produced by Cancer Care Ontario, and Ontario Health that included:
Cancer Care Ontario resources
The Right Time for the Pap Test brochure
Videos from Ontario Health: Hamilton FHT
Participants were then divided into two self-selected cohorts: Cohort A: Participants willing to use the HPV-SS and Cohort B: Participants who did not agree to use the kit but consented to participate in the study.
Partcipants in Cohort A were provided with a link to a short instructional video on how to collect their cervicovaginal specimen, produced by BC Cancer (HPV Test educational video). Members of this cohort were instructed to pick up the HPV-SS device (a dry Copan swab FLOQSswab® 552C.80, Copan Italia S.P.A., Brescia, IT) at one of the collaborating centers, where our Research Associate (RA) was present.
At the center, participants received an instruction sheet with a diagram illustrating the steps to obtain a cervicovaginal specimen. They were instructed to insert the swab intravaginally, rotate it three times, and collect the specimen. Participants could collect the sample in the nearest washroom or private room at the center, then place the swab in the provided tube and return it to the RA.
Once the sample was returned, the RA suspended the dry swab in a vial containing the medium (Roche Cell Collection Medium, Roche Molecular Systems, Inc., Branchburg, NJ, US). The vials were then transported to designated hospital microbiology lab in Toronto by the RA for analysis on the Roche cobas® 6800 System using the cobas® HPV test (Roche Molecular Systems, Inc., Branchburg, NJ, US). .
Test results were emailed directly to RA and the first author, who then informed the participants of their results, as well as their primary care provider if contact information was provided and they were willing for us to share the results with their primary care providers.
Negative Results: Participants with negative results received a letter from the research team, advising them to follow provincial guidelines and undergo routine screening in five years. If a primary care provider’s contact information was provided, they also received a notification.
Positive Results: Participants with positive results received a letter advising them to follow up with their primary care provider for a Pap test. A member of the research team also called them directly. If a primary care provider’s contact information was provided, they were sent a letter.
Participants with positive results who did not have a primary care provider or preferred not to see their regular provider were assisted in arranging a visit at a community health center collaborating with our two community partners. They were instructed to bring their results letter. Additionally, Participants were connected with the HealthCareConnect program to help them find a long-term primary care provider if they were interested.
Participants in Cohort B were also provided with a link to the instructional video on collecting their cervicovaginal specimen, in case they reconsidered their decision. All participants were compensated $70 for their time, internet usage costs, and travel expenses to the collaborating centers for HPV-SS.
3. Data Analysis
Participants completed online surveys via Qualtrics, which were subsequently exported to IBM SPSS Statistics version 28.0 for analysis. Descriptive statistics (e.g., frequencies, means, medians, modes, and standard deviations) were calculated to summarize sociodemographic and clinical characteristics, knowledge of cervical cancer and screening, perceived stigma related to sex work and incarceration, as well as intentions, perceived facilitators, and barriers to using HPV-SS.
For knowledge about cervical cancer and screening with Pap and HPV an overall score was calculated by summing correct responses. Correct answers were assigned a value of 1, while incorrect or "don't know" responses were assigned a value of 0. Each scale consisted of 8 questions, with total scores ranging from 0 to 8. For self-reported perceived stigma during medical and social encounters, a 5-point Likert scale was used across several domains. The overall score was calculated by summing the responses, where "strongly disagree" was assigned a score of 1 and "strongly agree" a score of 5. The scale included 10 questions, with participant scores ranging from 10 to 50.
4. Results
4.1. Section A: Participants Socio-Demographic and Clinical Characteristics
4.1.1. Sociodemographic Characteristics
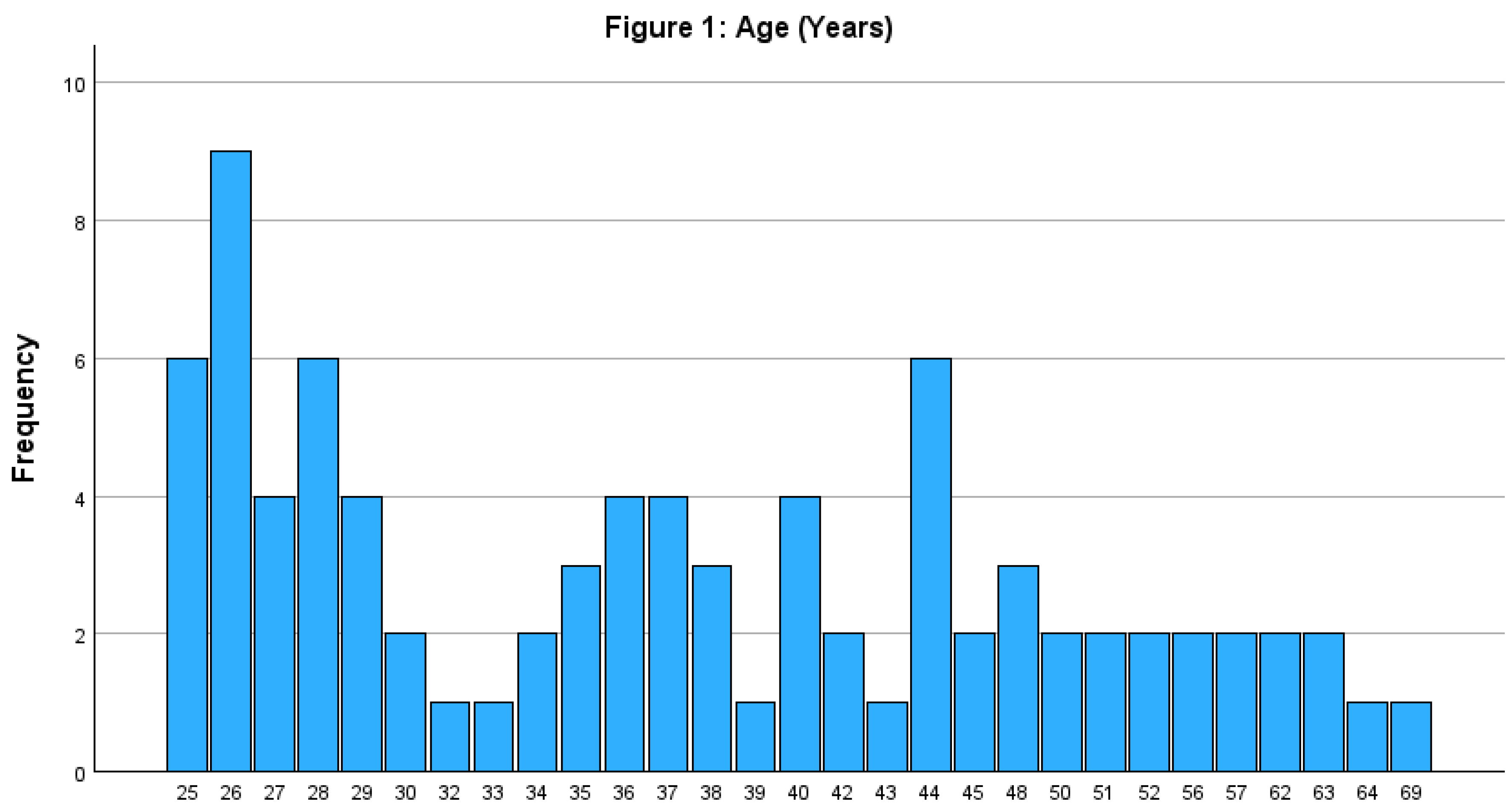
The study surveyed 84 participants, with 40 recruited by Maggie and 44 by Pasan. Participants' ages ranged from 25 to 69 years with a median age of 36.5 years and a standard deviation of 11.6 years. The age distribution was positively skewed (skewness = 0.76), indicating a younger cohort compared to the general population. Most participants were 45 years or younger (See
Figure 1).
Approximately 80% of participants (n=67) identified their gender as women, 13% as non-binary, and 5% as two-spirit or transgender masculine/genderqueer. Additionally, two respondents chose not to disclose their gender identity. Nearly 66% of the participants were single or never married and 16% were married. Additionally, about 46% of participants reported having children, with the number of children ranging from 1 to 6.
Regarding place of birth, 46.4% of the participants were not born in Canada. Of these, 32% had been living in Canada for less than five years, 5% for between five and nine years, and the remainder for more than ten years. Although 26% of participants who were born outside Canada preferred not to report their place of birth the region of origin for the remaining included Africa, East Asia, South Asia, West Asia, South America, and the Caribbean. Additionally, among those born outside Canada, 26% were refugee applicants, approximately 15% were landed immigrants or had obtained Canadian citizenship, and 5% were either temporary migrant workers or held visitor visas.
The majority of the participants completed some form of secondary education, with approximately 60% (n=50) having completed a college or university education. Conversely, a small fraction, around 7% (n=6), had education levels below high school (grade 9 or less).
Most participants have been involved in sex work (i.e., 91%) and 33% reported having a history of incarceration.
Table 1 indicates that 37% of participants have been working as sex workers for three years or less, while the remainder have been involved for a longer period.
Study participants were asked to identify the types of sex work they engage in. The most reported type was independent escorting, followed by working as sugar babies and Survival sex work (see
Table 2).
About half of the participants reported being unemployed in the past 3 months (
Table 3).
4.1.2. Clinical Characteristics
One third of participants (i.e., 33%) reported their health as poor to fair while about half of participants reported their health as good and only 18% as very good to excellent.
Common cancers reported among family members were breast cancer (n=20), Cervical cancer (n=13) and Colon Cancer (n=7) followed with other cancers (e.g., lung, liver, prostate, throat, bladder, brain, kidney, lymphoma, skin) (n=15). About 8% (n=7) of individuals reported having been diagnosed with breast, colon, or other cancers.
Approximately 50% of participants reported having their first sexual experience at age 16 or younger. About 25% indicated having had six or more sexual partners in the past three months. More than half of the participants did not regularly use condoms during sexual encounters, and nearly half had previously been diagnosed with an STI, ranging from chlamydia to gonorrhea and HIV/AIDS. Around 24% (n=20) of participants reported experiencing symptoms such as abnormal vaginal discharge, sores or ulcers on the vagina, or pelvic pain in the past six months. Surprisingly, only 40% (n=8) of those affected sought medical attention. Of those who did, half were subsequently diagnosed with an STI (see
Table 4)
4.2. Section B: Access to Health Services
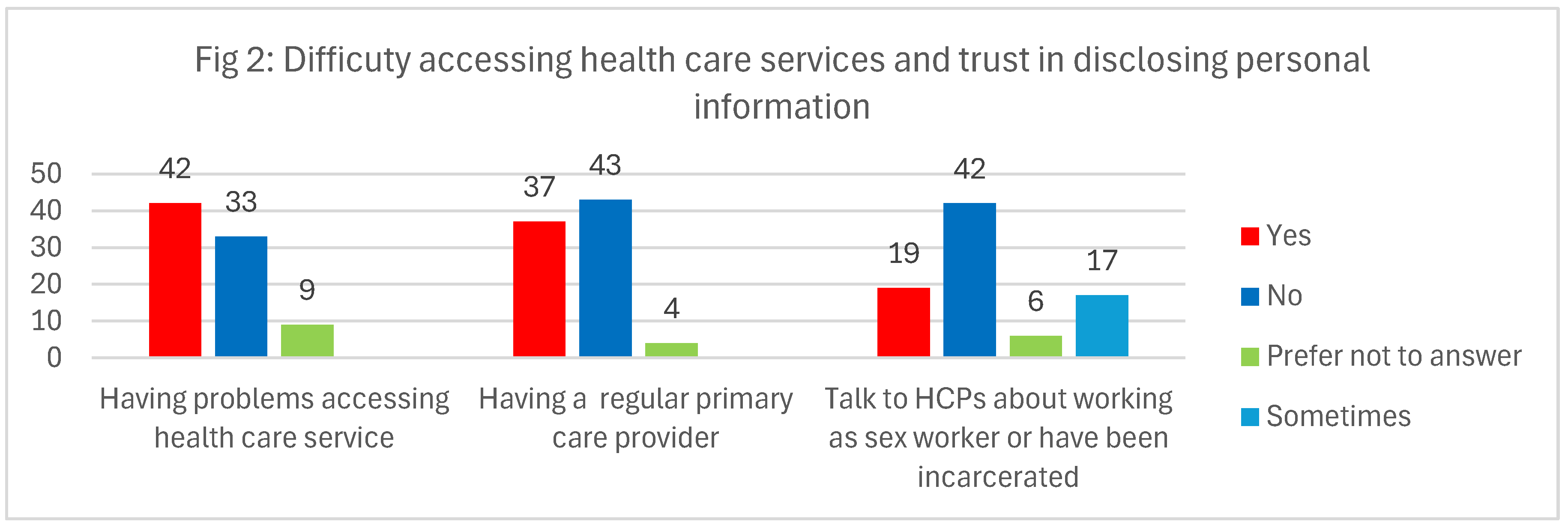
Half of the participants reported challenges in accessing healthcare services. Difficulties included long wait times to see family doctors, not having a regular family physician, lacking Ontario Health Insurance Plan (OHIP) coverage, and relying on walk-in clinics they believed were inadequately equipped to thoroughly address their health concerns or conduct follow-up tests. Participants also faced judgmental attitudes from healthcare providers and long delays in accessing mental health services. Many struggled to find healthcare providers who were sex-worker-friendly, 2SLGBTQ+ inclusive, and committed to anti-racist practices. Notably, about half of the participants did not have a consistent primary care provider and felt uncomfortable disclosing their sex work or criminal history to healthcare professionals (see
Figure 2).
It's noteworthy that individuals who did not report difficulty accessing healthcare services were often affiliated with community health organizations such as Hassle-Free Clinic, Women’s Health in Women’s Hands, the Black Coalition for AIDS Prevention (Black CAP), the Toronto People With AIDS Foundation (PWA), and Fairview Sexuality Clinics. Some emphasized their preference for these confidential community services, driven by concerns about potential prejudice related to their occupation. Additionally, others received care through Family Health Teams or maintained longstanding relationships with regular general practitioners. Interestingly, one participant shared that during her migration process, her immigration physician seamlessly referred her to a specialist, minimizing potential challenges. Furthermore, some participants reported maintaining good health and thus infrequently needing healthcare services. However, having access to the Ontario Health Insurance Plan (OHIP) facilitated their use of walk-in clinics when necessary.
4.3. Section C. Perceived Risk of Encountering Cervical Cancer
A quarter of participants (25%) perceived the risk of an average woman encountering cervical cancer as slim to nonexistent. However, a notable 43% expressed concerns about their own susceptibility to cervical cancer in the future. Additionally, a staggering 80% believed that their lives would be significantly impacted if they were to face a diagnosis of cervical cancer.
4.4. Section D. Knowledge of Cervical Cancer and Screening
4.4.1. Cervical Cancer
The participants' overall performance on the cervical cancer knowledge assessment, comprising 8 items, varied widely, with scores ranging from 0 to 8. The Cronbach's alpha reliability coefficient for our set of 8 items was calculated to be 0.635, indicating an acceptable level of internal consistency and reliability. On average, participants attained a mean score of 4.3, with a standard deviation of 2.1. The median and mode scores were both 5. Analysis of the data revealed that the distribution of knowledge levels among participants was as follows: 48% exhibited a low level of knowledge (total score of 0-4); 37% demonstrated a moderate level of knowledge (total score of 5-6); and 16% displayed a high level of knowledge (total score of 7-8).
4.4.2. Pap-Test
The participants' overall performance regarding knowledge of the Pap test as a method for cervical cancer screening, assessed through an 8-item scale, exhibited significant variability, with total scores spanning from 0 to 8. The Cronbach's alpha reliability coefficient was calculated to be 0.641, indicating an acceptable level of internal consistency and reliability. On average, participants achieved a mean score of 4.7, with a standard deviation of 1.6. The median and mode scores were 5 and 6, respectively. Analysis of the data revealed a distribution of knowledge levels among participants as follows: 43% demonstrated a low level of knowledge (total score of 0-4); 50% displayed a moderate level of knowledge (total score of 5-6); and 7% exhibited a high level of knowledge (total score of 7-8).
The findings indicated a notable gap in participants' awareness regarding the recommended screening guidelines: 43% were unaware of the recommended starting age for Pap test screening, while 73% were unaware of the recommended age for discontinuing screenings. Additionally, about one-third of participants (32%) were unaware of the recommended frequency of Pap tests (every 3 years). Furthermore, a quarter of the participants incorrectly believed that Pap tests were only recommended for older women, and 43% erroneously thought that Pap tests could lead to cervical cancer infection.
4.4.3. HPV Screening
Although HPV screening is not yet included in Ontario's cervical cancer screening guidelines and is not covered by OHIP, it is still available for those who are willing to pay out of pocket. The participants' overall performance in understanding the HPV test as a method for cervical cancer screening, assessed via an 8-item scale, showed considerable diversity, with total scores ranging from 0 to 8. The Cronbach's alpha reliability coefficient for our set of 8 items was calculated to be 0.625, indicating an acceptable level of internal consistency and reliability. On average, participants achieved a mean and median score of 4.5, with a standard deviation of approximately 2. The mode score was 4.0. Analysis of the data revealed a distribution of knowledge levels among participants as follows: 50% demonstrated a low level of knowledge, (total score of 0-4); 34.5% displayed a moderate level of knowledge (total score of 5-6); and 15.5% exhibited a high level of knowledge (total score of 7-8).
The findings underscored a notable gap in participants' awareness regarding HPV testing for cervical cancer screening: 47% were unaware that individuals who have received the HPV vaccine still need to undergo HPV testing, while 79% were unaware that a positive HPV test result does not equate to an abnormal Pap test result. Additionally, approximately one-third of participants (34.5%) were unaware that the HPV test sample can be self-collected by them using the HPV self-sampling kit. Furthermore, more than two-thirds of the participants incorrectly believed that the HPV test can determine how long a woman has had HPV, and 43% erroneously thought that a positive HPV test does not mean an increased risk of developing cervical cancer.
4.5. Section E. Perceived Stigma During Medical Encounters or Other Social Interactions
Table 5 displays 5-items Likert Scale responses ranging from Strongly disagree to strongly agree from the study participants regarding self-reported stigma experienced during medical encounters or other social interactions. The Cronbach's alpha reliability coefficient for our set of 10 items was calculated to be 0.85, indicating a high level of internal consistency and reliability.
The mean scores for items 4-5 and 8-10 exceed the weighted average, indicating that a considerable portion of participants reported internalized stigma and foresee enacting stigma from society, including their family and friends, due to their involvement in sex work or having a criminal record. Importantly, approximately a third of participants either agreed or strongly agreed that they had faced enacted stigma, such as being denied access to health services. Additionally, more than a third reported experiencing verbal harassment while receiving health or social care as well encountering judgmental attitudes from healthcare providers, characterized by negative remarks or gossip about them.
4.6. Section F: HPV-SS Uptake and Perceived Motivators and Barriers
4.6.1. HPV-SS Uptake
Initially, among the 84 participants enrolled in the study, 77showed interest in using the HPV-SS kit, while 7 declined. Of the 77 participants who opted to HPV-SS, 3 failed to attend their pre-scheduled testing appointments. Ultimately, among the 74 participants who successfully completed the test, 22 (29.7%) tested positive for HPV. Furthermore, 18% had high-risk HPV types 16/18.
4.6.2. Perceived Motivators for Utilizing HPV Self-Sampling
The aspects of HPV-SS that resonated with participants and were captured in respondents responses to open-ended questions on the survey questionnaire included its enabling feature for women and individuals with cervix to perform the sampling autonomously, without the presence and judgemental attitudes of health care providers. The accessibility of self-testing was highlighted as a boon, particularly for individuals with limited healthcare access or those who felt unprotected by their providers, providing them with a safe space and greater control. For those without regular health care providers, self-testing offered a convenient alternative, allowing them to take charge of their health and seek treatment as necessary.
Privacy and dignity were significant factors, with some feeling uncomfortable discussing sensitive topics with healthcare providers particularly when they had to disclose working as sex workers. Furthermore, traumatic or stressful experiences with traditional Pap tests led some to hope that the DIY (Do it yourself) approach would be more comfortable and less judgmental, enabling them to listen to their bodies without pressure. Moreover, some participants highlighted that HPV-SS facilitates screening for individuals with a history of sexual or medical abuse, as well as those experiencing body dysphoria. The following quotes vividly illustrate the appeal of HPV-SS from the perspective of women and individuals with a cervix:
“I love the idea of being able to do the test in a private space. My previous Pap test was traumatic and extremely stressful, so I hope the DIY [Do it Yourself]test will be more comfortable without a judgemental presence and the ability to take my time and listen to my body.” (sex worker, Age26)
“As a sex worker, there are several aspects of HPV test kits that I may find appealing: HPV test kits offer the convenience of testing for the virus in the privacy of my own home, without the need to visit a healthcare provider or clinic… Using an HPV test kit allows me to take control of my sexual health and make informed decisions about prevention and treatment. It empowers me to proactively monitor my HPV status and seek appropriate healthcare interventions if needed. … Overall, the convenience, control, confidentiality, accessibility, and peace of mind provided by HPV test kits are likely to be key reasons why women, including sex workers like me, may appreciate and benefit from using them as part of their sexual health.” (sex worker, Age 51)
“As a trans man, I think it's more accessible to do the kit on your own especially if you're body dysphoric and/or have history of sexual trauma.” (sex worker, Age 28)
“Able to be in control of the process and not have another touching my body. And being able to seek care for a diagnosis as opposed to having well-to-do doctors trying to impose their values or expectations upon you during their diagnosis/care process.” (sex worker, Age 34)
“It feels less invasive than having a healthcare practitioner do it. I am not against having a worker do it in and of itself but I do not want to risk being paired with one who will judge me.” (sex worker, Age 25)
4.6.3. Potential Barriers in Using HPV-SS
In addition to confidence in conducting the test correctly and cost considerations, participants identified a few other challenges which can contribute to underutilization of HPV-SS. These included a lack of awareness about the risks associated with HPV infection and the importance of screening, accessibility issues for nonbinary individuals like trans men due to the misconception that they do not require screening. privacy concerns about self-administering a test for a sensitive issue like HPV while residing in crowded households, fear of further being judged, blamed and stigmatized as carriers and transmitters of HPV. Additionally, follow-up care, counseling, and treatment after receiving a positive HPV result were considered particularly difficult for those without a regular primary care provider. Lastly, lack of emotional and psychological support while waiting for the test results was highlighted.
4.7. Section G: Reasons for Not Opting for HPV-SS
The main reason for the seven participants who declined to use HPV-SS were as follows: Some expressed preference for healthcare professionals (HCPs) to perform the test due to their expertise and skills, some felt unprepared or uncomfortable to conduct it themselves. Others mentioned they would consider self-sampling only if they received thorough explanations and instructions.
5. Discussion
Our study aimed to assess the feasibility of HPV-SS in increasing cervical cancer screening uptake among under-screened and never-screened sex workers, as well as individuals with a history of incarceration. We found that 88% of participants opted to use HPV-SS, with one-third harbouring high-risk HPV types. Our findings align with previous literature about the feasibility of this screening method for women and individuals with a cervix who lack access to regular cervical cancer screening due to systemic barriers [
28,
29,
30,
31,
33,
34]. For participants in our study who had avoided Pap tests due to fear of invasive procedures or had not undergone one for years because of a lack of a regular primary care provider, discomfort, or discriminatory attitudes in healthcare settings, self-sampling offered a less anxiety-inducing and more accessible alternative for cancer screening. Many participants felt that self-testing empowered them to take control of their sexual health, particularly those with a history of sexual or medical trauma, as it offered a private and anonymous option.
Moreover, we found that among those who tested positive, about a quarter of them carried high-risk HPV types 16/18, which are responsible for 74% of invasive cervical cancer cases among Canadian women [
41]. An estimated 6.2% of Canadian women are believed to harbor cervical HPV types 16/18 at any given time [
41]. Our findings suggest that this rate is nearly three times higher in our study population. The elevated prevalence of high-risk HPV types in the study population may be linked to factors such as multiple sexual partners, exposure to sexual violence, unstable living conditions, and a criminalized working environment, stigma within healthcare settings can further limit access to life-saving reproductive health services [
2,
3,
4,
5,
6,
7,
9,
10,
11,
12,
13,
14,
15]. This highlights the critical need for alternative screening methods, such as HPV-SS, particularly for structurally marginalized individuals with a cervix who face discrimination in accessing life-saving reproductive health services. The fact that nearly half of our participants lacked a regular primary care provider—delaying early detection of cervical cancer and access to medical treatments that could safeguard their reproductive health—further underscores the importance of HPV-SS in improving access to these essential services. Additionally, the discriminatory attitudes of healthcare providers pose a significant barrier for marginalized groups attempting to access available healthcare resources. It is therefore vital to educate healthcare providers and foster safe, inclusive environments that support sex workers and gender-diverse individuals. These efforts can reduce stigma and ensure that all individuals feel comfortable accessing essential sexual and reproductive health care.
Despite many participants having a high level of education, nearly half demonstrated limited knowledge about cervical cancer and screening. For example, approximately one-third of participants were unaware of the recommended frequency for Pap tests, which is every three years. Additionally, nearly half (43%) believed that Pap tests could cause cervical infection. These findings underscore the urgent need for co-creating visual, accessible, and art-based reproductive health education tailored to the specific learning needs of sex workers \ and delivered in formats (e.g., infographics, graphic novels, plain language fact sheets with art-work) and spaces that are easily accessible to them. This approach not only overcomes literacy barriers, which is crucial for sex workers with varying levels of formal education, but also provides Inclusive and community-centred way to learn [
42]. Such a format can be particularly important for those who may feel uncomfortable or unsafe in traditional healthcare settings.
It is noteworthy that approximately one-third of participants identified their sex work as “survival sex work,” which refers to engaging in sex work to meet basic human needs such as shelter, food, and clothing. This highlights the urgent need for adequate community resources and support services that address these fundamental needs, including access to housing, food security, and healthcare. Furthermore, additional research and advocacy are necessary to develop targeted interventions that can effectively address the social, economic and health disparities experience by this systematically marginalized population.
We found that over 60% of participants did not use protection during sexual intercourse, which further increases their risk of exposure to HPV and other STIs, including HIV. Recognizing that sex workers frequently have limited power or ability to require clients to use condoms, it is crucial to provide free access to female condoms, dental dams, and lubricants as protective barriers against STIs. In Ontario, these items can be accessed at no cost or at a reduced cost through harm reduction programs and services offered by community organizations, public health units, and sexual health clinics. Considering the Ontario government's move towards closing certain harm reduction services [
43], the potential ramifications for underserved populations could be devastating, further widening the health inequity gap. However, our study did not investigate the reasons behind participants' inconsistent use of protection during sexual intercourse or their awareness of existing harm reduction services, highlighting the need for further research in this area.
Our study also found that limited or inadequate access to mental health resources was a significant concern, particularly among equity-deserving groups with a history of sexual abuse and violence. Additionally, some participants highlighted the anxiety and stress associated with undergoing testing and receiving a diagnosis. It is imperative to ensure access to psychological and emotional support services both before and after cervical cancer screening for these groups.
While this study contributes to the existing literature on the subject, several limitations should be noted when interpreting the results. First, the small sample size and the absence of probability sampling may hinder the generalizability of the findings to the broader population of sex workers and individuals with a history of incarceration. Although not ideal, the use of nonprobability sampling was necessary due to the lack of a sampling frame for our target population. Second, the online design of our study may have restricted access for many potential and eligible participants who lacked access to computers or the internet. Although we provided the option for participants to use a designated computer at our collaborating agencies, this may still have discouraged some individuals from participating. Lastly, it is important to note that the study excluded immigrants and refugees who could not read, write, or converse in English. This limitation highlights the need for future research to include a more diverse population to better understand the experiences and barriers faced by all individuals in accessing cervical cancer screening.
6. Conclusion
Our study contributes to the growing literature on cervical cancer (CC) screening barriers among sex workers and individuals with a history of incarceration, highlighting the feasibility of HPV HPV-SS as an effective method to increase cervical cancer screening uptake. With 88% of participants opting for HPV-SS and one-third testing positive for high-risk HPV types, our findings underscore the importance of addressing the systemic barriers that prevent marginalized populations from accessing essential health services. HPV-SS not only serves as a viable screening approach to dismantle longstanding structural barriers but also empowers individuals to take control of their sexual health, providing a means to access screening without exposure to discriminatory attitudes in healthcare settings.
Author Contributions
MV took primary responsibility for the design of the study, analysis of survey, interpretation of data, drafting and revising the article. M.M, A.A., and N.K assisted with data collection. JW, JH., AL, NK., MM., and AA reviewed, revised the article critically for important intellectual content, and endorsed the final submission.
Funding
Financial support for this study was provided by Shoppers Foundation for Women’s Health-LOVE YOU by Shoppers Drug Mart™ Community Grants Program. The funding agreement ensured the author’s independence in designing the study, interpreting the data, writing, and publishing the report.
Data Availability
data supporting reported results can be found. All supporting data have been reported in the result section of the paper.
Acknowledgments
We would like to express our heartfelt thanks to the following individuals and organizations who played a crucial role in the successful completion of this study. First, we are deeply grateful to the study participants for finding time in their busy schedules to participate. We also extend our sincere thanks to our collaborating partners, Janet Rowe from PASAN and Jenna Hynes from Maggie’s Toronto, for their invaluable assistance in coordinating the study and supporting the training of community champions, data collection, and follow-up medical care. Our appreciation goes to our dedicated community champions, Nina Matijacic and Susan Shumba, for their efforts in recruiting participants and facilitating focus groups. Finally, we are thankful to Roche Diagnostics - Division Hoffmann-LaRoche Ltd. (Laval, Canada) for providing the HPV self-sampling swabs and reagents, and to the Mount Sinai Microbiology Lab, especially Dr. Tony Mazzulli and Jessica Bourke for coordinating the processing of the test results and Analiza Aquino for processing and analyzing the results.
Competing interests
The author(s) declared no actual or potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Abbreviations
| CC: Cervical cancer |
| Greater Toronto Area: GTA |
| HPV: Human papillomavirus |
| HPV-SS: Human papillomavirus Self-Sampling |
| Pap test: Papanicolaou test |
| RA: Research Associate |
| STIs: Sexually transmissible/transmitted infections |
| UNS: Under or never screened |
References
- Canadian Cancer Society - The annual report – Canadian Cancer Statistics 2023. p. 20. https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2023-statistics/2023_pdf_en.pdf?rev=7e0c86ef787d425081008ed22377754d&hash=DBD6818195657364D831AF0641C4B45C&_gl=1*120rhkl*_gcl_au*ODg5OTY4NDgyLjE3MTMyMTQ2NTAC.
- Canadian Cancer Society, Risk factors for Cervical Cancer. https://cancer.ca/en/cancer-information/cancer-types/cervical/risks.
- Canadian Cancer Society, Cervical Cancer Statistics. https://cancer.ca/en/cancer-information/cancer-types/cervical/statistics.
- Cervical Screening: Cancer Care Ontario; https://www.cancercareontario.ca/en/types-of-cancer/cervical/screening.
- Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecologic oncology reports. 2017 Aug 1;21:101-8. [CrossRef]
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. The Lancet. 2019 Jan 12;393(10167):169-82.
- Roura E, Castellsague X, Pawlita M, Travier N, Waterboer T, Margall N, Bosch FX, De Sanjosé S, Dillner J, Gram IT, Tjønneland A. Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. International journal of cancer. 2014 Jul 15;135(2):453-66. [CrossRef]
- Brenner DR, Gillis JL, Demers A, Ellison LF, Billette JM, Zhang SX, Liu J, Woods, RR, Finley C, Fitzgerald N, Saint-Jacques N, Shack L, Turner D, for the Canadian Cancer Statistics Advisory Committee. Projected estimates of cancer in Canada in 2024. CMAJ 2024 May 13;196:E615-23. [CrossRef]
- Vahabi, M., Lofters, A, Kim, E., Wong, J., Ellison,L, Graves, E, Glazier R.H. (2017).Breast cancer screening among women from Muslim-majority countries in Ontario, Canada. Preventive Medicine, 105:176-183. Published online September 13, 2017. [CrossRef]
- Vahabi, M., Lofters, A. (2018). HPV self-sampling: A promising approach to reduce cervical cancer screening disparities in Canada, Current Oncology, Special issue (HPV self-sampling in promoting cervical cancer screening), 25 (1):13-18, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5832271/. http://doi.org/10.3747/co.25.3845.
- Lofters, A., Kopp, A., Vahabi, M., Glazier R.H. (2019) Understanding Those Overdue For Cancer Screening By Five Years Or More: A Retrospective Cohort Study In Ontario, Canada. Preventive Medicine. 26(10):1493-1499. [CrossRef]
- Wong, J. Vahabi, M., Miholjcic, J., Tan, V., Owino, M., Li, A., Poon, M. (2018). Knowledge of HPV/cervical cancer and acceptability of HPV self-sampling among women living with HIV: A scoping review. Current Oncology, Special issue (HPV self-sampling in promoting cervical cancer screening), 25 (1): e73-e82. [CrossRef]
- Lofters, A., Vahabi, M., Kim, E., Ellison, L, Graves, E, Glazier R.H. (2017) Cervical cancer screening among women from Muslim majority countries in Ontario, Canada. Cancer Epidemiology, Biomarkers & Prevention, 26(10):1493-9. [CrossRef]
- McDonald, J.T., Kennedy, S. (2007). Cervical cancer screening by immigrant and minority women in Canada. Journal of Immigrant Minority Health, 9: 323-334. [CrossRef]
- Ross, L. E., Sterling, A., Dobinson, C., Logie, C. H., & D'Souza, S. (2021). Access to sexual and reproductive health care among young adult sex workers in Toronto, Ontario: a mixed-methods study. CMAJ Open, 9(2), E482–E490. [CrossRef]
- Duff, P., Ogilvie, G., Shoveller, J., Amram, O., Chettiar, J., Nguyen, P., Dobrer, S., Montaner, J., & Shannon, K. (2016). Barriers to Cervical Screening Among Sex Workers in Vancouver. American Journal of Public Health (1971), 106(2), 366–373. [CrossRef]
- Sex work in Canada: the public health perspective. Ottawa: Canadian Public Health Association; 2014. Available: www.cpha.ca/sites/default/files/assets/policy/sex-work_e.pdf.(accessed 2022 Dec. 15).
- UNAIDS welcomes the decision by the Northern Territory of Australia to decriminalize sex work [news release]. Geneva: UNAIDS; 2019. Available: www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2019/december/decision-northern-territory-australia-decriminalize-sex-work.(accessed 2022 Dec. 5).
- Lazarus, L., Deering, K. N., Nabess, R., Gibson, K., Tyndall, M. W., & Shannon, K. (2012). Occupational stigma as a primary barrier to health care for street-based sex workers in Canada. Culture, Health & Sexuality, 14(2), 139–150. [CrossRef]
- Benoit, C., Jansson, S. M., Smith, M., & Flagg, J. (2018). Prostitution Stigma and Its Effect on the Working Conditions, Personal Lives, and Health of Sex Workers. The Journal of Sex Research, 55(4-5), 457–471. [CrossRef]
- Saslaw, D., Solomon, D., Lawson, H.W., Killackey, M., Kulasingam, S.L., Cain, J. et al., (2012). American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American for Clinical Pathology Screening guidelines for the prevention and early detection of cervical cancer. American Journal of Pathology, 137(4): 516-542.
- Petignant, P., Vassilakos, P. (2012). Is it time to introduce HPV self-sampling for primary cervical cancer screening? Journal of National Cancer Institute, 104(3): 166-167.
- Agorastos, T., Sotiriadis, A., Chatzigeorgiou, K. (2010). Can HPV testing replace the Pap smear? Annals of the New York Academy of Sciences, 1205: 51-56.
- Bhatla, N., Dar, L., Patro, A.R., Kumar, P., Kriplani, A., Gulati, A, et Al., (2009). Can human papilliomavirus DNA testing of self-collected vaginal samples compares with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiology, 33(6): 446-450.
- Schiffman, M., Wentzensen, N., Wacholders, S., Kinney, W., Gage,J.C., Castle, P.E. (2011). Human papilliomavirus testing in the prevention of cervical cancer, Journal of National Cancer Institute, 103(5): 368-383.
- Wright, T.C., (2007). Cervical cancer screening in the 21st century: Is it time to retire the Pap smear? Clinical Obstetrics & Gynecology, 50(2): 313-323.
- Ogilvie, G., Krajden, M., Maginley, J., Isaac-Renton, J., Hislop,, G., Elwood-Martin, R., et al. (2007). Feasibility of self-collected of specimens for human papillomavirus testing in hard to reach women. Canadian Medical Association Journal, 177(5): 480-483. [CrossRef]
- Khoo, S. P., Lim, W. T., Rajasuriar, R., Nasir, N. H., Gravitt, P., & Woo, Y. L. (2021). The acceptability and preference of vaginal self-sampling for human papillomavirus (HPV) testing among a multi-ethnic Asian female population. Cancer Prevention Research, 14(1), 105-112. [CrossRef]
- Vahabi, M, Kithulegoda, N., Wong, J, Lofters, A.K., (2024) Knowledge and Attitudes towards cervical cancer screening and acceptability of HPV self-sampling (HPV-SS) among under or never screened racialized immigrant women in GTA, Ontario, Canada, Journal of Environmental Science and Public Health. 2024; 8(2), 32-48, . [CrossRef]
- Lofters A, Devotta K, Prakash V, Vahabi M. Understanding the Acceptability and Uptake of HPV Self-Sampling Amongst Women Under- or Never-Screened for Cervical Cancer in Toronto (Ontario, Canada): An Intervention Study Protocol. Int J Environ Res Public Health. 2021;18(17):9114. Published 2021 Aug 29. [CrossRef]
- Vahabi, M., Mishra, G., Pimple, S., Wong, J. P.H., Khan, M., Prakash, V., Anand, K., Narushima, M., Lofters, A.K. 2023, Effectiveness of family-centred sexual health education and HPV self-sampling in promoting cervical cancer screening among hard-to-reach Indian women in rural and tribal areas: a community-based pilot study. BMC Public Health 23, 671 (2023). [CrossRef]
- Nelson, E. J., Maynard, B. R., Loux, T., Fatla, J., Gordon, R., & Arnold, L. D. (2017). The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sexually transmitted infections, 93(1), 56-61. [CrossRef]
- Mitchelle, S., Ogilvie, G., Steinberg, M., Sekikubo, M., Biryabarema, C., Money, D. (2011). Assessing women’s willingness to collect their own cervical samples for HPV testing as part of ASPIRE cervical cancer screening project in Uganda. International Journal of Gynecology & Obstetrics, 114(2): 111-115. [CrossRef]
- Ogilvie G, Krajden M, Maginley J, Isaac-Renton J, Hislop G, Elwood-Martin R, Sherlock C, Taylor D, Rekart M. Feasibility of self-collection of specimens for human papillomavirus testing in hard-to-reach women. CMAJ. 2007 Aug 28;177(5):480-3. [CrossRef]
- Zhao, F.H., Lewkowitz, A.K., Chen, F., Lin, M.J., Hu, S.Y., Zhang, X., et al. (2012). Pooled analysis of a self-sampling HPV DNA test as a cervical cancer primary screening method, Journal of National Cancer Institute, 104(3): 178-188. [CrossRef]
- Virtanen, A., Nieminen, P., Lustarinen, T., Anttila, A. (2011). Self-sample HPV tests as an intervention for non-attendees of cervical cancer screening in Finland :A randomized trial, Cancer Epidemiology Biomarkers & Prevention, 20(9): 1960-1969.
- Ogilvie, G., Partrick, D.M., Schulzer, M., Sellors, J.W., Petric, M., Chambers, K., et al. (2005). Diagnostic accuracy of self-collected vaginal specimens for human papillomavirus testing compared to clinician collected human papillomavirus specimens: A meta-analysis. Sexually Transmitted Infection, 81 (3): 207-2012.
- Fernandes B, Dworkin S, Bunzeluk K, Turner D, Baldry L, Coulter L, et al. Elimination of cervical cancer in Canada: exploring invitation strategies for HPV self-sampling among under screened women [Poster Presentation]. ICSN 2023, Turin, Italy. 2023.
- Jalili F, O'Conaill C, Templeton K, Lotocki R, Fischer G, Manning L, Cormier K, Decker K. Assessing the impact of mailing self-sampling kits for human papillomavirus testing to unscreened non-responder women in Manitoba. Current Oncology. 2019 Jun;26(3):167-172. [CrossRef]
- Devotta, K., Vahabi, M., Prakash, V., Lofters, AK. 2023, Implementation of a Cervical Cancer Screening Intervention for Under- or Never-Screened Women in Ontario, Canada: Understanding the Acceptability of HPV Self-Sampling. Current Oncology. 30(7):6786-6804. [CrossRef]
- ICO/IARC Information Center on HPV and Cancer. Canada: Human Papillomavirus and related Cancer , Fact sheet 2023. https://hpvcentre.net/statistics/reports/CAN_FS.pdf.
- Kerrigan D, Kennedy CE, Morgan-Thomas R, Reza-Paul S, Mwangi P, Win KT, McFall A, Fonner VA, Butler J. A community empowerment approach to the HIV response among sex workers: effectiveness, challenges, and considerations for implementation and scale-up. Lancet. 2015 Jan 10;385(9963):172-85.
- Province to close 5 Toronto supervised drug consumption sites, CBC, https://www.cbc.ca/news/canada/toronto/toronto-supervised-injection-sites-ontario-restrictions-1.7299398.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).