Introduction
Over recent years, the term “Artificial Intelligence” (AI) has become a household word. It was first introduced in a workshop proposal in 1950, driven by neuro-robotic and brain mechanisms envisioned for tomography image analysis [
1]. In the 1980s, Fletcher and Doi at the University of Chicago suggested the potential applicability of AI/machine learning (AI/ML) in the medical field for the first time. They widened systematic medical image analysis by AI for computer-guided diagnosis and physician labor reduction [
2]. Over time, AI has remarkably evolved from theory to practicing machine learning, expert systems, machine logic, neural networks, and the paradigm shift from “Rule-Based Systems” to “Data-Driven approaches” enabled supervised learning AI [
3,
4,
5,
6]. Notable global investments have been in advancing this disruptive technology [
7]. The term “AI” is a diverse set of inquiries and development from various disciplines and conceptual strategies with no universally accepted definition, often seen as research that creates technologies capable of tasks requiring human-like intelligence [
8]. Several cognitive scientists, AI experts, and philosophers suggest that AI research can provide insights into the workings of the human mind [
9,
10]. Some, like Turing, argue that if an AI behaves indistinguishably from a human, its intelligence should be considered natural. Other key AI pioneers suggested alternative terms and definitions, such as “A thinking machine” [
11] and “A general problem solver” [
12].
AI is increasingly impacting all sectors of the economy, fueled by swift advancements in information processing and rising consumer expectations for competitive products and services. As AI is transforming the global economy, AI definitions have evolved to balance technical precision and accessibility. The Organization for Economic Co-operation and Development [
13] defines AI as ‘systems that can be regulated, certified, and put on the market, with a key focus on their disruptive economic potential’. The EU’s High-Level Expert Group on AI builds on this by highlighting the ability of AI to learn and adapt based on outcomes. The EU defines AI as ‘A system designed to operate autonomously, potentially showing adaptiveness post-deployment, by using machine or human-provided data to infer how to meet human-defined objectives through machine learning and logic approaches’ [
14]. A more user-friendly definition offered by UNICEF describes AI as ‘machine-based systems that make predictions, recommendations, or decisions to influence environments, often appearing autonomous while still relying on human-defined objectives’ [
15]. This definition is very close to the US FDA definition of FDA [
16]. This broader view accommodates data-driven, symbolic, and future AI paradigms, emphasizing the critical role of human oversight at every stage of AI-enabled development. As AI regulation progresses, debates over legal definitions and scope continue, reflecting the diverse techniques and technologies that comprise AI systems today. Regulatory agencies have issued papers addressing challenges in AI-driven therapeutic product manufacturing [
17,
18].
AI applications have been expanded into various domains and interdisciplinary fields, from computer sciences, languages, and statistical modeling to biology and healthcare, education, the pharmaceuticals industry, drug discovery and development, sales and marketing, business decision-making, finance, and beyond [
19]. AI’s application in pharmaceuticals began in earnest in the late 1990s when advancements in machine learning and deep learning showed promise in drug discovery. Initially, AI was used primarily for data analysis and identifying potential drug targets. However, as algorithms became more sophisticated, AI’s role expanded to include compound screening, molecular design, and clinical trial design [
20,
21,
22]. Pfizer and AstraZeneca are harnessing AI to accelerate drug discovery and improve patient outcomes. In collaboration with CytoReason, Pfizer is building a simulated immune system model to uncover new medicines and match treatments to patients more efficiently [
23]. Similarly, AstraZeneca’s partnership with BenevolentAI focuses on using AI to identify drug targets in immunology and cardiovascular research [
24]. Both collaborations highlight the need for AI-human partnerships, combining biology and data science expertise to reduce costs, speed up development, and create transformative therapies.
Although AI holds great promise for advancing drug and biological product development, avoiding overreliance on these technologies in this highly regulated field is essential. While the health authorities’ approach supports AI innovation, AI systems are not infallible. They can make mistakes, and challenges persist, including the need for representative datasets, concerns about bias, and issues with interpretability [
5,
25,
26]. Human oversight remains essential to ensure that AI-driven decisions are accurate and appropriate. The shift from rule-based to data-driven methodologies has profoundly transformed the AI landscape, unlocked new potential and steering ongoing research and development [
27].
A well-structured governance framework for generative AI is critical for ethical and responsible technology use. Such a framework should include clear internal standards and guidelines prioritizing accountability, transparency, and regulatory compliance. By adopting best practices, organizations can establish a robust governance structure that supports responsible AI development and deployment. There is an ongoing need to ensure that the potential risks associated with AI are assessed and mitigated. Therefore, the FDA and EMA guidelines could benefit from greater specificity and clarity on potential biases or errors in data collection and analysis, providing more practical frameworks for careful monitoring and validation [
14,
26,
27].
The US FDA has been proactive in establishing regulatory frameworks to guide the application of AI in healthcare and the pharmaceutical industry. To address emerging challenges, the FDA’s Center for Drug Evaluation and Research (CDER) launched the Center for Clinical Trial Innovation (C3TI) to foster innovative approaches in clinical trials and precision medicine. Despite these advancements, the FDA has faced criticism for delays updating clinical trial guidance, highlighting the need for more robust and timely regulation in this rapidly evolving cyberspace [
14].
Privacy is a particularly pressing concern. AI algorithms often rely on large datasets that may include sensitive patient information. This data could be compromised without proper safeguards, leading to significant privacy breaches. Ensuring data security and anonymization is crucial for protecting patient confidentiality. Furthermore, AI algorithms are only as good as the data on which they are trained. If the training data is biased, the AI system may perpetuate these biases, leading to unfair or discriminatory outcomes. To mitigate this risk, it is essential to use diverse and representative datasets and to regularly audit AI systems for bias [
28]. The use of AI in healthcare also raises critical ethical questions. For instance, should AI be used to make life-or-death decisions? How can we ensure that AI systems are deployed relatively and equitably? Addressing these ethical concerns requires thoughtful consideration and collaboration among scientists, policymakers, and ethicists.
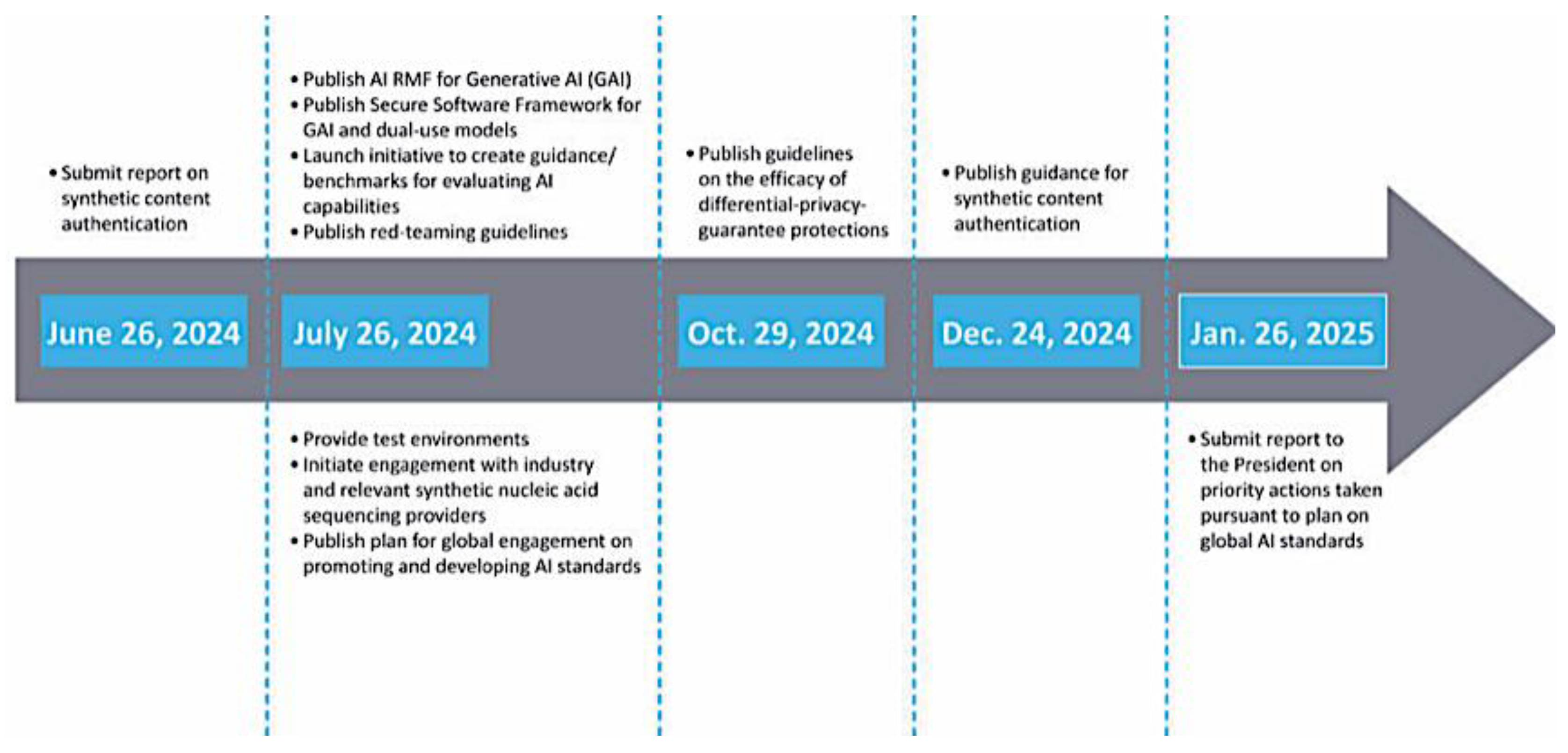
Navigating the complex ethical and regulatory landscape of AI and ML technologies in good manufacturing practices (GMP) and clinical practices (GCP) is indispensable for drug and biologics development organizations. Staying informed about evolving regulations, such as the EU’s AI Act and the US’s sector-based approach, is pivotal. Providing education and training to healthcare professionals and the public about AI’s benefits and risks is equally important. Ethical principles like fairness, transparency, and accountability are essential for responsible AI development. In August 2024, the FDA hosted a workshop on AI in drug and biological product development, co-organized with the Clinical Trials Transformation Initiative (CTTI). A group of regulatory and industry experts convened to underscore AI’s transformative potential in streamlining clinical trials, optimizing drug discovery, and improving patient outcomes [
29]. Key takeaways included the importance of transparency, data quality, management, and algorithmic fairness, though the workshop did not fully address ethical implications related to bias and privacy protection. Integrating AI across diverse clinical settings and the need for multidisciplinary collaboration also require further attention to practical instruction on implementation’ [
30,
31].
Here, we employ a comparative lens to overview the current regulatory landscape and a synopsis of the FDA workshop discussions on the use of AI in drug and biological product development. We discuss the promises, challenges, limitations, related practices adopted to overcome them, and our practical recommendation for regulatory oversight. Finally, the paper outlines a path forward and future opportunities.
The Broader Role and Applications of AI in Drug and Biologics Development: Lessons from the FDA Workshop and Industry
The pharmaceutical industry has long relied on trial-and-error methods for drug discovery, which, while sometimes effective, are often inefficient and costly. AI’s expanding portfolio of applications and methodologies is revolutionizing drug discovery and development, driving more efficient, precise, and cost-effective processes. Leading pharmaceutical companies have successfully integrated AI and ML across various stages of drug development, accelerating the identification of therapeutic targets, optimizing clinical trial design, and enhancing the drug pipeline. Below are critical real-world examples that highlight the impact of AI in drug discovery, followed by an exploration of the broader role AI plays in optimizing the drug development process. Moreover, AI’s ability to learn from existing data allows it to improve over time, making it an invaluable tool in the iterative drug development process. By continuously analyzing the outcomes of previous experiments, AI can refine its predictions, identifying more effective compounds with fewer side effects. This iterative learning process is a significant departure from traditional methods, where each new experiment often starts from scratch without the benefit of insights gained from previous failures [
35].
Several key pharmaceutical industry players have demonstrated AI’s applications in drug and Biologics discovery. Bristol-Myers Squibb, for instance, deployed an ML model to predict CYP450 enzyme inhibition, which is essential for drug metabolism. Their AI tool achieved 95% accuracy, reducing failure rates by sixfold compared to traditional methods [
22,
37]. This improvement accelerated the screening process and allowed researchers to focus on drug candidates with a higher likelihood of success during human trials and FDA approval. Merck and Bayer partnered with Cyclica to enhance drug candidate identification through AI. This collaboration led to discovery of a target protein linked to FDA-approved drugs for treating systemic scleroderma and the Ebola virus [
38]. The use of AI to repurpose existing drugs highlights the technology’s potential to expedite the drug development cycle. In another example, GlaxoSmithKline (GSK) worked with Exscientia to discover a novel molecule targeting a pathway involved in chronic obstructive pulmonary disease (COPD). This collaboration demonstrated AI’s ability to identify innovative therapeutic targets for complex diseases [
39].
Similarly, Exscientia and Evotec applied AI to discover a cancer treatment that targets the A2a receptor rapidly. Within just eight months, the drug candidate entered clinical trials in 2021, showcasing how AI can significantly reduce the discovery phase in drug development [
40]. Berg’s AI platform identified BPM31510 as a promising treatment for advanced pancreatic cancer, one of the most challenging diseases. The platform’s capability to predict patient responses and potential adverse effects underscores AI’s growing role in enabling personalized medicine [
41]. Rapid advancements in the use of AI in cancer research, diagnosis, and treatment have spanned a diverse range of indications. Numerous startups are leveraging AI to offer innovative approaches to transforming the field [
42]. MultipleAI, for example, provides a comprehensive whole blood screening test that utilizes RNA sequencing technology and AI to detect a wide range of complex diseases, including cardiovascular and cancer [
43]. Another notable achievement in AI-driven drug discovery came from BenevolentAI, which used its platform to identify baricitinib, initially developed by Eli Lilly for rheumatoid arthritis, as a potential treatment for COVID-19. The drug was subsequently approved for emergency use in the U.S. and Japan, illustrating how AI can rapidly repurpose existing drugs in response to global health crises [
43]. Lastly, in collaboration with Insilico Medicine, Taisho Pharmaceutical utilized AI to identify compounds targeting cellular aging. Insilico’s AI system helped discover drug-like molecules that target senescent cells, which play a role in aging-related diseases [
44]. This project underscores AI’s potential to pioneer new therapeutic areas, such as anti-aging therapeutics.
This rapid integration of AI and ML technologies into drug development has the potential to revolutionize the pharmaceutical industry. However, adopting these advanced tools is fraught with complex technical, regulatory, clinical, and ethical challenges. The recent FDA workshop provided a forum for leading experts across academia, industry, and regulatory bodies to discuss these challenges and propose actionable pathways forward [
45]. The workshop discussions focused on four major challenge areas that are critical to ensuring the safe, effective, and equitable deployment of AI/ML technologies in drug development:
Optimizing Model Design Through Multidisciplinary Expertise
A key theme of the workshop was the importance of integrating diverse expertise in designing AI/ML models. Developing AI solutions for drug development requires the collaboration of professionals from computational sciences, clinical research, regulatory affairs, and ethics to ensure that models are technically sound, clinically relevant, and compliant with regulatory standards. Several speakers emphasized that this multidisciplinary approach is vital to creating models that align with the realities of clinical practice and regulatory expectations [
30,
46].
Using the Data We Have, Creating the Data We Need: Clinical Development, Clinical Data Management, and Analysis
AI/ML technologies are poised to transform clinical trial design, patient recruitment, and data analysis, yet these advancements depend heavily on the integrity and management of clinical data. Workshop participants highlighted the need for rigorous data governance practices, including standardization, harmonization across regulatory frameworks, and fit-for-purpose approaches tailored to specific clinical applications. Effective clinical data management is essential to unlocking the full potential of AI-driven insights and ensuring that AI/ML models can be integrated into regulatory submissions [
47,
48]. The session addressed selection and algorithmic biases that can affect outcomes in clinical data analysis. And focused on ensuring clinical data integrity and model validation concerning FDA regulations on Good Clinical Practice (2021) [
49]. AI aids in target identification, dose optimization, and understanding pharmacokinetics and pharmacodynamics [
50]. Moreover, different datasets aggregate single-cell data from various research efforts, enabling in silico drug targeting and enhancing the precision of drug development and therapeutic targeting
[51,52]. These predictions depend significantly on the volume and quality of the data used.
Identifying Gaps, Addressing Challenges, and Charting the Path Forward
Despite the progress made in AI/ML regulatory frameworks, significant gaps remain, particularly in the harmonization of global policies and the continuous validation of AI models post-market. The workshop discussions called for a coordinated, international effort to develop more consistent and fit-for-purpose pathways for AI applications in drug development. Regular model monitoring, the integration of real-world data, and clear guidelines for risk-based approaches were identified as critical components for advancing AI’s potential while safeguarding patient safety.
Through these four challenge areas, the FDA workshop provided a comprehensive overview of the current state of AI/ML in drug development and identified key priorities for future research, policy development, and cross-sector collaboration. By addressing these challenges, the AI community can work toward a future where AI/ML technologies contribute meaningfully to faster, safer, and more personalized drug development. We will discuss some of these challenges in the following sections.
Data Integrity and Quality Challenges in AI-Driven Drug Development Governance Considerations: Practical Guidelines for AI Implementation
Data integrity and quality are critical factors in the success of AI-driven drug development. Data accuracy, consistency, and completeness are essential for training effective AI models and generating reliable results [
55]. Data integrity and quality challenges can arise from various sources, including data collection errors, biases, and inconsistencies across different datasets [
49,
57,
58,
59,
60]. Addressing the following data-related challenges is crucial for developing AI models that can provide meaningful insights and support informed decision-making throughout the drug development process:
One of the primary data-related challenges in leveraging AI for drug development is the absence of standardized sources for patient demographic data. This lack of standardization complicates efforts to set accurate enrollment goals for underrepresented groups in clinical trials, a crucial aspect of ensuring that trials are diverse and equitable. For AI applications, standardized data is essential for training models representative of the target populations. However, disparate and often incomplete data sources significantly hinder the integration and analysis processes required for effective AI-driven drug development. The resulting gaps in data availability can lead to models that fail to generalize across diverse groups, limiting their potential to drive genuinely inclusive innovations.
A related challenge is the insufficient biomarker data across demographic groups, further hampers the development of AI systems capable of predicting drug efficacy and safety for diverse populations. AI models rely on extensive and diverse biomarker data to accurately generalize their predictions across various population subsets. The current gap in biomarker data affects clinical endpoint assessments and impedes the generation of accurate individual case safety reports (ICSR). This lack of comprehensive biomarker data hampers the ability of AI systems to predict drug efficacy and safety for diverse populations accurately. AI models require extensive and diverse biomarker data to generalize across various groups and provide accurate insights on the adverse effects of drugs, as well as automated case submission and evaluation.
Another significant obstacle is the inconsistency in the definitions of race and ethnicity across different datasets. This lack of uniformity complicates data integration and analysis, which is particularly problematic for AI applications in drug development. Consistent and clear definitions of race and ethnicity are critical for ensuring that AI algorithms are trained on relevant and comparable data, which helps to avoid perpetuating existing biases. Inconsistent definitions increase the risk of reinforcing these biases in AI models, potentially leading to skewed outcomes that fail to address the needs of diverse populations. Moreover, the inadequate collection of social determinants of health (SDoH) data further limits the ability of AI models to account for the vast array of factors that influence drug responses and overall health outcomes. Social determinants of health, such as socioeconomic status, education, and access to healthcare, play a critical role in determining patient outcomes. Incorporating SDoH into AI models is essential for understanding the broader context of patient health and ensuring that drug development efforts are inclusive and effective across diverse populations. Unfortunately, this data is not routinely collected, presenting a substantial barrier to developing AI systems that reflect real-world complexities.
Additionally, the limited availability of robust data on populations outside the United States poses a significant challenge to the global applicability of AI models in drug development. For AI systems to drive innovation worldwide, they must be trained on diverse datasets that include international populations. Without such data, the findings produced by AI models risk being overly specific to U.S. healthcare systems. They may fail to generalize to other regions, limiting the broader utility of these models in international drug development efforts. Addressing these data challenges is critical for the effective implementation of AI in drug development. One proposed strategy involves creating a centralized repository for biomarker data, which would serve as a standardized source of nationally representative data for various disease areas. This repository could integrate race and ethnicity data, thereby enhancing AI models’ ability to generate inclusive and accurate insights. By consolidating existing data sources and developing integration standards, researchers can overcome inconsistencies and support the development of more robust AI models. This would provide a more comprehensive understanding of disease biology and facilitate the identification of promising drug targets.
Beyond these strategies, additional challenges must be addressed to optimize AI use in drug development. Data privacy and security are paramount, particularly given the sensitive nature of patient data used in training AI models. Ensuring compliance with data protection regulations requires the implementation of robust encryption, anonymization techniques, and transparent data governance policies. Furthermore, navigating the evolving regulatory landscape for AI applications remains a significant challenge. Proactive engagement with regulatory agencies and participation in industry forums are necessary to stay abreast of changes and ensure that AI innovations are aligned with regulatory expectations. Integrating AI tools into existing drug development processes and scaling them across different markets presents further obstacles. For AI to reach its full potential, solutions must be scalable and adaptable to various production environments, including diverse regulatory and healthcare systems. The FDA’s discussion paper on AI and ML in drug development underscores the agency’s commitment to a risk-based regulatory framework that fosters innovation while prioritizing patient safety. This framework spans the entire drug development lifecycle, covering drug discovery, non-clinical research, clinical research, post-market safety surveillance, and advanced manufacturing technologies (AMT).
Several key considerations are vital for successfully implementing AI/ML in drug development. First, AI systems must be developed and deployed with human-led governance, accountability, and transparency to ensure responsible and ethical use. Additionally, the quality, reliability, and representativeness of data used in AI models must be guaranteed to ensure that the models reflect the diverse populations they aim to serve. Lastly, rigorous model development, performance monitoring, and validation processes are essential to ensure that AI systems deliver accurate, reliable, and actionable insights throughout the drug development process [
49,
50,
51,
52,
53].
The successful integration of AI in these areas requires adherence to best practices in data management, model validation, transparency, risk assessment, management approaches, continuous monitoring, and strategic collaboration. By addressing these fundamental considerations, organizations can harness AI’s potential to accelerate drug discovery, improve patient outcomes, and drive innovation in the healthcare industry. As an example of successful AI tools in drug development, Cassandra- an ICON’s advanced AI prediction system- demonstrates how integrating real-world evidence with regulatory insights can streamline postmarking requirements, enhance regulatory approval, and facilitate a more efficient path to market [
61].
Access, Fairness, and Accountability: Lessons from Economic, Law, Ethics, and Politics:
Integrating AI into drug development and manufacturing offers transformative economic potential, reducing the cost and time to market for new therapies. AI algorithms can optimize drug formulation and predict clinical trial outcomes, cutting research and development costs. In manufacturing, AI-driven platforms enhance efficiency, improve quality control, and support real-time monitoring. These platforms, recognized by the FDA’s advanced manufacturing platform designation [
62], enable cost-effective, flexible production even for complex biologics [
22,
63]. However, AI/ML systems and algorithms used in drug development and manufacturing can resemble the economic profile of a “natural monopoly,” akin to railroads or telecommunications [
64,
65,
66]. The high fixed costs of AI development, low marginal deployment costs, and network effects allow companies controlling these platforms to dominate the market [
67,
68]. This economic structure poses regulatory challenges, requiring thoughtful oversight to prevent monopolistic pricing and ensure access [
68,
69,
70,
71].
AI/ML can be thought of as two distinct workflows: first, the design, development, and training of a model, and second, the deployment of that model to make decisions in real-world scenarios [
72]. For companies, there are no disadvantages to collecting unlimited data; in the United States, few restrictions prevent them from doing so. As AI becomes central to biopharmaceutical production, regulatory oversight, not competition, may be needed to maintain fair pricing and equitable access to balance monopolism in this data-intensive industry [
72]. Drawing on historical lessons from other monopolies, regulation can prevent practices like inflated pricing or restricted access. Moreover, it can address issues like data privacy, algorithmic bias, and prioritizing profit over patient care [
66,
69,
73]. We will discuss HIPAA and GDRP as examples of such a provision. Public oversight will ensure that AI’s benefits are distributed across the healthcare ecosystem, not concentrated in a few dominant entities [
74].
One primary concern with natural monopolies, including AI, is the potential for inefficiently high pricing. AI-driven drug development could result in exorbitant fees for access to platforms or data. Regulators can mitigate this by implementing rate regulation—similar to traditional approaches with natural monopolists—to protect consumers and limit data collection to what’s necessary for system improvement. This would address privacy concerns and prevent developers from extracting personal information in exchange for services, which could deter privacy-conscious users from benefiting from AI [
75]. Another challenge is the risk of underinvestment in areas like safety, security, and bias prevention. Monopolists in concentrated markets have less incentive to improve safety or address discrimination, which is especially dangerous in healthcare. Regulators could impose service-quality standards, requiring AI systems in drug development to meet accuracy, reliability, and fairness benchmarks, ensuring AI aligns with social goals like accountability and equity [
74]. AI monopolies can also lead to inefficiencies in competition, mainly through wasteful duplication of data collection and infrastructure investments. To promote competition without this waste, regulators could adopt franchise bidding or mandate data-sharing and federated learning, allowing multiple competitors to access shared datasets and infrastructure.
While AI has the potential to revolutionize drug development and lower therapeutic costs, its monopolistic tendencies demand robust regulation. Policymakers can ensure AI-driven innovations serve the public good without harming consumer welfare by addressing concerns over data privacy, underinvestment, and inefficient competition. AI technologies should primarily operate under a policy framework that encourages permissionless innovation while counseling against “command-and-control regulation,” enabling humanity to fully harness the opportunities and benefits they offer [
76,
77]. With appropriate oversight, the benefits of AI in FDA-recognized manufacturing platforms can be realized while preventing the downsides of unchecked monopolies.
Navigating the Future - GDPR Compliance and Harmonization in Clinical Trials
The regulatory landscape for generative AI is rapidly evolving, with different countries and regions adopting varying approaches. In the United States, the FDA has issued guidance on using AI in medical device development [
78], emphasizing the need for transparency, validation, and clinical evidence. The European Union has proposed the AI Act [
46], establishing a risk-based framework for regulating AI systems. The landscape of clinical trials is undergoing a profound transformation with the integration of AI. This technology promises to enhance drug development processes but also introduces complex challenges related to data protection, regulatory compliance, and harmonization across jurisdictions.
Several emerging regulations and policy initiatives are shaping the future of generative AI in drug and biologics development [
79]. These include addressing biases in AI algorithms to ensure equitable outcomes, protecting intellectual property rights related to AI-generated content, ensuring AI systems can provide understandable explanations for their decisions, and fostering collaboration between regulators, industry, and academia to develop effective governance and regulatory frameworks.
Ethical and Compliance Challenges on AI’s Expanding Role in Clinical Trials
AI is increasingly used to optimize various aspects of clinical trials, from study design to patient recruitment. By analyzing large datasets, AI can help researchers design more effective trials, predict outcomes, and refine protocols. In patient recruitment, AI’s ability to process electronic health records and other data sources streamlines the identification of eligible participants. Additionally, AI-powered digital health technologies (DHTs) facilitate continuous monitoring, making decentralized trials more feasible and inclusive.
Despite its potential, the integration of AI raises significant ethical and regulatory concerns, particularly around data privacy and security [
28]. Ensuring compliance with existing regulations is crucial to protect patient information and maintain trust in the research process.
Ethical and Legal Considerations of Privacy and Nondiscrimination
Fundamental ethical principles, including transparency, fairness, and accountability, must govern AI’s application in clinical trials. Compliance with regulations such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) is essential for protecting patient privacy [
31,
80]. GDPR, with its rigorous data protection standards, influences global AI governance and sets a benchmark for data privacy. Data privacy and security are paramount in clinical research. The collection and use of personal health information require strict adherence to regulations to mitigate legal risks and maintain participant trust [
81]. The executive order prioritizes individual privacy and non-discrimination in AI-enabled technology. It calls for comprehensive privacy legislation and emphasizes the need to mitigate biases and prevent discrimination in AI applications.
Regulatory and Compliance Framework for AI in Drug Development
The regulatory environment for using AI in drug development and clinical trials is rapidly evolving to address these technologies’ unique challenges and opportunities. In the European Union, the EU Artificial Intelligence Act classifies AI systems according to their risk levels and sets stringent requirements for high-risk applications [
82]. This framework emphasizes vital principles such as transparency, accountability, and data protection, which are essential for the ethical application of AI in healthcare settings.
The FDA’s discussion paper on AI and ML applications in drug development and clinical trials in the United States outlines a comprehensive approach to regulating these technologies. The FDA defines AI as a broad category encompassing algorithms and models capable of learning, decision-making, and prediction [
79]. ML, a subset of AI, creates models through data analysis. The FDA’s discussion paper articulates a commitment to a risk-based regulatory framework that supports innovation while prioritizing patient safety. It covers a wide array of drug development activities—from initial discovery through post-market surveillance—and explores how AI/ML can be integrated across various stages, including drug discovery, non-clinical and clinical research, post-marketing safety monitoring, and advanced manufacturing. While recognizing the potential benefits of AI and ML, the FDA also addresses the risks associated with these technologies, such as data biases and limited explainability of models. The discussion paper calls for clear guidance and standards for the diverse applications of AI throughout the drug development process. It identifies critical concerns such as data quality, model reliability, privacy, and transparency and emphasizes the need for stakeholder engagement to address these issues. The FDA’s approach includes fostering dialogue with industry, academia, and other stakeholders to develop guidelines that ensure AI’s ethical and practical use in drug development [
28,
83,
84].
The discussion paper also highlights the transformative potential of AI and ML to enhance various aspects of clinical trials, including participant selection, data management, and trial design [
83]. AI technologies can optimize participant selection by analyzing vast datasets to identify suitable candidates, thereby improving the diversity and representativeness of trial populations [
83]. This is crucial for addressing historical bottlenecks in patient recruitment and retention, which have often slowed the completion of clinical trials. Additionally, AI/ML can support innovative trial formats, such as decentralized clinical trials (DCTs), which utilize digital health technologies (DHTs) to facilitate more participant-centric approaches. These non-traditional trial designs can increase accessibility and reduce participant burden, leading to more efficient and inclusive trials [
85].
The FDA acknowledges the potential of AI/ML to improve trial design efficiency by using real-world data (RWD) and other data sources to predict effective study protocols and identify optimal participants. However, the FDA also emphasizes the need to maintain high standards for data quality and ensure robust oversight to mitigate bias and data reliability risks. The agency stresses the importance of ethical considerations, privacy protections, and transparency in developing and using AI technologies in clinical trials [
28,
82,
86]. AI technologies have the potential to revolutionize clinical trials by extracting valuable data from unstructured reports and automatically annotating images or lab results. AI can fill in missing data points through predictive modeling, allowing researchers to identify unique subgroups within a population that respond differently to treatments.
Additionally, AI techniques can extract critical information from clinical trial reports, including recovery outcomes, symptoms, side effects, and adverse incidents. This data extraction process can streamline the formatting of eligibility requirements from trial descriptions into structured tables, optimize site selection, refine eligibility criteria, and predict trial outcomes. Tasks traditionally requiring a team of data scientists, such as data analysis or visualization coding, can now be automated with AI, significantly enhancing efficiency and reducing costs [
28].
Integrating AI into healthcare brings significant benefits but poses serious privacy concerns. The rise of large language models further expands the possibilities for AI in clinical trials. These models can support healthcare professionals in various capacities, such as early disease detection, medical image interpretation, drug discovery, treatment recommendations, and remote patient monitoring. By automating repetitive tasks and providing decision support, AI can enable more accurate and timely diagnoses, ultimately improving patient care and optimizing healthcare resource allocation. This technological advancement facilitates a more personalized approach to medicine, where treatments can be tailored to the specific needs of patients, enhancing both efficacy and safety. As commercial entities gain increased access to patient health data, there is a growing risk of misuse or insufficient protection of this sensitive information. The “black box” nature of many AI algorithms complicates oversight, as their decision-making processes can be opaque [
87]. Regulations must emphasize patient agency to address these challenges, including recurrent informed consent and the right to withdraw data. Additionally, innovative data protection measures and strict jurisdictional controls are needed to safeguard privacy in an evolving technological landscape. Without robust oversight, we risk falling behind the rapid pace of AI development.
The FDA has approved AI/ML applications in clinical care, such as software to detect diabetic retinopathy from diagnostic images. This approval marks a significant step towards integrating AI into everyday medical practices. Despite the promising capabilities of AI in healthcare, many technological advancements still originate in academic research settings. Partnerships with commercial entities are often necessary to bring these innovations from the lab to real-world application. These collaborations can help scale AI technologies and ensure they are accessible and beneficial to the broader healthcare community [
81,
88].
The European Medicines Agency (EMA) has also been proactive in this area, issuing a draft reflection paper on the application of AI and ML in developing, regulating, and using human and veterinary medicines. This document, part of the HMA-EMA joint Big Data Steering Group’s Workplan 2022-2025, explores the role of AI across the entire lifecycle of medicinal products—from drug discovery to post-authorization. The reflection paper outlines various AI applications, such as replacing animal models in preclinical development, optimizing patient selection in clinical trials, and enhancing post-authorization pharmacovigilance activities. However, it also highlights challenges like understanding algorithmic design, managing biases, and mitigating risks of technical failures. The EMA advocates for a human-centric approach to AI development and deployment, emphasizing compliance with existing legal requirements, ethical standards, and fundamental rights. Developers are encouraged to seek early regulatory support, particularly if AI/ML systems could impact the benefit-risk balance of a medicine.
Overall, both the FDA and EMA recognize the need for a balanced approach that fosters innovation in AI applications while ensuring patient safety, ethical use, and regulatory compliance. As AI technologies evolve, these regulatory bodies will likely continue to refine their frameworks and guidelines to address emerging challenges and ensure the responsible use of AI in drug development and clinical trials.
A Global Regulatory Landscape: EU vs. US and Industry Initiatives
AI represents an emerging and transformative force in global healthcare, yet it still operates in a landscape lacking a unified international legal and regulatory framework. The Global Initiative on Ethics of Autonomous and Intelligent Systems [
89] aims to establish a comprehensive set of standards and principles designed to ensure that autonomous and intelligent systems are developed in a secure, ethical, and beneficial manner to society. This initiative also seeks to engage the public in formulating ethical frameworks, thereby enhancing societal understanding and awareness of the ethical issues associated with these technologies. By fostering public participation, the initiative encourages a more inclusive approach to addressing autonomous and intelligent systems’ moral, social, and economic implications, ensuring that these technologies serve the common good and reflect diverse societal values and perspectives [
90].
While AI offers immense potential, its rapid development has raised concerns about its ethical and societal implications. As a result, governments worldwide are grappling with how to regulate this powerful technology. The European Union (EU) and the United States (US) have taken distinct approaches, each with strengths and weaknesses. EU law could guide the WHO in reforming the International Health Regulations (IHR). The EU has adopted a risk-based approach to AI regulation, embodied in the AI Act. This framework categorizes AI systems based on their potential risks to individuals and society. High-risk AI systems, such as those used in critical infrastructure or law enforcement, face stricter requirements, including mandatory human oversight and transparency. In contrast, the US has taken a more sector-based approach, relying on existing regulations in areas like healthcare, finance, and transportation to address AI-related concerns. This approach offers flexibility but can also lead to fragmentation and inconsistencies.
Beyond government regulation, individual companies and industry associations have also taken steps to address AI-related risks. Microsoft, for example, has developed its own internal AI principles to guide its research and development. However, there is a growing recognition that broader, industry-wide initiatives are needed to establish common guidelines and principles. Organizations should develop internal standards and guidelines aligning with ethical principles and regulatory requirements. These standards should cover various AI development and use aspects, including data governance, risk assessment, and transparency. By establishing clear internal rules, organizations can ensure that their AI activities are conducted ethically and responsibly.
The EU’s AI Act establishes a robust enforcement mechanism, with significant fines for non-compliance. While lacking a centralized regulatory body, the US relies on a combination of federal agencies and industry self-regulation to oversee AI development and deployment.
The rapid pace of AI development and its global reach necessitate international cooperation. While the EU and US have taken different approaches, a growing consensus exists on the need for global standards and best practices. International organizations like the Organization for Economic Co-operation and Development [
91] and the Group of Seven (G7) are promoting responsible AI development and addressing shared challenges. While the EU and US have adopted distinct approaches, both recognize the importance of balancing innovation with ethical considerations. As AI continues to advance, it will be crucial for governments, industry, and civil society to work together to ensure that this powerful technology is developed and used responsibly for all benefit.
Comparative Perspective on AI in Clinical Manufacturing and Commercialization
When comparing the FDA’s guidance on the use of AI in clinical trials with that of the European Medicines Agency (EMA), several key differences and similarities emerge, reflecting their respective regulatory philosophies and priorities [
13,
83]
.
The FDA has taken a proactive approach in addressing the integration of AI in clinical trials, recognizing the potential of AI to enhance drug development and patient outcomes. However, the agency’s guidance has often been criticized for being broad and high-level, focusing more on outlining expectations and concerns rather than offering specific, practical instructions for implementation. While encouraging innovation, this approach has left some industry stakeholders seeking more concrete direction on complying with regulatory standards when utilizing AI in clinical research. The FDA has also emphasized the importance of data quality, transparency, and the need for AI systems to be interpretable, ensuring that the technology’s use does not compromise patient safety or trial integrity.
On the other hand, the EMA has been somewhat more cautious in its approach to AI in clinical trials. The EMA’s guidance tends to be more detailed and prescriptive, ensuring that AI applications in clinical trials adhere to stringent data protection and ethical standards, particularly in the context of the General Data Protection Regulation (GDPR). The EMA has emphasized the importance of maintaining patient data privacy, informed consent, and the ethical use of AI, with a strong focus on ensuring that AI systems do not introduce bias or compromise the validity of clinical trial results. The EMA also emphasizes the need for transparency and explainability of AI algorithms, ensuring that all stakeholders, including regulators, can understand how AI-driven decisions are made.
The FDA’s guidance is generally more high-level and flexible, allowing for a broader interpretation of how AI can be integrated into clinical trials. In contrast, the EMA’s guidance is more detailed, focusing more on specific regulatory compliance, particularly regarding data protection under GDPR.
The FDA and EMA prioritize transparency and explainability in AI systems, but the EMA places a heavier emphasis on these aspects, especially regarding ethical considerations and patient safety. The opaque nature of many AI models, particularly those based on deep learning, makes it challenging for regulators to assess and verify the decision-making processes. The EMA has stated that transparent AI systems should be preferred, emphasizing the need for companies to invest in explainable AI technologies and maintain detailed documentation of AI decision-making processes.
Regarding data integrity and privacy, the EMA’s guidance is more stringent due to GDPR requirements, while the FDA has a slightly different focus despite similar concerns. Both agencies recognize the importance of data reliability and accuracy, and manufacturers should establish procedures for data collection and selection. Companies should consider improving traditional tools like encryption, access controls, and audit trails to ensure data integrity and handle more extensive and complex datasets. Investing in advanced cybersecurity measures and establishing rigorous data governance protocols is also essential.
Manufacturers must validate critical aspects of their operations through product and process lifecycle validation. AI/ML-specific features, such as continuous learning, pose challenges as AI systems evolve based on new data. We anticipate regulators will update their frameworks to encompass continuous monitoring and revalidation protocols for AI systems. Companies should implement robust change control systems to manage updates to AI algorithms and consider developing validation protocols that define objectives, scope, and acceptance criteria. These may include performance testing, comparison against reference methods, evaluation of algorithm robustness, and maintaining detailed records of algorithmic changes and performance metrics.
Using AI brings new and unknown risks, such as unfair or unreliable results due to untested algorithms. Manufacturers should implement thorough risk assessment procedures, adopt specific controls, enhance cybersecurity measures, and establish contingency plans for system failures.
While both the FDA and EMA recognize the transformative potential of AI in clinical trials, their approaches differ in terms of the level of detail, focus on data privacy, and the provision of practical guidance. The FDA’s more flexible, high-level guidance contrasts with the EMA’s detailed, prescriptive approach, reflecting the U.S. and Europe’s different regulatory landscapes and priorities. Understanding these differences is crucial for companies operating in both regions to navigate the regulatory environment and successfully integrate AI into clinical trials.
WHO Guidelines and Perspectives on AI
The WHO has released multilateral reports in 2021 and 2023 emphasizing the need for ethical and responsible use of AI in healthcare. These reports advocate for AI systems that uphold human dignity, equity, fairness, and accountability while highlighting significant legal and ethical challenges. The WHO highlights the need to ensure AI systems are safe and effective, quickly provide these systems to those in need, and encourage dialogue among all stakeholders, including developers, regulators, manufacturers, health workers, and patients. A key issue identified is the lack of harmonized standards and coordination among countries and stakeholders, particularly concerning data privacy and governance, underscoring the need for global collaboration to ensure AI benefits all fairly and inclusively [
33,
92].
The WHO guidelines on AI in clinical trials, drug and biologics product development, and manufacturing emphasize the importance of ethical considerations, data privacy, and addressing biases to prevent health disparities. AI should augment rather than replace human decision-making, and robust validation is crucial to ensure AI systems are accurate, reliable, and meet safety and efficacy standards. Transparency in AI algorithms, with clear documentation of their development and usage, is strongly advocated. In manufacturing, WHO recommends that AI be integrated within existing Good Manufacturing Practices (GMP), ensuring it enhances rather than compromises product quality, with continuous monitoring to detect and resolve any issues. Compared to the FDA and EMA, the WHO’s guidelines align closely, with all three organizations emphasizing ethics, validation, transparency, and the integration of AI within existing regulatory frameworks. However, the specific approaches and requirements differ slightly, reflecting each organization’s regulatory context.
The FDA and EMA provide comprehensive guidelines for integrating AI in clinical trials, drug and biologics product development, and manufacturing processes, with a strong emphasis on ethics, validation, and transparency. The FDA’s guidelines specifically focus on the validation and verification of AI algorithms to ensure they meet clinical decision support standards, and it integrates AI into GMP frameworks to maintain human oversight in manufacturing. Similarly, the EMA aligns its guidance with WHO’s principles, stressing the importance of rigorous validation, documentation, and continuous monitoring of AI systems to ensure accuracy, reliability, and safety in drug development and manufacturing. While both agencies emphasize ethical considerations and robust validation, their guidelines reflect nuanced differences due to their distinct regulatory environments. Many AI applications and methods do not significantly affect how current regulations or policies are interpreted or applied, making them largely policy agnostic. However, the advancements toward achieving parity between machine processing and human cognition have revealed instances where existing public policies fall short in addressing societal challenges, a phenomenon known as regulatory gaps.
Despite its expertise and constitutional mandate to regulate global health, the WHO’s role in setting new norms is limited to issuing non-binding guidelines and recommendations. Although these “soft” standards are influential and can shape national legislation and regulations, they fail to create enforceable legal norms. In contrast, the International Health Regulations (IHR) are an essential advancement in international law, providing a more formal framework for global health governance. Since states have a general obligation to cooperate under the UN Charter, including in health matters, the WHO should be empowered with enforcement authority to ensure compliance with the International Health Regulations (IHR), which should be amended to address the integration of AI in healthcare.
The current legal framework reveals the limitations of global public health and the relatively constrained role of the WHO despite its historical regulatory efforts. With the rise of international tech companies and evolving healthcare technologies, there is a pressing need for a new paradigm to address these global expansions. The WHO must enhance its authority and normative powers to address emerging issues related to AI in healthcare. AI can improve healthcare access and strengthen health systems, particularly in developing countries. However, challenges such as bias, data protection, and explainability must be addressed [
93]. European regulations like GDPR, the Data Act, and the AI Act offer robust frameworks for ethical AI use [
33]. WHO members need to collaborate on developing new, legally binding guidelines under the International Health Regulations (IHR) to ensure effective and responsible AI integration in healthcare.
Integration and Regulation of AL/ML in Pharmaceutical Manufacturing
Integrating AI and ML technologies into pharmaceutical manufacturing has introduced significant quality control and operational efficiency advancements. The rapid rise and expansion of biologics, including living drugs such as cell and gene therapy (CGT), presents additional opportunities and challenges in this area. For example, manufacturing engineered T-cells, a promising cancer therapy, faces unique challenges due to their complexity and regulatory requirements [
79]. These products face lengthy approval times in both the US and EU, with the timeline expected to worsen due to a technological shift in CGT drugs, especially for cancer. Using mathematical modeling and AI, we can simulate product behaviors, quantify the relationship between product attributes, patient physiology, and clinical outcomes, optimize treatments, and accelerate personalized medicine development [
94]. As the field grows, regulatory frameworks must continuously adapt to manage emerging technologies effectively, balancing risks, benefits, and unique characteristics for traditional biologics and advanced medicinal product manufacturing. Although several guidelines exist for establishing these programs, they do not address the specific challenges of manufacturing CGT products or offer real-world evidence of the program’s effectiveness. In a GxP-regulated environment—including that regulating CGT, where Good Practice guidelines are essential to ensure product quality and patient safety—AI/ML tools have demonstrated their ability to enhance compliance by automating critical tasks and reducing human error. For instance, AI systems can scrutinize every step of the manual vial-filling process in aseptic processing, analyzing each segment to detect potential contamination risks. Similarly, AI introduces precision through image recognition technologies in environmental monitoring, moving beyond the traditional manual counting of microbial colonies [
95]. In quality control testing, AI is now being used to automate the integration of chromatographic peaks and the visual inspection of injectable drugs, ensuring adherence to stringent regulatory standards [
96].
AI’s impact extends beyond routine manufacturing processes to producing advanced therapy medicinal products (ATMP), medicines based on genes, tissue, cells, or combination, such as CGT. Its ability to automate critical steps in the manufacturing process improves the scalability and reliability of treatments, which is particularly beneficial in hospital settings, making personalized medicine more accessible and practical. However, adopting AI/ML in GxP environments requires careful consideration of regulatory requirements and validation processes.
As regulatory agencies work to develop and update guidelines that address the unique challenges posed by AI/ML, particularly in the context of generative AI, stakeholders must ensure that AI-driven processes comply with current Good Manufacturing Practices (GMP) and other GxP standards. Efforts to harmonize AI-related regulations across global platforms, such as those by the International Society for Pharmaceutical Engineering (ISPE) and the International Council on Harmonization (ICH), aim to reduce complexity for multinational companies and enhance operational efficiencies while maintaining compliance. The evolving regulatory landscape underscores the need for clear guidance on AI applications in Chemistry, Manufacturing, and Controls (CMC), process control, and quality assurance, ensuring these technologies can be safely integrated into pharmaceutical production [
97].
Regulators are already gathering experience with AI/ML applications in the pharmaceutical industry. For example, the FDA has published a white paper describing how various centers, including CBER, CDER, CDRH, and OCP, collaborate to safeguard public health while fostering responsible and ethical innovation. The EMA has established a Quality Innovation Group (QIG) to support innovative medicine design, manufacturing, and quality control approaches. Both agencies are updating or creating new guidelines to align with the digital age, such as the FDA’s draft guidance on “Computer Software Assurance for Production and Quality System Software” and the EMA’s concept paper for revising GMP Annex 11 (computerized systems). Despite these efforts, the regulatory framework has not kept pace with the rapidly advancing field of AI/ML applications in GMP settings, especially in the pharmaceutical context, creating a need for continuous monitoring and adaptation.
The FDA and EMA also focus on specific guidance for the life sciences sector. The EMA has issued a draft reflection paper on using AI in the drug life cycle, which includes recommendations for model development and performance assessment based on quality risk management principles and ICH Q8, Q9, and Q10 guidelines. The FDA has initiated discussions with stakeholders to gather feedback on areas requiring clarity, such as regulatory oversight of AI in drug manufacturing and standards for developing and validating AI models used for process control [
97,
83]
As AI continues to evolve, its applications in pharmaceutical manufacturing will require ongoing regulatory attention and adaptation. Engaging with regulators early and shaping these evolving guidelines will be crucial for stakeholders to navigate the complexities and harness the full potential of AI in this highly regulated industry.
5.3.1. Digital Twins and Predictive Modeling
The pharmaceutical industry is increasingly adopting AI to enhance various stages of drug development, from discovery to post-marketing surveillance. Digital twins represent a cutting-edge application of AI in manufacturing process control by creating virtual simulations of physical systems based on real-time sensor data [
98,
99]. A DT is a virtual representation of a real-world object or system designed to reflect its behavior in real time and is continuously updated based on historical data [
100]. Unlike traditional simulations, DTs run in parallel with their real-world counterparts and can simultaneously simulate multiple processes, allowing for enhanced process optimization and development. Advanced cloud technologies support DTs in managing highly controlled industrial environments, facilitating continuous data exchange and self-monitoring. This multidirectional information flow enables DTs to provide comprehensive and accurate representations of a system’s status, predict outcomes, suggest necessary actions, and even support closed-loop process control, making them more effective than classical simulations [
5]. A key advantage of AI-driven models is their ability to perform counterfactual analyses, which involve rapidly simulating hypothetical scenarios to predict possible outcomes. This capability allows pharmaceutical companies to conduct virtual experiments, explore different manufacturing conditions, and understand the potential impacts of various interventions without requiring extensive physical trials. By simulating “what-if” scenarios, these models can identify the optimal process parameters that minimize waste, reduce costs, and improve overall efficiency.
Furthermore, AI models enhance decision-making by integrating vast amounts of data from diverse sources, such as sensor networks and historical databases, enabling a more holistic and data-driven approach to process optimization [
101]. Critical challenges in applying AI in biopharmaceutical manufacturing include the absence of clear regulatory guidelines, gaps in data completeness, complexities in conducting risk assessments, a lack of specialized tools tailored for biopharma, and a shortage of skilled professionals. These issues are compounded by the rapidly evolving landscape of AI technologies, which demands continuous updates to regulatory frameworks and increased training for personnel to utilize AI tools effectively [
102].
Integrating DT technology early in product development, with a phase-appropriate and change management approach, enables pharmaceuticals to adopt faster processes, achieve higher efficiencies, and enhance customer engagement. As DTs and patient-specific data drive advancements in clinical trials, they accelerate ATMP/CGT product development processes such as safety and efficacy assessments, improve outcomes, and reduce costs. A key example is in silico clinical trials for TCR-engineered T-cell therapies for cancer. Using a quantitative system pharmacology model, patient-specific digital twins were developed to stimulate T cell kinetics, created for patients with metastatic HPV-associated cancers, and identified stem cell-like memory T cells (Tscm) as crucial for persistence and functional outcomes. Simulating the in silico trials, the model predicted that enriching Tscm in the infused product could improve persistence and allow for lower dosing [
103]. This application illustrates how digital twins can optimize therapeutic strategies, reduce variability, and accelerate drug development.
DT models face significant challenges, particularly regarding the availability of clinical data necessary for algorithmically generating clinical decision support and trust reliability of the treatment options evaluated [
104,
105,
106]. To tackle these issues, a collaborative approach can effectively mitigate the regulatory and ethical concerns of integrating AI-based decision-making tools into clinical practices. This strategy emphasizes the need for high-quality data, effective historical data management and awareness of synthetic biases, and thorough documentation and evaluation of model validation to ensure effectiveness and equitable outcomes in drug development.
In biomanufacturing, AI-driven digital twins are precious for optimizing complex and sensitive processes [
107]. For example, these tools can precisely control bioreactor conditions by continuously analyzing data streams related to temperature, pH, and nutrient levels. The models can predict the impact of slight variations on the quality and yield of the biological product, allowing for real-time adjustments that maintain optimal production conditions [
108]. This intelligent closed-loop control ensures consistent product quality and significantly reduces the likelihood of batch failures, which can be costly and time-consuming. By providing real-time insights and recommendations, AI-driven digital twins enable pharmaceutical manufacturers to maintain high precision and reliability in their operations [
109].
Additionally, integrating AI in biomanufacturing fosters innovation by accelerating the development of new therapies and enhancing the scalability of production processes. AI models can analyze patterns and trends in vast datasets to identify novel drug candidates, optimize formulation compositions, and predict how changes in the manufacturing process could affect drug efficacy and safety. This capability is critical as the industry shifts towards more personalized medicine approaches, where small-batch production and rapid adjustments to manufacturing processes are crucial. By optimizing these processes, AI helps bring new therapies to market faster and supports the production of complex biologics tailored to individual patient needs [
110]. Integrating AI and digital twins into the pharmaceutical industry revolutionizes how complex data is handled, analyzed, and utilized. It enables more precise and efficient manufacturing processes, reduces time-to-market for new drugs, and ultimately leads to better patient outcomes. As AI technology advances, its role in the pharmaceutical landscape is expected to expand, driving further innovations and setting new standards for drug development and production [
111].
5.3.2. Emerging AI-Focused Standards for Advanced Manufacturing Technologies
Integrating AI into advanced manufacturing technologies (AMT) has been transformative in healthcare drug and biological product development. AI enhances precision, efficiency, and adaptability, addressing critical challenges in producing complex therapeutics such as biologics, gene therapies, and personalized medicine. Regulatory bodies, including the U.S. Food and Drug Administration (FDA) and organizations like the National Institute of Standards and Technology (NIST), play a crucial role in guiding the effective and safe implementation of AI in these processes [
108].
AI algorithms are increasingly employed to monitor and optimize manufacturing processes in real time. Continuous data collection and analysis enable AI to predict potential deviations and adjust parameters to maintain optimal conditions, ensuring consistent product quality. This capability is essential in biologics production, where even minor process variations can significantly affect product efficacy and safety. The FDA’s guidelines stress the importance of AI-driven process control, advocating for AI to complement existing technologies such as Process Analytical Technology (PAT) and continuous manufacturing systems [
112]. AI also facilitates the transition from traditional batch manufacturing to constant manufacturing—a method endorsed by the FDA for its efficiency and flexibility. Continuous manufacturing allows uninterrupted production, reducing lead times and minimizing human error. AI systems are integral to monitoring and controlling material flow maintaining quality throughout the process. The FDA’s guidance highlights AI’s role in enabling rapid scaling to meet market demands, particularly for vaccines and other time-sensitive therapies [
84,
113].
The ability of AI to analyze extensive datasets enhances the sophistication of quality assurance measures. Machine learning models can identify patterns and anomalies that traditional methods might overlook, allowing for early detection of potential quality issues. The FDA and NIST emphasize AI’s role in quality assurance, especially in preventing defects and ensuring compliance with regulatory standards. Additionally, AI is pivotal in predictive maintenance, analyzing equipment data to forecast and prevent failures, thus minimizing downtime and maintaining operational efficiency. In the highly regulated drug and biological product manufacturing field, adherence to regulatory standards is critical. AI can streamline documentation processes, ensuring all manufacturing steps are accurately recorded in real time. This digital traceability simplifies audits and provides a robust framework for demonstrating compliance with regulations from agencies like the FDA and the European Medicines Agency (EMA). AI-driven systems also aid in preparing regulatory submissions by automatically generating reports that meet required data standards and formatting. AI is incredibly transformative in producing personalized medicine and advanced therapeutics, such as CAR-T cell therapies and gene editing technologies. These treatments demand highly specialized manufacturing processes tailored to individual patients. AI helps manage the complexity of these processes, ensuring that each therapeutic product meets stringent quality and safety standards. The FDA’s guidelines on advanced therapeutics underscore AI’s role in enabling scalable production of personalized treatments, thereby increasing accessibility to these advanced therapies. As AI technology continues to evolve, its integration into manufacturing processes is expected to deepen, leading to the developing of “smart” manufacturing environments. These environments will feature fully automated, AI-driven production lines capable of quickly adapting to new challenges and innovations. Collaborative efforts by the FDA, NIST, and the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL) are crucial in shaping these advancements. NIST’s development of AI standards and performance metrics, combined with the FDA’s regulatory frameworks, will ensure that intelligent manufacturing in drug and biologic production adheres to the highest safety and quality standards [
84].
The FDA has also addressed the potential of AI/ML to enhance pharmaceutical manufacturing, as highlighted in its Second Discussion Paper. This paper elaborates on how advanced analytics using AI/ML can support various aspects of manufacturing, including process controls, equipment reliability, and early warning systems for process deviations. The FDA identifies four critical areas for AI/ML applications: process design optimization, advanced process control implementation, intelligent monitoring and maintenance, and trending activities [
83]. The FDA acknowledges the need for robust standards for trustworthy AI, focusing on characteristics such as explainability, reliability, privacy, safety, security, and bias mitigation. The agency seeks feedback from stakeholders to refine these standards and ensure they address specific concerns in drug development, including governance, data quality, and model performance. While AI and ML hold immense potential for revolutionizing drug development and manufacturing, realizing this potential requires a balanced approach to regulatory compliance and ethical standards. Addressing these challenges head-on, coupled with global harmonization efforts, will enable the industry to harness the benefits of AI while maintaining the highest standards of data protection and patient care.
5.3.3. AI-Enhanced Manufacturing Processes Monitoring
AI technologies rapidly advance in pharmaceutical manufacturing, offering significant potential to enhance process monitoring, quality assurance, and operational efficiency. NIST has been at the forefront of exploring AI-enhanced monitoring to optimize manufacturing processes, providing real-time insights and predictive analytics that traditional methods could not achieve. AI systems can analyze vast amounts of data from sensors and control systems, enabling continuous real-time monitoring and adjustments to critical parameters, which is crucial for maintaining quality and compliance in pharmaceutical production.
To further advance the integration of AI in manufacturing, NIST announced plans to establish a Manufacturing USA institute in Spring 2024. The agency aims to select an applicant team “most capable of establishing and leading a Manufacturing USA institute to accelerate the use of AI for strengthening the resilience of manufacturing processes for the nation’s manufacturers.” This initiative encourages collaboration among industry stakeholders, academia, federal laboratories, and state and local governments, ensuring a broad base of expertise and resources to drive innovation in AI applications. A key component of AI-focused manufacturing process monitoring is testing and validating new data streams and system enhancements in a controlled, low-risk environment. Platforms like CROW (Cyber-physical Research on Working setups) provide a benchtop setup where pharmaceutical manufacturers can evaluate various AI tools and systems without fearing losing time or resources in a full-scale production facility. This “try before you buy” philosophy is crucial for facilities with limited resources, allowing them to invest confidently in technologies that promise the highest returns.Experiments conducted on setups like CROW enable developers to test and benchmark AI-based products and allow manufacturers to understand these products’ effects better, compare solutions, and identify potential pain points. These efforts also help develop best practice guides and standard operating procedures (SOPs) to manage, maintain, and sustain intelligent automation now and in the futureSpecific areas targeted by AI-enhanced monitoring efforts include:
Implementation of manufacturing data exchange standards
Cybersecurity monitoring to safeguard network and information integrity
Digital twin or digital surrogate simulations for process testing and control
Reliability, prognostics, and health management of manufacturing equipment
Product quality monitoring to ensure compliance with regulatory standards
System-level evaluations to assess overall process efficiency and effectiveness
Human interactivity and feedback mechanisms through natural language processing
Trust and trustworthiness requirements for AI systems
Future initiatives also anticipate enhancements in robot and co-bot control, advanced material handling, standardization and implementation of data exchange protocols, and digital thread mapping and utilization. By leveraging these advanced monitoring capabilities, pharmaceutical manufacturers can detect anomalies or deviations from standard operating procedures more quickly than human inspectors, reducing waste and preventing costly recalls or compliance violations.
Moreover, AI-enhanced monitoring supports predictive maintenance strategies by anticipating equipment failures before they occur, minimizing downtime, and extending the lifecycle of critical manufacturing equipment. In a highly regulated environment, such as pharmaceutical manufacturing, these capabilities help maintain compliance with GMP by providing continuous oversight and control over the production environment. As regulatory bodies continue to develop guidelines for AI applications in manufacturing, the pharmaceutical industry must engage in collaborative efforts, such as those encouraged by NIST’s upcoming Manufacturing USA institute. This collaboration will be crucial in establishing best practices and standards for AI-enhanced monitoring technologies, ensuring these tools are effective and compliant with regulatory requirements. Proactively integrating AI technologies into their monitoring systems, pharmaceutical manufacturers can enhance their process monitoring capabilities and maintain a competitive edge in a rapidly evolving industry.
Conclusions and Path Forward
Integrating AI and ML in drug development and clinical trials presents a double-edged sword, offering transformative potential while posing significant challenges. On one hand, AI technologies promise to enhance operational efficiency, improve trial design, and accelerate the development of new therapies. On the other hand, they introduce ethical, regulatory, and data protection challenges that must be carefully managed to ensure patient safety and data integrity.
Rules-based AI, operating on predefined rules and requirements, provides stability and transparency but is limited by the knowledge and capabilities of its creators. In contrast, data-driven responsible AI, such as machine learning algorithms, adapts and learns from data, offering flexibility and the ability to handle complex scenarios. However, their effectiveness depends on the quality of data and the system’s ability to manage and interpret it correctly. Misapplications or data issues can lead to poor results, underscoring the need for rigorous testing and validation. Using a risk assessment framework in model development helps determine the appropriate balance between automation and human oversight, enhancing safety and overall system performance.
The FDA and EMA have proactively addressed these challenges, highlighting the importance of collaboration among regulators, industry stakeholders, and data protection experts. The recent FDA workshop emphasized the need to manage biases, ensure data quality, and maintain rigorous validation and transparency of AI algorithms. Continuous monitoring and adaptation are essential to navigate the evolving landscape of AI in drug development, along with robust regulatory frameworks that prioritize ethical considerations related to data integrity, privacy, and risk-based decision-making.
Key areas of focus moving forward should include:
Personalization: Leveraging AI to advance personalized medicine, ensuring treatments are tailored to individual patient profiles.
Regulatory Frameworks: Developing and refining robust AI validation and monitoring frameworks to ensure compliance and safety.
Ethical Considerations: Addressing data privacy, security, and decision-making issues to maintain public trust and uphold patient rights.
The National Institute of Standards and Technology (NIST) has been instrumental in developing testing methods and metrics to differentiate useful AI tools from ineffective ones, emphasizing the importance of good data and community feedback in this process. Our regulatory frameworks must adapt as AI technologies evolve to ensure innovation is pursued ethically and with patient safety at the forefront.
In conclusion, while AI and ML are promising to revolutionize drug development and clinical trials, realizing this potential requires a balanced approach to regulatory compliance and ethical standards. By addressing these challenges head-on and working towards global harmonization, the industry can harness the benefits of AI while upholding the highest standards of data protection and patient care. As we look to the future, the role of AI and ML in clinical trials and drug development is poised for significant growth, ushering in an exciting new era of innovation and enhanced patient outcomes.