1. Introduction
One of the primary challenges in cancer treatment is the uncontrolled proliferation of tumor cells and the demand for more selective therapies. Conventional anticancer treatments are often limited by toxicity and the development of drug resistance, partly due to the complexity of the tumor microenvironment. This underscores the importance of ongoing research to discover or develop new compounds that can enhance therapeutic efficacy while reducing adverse effects. Historically, many potent drugs have been derived from natural sources and subsequently optimized for improved effectiveness [
1].
Combretastatin A4 (CA-4), a plant-derived compound isolated from the African willow (
Combretum caffrum (Eckl. & Zeyh.) Kuntze) in 1989 [
2], belongs to a family of structurally related compounds known for their potent antimitotic and antiproliferative activities [
3,
4,
5]. These compounds include stilbenes (combretastatin A), dihydrostilbenes (combretastatin B), phenanthrenes (combretastatin C), and macrocyclic lactones (combretastatin D) [
4]. Their biological activity is closely tied to their stereoisomeric configurations, and both natural and synthetic derivatives are of significant interest in cancer chemotherapy clinical trials [
6,
7,
8]. Notably, despite the name, combretastatins are structurally unrelated to statins.
In clinical trials involving patients with advanced solid tumors, CA-4 has shown a “vascularly active” profile with minimal cytotoxic side effects [
7,
8]. Early studies in murine models of melanoma, lung, ovarian, and colon cancer revealed that CA-4’s anticancer effects are primarily due to its inhibition of angiogenesis, reduction of tumor tissue perfusion, and damage to the blood vessel endothelium supplying the tumor [
5,
9]. Mechanistically, CA-4 binds to the colchicine-binding site on tubulin, inhibiting its polymerization, disrupting the cell’s structural integrity, and arresting the cell cycle at the G2M phase. This leads to mitotic catastrophe and the activation of apoptosis pathways, ultimately inducing cell death [
10,
11].
Additionally, CA-4 has been studied for its effects on reactive oxygen species (ROS) production in cancer cells [
6,
12,
13]. Cancer cells typically exhibit higher basal levels of ROS compared to normal cells due to their altered metabolism and rapid proliferation [
14,
15]. CA-4 further elevates ROS levels, aggravating the oxidative stress in cancer cells. This excessive ROS production induces oxidative damage, disrupting critical cellular functions and leading to cell death. Interestingly, while CA-4 disrupts antioxidant defences in cancer cells, it has also been reported to exhibit strong antioxidant properties compared to other analogue compounds [
4,
16].
CA-4 can also exacerbate DNA damage in cancer cells, which, coupled with their impaired DNA repair mechanisms, increases their susceptibility to apoptosis [
17,
18]. Single-strand breaks (SSBs) are the most common form of DNA damage, occurring approximately 10,000 times per day due to endogenous ROS. Typically, these breaks are efficiently repaired by the base excision repair (BER) mechanism. However, if left unrepaired, especially in proliferating cells, SSBs can cause replication fork collapse, resulting in double-strand breaks (DSBs)—the most lethal form of DNA damage [
19]. In many cancers, defective DNA repair mechanisms elevate the baseline level of DNA damage [
20]. By exacerbating this stress, CA-4 further increases DNA damage beyond the cells’ capacity for repair, ultimately pushing them past their homeostatic threshold and leading to cell death [
18].
Lymphocytes, comprising 20-40% of leukocytes, play a pivotal role in the adaptive immune response, defending the body against pathogens and eliminating abnormal cells, including tumor cells. Healthy lymphocytes are crucial for effective immune surveillance, a process by which the immune system continuously monitors the body for cancerous cells. Key lymphocyte subsets, such as cytotoxic T cells and natural killer (NK) cells, directly contribute to tumor cell elimination. Cytotoxic T cells recognize specific antigens on tumor cells and initiate their destruction, while NK cells target tumor cells that evade the adaptive immune system. Without a robust population of healthy lymphocytes, the immune system’s ability to control tumor growth is compromised, increasing the risk of cancer progression and metastasis [
21,
22].
This research aimed to evaluate the in vitro effects of CA-4 on the survival and function of healthy human peripheral blood mononuclear cells (PBMCs), primarily lymphocytes, with a focus on its potential cytotoxic, antioxidant, and antigenotoxic properties. Additionally, for the first time, the cytotoxic effects of CA-4 were assessed in both normal human trophoblast cells and malignant trophoblast cells (choriocarcinoma cells). Given the critical role of trophoblast cells in pregnancy, understanding the safety profile of CA-4 in these cells is essential for evaluating its potential risks during pregnancy, thereby expanding our understanding of its broader impact. HeLa cell line was used as a reference cancer cell line that is the most widely used model for studying human cancer biology.
2. Materials and Methods
2.1. Treatment Preparation
For the cytotoxic evaluation stock solution was prepared by dissolving CA-4 in DMSO at concentration of 100 mM. For the experiments, serial dilutions were prepared from stock solution, and control cells were exposed to an equivalent concentration of DMSO (not exceeding 0.2% at the highest treatment concentration).
2.2. Subjects and PBMC Isolation
Peripheral blood samples from four healthy donors (two males and two females) who reported not using drugs, cigarettes, or alcohol or receiving medical therapy before the study were collected in heparinized containers. Participants gave informed consent in accordance with the regulations of the Clinical Trials Ethics Committee of the University of Belgrade - Faculty of Pharmacy.
Samples were collected in vacutainer tubes with lithium heparin (Vacuette, Greiner Bio-One, Kremsmuenster, Austria). A total of 5 mL of blood was collected per participant for the analyses and lymphocytes separation was performed immediately. A volume of 4 mL of anticoagulant blood was used for lymphocyte separation on Ficoll-Pacque medium (GE Healthcare Bio-Sciences, Uppsala, Sweden) and centrifuged at 450 g for 15 min. Lymphocytes in the ring-shaped layer formed directly above the Ficoll-Pacque medium were collected by pipette and washed twice, first in phosphate-buffered saline (PBS, Fisher Scientific, Pittsburgh, PA) and then in RPMI 1640 medium (Capricorn Scientific, Ebsdorfergrund, Germany). Each wash was followed by centrifugation for 5 min at 300 g. After the last wash and centrifugation, the supernatant was carefully removed so as not to disturb the lymphocyte pellet at the bottom of the tube. The pellet was suspended in 1 ml of complete medium (RPMI 1640 medium + 10% fetal calf serum + 1% penicillin/streptomycin) by gentle mixing with a pipette, and were used immediately for further analysis.
2.3. Cytotoxic Activity
The cytotoxic effects of CA-4 were evaluated against human lymphocyte cells, as well as in HTR-8/SVneo (human trophoblast), JAR (human choriocarcinoma), and HeLa (human cervical carcinoma) cells by MTT assay.
2.4. Cell Culture
Three different cell lines, one non-malignant, and two cancer cell lines were used to evaluate the cytotoxicity of the combratestatin A4. The non-malignant HTR-8/SVneo extravillous trophoblast cell line, established from the human first-trimester placenta explant cultures immortalized by SV40 large T antigen were obtained by courtesy of Dr. Charles H. Graham, Queen’s University, Kingston, Canada. Cells were cultured in RPMI 1640 (Gibco, Waltham, MA, USA), 10% fetal calf serum (FCS, Pan Biotech, Aidenbach, Germany), and 1% antibiotic–antimycotic solution (Capricorn Scientific GmbH, Ebsdorfergrund, Germany) - complete RPMI medium. JAR choriocarcinoma cell line (ATCC®, American Type Culture Collection, Virginia, USA) and human cervical adenocarcinoma (HeLa, ATCC® CCL-2™, American Type Culture Collection, Virginia, USA) cell lines were also cultured in complete RPMI medium. All cell lines were grown in 25 cm2 tissue culture flasks and kept at 37ºC, 5% CO2, in a humidified incubator.
2.5. MTT Assay
After reaching 70 % confluence, the cells were harvested from flasks using 0.25% trypsin-EDTA solution (Institute for Virology, Vaccines and Serum“Torlak”, Belgrade, Serbia) and seeded in 96 well plates (2×104 cells/well for HTR-8/SVneo and JAR, and 1.5 ×104 cells/well for HeLa cells) in 100 µl of the complete medium, and were left to adhere to the plates for 24h. Following day, the medium was removed, and a fresh control medium or the complete medium with the CA4 treatments, in a total culture volume of 100 µl per well were added. The cells were incubated for 24h with the treatments. For PBMC analysis, the isolated lymphocytes were seeded at 3 × 105 cells/well in 96 well plates and were incubated at 37°C for 24h with CA4 in complete medium. After incubation, medium was replaced with 100 μl/well of fresh complete medium containing thiazolyl blue tetrazolium bromide (MTT) in final concentration of 0.5 mg/ml (Acros Organics, Fisher Scientific Company, Fair Lawn, NJ, USA), and the cells were left for 4h in the dark at 37°C.The formed formazan crystals were further solubilized by adding sodium dodecyl sulfate (10% SDS in 0.01 M HCl, Sigma Aldrich, St. Louis, MO, USA) at 100 µl per well and left for 24h at 37°C. Next day, the plates were shaken for 5 min and absorbance was measured at 570 nm on a microplate reader (BioTek ELx800, VT, USA). Each experiment was performed three times in triplicate. Cell viability was represented as percentage of viable cells relative to control. Viability assay data were also used to calculate IC50 value, which represents the half-maximal inhibitory concentration, or the concentration that leads to 50% reduction in cell viability. Values were given in comparison to the reference compound cisplatin.
2.4. Cellular Reactive Oxygen Species Production
H2DCFDA (2’,7’-Dichlorofluorescin Diacetate) Assay
Isolated lymphocytes were seeded in complete RPMI medium in 96-well plates at a density of 3 × 105 cells per well, in a final volume of 100 µl per well, and CA4 treatments at final concentrations in complete medium were added to the cells. After 24 h, treatments were removed and cells were washed with PBS. Next, the H2DCFDA (2’,7’-dichlorofluorescin diacetate) assay was performed according to the manufacturer’s instructions. Using PBS as a solvent, a solution of 5 µM H2DCFDA (CAS 4091-99-0, Merck Millipore Calbiochem, USA) which is sensitive to oxidation in cells was added to each well and left for 45 min in the dark to the reaction takes place. Then, cells were washed with PBS and exposed to PBS alone (control) or 50 µM H2O2, used to induce ROS production in cells. After an incubation time of 30 minutes and the conversion of H2DCFDA to highly fluorescent 2’, 7’-dichlorofluorescin (DCF), the generation of intracellular ROS levels in cells was determined by measuring fluorescence on a plate reader (Wallac 1420 multilabel counter Victor 3V, USA) at wavelengths of 485 -535 nm. Data are expressed as relative fluorescence intensity which is directly proportional to the level of ROS. Analysis of four independent samples of isolated cells from volunteers were performed in triplicate (n=12).
2.6. Statistical Analysis
One-way analysis of variance (ANOVA) with Tukey post-hoc analysis was used to assess differences in treatments versus control, after data were tested for normality. All results are expressed as mean + standard error of the mean (mean + S.E.M). GraphPad Prism 6.0 (GraphPad Software, Inc., USA) was used for statistical analysis, and p<0.05 was considered significant.
3. Results
Considering the current understanding of CA-4’s bioactivity and efficacy, our research aimed to investigate its potential cytotoxic, antioxidant, and antigenotoxic effects on human PBMCs. We evaluated its selectivity by comparing the cytotoxic potency against healthy PBMCs and trophoblast cells (HTR-8/SVneo) versus tumor cell lines (JAR and HeLa).
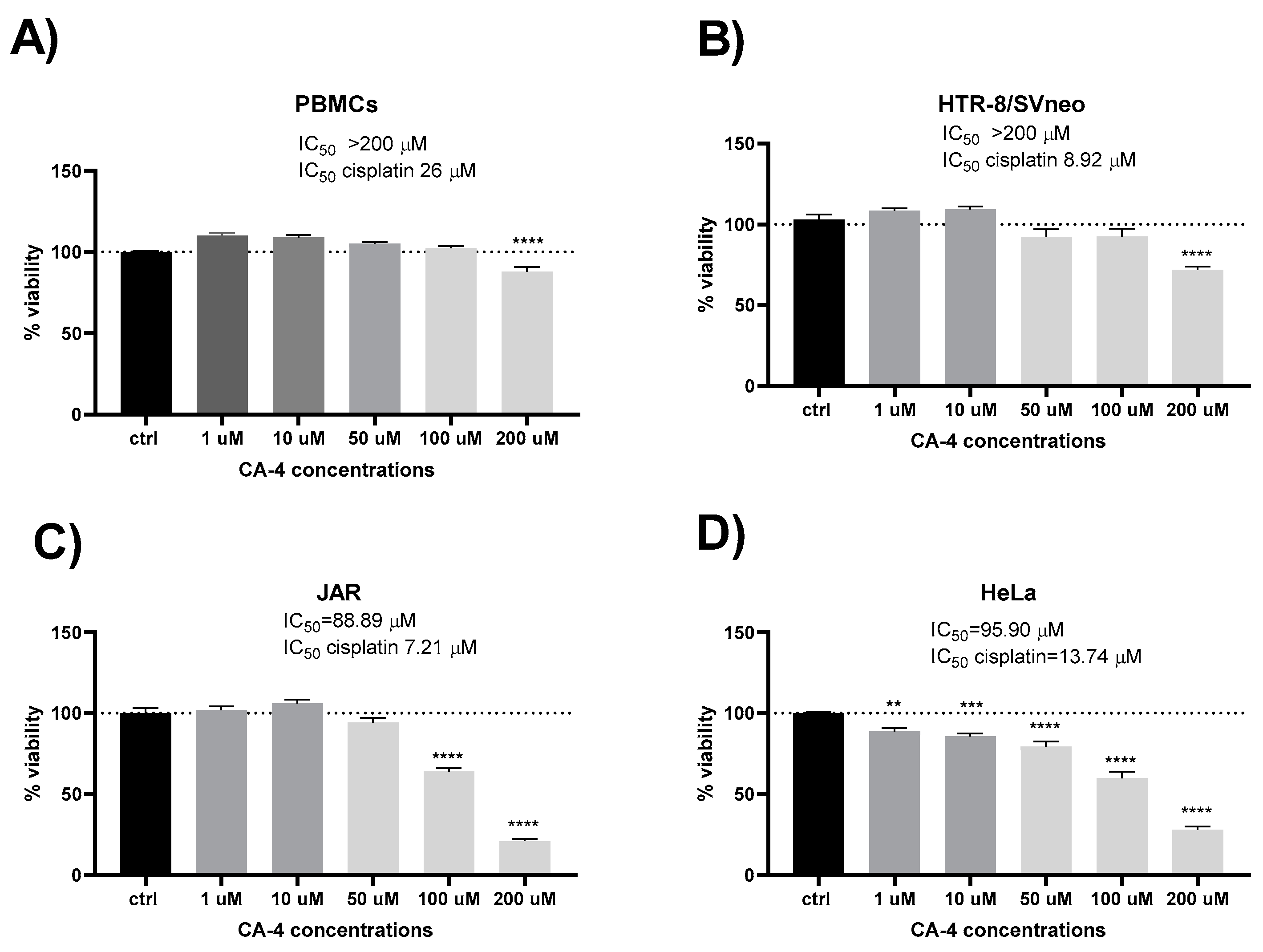
Initially, we examined the influence of CA-4 on the survival and metabolic activity of freshly isolated normal human PBMCs from healthy donors. The degree of reduction in MTT reagent reflects metabolic activity, which correlates with the number of viable cells. As illustrated in
Figure 1, CA-4 exhibited no statistically significant effect on viability or metabolic function at concentrations below 200 µM when compared to untreated control cells. A significant decrease in metabolic activity was observed only at the highest concentration (200 µM), while lower concentrations (1, 10, 50, 100 µM) either preserved or slightly enhanced metabolic activity. Similarly, normal human trophoblast cells (HTR-8/SVneo) tolerated CA-4 exposure up to 100 µM without significant effects on viability, with a notable decrease at 200 µM (IC
50 of CA-4 > 200 µM vs. IC
50 of cisplatin 8.92). Notably, HTR-8/SVneo, throfoblast cells exhibited different sensitivities to CA-4 compared to the malignant cell lines, as shown in
Figure 1.
CA-4 demonstrated moderate cytotoxicity compared to the reference compound cisplatin in both HeLa (IC50 of CA-4 95.90 vs. IC50 of cisplatin 13.74) and JAR cells (IC50 of CA-4 88.89 vs. IC50 of cisplatin 7.21). In JAR choriocarcinoma cells, a significant reduction in cell viability was noted with 100 µM CA-4. Only the two highest concentrations of CA-4 significantly decreased viability in the JAR cell line. In cervical carcinoma cells (HeLa), CA-4 reduced viability in a concentration-dependent manner, with significant reductions starting at 1 µM.
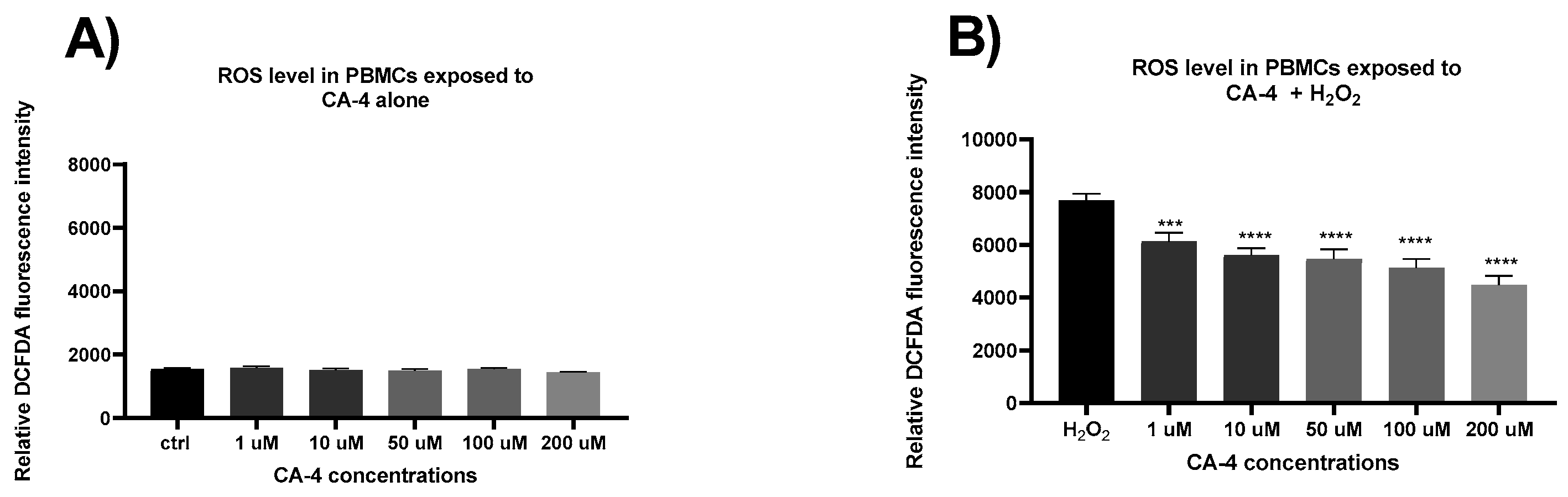
In the next phase of this study, we investigated the effects of CA-4 on the production of reactive oxygen species (ROS) in isolated PBMCs after 24 hours of treatment with concentrations ranging from 1 to 200 µM.
Figure 2A shows the fluorescence intensity, representing spontaneous ROS production without hydrogen peroxide exposure. No statistically significant differences were observed between the groups treated with varying concentrations of CA-4. However, when cells were exposed to oxidative stress (
Figure 2B) via hydrogen peroxide, a statistically significant, concentration-dependent reduction in fluorescence intensity was observed, indicating a corresponding decrease in ROS levels. These results suggest that CA-4 exerts an antioxidant and protective effect in healthy lymphocytes.
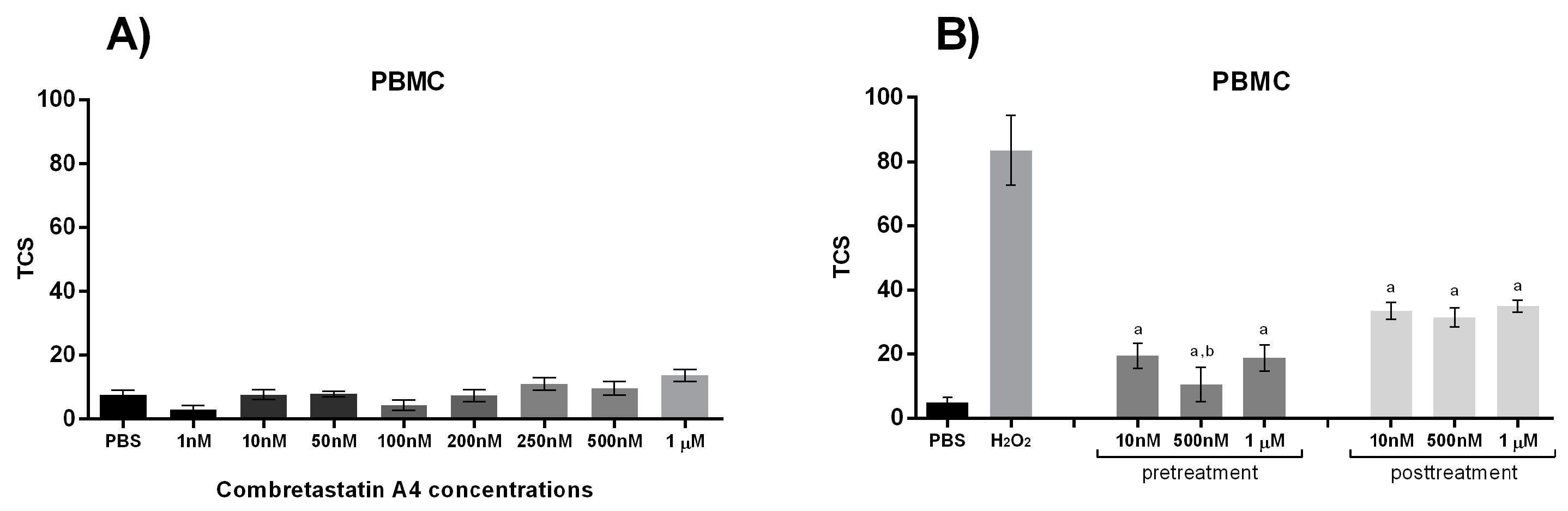
Finally, considering the distinct effects combretastatins may have on DNA damage in normal versus cancer cells, we evaluated the impact of CA-4 on DNA damage levels in PBMCs. DNA damage was assessed using the alkaline comet assay, and the results were expressed as total comet score (TCS), as described in the Materials and Methods section. As shown in
Figure 3A, no significant increase in TCS was observed following a 30 min exposure of PBMCs to CA-4 compared with solvent-only controls. Interestingly, in contrast to its pro-damaging effect on cancer cells, CA-4 exhibited a significant reduction in DNA damage in PBMCs under oxidative stress conditions. The antigenotoxic effect of CA-4 in PBMCs is presented in
Figure 3B.Both pre- and post-treatment with CA-4, compared to H
2O
2-treated controls, demonstrated a reduction in DNA damage, with pre-treatment at 500 nM proving significantly more effective than post-treatment at 10 nM and 1 µM concentrations.
4. Discussion
In this study, we have demonstrated significant differences in the sensitivity to CA-4 between healthy, normal cells and cancer cells. CA-4, along with its analogues, has been shown to exert cytotoxic effects on a range of tumor cells at nanomolar to micromolar concentrations, including cells resistant to other antitumor drugs [
4,
5,
12]. Previous studies documented the most potent antiproliferative activity of CA-4 against several cancer cell lines, with IC
50 values ranging from 0.08 to 35.6 µM [
25] and 0.36 to 7.08 µM [
26]. These concentrations are significantly lower than the 200 µM threshold at which we observed sensitivity in primary lymphocytes and normal trophoblast cells. Consistent with our findings, other studies have reported that CA-4 exhibits considerably lower cytotoxicity toward healthy cells compared to tumor cells [
25]. However, one study showed less selectivity, with CA-4 exhibiting antiproliferative effects on human lymphocytes, though it still displayed significantly higher cytotoxicity for HL-60 (human leukemia) and OVCAR-8 (ovarian adenocarcinoma) tumor cell lines [
27]. Collectively, our results, together with previous findings, indicate clear selectivity in CA-4’s cytotoxicity toward tumor cells in comparison to non-dividing healthy lymphocytes, with proliferating lymphocytes being more sensitive. Healthy trophoblast cells and PBMCs displayed at least 100 times lower sensitivity, becoming evident only at high micromolar doses, while HeLa tumor cells showed significant sensitivity even at concentrations as low as 1 µM.
Of particular importance is the relative resistance of lymphocytes to potential antitumor drugs, given their crucial role in combating tumor cells [
21,
22], which may contribute to improved healing and better long-term prognoses. Additionally, the observed resistance of normal trophoblast cells to CA-4 underscores its potential safety profile during pregnancy. However, it is noteworthy that JAR choriocarcinoma cells displayed greater resistance to CA-4 compared to cervical carcinoma HeLa cells, though they were still more sensitive than healthy trophoblasts. These findings provide initial data on the in vitro anticancer potential of CA-4 in human choriocarcinoma and normal trophoblast cells. Future research on CA-4’s application in treating gestational trophoblastic cancer will require further validation through in vivo studies, along with a thorough examination of safety implications and the molecular mechanisms involved.
Next, our results suggest that CA-4 exerts an antioxidant and protective effect in healthy lymphocytes. This aligns with previous studies demonstrating the inherent antioxidant properties of CA-4, with IC
50 scavenging values of 4.65 mg/mL and 5.71 mg/mL for its cis and trans isomers, respectively, compared to the IC
50 of ascorbic acid at 8.43 mg/mL [
28]. Interestingly, CA-4 has shown contrasting effects in cancer cells, where it significantly elevates ROS levels, pushing them beyond their oxidative stress threshold. By further increasing ROS production in cancer cells, CA-4 induces oxidative damage, disrupting key cellular processes and ultimately leading to cell death [
4,
12,
13]. This differential effect between normal and cancer cells highlights CA-4’s selective anticancer activity.
Previous studies have also demonstrated that this pro-oxidant effect in cancer cells is associated with reduced activity of key antioxidant enzymes such as catalase (CAT) and glutathione reductase (GR), while superoxide dismutase (SOD) activity remains unchanged. These findings suggest that CA-4 disrupts the cellular antioxidant defense system, leading to ROS accumulation and apoptosis at nanomolar concentrations (83, 130, 190 nM) [
29].
While the exact mechanism by which CA-4 reduces ROS in stimulated lymphocytes remains to be fully elucidated, the differential effects observed between healthy and tumor cells strongly support the compound’s selective antitumor activity. Combined with the metabolic activity assays and ROS production data, these findings underscore CA-4’s potential as an anticancer agent, particularly given its lack of cytotoxicity within the therapeutic concentration range and its protective effect on lymphocytes under oxidative stress [
4,
6,
14,
15,
16].
Finally, similar to our findings, previous studies have reported no clastogenicity of CA-4 in human peripheral blood lymphocytes in vitro. In these studies, no increase in structural chromosome aberrations was observed across a wide range of CA-4 concentrations (50 to 500 µg/mL), with or without metabolic activation [
30]. Notably, the lowest concentration tested in that study, 50 µg/mL, is equivalent to approximately 158 µM, which is much higher than the concentrations used in our comet assay. Conversely, in vivo studies on Swiss mice indicated a dose-dependent increase in DNA damage with increasing doses of CA-4 analogs [
31]. Another study showed that treatment with CA-4P in leukemia/lymphoma cell lines caused mitochondrial membrane disruption, increased intracellular ROS, and DNA fragmentation, ultimately leading to cell death, suggesting that ROS accumulation is a critical contributor to CA-4P-induced cytotoxicity [
32].
In normal cells, robust antioxidant defense mechanisms and efficient DNA repair systems, such as the base excision repair (BER) pathway, mitigate the effects of DNA damage. However, cancer cells are more vulnerable due to compromised DNA repair pathways and their elevated baseline oxidative stress levels [
20,
24]. Defective DNA repair mechanisms in cancer cells lead to increased constitutive DNA damage [
19]. Combretastatins, including CA-4, exacerbate this stress, pushing cancer cells beyond their threshold for tolerable DNA damage, leading to apoptosis [
4,
17,
18].
5. Conclusions
In this study, Combretastatin A-4 (CA-4) demonstrated selective cytotoxicity, exerting significant cytotoxic effects on cancer cell lines while sparing healthy human PBMCs and trophoblast cells. CA-4 exhibited minimal cytotoxicity in normal cells at therapeutic concentrations, preserving metabolic activity and even reducing oxidative stress-induced DNA damage and levels of ROS. These results highlight CA-4’s potential as a selective anticancer agent with antioxidant and antigenotoxic properties in normal cells, warranting further investigation into its application in cancer therapy, particularly for gestational trophoblastic cancer.
Author Contributions
Conceptualization, B.S-P. and P.P.; methodology, A.P. and D.T.; software, A.P., D.T. and M.M.; validation, P.P., and L.Z.; formal analysis, A.P. and D.T.; investigation, L.Z.; resources, B.S-P.; data curation, D.T.; writing—original draft preparation, P.P., A.P. and D.T.; writing—review and editing, L.Z., M.M., B.S-P.; visualization, A.P. and D.T.; supervision, P.P., L.Z. and B.S-P.; All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia through Grant Agreement with University of Belgrade-Faculty of Pharmacy and University of Belgrade-Institute for the Application of Nuclear Energy-INEP [Grant No 451-03-65/2024-03/200161; Grant No 451-03-66/2024-03/200019].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Faculty of Pharmacy, University of Belgrade (approval no. approval no. 1769/2).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors report no competing interests.
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Singh, S.B.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendal, D. Isolation and Structure of the Strong Cell Growth and Tubulin Inhibitor Combretastatin A-4. Experientia 1989, 45, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Nam, N.-H. Combretastatin A-4 Analogues as Antimitotic Antitumor Agents. Curr. Med. Chem. 2003, 10, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, G.Ş.; Küpeli Akkol, E.; Genç, Y.; Bardakcı, H.; Yücel, Ç.; Sobarzo-Sánchez, E. Combretastatins: An Overview of Structure, Probable Mechanisms of Action and Potential Applications. Molecules 2020, 25, 2560. [Google Scholar] [CrossRef] [PubMed]
- Dark, G.G.; Hill, S.A.; Prise, V.E.; Tozer, G.M.; Pettit, G.R.; Chaplin, D.J. Combretastatin A-4, an Agent That Displays Potent and Selective Toxicity toward Tumor Vasculature. Cancer Res. 1997, 57, 1829–1834. [Google Scholar]
- Guo, K.; Ma, X.; Li, J.; Zhang, C.; Wu, L. Recent Advances in Combretastatin A-4 Codrugs for Cancer Therapy. Eur. J. Med. Chem. 2022, 241, 114660. [Google Scholar] [CrossRef]
- Dowlati, A.; Robertson, K.; Cooney, M.; Petros, W.P.; Stratford, M.; Jesberger, J.; Rafie, N.; Overmoyer, B.; Makkar, V.; Stambler, B.; et al. A Phase I Pharmacokinetic and Translational Study of the Novel Vascular Targeting Agent Combretastatin A-4 Phosphate on a Single-Dose Intravenous Schedule in Patients with Advanced Cancer. Cancer Res. 2002, 62, 3408–3416. [Google Scholar]
- Grisham, R.; Ky, B.; Tewari, K.S.; Chaplin, D.J.; Walker, J. Clinical Trial Experience with CA4P Anticancer Therapy: Focus on Efficacy, Cardiovascular Adverse Events, and Hypertension Management. Gynecol. Oncol. Res. Pract. 2018, 5, 1. [Google Scholar] [CrossRef]
- Tozer, G.M.; Prise, V.E.; Wilson, J.; Cemazar, M.; Shan, S.; Dewhirst, M.W.; Barber, P.R.; Vojnovic, B.; Chaplin, D.J. Mechanisms Associated with Tumor Vascular Shut-down Induced by Combretastatin A-4 Phosphate: Intravital Microscopy and Measurement of Vascular Permeability. Cancer Res. 2001, 61, 6413–6422. [Google Scholar]
- McGown, A.T.; Fox, B.W. Structural and Biochemical Comparison of the Anti-Mitotic Agents Colchicine, Combretastatin A4 and Amphethinile. Anticancer. Drug Des. 1989, 3, 249–254. [Google Scholar]
- Griggs, J.; Metcalfe, J.C.; Hesketh, R. Targeting Tumour Vasculature: The Development of Combretastatin A4. Lancet Oncol. 2001, 2, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.F. Combretastatin A4-Based Coumarins: Synthesis, Anticancer, Oxidative Stress-Relieving, Anti-Inflammatory, Biosafety, and in Silico Analysis. Chem. Pap. 2024, 78, 3705–3720. [Google Scholar] [CrossRef]
- Kumar, B.; Sharma, P.; Gupta, V.P.; Khullar, M.; Singh, S.; Dogra, N.; Kumar, V. Synthesis and Biological Evaluation of Pyrimidine Bridged Combretastatin Derivatives as Potential Anticancer Agents and Mechanistic Studies. Bioorg. Chem. 2018, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Chandel, N.S. Targeting Mitochondria Metabolism for Cancer Therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef]
- Aziz, G.; Odlo, K.; Hansen, T.V.; Paulsen, R.E.; Mathisen, G.H. Combretastatin A-4 and Structurally Related Triazole Analogues Induce Caspase-3 and Reactive Oxygen Species-Dependent Cell Death in PC12 Cells. Eur. J. Pharmacol. 2013, 703, 25–32. [Google Scholar] [CrossRef]
- Billard, C.; Menasria, F.; Quiney, C.; Faussat, A.-M.; Finet, J.-P.; Combes, S.; Kolb, J.-P. 4-Arylcoumarin Analogues of Combretastatins Stimulate Apoptosis of Leukemic Cells from Chronic Lymphocytic Leukemia Patients. Exp. Hematol. 2008, 36, 1625–1633. [Google Scholar] [CrossRef]
- ZHANG, C.; ZHOU, S.-S.; LI, X.-R.; WANG, B.-M.; LIN, N.-M.; FENG, L.-Y.; ZHANG, D.-Y.; ZHANG, L.-H.; WANG, J.-B.; PAN, J.-P. Enhanced Antitumor Activity by the Combination of Dasatinib and Combretastatin A-4 in Vitro and in Vivo. Oncol. Rep. 2013, 29, 2275–2282. [Google Scholar] [CrossRef]
- Kuzminov, A. Single-Strand Interruptions in Replicating Chromosomes Cause Double-Strand Breaks. Proc. Natl. Acad. Sci. USA 2001, 98, 8241–8246. [Google Scholar] [CrossRef]
- Moretton, A.; Loizou, J.I. Interplay between Cellular Metabolism and the DNA Damage Response in Cancer. Cancers 2020, 12, 2051. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New Insights into Cancer Immunoediting and Its Three Component Phases—Elimination, Equilibrium and Escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Dobson, V.L.; Dušinská, M.; Kennedy, G.; Štětina, R. The Comet Assay: What Can It Really Tell Us? Mutat. Res. Mol. Mech. Mutagen. 1997, 375, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Duan, Y.-T.; Man, R.-J.; Tang, D.-J.; Yao, Y.-F.; Tao, X.-X.; Yu, C.; Liang, X.-Y.; Makawana, J.A.; Zou, M.-J.; Wang, Z.-C.; et al. Design, Synthesis and Antitumor Activity of Novel Link-Bridge and B-Ring Modified Combretastatin A-4 (CA-4) Analogues as Potent Antitubulin Agents. Sci. Rep. 2016, 6, 25387. [Google Scholar] [CrossRef]
- Jadala, C.; Sathish, M.; Anchi, P.; Tokala, R.; Lakshmi, U.J.; Reddy, V.G.; Shankaraiah, N.; Godugu, C.; Kamal, A. Synthesis of Combretastatin-A4 Carboxamidest That Mimic Sulfonyl Piperazines by a Molecular Hybridization Approach: In Vitro Cytotoxicity Evaluation and Inhibition of Tubulin Polymerization. ChemMedChem 2019, 14, 2052–2060. [Google Scholar] [CrossRef]
- do Amaral, D.N.; Cavalcanti, B.C.; Bezerra, D.P.; Ferreira, P.M.P.; Castro, R.d.P.; Sabino, J.R.; Machado, C.M.L.; Chammas, R.; Pessoa, C.; Sant’Anna, C.M.R.; et al. Docking, Synthesis and Antiproliferative Activity of N-Acylhydrazone Derivatives Designed as Combretastatin A4 Analogues. PLoS ONE 2014, 9, e85380. [Google Scholar] [CrossRef]
- Kwak, Y.-S.; Joo, S.-H.; Gansukh, E.; Mistry, B.M.; Keum, Y.S. Synthesis and Anticancer Activities of Polymethylenedioxy Analogues of Combretastatin A-2. Appl. Biol. Chem. 2019, 62, 25. [Google Scholar] [CrossRef]
- Song, M.-Y.; He, Q.-R.; Wang, Y.-L.; Wang, H.-R.; Jiang, T.-C.; Tang, J.-J.; Gao, J.-M. Exploring Diverse-Ring Analogues on Combretastatin A4 (CA-4) Olefin as Microtubule-Targeting Agents. Int. J. Mol. Sci. 2020, 21, 1817. [Google Scholar] [CrossRef]
- Sadhu, D.N.; Desai, L.; Young, S.; Randall, J.C. Lack of Clastogenicity of Combretastatin A-4 (CA4) - Microtubule Destabilizing Agent, in Human Peripheral Blood Lymphocytes in Vitro. Cancer Res. 2005, 65, 809. [Google Scholar]
- Carvalho, P.C.; Santos, E.A.; Schneider, B.U.C.; Matuo, R.; Pesarini, J.R.; Cunha-Laura, A.L.; Monreal, A.C.D.; Lima, D.P.; Antoniolli, A.C.M.B.; Oliveira, R.J. Diaryl Sulfide Analogs of Combretastatin A-4: Toxicogenetic, Immunomodulatory and Apoptotic Evaluations and Prospects for Use as a New Chemotherapeutic Drug. Environ. Toxicol. Pharmacol. 2015, 40, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Petit, I.; Karajannis, M.A.; Vincent, L.; Young, L.; Butler, J.; Hooper, A.T.; Shido, K.; Steller, H.; Chaplin, D.J.; Feldman, E.; et al. The Microtubule-Targeting Agent CA4P Regresses Leukemic Xenografts by Disrupting Interaction with Vascular Cells and Mitochondrial-Dependent Cell Death. Blood 2008, 111, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).