Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Animals and Cell Lines
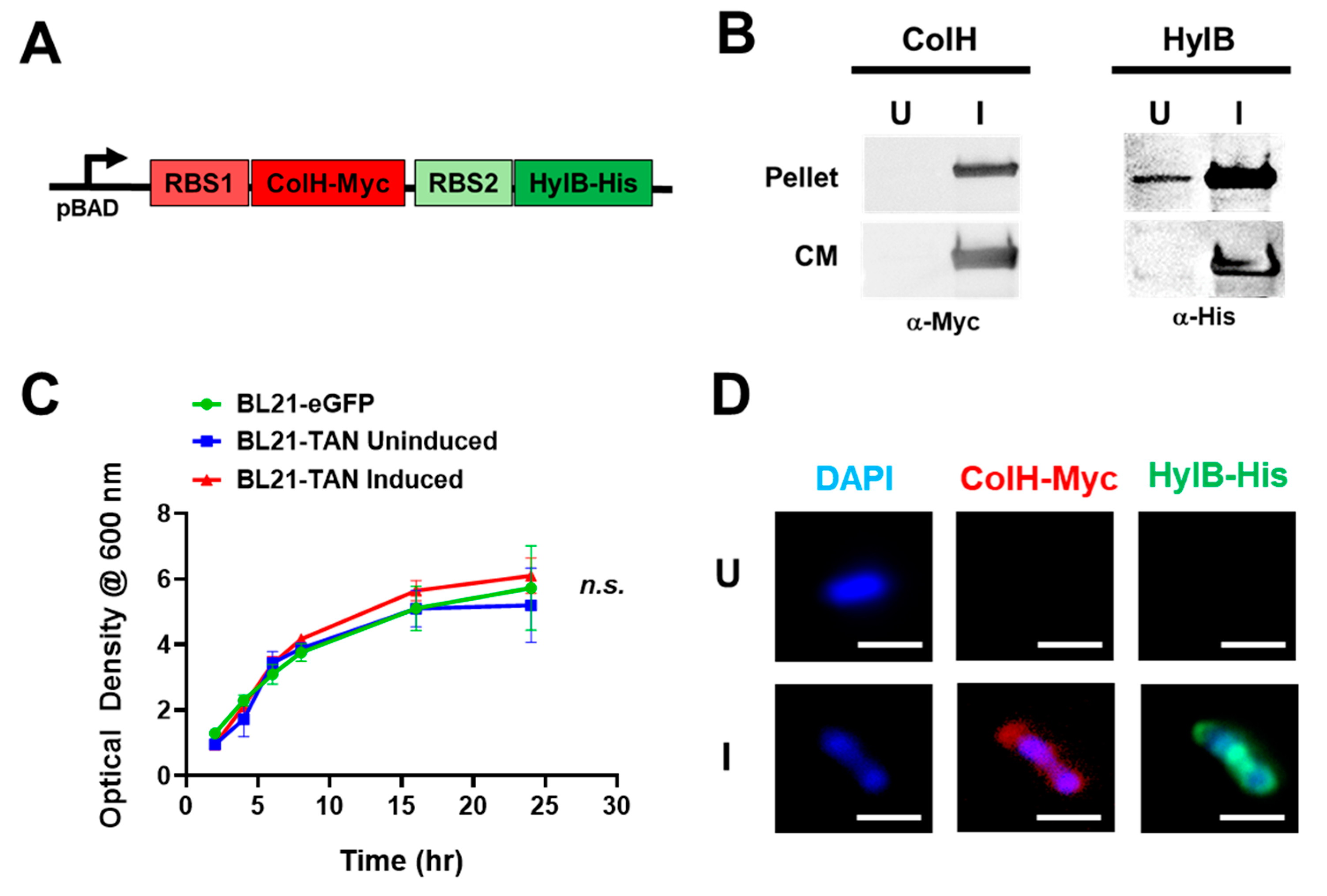
E.coli Strains and Generation of BL21-TAN
Bacterial Growth, Viability, and Analysis of TAN Expression
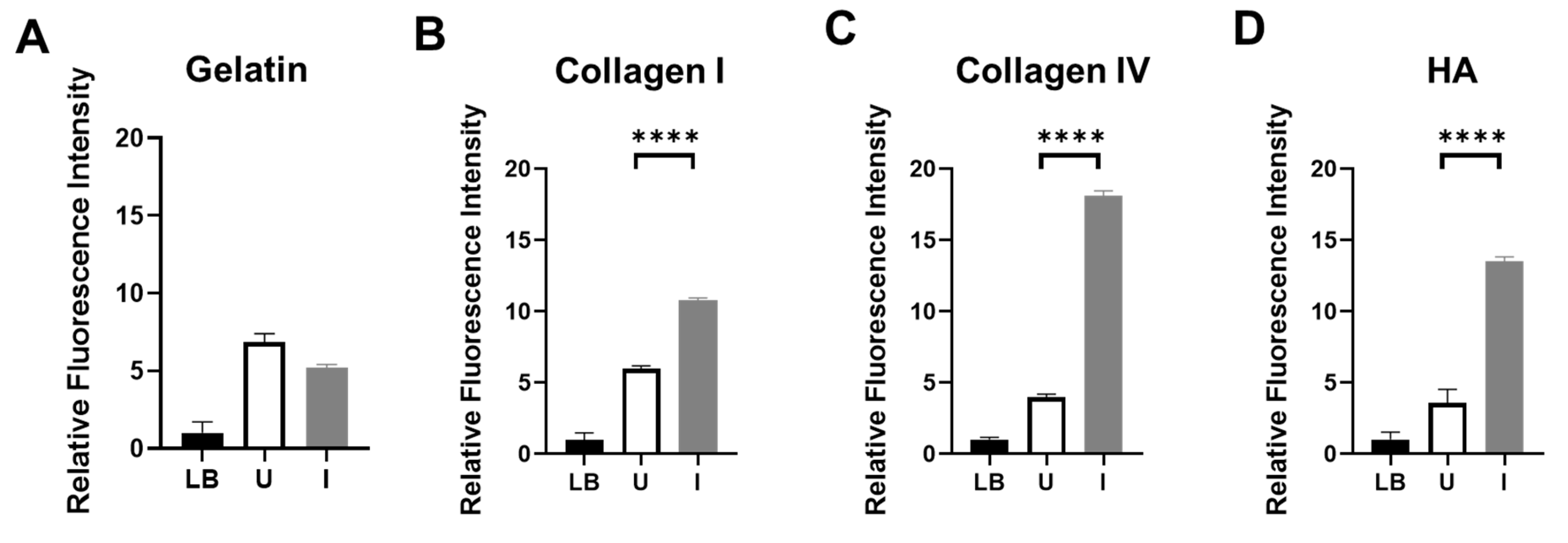
Fluorometric Substrate Assays
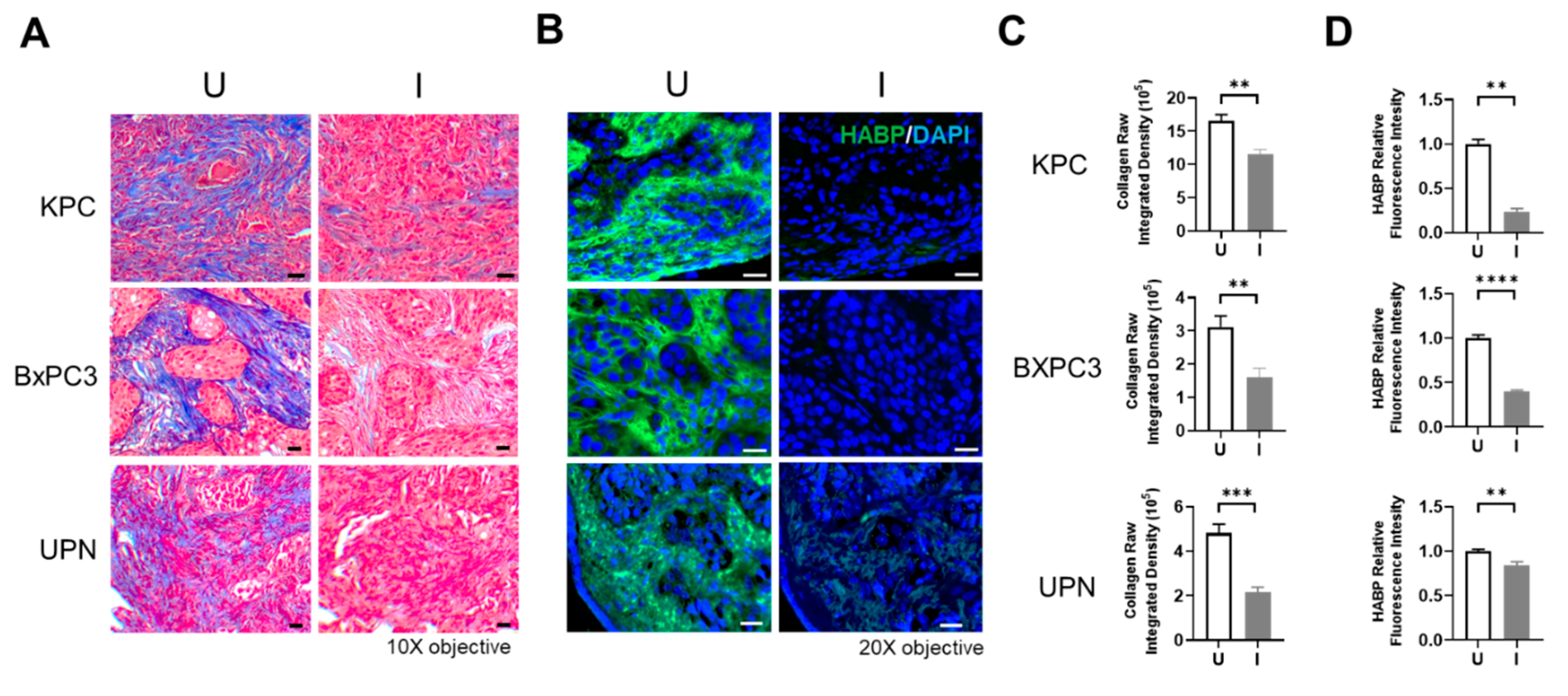
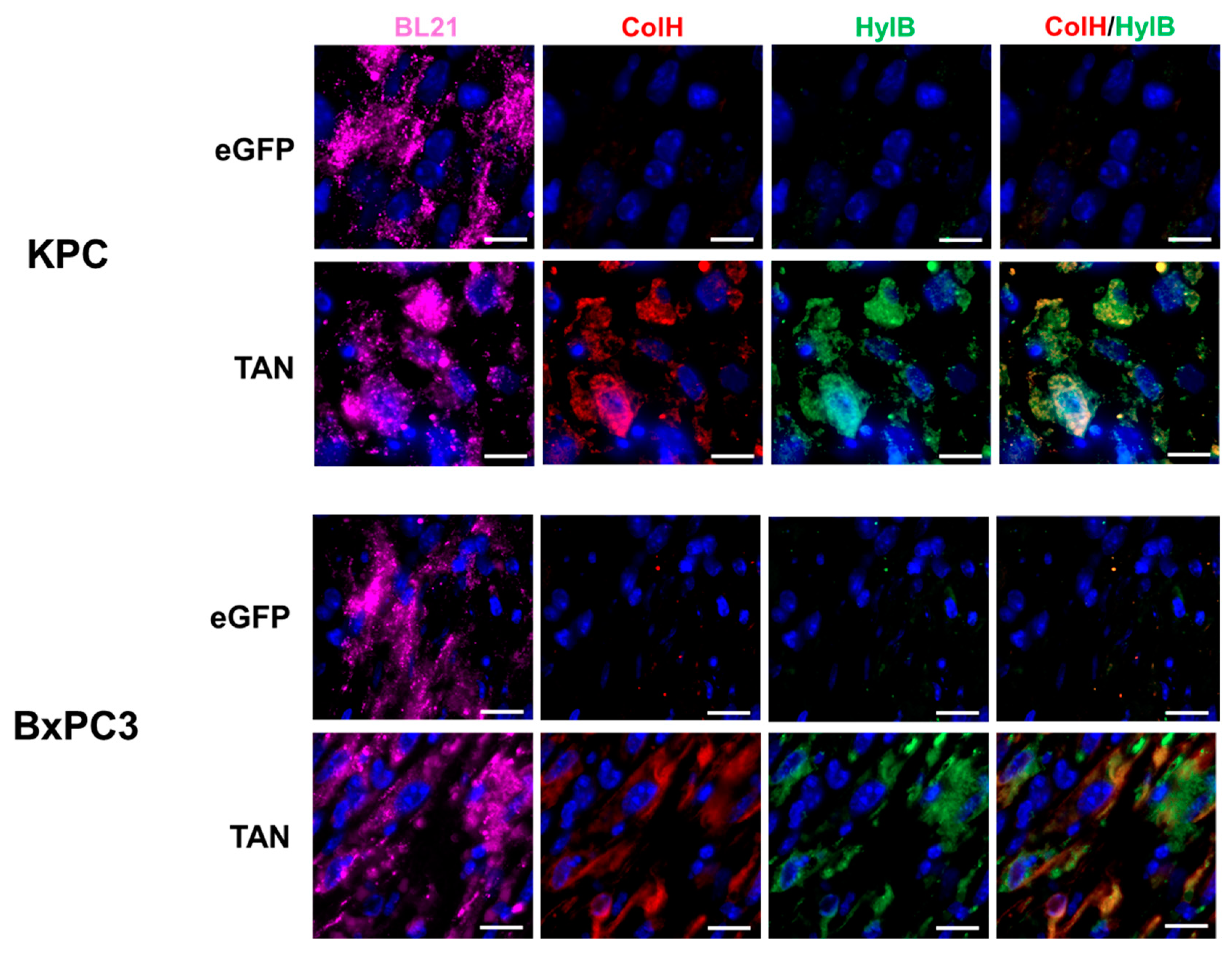
Immunohistochemistry/Immunofluorescence (IHC/IF)
Tumor Implantation, Administration, and Induction of BL21-TAN
Combination Treatment Studies with Gemcitabine
Statistics
3. Results
3.1. Construction and Inducible Expression of BL21-TAN
3.2. BL21-TAN Degrades Collagen I, Collagen IV, and Hyaluronic acid In Vitro
3.3. BL21-TAN Effectively Depletes PDAC Tumor-Derived Collagen and Hyaluronic Acid
3.4. BL21-TAN Expresses Both ColH and HylB in PDAC Murine Models
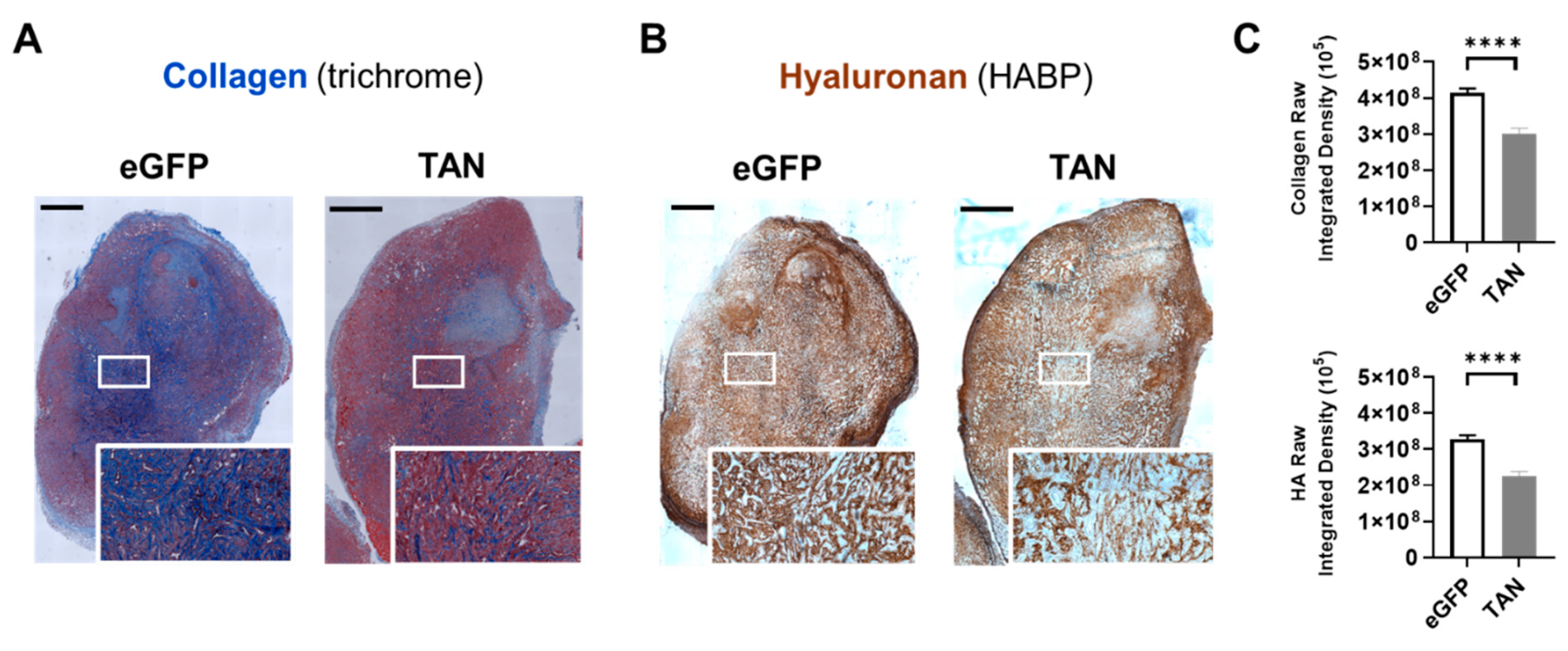
3.5. BL21-TAN Depletes Collagen and Hyaluronan in Murine Model of PDAC
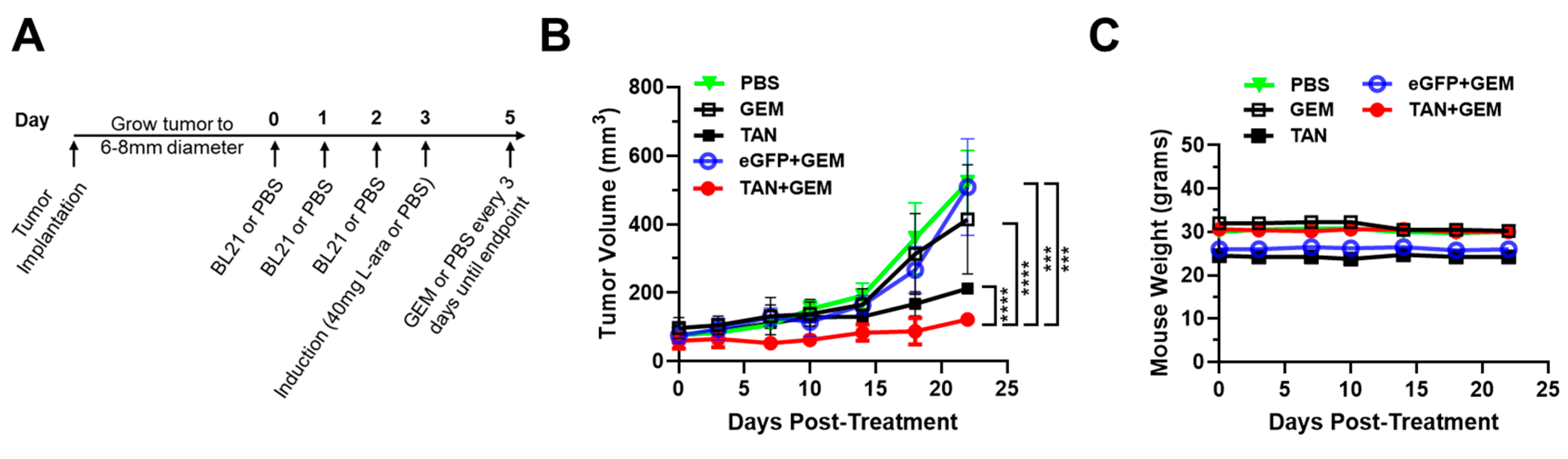
3.6. Pre-Treatment with BL21-TAN Increases Gemcitabine Efficacy in Murine Model of PDAC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. Epub 2024/01/17. [CrossRef] [PubMed]
- Duan X, Zhu Q, Zhang X, Shen Z, Huang Y. Expression, biochemical and structural characterization of high-specific-activity beta-amylase from Bacillus aryabhattai GEL-09 for application in starch hydrolysis. Microb Cell Fact. 2021;20(1):182. Epub 2021/09/20. [CrossRef] [PubMed] [PubMed Central]
- Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology. 2022;163(2):386-402 e1. Epub 2022/04/11. [CrossRef] [PubMed] [PubMed Central]
- Kang J, Hwang I, Yoo C, Kim KP, Jeong JH, Chang HM, Lee SS, Park DH, Song TJ, Seo DW, Lee SK, Kim MH, Hong SM, Shin SH, Hwang DW, Song KB, Lee JH, Kim SC, Ryoo BY. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs. 2018;36(4):732-41. Epub 2018/04/05. [CrossRef] [PubMed]
- Hwang HJ, Oh MS, Lee DW, Kuh HJ. Multiplex quantitative analysis of stroma-mediated cancer cell invasion, matrix remodeling, and drug response in a 3D co-culture model of pancreatic tumor spheroids and stellate cells. J Exp Clin Cancer Res. 2019;38(1):258. Epub 2019/06/16. [CrossRef] [PubMed] [PubMed Central]
- Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62(1):112-20. Epub 2012/04/03. [CrossRef] [PubMed] [PubMed Central]
- Peng DH, Rodriguez BL, Diao L, Chen L, Wang J, Byers LA, Wei Y, Chapman HA, Yamauchi M, Behrens C, Raso G, Soto LMS, Cuentes ERP, Wistuba, II, Kurie JM, Gibbons DL. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat Commun. 2020;11(1):4520. Epub 2020/09/11. GlaxoSmithKline, Astellas, Ribon Therapeutics and Sanofi. D.L.G. receives research grant funding from AstraZeneca, Janssen, Ribon Therapeutics, Astellas and Takeda. L.A.B. declares consulting work for AstraZeneca, AbbVie, GenMab, BergenBio, Pharma Mar, SA. L.A.B. receives research grant funding from AbbVie, AstraZeneca, GenMab, Tolero Pharmaceuticals. All other authors declare that they have no conflict of interests. [CrossRef] [PubMed] [PubMed Central]
- Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110(2):587-95. Epub 2007/03/31. [CrossRef] [PubMed]
- Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, Siebuhr A, Gudmann NS, Ronnow S, Sand JM, Daniels SJ, Mortensen JH, Schuppan D. The good and the bad collagens of fibrosis - Their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43-56. Epub 2017/07/25. [CrossRef] [PubMed]
- Palka J, Oscilowska I, Szoka L. Collagen metabolism as a regulator of proline dehydrogenase/proline oxidase-dependent apoptosis/autophagy. Amino Acids. 2021;53(12):1917-25. Epub 2021/04/06. [CrossRef] [PubMed] [PubMed Central]
- Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezene P, Rubis M, Secq V, Garcia S, Moutardier V, Lombardo D, Iovanna JL, Tomasini R, Guillaumond F, Vander Heiden MG, Vasseur S. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017;8:16031. Epub 2017/07/08. [CrossRef] [PubMed] [PubMed Central]
- Su H, Yang F, Fu R, Trinh B, Sun N, Liu J, Kumar A, Baglieri J, Siruno J, Le M, Li Y, Dozier S, Nair A, Filliol A, Sinchai N, Rosenthal SB, Santini J, Metallo CM, Molina A, Schwabe RF, Lowy AM, Brenner D, Sun B, Karin M. Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome. Nature. 2022;610(7931):366-72. Epub 2022/10/06. [CrossRef] [PubMed] [PubMed Central]
- Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, Mahadevan KK, Wu CJ, Sugimoto H, Chang CC, Jenq RR, McAndrews KM, Kalluri R. Oncogenic collagen I homotrimers from cancer cells bind to alpha3beta1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022;40(8):818-34 e9. Epub 2022/07/23. [CrossRef] [PubMed] [PubMed Central]
- Ohlund D, Lundin C, Ardnor B, Oman M, Naredi P, Sund M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br J Cancer. 2009;101(1):91-7. Epub 2009/06/06. [CrossRef] [PubMed] [PubMed Central]
- Ohlund D, Franklin O, Lundberg E, Lundin C, Sund M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer. 2013;13:154. Epub 2013/03/28. [CrossRef] [PubMed] [PubMed Central]
- Burnier JV, Wang N, Michel RP, Hassanain M, Li S, Lu Y, Metrakos P, Antecka E, Burnier MN, Ponton A, Gallinger S, Brodt P. Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene. 2011;30(35):3766-83. Epub 2011/04/12. [CrossRef] [PubMed]
- Markowska A, Antoszczak M, Markowska J, Huczynski A. Role of Hyaluronic Acid in Selected Malignant Neoplasms in Women. Biomedicines. 2023;11(2). Epub 2023/02/26. [CrossRef] [PubMed] [PubMed Central]
- Michalczyk M, Humeniuk E, Adamczuk G, Korga-Plewko A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int J Mol Sci. 2022;24(1). Epub 2023/01/09. [CrossRef] [PubMed] [PubMed Central]
- Inokoshi Y, Tanino Y, Wang X, Sato S, Fukuhara N, Nikaido T, Fukuhara A, Saito J, Frevert CW, Munakata M. Clinical significance of serum hyaluronan in chronic fibrotic interstitial pneumonia. Respirology. 2013;18(8):1236-43. Epub 2013/06/26. [CrossRef] [PubMed]
- Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22(1):276-84. Epub 2007/09/01. [CrossRef] [PubMed]
- Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res. 2015;21(15):3561-8. Epub 2015/02/20. [CrossRef] [PubMed] [PubMed Central]
- Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326(9):851-62. Epub 2021/09/22. [CrossRef] [PubMed] [PubMed Central]
- Maclennan JD, Mandl I, Howes EL. Bacterial digestion of collagen. J Clin Invest. 1953;32(12):1317-22. Epub 1953/12/01. [CrossRef] [PubMed] [PubMed Central]
- Harrington DJ. Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun. 1996;64(6):1885-91. Epub 1996/06/01. [CrossRef] [PubMed] [PubMed Central]
- Jedrzejas MJ. Structural and functional comparison of polysaccharide-degrading enzymes. Crit Rev Biochem Mol Biol. 2000;35(3):221-51. Epub 2000/07/25. [CrossRef] [PubMed]
- Burgeson RE, Nimni ME. Collagen types. Molecular structure and tissue distribution. Clin Orthop Relat Res. 1992(282):250-72. Epub 1992/09/01. [PubMed]
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242(1):27-33. Epub 1997/07/01. [CrossRef] [PubMed]
- Lian C, Wang X, Qiu X, Wu Z, Gao B, Liu L, Liang G, Zhou H, Yang X, Peng Y, Liang A, Xu C, Huang D, Su P. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin beta1-SMAD1 interaction. Bone Res. 2019;7:8. Epub 2019/03/12. [CrossRef] [PubMed] [PubMed Central]
- Ebelt ND, Zamloot V, Zuniga E, Passi KB, Sobocinski LJ, Young CA, Blazar BR, Manuel ER. Collagenase-Expressing Salmonella Targets Major Collagens in Pancreatic Cancer Leading to Reductions in Immunosuppressive Subsets and Tumor Growth. Cancers (Basel). 2021;13(14). Epub 2021/07/25. [CrossRef] [PubMed] [PubMed Central]
- Ebelt ND, Zuniga E, Passi KB, Sobocinski LJ, Manuel ER. Hyaluronidase-Expressing Salmonella Effectively Targets Tumor-Associated Hyaluronic Acid in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2020;19(2):706-16. Epub 2019/11/07. [CrossRef] [PubMed] [PubMed Central]
- Zhang YZ, Ran LY, Li CY, Chen XL. Diversity, Structures, and Collagen-Degrading Mechanisms of Bacterial Collagenolytic Proteases. Appl Environ Microbiol. 2015;81(18):6098-107. Epub 2015/07/08. [CrossRef] [PubMed] [PubMed Central]
- Wang Z, Guo C, Xu Y, Liu G, Lu C, Liu Y. Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression. Infect Immun. 2014;82(6):2615-25. Epub 2014/04/09. [CrossRef] [PubMed] [PubMed Central]
- Bankhead P, Loughrey MB, Fernandez JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. Epub 2017/12/06. [CrossRef] [PubMed] [PubMed Central]
- Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177(14):4121-30. Epub 1995/07/01. [CrossRef] [PubMed] [PubMed Central]
- Whatcott CJ, Han H, Posner RG, Hostetter G, Von Hoff DD. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Cancer Discov. 2011;1(4):291-6. Epub 2011/11/05. [CrossRef] [PubMed] [PubMed Central]
- Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191-200. Epub 2012/06/19. [CrossRef] [PubMed]
- Ouyang Z, Dong L, Yao F, Wang K, Chen Y, Li S, Zhou R, Zhao Y, Hu W. Cartilage-Related Collagens in Osteoarthritis and Rheumatoid Arthritis: From Pathogenesis to Therapeutics. Int J Mol Sci. 2023;24(12). Epub 2023/06/28. [CrossRef] [PubMed] [PubMed Central]
- Jiang SN, Phan TX, Nam TK, Nguyen VH, Kim HS, Bom HS, Choy HE, Hong Y, Min JJ. Inhibition of tumor growth and metastasis by a combination of Escherichia coli-mediated cytolytic therapy and radiotherapy. Mol Ther. 2010;18(3):635-42. Epub 2010/01/07. [CrossRef] [PubMed] [PubMed Central]
- Gentschev I, Petrov I, Ye M, Kafuri Cifuentes L, Toews R, Cecil A, Oelschaeger TA, Szalay AA. Tumor Colonization and Therapy by Escherichia coli Nissle 1917 Strain in Syngeneic Tumor-Bearing Mice Is Strongly Affected by the Gut Microbiome. Cancers (Basel). 2022;14(24). Epub 2022/12/24. [CrossRef] [PubMed] [PubMed Central]
- Sun XM, Zhang ZX, Wang LR, Wang JG, Liang Y, Yang HF, Tao RS, Jiang Y, Yang JJ, Yang S. Downregulation of T7 RNA polymerase transcription enhances pET-based recombinant protein production in Escherichia coli BL21 (DE3) by suppressing autolysis. Biotechnol Bioeng. 2021;118(1):153-63. Epub 2020/09/09. [CrossRef] [PubMed]
- Popova NV, Jucker M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers (Basel). 2022;14(1). Epub 2022/01/12. [CrossRef] [PubMed] [PubMed Central]
- Song K, Yu Z, Zu X, Li G, Hu Z, Xue Y. Collagen Remodeling along Cancer Progression Providing a Novel Opportunity for Cancer Diagnosis and Treatment. Int J Mol Sci. 2022;23(18). Epub 2022/09/24. [CrossRef] [PubMed] [PubMed Central]
- Donelan W, Dominguez-Gutierrez PR, Kusmartsev S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front Immunol. 2022;13:971278. Epub 2022/10/15. [CrossRef] [PubMed] [PubMed Central]
- Siggins MK, Sriskandan S. Bacterial Lymphatic Metastasis in Infection and Immunity. Cells. 2021;11(1). Epub 2022/01/12. [CrossRef] [PubMed] [PubMed Central]
- DuFort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, Thompson CB, Connor RJ, Thanos CD, Scott Brockenbrough J, Provenzano PP, Frost GI, Michael Shepard H, Hingorani SR. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys J. 2016;110(9):2106-19. Epub 2016/05/12. [CrossRef] [PubMed] [PubMed Central]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497-503. Epub 2000/05/16. [PubMed]
- Chaudhry GE, Akim A, Naveed Zafar M, Safdar N, Sung YY, Muhammad TST. Understanding Hyaluronan Receptor (CD44) Interaction, HA-CD44 Activated Potential Targets in Cancer Therapeutics. Adv Pharm Bull. 2021;11(3):426-38. Epub 2021/09/14. [CrossRef] [PubMed] [PubMed Central]
- Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun. 2018;9(1):4692. Epub 2018/11/10. [CrossRef] [PubMed] [PubMed Central]
- Xu S, Xu H, Wang W, Li S, Li H, Li T, Zhang W, Yu X, Liu L. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17(1):309. Epub 2019/09/16. [CrossRef] [PubMed] [PubMed Central]
- Ji T, Feng W, Zhang X, Zang K, Zhu X, Shang F. HDAC inhibitors promote pancreatic stellate cell apoptosis and relieve pancreatic fibrosis by upregulating miR-15/16 in chronic pancreatitis. Hum Cell. 2020;33(4):1006-16. Epub 2020/06/12. [CrossRef] [PubMed] [PubMed Central]
- Ferrara B, Pignatelli C, Cossutta M, Citro A, Courty J, Piemonti L. The Extracellular Matrix in Pancreatic Cancer: Description of a Complex Network and Promising Therapeutic Options. Cancers (Basel). 2021;13(17). Epub 2021/09/11. [CrossRef] [PubMed] [PubMed Central]
- Zamloot V, Ebelt ND, Soo C, Jinka S, Manuel ER. Targeted Depletion of Hyaluronic Acid Mitigates Murine Breast Cancer Growth. Cancers (Basel). 2022;14(19). Epub 2022/10/15. [CrossRef] [PubMed] [PubMed Central]
- Kim JS, Park JE, Choi SH, Kang SW, Lee JH, Lee JS, Shin M, Park SH. ECM-targeting bacteria enhance chemotherapeutic drug efficacy by lowering IFP in tumor mouse models. J Control Release. 2023;355:199-210. Epub 2023/02/08. [CrossRef] [PubMed]
- McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509-13. Epub 2006/03/03. [CrossRef] [PubMed]
- Eikenes L, Tufto I, Schnell EA, Bjorkoy A, De Lange Davies C. Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer Res. 2010;30(2):359-68. Epub 2010/03/25. [PubMed]
- Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomedicine. 2007;2(2):265-74. Epub 2007/08/29. [PubMed] [PubMed Central]
- Kohli AG, Kivimae S, Tiffany MR, Szoka FC. Improving the distribution of Doxil(R) in the tumor matrix by depletion of tumor hyaluronan. J Control Release. 2014;191:105-14. Epub 2014/05/24. [CrossRef] [PubMed] [PubMed Central]
- Larsen AMH, Kuczek DE, Kalvisa A, Siersbaek MS, Thorseth ML, Johansen AZ, Carretta M, Grontved L, Vang O, Madsen DH. Collagen Density Modulates the Immunosuppressive Functions of Macrophages. J Immunol. 2020;205(5):1461-72. Epub 2020/08/26. [CrossRef] [PubMed]
- Gong D, Shi W, Yi SJ, Chen H, Groffen J, Heisterkamp N. TGFbeta signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. Epub 2012/06/19. [CrossRef] [PubMed] [PubMed Central]
- Jacob SS, Shastry P, Sudhakaran PR. Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem. 2002;233(1-2):9-17. Epub 2002/06/27. [CrossRef] [PubMed]
- Huang Y, Lei X, Sun L, Liu Y, Yang J. Leveraging various extracellular matrix levels to assess prognosis and sensitivity to immunotherapy in patients with ovarian cancer. Front Oncol. 2023;13:1163695. Epub 2023/05/25. [CrossRef] [PubMed] [PubMed Central]
- Yuan G, Xie F, Song Y, Li Q, Li R, Hu X, Zang M, Cheng X, Lu G, Huang J, Fan W, Rong X, Sun J, Chen J. Hepatic Tumor Stiffness Measured by Shear Wave Elastography Is Prognostic for HCC Progression Following Treatment With Anti-PD-1 Antibodies Plus Lenvatinib: A Retrospective Analysis of Two Independent Cohorts. Front Immunol. 2022;13:868809. Epub 2022/06/28. [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




