Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Biomass Collection
2.2. Alkaloid Extraction
2.3. Totals Alkaloids Content
2.4. Antioxidant Capacity Assay
2.5. Identification and Quantification of Alkaloids by Ultra High-Resolution Liquid Chromatography/Mass Spectrometry (UPLC/MS)
2.6. Alkaloid Microencapsulation
2.7. Physical Characterization of the Microcapsules
2.7.1. Morphology
2.7.2. Moisture
2.7.3. Process Yield
2.7.4. Encapsulation Efficiency (EE)
2.8. Bioaccessibility Assay
2.9. Statistical Analysis
3. Results
3.1. Total Alkaloid Content of Eggplant Biomass Extract
3.2. Antioxidant Capacity of Eggplant Alkaloids Fruit Extract
3.3. Identification and Quantification of Alkaloids by Ultra High-Resolution Liquid Chromatography Coupled to Mass Spectrometry (UPLC/MS) in Extract
3.4. Physical Characterization of Microcapsules
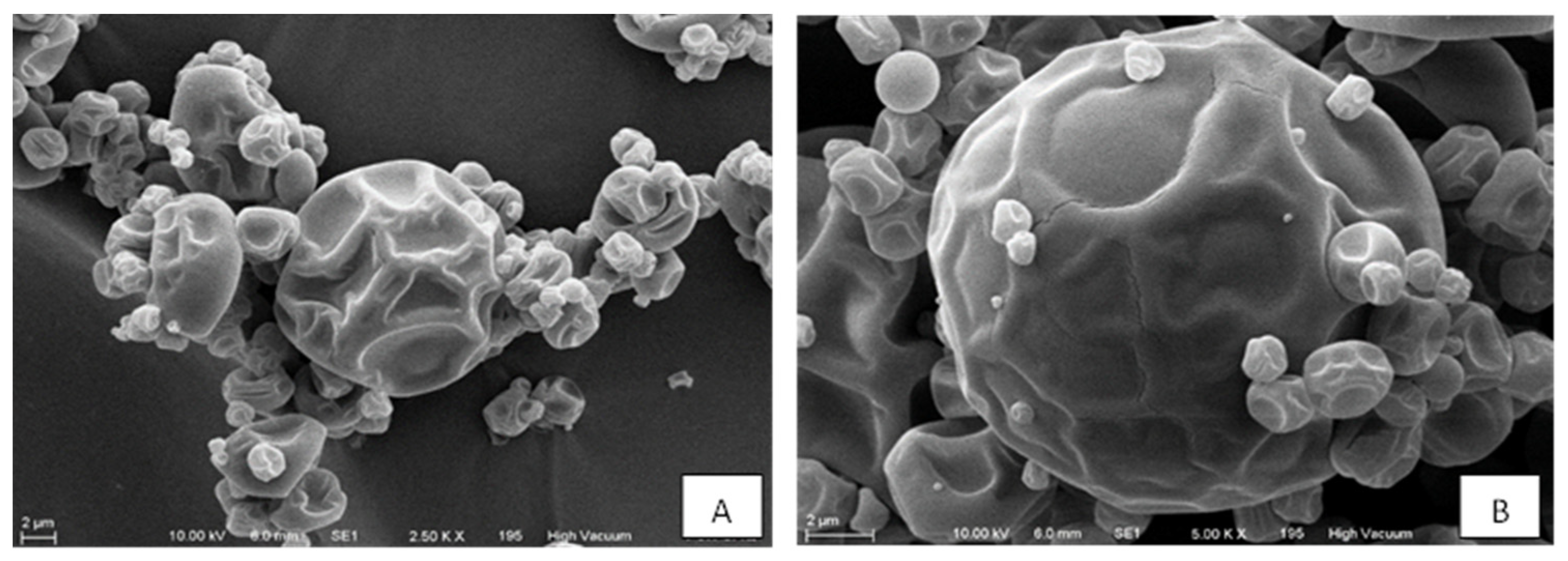
3.4.1. Morphology
3.4.2. Moisture and Yield
3.4.3. Encapsulation Efficiency
3.5. Total Alkaloid Content of Encapsulated Eggplant Alkaloids Fruit
3.6. Antioxidant Capacity of Encapsulated Eggplant Alkaloids Fruit
3.7. Identification and Quantification of Alkaloids by Ultra High-Resolution Liquid Chromatography Coupled to Mass Spectrometry (UPLC/MS) of Encapsulated Eggplant Alkaloids Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agoreyo, B.O.; Obansa, E.S.; Obanor, E.O. Comparative nutritional and phytochemical analyses of two varieties of Solanum melongena. Sci. World J. 2012, 7, 5-8.
- SIAP. Panorama agroalimentario 2022. Servicio de información agroalimentaria y pesquera 2022, 42-43.
- Mauro, R.P.; Agnello, M.; Rizzo, V.; Graziani, G.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Valorización de residuos de campo de berenjena como fuente de fitoquímicos. Sci Horticulturae 2020. [CrossRef]
- Maurya, R.; Bharti, C.; Singh, T.D.; Pratap, V. Crop Residue Management for Sustainable Agriculture. Int J Curr Microbiol Appl Sci 2020, 9, 2020. [CrossRef]
- Lobo, M.G.; Dorta, E. Utilization and management of horticultural waste. In Postharvest Technology of Perishable Horticultural Commodities; Elsevier: 2019; pp. 639-666.
- Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative technologies for extraction and microencapsulation of bioactives from plant-based food waste and their applications in functional food development. Foods 2021, 10, 279. [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S.; Najari, Z. An integrated valorization of industrial waste of eggplant: Simultaneous recovery of pectin, phenolics and sequential production of pullulan. J. Waste Manag. 2019, 100, 101-111. [CrossRef]
- Contreras-Angulo, L.A.; Moreno-Ulloa, A.; Carballo-Castañeda, R.A.; León-Felix, J.; Romero-Quintana, J.G.; Aguilar-Medina, M.; Ramos-Payán, R.; Heredia, J.B. Metabolomic analysis of phytochemical compounds from agricultural residues of eggplant (Solanum melongena L.). Molecules 2022, 27, 7013. [CrossRef]
- Distl, M.; Wink, M. Identification and quantification of steroidal alkaloids from wild tuber-bearing Solanum species by HPLC and LC-ESI-MS. Potato Res. 2009, 52, 79-104. [CrossRef]
- Matias, L.J.; Mercadante-Simões, M.O.; Royo, V.A.; Ribeiro, L.M.; Santos, A.C.; Fonseca, J. Structure and histochemistry of medicinal species of Solanum. Rev. bras. farmacogn. 2016, 26, 147-160. [CrossRef]
- Morillo, M.; Rojas, J.; Lequart, V.; Lamarti, A.; Martin, P. Natural and synthetic derivatives of the steroidal glycoalkaloids of Solanum genus and biological activity. Nat Prod Res 2020. [CrossRef]
- Lelario, F.; De Maria, S.; Rivelli, A.R.; Russo, D.; Milella, L.; Bufo, S.A.; Scrano, L. A complete survey of glycoalkaloids using LC-FTICR-MS and IRMPD in a commercial variety and a local landrace of eggplant (Solanum melongena L.) and their anticholinesterase and antioxidant activities. Toxins (Basel) 2019, 11, 230. [CrossRef]
- Chang, L.C.; Tsai, T.R.; Wang, J.J.; Lin, C.N.; Kuo, K.W. The rhamnose moiety of solamargine plays a crucial role in triggering cell death by apoptosis. Biochem. Biophys. Res. Commun. 1998, 242, 21-25. [CrossRef]
- Friedman, M. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. J. Agric. Food Chem. 2015, 63, 3323-3337. [CrossRef]
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O'Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactividades de glicoalcaloides y sus agliconas de especies de Solanum. J. Agric. Food Chem. 2011, 59 (8), 3454-3484. [CrossRef]
- Xie, X.; Zhu, H.; Yang, H.; Huang, W.; Wu, Y.; Wang, Y.; Luo, Y.; Wang, D.; Shao, G. Solamargine triggers hepatoma cell death through apoptosis. Oncol. Lett. 2015, 10, 168-174. [CrossRef]
- Zhao, D.K.; Zhao, Y.; Chen, S.Y.; Kennelly, E. Solanum steroidal glycoalkaloids: Structural diversity, biological activities, and biosynthesis. Natural Product Reports 2021, 38, 1423-1444. [CrossRef]
- Sharma, T.; Airao, V.; Panara, N.; Vaishnav, D.; Ranpariya, V.; Sheth, N.; Parmar, S. Solasodine protects rat brain against ischemia/reperfusion injury through its antioxidant activity. Eur. J. Pharmacol. 2014, 7. [CrossRef]
- Chauhan, K.; Sheth, N.; Ranpariya, V.; Parmar, S. Anticonvulsant activity of solasodine isolated from Solanum sisymbriifolium fruits in rodents. Pharm. Biol. 2011, 49, 194-199. [CrossRef]
- Das, M.; Barua, N. Pharmacological activities of Solanum melongena Linn.(Brinjal plant). Int. J. Green Pharm. 2013, 7. [CrossRef]
- Vohora, S.B.; Kumar, I.; Khan, M.S.Y. Effect of alkaloids of Solanum melongena on the central nervous system. J. Ethnopharmacol. 1984, 11, 331-336. [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. JFB 2018, 4, 11–68-11–68. [CrossRef]
- Shahidi, F.; Pan, Y. Influence of food matrix and food processing on the chemical interaction and bioaccessibility of dietary phytochemicals: A review. Crit. Rev. Food Sci. Nutr 2022, 62, 6421-6445. [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366-380. [CrossRef]
- Azevedo, M.; Leite, I.B.; Queiroz, C.; Fialho, E. Spiced risotto: Cooking processing and simulated in vitro digestion on curcuminoids, capsaicin and piperine. J CULIN. 2019, 17, 256-267. [CrossRef]
- Pasli, A.A.; Yavuz-Düzgün, M.; Altuntas, U.; Altin, G.; Özçelik, B.; Firatligil, E. In vitro bioaccessibility of phenolics and flavonoids in various dried vegetables, and the determination of their antioxidant capacity via different spectrophotometric assays. Food Res Int 2019, 26, 793-800.
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of bioactive compounds from fruit and vegetable by-products for food application–A review. Trends Food Sci Tech 2021, 116, 11-23. [CrossRef]
- Srinivasan, K.; Shanmughasundaram, S. Microencapsulation of vasicine alkaloids through spray drying. IAJPR 2016. [CrossRef]
- Lehotay, S. AOAC official method 2007.01 pesticide residues in foods by acetonitrile extraction and partitioning with Magnesium Sulfate. J. AOAC Int. 2007, 90, 485-520. [CrossRef]
- Shamsa, F.; Monsef, H.; Ghamooshi, R.; Verdian-rizi, M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. TJPS 2008, 32, 17-20. [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41-60. [CrossRef]
- Benzie, I.F.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70-76. [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437-4444. [CrossRef]
- Bernal-Millán, M.J.; Gutiérrez-Grijalva, E.P.; Contreras-Angulo, L.; Muy-Rangel, M.D.; López-Martínez, L.X.; Heredia, J.B. Spray-dried microencapsulation of oregano (Lippia graveolens) polyphenols with maltodextrin enhances their stability during in vitro digestion. J. Chem. 2022, 2022. [CrossRef]
- AOAC. Association of official analytical chemists. Available online: https://www.aoac.org/ (accessed on.
- Al-Mubarak, A.; Hamid, N.; Kam, R.; Chan, H. The effects of spray drying conditions on the physical and bioactive properties of New Zealand Tamarillo (Solanum betaceum) powder. Act. Sci. Nutr. Health. 2019, 3, 121-131. [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991-1014. [CrossRef]
- Can-Karaca, A.; Guzel, O.; Ak, M.M. Effects of processing conditions and formulation on spray drying of sour cherry juice concentrate. J Sci Food Agric. 2016, 96, 449-455. [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food front 2021, 2, 426-442. [CrossRef]
- Manoharan, R.; Nair, C.S.; Eissa, N.; Cheng, H.; Ge, P.; Ren, M.; Jaleel, A. Therapeutic Potential of Solanum Alkaloids with Special Emphasis on Cancer: A Comprehensive Review. Drug Des Devel Ther. 2024, 3063-3074. [CrossRef]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O. Biological activities of some isoquinoline alkaloids from Fumaria schleicheri Soy. Will. Plants 2022, 11, 1202. [CrossRef]
- Martini, S.; Conte, A.; Cattivelli, A.; Tagliazucchi, D. Domestic cooking methods affect the stability and bioaccessibility of dark purple eggplant (Solanum melongena) phenolic compounds. Food Chem 2021, 341, 128298. [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. J. Nutr. 2020, 12, 1401. [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827-835. [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Assessing the activity of natural food antioxidants. In Oxidation in Foods and Beverages and Antioxidant Applications; Elsevier: 2010; pp. 332-367.
- Elizalde-Romero, C.A.; Basilio-Heredia, J.; Contreras-Angulo, L.A.; Gutiérrez-Grijalva, E.P.; Leyva-López, N. Bioaccesibilidad de extractos hidrofílicos de berenjena (Solanum melongena L.) y su potencial como inhibidor de lipasa Centro de Investigación en Alimentación y Desarrollo A.C., 2020.
- Ogunsuyi, O.B.; Ademiluyi, A.O.; Oboh, G. Solanum leaves extracts exhibit antioxidant properties and inhibit monoamine oxidase and acetylcholinesterase activities (in vitro) in Drosophila melanogaster. JBCPP 2020, 31. [CrossRef]
- Moyo, S.M.; Serem, J.C.; Bester, M.J.; Mavumengwana, V.; Kayitesi, E. The impact of boiling and in vitro human digestion of Solanum nigrum complex (Black nightshade) on phenolic compounds bioactivity and bioaccessibility. Food Res Int 2020, 137, 109720. [CrossRef]
- Izzo, L.; Castaldo, L.; Lombardi, S.; Gaspari, A.; Grosso, M.; Ritieni, A. Bioaccessibility and antioxidant capacity of bioactive compounds from various typologies of canned tomatoes. Front. Nutr. 2022, 9, 849163. [CrossRef]
- Kaur, C.; Nagal, S.; Nishad, J.; Kumar, R. Evaluating eggplant (Solanum melongena L) genotypes for bioactive properties: A chemometric approach. Food Res Int 2014, 60, 205-211. [CrossRef]
- Ng, R.C.; Kassim, N.K.; Yeap, Y.S.; Ee, G.C.L.; Yazan, S.L.; Musa, K.H. Isolation of carbazole alkaloids and coumarins from Aegle marmelos and Murraya koenigii and their antioxidant properties. Sains Malays 2018, 47, 1749-1756. [CrossRef]
- Benítez-Estrada, A.; Villanueva-Sánchez, J.; González-Rosendo, G.; Alcántar-Rodríguez, V.E.; Puga-Díaz, R.; Quintero-Gutiérrez, A.G. Determinación de la capacidad antioxidante total de alimentos y plasma humano por fotoquimioluminiscencia: Correlación con ensayos fluorométricos (ORAC) y espectrofotométricos (FRAP). Tip rev. espec. cienc. quím. -biol. 2020, 23. [CrossRef]
- Bisby, R.H.; Brooke, R.; Navaratnam, S. Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay. Food Chem. 2008, 108, 1002-1007. [CrossRef]
- Tian, W.; Zhi, H.; Yang, C.; Wang, L.; Long, J.; Xiao, L.; Liang, J.; Huang, Y.; Zheng, X.; Zhao, S. Chemical composition of alkaloids of Plumula nelumbinis and their antioxidant activity from different habitats in China. IC&P 2018, 125, 537-548. [CrossRef]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.C.; Mustafa, M.R.; Awang, K. Antidiabetic and antioxidant properties of alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770-9784. [CrossRef]
- Goboza, M.; Meyer, M.; Aboua, Y.G.; Oguntibeju, O.O. In vitro antidiabetic and antioxidant effects of different extracts of Catharanthus roseus and its indole alkaloid, vindoline. Molecules 2020, 25, 5546. [CrossRef]
- Siddique, M.A.B.; Brunton, N. Food glycoalkaloids: distribution, structure, cytotoxicity, extraction, and biological activity. In Alkaloids, Kurek, J., Ed.; IntechOpen: Rijeka, 2019.
- Li, H.; Deng, Z.; Liu, R.; Loewen, S.; Tsao, R. Bioaccessibility, in vitro antioxidant activities and in vivo anti-inflammatory activities of a purple tomato (Solanum lycopersicum L.). Food Chem. 2014, 159, 353-360. [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.-Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: their journey after intake. J. Funct. Foods 2014, 5, 189-197. [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the food industry: A brief historical overview to recent developments. Food sci. nutr. 2020, 11, 481-508. [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59-68. [CrossRef]
- Arrazola, G.; Herazo, I.; Alvis, A. Microencapsulación de Antocianinas de Berenjena (Solanum melongena L.) mediante Secado por Aspersión y Evaluación de la Estabilidad de su Color y Capacidad Antioxidante. Inf. Tecnol. 2014, 25, 31-42. [CrossRef]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209-215. [CrossRef]
- Vergara, C.; Pino, M.T.; Zamora, O.; Parada, J.; Pérez, R.; Uribe, M.; Kalazich, J. Microencapsulation of anthocyanin extracted from purple flesh cultivated potatoes by spray drying and its effects on in vitro gastrointestinal digestion. Molecules 2020, 25, 722. [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem 2018, 254, 281-291. [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452-490. [CrossRef]
- Santos-Silva, G.; Gouveia-Gomes, M.H.; Moreira de Carvalho, L.; Leite-Abreu, T.; dos Santos Lima, M.; Suely-Madruga, M.; Kurozawa, L.E.; Alencar-Bezerra, T.K. Microencapsulation of organic coffee husk polyphenols: Effects on release, bioaccessibility, and antioxidant capacity of phenolics in a simulated gastrointestinal tract. Food Chem 2023, 137435. [CrossRef]
- Ortega-Hernández, E.; Camero-Maldonado, A.V.; Acevedo-Pacheco, L.; Jacobo-Velázquez, D.A.; Antunes-Ricardo, M. Immunomodulatory and Antioxidant Effects of Spray-Dried Encapsulated Kale Sprouts after In Vitro Gastrointestinal Digestion. Foods 2023, 12, 2149. [CrossRef]
- Cardona-Tangarife, D.P.; Patiño-Arias, L.P.; Ormaza-Zapata, A.M. Aspectos tecnológicos de la microencapsulación de compuestos bioactivos en alimentos mediante secado por aspersión. Cienc. Tecnol. Agropecuaria. 2021, 22, 1-21. [CrossRef]
- Iturri, M.S.; Barros-Calado, C.M.; Prentice, C. Microparticles of Eugenia stipitata pulp obtained by spray-drying guided by DSC: An analysis of bioactivity and in vitro gastrointestinal digestion. Food Chem. 2021, 334, 127557. [CrossRef]
- Tomé-Sánchez, I.; Martín-Diana, A.B.; Peñas, E.; Frias, J.; Rico, D.; Jiménez-Pulido, I.; Martínez-Villaluenga, C. Bioprocessed wheat ingredients: Characterization, bioaccessibility of phenolic compounds, and bioactivity during in vitro digestion. Front. Plant Sci. 2021, 12, 790898. [CrossRef]
- Hameed, A.; Ijaz, S.; Mohammad, I.S.; Muhammad, K.S.; Akhtar, N.; Khan, H.M.S. Aglycone solanidine and solasodine derivatives: A natural approach towards cancer. Biomed. Pharmacother. 2017, 94, 446-457. [CrossRef]
- Merkel, S.; Dib, B.; Maul, R.; Köppen, R.; Koch, M.; Nehls, I. Degradation and epimerization of ergot alkaloids after baking and in vitro digestion. Anal. Bioanal. Chem. 2012, 404, 2489-2497. [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; McDougall, G.J.; Requena, T. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413-436. [CrossRef]
- Vronen, P.J.E. Potato glycoalkaloids as starting material for the synthesis of steroid hormones; Wageningen University and Research: 2003.
| Method | Undigested | Digested | % BA |
|---|---|---|---|
| Total alkaloid content* | 173 ± 8.6a | 7 ±0.8 b | 4 |
| TEAC** | 1195 ± 10a | 141 ± 16b | 12 |
| FRAP** | 800 ± 21a | 50 ± 2b | 6 |
| ORAC** | 1778 ± 43a | 545 ± 5b | 31 |
| Compound | Compound type | Molecular mass [M+H]+ | Retention time (min) | Undigested (ng/g) |
Digested (ng/g) |
% BA |
|---|---|---|---|---|---|---|
| Solamargine | Glycoalkaloid | 867.49 | 3.86 | 2485 ± 6a | 431 ± 11b | 17.34 |
| Solasonine | Glycoalkaloid | 883.49 | 3.81 | 1724 ± 35a | 263 ± 9b | 15.26 |
| Method | Undigested | Digested | %BA |
|---|---|---|---|
| Total alkaloid content* | 1.59 ± 0.06a | 0.185 ± 0.04b | 12 |
| TEAC** | 3.90 ± 0.05b | 8 ± 0a | >100 |
| FRAP** | 3.46 ± 0.22a | 2.75 ± 0b | 79 |
| ORAC** | 16 ± 0b | 30 ± 1a | >100 |
| Compound | Alkaloid type | Molecular mass [M+H]+ | Retention time (min) | Undigested (ng/g) |
Digested (ng/g) |
|---|---|---|---|---|---|
| Solamargine | Glycoalkaloid | 867.49 | 3.86 | 6.111 ± 1b | 12.74 ± 0a |
| Solasonine | Glycoalkaloid | 883.49 | 3.81 | 5.169 ± 1b | 9.743 ± 0a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





