Submitted:
30 October 2024
Posted:
31 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Depression Overview
3. Mechanisms of Action of Antidepressants and Neuroplasticity
4. Pharmacological Treatment of Depression
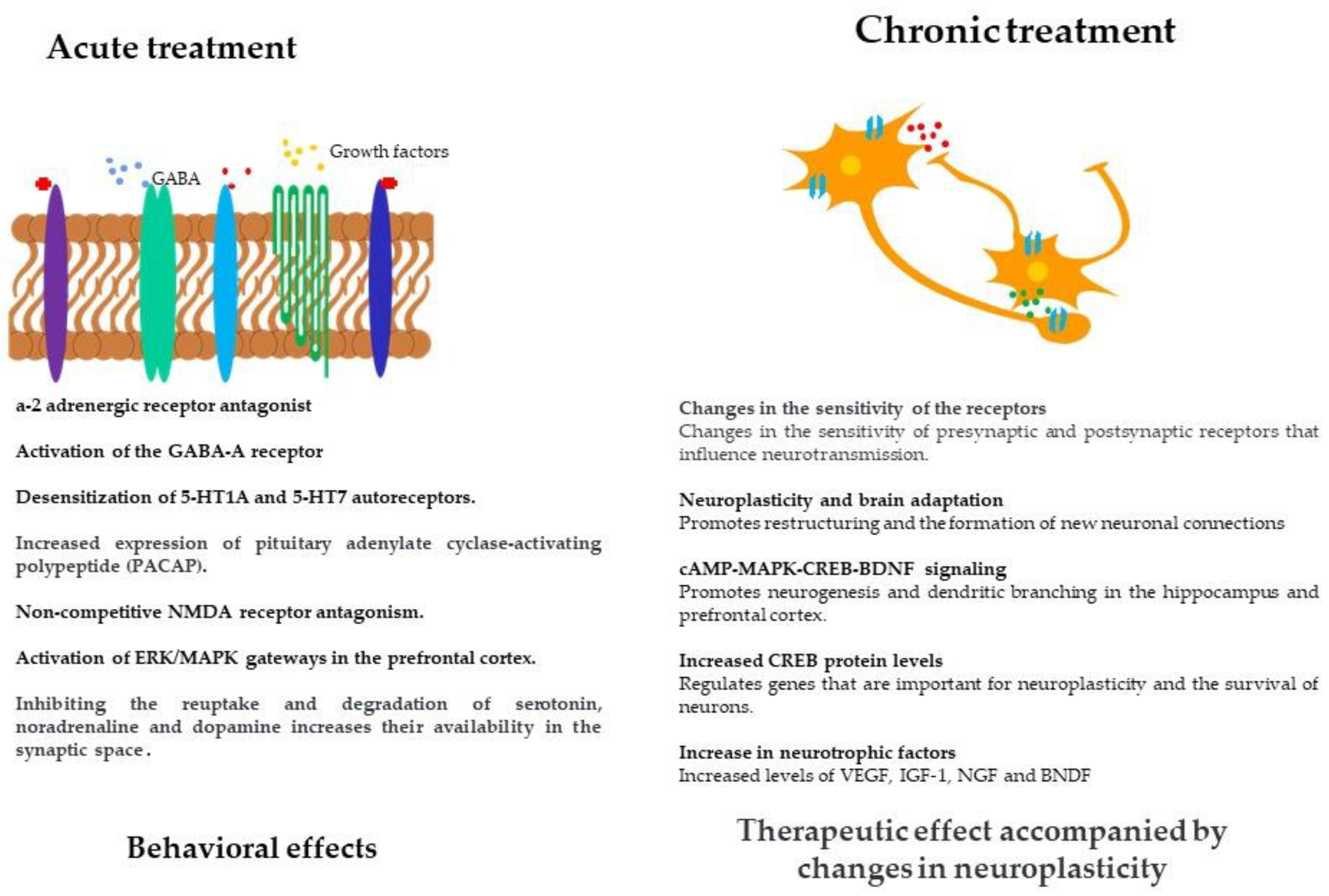
5. Effect of Acute Antidepressant Treatment on an Experimental Level
6. Effect of Chronic Antidepressant Treatment on an Experimental Level
7. Pharmacological alternatives in the treatment of depression
8. Conclusions
9. Future Directions
Acknowledgments
Conflicts of Interest
References
- Tardito, D.; Perez, J.; Tiraboschi, E.; Musazzi, L.; Racagni, G.; Popoli, M. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmrev. 2006, 58, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Racagni, G.; Popoli, M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin. Neurosci. 2008, 10, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Pompili, M.; Innamorati, M.; Giordano, G.; Montebovi, F.; Sher, L.; Girardi, P. The role of microRNAs in synaptic plasticity, major affective disorders and suicidal behavior. Neurosci. Res. 2012, 73, 179–190. [Google Scholar] [CrossRef]
- Arantes-Gonçalves, F.; Coelho, R. Depressão e tratamento. Apoptose, neuroplasticidade e antidepressivos. Acta Med. 2006, 19, 9–20. [Google Scholar]
- Gray, J.D.; Milner, T.A.; McEwen, B.S. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013, 239, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Anilkumar, S.; Chattarji, S.; Buwalda, B. Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav. Brain Res. 2018, 347, 314–324. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef]
- Contreras, C.M.; Rodríguez-Landa, J.F.; Gutiérrez-García, A.G.; Bernal-Morales, B. The lowest effective dose of fluoxetine in the forced swim test significantly affects the firing rate of lateral septal nucleus neurones in the rat. J. Psychopharmacol. 2001, 15, 231–236. [Google Scholar] [CrossRef]
- Lozano-Hernandez, R.; Rodríguez-Landa, J.F.; Hernández-Figueroa, J.D.; Saavedra, M.; Ramos-Morales, F.R.; Cruz-Sánchez, J.S. Antidepressant-like effects of two commercially available products of Hypericum perforatum in the forced swim test: a long-term study. J. Med. Plants Res. 2010, 4, 131–137. [Google Scholar]
- Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; De Gomes, M.G.; Souza, L.C. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+, K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience. 2015, 289, 367–380. [Google Scholar] [CrossRef]
- German-Ponciano, L.J.; Rosas-Sánchez, G.U.; Ortiz-Guerra, S.I.; Soria-Fregozo, C.; Rodríguez-Landa, J.F. Effects of chrysin on mRNA expression of 5-HT 1A and 5-HT 2A receptors in the raphe nuclei and hippocampus. Rev. Bras. Farmacogn. 2021, 31, 353–360. [Google Scholar] [CrossRef]
- Page, M.E.; Detke, M.J.; Dalvi, A.; Kirby, L.G.; Lucki, I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999, 147, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Guasti, A.; Olivares-Nazario, M.; Reyes, R.; Martínez-Mota, L. Sex and age differences in the antidepressant-like effect of fluoxetine in the forced swim test. Pharmacol. Biochem. Behav. 2017, 152, 81–89. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression. In WHO fact sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 28 May 2024).
- Clack, S.; Ward, T. The classification and explanation of depression. Behav. Chang. 2019, 36, 41–55. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 4 March 2023).
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Woody, C. A.; Ferrari, A. J.; Siskind, D. J.; Whiteford, H. A.; Harris, M. G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef]
- Ribeiro, J.D.; Huang, X.; Fox, K.R.; Franklin, J.C. Depression and hopelessness as risk factors for suicide ideation, attempts and death: meta-analysis of longitudinal studies. Br. J. Psychiatry. 2018, 212, 279–286. [Google Scholar] [CrossRef]
- Puyat, J.H.; Kazanjian, A.; Wong, H.; Goldner, E. Comorbid chronic general health conditions and depression care: a population-based analysis. Psychiatr. Serv. 2017, 68, 907–915. [Google Scholar] [CrossRef]
- Walker, J.; Burke, K.; Wanat, M.; Fisher, C.; Fielding, J.; Quinlan, R.; Coker, O.; Murray, G.; Sharpe, M. The prevalence of depression in general hospital inpatients: a systematic review and meta-analysis of interview-based studies. Psychol. Med. 2018, 48, 2285–2298. [Google Scholar] [CrossRef]
- Guo, Y.; Sims, O.T.; Qin, W.; Yang, F. Factors associated with symptoms of depression and psychological distress during the COVID-19 pandemic. Behav. Sci. 2021, 11, 13. [Google Scholar] [CrossRef]
- Ustun, G. Determining depression and related factors in a society affected by COVID-19 pandemic. Int. J. Soc. Psychiatry. 2021, 67, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Notivol, J.; Gracia-García, P.; Olaya, B.; Lasheras, I.; López-Antón, R.; Santabárbara, J. Prevalence of depression during the COVID-19 outbreak: A meta-analysis of community-based studies. Int. J. Clin. Health Psychol. 2021, 21, 100196. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Sanacora, G.; Duman, R.S.; Krystal, J.H. Ketamine and rapid-acting antidepressants: A window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 2015, 66, 509–523. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef]
- Sisay, T.; Wami, R. Adverse drug reactions among major depressive disorders: Patterns by age and gender. Heliyon. 2021, 7, e08655. [Google Scholar] [CrossRef]
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; de Girolamo, G.; Florescu, S.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef]
- Keyloun, K.R.; Hansen, R.N.; Hepp, Z.; Gillard, P.; Thase, M.E.; Devine, E.B. Adherence and persistence across antidepressant therapeutic classes: A retrospective claims analysis among insured US patients with major depressive disorder (MDD). CNS Drugs. 2017, 31, 421–432. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and emerging treatments for major depression. Lancet. 2023, 401, 141–153. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef]
- Elhwuegi, A. S. Central monoamines and their role in major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 435–451. [Google Scholar] [CrossRef]
- Kusmider, M.; Faron-Górecka, A.; Solich, J.; Pabian, P.; Dziedzicka-Wasylewska, M. Time-course of changes in key catecholaminergic receptors and trophic systems in rat brain after antidepressant administration. Neurochem. Int. 2020, 141, 104885. [Google Scholar] [CrossRef] [PubMed]
- Owens, M. J. Molecular and cellular mechanisms of antidepressant drugs. Depress. Anxiety. 1996, 4, 153–159. [Google Scholar] [CrossRef]
- Shelton, R. C. Cellular mechanisms in the vulnerability to depression and response to antidepressants. Psychiatr. Clin. North Am. 2000, 23, 713–729. [Google Scholar] [CrossRef]
- Jarończyk, M.; Walory, J. Novel molecular targets of antidepressants. Molecules. 2022, 27, 533. [Google Scholar] [CrossRef]
- Sharp, T. Molecular and cellular mechanisms of antidepressant action. Behav. Neurobiol. Depress. Treat. 2013, 309–325. [Google Scholar] [CrossRef]
- Di Benedetto, B.; Radecke, J.; Schmidt, M.V.; Rupprecht, R. Acute antidepressant treatment differently modulates ERK/MAPK activation in neurons and astrocytes of the adult mouse prefrontal cortex. Neuroscience. 2013, 232, 161–168. [Google Scholar] [CrossRef]
- Thompson, S. M. Plasticity of synapses and reward circuit function in the genesis and treatment of depression. Neuropsychopharmacology. 2023, 48, 90–103. [Google Scholar] [CrossRef]
- Duman, R. S.; Heninger, G. R.; Nestler, E. J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Neumann, M.; Hansen, K.; Hong, S. M.; Kim, S.; Noble-Haeusslein, L. J.; Liu, J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J. Neurotrauma. 2011, 28, 259–268. [Google Scholar] [CrossRef]
- Song, T.; Wu, H.; Li, R.; Xu, H.; Rao, X.; Gao, L. ;... & Lei, H. Repeated fluoxetine treatment induces long-lasting neurotrophic changes in the medial prefrontal cortex of adult rats. Behav. Brain Res. 2019, 365, 114–124. [Google Scholar] [CrossRef]
- Guirado, R.; Varea, E.; Castillo-Gómez, E.; Gómez-Climent, M. A.; Rovira-Esteban, L.; Blasco-Ibáñez, J. M.; Nacher, J. Effects of chronic fluoxetine treatment on the rat somatosensory cortex: activation and induction of neuronal structural plasticity. Neurosci. Lett. 2009, 457, 12–15. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, Y.; Tan, H. J. Chronic fluoxetine treatment reverses depressive-like behaviors in mice via enhancing neuroplasticity. J. Pharmacol. Pharmacother. 2023, 14, 259–267. [Google Scholar] [CrossRef]
- Djordjevic, A.; Djordjevic, J.; Elaković, I.; Adzic, M.; Matić, G.; Radojcic, M. B. Fluoxetine affects hippocampal plasticity, apoptosis and depressive-like behavior of chronically isolated rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Varea, E.; Guirado, R.; Gilabert-Juan, J.; Martí, U.; Castillo-Gómez, E.; Blasco-Ibáñez, J. M. ;... & Nacher, J. Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. J. Psychiatr. Res. 2012, 46, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zavvari, F.; Nahavandi, A.; Goudarzi, M. Fluoxetine attenuates stress-induced depressive-like behavior through modulation of hippocampal GAP43 and neurogenesis in male rats. J. Chem. Neuroanat. 2020, 103, 101711. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Page, M.E.; Lucki, I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology. 2005, 182, 335–344. [Google Scholar] [CrossRef]
- Tsugiyama, L.E.; Moraes, R.C.M.; Moraes, Y.A.C.; Francis-Oliveira, J. Promising new pharmacological targets for depression: The search for efficacy. Drug Discov. Today. 2023, 28, 103804. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Hollander, E.; Nutt, D.; Blier, P. Clinical evidence and potential neurobiological underpinnings of unresolved symptoms of depression. J. Clin. Psychiatry. 2008, 69, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; StarD Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STARD: implications for clinical practice. Am. J. Psychiatry. 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Sharpley, A.L.; Cowen, P.J. Effect of pharmacologic treatments on the sleep of depressed patients. Biol. Psychiatry. 1995, 37, 85–98. [Google Scholar] [CrossRef]
- Harrigan, R.A.; Brady, W.J. ECG abnormalities in tricyclic antidepressant ingestion. Am. J. Emerg. Med. 1999, 17, 387–393. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Baumann, J.; Wheeler-Castillo, C.; Latov, D.; Henter, I.D.; Salvadore, G.; Zarate Jr, C.A. The timing of antidepressant effects: A comparison of diverse pharmacological and somatic treatments. Pharmaceuticals. 2010, 3, 19–41. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry. 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61, 4–6. [Google Scholar] [PubMed]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013, 27, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1. [Google Scholar] [CrossRef]
- Vaishnavi, S.N.; Nemeroff, C.B.; Plott, S.J.; Rao, S.G.; Kranzler, J.; Owens, M.J. Milnacipran: a comparative analysis of human monoamine uptake and transporter binding affinity. Biol. Psychiatry. 2004, 55, 320–322. [Google Scholar] [CrossRef]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef]
- Stahl, S.M.; Grady, M.M.; Moret, C.; Briley, M. SNRIs: the pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005, 10, 732–747. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Thase, M.E.; Fava, M.; Nelson, J.C.; Shelton, R.C. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol. Psychiatry. 2007, 62, 1217–1227. [Google Scholar] [CrossRef]
- de Silva, V.A.; Hanwella, R. Efficacy and tolerability of venlafaxine versus specific serotonin reuptake inhibitors in treatment of major depressive disorder: A meta-analysis of published studies. Int. Clin. Psychopharmacol. 2012, 27, 8–16. [Google Scholar] [CrossRef]
- Fava, M.; Rush, A.J.; Thase, M.E.; Clayton, A.; Stahl, S.M.; Pradko, J.F.; Johnston, J.A. Fifteen years of clinical experience with bupropion HCl: From bupropion to bupropion SR to bupropion XL. Prim. Care Companion J. Clin. Psychiatry. 2005, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Laudon, M.; Frydman-Marom, A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int. J. Mol. Sci. 2014, 15, 15924–15950. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhu, X.; Gillespie, A.; Feng, Y.; Zhou, J.; Chen, X. ;... & Chen, R. Effectiveness of mirtazapine as add-on to paroxetine v. paroxetine or mirtazapine monotherapy in patients with major depressive disorder with early non-response to paroxetine: a two-phase, multicentre, randomized, double-blind clinical trial. Psychol. Med. 2021, 51, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Bang-Andersen, B.; Ruhland, T.; Jørgensen, M.; Smith, G.; Frederiksen, K.; Jensen, K.G.; Stensbøl, T.B. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl) phenyl] piperazine (Lu AA21004): A novel multimodal compound for the treatment of major depressive disorder. J. Med. Chem. 2011, 54, 3206–3221. [Google Scholar] [CrossRef] [PubMed]
- Nikayin, S.; Murphy, E.; Krystal, J.H.; Wilkinson, S.T. Long-term safety of ketamine and esketamine in treatment of depression. Expert Opin. Drug Saf. 2022, 21, 777–787. [Google Scholar] [CrossRef]
- Khabir, Y.; Hashmi, M.R.; Asghar, A.A. Rapid-acting oral drug (Auvelity) for major depressive disorder. Ann. Med. Surg. 2022, 82. [Google Scholar] [CrossRef]
- Cornett, E.M.; Rando, L.; Labbé, A.M.; Perkins, W.; Kaye, A.M.; Kaye, A.D.; Urits, I. Brexanolone to treat postpartum depression in adult women. Psychopharmacol. Bull. 2021, 51, 115. [Google Scholar]
- Blier, P.; Ward, H.E.; Tremblay, P.; Laberge, L.; Hébert, C.; Bergeron, R. Combination of antidepressant medications from treatment initiation for major depressive disorder: A double-blind randomized study. Am. J. Psychiatry. 2010, 167, 281–288. [Google Scholar] [CrossRef]
- Kato, T.; Furukawa, T.A.; Mantani, A.; Kurata, K.I.; Kubouchi, H.; Hirota, S. ; SUN☺ D Investigators. Optimising first-and second-line treatment strategies for untreated major depressive disorder—the SUN☺ D study: A pragmatic, multi-centre, assessor-blinded randomised controlled trial. BMC Med. 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Henssler, J.; Alexander, D.; Schwarzer, G.; Bschor, T.; Baethge, C. Combining antidepressants vs antidepressant monotherapy for treatment of patients with acute depression: A systematic review and meta-analysis. JAMA Psychiatry. 2022, 79, 300–312. [Google Scholar] [CrossRef]
- Fornaro, M.; Martino, M.; Mattei, C.; Prestia, D.; Vinciguerra, V.; De Berardis, D.; Fornaro, P. Duloxetine-bupropion combination for treatment-resistant atypical depression: A double-blind, randomized, placebo-controlled trial. Eur. Neuropsychopharmacol. 2014, 24, 1269–1278. [Google Scholar] [CrossRef]
- Mørk, A.; Pehrson, A.; Brennum, L.T.; Nielsen, S.M.; Zhong, H.; Lassen, A.B.; Stensbøl, T.B. Pharmacological effects of Lu AA21004: A novel multimodal compound for the treatment of major depressive disorder. J. Pharmacol. Exp. Ther. 2012, 340, 666–675. [Google Scholar] [CrossRef]
- Kanes, S.J.; Colquhoun, H.; Doherty, J.; Raines, S.; Hoffmann, E.; Rubinow, D.R.; Meltzer-Brody, S. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum. Psychopharmacol. 2017, 32, e2576. [Google Scholar] [CrossRef] [PubMed]
- Melón, L.; Hammond, R.; Lewis, M.; Maguire, J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front. Endocrinol. 2018, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Briefing document: New Drug Application 211371/New Drug Application, brexanolone for the treatment of postpartum depression. 2018. (accessed on 30 May 2024).
- Patterson, R.; Balan, I.; Morrow, A.L.; Meltzer-Brody, S. Novel neurosteroid therapeutics for post-partum depression: perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology. 2024, 49, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.M.; Bowden, C.L.; Frazer, A. Rethinking depression and the actions of antidepressants: uncovering the links between the neural and behavioral elements. J. Affect. Disord. 2010, 120, 16–23. [Google Scholar] [CrossRef]
- Feighner, J.P.; Entsuah, A.R.; McPherson, M.K. Efficacy of once-daily venlafaxine extended release (XR) for symptoms of anxiety in depressed outpatients. J. Affect. Disord. 1998, 47, 55–62. [Google Scholar] [CrossRef]
- Cryan, J.F.; Lucki, I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine2C receptors. J. Pharmacol. Exp. Ther. 2000, 295, 1120–1126. [Google Scholar] [PubMed]
- Estrada-Camarena, E.; Fernández-Guasti, A.; López-Rubalcava, C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003, 28, 830–838. [Google Scholar] [CrossRef]
- Detke, M.J.; Johnson, J.; Lucki, I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp. Clin. Psychopharmacol. 1997, 5, 107. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J. F.; Cueto-Escobedo, J.; Flores-Aguilar, L. Á.; Rosas-Sánchez, G. U.; Rovirosa-Hernández, M. D. J.; García-Orduña, F.; Carro-Juárez, M. The aqueous crude extracts of Montanoa frutescens and Montanoa grandiflora reduce immobility faster than fluoxetine through GABAA receptors in rats forced to swim. J. Evid.-Based Integr. Med. 2018, 23, 2515690X18762953. [Google Scholar] [CrossRef]
- Mombereau, C.; Gur, T.L.; Onksen, J.; Blendy, J.A. Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int. J. Neuropsychopharmacol. 2010, 13, 321–334. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Yan, L.; Zhang, Y.; Yang, Z.; Wang, J.; Song, C. EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction, and hippocampal apoptosis signaling in a chronic stress-induced rat model of depression. Int. J. Mol. Sci. 2020, 21, 1769. [Google Scholar] [CrossRef]
- Kang, Z.; Ye, H.; Chen, T.; Zhang, P. Effect of electroacupuncture at siguan acupoints on expression of BDNF and TrkB proteins in the hippocampus of post-stroke depression rats. J. Mol. Neurosci. 2021, 71, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Atli, M.; Bennett, J.C.; Croal, M.; DeBattista, C.; Dunlop, B.W.; Feifel, D.; Hellerstein, D.J.; Husain, M.I.; Kelly, J.R.; Lennard-Jones, M.R.; Licht, R.W.; Marwood, L.; Mistry, S.; Páleníček, T.; Redjep, O.; Repantis, D.; Schoevers, R.A.; Malievskaia, E. Single-dose psilocybin for a treatment-resistant episode of major depression: Impact on patient-reported depression severity, anxiety, function, and quality of life. J. Affect. Disord. 2023, 327, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Kavalali, E.T.; Monteggia, L.M. Ketamine and rapid antidepressant action: New treatments and novel synaptic signaling mechanisms. Neuropsychopharmacology. 2024, 49, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.I.T.C.; Cavalcanti-Ribeiro, P.; Palhano-Fontes, F.; Gonçalves, K.T.D.C.; Nunes, E.A.; Lima, N.B.M.; Santos, N.C.; Brito, A.J.C.; de Araujo, D.B.; Galvão-Coelho, N.L. Rapid and long-lasting effects of subcutaneous esketamine on suicidality: An open-label study in patients with treatment-resistant depression. J. Psychiatr. Res. 2024, 176, 254–258. [Google Scholar] [CrossRef]
- Chrenek, C.; Duong, B.; Khullar, A.; McRee, C.; Thomas, R.; Swainson, J. Use of ketamine for treatment resistant depression: Updated review of literature and practical applications to a community ketamine program in Edmonton, Alberta, Canada. Front. Psychiatry. 2024, 14, 1283733. [Google Scholar] [CrossRef]
- Epperson, C.N.; Rubinow, D.R.; Meltzer-Brody, S.; Deligiannidis, K.M.; Riesenberg, R.; Krystal, A.D.; Bankole, K.; Huang, M.Y.; Li, H.; Brown, C.; Kanes, S.J.; Lasser, R. Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. J. Affect. Disord. 2023, 320, 353–359. [Google Scholar] [CrossRef]
- Hjorth, S.; Bengtsson, H.J.; Kullberg, A.; Carlzon, D.; Peilot, H.; Auerbach, S.B. Serotonin autoreceptor function and antidepressant drug action. J. Psychopharmacol. 2000, 14, 177–185. [Google Scholar] [CrossRef]
- Lino-de-Oliveira, C.; Sales, A.J.; Del Bel, E.A.; Silveira, M.C.; Guimarães, F.S. Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res. Bull. 2001, 55, 747–754. [Google Scholar] [CrossRef]
- Possamai-Della, T.; Dal-Pont, G.C.; Resende, W.R.; Aguiar-Geraldo, J.M.; Peper-Nascimento, J.; Quevedo, J.; Valvassori, S.S. Imipramine can be effective on depressive-like behaviors, but not on neurotrophic factor levels in an animal model for bipolar disorder induced by ouabain. Mol. Neurobiol. 2022, 59, 7170–7181. [Google Scholar] [CrossRef]
- Cryan, J.F.; O'Leary, O.F.; Jin, S.H.; Friedland, J.C.; Ouyang, M.; Hirsch, B.R.; Page, M.E.; Dalvi, A.; Thomas, S.A.; Lucki, I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 8186–8191. [Google Scholar] [CrossRef]
- Sgambato, V. The Serotonin 4 Receptor Subtype: A Target of Particular Interest, Especially for Brain Disorders. Int. J. Mol. Sci. 2024, 25, 5245. [Google Scholar] [CrossRef] [PubMed]
- Koncz, S.; Papp, N.; Pothorszki, D.; Bagdy, G. (S)-Ketamine but Not (R)-Ketamine Shows Acute Effects on Depression-Like Behavior and Sleep-Wake Architecture in Rats. Int. J. Neuropsychopharmacol. 2023, 26, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Siwek, A.; Starowicz, G.; Librowski, T.; Nowak, G.; Drabik, U.; Gajdosz, R.; Popik, P. Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor. Neuropharmacology. 2015, 99, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Kounelis-Wuillaume, S.K.; Gheidi, A.; Morrow, J.D.; Spencer-Segal, J.L.; Watson, B.O. Sex- and stress-dependent effects of a single injection of ketamine on open field and forced swim behavior. Stress. 2021, 24, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.J.; Yen, J.Y.; Watson, B.O. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE. 2019, 14, e0215554. [Google Scholar] [CrossRef]
- Assis, L.C.; Rezin, G.T.; Comim, C.M.; Valvassori, S.S.; Jeremias, I.C.; Zugno, A.I.; Quevedo, J.; Streck, E.L. Effect of acute administration of ketamine and imipramine on creatine kinase activity in the brain of rats. Rev. Bras. Psiquiatr. 2009, 31, 247–252. [Google Scholar] [CrossRef]
- Garcia, L.S.; Comim, C.M.; Valvassori, S.S.; Réus, G.Z.; Barbosa, L.M.; Andreazza, A.C.; Stertz, L.; Fries, G.R.; Gavioli, E.C.; Kapczinski, F.; Quevedo, J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008, 32, 140–144. [Google Scholar] [CrossRef]
- Hernández-Hernández, E.; Ledesma-Corvi, S.; Jornet-Plaza, J.; García-Fuster, M.J. Fast-acting antidepressant-like effects of ketamine in aged male rats. Pharmacol. Rep. 2024. [Google Scholar] [CrossRef]
- Zhang, G.F.; Wang, J.; Han, J.F.; Guo, J.; Xie, Z. M.; Pan, W.; Yang, J.J.; Sun, K.J. Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model. Neurosci. Lett. 2016, 631, 7–12. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sun, Y.; Wu, Z.J.; Yin, Y.; Liu, R.Y.; Zhang, J.C.; Zhang, Z.J.; Yau, S.Y.; Wu, H.X.; Yuan, T.F.; Zhang, L.; Adzic, M.; Chen, G. Hippocampal PACAP signaling activation triggers a rapid antidepressant response. Mil. Med. Res. 2024, 11, 49. [Google Scholar] [CrossRef]
- Acevedo, J.; Mugarura, N.E.; Welter, A.L.; Johnson, E.M.; Siegel, J.A. The Effects of Acute and Repeated Administration of Ketamine on Memory, Behavior, and Plasma Corticosterone Levels in Female Mice. Neuroscience. 2023, 512, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Gardier, A.M. Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol. Ther. 2019, 199, 58–90. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, J.P.; Nguyen, T.M.L.; Gardier, A.M. Ketamine: A neuropsychotropic drug with an innovative mechanism of action. Biol. Aujourd'hui. 2023, 217, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Tunc-Ozcan, E.; Dunlop, S.; Tsai, Y.H.; Li, F.; Bertossi, R.; Peng, C.Y.; Kessler, J.A. Ketamine's rapid and sustained antidepressant effects are driven by distinct mechanisms. Cell. Mol. Life Sci. 2024, 81, 105. [Google Scholar] [CrossRef]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Chem. Neurosci. 2020, 11, 864–871. [Google Scholar] [CrossRef]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Maćkowiak, M.; Gołembiowska, K. Limbic System Response to Psilocybin and Ketamine Administration in Rats: A Neurochemical and Behavioral Study. Int. J. Mol. Sci. 2023, 25, 100. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Y.; Yao, Y.; Dai, W.; Yin, Y.; Wang, G.; Li, Y.; Zhang, L. Psilocybin promotes neuroplasticity and induces rapid and sustained antidepressant-like effects in mice. J. Psychopharmacol (Oxford, England) 2024, 38, 489–499. [Google Scholar] [CrossRef]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; Kuutti, M.; Fred, S.M.; Elsilä, L.V.; Sakson, S.; Cannarozzo, C.; Diniz, C.R.A.F.; Seiffert, N.; Rubiolo, A.; Haapaniemi, H.; Meshi, E.; Castrén, E. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Jefsen, O.; Højgaard, K.; Christiansen, S.L.; Elfving, B.; Nutt, D.J.; Wegener, G.; Müller, H.K. Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatr. 2019, 31, 213–219. [Google Scholar] [CrossRef]
- Kolasa, M.; Nikiforuk, A.; Korlatowicz, A.; Solich, J.; Potasiewicz, A.; Dziedzicka-Wasylewska, M.; Bugno, R.; Hogendorf, A.; Bojarski, A.; Faron-Górecka, A. Unraveling psilocybin's therapeutic potential: Behavioral and neuroplasticity insights in Wistar-Kyoto and Wistar male rat models of treatment-resistant depression. Psychopharmacology. 2024. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroid replacement therapy for catamenial epilepsy, postpartum depression and neuroendocrine disorders in women. J. Neuroendocrinol. 2022, 34, e13028. [Google Scholar] [CrossRef]
- Contreras, C.M.; Azamar-Arizmendi, G.; Saavedra, M.; Hernández-Lozano, M. A five-day gradual reduction regimen of chlormadinone reduces premenstrual anxiety and depression: A pilot study. Arch. Med. Res. 2006, 37, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Kaur, G.; Kulkarni, S.K. Sigma (sigma1) receptor mediated anti-depressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport. 1998, 9, 3069–3073. [Google Scholar] [CrossRef]
- Khisti, R.T.; Chopde, C.T.; Jain, S.P. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol. Biochem. Behav. 2000, 67, 137–143. [Google Scholar] [CrossRef]
- Rodríguez-Landa, J.F.; Contreras, C.M.; García-Ríos, R.I. Allopregnanolone microinjected into the lateral septum or dorsal hippocampus reduces immobility in the forced swim test: participation of the GABAA receptor. Behav. Pharmacol. 2009, 20, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Landa, J.F.; Contreras, C.M.; Bernal-Morales, B.; Gutiérrez-García, A.G.; Saavedra, M. Allopregnanolone reduces immobility in the forced swimming test and increases the firing rate of lateral septal neurons through actions on the GABAA receptor in the rat. J. Psychopharmacol. (Oxf.) 2007, 21, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S.; Mbilinyi, R.H.; Estes, E. Preclinical and clinical pharmacology of brexanolone (allopregnanolone) for postpartum depression: a landmark journey from concept to clinic in neurosteroid replacement therapy. Psychopharmacology. 2023, 240, 1841–1863. [Google Scholar] [CrossRef]
- Shirayama, Y.; Fujita, Y.; Oda, Y.; Iwata, M.; Muneoka, K.; Hashimoto, K. Allopregnanolone induces antidepressant-like effects through BDNF-TrkB signaling independent from AMPA receptor activation in a rat learned helplessness model of depression. Behav. Brain Res. 2020, 390, 112670. [Google Scholar] [CrossRef] [PubMed]
- Cueto-Escobedo, J.; Andrade-Soto, J.; Lima-Maximino, M.; Maximino, C.; Hernández-López, F.; Rodríguez-Landa, J.F. Involvement of GABAergic system in the antidepressant-like effects of chrysin (5,7-dihydroxyflavone) in ovariectomized rats in the forced swim test: Comparison with neurosteroids. Behav. Brain Res. 2020, 386, 112590. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Salvadore, G.; Luckenbaugh, D.A.; Manji, H.K.; Zarate, C.A., Jr. Rapid onset of antidepressant action: A new paradigm in the research and treatment of major depressive disorder. J. Clin. Psychiatry. 2008, 69, 946–958. [Google Scholar] [CrossRef]
- Brymer, K.J.; Johnston, J.; Botterill, J.J.; Romay-Tallon, R.; Mitchell, M.A.; Allen, J.; Pinna, G.; Caruncho, H.J.; Kalynchuk, L.E. Fast-acting antidepressant-like effects of Reelin evaluated in the repeated-corticosterone chronic stress paradigm. Neuropsychopharmacology. 2020, 45, 1707–1716. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, Y.; Shanley, M.R.; Morel, C.; Ku, S.M.; Zhang, H.; Shen, Y.; Friedman, A.K.; Han, M.H. HCN channel inhibitor induces ketamine-like rapid and sustained antidepressant effects in chronic social defeat stress model. Neurobiol. Stress. 2023, 26, 100565. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet. 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, H.; Nie, K.; Gao, Y.; Su, H.; Wang, Z.; Dong, H. Traditional herbal formula Jiao-tai-wan improves chronic restrain stress-induced depression-like behaviors in mice. Biomed. Pharmacother. 2022, 153, 113284. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Suckow, R.F.; Cooper, T.B.; O’Connor, C.M.; Malinin, A.I.; Krishnan, K.R.R. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. Am. J. Psychiatry. 2005, 162, 1165–1170. [Google Scholar] [CrossRef]
- DeLucia, V.; Kelsberg, G.; Safranek, S. Which SSRIs most effectively treat depression in adolescents? J. Fam. Pract. 2016, 65, 632–634. [Google Scholar]
- Vermetten, E.; Vythilingam, M.; Schmahl, C.; De Kloet, C.; Southwick, S.M.; Charney, D.S.; Bremner, J.D. Alterations in stress reactivity after long-term treatment with paroxetine in women with posttraumatic stress disorder. Ann. N.Y. Acad. Sci. 2006, 1071, 184–202. [Google Scholar] [CrossRef]
- Chu, A.; Wadhwa, R. Selective serotonin reuptake inhibitors. In StatPearls [Internet]. StatPearls Publishing 2022.
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.M. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Hu, B.; Doods, H.; Treede, R.D.; Ceci, A. Duloxetine and 8-OH-DPAT, but not fluoxetine, reduce depression-like behaviour in an animal model of chronic neuropathic pain. Neurosci. Lett. 2016, 619, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, X.; Yu, P.; Sheng, T.; Wang, Y.; Ye, Y. Crocin ameliorates depressive-like behaviors induced by chronic restraint stress via the NAMPT-NAD+-SIRT1 pathway in mice. Neurochem. Int. 2022, 157, 105343. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Mosalam, E.M.; Zidan, A.A.A.; Elattar, K.S.; Zaki, S.A.; Ramadan, A.N.; Ebeid, A.M. RETRACTED ARTICLE: The Antidiabetic Metformin as an Adjunct to Antidepressants in Patients with Major Depressive Disorder: A Proof-of-Concept, Randomized, Double-Blind, Placebo-Controlled Trial. Neurotherapeutics. 2020, 17, 1897–1906. [Google Scholar] [CrossRef]
- Hayley, S.; Litteljohn, D. Neuroplasticity and the next wave of antidepressant strategies. Front. Cell. Neurosci. 2013, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Levy, M. J.; Boulle, F.; Steinbusch, H. W.; van den Hove, D. L.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology. 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, N.; Li, T. VEGF regulates antidepressant effects of lamotrigine. Eur. Neuropsychopharmacol. 2012, 22, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Dantzer, R.; Kelley, K.W.; McCusker, R.H. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflamm. 2011, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C. ;... & Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol 2015, 18, pyu077. [Google Scholar] [CrossRef]
- Huang, Y.J.; Lane, H.Y.; Lin, C.H. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural Plast. 2017, 2017, 4605971. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Corvi, S.; Jornet-Plaza, J.; García-Fuster, M.J. Aromatase inhibition and ketamine in rats: Sex-differences in antidepressant-like efficacy. Biol. Sex Differ. 2023, 14, 73. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Z.; Lan, T.; Wu, Y.; Li, Y.; Wang, C.; Jian, W.; Yu, S.Y. Levomilnacipran ameliorates lipopolysaccharide-induced depression-like behaviors and suppressed the TLR4/Ras signaling pathway. Int. Immunopharmacol. 2023, 122, 110595. [Google Scholar] [CrossRef]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Yeoh, K.; Turck, C.W. Chronic fluoxetine treatment of socially isolated rats modulates prefrontal cortex proteome. Neuroscience. 2022, 501, 52–71. [Google Scholar] [CrossRef]
- Seo, M.K.; Lee, J.G.; Park, S.W. Effects of escitalopram and ibuprofen on a depression-like phenotype induced by chronic stress in rats. Neurosci. Lett. 2019, 696, 168–173. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, L.; Yang, J.Q.; Luo, Y.; Cui, T.; Du, T.T.; Jiang, X.H. Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid. Genes Dis. 2018, 6, 167–175. [Google Scholar] [CrossRef]
- Alkon, D.L.; Hongpaisan, J.; Sun, M.K. Effects of chronic bryostatin-1 on treatment-resistant depression in rats. Eur. J. Pharmacol. 2017, 807, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Elias, E.; Zhang, A.Y.; Manners, M.T. Novel pharmacological approaches to the treatment of depression. Life. 2022, 12, 196. [Google Scholar] [CrossRef]
- Pedersen, M. E.; Szewczyk, B.; Stachowicz, K.; Wieronska, J.; Andersen, J.; Stafford, G. I.; van Staden, J.; Pilc, A.; Jäger, A. K. Effects of South African traditional medicine in animal models for depression. J. Ethnopharmacol. 2008, 119, 542–548. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin improves anxiety and reserpine-induced depression in rats. Molecules. 2022, 27, 7313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, X.M.; Tian, J.S.; Gao, X.X.; Du, G.H.; Zhou, Y.Z. Integrated network pharmacology and metabolomics to dissect the combination mechanisms of Bupleurum chinense DC-Paeonia lactiflora Pall herb pair for treating depression. J. Ethnopharmacol. 2021, 264, 113281. [Google Scholar] [CrossRef]
- Luo, Y.; Zhong, Z.; Li, H.; Wang, L.; Guo, D.; Dong, X.; Liu, J.; Xie, M.; Wu, M.; Xiang, Y.; Zhang, X.; Meng, P. Integrating serum metabolomics and network analysis to explore the antidepressant activity of crocin in rats with chronic unexpected mild stress-induced depression. Pharm. Biol. 2023, 61, 1414–1430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, M.; Lu, X.; Wei, R.; Xu, J. Ziziphi spinosae lily powder suspension in the treatment of depression-like behaviors in rats. BMC Complement. Altern. Med. 2017, 17, 238. [Google Scholar] [CrossRef]
- Adeoluwa, O.A.; Olayinka, J.N.; Adeoluwa, G.O.; Akinluyi, E.T.; Adeniyi, F.R.; Fafure, A.; Nebo, K.; Edem, E.E.; Eduviere, A.T.; Abubakar, B. Quercetin abrogates lipopolysaccharide-induced depressive-like symptoms by inhibiting neuroinflammation via microglial NLRP3/NFκB/iNOS signaling pathway. Behav. Brain Res. 2023, 450, 114503. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017, 79, 40–48. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Niu, X.; Tang, C.; Wang, H.; Gao, J.; Hu, J. Probiotics alleviate depressive behavior in chronic unpredictable mild stress rat models by remodeling intestinal flora. NeuroReport. 2021, 32, 686–693. [Google Scholar] [CrossRef]
- Daugé, V.; Philippe, C.; Mariadassou, M.; Rué, O.; Martin, J.C.; Rossignol, M.N. ;... & Naudon, L. A probiotic mixture induces anxiolytic-and antidepressive-like effects in fischer and maternally deprived long evans rats. Front. Behav. Neurosci. 2020, 14, 581296. [Google Scholar] [CrossRef]
| Class | Pharmacological target | Mechanism of action | Reference |
| Monoamine Oxidase Inhibitors (IMAO) | |||
| Selegiline | Monoamine Oxidase Enzyme Type A and B. | Prevents the degradation of 5-hydroxytryptamine (5-HT); increases the availability of 5-HT in the synapses. | [59,60]. |
| Fenelzina | |||
| Tricyclic antidepressants (TCA) | |||
| Amitriptyline | 5-HT reuptake transporter and norepinephrine (NE) reuptake transporter. | Inhibits NE and 5-HT reuptake transporters; increases the availability of 5-HT and NE at the synapses; binds to postsynaptic noradrenaline, histamine and acetylcholine receptors. | [58,59]. |
| Imipramine | |||
| Nortriptyline | |||
| Selective serotonin reuptake inhibitors (SSRIs) | |||
| Fluoxetine | 5-HT reuptake transporters | In particular, they inhibit the reuptake of 5-HT, which increases the availability of serotonin in the synapse. | [58,60,64]. |
| Paroxetine | |||
| Escitalopram | |||
| Sertraline | |||
| Specific noradrenergic and serotonergic antidepressants | |||
| Mirtazapine | α2 NE receptors and 5-HT receptors | α-2 NE receptor antagonists that cause an increased release of 5-HT and NE; they act as antagonists/agonists of several specific 5-HT receptors. | [68,73,74,75]. |
| Mianserin | |||
| Norepinephrine and serotonin reuptake inhibitors | |||
| Venlafaxine | 5-HT receptors and NE uptake transporters | Inhibits the reuptake of 5-HT and NE and increases their availability in the synapses. | [75,76]. |
| Duloxetine | |||
| Atypical antidepressants | |||
| Agomelatine | 5-HT receptors, NE receptors and melatonin receptors | Bupropion acts as a DA and NE reuptake inhibitor; vortioxetine acts as an agonist/antagonist of several 5-HT and NE receptors; agomelatine activates melatonin receptors and antagonizes some 5-HT receptors. | [76,77]. |
| Bupropion | |||
| Vortioxetine | |||
| New antidepressants | |||
| Ketamine | Antagonist of the ionotropic glutamate receptor, NMDA 3A. Potentiator of the 5-hydroxytryptamine receptor 3A. Antagonist of the neuronal acetylcholine receptor subunit alpha-7. Inhibitor of nitric oxide synthase brain. Agonist and partial agonist of the dopamine D2 receptor. Agonist of the kappa-type opioid receptor. Antagonist of 5-hydroxytryptamine receptor 2 and 5-hydroxytryptamine receptor 1. |
Ketamine interacts with N-methyl-D-aspartate (NMDA) receptors, opioid receptors, monoaminergic receptors, muscarinic receptors and voltage sensitive Ca ion channels. Unlike other general anaesthetic agents, ketamine does not interact with GABA receptors | [70,71]. |
| Brexenolone | Positive allosteric modulator GABA(A) Receptor | Brexanolone is a neuroactive steroid that occurs naturally in the body (as natural allopregnanolone) when the female sex hormone progesterone is metabolized. This steroid compound is also thought to act as a barbitu-like positive allosteric modulator of synaptic and extrasynaptic GABAA receptors. In this way, brexanolone may enhance the activity of GABA at these receptors by opening the calcium channels of GABAA receptors more frequently and for longer periods of time. It is also thought that brexanolone triggers this effect on GABAA receptors at a binding site that differs from those of benzodiazepines. |
[78,79,80,81]. |
| Drug | Dosage (route of administration, dose and duration of treatment) | Experimental subject | Experimental model | Identified effect | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Ketamine Letrozole |
Ketamine 5 mg/kg. Letrozole 1 mg/kg. 7 days of treatment Intraperitoneal route of administration |
Adult female and male rats of the Sprague-Dawley strain. | Forced Swim Test (FST) | Reduction of FST immobility in men with ketamine and reduction of FST immobility in women with letrozole. The combination of ketamine and letrozole reduces FST immobility in men. | It is assumed that they act via NMDA receptors and could modulate neurotrophic factors in the prefrontal cortex. | [148] |
| Levomilnacipran | 30 mg/kg. 14 days of treatment Intraperitoneal administration route |
Male Wistar rats. | Depression induced with lipopolysaccharides (LPS) 0.5 mg/kg for 2 weeks. Sucrose preference. FST. Open Field Test. |
Levomilnacipran reduces immobility behavior in the FST. It reverses the increase in transcript levels of the proinflammatory cytokines IL-1β, INF-γ and TNF-α. | Levomilnacipran inhibits the activation of the TLR4/NF-κB and Ras/p38 signaling pathways and modulates the ERK/CREB/BDNF pathway. | [149] |
| Fluoxetine | 15 mg/kg/day. 6 weeks Intraperitoneal administration route |
Male Wistar Rats | Chronic social isolation (CSIS) (6 weeks) Sucrose preference FST |
Increased sucrose consumption. Reduction of immobility time in the FST | Expression of calcium/calmodulin-dependent protein kinase 1 (CaMKK1). Phosphorylation of the cAMP-responsive element-binding protein (CREB). Expression of BDNF. |
[150] |
| Escitalopram Ibuprofen |
Escitalopram 10 mg/kg Ibuprofen 40 mg/kg Combination of both 21 days of treatment Intraperitoneal administration route |
Adult male Sprague-Dawley rats | Stress from restriction FST |
Reduction of immobility in FST with individual and combined treatment. Reduction in corticosterone levels. Increase in BDNF and p11 levels. |
Positive regulation of BDNF and p11 | [151] |
| Meloxicam Caffeic acid Sertraline |
Meloxicam 3 mg/kg – 1mg/kg Caffeic acid 30 mg/kg – 10 mg/kg Sertraline 5mg/kg Meloxicam 1mg/kg + Caffeic acid 10mg/kg 21 days of treatment Intraperitoneal administration route |
Adult male Sprague-Dawley rats | CUMS 6 weeks Open Field Test FST |
All treatments reduced immobility in FST. Caffeic acid inhibits NA reduction and increases Trp and MHGP. Meloxicam inhibits NA reduction and increases Trp, MHGP and Tyr. |
Inhibition of COX-2 and reduction of 5-HIAA. | [152] |
| Bryostatin-1 Imipramine |
Bryostatin-1 (20 µg/m2) Intravenous administration by tail Imipramine (15 mg/kg) intraperitoneal administration 5.5 weeks of treatment |
Male Wistar Rats | Open space swimming test Morris water maze Visible platform test. |
Bryostatin-1 reduces immobility after 2 weeks of treatment. Bryostatin-1 restored the rats' ability in spatial learning and spatial memory recall. |
Protein kinase C (PKC)ε activation | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





