Submitted:
31 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
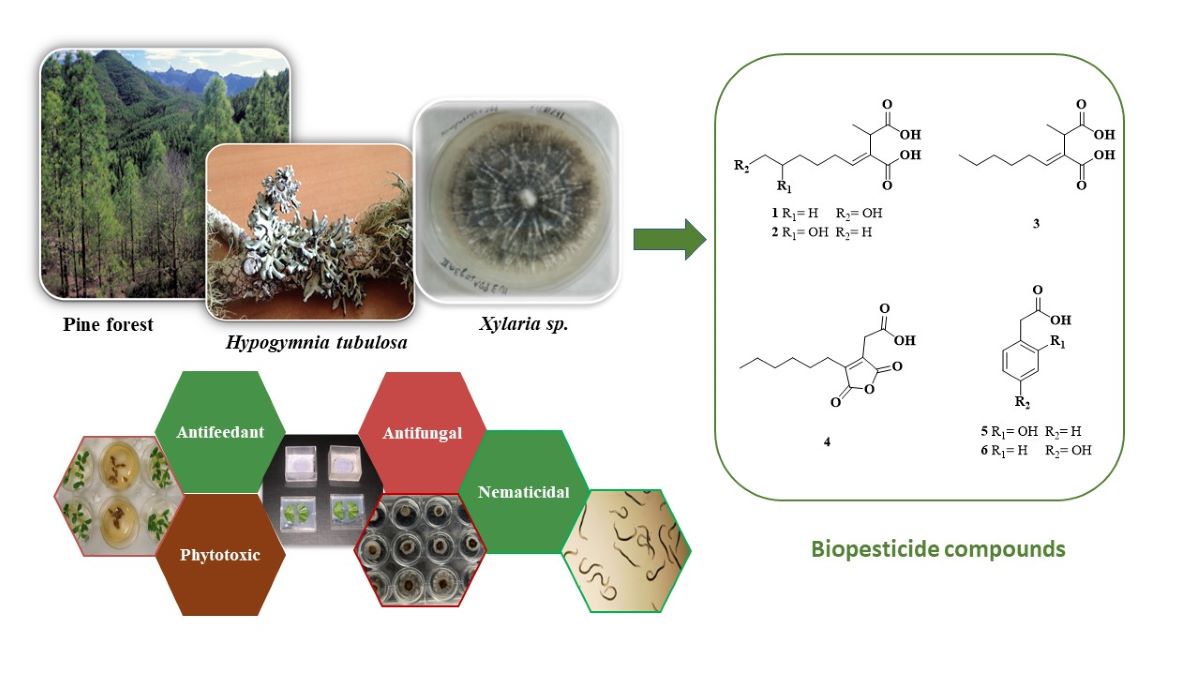
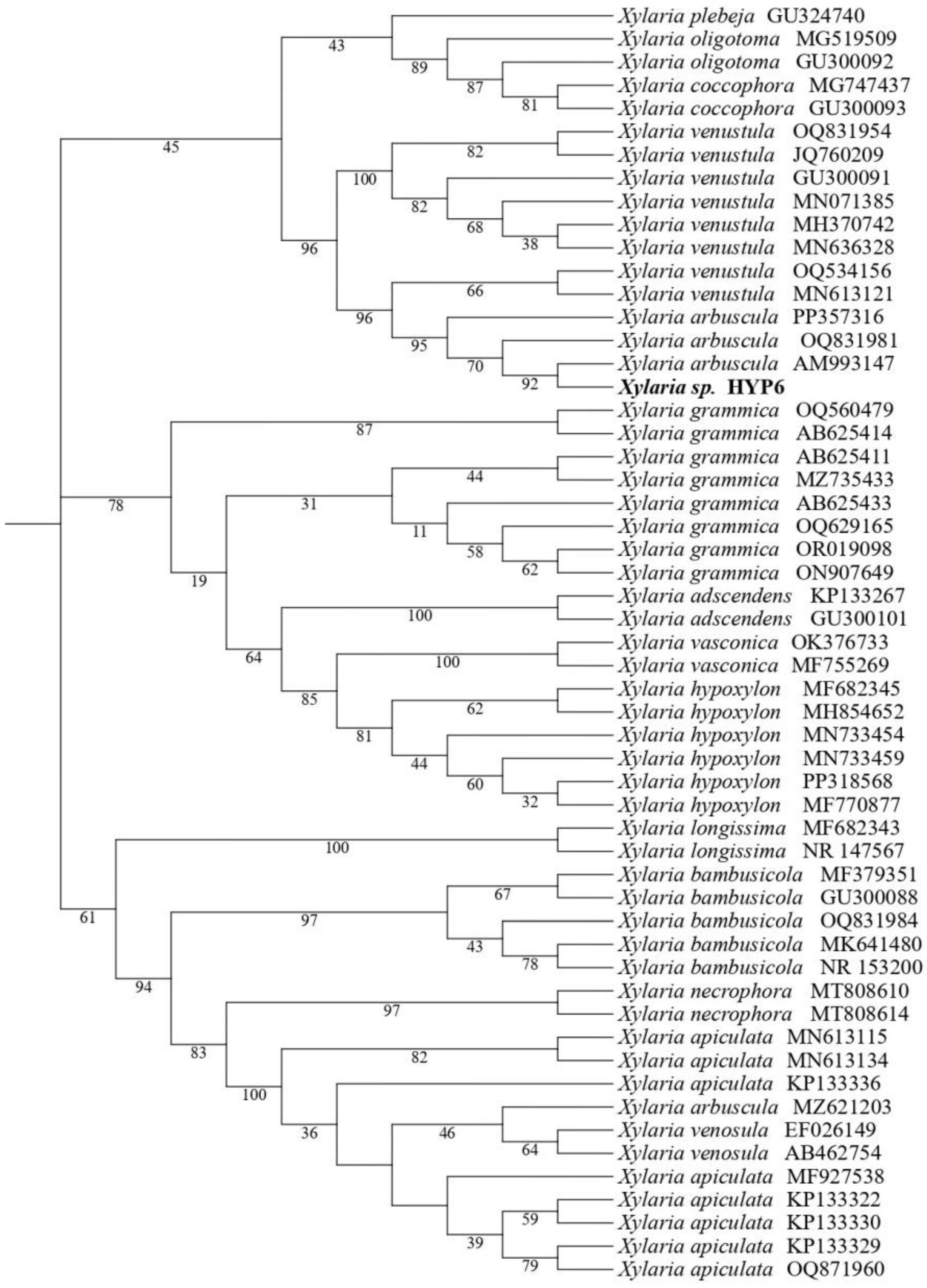
2.1. Fungal Identification
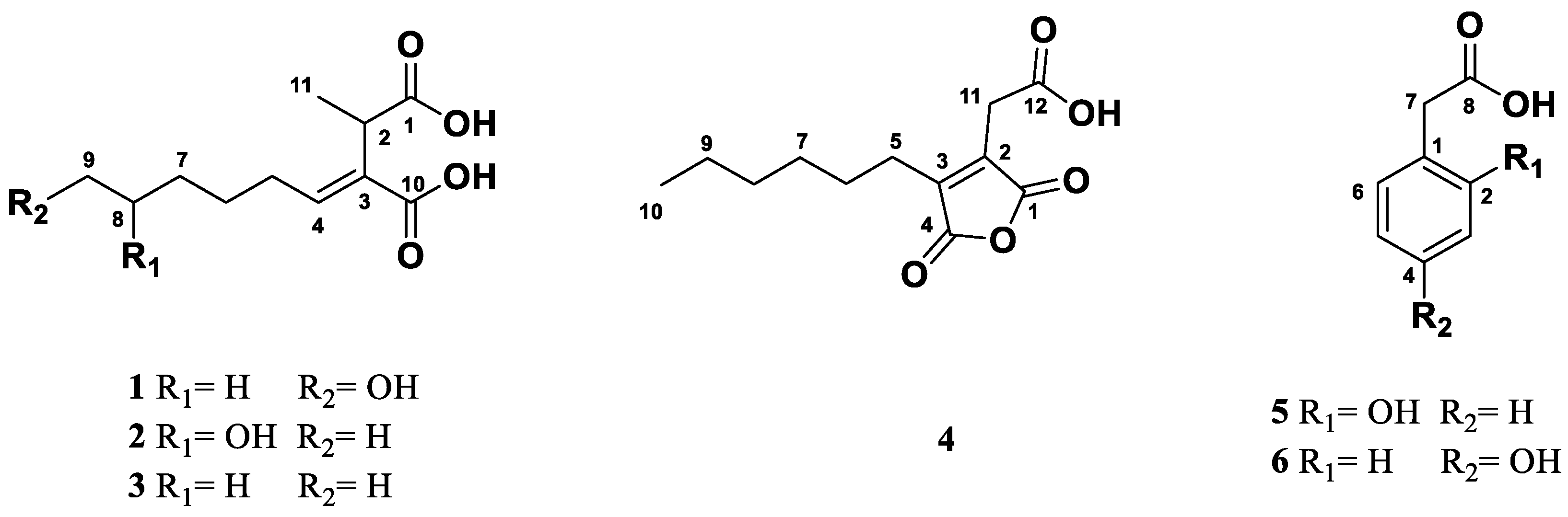
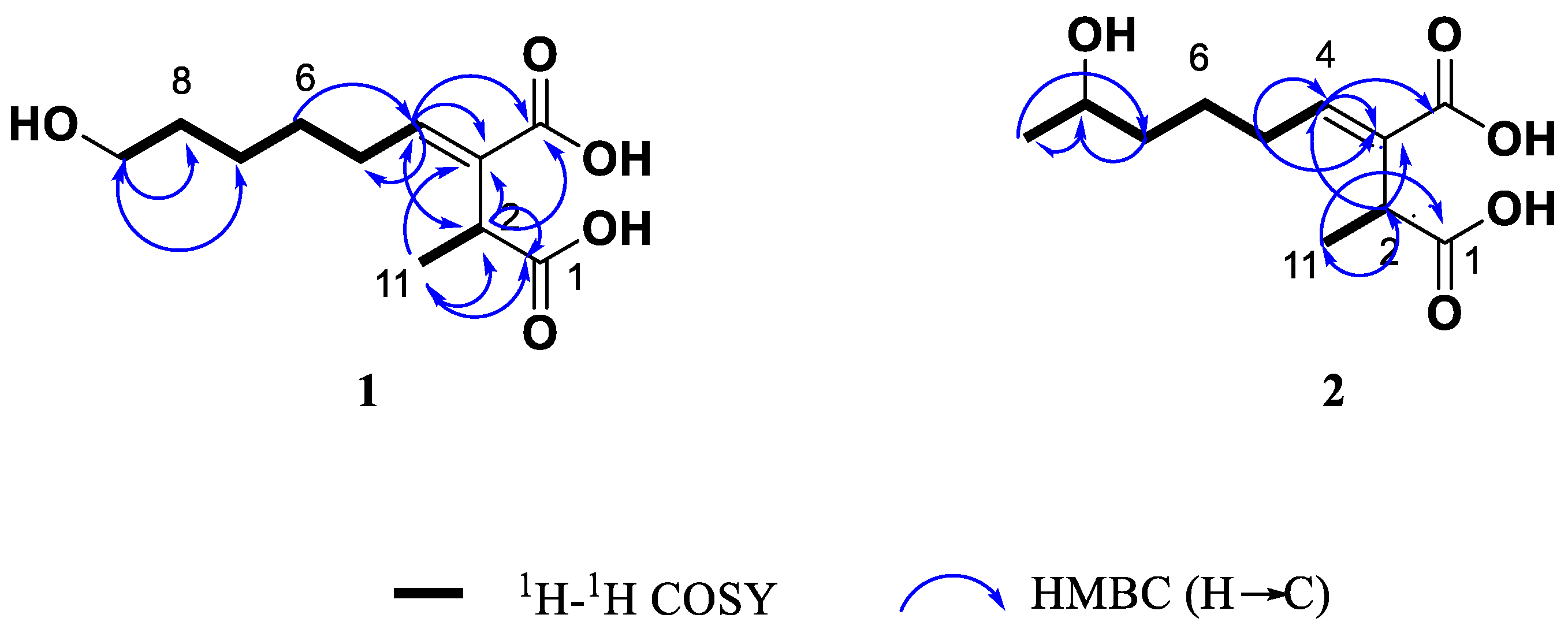
2.2. Structure Elucidation
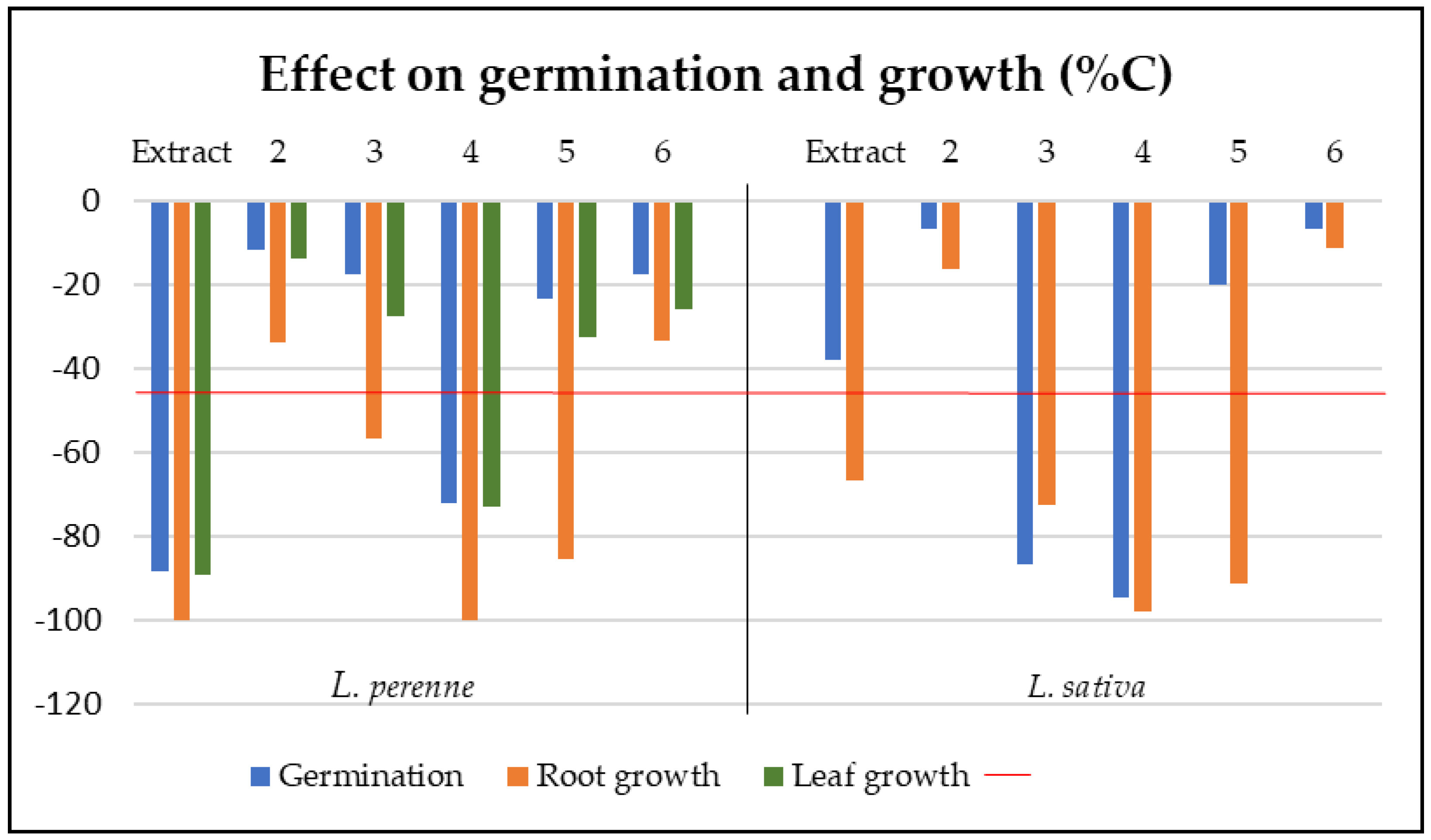
2.3. Biopesticide Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Lichen Material and Isolation of the Endolichenic Fungus HYP6
3.3. Molecular Characterization of HYP6 Strain
3.4. Cultivation of HYP6
3.5. Extraction and Isolation of Compounds
3.6. Spectroscopic Data of Compounds
3.6.1. (+)-9-. Hydroxypiliformic Acid (1)
3.6.2. (+)-8-. Hydroxypiliformic Acid (2)
3.6.3. (+)-Piliformic Acid (2-hexylidene-3-methylsuccinic Acid) (3)
3.6.4. Hexylaconitic Acid A Anhydride (2-carboxymethyl-3-n-hexyl-maleic acid anhydride) (4)
3.6.5. 2-Hydroxyphenylacetic Acid (5)
3.6.6. 4-Hydroxyphenylacetic Acid (6)
3.7. Antifungal Bioassay
3.8. Antifeedant Bioassay
3.9. Nematicidal Bioassay
3.10. Phytotoxic Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kellogg, J.; Raja, H. Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon. Phytochem. Rev. 2017, 16, 271–293. [Google Scholar] [CrossRef]
- Agrawal, S.; Deshmukh, S.K.; Reddy, M.S.; Prasad, R.; Goel, M. Endolichenic fungi: A hidden source of bioactive metabolites, S. Afr. J. Bot. 2020, 134, 163–186. [Google Scholar] [CrossRef]
- Zhang, W.; Ran, Q.; Li, H.; Lou, H. Endolichenic Fungi: A promising medicinal microbial resource to discover bioactive natural molecules-An update. J. Fungi 2024, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Pushpavathi, D.; Krishnamurthy, Y.L. Study on endolichenic fungal assemblage in Parmotrema and Heterodermia lichens of Shivamoga, Karnataka. Mol. Biol. Rep. 2024, 51, 549. [Google Scholar] [CrossRef]
- Miral, A.; Jargeat, P.; Mambu, L.; Rouaud, I.; Tranchimand, S.; Tomasi, S. Microbial community associated with the crustose lichen Rhizocarpon geographicum L. (DC.) living on oceanic seashore: A large source of diversity revealed by using multiple isolation methods. Environ. Microbiol. Rep. 2022, 14, 856–872. [Google Scholar] [CrossRef]
- Santiago, K.A.A; dela Cruz, T.E.E.; Ting, A.S.Y. Endolichenic fungi from common Usnea lichens found in a montane forest in Malaysia: a study on diversity and bioactivity profiling. Asian J. Mycol. 2022, 5, 18–37. [Google Scholar] [CrossRef]
- Vandegrift, R. Xylariales (sordariomycetes, Ascomycota) of the Boston Harbor Islands. Northeastern Nat. 2021, 25, 150–199. [Google Scholar] [CrossRef]
- Thomas, D.C.; Vandegrift, R.; Ludden, A.; Carroll, G.C.; Roy, B.A. Spatial ecology of the fungal genus Xylaria in a tropical cloud forest. Biotropica 2016, 48, 381–393. [Google Scholar] [CrossRef]
- Franco, M.E.E.; Wisecaver, J.H.; Arnold, A.E.; Ju, Y.M.; Slot, J.C.; Ahrendt, S.; Moore, L.P.; Eastman, K.E.; Scott, K.; Konkel, Z.; Mondo, S.J.; Kuo, A.; Hayes, R.D.; Haridas, S.; Andreopoulos, B.; Riley, R.; LaButti, K.; Pangilinan, J.; Lipzen, A.; Amirebrahimi, M.; U'Ren, J.M. Ecological generalism drives hyperdiversity of secondary metabolite gene clusters in xylarialean endophytes. The New phytol. 2022, 233, 1317–1330. [Google Scholar] [CrossRef]
- Miral, A.; Ferron, S.; Rouaud, I.; Slyambayev, D.; Bousarghin, L.; Camuzet, C.; Belouzard, S.; Séron, K.; Le Pogam, P.; Tranchimand, S.; Tomasi, S. Eremoxylarins D-J, antibacterial eremophilane sesquiterpenes discovered from an endolichenic strain of Xylaria hypoxylon. J. Nat. Prod. 2023, 86, 730–738. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jang, J.Y.; Yu, N.H.; Chi, W.J.; Bae, C.H.; Yeo, J.H.; Park, A.R.; Hur, J.S.; Park, H.W.; Park, J.Y.; Park, J.H.; Lee, S.K.; Kim, J.C. Nematicidal activity of grammicin produced by Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Pest Manag. Sci. 2018, 74, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Chang, S.; Li, Y.; Xi, X.; Chen, M.; He, N.; Wang, M.; Zhao, W.; Xie, Y. Molecular networking-based screening led to the discovery of a cyclic heptadepsipeptide from an endolichenic Xylaria sp. J. Nat. Prod., 2022, 85, 972–979. [Google Scholar] [CrossRef]
- Santhirasegaram, S.; Wickramarachchi, S.R.; Attanayake, R.N.; Weerakoon, G.; Samarakoon, S.; Wijeratne, K.; Paranagama, P.A. A novel cytotoxic compound from the endolichenic fungus, Xylaria psidii inhabiting the lichen, Amandinea medusulina. Nat. Prod. Commun. 2020, 15, 1934578X20933017. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Sánchez-Fernández, R.E. Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World J. Microbiol. Biotechnol. 2017, 33, 15. [Google Scholar] [CrossRef]
- Sica, V.P; Rees, E.R.; Tchegnon, E.; Bardsley, R.H.; Raja, H.A.; Oberlies, N.H. Spatial and temporal profiling of griseofulvin production in Xylaria cubensis using mass spectrometry mapping. Front. Microbiol. 2016, 7, 544. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.N.; Walker, A.K.; Nsiama, T.K.; McFarlane, J.; Sumarah, M.W.; Ibrahim, A.; Miller J., D. Griseofulvin-producing Xylaria endophytes of Pinus strobus and Vaccinium angustifolium: evidence for a conifer-understory species endophyte ecology. Fungal Ecology 2014, 11, 107–113. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, G.J.; Lee, H.B.; Kim, K.M.; Jung, H.S.; Lee, S.W.; Jang, K.S; Cho, K.Y.; Kim, J.C. Griseofulvin from Xylaria sp. Strain F0010, an endophytic fungus of Abies holophylla and its antifungal activity against plant pathogenic fungi. J. Microbiol. Biotechnol. 2005, 15, 112–117. [Google Scholar]
- Ibrahim, A.; Sørensen, D.; Jenkins, H.A.; McCarry, B.E.; Sumarah, M.W. New diplosporin and agistatine derivatives produced by the fungal endophyte Xylaria sp. isolated from Vitis labrusca, Phytochem. Lett. 2014, 9, 179-183. [CrossRef]
- Wicklow, D.T.; Rogers, K.D.; Dowd, P.F.; Gloer, J.B. Bioactive metabolites from Stenocarpella maydis, a stalk and ear rot pathogen of maize. Fungal Biol. 2011, 115, 133–142. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Sun, Q.Q.; Qin, J.C.; Pescitelli, G.; Gao, J.M. Characterization of cytochalasins from the endophytic Xylaria sp. and their biological functions. J. Agric. Food Chem. 2014, 62, 10962–10969. [Google Scholar] [CrossRef]
- Fenta, L.; Mekonnen, H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: current perspectives and research directions. Scientifica, 2024, 2024, 12. [Google Scholar] [CrossRef]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S.S.; Singh, V.; Ahmad, M.A.; Rani, N.; Singh, T.G.; Chandrasekaran, B. Pesticides: An alarming detrimental to health and environment. Sci. Total Environ 2024, 915, 170113. [Google Scholar] [CrossRef] [PubMed]
- Daraban, G.M.; Hlihor, R.M.; Suteu, D. Pesticides vs. Biopesticides: From pest management to toxicity and impacts on the environment and human health. Toxics 2023, 11, 983. [CrossRef]
- Hsieh, H.M.; Lin, C.R.; Fang, M.J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenet. Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.; Flessa, F.; Peršoh, D.; Stadler, M. Three new Xylaria species from Southwestern Europe. Mycol. Prog. 2010, 10, 33–52. [Google Scholar] [CrossRef]
- Sonyot, W.; Lamlertthon, S.; Luangsa-Ard, J.J.; Mongkolsamrit, S.; Usuwanthim, K.; Ingkaninan, K.; Waranuch, N.; Suphrom, N. In vitro antibacterial and anti-inflammatory effects of novel insect fungus Polycephalomyces phaothaiensis extract and its constituents against Propionibacterium acnes. Antibiotics 2020, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Chesters, N.; O´Hagan, D. Biosynthesis of the fungal metabolite, piliformic acid (2-hexylidene-3-methylsuccinic acid). J. Chem. Soc., Perkin Trans. 1997, 6, 827–834. [Google Scholar] [CrossRef]
- Mangaleswaran, S.; Argade, N. An efficient synthesis of (±)-piliformic acid †. J. Chem. Soc., Perkin Trans. 1, 2000, 3290-3291. [CrossRef]
- Weidenmüller, H.L.; Cavagna, F.; Fehlhaber, H.W.; Präve, P. 2-carboxymethyl-3-n-hexyl-maleic acid anhydride, a novel metabolite from an Aspergillus. Tetrahedron Lett. 1972, 13, 3519–3522. [Google Scholar] [CrossRef]
- Viveki, A.B.; Pol, M.D.; Halder, P.; Sonavane, S.R.; Mhaske, S.B. Annulation of enals with carbamoylpropiolates via NHC-catalyzed enolate pathway: Access to functionalized maleimides/iso-maleimides and synthesis of Aspergillus FH-X-213. J. Org. Chem 2021, 86, 9466–9477. [Google Scholar] [CrossRef]
- Boukouvalas, J.; Thibault, C.; Loach, R. Expedient assembly of bioactive maleic anhydrides using click Diels–Alder chemistry. Sylett. 2014, 25, 2139–2142. [Google Scholar] [CrossRef]
- Becker, K.; Stadler, M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J. Antibiot. 2021, 74, 1–23. [Google Scholar] [CrossRef]
- Li, Z.; Park, H.S.; Qiao, J.X.; Yeung, K.S.; Yu, J.Q. Ligand-enabled C–H hydroxylation with aqueous H2O2 at room temperature. ACS. 2022, 144, 18109–18116. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.F.; Chen, Y.; Li, M.S.; Zhang, B.; Luo, J.Z.; Song, X.X.; Li, J.J.; Qin, F. Chemical constituents of Corydalis saxicola. Chem. Nat. Compd. 2022, 58, 119–121. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.L.; Van, Q.N.; Tuan, N.L.; Dang, X.T. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Elias, L.M.; Fortkamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, Q.V.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Braz. J. Microbiol. 2018, 49, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Almassi, F.; Ghisalberti, E.L.; Rowland, C.Y. Alkylcitrate-derived metabolites from Aspergillus niger. J. Nat. Prod., 1994, 57, 833–836. [Google Scholar] [CrossRef]
- Koch, L.; Lodin, A.; Herold, I.; Ilan, M.; Carmeli, S.; Yarden, O. Sensitivity of Neurospora crassa to a marine-derived Aspergillus tubingensis anhydride exhibiting antifungal activity that is mediated by the MAS1 protein. Mar. Drugs 2014, 12, 4713–4731. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sytykiewicz, H.; Sprawka, I.; Leszczynski, B. Aphidicidal and deterrent activity of phenolic acid extracts from some herbal plants towards Myzus persicae Sulz. and Rhopalosiphum padi L. Fresenius Environ. Bull. 2016, 25, 5714–5721. [Google Scholar]
- Singh, S.; Kaur, S.; Kaur, R.; Kaur, A. Impact of plant symbiotic endophytic fungus, Aspergillus terreus on insect herbivore Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2023, 52, 932–944. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, D.H.; Choi, J.Y.; Wang, M.; Liu, S.; Je, Y.H. Characterization of insecticidal compound from Streptomyces gramineus against Thrips palmi. , J. Asia. Pac. Entomol. 2023, 26, 102166. [Google Scholar] [CrossRef]
- Cao, L.; Yan, W.; Gu, C.; Wang, Z.; Zhao, S.; Kang, S.; Khan, B.; Zhu, H.; Li, J.; Ye, Y. New alkylitaconic acid derivatives from Nodulisporium sp. A21 and their auxin herbicidal activities on weed seeds. J. Agric. Food Chem. 2019, 67, 2811–2817. [Google Scholar] [CrossRef]
- Mondal, G.; Dureja, P.; Sen, B. Fungal metabolites from Aspergillus niger AN27 related to plant growth promotion. Indian J. Exp. Biol. 2000, 38, 84–87. [Google Scholar] [PubMed]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant Sci. 2017, 8, 1762. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Morales-Sánchez, V.; Díaz, C. E.; Trujillo, E.; Olmeda, S.A.; Valcarcel, F.; Muñoz, R.; Andrés, M.F.; González-Coloma, A. Bioactive metabolites from the endophytic fungus Aspergillus sp. SPH2. J. Fungi. 2021, 7, 109. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC bioinformatics 2017, 18, 529. [Google Scholar] [CrossRef]
- Truzi, C.C.; Vieira, N.F.; De Souza, J.M.; De Bortoli, S.A. Artificial diets with different protein levels for rearing Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Insect Sci. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- González-Coloma, A.; Andres, M.F.; Contreras, R.; Zúñiga, G.E.; Díaz, C.E. Sustainable production of insecticidal com-pounds from Persea indica. Plants 2022, 11, 418. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Sanz, J. ; Burillo, J; & Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; González-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crops Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH, mult (J in Hz) |
δC | δH, mult (J in Hz) |
δC | |
| 1 | - | 178.0 | - | 177.8 |
| 2 | 3.63, q (7.1) | 39.0 | 3.64, qd (7.1, 1.9) | 38.7 |
| 3 | - | 134.5 | 134.3 | |
| 4 | 6.82, t (7.6) | 144.7 | 6.85, t (7.6) | 145.0 |
| 5 | 2.26, m | 29.4 | 2.26, m | 29.4, (29.4)a |
| 6 | 1.51, m | 29.6 | 1.61, m 1.52, m |
26.0, (26.0) a |
| 7 | 1.43, m | 26.7 | 1.50, m | 39.6, (39.7) a |
| 8 | 1.56, m | 33.4 | 3.74, sext (6.1) | 68.2,(68.3) a |
| 9 | 3.55, t (6.6) | 62.8 | 1.16, d (6.1) | 23.5 |
| 10 | - | 170.3 | - | 169.9 |
| 11 | 1.31, d (7.2) | 16.4 | 1.31, d (7.1) | 16.3 |
| Target | Effect | Treatment | Activity (%) | EC50c/LD50d |
|---|---|---|---|---|
| S. littoralis | %FIa | Extract | 42.9±11.4 | |
| 2 | 33.0±14.9 | |||
| 3 | 18.0±8.4 | |||
| 4 | 37.8±18.7 | |||
| 5 | 9.1±6.3 | |||
| 6 | 17.7±11.7 | |||
| M. persicae | %SIa | Extract | 81.5±6.8 | 10.9 (7.2-16.4) |
| 2 | 25.5±6.3 | |||
| 3 | 50.4±8.9 | |||
| 4 | 100.0±0.0 | 1.6 (0.5-4.9) | ||
| 5 | 98.9±0.6 | 4.5 (2.0-10.0) | ||
| 6 | 91.9±2.3 | 15.5 (12.0-20.0) | ||
| R. padi | %SIa | Extract | 61.5±6.0 | |
| 2 | 20.6±6.4 | |||
| 3 | 30.3±8.8 | |||
| 4 | 91.0±3.8 | 8.9 (4.6-7.5) | ||
| 5 | 61.2±9.2 | |||
| 6 | 43.0±7.9 | |||
| M. javanica | %Mortalityb | Extract | 2.0±0.7 | |
| 2 | 2.2±0.7 | |||
| 3 | 28.4±6.6 | |||
| 4 | 100.0±0.0 | 0.10 (0.10-0.11) | ||
| 5 | 26.4±5.8 | |||
| 6 | 29.5±5.6 | |||
| A. alternata | % MGIb | Extract | 58.5±2.4 | |
| 2 | 27.3±3.7 | |||
| 3 | 47.3±5.1 | |||
| 4 | 63.7±1.4 | 0.24 (0.20-0.28) | ||
| 5 | 32.2±5.6 | |||
| 6 | 42.5±6.0 | |||
| B. cinerea | % MGIb | Extract | 75.9±9.7 | |
| 2 | 19.7±6.2 | |||
| 3 | 62.1±7.6 | 0.36 (0.25-0.51) | ||
| 4 | 84.3±4.5 | 0.12 (0.09-0.15) | ||
| 5 | 0.5±8.8 | |||
| 6 | 5.8±6.2 | |||
| F. oxysporum | % MGIb | Extract | 61.9±6.5 | |
| 2 | 18.7±3.1 | |||
| 3 | 42.3±3.6 | |||
| 4 | 52.3±2.6 | |||
| 5 | 32.6±9.1 | |||
| 6 | 35.7±3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





