1. Introduction
Probiotics are non-pathogenic microorganisms that, when administered in adequate amounts, exert a positive influence on the health or physiology of the host (WGO 2011; Hill et al. 2014).
Microorganisms with probiotic effects include bacteria of the genera Lactobacillus and Bifidobacterium in addition to the yeast Saccharomyces boulardii (McFarland 2017). S. boulardii is a nonpathogenic thermophilic yeast that is resistant to gastric acid and proteolysis and was isolated from lychee in Indochina (Fratianni et al. 2014; Czerucka et al. 2007). It is not part of the intestinal microbiota; it does not colonize the intestine, and its concentration decreases after the end of administration (Czeruca et al. 2007; Pais et al. 2020). This yeast has been used to treat traveller's diarrhoea, antibiotic-associated diarrhoea, acute gastroenteritis in adults and children, diarrhoea in intensive care unit patients, and chronic diarrhoea in HIV-positive patients. It has a protective effect against Clostridium difficile, Vibrio cholerae, Shigella and E. coli (EPEC), and Salmonella enterica Typhimurium (Capece et al. 2018; Datta et al. 2017; Czeruca et al. 2007).
Probiotication is the process of inoculating beneficial microorganisms into a liquid substrate to produce functional beverages, which subsequently adds market value due to the various health benefits of probiotics (Czeruca & Rampal, 2002; Mojikon et al 2022).
Fruit and vegetable juices are appropriate as a matrix for probiotics because they do not contain dairy allergens that prevent consumption by the population (Değirmencioğlu et al. 2016). Despite providing products with probiotic potential and being sensorially accepted, the use of the yeast S. boulardii in fruit juices and other beverages has not been commercially explored (Czerucka et al. 2007). Several authors have noted the possibility of applying S. boulardii in different food matrices, such as tomato and carrot juice (Sivudu et al. 2016); craft beer (Capece et al. 2018); grape juice with apple pieces (Gallo et al. 2014); beverages made from radish, beetroot and carrot (Değirmencioğlu et al., 2016); apple pulp (Farinazzo et al., 2017); beetroot, carrot and radish beverages (Profir & Vizireanu, 2013); tomato juice (Fratianni et al. 2013); milk and dairy drinks (Lourens-Hattingh & Viljoen, 2001); goat milk yogurt (Karaolis et al., 2013); berry juice (Fratianni et al., 2014); and soymilk ice cream (Heenan et al., 2004), among others.
Lychee (Litchi chinensis Sonn.), which was originally obtained from China (Zhang et al., 2013), is a sweet fruit that is rich in sugars, has fleshy pulp, an attractive colour, and an aroma, and can be consumed in syrup, powdered pulp, dehydrated, and in the form of juice (Zeng et al., 2008).
In addition to presenting bioactive compounds with antioxidant, antibacterial, anti-inflammatory, antiallergic, hepatoprotective, antiviral, anticancer, and vasodilator properties (Zhang et al., 2013; Su et al., 2014), lychee has a rich and pleasant flavour, with good sensory characteristics and potential application in fermented and probiotic beverages (Alves et al. 2011; Zhang et al. 2013; Su et al. 2014).
Thus, the objective of this study was to develop a lychee probiotic beverage using the yeast Saccharomyces boulardii and to evaluate the effect of refrigerated storage on cell viability, physicochemical characteristics, and acceptance.
2. Materials and Methods
2.1. Material
2.1.1. Reagents
The following reagents from Sigma‒Aldrich (St. Louis, USA) (purity ≥99%) were used: glucose, fructose, sucrose, stachyose, mannitol, citric acid, malic acid, tartaric acid, lactic acid, succinic acid, acetic acid, ascorbic acid, DPPH, TPTZ (2,4,6-tripyridyl-S-triazine), ABTS, Folin-Ciocalteu, Trolox, gallic acid, protocatechuic acid, theobromine, paraxanthin, epigallocatechin, catechin, epicatechin, quercetin, caffeine, caffeine, rutin, kaempferol, chlorogenic acid, p-coumaric acid, ferrulic acid, synapic acid, myricetin and trigonelline. Acetonitrile (HPLC grade) was obtained from J.T. Baker (Xalostoc, Mexico). Ethanol and other reagents were obtained from Merck (Darmstadt, Germany). We used ultrapure water (Simplicity 185, Millipore, MA, USA) for the extractions and standard solutions.
2.1.2. Lychee Juice
Lychees of the Bengal cultivar (23°45'57"S; 53°19'30"O; altitude of 442 m) were sanitized (NaClO 0.01% per 5 min), peeled and pulped (F. Silva, model MS-200, Brazil). The pulp (15.8 °Brix) was packed in polyethylene bags, stored at -15°C and filtered through 200 mesh to obtain the lychee juice.
2.1.3. Probiotic Culture
S. boulardii CNCM I-745 was obtained from a commercial lyophilized product (Floratil® Merck S.A., Brazil). Each 100 mg capsule contained at least 8.66 log S. boulardii-17 cells.
2.2. Methods
2.2.1. Preparation of S. boulardii Inoculum
The lyophilized yeast was reactivated in 100 mL of YPD broth (1:2:2:95, yeast extract, bacteriological peptone, dextrose, and water) and stirred (120 rpm) at 30°C for 24 h (CT-712 Cientec, Brazil).
After incubation, the yeast biomass was centrifuged at 14000 × g for 15 min (Eppendorf 5804 R, Germany) and washed twice with NaCl 0.85% (w/v) to remove residual YPD. The biomass was resuspended in 50 mL of 0.85% NaCl (w/v), resulting in the activated S. boulardii culture.
2.2.2. Probiotication of Lychee Juice
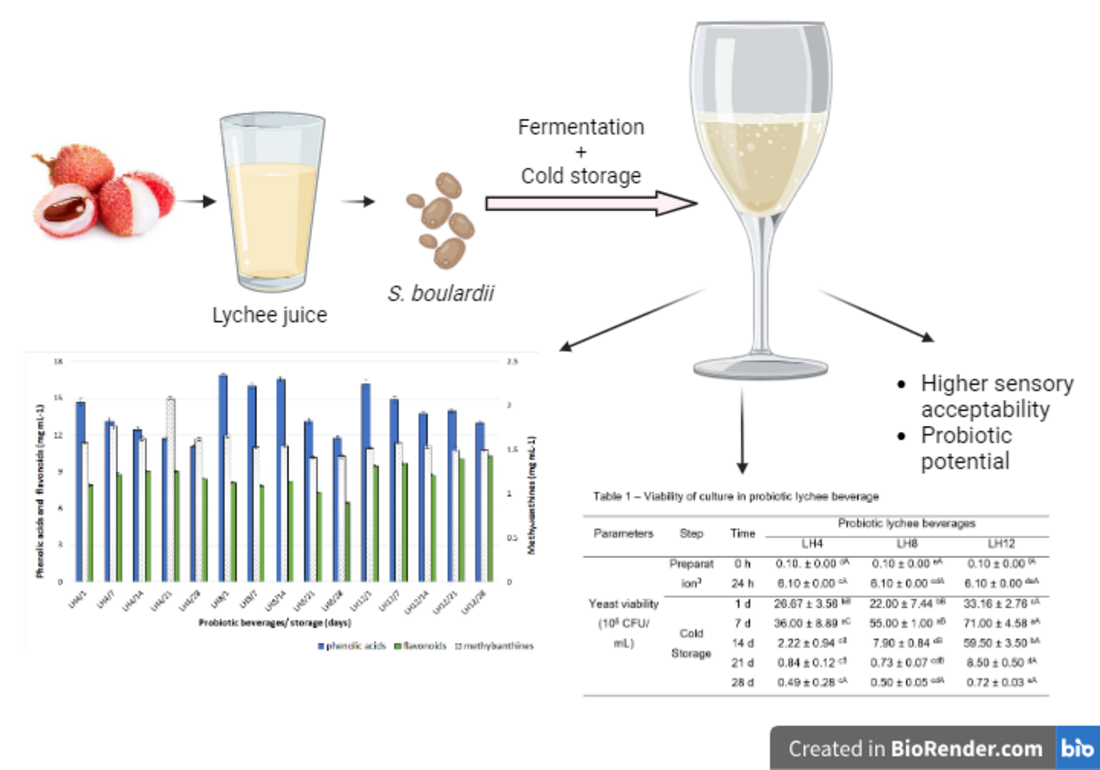
Figure 1 shows the main stages of the probiotication of lychee juice, as proposed from preliminary tests. Water and lychee juice were combined until a total soluble solid (TSS) concentration of 12 °Brix was reached. The pH was adjusted to 4.5, and 20% citric acid was added to the juice. The juices were glass bottled and pasteurized in a water bath (20 min/80°C).
Lychee juice was inoculated with S. boulardii to reach 5 log cells/mL of juice and fermented in an oven with agitation (120 rpm) at 24 h/30°C. The probioticed juice (LB) (4.07 °Brix) was separated into three portions: (a) the first remaining with the original TSS content (~4 °Brix), named LB-4; (b) another receiving sucrose to reach 8 °Brix, denominated LB-8; and the last portion (c) receiving the addition of sucrose up to 12 °Brix, denominated LB-12. These beverages were stored in a refrigerator at 4°C for 28 days and analysed every seven days. All the juices were prepared twice.
2.2.3. Viability of S. boulardii
The viability of S. boulardii was determined after dilution of the beverages in 0.1% (w/v) peptone water (Oxoid®), followed by plating on YPD agar at 37°C for 48 h. The results are expressed as log CFU/mL.
2.2.4. Physicochemical Analysis
The pH was determined using a pH meter (model HI 3221; Hanna Instruments Inc., Woonsocket, USA). The content of total soluble solids (TSSs) in °Brix was determined by a digital refractometer (Pocket Pal-1, Atago, Tokyo, Japan).
The total phenolic compound (TPC) content was determined according to the methods of Singleton, Orthofer, and Lamuela-Raventos (1999), and the results are expressed as µg of gallic acid equivalents (GAE) per mL.
Antioxidant activity (AA) was evaluated by measuring (a) reducing the free radical DPPH, (b) reducing the ABTS●+ and (c) reducing Fe3+ to Fe2+ according to Sánchez-González et al. (2005) using standard curves of Trolox. The AA results were expressed in each method in μmol of Trolox equivalents (TE) per mL.
2.2.3.1. Chromatographic Analyses by HPLC and UHPLC
The identification and quantification of sugars, organic acids, phenolic acids, methylxanthines, and flavonoids were performed by chromatographic analysis. An aliquot (1 mL) of each sample was homogenized in 10 mL of water and centrifuged (9056 g/15 min). The supernatant was collected and filtered through a 0.22 µm PVDF filter (Millipore, Cork, Ireland).
For the determination of sugars and organic acids, a Shimadzu LC 20A HPLC (Shimadzu Co., Kyoto, Japan) composed of a pump (LC-20AT), automatic injector (SIL-20AC HT), column oven (CTO-20A) and photodiode array (SPD-M20A) and refractive index (RID-10A) detectors coupled in series (HPLC-PDA-RID) was used. The chromatographic analyses were isocratic.
The chromatographic conditions for sugar and organic acid determination are described in the Supplementary Material - Table S1, according to Pauli et al. (2011).
The phenolic compounds and methylxanthines were determined according to Terhaag et al. (2021). A gas chromatograph coupled with mass spectrometry (CG-MS, QP2010-SE-Shimadizu) was used to determine the ethanol content. The chromatographic conditions used were as follows: capillary column, Restek Rtx-5MS (30 m × 0.25 mm; i.d. 0.25 μm); helium as the carrier gas with a flow of 0.7 mL/min; and column pressure and temperature of 23.3 kPa and 35°C, respectively. The oven temperature ramp was 35-280°C (1-5 min at 30°C/min; 5-7.5 min at 50°C/min; and a constant temperature maintained for 7.5-9.43 min); the injector, detector, and interface temperatures were 150, 200 and 280°C, respectively; and the system was operated in Scan mode 30-100 (m/z) with an electronic impact of 70 eV. The injection volume was 500 μL at a 1:20 split ratio 32.
The results were obtained by analysing the retention times and areas of the peaks in the chromatograms after comparison with the standard curves of some phenolics and methylxanthines. The results are expressed in μg/mL. The ethanol content was calculated after comparison with the standard. These results are expressed in mL/100 mL of juice.
Ethanol content was determined by gas chromatograph coupled to mass spectrometry (GC-MS, QP2010-SE-SHIMADIZU). The chromatographic conditions employed were: Restek Rtx-5MS capillary column, (30 m x 0.25 mm; internal diameter of 0.25 μm); helium as a carrier gas with a flow rate of 0.7 mL/min; column pressure and temperature of 23.3 kPa and 35 °C, respectively. The oven temperature ramp was 35-280 °C, being: 1-5 min at a rate of 30 °C/min; and from 5-7.5 min at 50 °C/min, and constant temperature maintained for 7.5-9.43 min; injector temperature of 150 °C; detector and interface temperature of 200 °C and 280 °C, respectively, operating in Scan mode 30-100 (m/z) with electronic impact of 70 eV. Injection of a volume of 500 μL at a split ratio of 1:20 (Bagewadi, 2016). The results were obtained by analyzing the retention times and peak areas of the chromatograms of the samples, after comparison with a standard curve of HPLC-grade ethanol, and expressed as a percentage of the volume of probiotic lychee beverage (v/v)
2.2.5. Sensory Acceptance
The LB4, LB8, and LB12 beverages on the 1st, 7th, 14th, 21st, and 28th days of cold storage were subjected to descriptive and acceptance tests. Sensory tests were carried out in individual booths, with procedures approved by the Committee of Ethics in Research Involving Humans (certificate CAAE n° 56478316.8.0000.5231).
To assess acceptance, the team consisted of 53 usual or potential consumers of fruit-based beverages and/or functional beverages (they drank approximately 2.5 litres of fruit-based beverages per month). The team consisted of 33 women and 20 men who were younger (76% aged between 18 and 39 years) and had a high level of education (85% undergraduate or graduate).
The evaluators evaluated the colour, aroma, flavour, texture, and global acceptability of the beverages through a structured hybrid scale of ten centimetres (0 = dislike extremely; 10 = like extremely).
2.3. Statistical Analysis
The experiment was conducted twice in accordance with a completely randomized design (CRD). The physicochemical and microbiological parameters were analysed weekly for 28 days in triplicate. A split-plot treatment was employed, and sugar content and storage were the main and secondary treatments, respectively. The preparation and storage data were analysed by ANOVA and Tukey's test (p ≤ 0.05) (Sisvar 5.6 program; Ferreira, 2014).
The sensory analysis data were subjected to two-way ANOVA (for beverages and consumers). Tukey’s test was used to compare means (p ≤ 0.05). The Statistica 7.1 program (Statsoft, 2006) was used in these analyses.
3. Results and Discussion
3.1. Effect of S. boulardii Probiotic on the Physicochemical Characteristics of Lychee Beverages
The culture viability, TSS, and ethanol content after beverage preparation and fermentation are shown in
Table 1. The addition of inoculum, followed by fermentation for 24 hours, increased (p ≤ 0.05)
S. boulardii viability in LB (6-7 log CFU/mL). Lee and Salminen (1995) reported a minimum daily consumption of 6 to 9 log CFU of probiotic microorganisms to obtain some health-beneficial effects. According to some authors, this amount varies depending on the type of probiotic microorganism and the desired health effect (Mc Farland, 2017; Hill et al. 2014). In the LB probiotication, the fermentation stage promoted an increase in cell viability, and the beverage became viable enough to be probiotic and promoted a beneficial effect on health.
The probiotication of S. boulardii promoted a decrease in TSS because yeasts use sugars as the primary energy source for their metabolism (Nelson & Cox, 2017). The fermentation stage was carried out under agitation to provide greater oxygenation in the lychee juice, which contributed to the increase in yeast viability.
During fermentation, ethanol and CO2 were produced, in addition to a slight reduction in the pH of the LB. This result is similar to those reported by Estela-Escalante et al. (2012) and Nelson and Cox (2017), who reported that S. boulardii metabolizes sugars, producing mainly ethanol and CO2 in addition to small amounts of organic acids.
3.2. Effect of TSS and Cold Storage in Lychee Probiotic Beverages
3.2.1. S. boulardii Viability
Even when stored in refrigerated conditions, LB increased the viability of
S. boulardii yeast. After the 1st day of cold storage, the CFUs ranged from 7.34 to 7.52 log CFU/mL (
Table 1). As the lychee juice used to prepare LB contained 4.2, 3.7, and 0.1 g/100 mL fructose, glucose, and sucrose, respectively, the multiplication of yeasts in LB-4 occurred due to the natural presence of these fermentable carbohydrates
36,39. In addition to the sugars from the lychee juice, LB-8 and LB-12 received sucrose supplementation, which increased the yeast viability compared to that of LB-4. Gaboardi et al. (2018) reported an increase in
S. boulardii viability in effluents supplemented with sucrose.
All beverages presented greater viability after the 7th day of cold storage. On the 21st day, LB-4 and LB-8 had viabilities < 5.8 log CFU/mL. On the same day, LB-12 showed the greatest increase in viability (6.9 log CFU/mL), decreasing to 5.8 log CFU/mL on the 28th day. Considering that the viability of LH-12 was > 6.9 log CFU/mL and that this parameter is a prerequisite for its use as a probiotic, LH-12 was considered adequate until the 21st day.
3.2.2. Total Soluble Solids (TSS) and pH
Even under refrigeration,
S. boulardii continued metabolizing mono- and disaccharides as primary carbon sources, increasing cell biomass (Nelson & Cox, 2017). In LB-4, the TSS concentration remained constant throughout cold storage (
Table 1 ). LB-8 and LB-12 showed a gradual decrease in TSS content during cold storage. During cold storage, LB-8 presented a decrease in TSS (13% and 43% on the 1st and 28th days, respectively). The same reduction occurred with LB-12 (approximately 16% and 45% on the 1st and 28th days, respectively). Greater viability is related to the TSS, as proven by Pearson correlation (r = 0,37, p ≤ 0,05).
Regarding the pH, on the 1st day of cold storage, the beverages had different pH values (3.74 (LB-4) and 3.59 (LB-12)). In LB-4, the pH remained constant from the 7th to the 21st day and decreased on the 28th day of storage. The pH in both LB-8 and LB-12 increased after seven days, followed by a constant decrease until the end of the storage period. Cold storage does not promote the synthesis of large amounts of organic acids, as this is not the ideal growth temperature for S. boulardi (Lazo-Vélez et al. 2018; Nelson & Cox, 2017). Hedin (2022) pointed that there is positive correlation between the growth of the S. boulardii and the organic acids synthesis with an inverse correlation of glucose level.
3.2.3. Ethanol Content
Like other yeasts of the genus
Saccharomyces,
S. boulardii is a yeast that produces ethanol and CO
2 from the fermentation of glucose 36, 39. Because they contain different TSSs (
Table 1), LB-4, LB-8, and LB-12 had different ethanol concentrations at the beginning of storage (5.5, 7.5, and 6.2%, respectively). The ethanol content remained constant throughout cold storage in LB-4 and LB-8. Factors such as the composition of the medium (mainly the TSS level) and the accumulation of ethanol interfere with the viability and fermentative capacity of the yeast (Bai et al., 2008), which explains the ethanol constancy level of LB-4 and LB-8.
For LB-12, there was an increase in the ethanol content of 40% and 63% at the 21st and 28th days of storage, respectively, due to the continuity of S. boulardii metabolism, even during refrigerated storage. On the 28th day of cold storage, LB-12 contained 10.1% ethanol, probably due to the metabolism of the remaining sugars and their conversion to alcohol. Cold storage does not prevent ethanol synthesis in these beverages. There was a significant and positive correlation (r = 0.69, p ≤ 0.05) between the SSTs before fermentation and the alcohol content of the beverages and between the storage time and the alcohol content (r = 0.45, p ≤ 0.05).
3.2.4. Sugars Content
There were no sucrose or small sucrose molecules in LB-4 (
Figure 2A and 2B), demonstrating that these nutrients had already been consumed by the yeast during fermentation before cold storage.
On the 1st day of cold storage, 0.08 and 0.78 mg/mL sucrose was added to LB-8 and LB-12, respectively (
Figure 2A). There were decreases of approximately 63% and 27%, respectively, until the 28th day. Similarly, for LB-8 and LB-12, there was a decrease in glucose content (
Figure 2B), with a maximum on the 1st day and a minimum on the 14th day. At lower glucose levels,
S. boulardii preferred sucrose to other sugars. Gaboardi et al.
40 reported similar results in rice effluent when observing a preference for
S. boulardii to use sucrose over other organic matter.
Among the sugars detected and quantified, fructose was the only one that remained present in the LB-4 beverage until the end of cold storage (
Figure 2C). At the start of cold storage, LB-8 and LB-12 presented 24.9 and 29.3 mg/ml fructose, representing decreases of 27.5% and 31.1%, respectively, throughout the storage period. Thus,
S. boulardii did not prefer to metabolize fructose in the probiotication of the lychee beverage since this sugar remained detrimental to the decay of other sugars.
Sulieman et al. 2018 verified similar results in sugary date palm syrups fermented by S. cerevisiae. These authors reported a significant reduction in sucrose and glucose content and maintenance of fructose in the syrup fermented for 72 h. This type of metabolic preference is typical of the Saccharomyces genus (Nelson and Cox, 2017; Lazo-Vélez et al., 2018).
The presence of fructose in probioticated LB can be desirable for sensory and nutritional reasons since this monosaccharide has a relative sweetness of 1.3 relative to that of sucrose (Parker et al. 2010). Fructose can increase sweetness and make beverages more accepted by consumers, in addition to providing a lower caloric value per gram.
3.2.5. Organic Acid Content
Some acids identified in LB are malic, acetic, and citric acids. These organic acids naturally occur in fruit and are also derived from substances involved in the glycolysis of yeast cells (Nelson and Cox, 2017; Datta et al., 2017).
During cold storage, there was an increase in the organic acid content (
Figure 3A, 3b, and 3c) in the lychee probioticated beverages. The acetic acid content (
Figure 3B) in LB-4 remained constant, with increases in LB-8 and LB-12 (41% and 56%, respectively) at the end of cold storage. There was an increase of 27% (LB-4), 52% (LB-8), and 69% (LB-12) in citric acid during storage (
Figure 3C). The addition of lychee probiotic beverages containing more sucrose resulted in a higher organic acid content.
3.2.6. Total Phenolic Compounds (TPC) and Antioxidant Activity (AA)
On the 1
st day of cold storage, the concentrations of the lychee probiotic beverages were 151.7, 133.7, and 190.1 µg GAE/Ml (LB-4, LB-8, and LB-12, respectively). Similar results were reported by Değirmencioğlu et al. (2016) for radish juice fermented by
S. boulardii and other fungi. In general, there was a decrease in the TPC and AA content in LB during cold storage (
Table 2).
Despite LB-12 having a greater TPC than the other beverages, at the end of cold storage, all beverages had similar phenolic contents.
Some factors, such as nutrient levels, fermentation conduction, exposure to oxygen and light, and intermolecular interactions, can interfere with the TPC content and (probably) the AA content 46. There was a reduction in TPC during cold storage of approximately 20% (LB-4), 8% (LB-8), and 26% (LB-12). The same reduction in the TPC of juices probioticated by S. boulardii during cold storage was observed in berry juice (7.8%) (Fratianni et al. 2014) and tomato juice (3.6%) (Fratianni et al. 2013).
The AA content measured by the DPPH assay decreased during cold storage (57.5%, 68.8%, and 41.2% for LB-4, LB-8, and LB-12, respectively). Additionally, a decrease in AA was detected by the FRAP and ABTS methods. The AA content of the probiotic beverages was similar according to the DPPH, FRAP, and ABTS assays at the end of cold storage.
There was also a significant (p ≤ 0.05) positive correlation between TPC and AA according to the DPPH, FRAP, and ABTS assays (r = 0.39; r = 0.66; r = 59, respectively). Therefore, most of the phenolic compounds in LB are related to its antioxidant activity.
3.2.7. Phenolic Acid, Flavonoid and Methylxanthine Contents
Through chromatographic analysis, 21 bioactive compounds were separated from probiotic lychee beverages. The following were identified in ascending order of retention time: trigonelline, ascorbic acid, nicotinic acid, gallic acid, protocatechuic acid, theobromine, paraxanthine, theophylline, epigallocatechin, catechin, chlorogenic acid, caffeic acid, caffeine, epicatechin, p-coumaric acid, ferulic acid, sinapic acid, rutin, myricetin, quercetin and kaempferol.
Except for trigonelline (9.36, 9.26, and 8.56 mg/Ml in LB4, LB8, and LB12, respectively), ascorbic acid (213.1, 95.1, and 110.7 mg/Ml, in LB4, LB8 and LB12, respectively), and nicotinic acid (0.24, 0.25, 0.18 mg/Ml, in LB4, LB8 and LB12, respectively) the other compounds were grouped into three classes — phenolic acids, flavonoids, and methylxanthines —to facilitate the discussion of the results. The concentrations belonging to each class were summed (
Figure 4). The compounds classified as phenolic acids are gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, and sinapic acid. The flavonoids were epigallocatechin, catechin, epicatechin, rutin, myricetin, quercetin and kaempferol. The methylxanthines used were caffeine, theobromine, paraxanthine, and theophylline.
In LB-4, there was a decrease in phenolic acid content and a slight increase in methylxanthine and flavonoid content during storage. The levels of phenolic acids, flavonoids, and methylxanthines decreased in LB-8 during storage (30%, 14%, and 19%, respectively). For LB-12, there was an increase in the flavonoid content of approximately 9% over the 28 days of storage and, on the other hand, a 19% decrease in the phenolic acid content and maintenance of the methylxanthine content.
Research indicates that the action of microorganisms during fermentation—metabolizing the substrate and providing molecular interactions, including with the oxygen present in the medium—can interfere with the profile and content of phenolic acids, methylxanthines, and flavonoids (Değirmencioğlu et al. 2016; Tiwari & Cummins, 2013; Wiczkowski et al. 2015). Thus, the different sucrose contents of the lychee probiotic beverages differed during storage in terms of yeast viability, ethanol content, sugar content, organic acid content, and phenolic compound content.
3.3. Sensory Acceptability of Lychee Probiotic Beverages
The lychee probiotic beverage LH12 was chosen for sensory acceptability because it showed greater viability of S. boulardii and higher TPC and TSS at the end of the cold storage period. To meet the requirement of being probiotic, LB-12 samples whose viability was < 6 log CFU/mL on the date of sample collection were excluded. Due to the viability > 6 log CFU/mL up to the 21st day, LB-12 acceptance on the 1st, 7th, 14th, and 21st days of cold storage was determined. These beverages were renamed LB-12/1, LB-12/7, LB-12/14, and LB-12/21 (collected on the 1st, 7th, 14th and 21st days of cold storage, respectively).
Table 3 shows the sensory acceptance of the lychee probiotics LB-12/1, LB-12/7, LB-12/14, and LB-12/21 for color, aroma, flavour, texture attributes, and global acceptability.
In general, all beverages were sensorially accepted (mean scores > 6.6,
Table 3). The beverages had similar (p ≤ 0.05) aromas, flavours, textures, and overall acceptability. LB-12/14 presented a similar color to that of LB-12/1, and both had better colors than LB-12/7 and LB-12/21. These results indicate that the physicochemical characteristics of LB-12 differed during storage but did not affect consumer preference.
The consumers highlighted several characteristics throughout the sensory evaluations: (a) the appreciation of the texture due to the presence of bubbles from the CO2 produced by the action of S. boulardii and (b) the aroma and global acceptance positively related to the “attractive aroma” and aroma “very similar to a sparkling wine”. These reports point to the positive effect of lychee juice probiotication by S. boulardii, as it can make it more sensitive to products already accepted and known by consumers.
Conversely, Heenan et al. (2004) noted that sensory consumers reported the presence of undesirable flavours in probiotic soy ice cream supplemented with S. boulardii. Lourens-Hattingh and Viljoen (2001) pointed out that S. boulardii is unfeasible as a probiotic agent in flavoured yogurts and fruit yogurts because it causes the formation of excessive amounts of ethanol and CO2, which are considered undesirable by the sensory evaluators of this product.
4. Conclusions
The probiotication of lychee beverages by S. boulardii promotes a suitable health-beverage alternative to consumers.
The sucrose content added to the probiotic lychee beverage results in different physicochemical characteristics, antioxidant properties, and phenolic compound contents.
The probioticated lychee beverage can be cold stored because it allows S. boulardii biomass to increase, maintaining culture viability. This beverage, which adds sucrose, promotes S. boulardii viability >7.8 log CFU/mL until 21 days of cold storage.
Acknowledgments
This work was supported by the Coordination for the Improvement of Higher Education Personnel - Ministry of Education (Capes – MEC), National Council for Scientific and Technological Development (CNPQ) and Federal Institute of Paraná (IFPR). We thank the Laboratory of Materials and Molecules (LAMM) and the Laboratory for Support of Agricultural Research (LAPA) for their collaboration in conducting gas chromatography and liquid chromatography analyses, respectively, both part of the Multi-User Central of PROPPG-UEL/FINEP.
References
- ALVES JA, LIMA LCO, DIAS D R, NUNES CA & SCHWAN RF. 2010. Effects of spontaneus and inoculated fermentation on the volatile profile of lychee (Litchi chinensis Sonn) fermented beverages. Int. J. Food Sci. Technol. 45: 2358-2365. [CrossRef]
- ALVES JA, LIMA LCO, DIAS D R, NUNES CA & SCHWAN RF. 2011. Chemical, Physical–Chemical, and Sensory Characteristics of Lychee (Litchi chinensis Sonn) Wines. J. Food Sci. 76 (5): s330-s336. [CrossRef]
- BAGEWADI Z, MULLA S & NINNEKAR HZ. 2016. Purification and characterization of endo b-1,4-D-glucanase from Trichoderma harzianum strain HZN11 and its application in production of bioethanol from sweet sorghum bagasse. 3 Biotech 6:101. [CrossRef]
- BAI FW, ANDERSON WA & WOO-YOUNG M. 2008. Ethanol fermentation Technologies from sugar and starch feedstocks. Biot Adv 26:89-105. [CrossRef]
- BRASIL. Resolução RDC n° 12, de 2 de janeiro de 2001. Aprova o Regulamento técnico sobre padrões microbiológicos para alimentos. ANVISA- Agência Nacional de Vigilância Sanitária. Avaliable at: https://antigo.anvisa.gov.br/documents/10181/2718376/IN_161_2022_.pdf/b08d70cb-add6-47e3-a5d3-fa317c2d54b2. Accessed in: June 10 2024.
- DATTA S, TIMSON DJ & ANNAPURE US. 2017. Antioxidant properties and global metabolite screening of the probiotic yeast Saccharomyces cerevisiae var. boulardii. J. Sci. Food Agric. 97 (9):3039-3049. [CrossRef]
- CAPECE A, ROMANIELLO R, PIETRAFESA A, GABRIELLA S, PIETRAFESA R, ZAMBUTO M & ROMANO P. 2018. Use of Saccharomyces cerevisae var. boulardii in co-fermentations with S. cerevisae for the production of craft beers with potential healthy value added. Int. J. Food Microbiol. 284:22-30. [CrossRef]
- CZERUCA D, PICHE T & RAMPAL P. 2007. Review article: yeast as probiotics – Saccharomyces boulardii. Aliment Pharmacol Ther 26:767-778. [CrossRef]
- CZERUCA D & RAMPAL P. 2002. Experimental effects of Saccharomices boulardii on diarrheal pathogens. Microbes and Infect 4 (7): 733-729. [CrossRef]
- DEĞIRMENCIOĞLU N, GURBUZ O & AHAN YS. 2016. The monitoring, via an in vitro digestion system, of the bioactive content of vegetable juice fermented with Saccharomyces cerevisiae and Saccharomyces boulardii. J. Food Process. Preserv. 40:798-811. [CrossRef]
- ESTELA-ESCALANTE W, RYCHTERA M, MELZOCH K & HATTA-SAKODA B. 2012. Effect of aeration on the fermentative activity of Saccharomyces cerevisiae cultured in apple juice. Rev. Mex. Ing. Quím 11(2):211-226.
- FRATIANNI F, CARDINALE F, RUSSO I, IULIANO C, CUCCINIELLO AC, MAIONE M, D´ACIERNO A & NAZZARO F. 2013. Fermentation of tomato juice with the probiotic yeast Saccharomyces boulardii. In: ROBINSON A & EMERSON D (Eds), Functional Foods: Sources, Biotechnology Applications, and Health Challenges, New York: Nova Science Publisher; New York, USA, p. 143–152.
- FRATIANNI F, CARDINALE F, RUSSO I., IULIANO C, TREMONTE P, COPPOLA R & NAZZARO F. 2014. Ability of symbiotic encapsulated Saccharomyces cerevisiae boulardii to grow in berry juice and to survive under simulated gastrointestinal conditions, J. Microencapsul 31 (3):299-305. [CrossRef]
- FARINAZZO FS, FARINAZZO ES, SPINOSA WA, GARCIA S. 2017. Saccharomyces boulardii: optimization of simultaneous saccharification and fermentation of cell production in organic and conventional apple substrate pulp. Food Sci Biotechnol 26 (4):969-977. [CrossRef]
- FERREIRA DF. 2014. Sisvar: a Guide for its Bootstrap procedures in multiple comparisons. Cienc. Agrotecnologia. 38:109-112. [CrossRef]
- GALLO M, BEVILACQUA A, SPERANZA B, SINIGAGLIA M & CORBO MR. 2014. Alginate beds and apple pieces as carriers for Saccharomyces ceverisae var. boulardii, as representative of yeast functional starter cultures. Int. J. Food Sci. 49:2092-2100. [CrossRef]
- GABOARDI G, DOS SANTOS DG, MENDES L, CENTENO L, MEIRELES T, VARGAS S, GRIEP E, SILVA ACJ, MOREIRA NA & CONCEIÇÃO FR. 2018. Bioremediation and biomass production from the cultivation of probiotic Saccharomyces boulardii in parboiled rice effluent. J Environ Manage. 226:180–186. [CrossRef]
- HEDIN, KA. 2022. Establishing Saccharomyces boulardii as an advanced microbiome therapeutic platform. Technical University of Denmark. Available at: https://backend.orbit.dtu.dk/ws/portalfiles/portal/351170515/PhD_Thesis_Karl_Alex_Hedin.pdf. Accessed at: June 10 2024.
- HEENAN CN, ADAMS MC, HOSKEN RW & FLEET GH. 2004. Survival and sensory acceptabilityof probiotic microorganisms in a nonfermented frozen vegetarian dessert. LWT 37 (4):461–466. [CrossRef]
- HILL C, GUARNER F, REID G, GIBSON GR, MERENSTEIN DJ, POT B, MORELLI, L, CANANI RB, FLINT HJ, SALMINEN S, CALDER PC & SANDERS ME. 2014. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11 (8):506–514. [CrossRef]
- HISS H. Cinética de Processos Fermentativos. In: LIMA, UA, AQUARONE E, BORZANI WE & SCHMIDELL W. (Eds), Processos Fermentativos e Enzimáticos (2001) Série Biotecnologia Industrial, n.2, 1st ed, São Paulo: Ed. Edgard Blücher Ltda, p.93-121.
- HOLZAPFEL WH & SCHILLINGER U. 2002. Introduction to pre- and probiotics. Food Res. Int. 35:109-116. [CrossRef]
- JAYACHANDRAN LE, CHAKRABORTY S & RAO PS. 2015. Effect of high-pressure processing on physicochemical properties and bioactive compounds in litchi based mixed fruit beverage. Innov. Food Sci. Emerg. Technol. 28: 1–9. [CrossRef]
- JAKOBEK L. 2015. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 175:556-567. [CrossRef]
- KALITA D, SAIKIA S, GAUTAM G &, MUKHOPADHYAY R. 2018. Characteristics of synbiotic spray dried powder of litchi juice with Lactobacillus plantarum and different carrier materials. LWT 87: 351-360. [CrossRef]
- KARAOLIS C, BOTSARIS G, PANTELIDES I & TSALTAS D. 2013. Potential application of Saccharomyces boulardii as a probiotic in goat´s yoghurt: survival and organoleptic effects. Int. J. Food Sci., 48:1445-1452. [CrossRef]
- KÜHLE A, SKOVGAARD K & JESPERSEN L. 2005. In vitro screening of probiotic properties of Saccharomyces cerevisae var boulardii and food-borne Saccharomyces cerevisae strains. Int J. Food Microbiol. 101:29-39. [CrossRef]
- LAZO-VÉLEZ MA, SERNA-SALDÍVAR SO, ROSALES-MEDINA MF, TINOCO-ALVEAR M & BRIONES-GARCIA M. 2018. Application of Saccharomyces cereviasae var. boulardii in food processing: a review. J. Appl. Microbiol. 125 (4): 943-951. [CrossRef]
- LEE YL & SALMINEN S. 1996. The coming of age of probiotics. Trends Food Sci Technol 6:241–245.
- LOURENS-HATTINGH A & VILJOEN BC. 2011. Growth and survival of a probiotic yeast in dairy products. Food Res. Int. 34:791-796. [CrossRef]
- LUCKOW T & DELAHUNTY C. 2004. Which juice is ‘healthier’? A consumer study of probiotic non-dairy- juice drinks. Food Qual. 15:751-759. [CrossRef]
- MCFARLAND LV. 2017. Common Organisms and Probiotics: Saccharomyces boulardii. In: FLOCH Mh, Ringel Y & Walker WA. The Microbiota in Gastrointestinal Pathophysiology. Cambridge: Academic Press; Cambridge, USA p. 145–164.
- MARTINS FS, VIEIRA AT, ELIAN DAS, ARANTES RME, TIAGO FCP, SOUSA LP, ARAUJO HRC, PIMENTA PF, BONJARDIM CA, NICOLI JR & TEIXEIRA MM. 2013. Inhibition of tissue inflammation and bacterial translocation as one of the protective mechanisms of Saccharomyces boulardii against Salmonella infection in mice. Microbes Infec. 15 (4):270-279.
- MOJIKON FD, KASIMIN ME, MOLUJIN AM, GANSAU JA & JAWAN R. 2022. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutr. 14 (17):3457. [CrossRef]
- NELSON DL & COX MM. 2017. Lehninger Principles of Biochemistry. 7th ed. New York: W.H. Freeman,1308 p.
- PAIS P, ALMEIDA V, YILMAZ M & TEIXEIRA MC. 2020. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J Fungi 6(2):78. [CrossRef]
- PAULI. ED, CRISTIANO V & NIXDORF SL. 2011. Method for determining carbohydrates used in screening for adulterations in coffee. Quim. Nova. 34: 689-694. [CrossRef]
- PROFIR AG, VIZIREANU C. 2013. Sensorial analysis of a functional beverage based on vegetables juice. Acta Biol. 57(2):145-148.
- PARKER K, SALAS M, NWOSU VC. 2010. High fructose corn syrup: production, uses and public health concerns. Biotechnol. Mol. Biol. Rev. 5:71-78. [CrossRef]
- SIVUDU N, RAMESH B, UMAMAHESH K, REDDY OVS. 2016. Probiotication of tomato and carrot juices for shelf-life enhancement using microencapsulation. J. Food Biosci. Technol. 6(2):13-22.
- STATSOFT. STATISTIC for Windows: computer program manual. 2006. 7.1 version. Tulsa: Software Inc.
- SU D, TI H, ZHANG R, ZHANG M, WEI Z, DENG Y, GUO J. 2014. Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chem. 158:385–391. [CrossRef]
- SULIEMAN AK, PUTRA MD, ABASAEED AE, GAILY MH, AL-ZAHRANI SM & ZEINELABDEEN MA. 2018. Kinetic modelling of the simultaneous production of ethanol and fructose by Saccharomyces cerevisiae. Electron. J. Biotechnol. 34:1–8. [CrossRef]
- TERHAAG MM CONSTATINTO LV WATANABE LS MADEIRA TB NIXDORF S & PRUDENCIO SH. 2021. Carbohydrates, organic acids and phenolic compounds in yerba mate leaves and infusion. Rev. Mundi Amb. Agr. 61-20. [CrossRef]
- VILLANUEVA NDM, PETENATE AJ & DA SILVA MAAP. 2005. Performance of hybrid hedonic scale as compared to the traditional hedonic, self-adjusting and ranking scales. Food Qual. 16:691−703. [CrossRef]
- WGO. Organização Mundial de Gastroenterologia. Diretrizes Mundiais da Organização Mundial de Gastroenterologia: Probióticos e prebióticos. 2011. Available at: http://www.worldgastroenterology.org/assets/export/userfiles/Probiotics_FINAL_pt_2012.pdf. Acessed in: June 11 jun. 2024.
- YOON YK, WOODAMS EE &, HANG YD. 2004. Probiotication of tomato juice lactic acid bacteria. J. Microbiol. 42:315–318.
- ZENG XA, CHEN XD, QIN FGF & ZHANG L. 2008. Composition analysis of litchi juice and litchi wine. Int. J. Food Eng. 4 (4):1-16. [CrossRef]
- ZHANG RF, ZENG QS, DENG YY, ZHANG MW, WEI ZC, ZHANG Y & TANG XJ. 2013. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 136:1169–1176. [CrossRef]
- ZHENG X, YU Y, XIAO G, XU Y, WU J, TANG D & ZHANG Y. 2014. Comparing product stability of probiotic beverages using litchi juice treated by high hydrostatic pressure and heat as substrates. Innov. Food Sci. Emerg. Technol. 23:61–67. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).