1. Introduction
Pancreatic cancer is one of the most lethal malignancies, ranking as the seventh leading cause of cancer mortality globally and the second most common cause of death among gastrointestinal cancers [
1]. Despite advancements in diagnostics and treatments, the prognosis for pancreatic cancer remains poor, with an actuarial five-year survival rate between 15% and 25% even among patients undergoing resection. This poor outcome is primarily due to the advanced stage at which most patients present, rendering a minority eligible for curative surgery [
2,
3,
4]
Several histopathological factors have been identified as key prognostic indicators in pancreatic adenocarcinoma, including tumor size, lymph node (LN) status, tumor grade, stage, and margin status following resection [
5]. Among these, LN metastasis is particularly significant as it serves as a predictor of both overall survival and disease-free survival in non-metastatic patients undergoing surgery [
6,
7].The presence of LN involvement is assessed through the tumor-node-metastasis (TNM) classification system, which has evolved in recent editions of the American Joint Committee on Cancer (AJCC) guidelines to refine nodal categorization based on the number of positive LNs [
8].
Traditional methods for assessing the prognostic impact of LN involvement include evaluating the number of positive LNs or calculating the ratio of positive to total LNs (lymph node ratio or LNR) [
9]. However, relying solely on the number of positive nodes may be misleading due to variability in histopathological assessment and potential inadequacies in lymphadenectomy, which can result in stage migration. Emerging evidence has highlighted that LNR, defined as the proportion of metastatic LNs to the total harvested LNs, may provide a more reliable prognostic indicator than positive LN count alone, especially in terms of stratifying risk in node-positive pancreatic cancer patients [
10,
11].
The prognostic relevance of LNR has been widely studied in other malignancies, such as rectal cancer, where it has shown substantial value in predicting survival outcomes [
12,
13]. In pancreatic cancer, this approach holds potential to enhance the accuracy of survival predictions, informing better risk stratification and personalized treatment approaches. In this study, we aim to evaluate the impact of LNR and CA 19-9 levels on survival outcomes in pancreatic adenocarcinoma, contributing to a growing body of evidence that these markers can serve as independent prognostic factors in this aggressive disease. The aim of this study is to evaluate the prognostic impact of LNR and CA 19-9 levels on survival outcomes in patients with resected pancreatic adenocarcinoma, with the goal of refining risk stratification and improving predictive accuracy in this high-mortality disease.
2. Materials and Methods
2.1. Study Design and Setting
This retrospective cohort study was conducted at the General Surgery Clinic of Haydarpaşa Numune Training and Research Hospital. The study included patients who underwent surgical resection for pancreatic adenocarcinoma between January 2013 and January 2023. The study protocol was approved by the institutional review board, and patient data were collected in compliance with ethical standards and patient confidentiality protocols.
2.2. Patient Selection
A total of 146 patients who underwent surgery for pancreatic cancer were initially considered. However, only cases with histopathologically confirmed adenocarcinoma were included in the study, resulting in a final cohort of 123 patients. Exclusion criteria included:
Patients with histopathological diagnoses other than adenocarcinoma.
Patients who were found to have unresectable tumors during surgery.
Cases where palliative surgical procedures were performed.
2.3. Data Collection
Patient data were collected from medical records, including demographic information, tumor characteristics, surgical details, and survival outcomes. Specific parameters collected included:
Demographic Data : Age, gender, and relevant comorbidities.
Tumor Characteristics : Tumor location (pancreatic head, periampullary region, or distal pancreas), T stage, tumor differentiation (well, moderately, and poorly differentiated), perineural invasion, lymphovascular invasion, and organ invasion status.
Surgical Details : Type of resection (Whipple procedure, distal pancreatectomy, or total pancreatectomy) and resection margin status (R0, R1, R2).
Laboratory Data : Preoperative serum CA 19-9 levels.
Lymph Node Evaluation : Total number of lymph nodes (LNs) harvested, number of positive LNs, and calculation of the lymph node ratio (LNR) as the ratio of positive LNs to the total number of LNs harvested.
2.4. Outcomes and Definitions
The primary outcome of interest was overall survival (OS), defined as the time from the date of surgery to the date of death or last follow-up. Mortality was categorized as either alive or deceased at the last follow-up. Positive LNR and CA 19-9 levels were investigated as potential prognostic indicators for OS.
2.5. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics, Version 22. Descriptive statistics were used to summarize the characteristics of the study population, with continuous variables expressed as means ± standard deviations (SD) or medians (for non-normally distributed data) and categorical variables as frequencies and percentages. The Kolmogorov-Smirnov test was applied to assess the normality of continuous data. For comparisons of continuous variables between two groups, the Mann-Whitney U test was used. For comparisons among more than two groups, the Kruskal-Walli’s test was applied. Categorical data were analyzed using the chi-square test, Fisher Freeman Halton Exact test, and continuity (Yates) correction, as appropriate. ROC Curve Analysis : Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values for LNR and CA 19-9 levels for predicting mortality. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each cutoff. Kaplan-Meier analysis was conducted to estimate survival curves, and the log-rank test was used to compare survival distributions between groups. A p-value of <0.05 was considered statistically significant.
2.6. Ethical Considerations
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. The ethical committee of Haydarpaşa Numune Training and Research Hospital approved the study (2023/16-27). Patient confidentiality was strictly maintained, and data were de-identified to prevent any disclosure of personal information. Given the retrospective nature of the study, informed consent was waived by the ethics committee.
3. Results
In this study, a total of 123 patients with histopathologically confirmed pancreatic adenocarcinoma. The majority of tumors were located in the pancreatic head (63.4%), followed by the periampullary region (19.5%) and the distal pancreas (17.1%). Most patients (91.9%) underwent the Whipple procedure, with smaller percentages undergoing distal (6.5%) and total pancreatectomy (1.6%). In terms of T staging, T3 was the most common (67.5%), while T1, T2, and T4 represented smaller groups (7.3%, 22.8%, and 2.4%, respectively). Most tumors were moderately differentiated (62.6%), with lower rates of well-differentiated (10.6%) and poorly differentiated (26.8%) tumors. High percentages of patients presented with perineural invasion (71.5%) and lymphovascular invasion (76.4%), whereas organ invasion was relatively rare (3.3%). Regarding resectability, 75.6% achieved R0 status. Mortality was recorded in 72.4% of cases (
Table 1).
The positive LNR was significantly higher in patients who succumbed to the disease (median 0.14 ± 0.16) compared to survivors (median 0.08 ± 0.13) (p=0.011). Patients with an LNR ≤ 0.14 had a mortality rate of 63%, while those with an LNR > 0.14 had a significantly higher mortality rate of 90.5% (p = 0.003). Patients with levels < 39 had a lower mortality rate (63.3%), with no significant difference (p = 0.103) (
Table 2).
Positive LNR levels varied across T stages, with lower ratios observed in earlier stages (T1 median 0.11 ± 0.15, T2 median 0.14 ± 0.16) and higher ratios in more advanced stages (T3 median 0.07 ± 0.08 and T4 median 0.10 ± 0.12). The association between T stage and LNR was not statistically significant (p = 0.334). Patients with CA 19-9levels < 39 had a median LNR of 0.14 ± 0.17, while those with levels ≥ 39 had a slightly lower median LNR of 0.11 ± 0.15, with no significant difference (p = 0.386) (
Table 3).
Tumor location varied by T stage and CA 19-9 level. The majority of tumors were located in the head of the pancreas across all T stages (66.7% in T1, 50% in T2, 67.5% in T3, and 66.7% in T4). Patients with CA 19-9 levels < 39 also had a majority of tumors in the pancreatic head (59.2%), as did those with levels ≥ 39 (66.2%). There was no statistically significant association between tumor location and either T stage (p = 0.358) or CA 19-9 level (p = 0.672) (
Table 4).
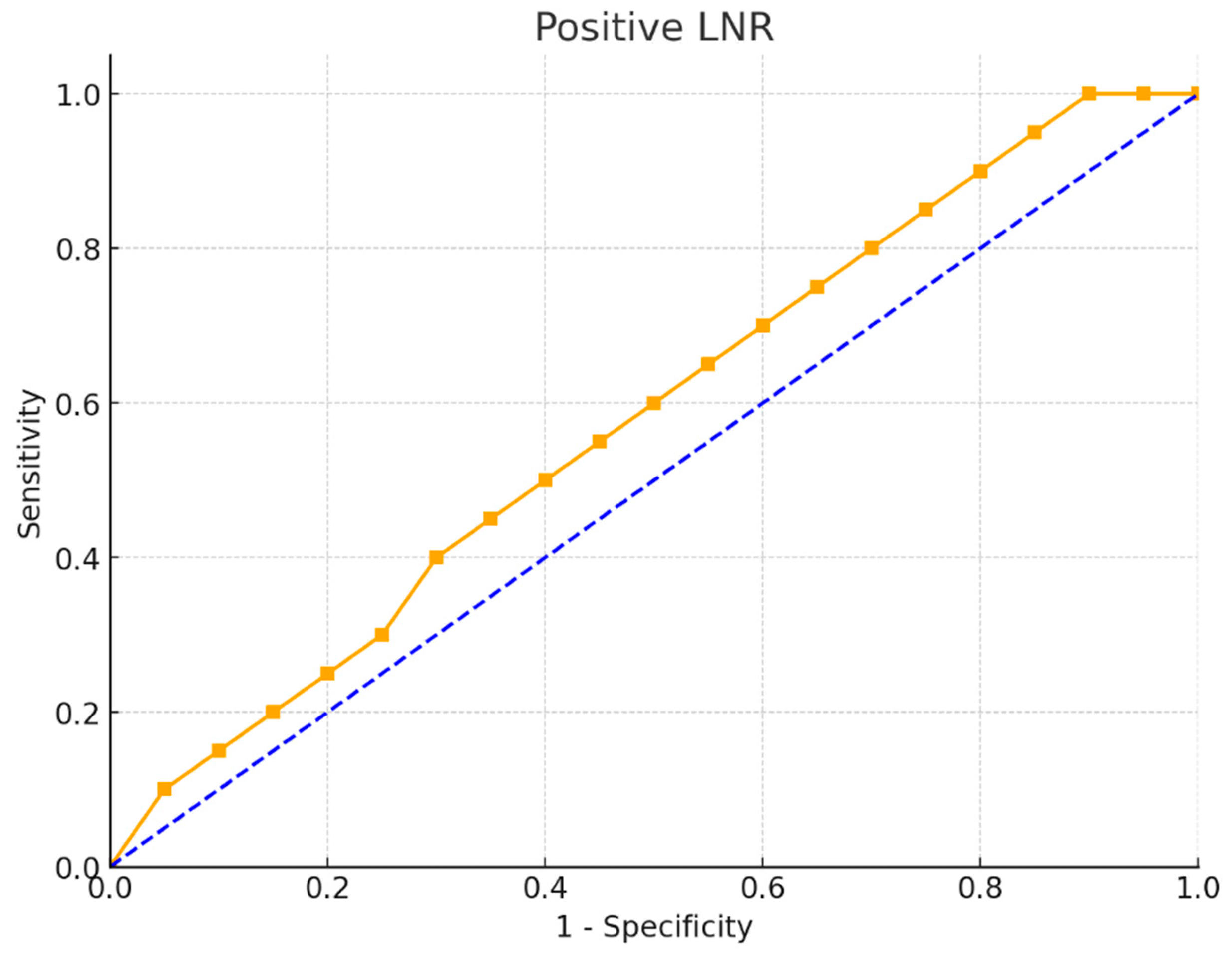
The ROC curve for the positive LNR as a diagnostic tool for mortality revealed an area under the curve (AUC) of 0.646 with a standard error of 0.05, which was significantly higher than 0.5 (p = 0.007). This indicates that LNR has a moderate ability to predict mortality. The optimal cut-off point for LNR in predicting mortality was determined to be >0.14, yielding a sensitivity of 88.2% and specificity of 42.7%. The positive predictive value was 90.5%, and the negative predictive value was 37%, highlighting LNR’s potential as a mortality predictor in this patient group (
Figure 1.).
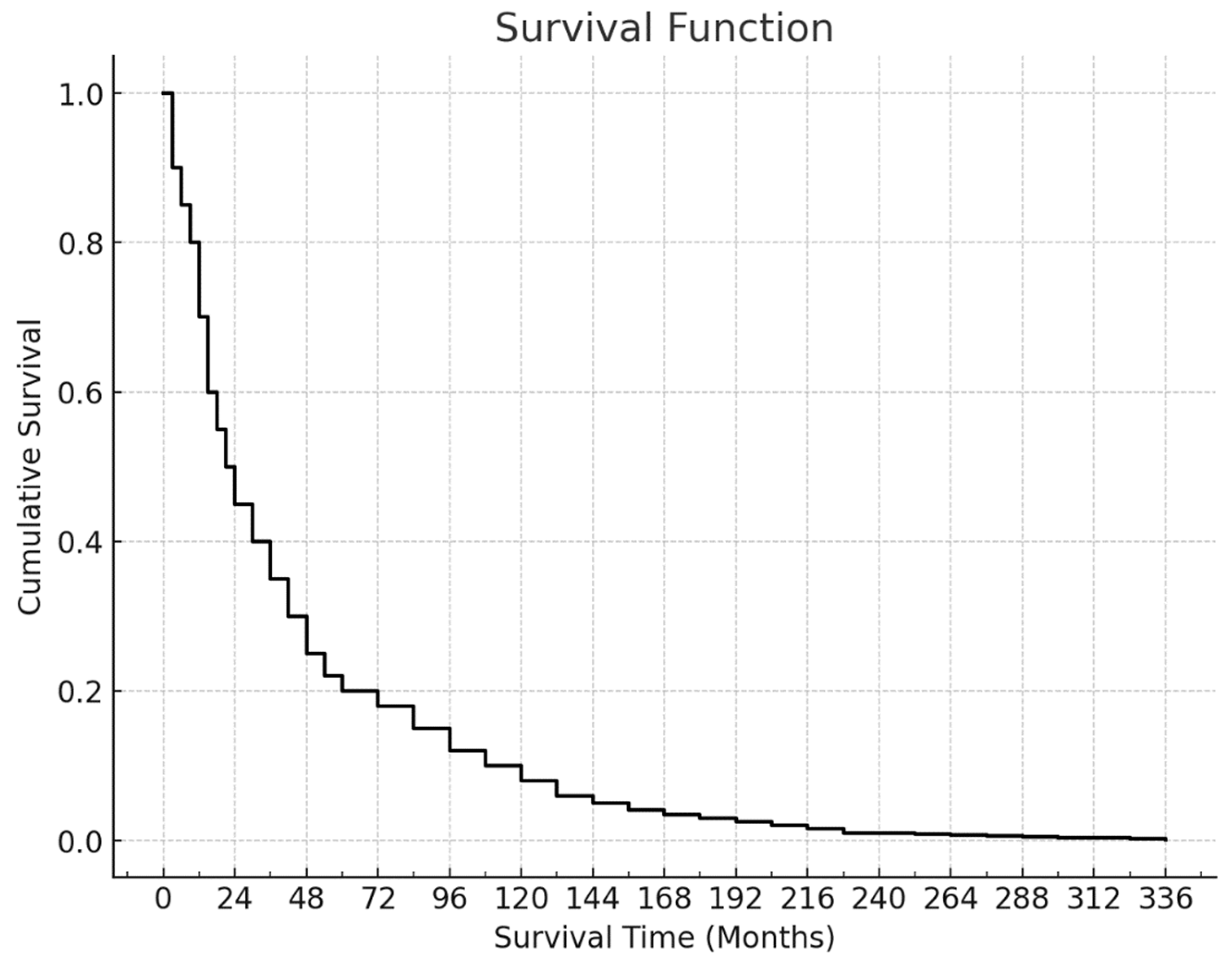
Among the 123 patients included in the survival analysis, 89 (72.4%) had died by the end of the follow-up period. The mean survival time was 45.36 ± 4.80 months, while the median survival time was 22 months. The last death was recorded at the 73rd month, resulting in a cumulative survival rate of 23.3% with a standard error of 4.3%. This curve illustrates the aggressive nature of pancreatic adenocarcinoma, with a high mortality rate and limited long-term survival (
Figure 2).
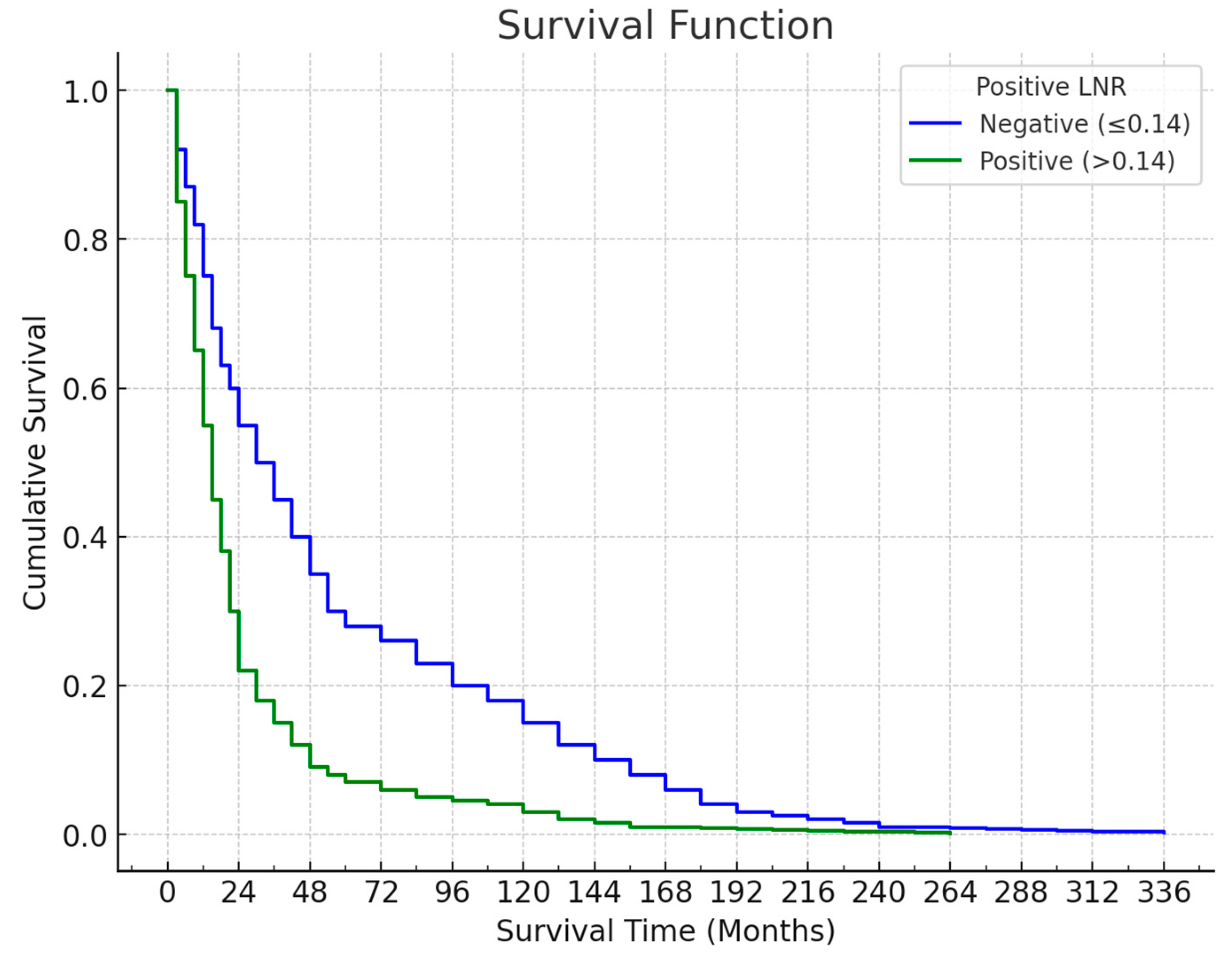
Survival outcomes varied significantly according to the LNR cut-off of 0.14. Among the 81 patients with an LNR ≤ 0.14, 51 (63%) died, with a mean survival time of 57.04 ± 6.47 months and a median survival time of 30.2 months. The last death in this group occurred at the 73rd month, yielding a cumulative survival rate of 33.6% (standard error: 5.8%). Conversely, of the 42 patients with an LNR > 0.14, 38 (90.5%) died, with a mean survival time of 19.65 ± 2.37 months and a median survival time of 17 months. The last death in this group occurred at the 60th month, and the cumulative survival rate was 0%. This significant difference in survival (p = 0.001) indicates that patients with an LNR > 0.14 had notably shorter survival times (
Figure 3).
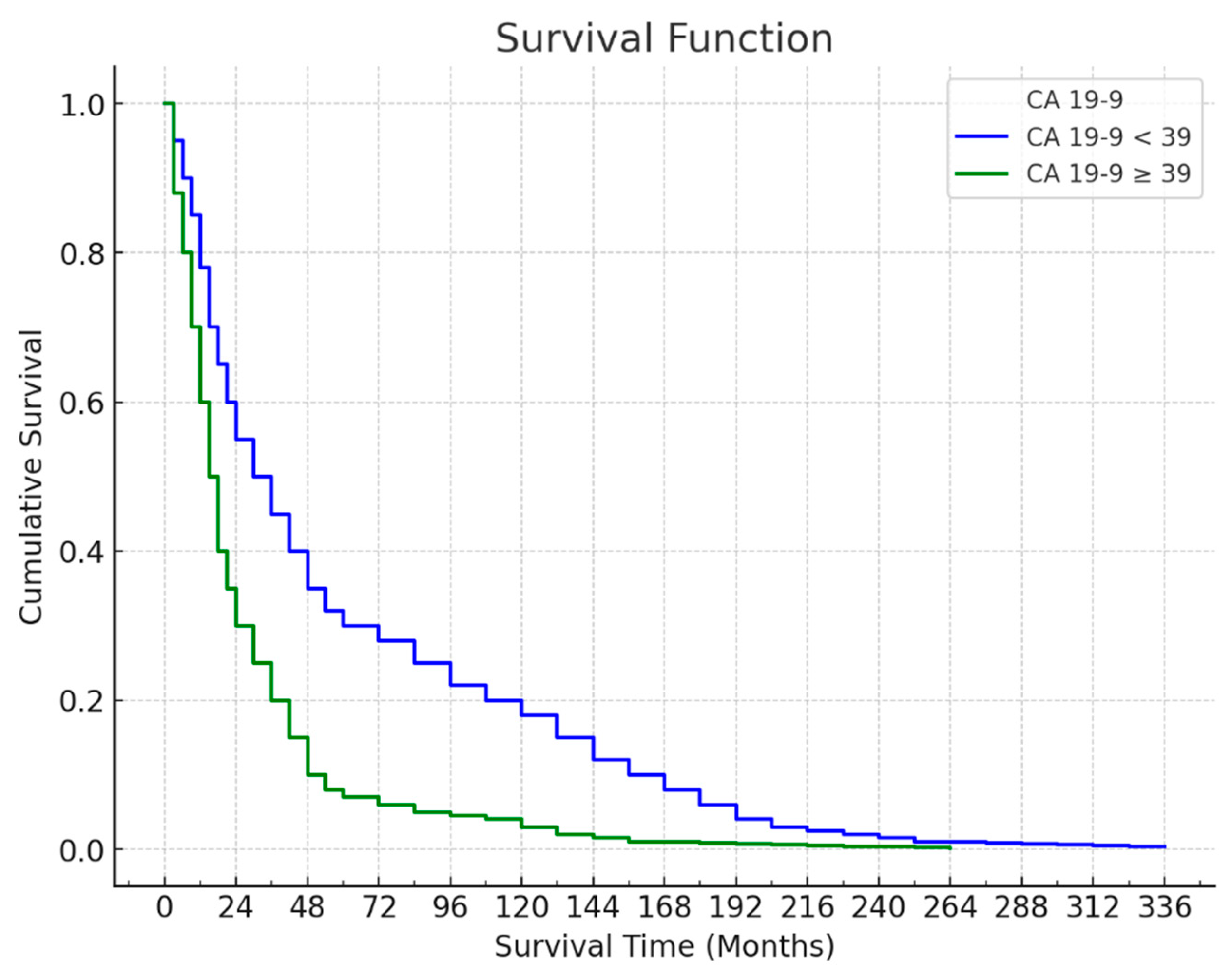
The survival curve based on CA 19-9 levels shows a difference in survival between patients with CA 19-9 levels below and above the 39 cut-offs. Among the 49 patients with CA 19-9 levels < 39, 31 (63.3%) died, with a mean survival time of 55.59 ± 7.93 months and a median survival time of 29 months. The last death in this group occurred at the 73rd month, resulting in a cumulative survival rate of 31.5% (standard error: 8.2%). For the 74 patients with CA 19-9 levels ≥ 39, 58 (78.4%) died, with a mean survival time of 36.91 ± 5.60 months and a median survival time of 17 months. The last death occurred at the 60th month, yielding a cumulative survival rate of 16.9% (standard error: 4.9%). The difference in survival between patients with CA 19-9 levels < 39 and those with levels ≥ 39 was statistically significant (p = 0.034), indicating that lower CA 19-9 levels were associated with longer survival (
Figure 4).
4. Discussion
Our study investigated the prognostic impact of LNR and CA 19-9 levels on survival in patients with pancreatic adenocarcinoma, revealing that both markers independently correlate with survival outcomes. Patients with an LNR > 0.14 and CA 19-9 levels ≥ 39 U/mL experienced significantly poorer survival compared to their counterparts with lower values. This aligns with existing literature and highlights the relevance of these markers in stratifying risk in pancreatic cancer, a malignancy known for its poor prognosis.
The prognostic value of LNR has been increasingly recognized in pancreatic cancer. In our cohort, an LNR cut-off of > 0.14 corresponded with a median survival of only 17 months, contrasting starkly with the 30.2 months observed in patients with an LNR ≤ 0.14. This is consistent with the findings by Karjol et al., who, through a systematic review and meta-analysis, determined that an LNR > 0.2 was associated with a hazard ratio of 1.84, indicating nearly double the risk of mortality compared to patients with lower LNRs [
14]. Similarly, Manen et al. observed that pancreatic cancer patients with LNRs > 0.15 had median survival times approximately 14 months shorter than those with lower LNRs (23 vs. 37 months) [
15]. The results of our study further strengthen the argument that LNR provides superior prognostic accuracy over absolute lymph node counts, which are prone to variability based on surgical and histopathological factors.
Furthermore, Coppola et al. reported that LNR not only predicts overall survival but also correlates with recurrence patterns in resectable pancreatic ductal adenocarcinoma (PDAC) patients [
16]. The higher LNR values in our study were likewise associated with lower cumulative survival rates, underscoring the increased likelihood of recurrent disease in patients with extensive nodal involvement. This cumulative evidence suggests that LNR should be integrated into clinical staging systems, potentially informing decisions on adjuvant treatment and more intensive follow-up strategies for patients at higher risk of recurrence.
CA 19-9 has long been recognized as a valuable marker in pancreatic cancer, both for diagnosis and prognosis. In our study, CA 19-9 levels ≥ 39 U/mL were significantly associated with reduced survival, with a median survival of 17 months compared to 29 months in patients with CA 19-9 < 39 U/mL. This aligns with findings by Luo et al., who documented those patients with CA 19-9 levels above 37 U/mL had a mean survival of only 15 months, compared to 25 months for those with lower levels [
17]. The Libyan cohort study by Eramiah et al. also observed similar trends, where elevated CA 19-9 levels (> 400 U/mL) were linked to a decrease in survival from 18 to 9 months (p < 0.05) [
18]. Such consistency across studies demonstrates the robust prognostic significance of CA 19-9 in pancreatic cancer, making it indispensable for clinical decision-making.
Xu et al. further refined CA 19-9’s prognostic utility by calculating a ratio of CA 19-9 to tumor volume, identifying a threshold of > 5.62 for predicting poor outcomes, with an AUC of 0.633. Although our study did not assess tumor volume, the findings suggest that combining CA 19-9 with additional tumor metrics may enhance prognostic accuracy [
19]. Integrating CA 19-9 with LNR, as done in our study, could represent a feasible alternative in routine clinical practice, allowing for more nuanced risk assessment without requiring complex tumor measurements.
The combination of high LNR and elevated CA 19-9 levels provides a dual assessment of tumor burden and metastatic potential, which can more accurately predict survival than either marker alone. In our study, patients with both an LNR > 0.14 and CA 19-9 ≥ 39 U/mL demonstrated the worst survival outcomes, with a cumulative survival rate of 0% by the 60th month, compared to 33.6% in patients with LNR ≤ 0.14 and CA 19-9 < 39 U/mL. This dual marker approach aligns with findings by Li et al., who reported that patients with elevated CA 19-9 and LNR levels had a 25% lower five-year survival rate compared to those with only one elevated marker (12% vs. 37%) [
20].
Moreover, Coppola et al. identified CA 19-9 as a predictor of positive nodal status in resectable PDAC, suggesting that elevated CA 19-9 levels may serve as a proxy for increased nodal involvement even before surgery [
16]. This predictive capacity aligns with our findings that higher CA 19-9 levels often accompany elevated LNRs, emphasizing the interconnectedness of these markers in reflecting advanced disease and poorer prognosis.
Our findings support the inclusion of LNR and CA 19-9 as part of the routine prognostic assessment for pancreatic adenocarcinoma patients. Their combined use could identify high-risk patients who may benefit from more aggressive treatment approaches, including adjuvant or neoadjuvant therapies. The work of Coppola et al. suggests that elevated CA 19-9 could identify candidates for neoadjuvant therapy, and incorporating LNR could further refine patient selection, potentially improving surgical outcomes by reducing the risk of residual nodal disease [
16]. Future studies should investigate the utility of preoperative LNR in predicting surgical outcomes and guiding neoadjuvant therapy, especially in borderline resectable patients.
Despite the promising implications, this study’s retrospective design and single-center setting limit the generalizability of our results. Additionally, the lack of data on postoperative therapy precludes assessment of how adjuvant treatments might modify the prognostic impact of LNR and CA 19-9. Multicenter prospective studies could address these limitations and validate our findings in diverse populations.
5. Limitations
This study is retrospective and limited by the single-center design, which may reduce the generalizability of our findings. Moreover, the absence of data on adjuvant therapy regimens limits our ability to assess the impact of post-operative treatment on survival outcomes. Future studies should explore prospective, multicenter trials to validate these findings further and consider a broader analysis involving additional biomarkers and tumor genetic profiles to improve prognostic accuracy.
6. Conclusions
This study underscores the prognostic utility of LNR and CA 19-9 in pancreatic adenocarcinoma. Patients with high LNR and elevated CA 19-9 levels are at a significantly increased risk of mortality, with markedly reduced survival rates. By combining these markers, clinicians can better stratify patients by risk, potentially guiding treatment decisions and improving long-term outcomes in this challenging cancer. Further research should explore the integration of LNR and CA 19-9 into staging systems and examine their role in tailoring multimodal therapeutic approaches for pancreatic cancer.
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. The Lancet 2020, 395, 2008–20. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. The global burden of pancreatic cancer. Archives of Medical Science 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Selek, U.; Pehlivan, B.; Kucuk, A.; Haksoyler, V.; Kilic Durankus, N.; et al. The prognostic significance of novel pancreas cancer prognostic index in unresectable locally advanced pancreas cancers treated with definitive concurrent chemoradiotherapy. Journal of Inflammation Research 2021, 4433–44. [Google Scholar] [CrossRef] [PubMed]
- Merlo, I.; Ardiles, V.; Sanchez-Clariá, R.; Fratantoni, E.; de Santibañes, E.; Pekolj, J.; et al. Prognostic factors in resected pancreatic ductal adenocarcinoma: is neutrophil-lymphocyte ratio a useful marker? Journal of Gastrointestinal Cancer 2023, 54, 580–8. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.; Nassour, I.; Hoehn, R.S.; Khan, S.; Althans, A.; Geller, D.A.; et al. Impact of resection margin status in patients with pancreatic cancer: a national cohort study. Journal of Gastrointestinal Surgery 2021, 25, 2307–16. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-J.; Mao, Y.-P.; Guo, R.; Huang, C.-L.; Fang, X.-L.; Ma, J.; et al. A nomogram based on serum biomarkers and clinical characteristics to predict survival in patients with non-metastatic nasopharyngeal carcinoma. Frontiers in Oncology. 2020, 10, 594363. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-N.; Jiang, J.; Jiang, L.-M.; Zhou, H.-T.; Li, N.; Lu, N.-N.; et al. Post-hoc analysis of clinicopathological factors affecting lateral lymph node metastasis based on STELLAR study for rectal cancer. Radiotherapy and Oncology. 2024, 200, 110512. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hu, C.; Yang, X.; Liu, Y.; Ji, G.; Ge, S.; et al. Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduction and Targeted Therapy 2023, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-L.; Gong, Y.; Pei, Y.-C.; Ji, P.; Hu, X.; Shao, Z.-M. Modified lymph node ratio improves the prognostic predictive ability for breast cancer patients compared with other lymph node staging systems. The Breast. 2020, 49, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Prassas, D.; Safi, S.A.; Stylianidi, M.C.; Telan, L.A.; Krieg, S.; Roderburg, C.; et al. N, LNR or LODDS: Which is the most appropriate lymph node classification scheme for patients with radically resected pancreatic cancer? Cancers 2022, 14, 1834. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Ray, S.; Behera, P.K.; Mohanty, L.; Das, P.; Biswal, P. Evaluation of lymph node ratios and log odds of positive nodes as prognostic indicators in primary organ malignancy. Medical Journal Armed Forces India. 2024. [CrossRef]
- Karjol, U.; Jonnada, P.; Chandranath, A.; Cherukuru, S. Lymph node ratio as a prognostic marker in rectal cancer survival: a systematic review and meta-analysis. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Sequeira, H.; Ladeira, K.; Bonito, N.; Viana, C.; Martins, S. Metastatic lymph node ratio as a better prognostic tool than the TNM system in colorectal cancer. Future Oncology 2021, 17, 1519–32. [Google Scholar] [CrossRef] [PubMed]
- Karjol, U.; Chandranath, A.; Jonnada, P.; Cherukuru, S.; Annavarjula, V.; Morla, S.A. Lymph node ratio as a prognostic marker in pancreatic cancer survival: a systematic review and meta-analysis. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- van Manen, L.; Groen, J.V.; Putter, H.; Pichler, M.; Vahrmeijer, A.L.; Bonsing, B.A.; et al. Stage-specific value of carbohydrate antigen 19-9 and carcinoembryonic antigen serum levels on survival and recurrence in pancreatic cancer: a single center study and meta-analysis. Cancers 2020, 12, 2970. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; La Vaccara, V.; Fiore, M.; Farolfi, T.; Ramella, S.; Angeletti, S.; et al. CA19. 9 serum level predicts lymph-nodes status in resectable pancreatic ductal adenocarcinoma: a retrospective single-center analysis. Frontiers in Oncology. 2021, 11, 690580. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef] [PubMed]
- Ermiah, E.; Eddfair, M.; Abdulrahman, O.; Elfagieh, M.; Jebriel, A.; Al-Sharif, M.; et al. Prognostic value of serum CEA and CA19-9 levels in pancreatic ductal adenocarcinoma. Molecular and clinical oncology 2022, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lyu, S.; Zhao, Y.; Zhang, X.; Liu, Z.; Zhao, X.; et al. Ratio of CA19-9 Level to Total Tumor Volume as a Prognostic Predictor of Pancreatic Carcinoma After Curative Resection. Technology in Cancer Research & Treatment. 2022, 21, 15330338221078438. [Google Scholar]
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and survival analysis of patients with pancreatic cancer: retrospective experience of a single institution. World Journal of Surgical Oncology 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).