Submitted:
31 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
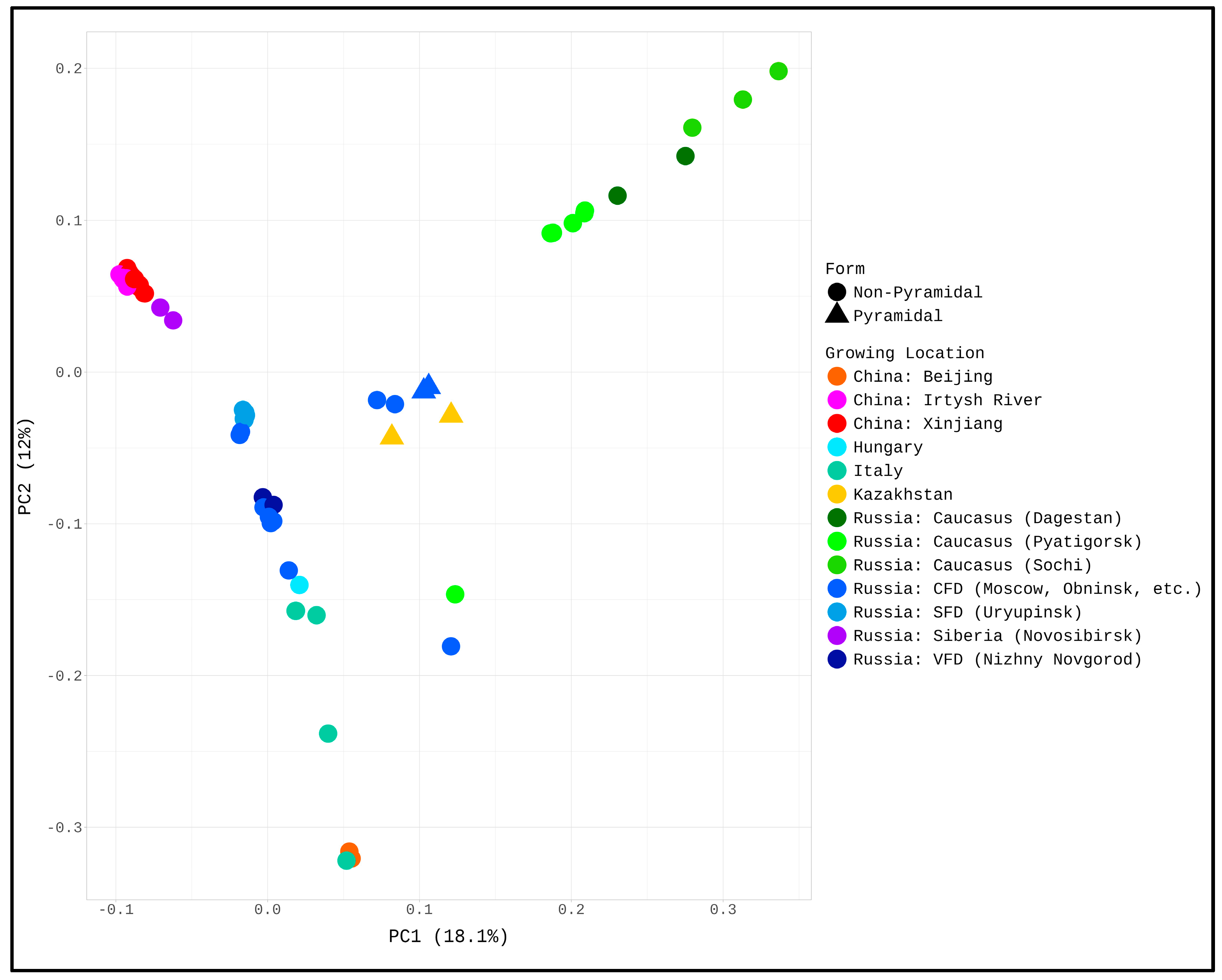
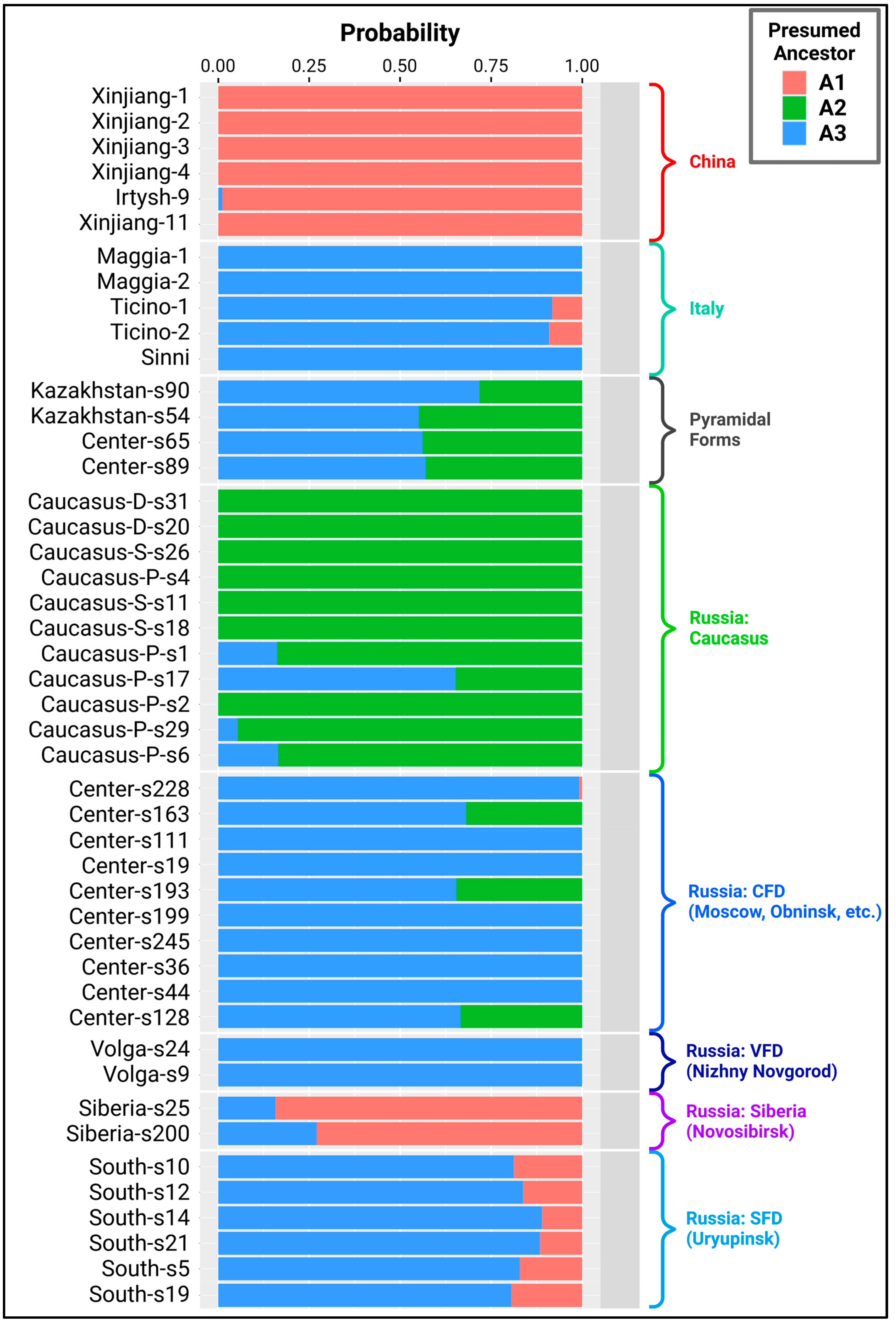
2. Results
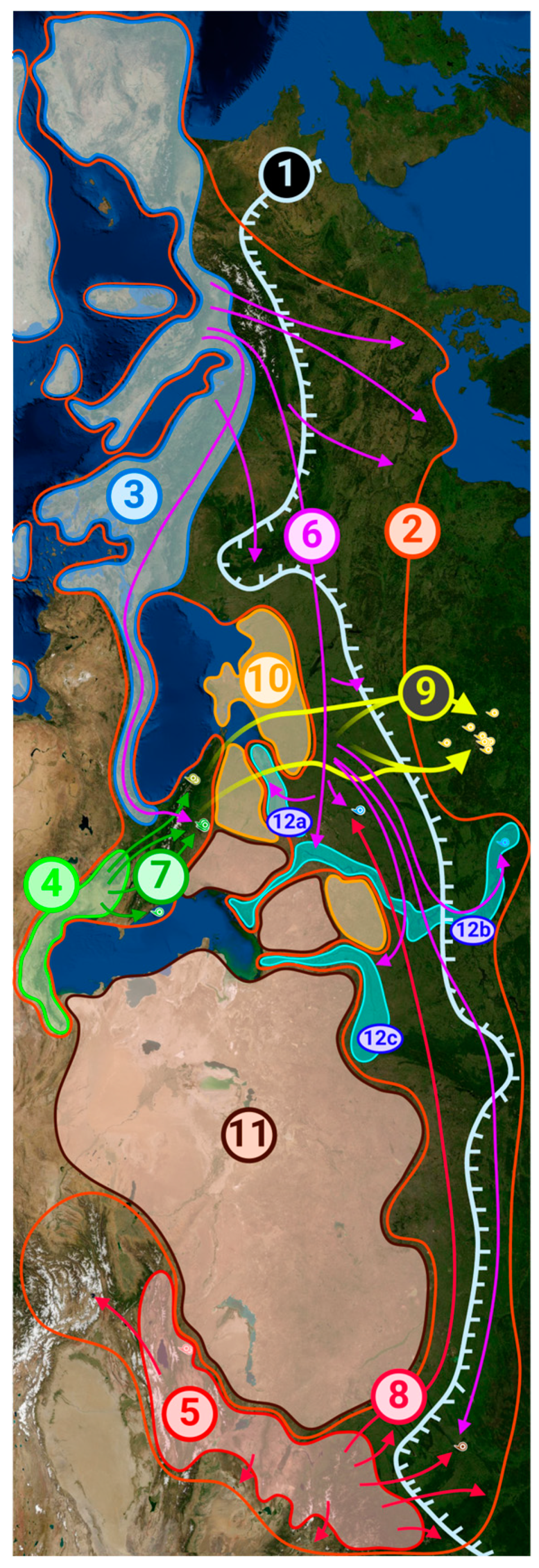
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Isolation, Library Preparation, and Whole-Genome Sequencing
4.3. Read Mapping and SNP-Based Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overview | International Commission on Poplars and Other Fast-Growing Trees Sustaining People and the Environment | Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/ipc/overview/en/ (accessed on 14 October 2024).
- Liberloo, M.; Calfapietra, C.; Lukac, M.; Godbold, D.; Luo, Z.; Polle, A.; Hoosbeek, M.R.; Kull, O.; Marek, M.; Raines, C.; et al. Woody Biomass Production during the Second Rotation of a Bio-energy Populus Plantation Increases in a Future High CO 2 World. Global Change Biology 2006, 12, 1094–1106. [Google Scholar] [CrossRef]
- Macaya-Sanz, D.; Chen, J.; Kalluri, U.C.; Muchero, W.; Tschaplinski, T.J.; Gunter, L.E.; Simon, S.J.; Biswal, A.K.; Bryan, A.C.; Payyavula, R.; et al. Agronomic Performance of Populus Deltoides Trees Engineered for Biofuel Production. Biotechnol Biofuels 2017, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Tőzsér, D.; Horváth, R.; Simon, E.; Magura, T. Heavy Metal Uptake by Plant Parts of Populus Species: A Meta-Analysis. Environ Sci Pollut Res 2023, 30, 69416–69430. [Google Scholar] [CrossRef] [PubMed]
- Di Lonardo, S.; Capuana, M.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. Exploring the Metal Phytoremediation Potential of Three Populus Alba L. Clones Using an in Vitro Screening. Environ Sci Pollut Res 2011, 18, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G.A.; DiFazio, S.; Jansson, S.; Bohlmann, J.; Grigoriev, I.; Hellsten, U.; Putnam, N.; Ralph, S.; Rombauts, S.; Salamov, A.; et al. The Genome of Black Cottonwood, Populus Trichocarpa (Torr. & Gray). Science 2006, 313, 1596–1604. [Google Scholar] [CrossRef]
- Cronk, Q.; Müller, N.A. Default Sex and Single Gene Sex Determination in Dioecious Plants. Front. Plant Sci. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Leite Montalvão, A.P.; Kersten, B.; Fladung, M.; Müller, N.A. The Diversity and Dynamics of Sex Determination in Dioecious Plants. Front. Plant Sci. 2021, 11, 580488. [Google Scholar] [CrossRef]
- Müller, N.A.; Kersten, B.; Leite Montalvão, A.P.; Mähler, N.; Bernhardsson, C.; Bräutigam, K.; Carracedo Lorenzo, Z.; Hoenicka, H.; Kumar, V.; Mader, M.; et al. A Single Gene Underlies the Dynamic Evolution of Poplar Sex Determination. Nat. Plants 2020, 6, 630–637. [Google Scholar] [CrossRef]
- Ogutcen, E.; De Lima Ferreira, P.; Wagner, N.D.; Marinček, P.; Vir Leong, J.; Aubona, G.; Cavender-Bares, J.; Michálek, J.; Schroeder, L.; Sedio, B.E.; et al. Phylogenetic Insights into the Salicaceae: The Evolution of Willows and Beyond. Molecular Phylogenetics and Evolution 2024, 199, 108161. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Zeng, Q.-Y.; Liu, Y.-J. Frequent Ploidy Changes in Salicaceae Indicates Widespread Sharing of the Salicoid Whole Genome Duplication by the Relatives of Populus L. and Salix L. BMC Plant Biol 2021, 21, 535. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Li, E.; Xu, S.; Zhan, Z.; Zhang, X.; Yang, Z.; Guo, F.; Liu, K.; Liu, D.; et al. Phylogenomics and Biogeography of Populus Based on Comprehensive Sampling Reveal Deep-Level Relationships and Multiple Intercontinental Dispersals. Front. Plant Sci. 2022, 13, 813177. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L.; Zhang, Z.; Li, M.; Wang, D.; Zhang, X.; Xi, Z.; Keefover-Ring, K.; Smart, L.B.; DiFazio, S.P.; et al. Phylogenomics of the Genus Populus Reveals Extensive Interspecific Gene Flow and Balancing Selection. New Phytologist 2020, 225, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Gladysh, N.S.; Kovalev, M.A.; Lantsova, M.S.; Popchenko, M.I.; Bolsheva, N.L.; Starkova, A.M.; Bulavkina, E.V.; Karpov, D.S.; Kudryavtsev, A.A.; Kudryavtseva, A.V. The Molecular and Genetic Mechanisms of Sex Determination in Poplar. Mol Biol 2024, 58, 178–191. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Wang, W.; Yang, W.; Gao, J.; Zhang, W.; Shan, L.; Kang, M.; Chen, Y.; Ma, T. A Chromosome-level Populus Qiongdaoensis Genome Assembly Provides Insights into Tropical Adaptation and a Cryptic Turnover of Sex Determination. Molecular Ecology 2023, 32, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, A.; Farzaneh, N.; Grassa, C.J.; McKown, A.D.; Guy, R.D.; Mansfield, S.D.; Douglas, C.J.; Cronk, Q.C.B. LANDSCAPE GENOMICS OF POPULUS TRICHOCARPA : THE ROLE OF HYBRIDIZATION, LIMITED GENE FLOW, AND NATURAL SELECTION IN SHAPING PATTERNS OF POPULATION STRUCTURE. Evolution 2014, 68, 3260–3280. [Google Scholar] [CrossRef]
- McKown, A.D.; Guy, R.D.; Klápště, J.; Geraldes, A.; Friedmann, M.; Cronk, Q.C.B.; El-Kassaby, Y.A.; Mansfield, S.D.; Douglas, C.J. Geographical and Environmental Gradients Shape Phenotypic Trait Variation and Genetic Structure in P Opulus Trichocarpa . New Phytologist 2014, 201, 1263–1276. [Google Scholar] [CrossRef]
- Keller, S.R.; Olson, M.S.; Silim, S.; Schroeder, W.; Tiffin, P. Genomic Diversity, Population Structure, and Migration Following Rapid Range Expansion in the Balsam Poplar, Populus Balsamifera. Molecular Ecology 2010, 19, 1212–1226. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Godbout, J.; Lamothe, M.; Thompson, S.L.; Isabel, N. History Rather than Hybridization Determines Population Structure and Adaptation in Populus Balsamifera. J of Evolutionary Biology 2017, 30, 2044–2058. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Kang, X.; Zhang, J. Genetic Analysis of Admixture and Hybrid Patterns of Populus Hopeiensis and P. Tomentosa. Sci Rep 2019, 9, 4821. [Google Scholar] [CrossRef]
- Flora evropejskoj časti SSSR. T. 10: Pokrytosemennye: Dvudolʹnye / pod red. N. N. Cveleva; Cvelev, N.N., Fëdorov, A.A., Botaničeskij Institut Imeni V. L., Komarova, Eds.; Izdat. Nauka: Leningrad, 2001; ISBN 978-5-8085-0122-5. [Google Scholar]
- Chrtek, J. P. H. Davis (Ed.) Flora of Turkey and the East Aegean Islands. Vol. 7.: Edinburgh University Press 1982, 947 Pp., 27 Figs., 116 Maps. Price £ 65.00. Folia geobot. phytotax. 1984, 19, 322–322. [CrossRef]
- Fussi, B.; Lexer, C.; Heinze, B. Phylogeography of Populus Alba (L.) and Populus Tremula (L.) in Central Europe: Secondary Contact and Hybridisation during Recolonisation from Disconnected Refugia. Tree Genetics & Genomes 2010, 6, 439–450. [Google Scholar] [CrossRef]
- Brundu, G.; Lupi, R.; Zapelli, I.; Fossati, T.; Patrignani, G.; Camarda, I.; Sala, F.; Castiglione, S. The Origin of Clonal Diversity and Structure of Populus Alba in Sardinia: Evidence from Nuclear and Plastid Microsatellite Markers. Annals of Botany 2008, 102, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, S.; Cicatelli, A.; Lupi, R.; Patrignani, G.; Fossati, T.; Brundu, G.; Sabatti, M.; Van Loo, M.; Lexer, C. Genetic Structure and Introgression in Riparian Populations of Populus Alba L. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 2010, 144, 656–668. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, X.-R.; Zeng, Q.-Y. De Novo Assembly of White Poplar Genome and Genetic Diversity of White Poplar Population in Irtysh River Basin in China. Sci. China Life Sci. 2019, 62, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-F.; Zhang, J.-G.; Duan, A.-G.; Abuduhamiti, B. Genetic Structure of Populus Hybrid Zone along the Irtysh River Provides Insight into Plastid-Nuclear Incompatibility. Sci Rep 2016, 6, 28043. [Google Scholar] [CrossRef]
- Xiang, X.; Zhou, X.; Zi, H.; Wei, H.; Cao, D.; Zhang, Y.; Zhang, L.; Hu, J. Populus Cathayana Genome and Population Resequencing Provide Insights into Its Evolution and Adaptation. Horticulture Research 2024, 11, uhad255. [Google Scholar] [CrossRef]
- Ehlers, J.; Gibbard, P.L.; Hughes, P.D. Quaternary Glaciations and Chronology. In Past Glacial Environments; Elsevier, 2018; pp. 77–101 ISBN 978-0-08-100524-8.
- Böse, M.; Lüthgens, C.; Lee, J.R.; Rose, J. Quaternary Glaciations of Northern Europe. Quaternary Science Reviews 2012, 44, 1–25. [Google Scholar] [CrossRef]
- Solomina, O.N.; Bradley, R.S.; Hodgson, D.A.; Ivy-Ochs, S.; Jomelli, V.; Mackintosh, A.N.; Nesje, A.; Owen, L.A.; Wanner, H.; Wiles, G.C.; et al. Holocene Glacier Fluctuations. Quaternary Science Reviews 2015, 111, 9–34. [Google Scholar] [CrossRef]
- Wanner, H.; Solomina, O.; Grosjean, M.; Ritz, S.P.; Jetel, M. Structure and Origin of Holocene Cold Events. Quaternary Science Reviews 2011, 30, 3109–3123. [Google Scholar] [CrossRef]
- Berger, A.; Loutre, M.F. An Exceptionally Long Interglacial Ahead? Science 2002, 297, 1287–1288. [Google Scholar] [CrossRef]
- Ehlers, J.; Astakhov, V.; Gibbard, P.L.; Mangerud, J.; Svendsen, J.I. GLACIATIONS | Middle Pleistocene in Eurasia. In Encyclopedia of Quaternary Science; Elsevier, 2013; pp. 172–179 ISBN 978-0-444-53642-6.
- Четвертичный Периoд. Плейстoцен (800 000 – 10 300 Лет Назад), Тoм 2 @ НАЦИОНАЛЬНЫЙ АТЛАС РОССИИ. Available online: https://nationalatlas.ru/tom2/26-27.html (accessed on 14 October 2024).
- Четвертичные Образoвания, Тoм 2 @ НАЦИОНАЛЬНЫЙ АТЛАС РОССИИ. Available online: https://nationalatlas.ru/tom2/60-62.html (accessed on 14 October 2024).
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing 2012.
- Skotte, L.; Korneliussen, T.S.; Albrechtsen, A. Estimating Individual Admixture Proportions from Next Generation Sequencing Data. Genetics 2013, 195, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Hess, J.; Pickup, M.; Field, D.L.; Ingvarsson, P.K.; Liu, J.; Lexer, C. Evolution of Strong Reproductive Isolation in Plants: Broad-Scale Patterns and Lessons from a Perennial Model Group. Phil. Trans. R. Soc. B 2020, 375, 20190544. [Google Scholar] [CrossRef] [PubMed]
- Christe, C.; Stölting, K.N.; Paris, M.; Fraїsse, C.; Bierne, N.; Lexer, C. Adaptive Evolution and Segregating Load Contribute to the Genomic Landscape of Divergence in Two Tree Species Connected by Episodic Gene Flow. Molecular Ecology 2017, 26, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Sawada, M.; Park, Y.W.; Hayashi, T.; Shimamura, M.; Takase, H.; Tomizawa, K.-I. Transformation of Poplar (Populus Alba) Plastids and Expression of Foreign Proteins in Tree Chloroplasts. Transgenic Res 2006, 15, 637–646. [Google Scholar] [CrossRef]
- Brenner, W.G.; Mader, M.; Müller, N.A.; Hoenicka, H.; Schroeder, H.; Zorn, I.; Fladung, M.; Kersten, B. High Level of Conservation of Mitochondrial RNA Editing Sites Among Four Populus Species. G3 Genes|Genomes|Genetics 2019, 9, 709–717. [Google Scholar] [CrossRef]
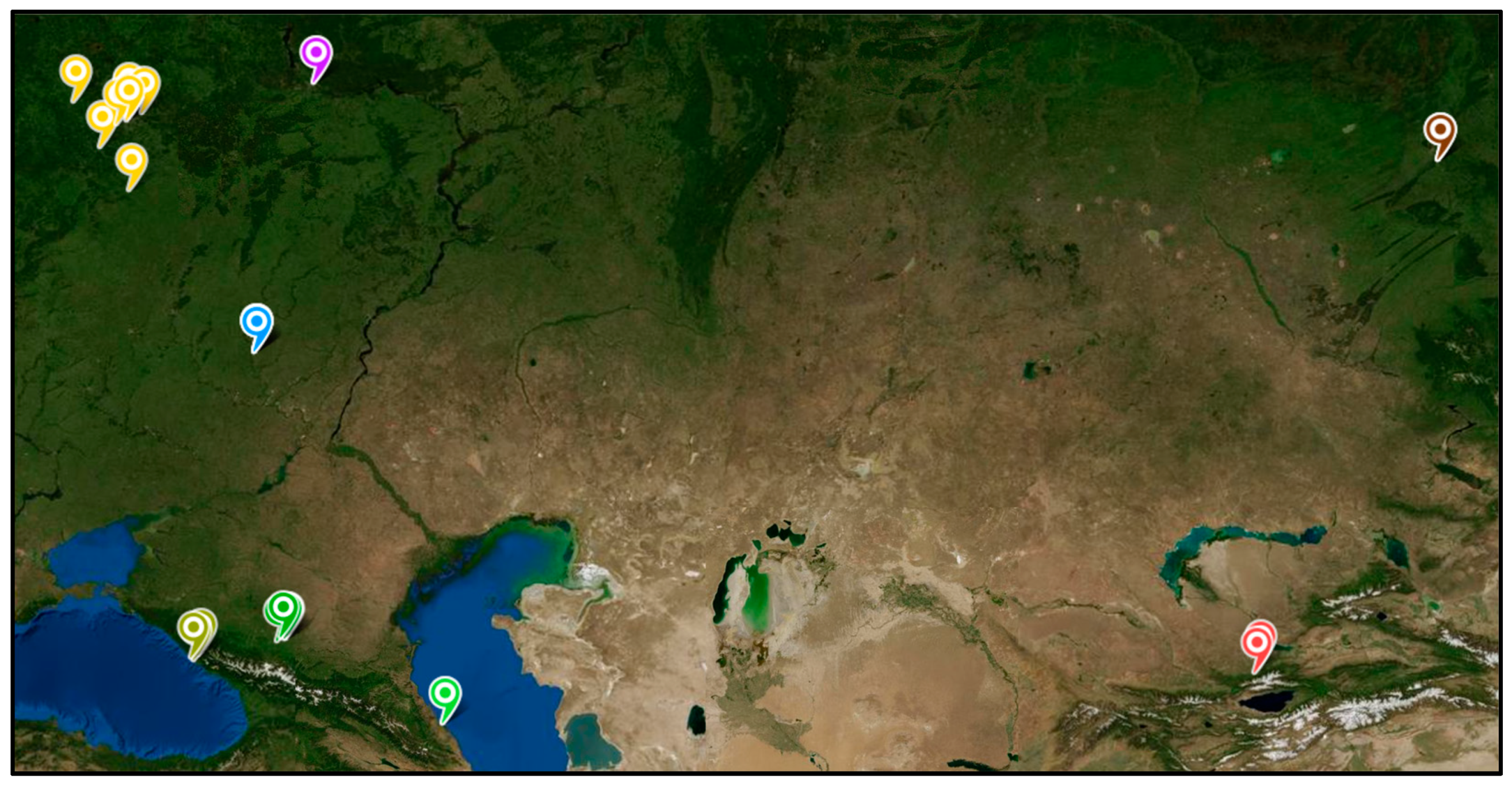
| Number | Growing region | Name | Coordinates | Sex of the tree |
|---|---|---|---|---|
| 1 | Central Federal District, Moscow | Center-s19 | 55.793039, 37.556753 | female |
| 2 | Central Federal District, Moscow | Center-s36 | 55.830375, 37.547010 | male |
| 3 | Central Federal District, Moscow | Center-s44 | 55.665872, 37.589880 | male |
| 4 | Central Federal District, Moscow | Center-s65 | 55.668454, 37.584296 | male |
| 5 | Central Federal District, Obninsk | Center-s89 | 55.093246, 36.618053 | female |
| 6 | Central Federal District, Moscow | Center-s111 | 55.674906, 37.558021 | male |
| 7 | Central Federal District, Moscow | Center-s163 | 55.750744, 38.061924 | female |
| 8 | Central Federal District, Moscow | Center-s193 | 55.668355, 37.614051 | female |
| 9 | Central Federal District, Moscow | Center-s199 | 55.528115, 37.196700 | female |
| 10 | Central Federal District, Tula | Center-s245 | 54.204123, 37.618254 | male |
| 11 | Central Federal District, Moscow | Center-s128 | 55.611070, 37.552098 | male |
| 12 | Central Federal District, Moscow** | Center-s228 | 55.969725, 35.691320 | female |
| 13 | Volga Federal District, Nizhny Novgorod | Volga-s9 | 56.349087, 44.059552 | male |
| 14 | Volga Federal District, Nizhny Novgorod | Volga-s24 | 56.346479, 44.061316 | male |
| 15 | Southern Federal District, Uryupinsk | South-s5 | 50.790670, 41.977279 | male |
| 16 | Southern Federal District, Uryupinsk | South-s10 | 50.813251, 41.992686 | female |
| 17 | Southern Federal District, Uryupinsk | South-s12 | 50.811208, 41.988316 | female |
| 18 | Southern Federal District, Uryupinsk | South-s14 | 50.810218, 41.979661 | female |
| 19 | Southern Federal District, Uryupinsk | South-s19 | 50.785039, 41.985022 | * |
| 20 | Southern Federal District, Uryupinsk | South-s21 | 50.787698, 41.985552 | female |
| 21 | North Caucasus Federal District, Pyatigorsk | Caucasus-P-s1 | 44.027603, 43.067865 | female |
| 22 | North Caucasus Federal District, Pyatigorsk | Caucasus-P-s2 | 44.027049, 43.063490 | female |
| 23 | North Caucasus Federal District, Pyatigorsk | Caucasus-P-s4 | 44.027473, 43.063530 | * |
| 24 | North Caucasus Federal District, Pyatigorsk** | Caucasus-P-s6 | 44.042940, 42.932274 | female |
| 25 | North Caucasus Federal District, Pyatigorsk** | Caucasus-P-s17 | 44.042521, 42.963468 | male |
| 26 | North Caucasus Federal District, Pyatigorsk** | Caucasus-P-s29 | 43.972696, 42.789123 | female |
| 27 | Southern Federal District, Sochi | Caucasus-S-s11 | 43.616446, 40.052978 | male |
| 28 | Southern Federal District, Sochi | Caucasus-S-s18 | 43.563056, 39.793006 | male |
| 29 | Southern Federal District, Sochi | Caucasus-S-s26 | 43.491915, 39.896625 | female |
| 30 | North Caucasus Federal District, Republic of Dagestan | Caucasus-D-s31 | 41.867246, 48.556359 | male |
| 31 | North Caucasus Federal District, Republic of Dagestan | Caucasus-D-s20 | 41.876808, 48.538710 | female |
| 32 | Republic of Kazakhstan, Almaty | Kazakhstan-s90 | 43.309556, 76.887625 | female |
| 33 | Republic of Kazakhstan, Almaty** | Kazakhstan-s54 | 43.164237, 76.760445 | female |
| 34 | Siberian Federal District, Novosibirsk** | Siberia-s13 | 54.820822, 83.120080 | male |
| 35 | Siberian Federal District, Novosibirsk** | Siberia-s25 | 54.819091, 83.117505 | female |
| 36 | Siberian Federal District, Novosibirsk** | Siberia-s200 | 54.819042, 83.114673 | female |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





