Introduction
Human serum sodium levels (SL) are tightly controlled, but individuals with traumatic brain injury (TBI) face an increased risk of dysnatremia [
1]. This is due to factors such as hyperosmolar treatment, diabetes insipidus, the use of fluids with high sodium content, and the choice of nutritional preparation by the provider. Some other factors are trauma-related, such as the occurrence of posterior pituitary dysfunction, and inappropriate water retention [
2,
3]. In TBI patients, the prevalence of hyponatremia and hypernatremia has been reported between 15-55% [
4,
5], and 30-50% [
6,
7], respectively. Both hypernatremia and hyponatremia have been individually linked to higher mortality and poorer outcomes in TBI cases [
4]. Fluctuations in SL as well as the absolute sodium concentrations can have significant pathophysiological impacts. Therefore, managing serum sodium is crucial, particularly for patients with elevated intracranial pressure.
Outside of the TBI context, recent studies have emphasized the importance of monitoring serum sodium variability over time to understand its relationship with patient outcomes. Evidence suggests that sodium level fluctuations contribute to increased mortality, independent of the actual sodium level [
8,
9]. This study investigates how variations in sodium levels (SV) and the overall SL affect clinical outcomes in TBI patients. Given the global prevalence of TBI and its substantial impact on physical and cognitive function, identifying early and reliable prognostic factors is essential to guide clinical decision-making, evaluation of quality of treatment, and medical resource allocation.
Method
Study Population
This is a retrospective, single-center study of patients with severe TBI admitted to a Level 1 trauma center in New York City from 2020 to 2023. This study has our center’s institutional review board (IRB) approval. Patients were identified based on the injury mechanism and AIS body region. We retrospectively collected data on previously managed patients encompassing both medical floors and ICU-level care capabilities. Ethics approval for contributing to this dataset was obtained locally. We studied patients admitted with severe TBI between January 1, 2020, and December 31, 2023, resulting in a total of 1,124 patients.
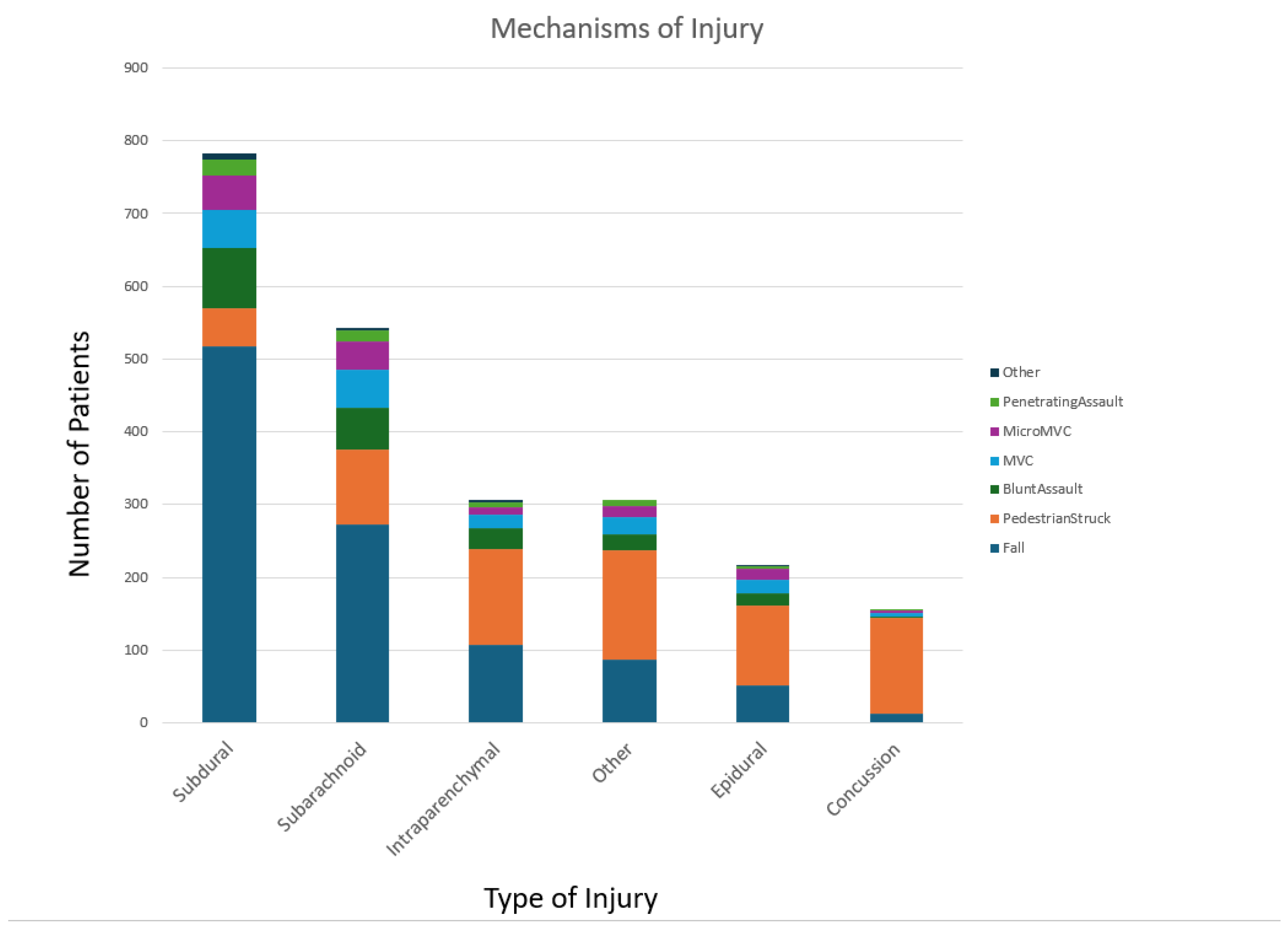
Inclusion criteria comprised patients with severe TBI, defined by an Abbreviated Injury Scale (AIS) of 3 or higher of the head and an International Classification of Diseases (ICD) injury description of an intracranial injury. The breakdown of the broad types of injury with subdural hemorrhage being the most prevalent, followed by subarachnoid, intraparenchymal, other injuries, epidural, and lastly concussions. Severe TBI is further defined by a Glasgow Coma Scale (GCS) score of 8 or less after resuscitation but before sedation, with an intracranial pressure (ICP) monitor in place for at least 72 hours. Exclusion criteria included patients with an ICD diagnosis of COVID-19 or those who died within 24 hours of arrival. Patients who were discharged or transferred and had SL collected were also included in the analysis.
Data Collection
In this study, demographic, clinical, and biochemical data were collected retrospectively. Baseline admission data included age, sex, GCS, and CT imaging, including that of the brain, which were required to calculate the AIS and determine the ICD injury description. Age ranges were divided into groups: pediatric (less than 18), young adults (ages 18 to 45), older adults (ages 46 to 75), and elderly (ages greater than 75). Sex was categorized as male and female.
We also investigated based on the types of injury- blunt versus penetrating, mechanism of injury- fall, pedestrian struck, blunt assault, motor vehicle accident (MVC), micro MVC, penetrating assault, and other, and broader classifications of intracranial injuries- subdural, subarachnoid, epidural, intraparenchymal, concussion. Further subset analysis was conducted based on a number of unique intracranial ICD-10 codes assigned to a patient.
The levels of various metabolites were obtained manually from charts at four pre-determined time frames of a patient’s hospital stay. These were hospital admission, ICU admission, ICU discharge, and then either death or hospital discharge. “Admission” was defined as the first measured level of a metabolite after admission to the trauma bay. “ICU admission” was defined as the first measured level of a metabolite after admission to the ICU while “ICU discharge” was the last measured level of a metabolite before arrival in a step-down or floor unit. “Hospital discharge” was the last measured value for a metabolite prior to discharge and “death” was the last measured value before the time of death. There were some unique cases where the lab value from one single blood draw was used for multiple time frames if it fit the above criteria.
Primary outcome variables in this data set were hospital length of stay, intensive care unit (ICU) length of stay, days requiring mechanical ventilation, and mortality. Statistical analysis was performed in both Excel using Visual Basic and R. A two-tailed T-test and single-factor ANOVA with significance levels of 0.05 were used to examine any potential differences in SL with demographic factors and injury patterns. To convert SL to a categorial variable for these analyses, patients were assigned a sodium range based on accepted levels in the literature. SL was categorized into the following ranges: extreme hyponatremia (less than 120 mEq/L), hyponatremia (120-135 mEq/L), normonatremia (135-145 mEq/L), hypernatremia (145-160 mEq/L), and extreme hypernatremia (>160mEq/L) [
10]. Primary outcome variables were studied using univariate linear regression models in R while subset analyses were performed on patients with one, two, three, and four or more assigned intracranial ICD-10 codes.
Results
There were multiple significant relationships found between Sodium Level (SL) and various prognostic factors and outcomes. In this study, an analysis of variance (ANOVA) was used to compare variance across means of distinct groups, and a linear regression analysis was used to predict outcomes based on dependent variables. We found statistically significant negative correlations between age and admission SL and ICU admission (day) SL (p = 8.79x10
-8, p = 0.000205). There was a positive correlation with ICU discharge levels (p = 0.0301) and no significance for hospital discharge or death. Differences in SL upon admission were statistically significant in four age ranges (pediatric, young adult, older adults, and elderly) (p = 2.394x10
-6). ICU admission (p = 0.00633), hospital discharge (p = 0.0266), and death (p = 0.0413) showed a significant difference, however, there was no statistical significance in ICU discharge (p=0.3358). No statistically significant difference was found in average SL during hospital stay in male versus female and blunt versus penetrating injury. The only exception was a difference in ICU discharge levels when comparing blunt versus penetrating injury (p = 1.304x10
-8) but the penetrating injury sample size was small which might have skewed the results as shown in
Table 1.
We compared ranges of SL (extreme hyponatremia, hyponatremia, normonatremia, hypernatremia, extreme hypernatremia) and found statistically significant differences in the sodium ranges at admission, ICU admission, and ICU discharge when compared to admission ISS scores and GCS scores.
Table 2 of this paper shows the two-tailed t-test comparing sodium ranges with ISS and GCS scores.
A linear regression analysis revealed a strong statistically significant positive correlation between ICU admission serum SL (SL) and Injury Severity Score (ISS) (p=6.927 X10
-9) and a negative correlation with GCS (p=1.7525x10
-11). Statistically significant differences were observed when comparing ICU admission SL to hospital length of stay (LOS) (p = 0.0367), ventilator days (p = 0.00201), ICU LOS (p=0.0233), and mortality (p = 0.000285).
Table 3. Shows the linear regression analysis comparing SL at 3-time frames (at admission, ICU admission, and ICU discharge) against outcome variables including subset analyses based on several diagnosed injuries (Hospital LOS, ICU LOS, Ventilator Days, and Mortality).
Linear regression analysis revealed a negative correlation between patient age and both admission SL and ICU admission SL. Furthermore, there was a negative correlation between ICU admission sodium and ICU discharge sodium with GCS scores.
When investigating the distinct types of injuries and the number of injury types using ANOVA, no statistically significant relationships were found between SL and the type of injury or the number of injury types. However, in a regression analysis examining the number of types of injuries, ICU admission sodium was positively correlated with ventilator days and mortality in patients with more than three types of injuries. ICU discharge sodium was positively correlated with mortality in patients with two, three, and more than three types of injuries. In patients with single injuries, discharge sodium was positively correlated with mortality in patients with subdural hematomas with p-values of 0.0002 (coefficient = 0.0141), respectively. Also, in single injuries, ICU admission sodium was positively correlated with mortality in patients with subdural hemorrhages with p-values of 0.0216 (coefficient = 0.0082) and negatively corrected with mortality in patients with subarachnoid hemorrhages with a p-value of 0.0342 (coefficient = -0.03395).
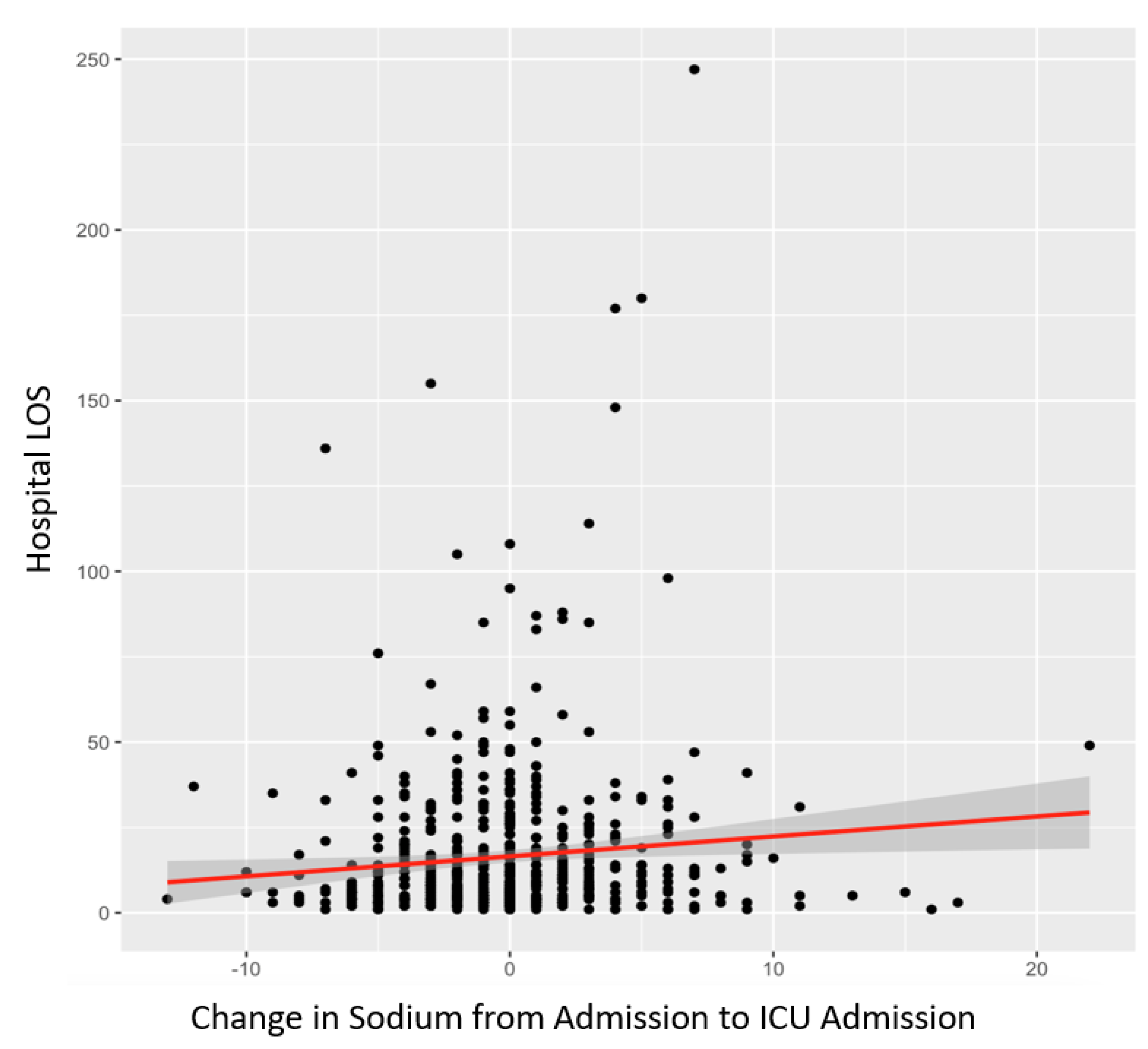
The sample sizes for subarachnoid, epidural, intraparenchymal, concussion, and other TBI groups were much smaller within the single injury pattern group; therefore, the regression analysis may have resulted in erroneous correlations. Finally, the variation in SL from hospital admission to ICU admission was positively correlated with hospital LOS (p=0.0148) as shown in
Figure 1, ventilator days (p=0.0261), and mortality (p=6.9x10
-8) as shown in
Table 4.
Figure 2 is a linear regression analysis between change of sodium from admission to ICU admission on hospital length of stay. There is a positive correlation with a p-value of 0.0148.
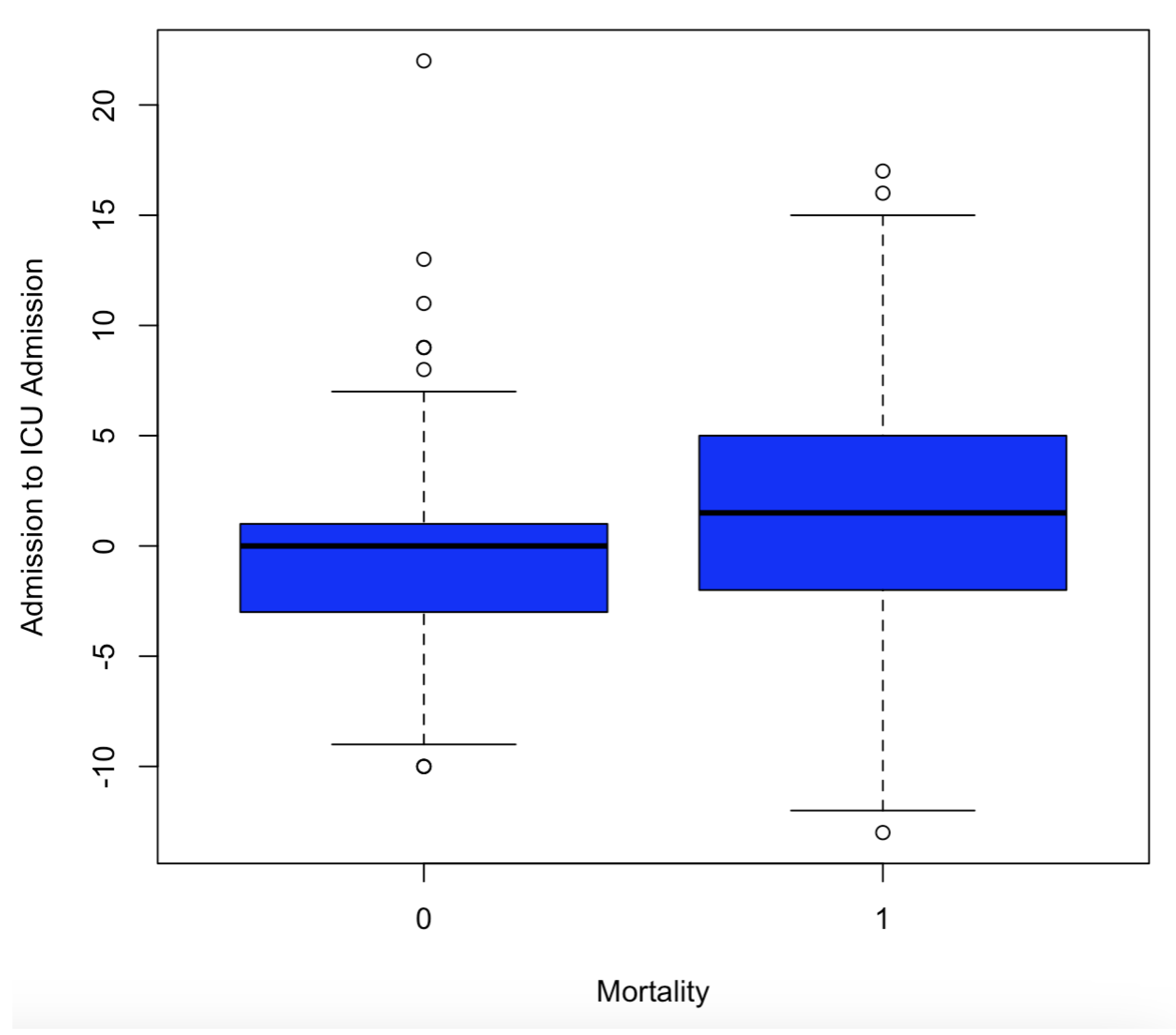
Figure 3 shows the Box plot of change in sodium from admission sodium to ICU admission sodium to mortality.
Table 4 represents the variation in SL from ICU admission to discharge was positively correlated with increased mortality (p-value = 1.1 × 10-5, coefficient = 0.0108). It also shows a linear regression analysis comparing changes in SL (changes in SL from hospital admission to ICU admission, as well as changes from ICU admission to discharge) to outcome variables (hospital LOS, ICU LOS, ventilator days, and mortality).
Discussion
Key Findings
This single-center study aimed to investigate the intricate relationship between SL at various points during hospitalization and the prognostic outcomes of patients suffering from TBI (TBI). Our analysis provided compelling evidence that hypernatremia is significantly associated with poorer outcomes in this vulnerable patient population. Hypernatremia, characterized by elevated SL in the blood, is a critical contributor to morbidity and mortality in patients with neurological diseases. For instance, in individuals with subarachnoid hemorrhage, hypernatremia has been linked to increased mortality rates and worse overall outcomes [
11]. Additionally, research has shown that hypernatremia in a heterogeneous group of patients treated in intensive care units is associated with higher rates of morbidity [
12].
Severe TBI (TBI) frequently results in complex disturbances in serum SL and water regulation, complicating patient management. Several factors influence SL, including the use of osmotherapy, the administration of high-sodium fluids, and the selection of nutritional preparations—all determined by the treatment provided [
2]. The management of SL can be particularly challenging due to the multifactorial nature of TBI. For instance, patients often present with a combination of cerebral edema, altered fluid balance, and endocrine dysfunction, all of which can influence sodium homeostasis. Additionally, some factors are related to the trauma itself. For example, posterior pituitary dysfunction can occur because of TBI, leading to a deficiency in the secretion of antidiuretic hormone (ADH). This deficiency results in excessive water loss and, subsequently, hypernatremia. Conversely, overproduction of ADH can cause water retention, heightening the risk of hyponatremia [
2,
3].
The exact etiology of hypernatremia in TBI patients is multifaceted and not strictly defined. It may arise from various causes, including hyperosmolar therapy, hypovolemia, insensible free water losses, or a high sodium load from intravenous fluids, nutritional feeds, or medications. This complexity necessitates a careful assessment of each patient's unique circumstances. Furthermore, hypothalamic dysfunction that does not lead to overt diabetes insipidus may also contribute to hypernatremia following TBI [
12]. Such dysfunction could be a result of direct neuronal injury or secondary to the systemic inflammatory response often seen in TBI, which further complicates fluid and electrolyte management.
The prevalence of hyponatremia and hypernatremia among TBI patients has been reported to range between 15% and 55% [
4,
5] and 30% to 50% [
6,
7] respectively. Notably, studies that have employed frequent sodium measurements indicate that as many as 64% of TBI patients experience hypernatremia [
13]. This alarmingly high prevalence underscores the clinical relevance of our findings and emphasizes the urgent need for increased awareness among healthcare providers regarding the implications of SL in the management of TBI. Continuous monitoring and early detection of sodium imbalances may be crucial for improving patient outcomes, as even mild disturbances can significantly affect neurological recovery and overall health.
Furthermore, our study revealed a strong correlation between SL recorded at the time of ICU admission and both the ISS and GCS. These two metrics are well-established indicators of injury severity and prognosis in TBI patients. Our findings support the notion that higher SL upon ICU admission is associated with greater injury severity and poorer neurological function. This relationship is particularly important, as it establishes SL as a potential predictive marker for adverse outcomes in TBI patients, aligning with previous literature that emphasizes the significance of early interventions based on initial lab results. Clinicians may need to consider SL alongside traditional scoring systems when assessing patient prognosis and determining treatment plans.
In addition to our primary findings, we discovered that increases in SL occurring from the time of initial hospital admission to the time of transfer to the ICU were significantly associated with several adverse clinical outcomes. Specifically, these increases correlated with prolonged hospital stays, extended durations of mechanical ventilation, and heightened mortality rates among patients. This notable association underscores the potential of SL to serve as critical prognostic indicators. By recognizing the implications of sodium fluctuations, healthcare professionals can gain valuable insights that may profoundly influence treatment decisions and patient management strategies within clinical settings.
The observed relationship between sodium level fluctuations and adverse outcomes strongly suggests that targeted interventions, implemented early—such as in the emergency room—aimed at normalizing these SL, could lead to improved prognoses for patients with TBI (TBI). For instance, implementing targeted therapies designed to effectively manage conditions such as hypernatremia or hyponatremia could optimize recovery trajectories for TBI patients. This approach has the potential not only to enhance individual patient outcomes but also to alleviate the overall burden on healthcare systems by reducing complications and associated healthcare costs.
By identifying these crucial relationships, our study contributes significantly to a deeper understanding of how sodium handling in TBI patients can inform better clinical practices. This understanding can lead to the development of strategies aimed at improving patient outcomes. Given the inherent complexities surrounding fluid and electrolyte management in patients with TBI, there is a compelling need for further research. Specifically, future studies should focus on the development and implementation of standardized protocols for sodium monitoring and intervention strategies to enhance patient mortality outcomes.
Implications of Study Findings
The implications of our study's findings present a significant challenge to the traditional paradigms that currently guide the management of traumatic brain injuries (TBI), particularly regarding the administration of hypertonic saline. Historically, hypertonic saline has been widely regarded as a beneficial treatment option for reducing intracranial pressure, which is a crucial factor in the management of TBI. This treatment has been integrated into clinical practice based on its perceived ability to effectively manage cerebral edema and improve patient outcomes. However, our findings, in conjunction with emerging research in the field, suggest that it may be time for a reevaluation of this long-standing approach. While hyperosmolar therapy can provide certain benefits in treating cerebral edema, the potential morbidity associated with hypernatremia—an elevation of SL in the blood—must be carefully weighed against these advantages. This balance necessitates frequent monitoring of serum SL during hyperosmolar therapy to prevent rapid and substantial increases in serum sodium, which can lead to serious complications [
14].
Furthermore, the landscape of TBI management is evolving, and there is a growing recognition of the complexity inherent in treating these patients. Recent studies have indicated that patients with TBI who received hypertonic saline did not demonstrate a statistically significant improvement in their likelihood of achieving favorable outcomes at discharge or even at the six-month follow-up when compared to those who received standard crystalloid solutions [
14]. This finding is particularly noteworthy as it suggests that hypertonic saline may not effectively reduce mortality rates; however, it is important to acknowledge that treatment with hypertonic saline did lead to a shorter LOS in the ICU [
14]. While a reduced LOS can be beneficial from a healthcare resource perspective, it raises questions about the overall efficacy of hypertonic saline in improving long-term patient outcomes.
Our data further proposes that a narrow therapeutic window exists for clinical interventions aimed at enhancing outcomes in critically ill patients with TBI. The observation that higher SL at the time of ICU admission, along with significant increases in SL during hospitalization, correlates with adverse outcomes presents a critical opportunity for targeted interventions. This insight emphasizes the need for a more nuanced approach to managing SL in TBI patients, especially early in the hospital course, advocating for treatment strategies tailored to individual patient responses and clinical conditions, such as expedited lab draws in emergency rooms. It is essential to recognize that TBI patients are not a homogeneous group; their clinical profiles can vary widely based on factors such as age, severity of injury, comorbid conditions, and individual responses to treatment.
This variability raises several vital questions for clinical practice. For example, could therapies specifically designed to lower SL during the critical period leading up to ICU admission significantly improve patient outcomes? Our results suggest that such interventions could lead to reductions in hospital length of stay, decreased reliance on mechanical ventilation, and lower overall mortality rates. This possibility underscores the necessity for ongoing dialogue and research concerning management protocols for TBI patients, as reevaluating sodium management could play a key role in enhancing patient-centered outcomes.
Moreover, understanding sodium's role extends beyond the mere measurement of its levels; it encompasses a broader physiological context in which electrolyte balance can profoundly affect neurological outcomes. The intricate interplay between SL and brain function indicates that careful monitoring and management of serum sodium may not only influence immediate clinical outcomes but also impact long-term recovery trajectories for patients with TBI [
12]. For instance, dysregulated SL can lead to neurological dysfunction, affecting cognitive recovery and overall rehabilitation success. This underscores the importance of considering SL as an integral component of a comprehensive approach to TBI management, which should also incorporate both medical and rehabilitative strategies to optimize recovery [
12].
In the context of trauma care, the roles of trauma teams and emergency room doctors are crucial, as they are often the first to assess and manage patients upon arrival, requiring rapid decision-making. These teams work closely with neurosurgeons to perform critical interventions like fluid resuscitation and medication administration, making effective communication and collaboration essential for optimizing patient outcomes, particularly for those with traumatic brain injuries (TBI). Once admitted, a multidisciplinary approach involving trauma physicians, neurosurgeons, intensivists, nursing staff, and rehabilitation specialists is vital for developing individualized treatment plans that address the complex factors influencing SL and overall patient care, ultimately leading to improved monitoring and interventions [
12].
In conclusion, our study highlights the urgent need to reevaluate current sodium management protocols for TBI patients, emphasizing its critical role in neurological health and patient outcomes. By fostering a dynamic understanding of sodium's impact, we can develop innovative, evidence-based treatment approaches that improve care quality. Collaborative efforts among clinicians, researchers, and healthcare systems are essential for establishing standardized protocols for sodium monitoring and intervention strategies, with future research focusing on multicenter studies to validate our findings. This comprehensive approach not only aims to enhance immediate survival but also prioritizes long-term recovery and quality of life for individuals with traumatic brain injuries, ultimately improving clinical outcomes and patient care.
Strengths and Limitations
While our study offers significant insights into the relationship between SL and outcomes in TBI (TBI) patients, it is essential to acknowledge certain limitations inherent in our research design that may affect the interpretation of our findings. Being a single-center study, the external validity of our results may be restricted, raising questions about their generalizability across diverse healthcare settings and populations. This limitation is particularly important in the context of TBI, as different institutions may employ varying protocols, treatment approaches, and patient management strategies. Consequently, the specific practices and outcomes observed in our study may not fully reflect those found in other clinical environments, which could impact the applicability of our findings.
Additionally, our study was conducted retrospectively, which introduces its own set of limitations. Retrospective studies often face potential biases related to data collection and interpretation, particularly concerning the accuracy and completeness of medical records. However, it is important to note that SL were objectively collected and consistently measured during patient hospitalization, allowing for a reliable analysis of these critical data points. We examined several important prognostic outcomes, such as mortality rates, hospital LOS, ICU LOS, and the number of days on mechanical ventilation among TBI patients. This comprehensive assessment helps to minimize concerns regarding selection bias and ascertainment bias, lending greater credibility to our findings.
A unique aspect of our study is its consideration of both the Injury Severity Scale (ISS) and the GCS as measures for assessing mortality. These metrics provide robust patient-centered outcome measures that enhance the validity of our findings. However, it is also important to recognize that our study did not include direct measurements of neurological outcomes for the patients. Such assessments would require longer follow-up periods, potentially extending from six months to one year after hospital discharge. This limitation highlights the need for future research to explore the long-term implications of SL on neurological recovery and overall quality of life for TBI patients. Longitudinal studies could offer valuable insights into how variations in sodium management impact recovery trajectories over time.
Furthermore, as our study was not designed as a randomized controlled trial, we must exercise caution when drawing conclusions about causality. While our findings suggest significant associations between SL and various patient outcomes, definitive causal relationships cannot be established based solely on this retrospective analysis. Correlation does not imply causation, and the relationships observed may be influenced by confounding variables not accounted for in our study. Future research, particularly prospective and randomized controlled trials, will be crucial to determine the directionality and underlying mechanisms of these associations. Such studies could help clarify whether interventions aimed at normalizing SL can lead to improved outcomes for TBI patients.
Given our findings, the importance of frequent monitoring of SL in TBI patients cannot be overstated. Rather than studying SL and changes at three specific points during hospitalization, a more dynamic approach involving frequent checks—such as every six hours—would provide more relevant data to study the impact of sodium level changes on patient outcomes. In practice, more frequent sodium checks allow for timely adjustments to therapies based on the patient's fluctuating SL, helping to prevent complications such as hypernatremia and hypovolemia. Routine assessments can facilitate proactive management strategies that enhance patient safety and improve overall outcomes.
In summary, while our study contributes valuable knowledge to the field of TBI management, the limitations inherent in our research design warrant careful consideration. By acknowledging these limitations, we can better understand the context of our findings and highlight the need for further investigation. Future studies should aim to address these gaps by employing larger, multicenter cohorts and utilizing rigorous methodologies that can strengthen the evidence base for sodium management in TBI patients. Ultimately, addressing these limitations will be key to advancing our understanding and improving clinical practices related to SL in this vulnerable population.
Future Directions
To build on our findings, further research is warranted to explore the intricate relationship between SL and various patient-centered outcomes in TBI (TBI) patients. Our study highlights the significant associations between sodium fluctuations and adverse outcomes, yet it raises several important questions that need to be addressed through more extensive investigations. Prospective studies are necessary to validate our findings in larger cohorts and diverse populations, allowing for more robust conclusions regarding the impact of sodium management on TBI outcomes. By including a broader spectrum of patients, researchers can better understand how different demographics and comorbidities might influence SL and, consequently, patient recovery.
Additionally, studying the effects of targeted interventions aimed at modulating SL could provide invaluable insights into optimizing TBI care. For instance, future studies could investigate the efficacy of specific treatment protocols designed to maintain sodium within an optimal range, thereby minimizing the risks associated with both hypernatremia and hyponatremia. These interventions may include protocols for fluid management, dietary sodium intake, and pharmacological approaches to regulate SL more effectively. By identifying which strategies yield the best outcomes, healthcare providers can refine their treatment plans and potentially improve patient prognoses.
Multicenter studies could facilitate a broader understanding of the complex pathophysiology associated with TBI and the role that SL play in influencing recovery. Collaborating across institutions would enable researchers to gather more extensive data, enhancing the generalizability of findings and informing clinical guidelines. Such collaborations could also allow for the pooling of resources, standardizing protocols for sodium monitoring and management, and creating a unified framework for evaluating treatment outcomes. By doing so, we can foster a more comprehensive understanding of how sodium handling affects recovery trajectories in diverse patient populations.
Moreover, it would be beneficial to investigate the long-term implications of sodium level management on neurological outcomes and overall quality of life for TBI patients. Longitudinal studies could help elucidate how fluctuations in SL during hospitalization influence not just immediate recovery but also long-term cognitive and functional outcomes. This comprehensive approach to studying TBI can reveal critical insights that extend beyond survival rates to include quality of life considerations, thus enriching our understanding of what it means to recover from such injuries.
In conclusion, our study advocates for a change in basic assumptions in TBI management, encouraging clinicians to view SL not merely as isolated laboratory values but as critical indicators that can guide treatment strategies. As our understanding of the interplay between SL and patient outcomes continues to evolve, it is imperative to integrate these insights into clinical practice to enhance care for individuals suffering from traumatic brain injuries. Clinicians must remain vigilant in monitoring SL frequently, utilizing dynamic assessment strategies, and adapting treatment plans based on real-time data. Through ongoing research and collaboration, we can aim for better patient outcomes and quality of care in this critical area of medicine.
Conclusions
In summary, this single-center study has demonstrated a significant association between hypernatremia and poorer outcomes in patients with TBI (TBI). Our findings indicate that SL upon ICU admission is correlated with both the ISS and the GCS. Specifically, elevated SL at admission was linked to adverse hospital outcomes, including prolonged lengths of stay (LOS), extended ICU stays, increased days of mechanical ventilation, and higher mortality rates.
Moreover, our results suggest that variability in serum SL is independently associated with mortality throughout the hospital stay, irrespective of the absolute serum sodium concentration. This highlights the importance of not only the sodium level itself but also its fluctuations during treatment. Further prospective investigations are essential to confirm the clinical significance of both SL and their variability in larger cohorts of TBI patients. Additionally, exploring whether interventions aimed at mitigating sodium variability can lead to improved patient outcomes would be valuable. A multicenter study would also provide a more comprehensive understanding of the complex pathophysiology associated with TBI and help validate our findings across diverse patient populations. This broader perspective could inform clinical practices and enhance patient care for this vulnerable group.